Abstract
In this research, a supercritical CO2-ethanol extraction was optimized to obtain a green coffee oil rich in bioactive compounds. A face-centered central composite design was used to evaluate the effect of temperature (50–70 °C), extraction pressure (15.0–30.0 MPa), and cosolvent content (5–20%) on the extraction yield and total phenolic compound content of green coffee supercritical extract (GCSE). The experimental data were fitted to a second-order polynomial model. According to the statistical analyses, the lack of fit was not significant for either mathematical model. From the response surface plots, the extraction pressure and cosolvent content significantly impacted the extraction yield, while the total phenolic compound content was impacted by temperature and cosolvent content. The optimal conditions were a 20% cosolvent content, a pressure of 30 MPa, and a temperature of 62 °C, which predicted an extraction yield of 7.7% with a total phenol content of 5.4 mg gallic acid equivalent g GCSE−1. The bioactive compounds included 5-caffeoylquinic acid (11.53–17.91 mg g GCSE−1), caffeine (44.76–79.51 mg g GCSE−1), linoleic acid (41.47–41.58%), and palmitic acid (36.07–36.18%). Our results showed that GCSE has the outstanding chemical quality and antioxidant potential, suggesting that GCSE can be used as a functional ingredient.
Keywords: Extraction yield, Total phenolic compound content, Fatty acids, Caffeine, 5-Caffeoylquinic acid
Introduction
Coffee is one of the most important industrial and economic products worldwide. In 2018, Mexico produced 860,000 tons of coffee beans, reaching 11th place worldwide in coffee production (Servicio de Información Agroalimentaria y Pesquera 2019). Green coffee beans are good sources of proteins, lipids, and bioactive compounds, such as polyphenols, diterpenes, and caffeine (De Oliveira et al. 2014; Bitencourt et al. 2018; Efthymiopoulos et al. 2019; Granados-Vallejo et al. 2019). Coffea arabica contains approximately 15% lipids, composed mainly of triacylglycerols, sterols, and tocopherols (Frost-Meyer and Logomarsino 2012). Palmitic, oleic, linoleic, stearic, arachidic, and behenic acids are the most prevalent fatty acids in green coffee beans (Andrade et al. 2012; Frost-Meyer and Logomarsino 2012; Hurtado-Benavides et al. 2016). The antioxidant activity of green coffee beans is attributed to phenolic compounds such as chlorogenic acids, caffeic acid, anthocyanins, tannins, and lignans (Farah and Donangelo 2006). The regular consumption of chlorogenic acids mainly regulates glucose metabolism and promotes a reduction in free radicals, visceral fat, body weight, and blood pressure (Onakpoya et al. 2011; Liang and Kitts 2015). Additionally, the chlorogenic acids were linked to cancer chemoprevention and the risk reduction of cardiovascular diseases (Palmioli et al. 2017; Martínez-López et al. 2019). The global market of chlorogenic acids was valued at 132.2 million USD in 2020 and is projected to reach USD 154.2 million USD by 2026 (Research-Reports 2020). Likewise, caffeine is the most popular alkaloid from green coffee beans. Caffeine consumption helps to reduce fatigue, enhance the capacity to remain awake, stimulate the central nervous system, increase blood pressure, and accelerate metabolism (Frost-Meyer and Logomarsino 2012; Babova et al. 2016; Ilgaz et al. 2018). Additionally, caffeine enhances long-term memory retention and reduces the symptoms associated with Parkinson’s disease (Ludwig et al. 2014).
Alkaloids, phenols, and oils from coffee beans are extracted by mechanical pressing, solvent extraction, or supercritical fluid extraction (Efthymiopoulos et al. 2019). Of these methods, supercritical fluid extraction is used to selectively remove chemical compounds using a solvent in its supercritical state, but this extraction process also reduces the undesirable organic pollutants, toxins, and pesticide residues present in natural products and food crops (Cavalcanti et al. 2012; Banchero et al. 2013; Jitrangsri et al. 2020). Carbon dioxide is an inexpensive, tasteless, and inert supercritical solvent used in pharmaceuticals, nutraceuticals, and food applications (Machmudah et al. 2011). The CO2 solvation power is modified by adding cosolvents and changing the temperature and pressure of the extraction (Akay et al. 2011). The most common cosolvents used are hexane, isopropanol, ethanol, ethyl acetate, or water (Couto et al. 2009; Andrade et al. 2012). However, the extraction yield of the bioactive compounds depends on the chemical parameters of the compound of interest (solubility, polarity, and molecular weight) and the extraction parameters (particle size, pressure, temperature, cosolvent concentration, time, and solvent flow rate). For example, the extraction yield of coffee oils with supercritical CO2-ethanol was higher than that obtained with supercritical CO2 alone (Couto et al. 2009). Bitencourt et al. (2018) found that supercritical extracts from crude green coffee oil contained free fatty acids and diterpenes.
Additionally, coffee oils extracted with supercritical CO2-ethanol contained phenolic compounds and caffeine (Andrade et al. 2012; Barbosa et al. 2014; Bitencourt et al. 2020). However, de Azevedo et al. (2008) reported that a low mass of caffeine and chlorogenic acids (approximately 30 mg) was extracted from green coffee beans using supercritical CO2-ethanol (5%) at 35.5 MPa and 60 °C. This result suggested inefficient extraction for chlorogenic acids under the supercritical conditions tested. In light of this, we decided to explore the impact of a higher cosolvent content that 5% and different supercritical conditions on the total phenolic compound content of green coffee extracts to obtain supercritical extracts with optimized amounts of chlorogenic acids and caffeine. This work aimed to investigate the impact of temperature, extraction pressure, and cosolvent content on the extraction yield and total phenolic compound content from green coffee beans according to response surface methodology. Then, the functional compounds, including 5-caffeoylquinic acid and caffeine and the fatty acid profile, were identified in the supercritical extract under the optimal extraction conditions.
Materials and methods
Materials
Green coffee beans (Coffea arabica) were harvested in the autumn of 2019 in Talpa de Allende, Jalisco, Mexico (20° 22′ 50″ N, 104° 49′ 19″ W). Fruits were husked and dried in an oven at a low temperature (approximately 50 °C). The proximate composition was 3.7% ash, 5.6% moisture, 13.3% proteins, 13.4% lipids, and 64.0% carbohydrates. Typically, the concentrations of caffeine and 5-caffeoylquinic acid in green coffee beans are ~ 10 mg/g−1 and ~ 29 mg/g−1, respectively (Farah 2012; Ruiz-Palomino et al. 2019). Folin-Ciocalteu reagent, 2,2-diphenyl-1-picrylhydrazyl hydrate (DPPH), 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), synthetic vitamin E, Trolox, (±)-6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid, gallic acid, potassium persulfate, sodium carbonate, ethanol, toluene, hexane, ethyl acetate, acetic acid, formic acid, and caffeine and 5-caffeoylquinic acid standards were purchased from Sigma-Aldrich (State of Mexico, Mexico). Dichloromethane was purchased from Fermont (Mexico City, Mexico). Carbon dioxide was acquired from Grupo-Infra (Jalisco, Mexico). The other chemical reagents purchased were of analytical grade.
Supercritical fluid optimization
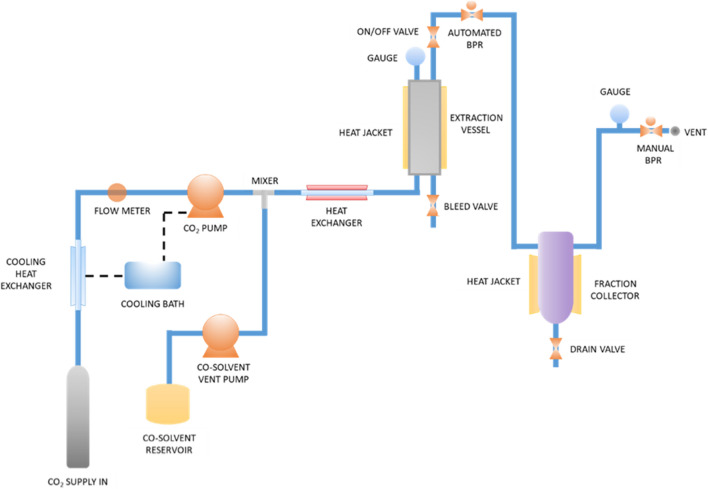
Dried green coffee beans were milled using a disk mill (Maren, Pulvex, City of Mexico, Mexico) and sifted through a 35-mesh sieve. The green coffee oil was extracted using a supercritical fluid extractor (SFE-500MR, Thar Designs, Inc., Pittsburgh, PA, USA), as shown in Fig. 1 (Waters 2018). One hundred forty-five grams of the milled beans were placed into the extraction vessel (500 mL) for each extraction. Carbon dioxide was mixed with ethanol (cosolvent) at predetermined ratios. The CO2-ethanol mixture was pumped into the extractor vessel with a constant total flow rate of 10 g min−1 for 180 min (Couto et al. 2009). Then, the extraction fluid was pressurized to the desired pressure and heated to the specified temperature to reach the supercritical state. The equipment software adjusted the solvent flow rates automatically (Table 1), i.e., the flow rate for CO2 varied from 8 to 9.5 g min−1, while the flow rate for ethanol was 0.5–2.0 g min−1. The impact of temperature (T, 50–70 °C), extraction pressure (P, 15.0–30.0 MPa), and cosolvent content (Co, 5–20 wt. %) on the extraction yield (EY) and total phenolic compound content (TPC) was evaluated using a face-centered central composite experimental design. The residual ethanol was removed after the supercritical extraction process by convection oven drying at 50 °C. The obtained GCSE was kept in amber flasks and stored at 4 °C until analysis.
Fig. 1.
Schematic diagram of the supercritical fluid extraction process
(Adapted from Waters (2018))
Table 1.
Experimental data of the face-center central composite design
| Name | T (°C) | P (MPa) | Co (%) | EY (%) | TPC (mg GAE g GCSE−1) |
|---|---|---|---|---|---|
| GCSE-1 | 50 | 15.0 | 5.0 | 0.8 | 0.15 |
| GCSE-2 | 70 | 15.0 | 5.0 | 0.6 | 0.49 |
| GCSE-3 | 50 | 30.0 | 5.0 | 4.0 | 0.01 |
| GCSE-4 | 70 | 30.0 | 5.0 | 4.1 | 0.58 |
| GCSE-5 | 50 | 15.0 | 20.0 | 6.1 | 3.85 |
| GCSE-6 | 70 | 15.0 | 20.0 | 7.1 | 1.36 |
| GCSE-7 | 50 | 30.0 | 20.0 | 8.0 | 3.74 |
| GCSE-8 | 70 | 30.0 | 20.0 | 8.1 | 4.95 |
| GCSE-9 | 50 | 22.5 | 12.5 | 5.5 | 2.34 |
| GCSE-10 | 70 | 22.5 | 12.5 | 6.1 | 2.03 |
| GCSE-11 | 60 | 15.0 | 12.5 | 5.4 | 3.85 |
| GCSE-12 | 60 | 30.0 | 12.5 | 5.5 | 3.62 |
| GCSE-13 | 60 | 22.5 | 5.0 | 2.9 | 0.24 |
| GCSE-14 | 60 | 22.5 | 20.0 | 6.6 | 5.82 |
| GCSE-15 | 60 | 22.5 | 12.5 | 5.4 | 2.46 |
| GCSE-16 | 60 | 22.5 | 12.5 | 5.3 | 2.62 |
| GCSE-17 | 60 | 22.5 | 12.5 | 5.9 | 2.78 |
The mean value is reported (n = 3). Green coffee supercritical extract (GCSE), temperature (T), pressure (P), cosolvent content (Co), extraction yield (EY), and total phenolic compound content (TPC)
Extraction yield (EY)
The extraction yield was estimated as the ratio of the GCSE mass recovered to the mass of the coffee beans by 100%.
Total phenolic compound content (TPC)
The total phenolic compound content was determined using Folin-Ciocalteu reagent (Jeszka-Skowron et al. 2016). Gallic acid was used as a standard (R2 = 0.995). Approximately 30 mg of GCSE was diluted in 2 mL of ethanol. An aliquot of 30 µL was mixed with 150 µL of 2 N Folin-Ciocalteu reagent (diluted with water, 1:10 v:v). After four minutes of reaction, 120 µL of Na2CO3 (0.71 M) was added, and the mixture was stored in the dark for one hour at 20 °C. The absorbance was measured spectrophotometrically at 765 nm (Multiskan™ GO, Thermo Scientific, Waltham, Massachusetts, USA). The total phenolic compound content was calculated and expressed as mg of gallic acid equivalent per g of the green coffee supercritical extract (mg GAE g GCSE−1).
Statistical analysis
The optimization conditions for supercritical CO2-ethanol extraction of oil from green coffee beans was carried out using RSM. A central composite face-centered 23 experimental design with three central points was used to evaluate the effects of the extraction pressure (P, MPa), temperature (T, °C), and cosolvent content (Co, %) on the extraction yield (EY) and total phenolic compound content (TPC). The experimental variables varied according to Table 1. All factors and levels tested were x1 for a low temperature (50 °C) and high temperature (70 °C), x2 for a low extraction pressure level (15.0 MPa) and high extraction pressure level (30.0 MPa), and, finally, x3 for a low cosolvent content (5%) and high content (20%). A second-order model fitted the experimental data, obtaining the regression coefficients:
| 1 |
where Y is the predictable response, xi is the code of the process variables, β0 is the interception value (constant), βi is the model coefficient linked to the linear effect, βii is a value related to the quadratic impacts, and βij is the coefficient for interaction effects. The quality of the adopted model fitting is expressed by the most important statistical factors, such as the coefficient of determination (R2), adjusted coefficient of determination (R2adj), lack of fit, model of F-value, and p values. The coefficient of determination was calculated according to .
Statgraphics Centurion XVII software (Version 17.0.16 Statpoint Technologies, Inc., Warrenton, VA, USA) was used for the statistical treatment of the results. The p values of the independent variables determined the regression coefficient importance. When the p value < 0.05, the examined factor showed statistical significance. The response surfaces were used to specify the interrelationships between significant variables. To determine the optimal extraction conditions for the GCSE, the study assessed the maximum values of the independent variables (T, P, and Co) and the responses (EY and TPC). The values of the determination coefficients (R2) and their adjusted values (R2adj) were used to assess the acceptability of regression models fit.
Analysis of variance (ANOVA) was performed to determine the differences between treatment means, according to Tukey’s test (p < 0.05).
Characterization of the GCSE
The DPPH and ABTS + radical scavenging activities, caffeine content, 5-caffeoylquinic acid content, and fatty acid composition of selected GCSEs were determined under the optimal conditions.
Free radical scavenging activity (DPPH)
The ability of the extracts to scavenge DPPH (2,2-diphenyl-1-picrylhydrazyl) radicals was measured according to a method reported by Jeszka-Skowron et al. (2016). Briefly, the GCSE was diluted in ethanol (15 mg mL−1). Then, the sample (20 μL) was mixed with 200 μL of an ethanolic DPPH solution (500 μM). The mixture was left to rest at room temperature in the dark for 30 min. The absorbance was evaluated spectrophotometrically at 516 nm. The results are expressed as the Trolox-equivalent antioxidant capacity (μM TEAC g GCSE−1). The synthetic vitamin E compound Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) was used as an antioxidant reference and was diluted in ethanol as a standard solution (R2 = 0.998).
ABTS+ radical scavenging activity
This assay was carried out according to a method reported by Jeszka-Skowron et al. (2016). Briefly, GCSE was diluted in ethanol (15 mg mL−1). Subsequently, an aliquot of 20 µL of the sample was mixed with 200 µL of the ABTS+ solution prepared 24 h earlier with 7 mM ABTS and 2.45 mM potassium persulfate. The solution was diluted in ethanol, reaching an absorbance of 0.70 ± 0.02 at 734 nm. The mixture was left for six minutes at room temperature in the dark. The absorbance was measured spectrophotometrically at 734 nm. The radical scavenging activity is expressed as the Trolox-equivalent antioxidant capacity (μM TEAC g GCSE−1).
Caffeine and 5-caffeoylquinic acid composition detection by high-performance thin-layer chromatography-UV (HPTLC-UV)
The caffeine and the 5-caffeoylquinic acid contents were estimated by HPTLC-UV equipment (Linomat 5, CAMAG, Muttenz, Switzerland). Samples and standards were applied on the surface of an HPTLC plate (20 cm × 10 cm). Application positions were at least 10 mm from the sides and 5 mm from the bottom of the HPTLC plate. A standard curve was generated with caffeine and 5-caffeoylquinic acid standards at concentrations from 0.5 to 7 µg mL−1. Five microliters of the samples were sprayed on the HPTLC plate with the help of a sample applicator under nitrogen gas flow. The mobile phase for caffeine consisted of a mixture of ethyl acetate/hexane/water/acetic acid (6:4:3:2 v/v/v/v). The mobile phase for 5-caffeoylquinic acid consisted of a mixture of ethyl acetate/toluene/dichloromethane/formic acid/water (11.00:1.95:0.73:0.65:0.65 v/v/v/v) (Ochoa Becerra 2020). The HPTLC plate was dried on a hot plate. Detection and densitometric scanning were performed by a TLC scanner (CAMAG, Muttenz, Switzerland) in adsorption mode at UV 273 nm for caffeine and 315 nm for 5-caffeoylquinic acid. The HPTLC equipment was controlled by winCATS software (CAMAG, Muttenz, Switzerland, version 1.4.4.6337).
Determination of fatty acid composition by GC
The fatty acid composition (linoleic, palmitic, oleic, stearic, and linolenic acids) of the GSCE was identified by GC. Samples were analyzed by a method described by Granados-Vallejo et al. (2019). The saponification reagent was a KOH solution (0.5 N). Boron trifluoride-methanol was used as the esterification reagent. The analysis was performed by gas chromatography (GC 7820, Agilent Technologies Inc., Palo Alto, CA, USA) equipment with a DB23 column (6 m × 0.25 i.d. × 0.25 μm of stationary phase) and a flame ionization detector. Quantification of the fatty acids was performed using external standards.
Results and discussion
Optimization of the supercritical extraction conditions
Supercritical fluid extraction is a useful technology for the extraction of oils from natural products. The response surface methodology evaluated the impact of the temperature, extraction pressure, and cosolvent content on the extraction yield and total phenolic compound content of the GCSE (Table 1). The extraction yield of the GCSEs ranged from 0.6–8.1%, while the total phenolic compound content ranged from 0.01–5.82 mg GAE g GCSE−1.
Extraction yield
The statistical significance of independent variables in the extraction yield was evaluated using ANOVA. The reduced ANOVA model for extraction yield is shown in Table 2. According to the p values obtained, only three factors significantly impacted extraction yield (p < 0.05): the extraction pressure, cosolvent content, and quadratic term of the cosolvent content. The other factors did not show significant effects on the extraction yield at the evaluated levels. However, the temperature and the interaction of P·Co were present in the mathematical model to maintain its robustness. In this sense, the lack of fit was not significant at the 0.05 level, and the experimental data fit well with the mathematical model. Additionally, the results showed a coefficient of determination of 0.9510, which means that the adopted quadratic model explained 95.10% of the data at the 95% confidence level. The small difference between the R2 and R2adj values suggested the adequacy of the reduced regression models for fitting the data. According to the coefficients of the mathematical model, the linear coefficients of the P, T, and Co content had positive effects on the extraction yield. However, the quadratic coefficient of Co content and the interaction P·Co showed a negative impact on the estimated maximum extraction yield value. The 3D surface plot displays the expected extraction yield as a function of extraction pressure and cosolvent content at 60 °C (Fig. 2a). We found that the cosolvent content increased the extraction yield from green coffee beans. The extraction yield predicted by the reduced regression model was approximately doubled when the cosolvent content increased from 5% to 20% at 30 MPa. For comparison purposes, the extraction yield was 0.9% using pure supercritical CO2, a pressure of 22.5 MPa, and a temperature of 60 °C. de Azevedo et al. (2008) and Ahangari and Sargolzaei (2013) suggested that the extraction pressure, rate flow, nature, and cosolvent content increased the extraction yield significantly in spent coffee grounds due to changes in the intermolecular interaction forces of the system. However, when the extraction pressure increased from 15 to 30 MPa, the extraction yield improved approximately four-fold at a low cosolvent content. Similar behavior was observed by Andrade et al. (2012) and Hurtado-Benavides et al. (2016). They suggested that the extraction yield increases at high extraction pressures due to an increase in the solvent density favoring the interaction between the solute and solvent. The mathematical model of the present work approached a maximum extraction yield at a high cosolvent content (approximately 20% ethanol). The optimal conditions predicted by the model for extraction yield (GCSEEY) were 7.68% using 20% cosolvent, a pressure of 30 MPa, and a temperature of 50 °C.
Table 2.
Reduced ANOVA and reduced regression model for extraction yield of the GCSE
| Source of variability | Sum of squares | Degree of freedom | Mean square | F ratio | p value |
|---|---|---|---|---|---|
| T | 0.256 | 1 | 0.256 | 2.48 | 0.2561 |
| P | 9.409 | 1 | 9.409 | 91.05 | 0.0108 |
| Co | 55.225 | 1 | 55.225 | 534.44 | 0.0019 |
| PCo | 1.805 | 1 | 1.805 | 17.47 | 0.0528 |
| Co2 | 2.35161 | 1 | 2.35161 | 22.76 | 0.0412 |
| Lack of fit | 3.3479 | 9 | 0.371989 | 3.60 | 0.2363 |
| Total error | 3.81057 | 2 | 0.103333 | ||
| Total (corr.) | 72.6012 | 16 | |||
| EY = − 6.68 + 1.6 × 10−2T + 2.35 × 10−1P + 8.39 × 10−1Co – 8.44 × 10−3PCo – 1.34 × 10−2Co2 | |||||
| R2 | 0.9510 | ||||
| R2adj | 0.9288 | ||||
T is the temperature (°C), P is the pressure (MPa), Co is the cosolvent concentration (%), EY is the extraction yield (%), and TPC is the total phenolic compound content (mg GAE g GCSE−1). Significant variables at the 0.05 level are presented in bold
Fig. 2.
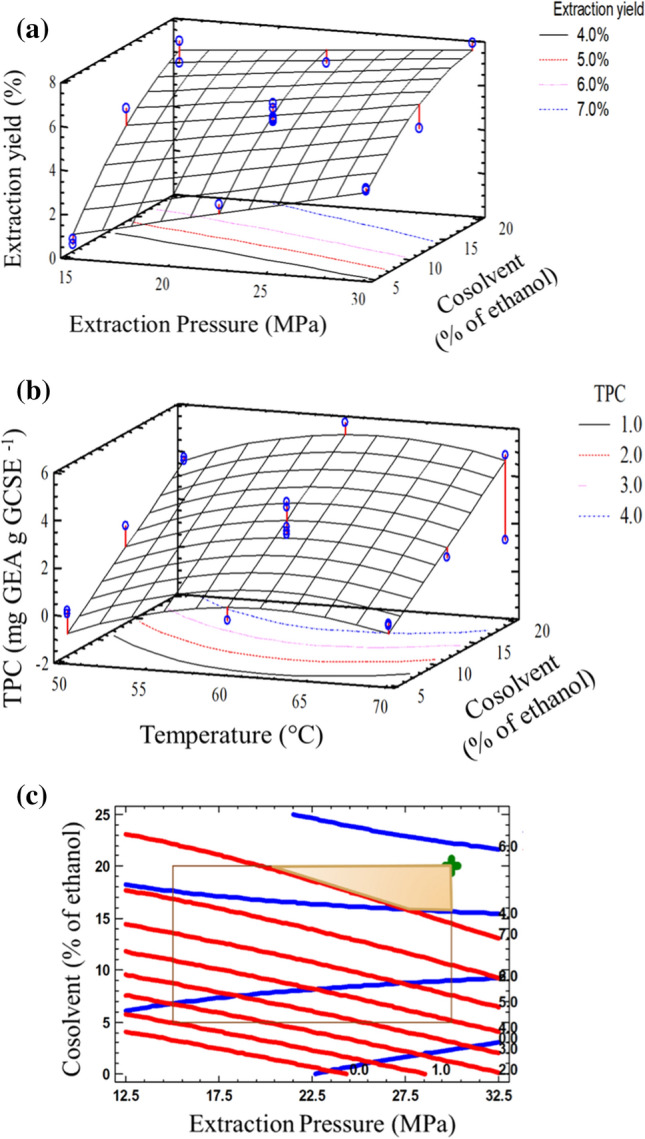
a Response surface plot of the extraction yield as a function of extraction pressure and cosolvent content at 60 °C, b Response surface plot of the total phenolic compound content as a function of the temperature and cosolvent content at 30 MPa, and c Overlaid contour plots of the extraction yield and total phenolic compound content at 60 °C. The shaded region is acceptable: total phenolic compound content > 4.0 and extraction yield > 7.0
Total phenolic compound content
The reduced ANOVA model for the total phenolic compound content is shown in Table 3. According to the p values, two factors significantly affected the total phenolic compound content (p < 0.05): the cosolvent content and the quadratic effect of the temperature. The other factors did not have a significant impact on the evaluated levels. However, the linear coefficients T, P and the interactions T·P and P·Co were maintained to preserve the robustness of the statistical model, making the lack of fit nonsignificant (p < 0.05). The reduced ANOVA model indicated a coefficient of determination of 0.8449, explaining 84.49% of the data by the quadratic model with a 95% confidence level. According to the mathematical model, the linear coefficients of the T and Co content and the interactions T·P and P·Co showed positive impacts on the total phenolic compound content, while the linear coefficient of P and the quadratic coefficient of T exhibited negative influences. The three-dimensional surface plot shows the estimated total phenolic compound content as a function of temperature and cosolvent content at 30 MPa (Fig. 2b). The addition of a cosolvent changes the solubility, density, transport properties, and intraparticle resistance in the green coffee beans, increasing the total phenolic compound content in the GCSE (de Azevedo et al. 2008; Akay et al. 2011). Akay et al. (2011) found that increasing the cosolvent content from 0% to 20% almost doubled the total phenolic compound content extracted from strawberries at 30 MPa and 80 °C. Similarly, Andrade et al. (2012) reported that the total phenolic compound content of spent coffee increased from approximately 24 to 57 mg chlorogenic acid equivalent g extract−1 with an increase in the ethanol content from 0% to 4% at 20 MPa and 50 °C. However, when the cosolvent content increased from 4% to 8%, a slight decrease was observed (approximately 42 mg chlorogenic acid equivalent g extract−1). Our results showed that the total phenolic compound content in the GCSE had a maximum value at 62 °C, independent of the cosolvent content (Fig. 2b). In general, when the temperature of extraction increases in the supercritical process, the solvation power increases. However, it has been reported that several bioactive compounds can be inactivated or degraded when the temperature reaches a critical value (Barbosa et al. 2014; Marques et al. 2016). The optimal condition predicted by the model for total phenolic compound content was 5.38 mg GAE g GCSE−1 using 20% cosolvent, a pressure of 30 MPa, and a temperature of 62 °C.
Table 3.
Reduced ANOVA and reduced regression model for TPC of the GCSE
| Source of variability | Sum of squares | Degree of freedom | Mean square | F ratio | p value |
|---|---|---|---|---|---|
| Co | 33.3282 | 1 | 33.3282 | 103.02 | 0.0002 |
| T2 | 5.03972 | 1 | 5.03972 | 15.58 | 0.0109 |
| T P | 1.93651 | 1 | 1.93651 | 5.99 | 0.0582 |
| PCo | 1.56291 | 1 | 1.56291 | 4.83 | 0.0793 |
| Lack of fit | 6.05054 | 7 | 0.764362 | 2.67 | 0.1484 |
| Total error | 1.61757 | 5 | 0.323514 | ||
| Total (corr.) | 49.5354 | 16 | |||
| TPC = − 28.75 + 1.18T – 4.92 × 10−1P + 6.66 × 10−2Co – 1.11 × 10−2T2 + 6.56 × 10−3TP + 7.86 × 10−3PCo | |||||
| R2 | 0.8449 | ||||
| R2adj | 0.7936 | ||||
T is the temperature (°C), P is the pressure (MPa), Co is the cosolvent concentration (%), EY is the extraction yield (%), and TPC is the total phenolic compound content (mg GAE g GCSE−1). Significant variables at the 0.05 level are presented in bold
The overlaid contour plots showed the impact of the pressure and cosolvent content on the extraction yield and total phenolic compound content of the GCSE at 60 °C (Fig. 2c). The shaded region shows that several combinations of pressure and cosolvent content were satisfactory to obtain a high extraction yield of GCSE rich in phenolic compounds. The symbol shows the desirability conditions (0.9374) within this region. The optimal extraction conditions were similar to the optimal total phenolic compound content conditions (GCSEOP, 20% cosolvent, a pressure of 30 MPa, and a temperature of 62 °C), predicting an extraction yield of 7.7% with a total phenol content of 5.4 mg gallic acid equivalent g GCSE−1.
Chemical characterization of the GCSE
The fatty acid composition of the GCSE was analyzed in samples from two different extraction conditions (Table 4): (a) GCSEEY (20% cosolvent, a pressure of 30 MPa, and a temperature of 50 °C) and (b) GCSEOP (20% cosolvent, a pressure of 30 MPa, and a temperature of 62 °C). The GSCEs contained significant amounts of unsaturated and polyunsaturated fatty acids, of which palmitic and linolenic acids were the most abundant. The GCSEs contained monounsaturated and polyunsaturated fatty acids of nutritional and health importance. Linoleic and α-linolenic acids are essential polyunsaturated fatty acids for human health, while palmitic, stearic, and oleic acids are important raw materials for the cosmetic industry (Hurtado-Benavides et al. 2016). Cornelio-Santiago et al. (2017) reported that the most abundant fatty acids in green coffee beans were linoleic (32–34%), palmitic (30–31%), and oleic (12–13%) acids. De Oliveira et al. (2014) showed a fatty acid composition of green coffee oil obtained by supercritical CO2 of 38% linoleic acid, 32% palmitic acid, and 12.8% oleic acid. The difference between our results and those of the other works could be due to the origin and harvest of the coffee beans.
Table 4.
Fatty acid, caffeine, and 5-caffeoylquinic acid contents and antioxidant activities of the green coffee oil obtained by supercritical fluid extraction under optimal conditions
| Compounds | GCSEEY | GCSEOP |
|---|---|---|
| Saturated fatty acids | 43.75 ± 0.02a | 43.64 ± 0.34a |
| Monounsaturated fatty acids | 7.52 ± 0.01a | 7.62 ± 0.04b |
| Polyunsaturated fatty acids | 42.85 ± 0.05a | 43.18 ± 0.31a |
| Palmitic acid (C16:0) | 36.18 ± 0.0a | 36.07 ± 0.28a |
| Stearic acid (C18:0) | 7.57 ± 0.02a | 7.57 ± 0.07a |
| Oleic acid (C18:1) | 7.52 ± 0.01a | 7.62 ± 0.04b |
| Linoleic acid (C18:2) | 41.47 ± 0.05a | 41.58 ± 0.29a |
| Linolenic acid (C18:3) | 1.39 ± 0.00a | 1.59 ± 0.01b |
| Caffeine (mg g−1) | 44.76 ± 5.02a | 79.51 ± 5.19b |
| 5-caffeoylquinic acid (mg g−1) | 11.53 ± 0.18a | 17.91 ± 0.72b |
| Antioxidant activity ABTS (TEAC) | 7.30 ± 0.19a | 10.98 ± 0.32b |
| Antioxidant activity DPPH (TEAC) | 41.42 ± 0.57a | 64.05 ± 0.97b |
The mean values are reported (± SD, n = 3). The same letter in a row indicates a significant difference, according to Tukey’s HSD test (p < 0.05). GCSEEY is the optimal conditions predicted for extraction yield, and GCSEOP is the extract with predicted optimal conditions
On the other hand, the concentrations of caffeine and 5-caffeoylquinic acid were quantified by using HPTLC (Table 4). The caffeine content varied between 44.76 and 79.51 mg g GCSE−1 (between approximately 35.8% and 61.2% of the caffeine in green coffee beans). The caffeine content in GCSEOP was 1.78 times higher than that in GCSEEY. Araújo et al. (2019) reported that the amount of caffeine in spent coffee ground extracts increased approximately 1.52 times at 80 °C compared with that in samples obtained at 40 °C. In this sense, according to Kopcak and Mohamed (2005), the positive effect of the cosolvent on the extraction of caffeine and 5-caffeoylquinic acid was enhanced with increasing temperature from 50 to 62 °C. This behavior is related to a decrease in the density of the solvents from 866 g/mL to 816 g/mL for GCSEEY and GCSEOP, respectively (estimated from Peng-Robinson equation state).
On the other hand, the 5-caffeoylquinic acid extracted content ranged from 11.53 to 17.91 mg g GCSE−1 (between approximately 3.1 and 4.7% of 5-caffeoylquinic acid in the green coffee beans). According to de Azevedo et al. (2008), chlorogenic-caffeine complexes are present in green coffee beans. These complexes are broken after the supercritical extraction process, increasing the concentration of the bioactive compound in the supercritical extract. However, these researchers reported traces of chlorogenic acids (lower than 0.5 mg) and a high amount of caffeine in green coffee supercritical extracts obtained with CO2-ethanol and CO2. Differences in the contents of bioactive compounds between this work and those reported in the literature were associated with the percent of cosolvent used. The solubility of 5-caffeoylquinic acid in CO2 is lower than that of caffeine due to its high molecular weight and its polar groups, which make caffeine easier to extract even with the addition of ethanol (Machmudah et al. 2011).
The antioxidant activities in GCSEOP were higher than those in GCSEEY. These compounds, such as chlorogenic acids and caffeine, could act as antioxidant agents, improving the chemical stability of green coffee oil and reducing the presence of free radicals. Similar results were found by Araújo et al. (2019), who reported that the antioxidant activity of spent coffee grounds increased with increasing temperature. Our results show that GCSEs could be used in human nutrition and cosmetic formulations due to the high amount of linoleic acid but also due to the presence of palmitic, stearic, and oleic acids and antioxidant compounds such as chlorogenic acids.
Conclusion
Green coffee oil was obtained using supercritical carbon dioxide with ethanol as a cosolvent. The extraction pressure presented a positive effect on the green coffee oil extraction yield. The addition of ethanol as a cosolvent significantly increased the extraction yield and the total phenol content. A second-order polynomial model predicted the extraction yield and total phenolic compound content with accuracies of 0.95 and 0.84, respectively, and the lack of fit was nonsignificant. The mathematical model obtained with the experimental design could predict the extraction yield and the total phenolic compound content of GCSE under the conditions analyzed. The optimum conditions to increase the extraction yield (7.7%) were obtained using a cosolvent content of 20%, 30.0 MPa, and 50 °C. A high total phenolic compound content (5.38 mg GAE g GCSE−1) was reached using cosolvent contents of 20%, 30 MPa, and 62 °C. The GCSE contained significant amounts of 5-caffeoylquinic acid (11.53–17.91 mg g GCSE−1), caffeine (44.76–79.51 mg g GCSE−1), linoleic acid (41.47–41.58%), and palmitic acid (36.07–36.18%). Although the use of cosolvent could increase the separation cost, the obtained GCSE contained several bioactive compounds with high commercial value. These results could promote the use of green coffee supercritical extract as a functional ingredient in the cosmetic and food industries.
Acknowledgements
This work was supported by the CONACYT [CB-2015-01-258118 and FORDECYT 292474]. The authors would like to thank CONACYT for scholarship No. #413405 (Barajas Álvarez, P.).
Abbreviations
- GCSE
Green coffee supercritical extract
- TEAC
Trolox equivalent antioxidant capacity
- TPC
Total phenolic compound content
- EY
Extraction yield
- Co
Cosolvent content
- GCSEEY
Green coffee supercritical extract at optimal yield extraction conditions
- GCSEOP
Green coffee supercritical extract under optimal conditions
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ahangari B, Sargolzaei J. Extraction of lipids from spent coffee grounds using organic solvents and supercritical carbon dioxide. J Food Process Preserv. 2013;37:1014–1021. doi: 10.1111/j.1745-4549.2012.00757.x. [DOI] [Google Scholar]
- Akay S, Alpak I, Yesil-Celiktas O. Effects of process parameters on supercritical CO2 extraction of total phenols from strawberry (Arbutus unedo L.) fruits: an optimization study. J Sep Sci. 2011;34:1925–1931. doi: 10.1002/jssc.201100361. [DOI] [PubMed] [Google Scholar]
- Andrade KS, Gonalvez RT, Maraschin M, et al. Supercritical fluid extraction from spent coffee grounds and coffee husks: antioxidant activity and effect of operational variables on extract composition. Talanta. 2012;88:544–552. doi: 10.1016/j.talanta.2011.11.031. [DOI] [PubMed] [Google Scholar]
- Araújo MN, Azevedo AQPL, Hamerski F, et al. Enhanced extraction of spent coffee grounds oil using high-pressure CO2 plus ethanol solvents. Ind Crops Prod. 2019;141:111723. doi: 10.1016/j.indcrop.2019.111723. [DOI] [Google Scholar]
- Babova O, Occhipinti A, Maffei ME. Chemical partitioning and antioxidant capacity of green coffee (Coffea arabica and Coffea canephora) of different geographical origin. Phytochemistry. 2016;123:33–39. doi: 10.1016/j.phytochem.2016.01.016. [DOI] [PubMed] [Google Scholar]
- Banchero M, Pellegrino G, Manna L. Supercritical fluid extraction as a potential mitigation strategy for the reduction of acrylamide level in coffee. J Food Eng. 2013;115:292–297. doi: 10.1016/j.jfoodeng.2012.10.045. [DOI] [Google Scholar]
- Barbosa HMA, de Melo MMR, Coimbra MA, et al. Optimization of the supercritical fluid coextraction of oil and diterpenes from spent coffee grounds using experimental design and response surface methodology. J Supercrit Fluids. 2014;85:165–172. doi: 10.1016/j.supflu.2013.11.011. [DOI] [Google Scholar]
- Bitencourt RG, Ferreira NJ, Oliveira AL, et al. High pressure phase equilibrium of the crude green coffee oil—CO2—ethanol system and the oil bioactive compounds. J Supercrit Fluids. 2018;133:49–57. doi: 10.1016/j.supflu.2017.09.017. [DOI] [Google Scholar]
- Bitencourt RG, Mello FMPA, Cabral FA, Meirelles AJA. High-pressure fractionation of spent coffee grounds oil using green solvents. J Supercrit Fluids. 2020 doi: 10.1016/j.supflu.2019.104689. [DOI] [Google Scholar]
- Cavalcanti RN, Meireles MAA, Sp C. Fundamentals of supercritical fluid extraction. Compr Sampl Sample Prep. 2012;2:117–133. doi: 10.1016/B978-0-12-381373-2.10039-0. [DOI] [Google Scholar]
- Cornelio-Santiago HP, Gonçalves CB, de Oliveira NA, de Oliveira AL. Supercritical CO2 extraction of oil from green coffee beans: Solubility, triacylglycerol composition, thermophysical properties and thermodynamic modelling. J Supercrit Fluids. 2017;128:386–394. doi: 10.1016/j.supflu.2017.05.030. [DOI] [Google Scholar]
- Couto RM, Fernandes J, da Silva MDRG, Simões PC. Supercritical fluid extraction of lipids from spent coffee grounds. J Supercrit Fluids. 2009;51:159–166. doi: 10.1016/j.supflu.2009.09.009. [DOI] [Google Scholar]
- de Azevedo ABA, Mazzafera P, Mohamed RS. Extraction of caffeine, chlorogenic acids and lipids from green coffee beans using supercritical carbon dioxide and cosolvents. Braz J Chem Eng. 2008;25:543–552. doi: 10.1590/S0104-66322008000300012. [DOI] [Google Scholar]
- De Oliveira PMA, De Almeida RH, De Oliveira NA, et al. Enrichment of diterpenes in green coffee oil using supercritical fluid extraction—characterization and comparison with green coffee oil from pressing. J Supercrit Fluids. 2014;95:137–145. doi: 10.1016/j.supflu.2014.08.016. [DOI] [Google Scholar]
- Efthymiopoulos I, Hellier P, Ladommatos N, et al. Effect of solvent extraction parameters on the recovery of oil from spent coffee grounds for biofuel production. Waste Biomass Valoriz. 2019;10:253–264. doi: 10.1007/s12649-017-0061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farah A. Coffee constituents. In: Chu Y-F, editor. Coffee: emerging health effects and disease prevention. 1. Hoboken: Blackwell Publishing Ltd; 2012. pp. 21–58. [Google Scholar]
- Farah A, Donangelo CM. Phenolic compounds in coffee. Braz J Plant Physiol. 2006;18:23–36. doi: 10.1590/S1677-04202006000100003. [DOI] [Google Scholar]
- Frost-Meyer NJ, Logomarsino JV. Impact of coffee components on inflammatory markers: a review. J Funct Foods. 2012;4:819–830. doi: 10.1016/j.jff.2012.05.010. [DOI] [Google Scholar]
- Granados-Vallejo M, Espinosa-Andrews H, Guatemala-Morales MG, et al. Oxidative stability of green coffee oil (Coffea arabica) microencapsulated by spray drying. Processes. 2019;7:734. doi: 10.3390/pr7100734. [DOI] [Google Scholar]
- Hurtado-Benavides A, Dorado DA, Sánchez-Camargo ADP. Study of the fatty acid profile and the aroma composition of oil obtained from roasted Colombian coffee beans by supercritical fluid extraction. J Supercrit Fluids. 2016;113:44–52. doi: 10.1016/j.supflu.2016.03.008. [DOI] [Google Scholar]
- Ilgaz S, Sat IG, Polat A. Effects of processing parameters on the caffeine extraction yield during decaffeination of black tea using pilot-scale supercritical carbon dioxide extraction technique. J Food Sci Technol. 2018;55:1407–1415. doi: 10.1007/s13197-018-3055-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeszka-Skowron M, Stanisz E, De Peña MP. Relationship between antioxidant capacity, chlorogenic acids and elemental composition of green coffee. LWT Food Sci Technol. 2016;73:243–250. doi: 10.1016/j.lwt.2016.06.018. [DOI] [Google Scholar]
- Jitrangsri K, Chaidedgumjorn A, Satiraphan M. Supercritical fluid extraction (SFE) optimization of trans-resveratrol from peanut kernels (Arachis hypogaea) by experimental design. J Food Sci Technol. 2020;57:1486–1494. doi: 10.1007/s13197-019-04184-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopcak U, Mohamed RS. Caffeine solubility in supercritical carbon dioxide/co-solvent mixtures. J Supercrit Fluids. 2005;34:209–214. doi: 10.1016/j.supflu.2004.11.016. [DOI] [Google Scholar]
- Liang N, Kitts DD. Role of chlorogenic acids in controlling oxidative and inflammatory stress conditions. Nutrients. 2015;8:16. doi: 10.3390/nu8010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig IA, Clifford MN, Lean MEJ, et al. Coffee: biochemistry and potential impact on health. Food Funct. 2014;5:1695–1717. doi: 10.1039/C4FO00042K. [DOI] [PubMed] [Google Scholar]
- Machmudah S, Kitada K, Sasaki M, et al. Simultaneous extraction and separation process for coffee beans with supercritical CO2 and water. Ind Eng Chem Res. 2011;50:2227–2235. doi: 10.1021/ie101252w. [DOI] [Google Scholar]
- Marques LLM, Panizzon GP, Aguiar BAA, et al. Guaraná (Paullinia cupana) seeds: selective supercritical extraction of phenolic compounds. Food Chem. 2016;212:703–711. doi: 10.1016/j.foodchem.2016.06.028. [DOI] [PubMed] [Google Scholar]
- Martínez-López S, Sarriá B, Mateos R, Bravo-Clemente L. Moderate consumption of a soluble green/roasted coffee rich in caffeoylquinic acids reduces cardiovascular risk markers: results from a randomized, cross-over, controlled trial in healthy and hypercholesterolemic subjects. Eur J Nutr. 2019;58:865–878. doi: 10.1007/s00394-018-1726-x. [DOI] [PubMed] [Google Scholar]
- Ochoa Becerra MA (2020) Extracción de cafeína y ácido clorogénico de la pulpa de café por medio de tecnologías no convencionales y su purificación mediante resinas poliméricas. Centro de Investigación y Asistencia en Tecnología y Diseño del Estado de Jalisco
- Onakpoya I, Terry R, Ernst E. The use of green coffee extract as a weight loss supplement: a systematic review and meta-analysis of randomised clinical trials. Gastroenterol Res Pract. 2011 doi: 10.1155/2011/382852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmioli A, Ciaramelli C, Tisi R, et al. Natural compounds in cancer prevention: effects of coffee extracts and their main polyphenolic component, 5-O-caffeoylquinic acid, on oncogenic Ras proteins. Chem Asian J. 2017;12:2457–2466. doi: 10.1002/asia.201700844. [DOI] [PubMed] [Google Scholar]
- Research-Reports 360 (2020) Global Chlorogenic Acid Market Research Report 2020. https://www.360researchreports.com/global-chlorogenic-acid-market-15083142. Accessed 28 Apr 2020
- Ruiz-Palomino P, Guatemala-Morales G, Mondragón-Cortéz PM, et al. Empirical model of the chlorogenic acid degradation kinetics during coffee roasting in a spouted bed. Rev Mex Ing Quim. 2019;18:387–396. doi: 10.24275/uam/izt/dcbi/revmexingquim/2019v18n2/Ruiz. [DOI] [Google Scholar]
- Servicio de Información Agroalimentaria y Pesquera (2019) Panorama Agroalimentario 2019. Ciudad de México
- Waters (2018) Supercritical Fluid Extraction (SFE) Systems. In: Waters Prep SFE Syst. http://www.waters.com/waters/en_US/SFE-extraction-equipment/nav.htm?locale=en_US&cid=134614431. Accessed 28 Apr 2020