Abstract
The aim of this study was to evaluate repeatability, reproducibility, and agreement of three commonly used tonometers in animal research (TonoLab, TonoVet, and TonoPEN AVIA) in a cohort of 24 rabbits. Additionally, the impact of sedation on IOP was investigated in 21 New Zealand White rabbits with the TonoVet tonometer. Repeatability was determined using the coefficient of variation (CoV) for two observers. For the TonoLab (6.55%) and TonoVet (6.38%) the CoV was lower than for the TonoPEN AVIA (10.88%). The reproducibility was highest for the TonoVet (0.2 ± 3.3 mmHg), followed by the TonoLab (0 ± 12.89 mmHg) and lowest for the TonoPEN AVIA (− 1.48 ± 10.3 mmHg). The TonoLab and TonoVet showed the highest agreement (r = 0.85, R2 = 0.73). After sedation, a significant IOP reduction (often > 25%) was observed. Our results show that among the three tonometers tested, the TonoVet tonometer is best for use in rabbits while the TonoLab should be avoided. The impact of sedation on IOP was substantial and should be taken into account during experimentation.
Subject terms: Medical research, Sensors and probes, Visual system
Introduction
Increased intraocular pressure (IOP) and fluctuations in IOP are important characteristics of glaucoma. Repeatable and reproducible objective measurement of IOP are of great importance for disease management. Animal models are routinely used to study underlying pathophysiology and are used in the development of new glaucoma therapies. For example, in glaucoma animal models the anterior chamber of the eye can be injected with microbeads to block the outflow of aqueous humor (AH), thus increasing IOP1–3.
IOP in animal experiments can be measured by manometry or tonometry. Although manometry is the most accurate method, it is invasive and requires trained personnel along with expensive and specialized equipment. Tonometry is an indirect non-invasive measuring method that can be divided into three different subcategories: indentation, applanation, and rebound tonometry. Indentation (also known as impression) tonometry uses a plunger to measure the depth of corneal indentation, as used in the Schiøtz tonometer4. In applanation tonometry, the force needed to flatten the cornea is used to calculate IOP. This method is routinely used in regular clinical care, where Goldmann applanation tonometry (GAT) is the gold standard5,6. Rebound tonometry determines the IOP via induction of a current generated by the rebound effect of a small probe onto the cornea. The use of rebound tonometry (e.g. iCare tonometers) is gaining popularity in the clinic, especially for children and non-cooperative patients, as the tonometers are handheld devices and no topical anesthesia is required for the procedure7.
In animal research, the most commonly used tonometers are the TonoLab (intended for mice and rats), TonoVet (intended for dogs, cats, and horses) and TonoPEN (intended for all animals) (Table 1). Although none of these tonometers have been specifically designed for rabbits and there may be substantial differences between the devices, they are commonly used on rabbits for research purposes.
Table 1.
Literature overview of the use of different tonometers in animal research.
| Animals | ICare TonoLab | ICare TonoVet (Plus) | Reichert TonoPEN (XL, VET, or AVIA) |
|---|---|---|---|
| Frogs | Determination of reference IOP23,24 | Determination of reference IOP23–25 | Determination of reference IOP25 |
| Turtles | Tonometer validation26 | Tonometer validation26,27 | |
| Mice |
Effect of general anesthesia on IOP28 Effect of CCT on IOP33 |
Glaucoma research31 |
|
| Chinchillas | Tonometer validation35 |
Tonometer validation35 Determination of reference IOP36 |
Tonometer validation35 |
| Rats |
Tonometer validation29,30,37–39 Effect of general anesthesia on IOP37 |
Tonometer validation30,34,39–41 Effect of general anesthetics on IOP42,43 Circadian variation44 |
|
| Guinea pigs |
Determination of reference IOP45 Effect of topical drugs on IOP46 |
||
| Ferrets | Determination of reference IOP47 | ||
| Hedgehogs | Determine prevalence of ocular diseases48 | Determine reference IOP49 | |
| Rabbits |
Effect of topical drugs on IOP55 |
||
| Dogs |
Effect of CCT on IOP60 |
Effect of CCT on IOP60 Effect of topical drugs on IOP66 Effect of age on the IOP67 |
|
| Cats |
Tonometer validation69 Effect of general anesthetics on IOP70 Effect of topical drugs on IOP55 |
Glaucoma research65 Tonometer validation69 |
|
| Birds |
Determination of reference |
Tonometer validation71 |
|
| Cows | Determination of reference IOP78 | ||
| Horses and donkeys |
Determination of reference IOP79 Effect of endurance training on IOP80 |
Determination of reference IOP79 | |
| Pigs | Tonometer validation81 | Tonometer validation81 | |
| Alpaca’s | Determination of reference IOP82 | Determination of reference IOP82 | |
| Goats and sheep | Determination of reference IOP83 | ||
| Non-human primates |
Determination of reference IOP86 |
Tonometer validation85 Determination of reference IOP86 Effect of general anesthetics on IOP87 Glaucoma research88 |
CCT, central corneal thickness; IOP, intraocular pressure.
Therefore, we wanted to investigate which tonometer is most suitable for research with rabbits. The TonoLab and TonoVet are both rebound tonometers, whereas the TonoPEN is an applanation tonometer. However, it is unknown which tonometer has the best repeatability and reproducibility when used by multiple observers. Biomechanical factors may also affect IOP readings, such as corneal thickness and stiffness8–10, mental stress11–14, circadian rhythm15,16, and the type of (e.g. general) anesthesia or sedation17–21. The use of sedatives is common practice in animal studies and clinical procedures22, but the effect of injectable sedatives on IOP has not been fully characterized. Furthermore, different absolute IOP values are commonly observed when various tonometers are compared. Hence, the aim of this study was to compare the repeatability, reproducibility and agreement of the TonoLab, TonoVet and TonoPEN AVIA tonometers, together with investigating the effect of the injectable sedative medetomidine (an α2 adrenergic agonist) on rabbit IOP.
Materials and methods
Animals and animal care
Animal procedures were conducted according to the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Visual Research, the Animal Research: Reporting of In Vivo Experiments (ARRIVE) 2.0 guidelines89, and the Guidelines of the Central Laboratory Animal Facility of Maastricht University. All protocols were approved by the Central Authority for Scientific Procedures on Animals (CCD, Den Haag, NL) and were in accordance with the European Guidelines (2010/63/EU) (Approved Dutch license number: AVD107002017829 and AVD1070020197464).
New Zealand White (NZW) rabbits (2.0 kg–2.5 kg, males and females) (Envigo (Horst, NL and Bicester, UK) and Charles River (Ecully, FR)) were group housed (maximum 7 animals per cage, males and females separated), and maintained under controlled conditions of temperature and humidity on a 12 h:12 h light–dark cycle. The rabbits had ad libitum access to water and 100 g dried chow per animal and all had a two-week acclimatization period before the start of the experiments. All rabbits were normotensive. At the end of the experiment the rabbits were euthanized with an overdose of pentobarbital sodium, 200 mg/kg (Euthasol 20, Produlab Pharma B.V., NL), intravenously injected.
Animals
24 NZW rabbits were used (12 males) for the IOP part of the study (AVD107002017829), whereas 21 NZW rabbits (10 males) were used for the sedation part (AVD1070020197464). The rabbits were trained for one week to get used to the restrainer and the IOP measurements. At the start of each experiment, the rabbits were intra muscularly (IM) sedated using medetomidine (0.5 mg/kg) (Sedator, A.S.T. Farma B.V., Oudewater, NL). Prior to the TonoPEN AVIA measurement, the eye was topically anesthetized with 0.4% Oxybuprocaine hydrochloride solution (MINIMS, Bausch & Lomb Pharma, Brussels, BE). After the measurements, the animals were recovered using 1 mg/kg i.m. atipamezole (Antisedan, ORION pharma, Mechelen, BE). For both studies, the left eye of the rabbits was used.
Tonometers
IOP was measured using the iCare TonoLab (iCare Finland Oy, Vantaa, FI) (in rat setting) (Fig. 1a), followed by the iCare TonoVet (iCare Finland Oy, Vantaa, FI) (in dog/cat setting) (Fig. 1b) and finally, Reichert TonoPEN AVIA (AMETEK Inc., Unterschleißheim, DE). An Ocu-Film tip-cover was used for the TonoPEN AVIA (Fig. 1c). The TonoPEN AVIA was used last, due to the potential effect of topical anesthesia on the TonoVET and TonoLab90.
Figure 1.
Measuring IOP in a rabbit using different tonometers. (a) TonoLab (rebound tonometer), (b) TonoVet (rebound tonometer), (c) TonoPEN AVIA with a single-use blue Ocu-Film tip-cover (applanation tonometer).
Repeatability, reproducibility, and agreement of three tonometers
To investigate the repeatability of IOP measurements, defined as the ability of the observer to produce similar results time after time, all measurements were performed in triplicate91. The average of six readings is reported by the tonometer. According to the manufacturer’s instructions, IOP measurements with a repetition deviation ≥ 1.0 mmHg (TonoLab and TonoVet) or a repetition confidence lower than 90% (TonoPEN AVIA) were discarded and the measurements were then repeated. The TonoLab, TonoVet, and TonoPEN AVIA had a detection limit of 7–60 mmHg, 10–60 mmHg, and 5–55 mmHg, respectively. Measurements were performed at baseline, 4 h, 8 h, 24 h, 4 days, 7 days, 14 days, 21 days, and 28 days. N equals the number of animals times the number of time points.
Reproducibility (also known as interobserver reproducibility) was defined as the ability to produce the same results for IOP measurements of identical samples under the same conditions by two different observers. Agreement (also known as intraobserver reproducibility) was defined as the ability of one observer to produce the same results of IOP in identical samples using different tonometers.
Effect of sedation on IOP
IOP was measured using the TonoVet tonometer before (awake) and after sedation. The TonoVet was selected based on the results obtained in paragraph 2.2. First, the IOP of the left eye was measured sixfold. Rabbits were then IM sedated with medetomidine (0.5 mg/kg). Within 15 min after induction of sedation, the IOP of the left eye was measured again sixfold. Measurements were performed 1, 5, 7, 11, 15, 25, and 40 days after acclimatization.
Sample size and statistical analysis
Sample size was determined using Meads resource equation92. IOP measurements were performed as part of another study93, hence the deviation in animal groups. All observed data were paired data between the two observers. Values were presented as mean IOP ± standard deviation (SD) for observer 1, observer 2, and both. To examine repeatability, IOP measurements were evaluated by coefficient of variation (CoV) as a normalized SD, as shown in;
A smaller CoV means better repeatability. A CoV < 10% was indicative of good repeatability and a CoV < 5% of very high repeatability94. The CoV was calculated per measurement with the average providing a mean CoV.
Reproducibility was visualized by plotting mean values of observer 1 over observer 2 and calculating a linear regression line with 95% confidence interval (CI). Pearson’s correlation analysis was applied between both observers, followed by Bland and Altman analysis95,96. The Bland and Altman analysis compares the difference of the measurements versus the mean. Agreement of the different tonometers was also visualized through this method.
In calculating the influence of sedation, a two-way repeated measures ANOVA test was performed with Bonferroni correction for multiple testing to compare sedated to awake situations. In addition, the repeatability of measurements in sedated animals were plotted as Bland and Altman plots, including the difference between the measurement and mean along with the percentage of equality (agreement, lower is better) between the values and mean.
Tests were performed using GraphPad Prism version 9 (GraphPad Software inc., San Diego, CA, USA).
Results
Repeatability
An overview of the IOP results for all three tonometers, performed by both observers, is shown in Table 2. Mean IOP measured by the TonoLab was approximately three times higher for both observers compared to the TonoVet and TonoPEN AVIA. Both observers showed good repeatability (CoV < 10%) by using the TonoLab and TonoVet, while TonoPEN AVIA use resulted in poorer repeatability.
Table 2.
Summary of IOP results of both observers. Measurements performed in triplicate, IOP expressed in mmHg.
| TonoLab | TonoVet | TonoPEN AVIA | |
|---|---|---|---|
| Observer 1 | |||
| n | 166 | 94 | 164 |
| Mean IOP (SD) | 37.00 (14.84) | 11.41 (3.98) | 11.76 (3.73) |
| Median IOP (25% PCTL − 75% PCTL) | 34.00 (26.92–44.25) | 11.00 (9.00–12.75) | 11.67 (10.00–13.33) |
| CoV% | 6.55 | 6.38 | 10.88 |
| Observer 2 | |||
| n | 166 | 94 | 164 |
| Mean IOP (SD) | 37.00 (14.24) | 11.38 (3.71) | 13.24 (5.78) |
| Median IOP (25% PCTL − 75% PCTL) | 34.83 (27.92–44.00) | 10.67 (9.33–13.08) | 12.00 (10.00–14.25) |
| CoV% | 7.04 | 7.08 | 14.78 |
Coefficient of variation (CoV), standard deviation (SD), percentile (PCTL).
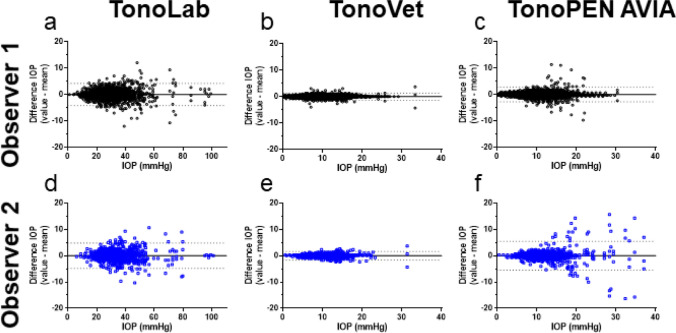
The repeatability of IOP measurements for each tonometer and for each observer was visualized in a Bland and Altman plot, showing the difference between the individual values and the mean for repeated measurements (Fig. 2). The smallest deviation was observed for the TonoVet by both observers (1.34 and 1.65 for observer 1 and observer 2, respectively, Fig. 2b,e, dashed lines). The TonoLab showed a deviation of 4.17 and 4.81 for observer 1 and observer 2, respectively (Fig. 2a,d). Observer 1 showed a lower deviation than observer 2 when using the TonoPEN AVIA (2.76 vs 5.50) (Fig. 2c,f). This higher deviation of observer 2 could be caused by the increased scattering in higher IOP values.
Figure 2.
Repeatability of IOP measurements in tonometers (TonoLab (a,d), TonoVet (b,e), and TonoPEN AVIA (c,f)) for both observers. Difference per measurement (y-axis) is plotted over the mean IOP (x-axis). Dotted lines represent 1.96 times the SD (95% confidence interval).
When plotted over time (Fig. 3a,d), the repeatability of the TonoLab remained stable for both observers. For the TonoVet (Fig. 3b,d), the repeatability of observer 1 was improved over time (measurement 1–300 showed good repeatability (CoV < 10%) while measurement 301–400 showed very high repeatability (CoV < 5%)).This trend was similar for observer 2. Furthermore, during the earlier IOP measurements with the TonoPEN AVIA, more values were outside the 95% confidence interval (limit of agreement) for observer 2 (see Fig. 3c, and this correlated with the higher CoV% for measurement 1–100 (see Fig. 3d)). In addition, CoV% of IOP measurements from observer 2 decreased from 18.25% (measurement 1–100) to 8.28% (measurement 401–500) (Fig. 3d) indicating a learning curve when using the TonoPEN AVIA (observable by the funnel structure in Fig. 3c).
Figure 3.
Repeatability of IOP measurements plotted over time. Intra-observer difference in IOP for the (a) TonoLab, (b) TonoVet, and (c) TonoPEN AVIA. (a–c) Dotted lines represent 1.96 times the SD (95% confidence interval (CI)). (d) Table summarizing the coefficient of variation (CoV) per range of measurements (per 100). Measurement #1 is the first measurement taken with the device, whereas #400 is the 400th measurement. na; not applicable.
Reproducibility
Figure 4 shows the reproducibility of the different tonometers of each observer. The TonoLab and TonoVet displayed a very strong correlation between observers (r = 0.90, p < 0.0001, R2 = 0.8, for both Fig. 4a,b, respectively), while the TonoPEN AVIA showed a moderate correlation (r = 0.45, p < 0.0001, R2 = 0.2 (Fig. 4c)).
Figure 4.
Reproducibility of different tonometers. (a–c) Show scatter plots with linear regressions (dashed lines is the 95% confidence interval (CI)). (d–f) Show a Bland and Altman plot expressing the difference of measurements by the observers plotted over the mean of the observers.
Both observers obtained similar results with the TonoLab (a bias of 0.0 with a deviation of ± 12.89 (Fig. 4d)). IOP values measured by observer 2 were on average 0.2 mmHg lower than those measured by observer 1 using the TonoVet, (a bias of 0.2 with a deviation of ± 3.3 (Fig. 4e)). Furthermore, IOP values measured by observer 1 were on average 1.48 mmHg lower than those measured by observer 2 with the TonoPEN AVIA (bias of − 1.48 with a deviation of ± 10.3 (Fig. 4f)). The negative bias was mainly caused by the large difference between both observers in the higher IOP values, as shown by the linear regression line (R2 = 0.20) in the plot.
Agreement between different tonometers
Agreement between the different tonometers was assessed by combining data of observer 1 and 2 (Fig. 5). Data for the individual observers is shown in Fig. S1. A strong correlation was observed between the TonoLab and TonoVet (r = 0.85, p < 0.0001, R2 = 0.73, Fig. 5a). A moderate correlation was found for the comparison of the TonoPEN AVIA with the TonoVet, and the TonoLab with TonoPEN AVIA (r = 0.53, p < 0.0001, R2 = 0.29, Fig. 5d and r = 0.58, p < 0.0001, R2 = 0.33, Fig. 5c, respectively). Due to the learning curve of observer 2 for the TonoPEN AVIA, a lower correlation and linear regression were obtained when compared to observer 1 (Fig. S1b, c, e, and f). A moderate correlation was found for the comparison of the TonoPEN AVIA with the TonoVet for observer 1, whereas this was low for observer 2 (r = 0.60, p < 0.0001, R2 = 0.36, Fig. S1b and r = 0.35, p < 0.0001, R2 = 0.12, Fig. S1e, respectively). A similar pattern was observed when comparing the TonoLab with TonoPEN AVIA for observer 1 and observer 2 (r = 0.63, p < 0.0001, R2 = 0.40, Fig. S1d and r = 0.40, p < 0.0001, R2 = 0.16, Fig. S1f, respectively).
Figure 5.
Agreement between tonometers. (a–c) Show a scatter plot with linear regression (dashed lines is the 95% confidence interval (CI)). (d–f) Show a Bland and Altman plot expressing the difference of measurements by the tonometers plotted over the mean values of the tonometers.
After plotting the difference between the tonometers, the TonoLab showed on average 22.7 ± 20.9 mmHg higher IOP values than the TonoVet (Fig. 5d). This difference was caused by the higher absolute IOP values of the TonoLab compared to the TonoVet, additionally confirmed by the linear regression line (R2 = 0.92).
The TonoVet and TonoPEN AVIA were more in agreement with a bias of 0.45 (the TonoVet provided a slightly higher IOP value than the TonoPEN AVIA) with a deviation of ± 6.5 (Fig. 5e). The TonoLab and TonoPEN AVIA showed a negative trend when differences were plotted against the mean values (R2 = 0.80, Fig. 5f). Similar to the comparison with the TonoVet, a bias of − 24.6 ± 24.2 mmHg was observed between the TonoLab and the TonoPEN AVIA, due to the high absolute values measured by the TonoLab. The findings were in line with individual differences between tonometers (Fig. S1g-l).
Effect of sedation on IOP
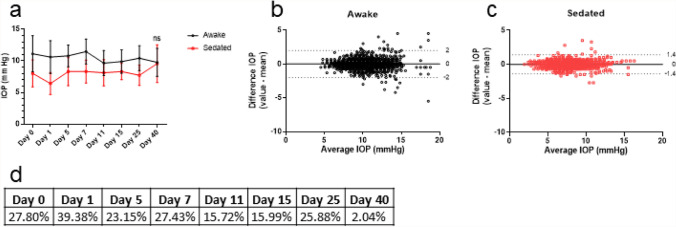
Figure 6a shows IOP over time in awake and sedated animals, measured with the TonoVet by observer 2. A two-way repeated measures ANOVA showed a significant difference between awake and sedated IOP measurement on all days, (Table 3, Fig. 6a) except for day 40 (p > 0.9999). For day 0 until day 25, the IOP of sedated animals was about 25% lower than of awake animals, while on day 40 this difference was only about 2% (Fig. 6d). The repeatability of the measurement was not affected by sedation, as shown in the Bland and Altman plots (Fig. 6b,c).
Figure 6.
Effect of sedation on IOP. Measurements taken by the same observer using the TonoVet. (a) IOP over time in both awake and sedated rabbits. (d) Percentage difference in IOP between awake and sedated rabbits. (b,c) Bland and Altman plot from the awake and sedated rabbits. Dotted lines show 1.96 times the SD (95% confidence interval). ns; not significant.
Table 3.
Results of the two-way repeated measures ANOVA with Bonferroni correction, N = 21.
| Mean awake | Mean sedated | Mean diff | SE of diff | 95% CI of diff | P Value | |
|---|---|---|---|---|---|---|
| Day 0 | 11.13 | 8.03 | 3.09 | 0.45 | 1.80 to 4.39 | < 0.0001 |
| Day 1 | 10.61 | 6.43 | 4.18 | 0.31 | 3.31 to 5.04 | < 0.0001 |
| Day 5 | 10.80 | 8.30 | 2.50 | 0.34 | 1.55 to 3.45 | < 0.0001 |
| Day 7 | 11.48 | 8.33 | 3.15 | 0.40 | 2.04 to 4.26 | < 0.0001 |
| Day 11 | 9.65 | 8.13 | 1.52 | 0.37 | 0.48 to 2.55 | 0.0007 |
| Day 15 | 9.90 | 8.32 | 1.58 | 0.29 | 0.77 to 2.40 | < 0.0001 |
| Day 25 | 10.43 | 7.73 | 2.70 | 0.33 | 1.78 to 3.62 | < 0.0001 |
| Day 40 | 9.78 | 9.58 | 0.20 | 0.48 | − 1.14 to 1.54 | > 0.9999 |
CI, confidence interval.
Discussion
In this study, we calculated the reproducibility, repeatability and agreement of three different tonometers (the TonoLab, the TonoVet and the TonoPEN AVIA) in a cohort of normotensive NZW rabbits, alongside the effect of sedation on IOP measurements.
Our results showed higher absolute IOP values when using the TonoLab compared to the TonoVet and the TonoPEN AVIA. Since the TonoLab is designed for use in mice and rats, the thicker cornea of the rabbit probably affected the readings of this device97,98. The TonoVet showed the highest repeatability (CoV of 6.38%), followed by the TonoLab and TonoPen AVIA (CoV of 6.55% and 10.88%, respectively). Furthermore, the repeatability was acceptable (defined as CoV < 10%)94 for the TonoVet and TonoLab and the measurements were in line with previous reports that found a CoV of 6.50% and 10.30% for the TonoVet and TonoPEN XL (an older version of the TonoPEN AVIA), respectively53.
Applanation tonometry is known to be sensitive to the technique used as well as the force applied6, while rebound tonometers can be easier to use. The ease of use for the TonoVet and TonoLab was comparable between the two, both allowing probes to be easily installed and correct usage of the device to be learned quickly. However, the TonoPEN AVIA showed to have a steeper learning curve. Applying the tip-cover of the TonoPEN AVIA may also introduce additional errors. Results showed that (for the TonoPEN AVIA in particular) extremely high IOP values (above 20 mmHg) are prone to larger error, in line with previous studies51,54. The highest correlation with regard to reproducibility was found with the two rebound tonometers, TonoLab and TonoVet, with a lower reproducibility of the TonoPEN AVIA. A possible explanation for this might be that using the TonoPEN AVIA is more difficult.
On agreement between the different tonometers, the TonoLab and TonoVet showed a strong correlation (r = 0.85, R2 = 0.73, p < 0.0001), in line with our expectations as both measure the IOP via rebound tonometry. When comparing the TonoPEN AVIA with the TonoVet, and the TonoLab with the TonoPEN AVIA, a more moderate agreement correlation was found (r = 0.53, p < 0.0001, and r = 0.58, p < 0.0001, respectively). Pereira et al. showed a correlation of r = 0.60 (R2 = 0.36) between the TonoVet and the TonoPEN AVIA in a cohort of 76 rabbit eyes51, in line with our findings. Ma et al. compared the TonoVet to the TonoPEN XL in rabbits. They found a high linear regression (R2 = 0.98) between both tonometers, but correlation was not tested separately53.
Recently, Gloe et al. examined the TonoVet Plus (a novel version of the TonoVet that has a rabbit setting, released after the onset of this study), TonoVet, TonoPEN Vet, and TonoPEN AVIA on post-mortem rabbit eyes. Their results showed a high linear regression of the tonometers when compared to manometry, the TonoVet Plus (R2 = 0.99), the TonoVet (R2 = 0.98), the TonoPEN Vet (R2 = 0.92), and the TonoPEN AVIA (R2 = 0.92). However, no correlation between the tonometers was done. Their findings indicate that all tonometers tend to underestimate IOP when compared to manometry54.
In the present study, the TonoVet and TonoPEN AVIA showed the highest agreement; however, the correlation is moderate due to the different working mechanisms of the tonometers (rebound versus applanation). The TonoLab and TonoVet demonstrated the best correlation, however their agreement was lower because the measurements of the TonoLab showed a much higher IOP than the TonoVet. Since the probe size of the TonoLab is specifically designed for use in small rodents, the system is not calibrated for the thick corneas of rabbits35. The average central corneal thickness (CCT) has been reported to be 105 µm for mice and 130 µm for rats35. The average CCT of New Zealand White rabbits is 407 ± 20 µm99, 3 to 4 times thicker than the reported values in rodents. The rabbit’s greater corneal thickness is likely responsible for inaccurate IOP values obtained with the TonoLab. Although we did not measure CCT in every animal and could therefore not correct for CCT differences, they were all New Zealand White rabbits from the same age, and differences in CCT were therefore likely to be limited . Additionally, all animals were measured with all three devices and the aim was to observe the differences between those devices .
Overall, the TonoVet was the best tonometer in terms of repeatability, reproducibility, and agreement and it was the most consistent tonometer in comparison to the TonoPEN AVIA and TonoLab. The TonoVet showed the highest agreement and strongest correlation to the other tonometers. The correlation between the TonoLab and TonoPEN AVIA was found to be moderate, presumably from them being different mechanisms of measurement, similar to the TonoPEN AVIA and the TonoVet having a moderate correlation.
The effect of the selective alpha 2-adrenoceptor agonist medetomidine on IOP was examined using the TonoVet. Our results indicated a ~ 25% reduction in IOP after IM sedation. In dogs, no reduction of IOP has been observed using a similar dose of 0.5 mg/kg medetomidine IM100. In rabbits, medetomidine has also topically been instilled18,19. Two studies found that a dose of 25 μg medetomidine reduced the IOP of the contralateral eye in 30 min by ~ 50%, while the treated eye was not affected18,19. This effect has also been observed in dogs101.
In contrast to other time points in our study, no difference in IOP between awake and sedated animals was observed at day 40. This might have been caused by elevated mental stress levels in the rabbits, caused by euthanasia of animals performed in the same room, an effect also observed in dogs101. Levels of mental stress were not assessed during the study but did not affect the repeatability and reproducibility of the measurements.
Because IOP fluctuates over short periods of time, similar to fluctuations in heart rate during the day15, any tonometer that records a near-instantaneous measurement of IOP is taking a sample from the IOP cycle causing measurements to only provide an estimation of the IOP at one time point. Variables such as fluctuating blood pressure, pulse, respiration, and anxiety could also account for discrepancies in IOP, along with the mental stress of repeated measures102.
Conclusion
Of the three tonometers tested, TonoVet was the most favorable as it showed the smallest inter- and intra-observer variations, without a learning curve. The TonoLab showed three-fold higher IOP values compared to the TonoVet, making it unsuitable for determining rabbit IOP. Additionally, when IM sedation is required in future experiments, it should be taken into account that it significantly reduces the IOP of rabbits (often by more than 25%).
Supplementary Information
Acknowledgements
This research was performed under the framework of the Chemelot Institute for Science and Technology (InSciTe). We would like to express our gratitude to F.A.M. Welten, B.A.Sc. for her technical support during the animal experiments and we would like to thank D. Crosbie, Ph.D. and J.T. Boonstra, M.Sc. for proofreading the manuscript.
Abbreviations
- ARRIVE
Animal Research: Reporting of In Vivo Experiments
- ARVO
Association for Research in Vision and Ophthalmology
- AH
Aqueous humor
- CI
Confidence Interval
- CCT
Central corneal thickness
- CoV
Coefficient of variation
- GAT
Goldmann applanation tonometry
- IM
Intramuscular
- IOP
Intraocular pressure
- NZW
New Zealand White
- ns
Not significant
- SD
Standard deviation
Author contributions
C.B.: Conceptualization, Investigation, Methodology, Data Curation, Formal analysis, Writing -Original draft R.M.: Investigation, Methodology, Data Curation, Formal analysis, Writing -Original draft T.B.: Methodology, Data Curation, Review & Editing M.G.: Data Curation, Review & Editing J.W.: Data Curation, Review & Editing T.G.: Supervision, Funding acquisition, Review & Editing R.N.: Conceptualization, Funding acquisition, Project administration, Supervision, Review & Editing H.B.: Conceptualization, Funding acquisition, Project administration, Supervision, Writing—Review & Editing All authors reviewed the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Christian J. F. Bertens and Ralph J. S. van Mechelen.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-98762-7.
References
- 1.Dang Y, et al. Intraocular pressure elevation precedes a phagocytosis decline in a model of pigmentary glaucoma. F1000Res. 2018;7:174. doi: 10.12688/f1000research.13797.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang Q, et al. Microbead-induced ocular hypertensive mouse model for screening and testing of aqueous production suppressants for glaucoma. Invest. Ophthalmol. Vis. Sci. 2012;53:3733–3741. doi: 10.1167/iovs.12-9814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mukai R, et al. Mouse model of ocular hypertension with retinal ganglion cell degeneration. PLoS ONE. 2019;14:e0208713. doi: 10.1371/journal.pone.0208713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alguire PC. Tonometry. In: Clinical Methods: The History, Physical, and Laboratory Examinations 3rd edn, (ed. Walker, HK, et al.) (Boston, Butterworths, 1990) Chapter 118. Available from: https://www.ncbi.nlm.nih.gov/books/NBK222/. [PubMed]
- 5.Pearce JG, Maddess T. The clinical interpretation of changes in intraocular pressure measurements using Goldmann applanation tonometry: a review. J Glaucoma. 2019;28:302–306. doi: 10.1097/IJG.0000000000001144. [DOI] [PubMed] [Google Scholar]
- 6.Lim KS, Wickremasinghe SS, Cordeiro MF, Bunce C, Khaw PT. Accuracy of intraocular pressure measurements in New Zealand white rabbits. Invest Ophthalmol Vis Sci. 2005;46:2419–2423. doi: 10.1167/iovs.04-0610. [DOI] [PubMed] [Google Scholar]
- 7.Davies LN, Bartlett H, Mallen EA, Wolffsohn JS. Clinical evaluation of rebound tonometer. Acta Ophthalmol. Scand. 2006;84:206–209. doi: 10.1111/j.1600-0420.2005.00610.x. [DOI] [PubMed] [Google Scholar]
- 8.Whitacre MM, Stein RA, Hassanein K. The effect of corneal thickness on applanation tonometry. Am. J. Ophthalmol. 1993;115:592–596. doi: 10.1016/S0002-9394(14)71455-2. [DOI] [PubMed] [Google Scholar]
- 9.Ko YC, Liu CJ, Hsu WM. Varying effects of corneal thickness on intraocular pressure measurements with different tonometers. Eye (Lond) 2005;19:327–332. doi: 10.1038/sj.eye.6701458. [DOI] [PubMed] [Google Scholar]
- 10.Ehlers N, Bramsen T, Sperling S. Applanation tonometry and central corneal thickness. Acta Ophthalmol. (Copenh) 1975;53:34–43. doi: 10.1111/j.1755-3768.1975.tb01135.x. [DOI] [PubMed] [Google Scholar]
- 11.Turner DC, Miranda M, Morris JS, Girkin CA, Downs JC. Acute stress increases intraocular pressure in nonhuman primates. Ophthalmol. Glaucoma. 2019;2:210–214. doi: 10.1016/j.ogla.2019.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gillmann K, Hoskens K, Mansouri K. Acute emotional stress as a trigger for intraocular pressure elevation in Glaucoma. BMC Ophthalmol. 2019;19:69. doi: 10.1186/s12886-019-1075-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jimenez R, Vera J. Effect of examination stress on intraocular pressure in university students. Appl. Ergon. 2018;67:252–258. doi: 10.1016/j.apergo.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 14.Dada T, et al. Mindfulness meditation reduces intraocular pressure, lowers stress biomarkers and modulates gene expression in glaucoma: a randomized controlled trial. J. Glaucoma. 2018;27:1061–1067. doi: 10.1097/IJG.0000000000001088. [DOI] [PubMed] [Google Scholar]
- 15.Rowland JM, Potter DE, Reiter RJ. Circadian rhythm in intraocular pressure: a rabbit model. Curr. Eye Res. 1981;1:169–173. doi: 10.3109/02713688109001822. [DOI] [PubMed] [Google Scholar]
- 16.Li R, Liu JH. Telemetric monitoring of 24 h intraocular pressure in conscious and freely moving C57BL/6J and CBA/CaJ mice. Mol. Vis. 2008;14:745–749. [PMC free article] [PubMed] [Google Scholar]
- 17.Dear GD, Hammerton M, Hatch DJ, Taylor D. Anaesthesia and intra-ocular pressure in young children. A study of three different techniques of anaesthesia. Anaesthesia. 1987;42:259–265. doi: 10.1111/j.1365-2044.1987.tb03036.x. [DOI] [PubMed] [Google Scholar]
- 18.Potter DE, Ogidigben MJ. Medetomidine-induced alterations of intraocular pressure and contraction of the nictitating membrane. Invest. Ophthalmol. Vis. Sci. 1991;32:2799–2805. [PubMed] [Google Scholar]
- 19.Ogidigben MJ, Potter DE. Comparative effects of alpha-2 and DA-2 agonists on intraocular pressure in pigmented and nonpigmented rabbits. J. Ocul. Pharmacol. 1993;9:187–199. doi: 10.1089/jop.1993.9.187. [DOI] [PubMed] [Google Scholar]
- 20.Kelly DJ, Farrell SM. Physiology and role of intraocular pressure in contemporary anesthesia. Anesth. Analg. 2018;126:1551–1562. doi: 10.1213/ANE.0000000000002544. [DOI] [PubMed] [Google Scholar]
- 21.Basaran B, Yilbas AA, Gultekin Z. Effect of interscalene block on intraocular pressure and ocular perfusion pressure. BMC Anesthesiol. 2017;17:144. doi: 10.1186/s12871-017-0436-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jasien JV, Girkin CA, Downs JC. Effect of anesthesia on intraocular pressure measured with continuous wireless telemetry in nonhuman primates. Invest. Ophthalmol. Vis. Sci. 2019;60:3830–3834. doi: 10.1167/iovs.19-27758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hausmann JC, Krisp A, Sladky K, Miller PE, Mans C. Measuring intraocular pressure in White's Tree Frogs (Litoria Caerulea) by rebound tonometry: Comparing device, time of day, and manual versus chemical restraint methods. J. Zoo Wildl. Med. 2017;48:413–419. doi: 10.1638/2016-0268R.1. [DOI] [PubMed] [Google Scholar]
- 24.Lewin AC, Hausmann JC, Miller PE. Intraocular pressure and examination findings in three species of central and South American Tree Frogs (Cruziohyla Craspedopus, Cruziohyla Calcarifer, and Anotheca Spinosa) J. Zoo Wildl. Med. 2017;48:688–693. doi: 10.1638/2016-0243.1. [DOI] [PubMed] [Google Scholar]
- 25.Cannizzo SA, Lewbart GA, Westermeyer HD. Intraocular pressure in American Bullfrogs (Rana catesbeiana) measured with rebound and applanation tonometry. Vet. Ophthalmol. 2017;20:526–532. doi: 10.1111/vop.12463. [DOI] [PubMed] [Google Scholar]
- 26.Delgado C, et al. Evaluation of rebound tonometry in red-eared slider turtles (Trachemys scripta elegans) Vet. Ophthalmol. 2014;17:261–267. doi: 10.1111/vop.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rajaei S, Ansari mood M, Sadjadi R, Azizi F. Measurement of intraocular pressure using Tonovet(R) in European Pond Turtle (Emys Orbicularis) J Zoo Wildl Med. 2015;46:421–422. doi: 10.1638/2014-0234R.1. [DOI] [PubMed] [Google Scholar]
- 28.Qiu Y, Yang H, Lei B. Effects of three commonly used anesthetics on intraocular pressure in mouse. Curr. Eye Res. 2014;39:365–369. doi: 10.3109/02713683.2013.845224. [DOI] [PubMed] [Google Scholar]
- 29.Wang WH, Millar JC, Pang IH, Wax MB, Clark AF. Noninvasive measurement of rodent intraocular pressure with a rebound tonometer. Invest. Ophthalmol. Vis. Sci. 2005;46:4617–4621. doi: 10.1167/iovs.05-0781. [DOI] [PubMed] [Google Scholar]
- 30.Saeki T, Aihara M, Ohashi M, Araie M. The efficacy of TonoLab in detecting physiological and pharmacological changes of mouse intraocular pressure–comparison with TonoPen and microneedle manometery. Curr. Eye Res. 2008;33:247–252. doi: 10.1080/02713680801919716. [DOI] [PubMed] [Google Scholar]
- 31.Pease ME, Hammond JC, Quigley HA. Manometric calibration and comparison of TonoLab and TonoPen tonometers in rats with experimental glaucoma and in normal mice. J. Glaucoma. 2006;15:512–519. doi: 10.1097/01.ijg.0000212276.57853.19. [DOI] [PubMed] [Google Scholar]
- 32.Pease ME, Cone FE, Gelman S, Son JL, Quigley HA. Calibration of the TonoLab tonometer in mice with spontaneous or experimental glaucoma. Invest. Ophthalmol. Vis. Sci. 2011;52:858–864. doi: 10.1167/iovs.10-5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chatterjee A, Oh DJ, Kang MH, Rhee DJ. Central corneal thickness does not correlate with TonoLab-measured IOP in several mouse strains with single transgenic mutations of matricellular proteins. Exp. Eye Res. 2013;115:106–112. doi: 10.1016/j.exer.2013.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reitsamer HA, Kiel JW, Harrison JM, Ransom NL, McKinnon SJ. Tonopen measurement of intraocular pressure in mice. Exp. Eye Res. 2004;78:799–804. doi: 10.1016/j.exer.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 35.Snyder KC, Lewin AC, Mans C, McLellan GJ. Tonometer validation and intraocular pressure reference values in the normal chinchilla (Chinchilla lanigera) Vet. Ophthalmol. 2018;21:4–9. doi: 10.1111/vop.12468. [DOI] [PubMed] [Google Scholar]
- 36.Muller K, Mauler DA, Eule JC. Reference values for selected ophthalmic diagnostic tests and clinical characteristics of chinchilla eyes (Chinchilla lanigera) Vet. Ophthalmol. 2010;13(Suppl):29–34. doi: 10.1111/j.1463-5224.2010.00801.x. [DOI] [PubMed] [Google Scholar]
- 37.Liu LF, Huang CK, Zhang MZ. Reliability of Tonolab measurements in rats. Int. J. Ophthalmol. 2014;7:930–934. doi: 10.3980/j.issn.2222-3959.2014.06.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee EJ, et al. Assessing intraocular pressure by rebound tonometer in rats with an air-filled anterior chamber. Jpn. J. Ophthalmol. 2008;52:500–503. doi: 10.1007/s10384-008-0591-1. [DOI] [PubMed] [Google Scholar]
- 39.Morrison JC, Jia L, Cepurna W, Guo Y, Johnson E. Reliability and sensitivity of the TonoLab rebound tonometer in awake Brown Norway rats. Invest. Ophthalmol. Vis. Sci. 2009;50:2802–2808. doi: 10.1167/iovs.08-2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moore CG, Milne ST, Morrison JC. Noninvasive measurement of rat intraocular pressure with the Tono-Pen. Invest. Ophthalmol. Vis. Sci. 1993;34:363–369. [PubMed] [Google Scholar]
- 41.Goldblum D, Kontiola AI, Mittag T, Chen B, Danias J. Non-invasive determination of intraocular pressure in the rat eye. Comparison of an electronic tonometer (TonoPen), and a rebound (impact probe) tonometer. Graefes Arch. Clin. Exp. Ophthalmol. 2002;240:942–946. doi: 10.1007/s00417-002-0571-y. [DOI] [PubMed] [Google Scholar]
- 42.Jia L, Cepurna WO, Johnson EC, Morrison JC. Effect of general anesthetics on IOP in rats with experimental aqueous outflow obstruction. Invest. Ophthalmol. Vis. Sci. 2000;41:3415–3419. [PubMed] [Google Scholar]
- 43.Moore CG, Epley D, Milne ST, Morrison JC. Long-term non-invasive measurement of intraocular pressure in the rat eye. Curr. Eye Res. 1995;14:711–717. doi: 10.3109/02713689508998499. [DOI] [PubMed] [Google Scholar]
- 44.Moore CG, Johnson EC, Morrison JC. Circadian rhythm of intraocular pressure in the rat. Curr. Eye Res. 1996;15:185–191. doi: 10.3109/02713689608997412. [DOI] [PubMed] [Google Scholar]
- 45.Williams D, Sullivan A. Ocular disease in the guinea pig (Cavia porcellus): a survey of 1000 animals. Vet. Ophthalmol. 2010;13(Suppl):54–62. doi: 10.1111/j.1463-5224.2010.00812.x. [DOI] [PubMed] [Google Scholar]
- 46.Tammewar AM, et al. Intraocular properties of an alkoxyalkyl derivative of cyclic 9-(S)-(3-hydroxyl-2-phosphonomehoxypropyl) adenine, an intravitreally injectable anti-HCMV drug in rabbit and guinea pig. J. Ocul. Pharmacol. Ther. 2007;23:433–444. doi: 10.1089/jop.2007.0018. [DOI] [PubMed] [Google Scholar]
- 47.Montiani-Ferreira F, Mattos BC, Russ HH. Reference values for selected ophthalmic diagnostic tests of the ferret (Mustela putorius furo) Vet. Ophthalmol. 2006;9:209–213. doi: 10.1111/j.1463-5224.2006.00475.x. [DOI] [PubMed] [Google Scholar]
- 48.Williams D, Adeyeye N, Visser E. Ophthalmological abnormalities in wild European hedgehogs (Erinaceus europaeus): a survey of 300 animals. Open Vet. J. 2017;7:261–267. doi: 10.4314/ovj.v7i3.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ghaffari MS, Hajikhani R, Sahebjam F, Akbarein H, Golezardy H. Intraocular pressure and Schirmer tear test results in clinically normal Long-Eared Hedgehogs (Hemiechinus auritus): reference values. Vet. Ophthalmol. 2012;15:206–209. doi: 10.1111/j.1463-5224.2011.00967.x. [DOI] [PubMed] [Google Scholar]
- 50.Kalesnykas G, Uusitalo H. Comparison of simultaneous readings of intraocular pressure in rabbits using Perkins handheld, Tono-Pen XL, and TonoVet tonometers. Graefes Arch. Clin. Exp. Ophthalmol. 2007;245:761–762. doi: 10.1007/s00417-006-0470-8. [DOI] [PubMed] [Google Scholar]
- 51.Pereira FQ, Bercht BS, Soares MG, da Mota MG, Pigatto JA. Comparison of a rebound and an applanation tonometer for measuring intraocular pressure in normal rabbits. Vet. Ophthalmol. 2011;14:321–326. doi: 10.1111/j.1463-5224.2011.00879.x. [DOI] [PubMed] [Google Scholar]
- 52.Zhang H, et al. Validation of rebound tonometry for intraocular pressure measurement in the rabbit. Exp. Eye Res. 2014;121:86–93. doi: 10.1016/j.exer.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 53.Ma D, et al. Repeatability, reproducibility and agreement of intraocular pressure measurement in rabbits by the TonoVet and Tono-Pen. Sci. Rep. 2016;6:35187. doi: 10.1038/srep35187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gloe S, Rothering A, Kiland JA, McLellan GJ. Validation of the Icare((R)) TONOVET plus rebound tonometer in normal rabbit eyes. Exp. Eye Res. 2019;185:107698. doi: 10.1016/j.exer.2019.107698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kovalcuka L, Nikolajenko M. Changes in intraocular pressure, horizontal pupil diameter, and tear production during the use of topical 1% cyclopentolate in cats and rabbits. Open Vet. J. 2020;10:59–67. doi: 10.4314/ovj.v10i1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Taskiran Comez A, Cakir DU, Tutunculer FK, Gencer B, Tufan HA. Relationship between raised intraocular pressure and ischemia-modified albumin in serum and humor aqueous: a pilot study in rabbits. Int. J. Ophthalmol. 2014;7:421–425. doi: 10.3980/j.issn.2222-3959.2014.03.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zagon IS, Sassani JW, Carroll MA, McLaughlin PJ. Topical application of naltrexone facilitates reepithelialization of the cornea in diabetic rabbits. Brain Res. Bull. 2010;81:248–255. doi: 10.1016/j.brainresbull.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goldblum D, Garweg JG, Bohnke M. Topical rivastigmine, a selective acetylcholinesterase inhibitor, lowers intraocular pressure in rabbits. J. Ocul. Pharmacol. Ther. 2000;16:29–35. doi: 10.1089/jop.2000.16.29. [DOI] [PubMed] [Google Scholar]
- 59.Fleischhauer JC, Liu R, Elena PP, Flammer J, Haefliger IO. Topical ocular instillation of nitric oxide synthase inhibitors and intraocular pressure in rabbits. Klin Monbl Augenheilkd. 2001;218:351–353. doi: 10.1055/s-2001-15898. [DOI] [PubMed] [Google Scholar]
- 60.Park YW, et al. Effect of central corneal thickness on intraocular pressure with the rebound tonometer and the applanation tonometer in normal dogs. Vet. Ophthalmol. 2011;14:169–173. doi: 10.1111/j.1463-5224.2010.00859.x. [DOI] [PubMed] [Google Scholar]
- 61.Nagata N, Yuki M, Hasegawa T. In vitro and in vivo comparison of applanation tonometry and rebound tonometry in dogs. J. Vet. Med. Sci. 2011;73:1585–1589. doi: 10.1292/jvms.11-0251. [DOI] [PubMed] [Google Scholar]
- 62.Ahn JT, et al. Accuracy of intraocular pressure measurements in dogs using two different tonometers and plano therapeutic soft contact lenses. Vet. Ophthalmol. 2012;15(Suppl 1):83–88. doi: 10.1111/j.1463-5224.2011.00979.x. [DOI] [PubMed] [Google Scholar]
- 63.de Oliveira JK, Montiani-Ferreira F, Williams DL. The influence of the tonometer position on canine intraocular pressure measurements using the Tonovet((R)) rebound tonometer. Open Vet. J. 2018;8:68–76. doi: 10.4314/ovj.v8i1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Slack JM, Stiles J, Moore GE. Comparison of a rebound tonometer with an applanation tonometer in dogs with glaucoma. Vet. Rec. 2012;171:373. doi: 10.1136/vr.100967. [DOI] [PubMed] [Google Scholar]
- 65.von Spiessen L, Karck J, Rohn K, Meyer-Lindenberg A. Clinical comparison of the TonoVet((R)) rebound tonometer and the Tono-Pen Vet((R)) applanation tonometer in dogs and cats with ocular disease: glaucoma or corneal pathology. Vet. Ophthalmol. 2015;18:20–27. doi: 10.1111/vop.12101. [DOI] [PubMed] [Google Scholar]
- 66.Taylor NR, Zele AJ, Vingrys AJ, Stanley RG. Variation in intraocular pressure following application of tropicamide in three different dog breeds. Vet. Ophthalmol. 2007;10(Suppl 1):8–11. doi: 10.1111/j.1463-5224.2007.00485.x. [DOI] [PubMed] [Google Scholar]
- 67.Mughannam AJ, Cook CS, Fritz CL. Change in intraocular pressure during maturation in Labrador Retriever dogs. Vet. Ophthalmol. 2004;7:87–89. doi: 10.1111/j.1463-5224.2004.04003.x. [DOI] [PubMed] [Google Scholar]
- 68.Adelman S, et al. The post-natal development of intraocular pressure in normal domestic cats (Felis catus) and in feline congenital glaucoma. Exp. Eye Res. 2018;166:70–73. doi: 10.1016/j.exer.2017.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rusanen E, Florin M, Hassig M, Spiess BM. Evaluation of a rebound tonometer (Tonovet) in clinically normal cat eyes. Vet. Ophthalmol. 2010;13:31–36. doi: 10.1111/j.1463-5224.2009.00752.x. [DOI] [PubMed] [Google Scholar]
- 70.Rodrigues BE, et al. Intraocular pressure and pupil diameter in healthy cats anesthetized with isoflurane and pre-medicated with isolated acepromazine or in combination with tramadol. Arquivo Brasileiro de Medicina Veterinária e Zootecnia. 2021;73:631–638. doi: 10.1590/1678-4162-12102. [DOI] [Google Scholar]
- 71.Jeong MB, et al. Comparison of the rebound tonometer (TonoVet) with the applanation tonometer (TonoPen XL) in normal Eurasian Eagle owls (Bubo bubo) Vet. Ophthalmol. 2007;10:376–379. doi: 10.1111/j.1463-5224.2007.00573.x. [DOI] [PubMed] [Google Scholar]
- 72.Reuter A, Muller K, Arndt G, Eule JC. Accuracy and reproducibility of the TonoVet rebound tonometer in birds of prey. Vet. Ophthalmol. 2010;13(Suppl):80–85. doi: 10.1111/j.1463-5224.2010.00817.x. [DOI] [PubMed] [Google Scholar]
- 73.Harris MC, et al. Ophthalmic examination findings in a colony of Screech owls (Megascops asio) Vet Ophthalmol. 2008;11:186–192. doi: 10.1111/j.1463-5224.2008.00618.x. [DOI] [PubMed] [Google Scholar]
- 74.Meekins JM, et al. Ophthalmic diagnostic tests and ocular findings in a flock of captive american flamingos (Phoenicopterus ruber ruber) J. Avian Med. Surg. 2015;29:95–105. doi: 10.1647/2014-021. [DOI] [PubMed] [Google Scholar]
- 75.Ansari Mood M, Rajaei SM, Ghazanfari Hashemi S, Williams DL, Sadjadi R. Measurement of tear production and intraocular pressure in ducks and geese. Vet. Ophthalmol. 2017;20:53–57. doi: 10.1111/vop.12351. [DOI] [PubMed] [Google Scholar]
- 76.Kovalcuka L, Boiko D, Williams DL. Tear production and intraocular pressure values in clinically normal eyes of whooper swans (Cygnus cygnus) Open Vet. J. 2018;8:335–339. doi: 10.4314/ovj.v8i3.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ansari Mood M, Rajaei SM, Hashemi SS, Williams DL. Measurement of intraocular pressure in the Domestic Pigeon (Columbia Livia) J. Zoo Wildl. Med. 2016;47:935–938. doi: 10.1638/2015-0102.1. [DOI] [PubMed] [Google Scholar]
- 78.Tofflemire KL, et al. Schirmer tear test I and rebound tonometry findings in healthy calves. Vet. Ophthalmol. 2015;18:147–151. doi: 10.1111/vop.12165. [DOI] [PubMed] [Google Scholar]
- 79.Hibbs CD, Barrett PM, Dees DD. Intraocular pressure reference intervals in eyes of clinically normal miniature donkeys (Equus africanus asinus) Vet. Ophthalmol. 2019;22:24–30. doi: 10.1111/vop.12561. [DOI] [PubMed] [Google Scholar]
- 80.Allbaugh RA, Keil SM, Ou Z, Bello NM. Intraocular pressure changes in equine athletes during endurance competitions. Vet. Ophthalmol. 2014;17(Suppl 1):154–159. doi: 10.1111/vop.12182. [DOI] [PubMed] [Google Scholar]
- 81.Lewin AC, Miller PE. Calibration of the TonoVet and Tono-Pen Vet tonometers in the porcine eye. Vet. Ophthalmol. 2017;20:571–573. doi: 10.1111/vop.12445. [DOI] [PubMed] [Google Scholar]
- 82.McDonald JE, Knollinger AM, Dustin-Dees D, MacLaren NE. Comparison of intraocular pressure measurements using rebound (TonoVet((R)) ) and applanation (TonoPen-XL((R))) tonometry in clinically normal alpacas (Vicugna pacos) Vet. Ophthalmol. 2017;20:155–159. doi: 10.1111/vop.12384. [DOI] [PubMed] [Google Scholar]
- 83.Peche N, Eule JC. Intraocular pressure measurements in cattle, sheep, and goats with 2 different types of tonometers. Can. J. Vet. Res. 2018;82:208–215. [PMC free article] [PubMed] [Google Scholar]
- 84.Yu W, et al. Evaluation of monkey intraocular pressure by rebound tonometer. Mol. Vis. 2009;15:2196–2201. [PMC free article] [PubMed] [Google Scholar]
- 85.McAllister F, Harwerth R, Patel N. Assessing the True Intraocular Pressure in the Non-human Primate. Optom. Vis. Sci. 2018;95:113–119. doi: 10.1097/OPX.0000000000001171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dubicanac M, et al. Intraocular pressure in the smallest primate aging model: the gray mouse lemur. Vet. Ophthalmol. 2018;21:319–327. doi: 10.1111/vop.12434. [DOI] [PubMed] [Google Scholar]
- 87.Kim J, et al. IOP change undergoing anesthesia in rhesus macaques (Macaca mulatta) with laser-induced ocular hypertension. J. Vet. Med. Sci. 2012;74:1359–1361. doi: 10.1292/jvms.12-0059. [DOI] [PubMed] [Google Scholar]
- 88.Ollivier FJ, et al. Time-specific intraocular pressure curves in Rhesus macaques (Macaca mulatta) with laser-induced ocular hypertension. Vet. Ophthalmol. 2004;7:23–27. doi: 10.1111/j.1463-5224.2004.00316.x. [DOI] [PubMed] [Google Scholar]
- 89.Percie du Sert N, et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. BMC Vet. Res. 2020;16:242. doi: 10.1186/s12917-020-02451-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Baudouin C, Gastaud P. Influence of topical anesthesia on tonometric values of intraocular pressure. Ophthalmologica. 1994;208:309–313. doi: 10.1159/000310527. [DOI] [PubMed] [Google Scholar]
- 91.British Standards Institution. Accuracy (trueness and precision) of measurement methods and results: basic methods for the determination of repeatability and reproducibility of a standard measurement method. ISO 5725 part 2 (1994).
- 92.Charan J, Kantharia ND. How to calculate sample size in animal studies? J. Pharmacol. Pharmacother. 2013;4:303–306. doi: 10.4103/0976-500X.119726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bertens CJF, et al. Pharmacokinetics and efficacy of a ketorolac-loaded ocular coil in New Zealand white rabbits. Drug Deliv. 2021;28:400–407. doi: 10.1080/10717544.2021.1883157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fujimura F, Kamiya K, Fujiwara K, Shoji N, Shimizu K. Repeatability and reproducibility of measurements using a NT-530P noncontact tono/pachymeter and correlation of central corneal thickness with intraocular pressure. Biomed. Res. Int. 2013;2013:370592. doi: 10.1155/2013/370592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. doi: 10.1016/S0140-6736(86)90837-8. [DOI] [PubMed] [Google Scholar]
- 96.Bland JM, Altman DG. Comparing methods of measurement: why plotting difference against standard method is misleading. Lancet. 1995;346:1085–1087. doi: 10.1016/S0140-6736(95)91748-9. [DOI] [PubMed] [Google Scholar]
- 97.Yildiz A, Yasar T. Comparison of Goldmann applanation, non-contact, dynamic contour and tonopen tonometry measurements in healthy and glaucomatous eyes, and effect of central corneal thickness on the measurement results. Med. Glas (Zenica) 2018;15:152–157. doi: 10.17392/960-18. [DOI] [PubMed] [Google Scholar]
- 98.Doughty MJ, Laiquzzaman M, Muller A, Oblak E, Button NF. Central corneal thickness in European (white) individuals, especially children and the elderly, and assessment of its possible importance in clinical measures of intra-ocular pressure. Ophthalmic Physiol. Opt. 2002;22:491–504. doi: 10.1046/j.1475-1313.2002.00053.x. [DOI] [PubMed] [Google Scholar]
- 99.Chan T, Payor S, Holden BA. Corneal thickness profiles in rabbits using an ultrasonic pachometer. Invest. Ophthalmol. Vis. Sci. 1983;24:1408–1410. [PubMed] [Google Scholar]
- 100.Kanda T, et al. Effects of medetomidine and xylazine on intraocular pressure and pupil size in healthy Beagle dogs. Vet. Anaesth Analg. 2015;42:623–628. doi: 10.1111/vaa.12249. [DOI] [PubMed] [Google Scholar]
- 101.Verbruggen AM, Akkerdaas LC, Hellebrekers LJ, Stades FC. The effect of intravenous medetomidine on pupil size and intraocular pressure in normotensive dogs. Vet. Q. 2000;22:179–180. doi: 10.1080/01652176.2000.9695052. [DOI] [PubMed] [Google Scholar]
- 102.Whitacre MM, Stein R. Sources of error with use of Goldmann-type tonometers. Surv. Ophthalmol. 1993;38:1–30. doi: 10.1016/0039-6257(93)90053-a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.