Abstract
We show that expression of p57Kip2, a potent tight-binding inhibitor of several G1 cyclin–cyclin-dependent kinase (Cdk) complexes, increases markedly during C2C12 myoblast differentiation. We examined the effect of p57Kip2 on the activity of the transcription factor MyoD. In transient transfection assays, transcriptional transactivation of the mouse muscle creatine kinase promoter by MyoD was enhanced by the Cdk inhibitors. In addition, p57Kip2, p21Cip1, and p27Kip1 but not p16Ink4a induced an increased level of MyoD protein, and we show that MyoD, an unstable nuclear protein, was stabilized by p57Kip2. Forced expression of p57Kip2 correlated with hypophosphorylation of MyoD in C2C12 myoblasts. A dominant-negative Cdk2 mutant arrested cells at the G1 phase transition and induced hypophosphorylation of MyoD. Furthermore, phosphorylation of MyoD by purified cyclin E-Cdk2 complexes was inhibited by p57Kip2. In addition, the NH2 domain of p57Kip2 necessary for inhibition of cyclin E-Cdk2 activity was sufficient to inhibit MyoD phosphorylation and to stabilize it, leading to its accumulation in proliferative myoblasts. Taken together, our data suggest that repression of cyclin E-Cdk2-mediated phosphorylation of MyoD by p57Kip2 could play an important role in the accumulation of MyoD at the onset of myoblast differentiation.
Cell cycle progression in eukaryotes is controlled by a series of cyclin-dependent kinases (Cdks) which are in turn modulated by binding to specific cyclins. D-type cyclins (D1, D2, and D3) and cyclin E, termed G1 cyclins (48), are involved in regulating G1 progression and S-phase entry. Complexes that control mammalian G1 progression include cyclin E-Cdk2 and Cdk4/Cdk6 associated with any D-type cyclin and become activated upon phosphorylation of the Cdk subunit by CAK (Cdk-activating kinase), itself a Cdk-related kinase complex (49). These cyclin-Cdk complexes can regulate positively the cell cycle by phosphorylating pRB and thereby inhibit the activity of this cell cycle regulator (48, 57). The discovery of proteins that bind to and inhibit the catalytic activity of cyclin-Cdk complexes has identified kinase inhibition as an intrinsic component of cell cycle control (50). These Cdk inhibitors (Ckis) induce cell cycle arrest in response to antiproliferative signals, including contact inhibition and serum deprivation (42), transforming growth factor β (44), and myogenic (41), myeloid (32), and neuronal (26) differentiation. Ckis can be divided in two families (50, 60). The Ink4 family includes p16Ink4a, p15Ink4b, p18Ink4c, and p19ARF. These proteins specifically bind and inhibit Cdk4 and Cdk6 and not other Cdks such as Cdk2 (45). p21Cip1, p27Kip1, and p57Kip2, members of the other family of inhibitors, the Cip/Kip family, have the ability to inhibit all G1/S-phase cyclin-Cdk complexes (19, 49, 56). Although p21Cip1 expression during development correlates with terminally differentiating tissues, mice lacking p21Cip1 develop normally (9, 39). Similarly, p27Kip1-deficient mice have a grossly normal development and display only phenotypes that seem to be linked to cell proliferation (13, 24, 38). These data suggest the existence of compensatory mechanisms between p21Cip1 and p27Kip1 during development. p57Kip2 is also a tight-binding inhibitor of cyclin A/E-Cdk2 and cyclin D-Cdk4/Cdk6 complexes and a negative regulator of cell proliferation (25, 33). The expression pattern of p57 mRNA in various adult human tissues indicates that its distribution is more restricted than that of p21Cip1 and p27Kip1 (25, 33), suggesting that p57Kip2 has an important role during development (61, 62).
To undergo differentiation, myogenic cells have to exit the cell cycle through the G1 checkpoint. Myogenic differentiation is under the control of a family of muscle-specific transcription factors (MRFs) which includes MyoD (7), myogenin (12, 59), Myf5 (4), and MRF4 (45), also known as herculin (34) or Myf6 (5). These proteins share a central basic helix-loop-helix (bHLH) domain that is involved in DNA binding and protein-protein interactions (8). This 70-amino-acid region accounts for their ability to form heterodimers with the E-protein bHLH factors (34, 35), to bind as heterodimers to an E-box DNA consensus sequence (CANNTG) (8), to transactivate muscle genes, and to efficiently convert nonmuscle cells to a myogenic lineage (55, 58). MyoD is expressed in proliferating myoblasts prior to terminal differentiation (55). A number of molecular mechanisms have been proposed to explain the functional inactivation of MyoD in proliferating myoblasts and the coupling of muscle differentiation with the cell cycle arrest (39, 40). These regulatory pathways modulate one or more aspects of myogenic bHLH protein functions such as dimerization with E-protein DNA binding, transactivation, and direct or indirect interaction with cofactors such as MEF-2 (35), pRB (14), p300/CBP (11), or the protein kinase Mos (29). Functional inactivation includes inhibitory phosphorylation of myogenic bHLH proteins (18, 30, 31), inhibition of the myogenic bHLH function via the Id family of dominant-negative HLH factors (2), and either direct or indirect inhibition by the cyclin D-dependent kinases (43, 51). It has been previously shown that overexpression of cyclinD-Cdk complexes inhibited myogenic transcriptional activation mediated by MyoD (15, 16). The role of Cdks in inhibiting muscle differentiation has been substantiated by the observation that forced expression of p21Cip1 or p16Ink4a in mitogen-stimulated myoblasts facilitates muscle differentiation in the absence of mitogen deprivation, suggesting that an active cyclin-Cdk complex suppresses MyoD function in proliferating myoblasts (51). It has been recently demonstrated that Cdk phosphorylation of MyoD can target this protein for rapid degradation (52). Indeed, recent data show that direct phosphorylation of MyoD Ser200 by Cdk1 or Cdk2 plays a crucial role in modulating MyoD half-life and myogenic activity (23). Although Ckis appear to be involved in both repression of cyclin-Cdk complexes and activation of MyoD in proliferating myoblasts, no direct relationship between these two events has been described to date.
In the present work, we show that p57Kip2 protein expression increases markedly during the early phases of myogenic differentiation. We show that in transient transfection assays, transcriptional transactivation of the mouse creatine kinase (MCK) promoter by MyoD is enhanced by p57Kip2. The Cip/Kip protein family but not p16Ink4a increases the expression level of MyoD. MyoD, an unstable nuclear protein, is stabilized by p57Kip2. In proliferating C2C12 myoblasts, forced expression of p57Kip2 represses phosphorylation of MyoD. A dominant-negative form of Cdk2 (Cdk2 DN) which arrests cells at the G1 phase also induces hypophosphorylation of MyoD. We demonstrate that phosphorylation by cyclin E-Cdk2 complexes of MyoD is inhibited by p57Kip2. In addition, the conserved cyclin and Cdk binding domain of p57Kip2 necessary for the inhibition of cyclin E-Cdk2 activity was sufficient to stabilize MyoD, leading to its accumulation in proliferative myoblasts. Taken together, our data suggest that repression of cyclin E-Cdk2-mediated phosphorylation of MyoD by p57Kip2 could play an important role in the stability and activity of MyoD during myoblast proliferation.
MATERIALS AND METHODS
Plasmids.
pEMSV-MyoD, pGEX-3X-MyoD, and pEMSV-E12 were generous gifts from the H. Weintraub laboratory. Expression vector pCMV-HA-MyoD was generated by cloning three hemagglutinin epitope (HA) tags at the amino terminus of the cDNA insert in pcDNA3 (InVitrogen). The MCK-chloramphenicol acetyltransferase (CAT) reporter plasmid (p1256MCK), generously provided by S. Hauschka, contains the mouse MCK promoter-enhancer region (6). pEX10X-p57Kip2 and pBIISK-p27Kip1 were kind gifts from J. Massagué. pCEP-WAF1 was a generous gift of B. Vogelstein. CMV-p16Ink4a was a kind gift from B. Heinglein. Cytomegalovirus (CMV)-Cdk2 and CMV-Cdk2 DN were generous gifts from the Ed Harlow laboratory. Cyclin E-Cdk2 was a kind gift from B. Ducommun. To create expression vectors, fragments containing the entire coding sequences were cloned into expression vectors pcDNA3 and/or pEMSV.
pEMSV-p57Kip2 was obtained by inserting the NcoI-HindIII fragment (filled in with Klenow polymerase) from p57Kip2 cDNA into expression plasmid pEMSV at the EcoRI site, which was filled in with Klenow polymerase. pEMSV-p57ΔQT was obtained by inserting the EcoRI-SmaI fragment from pEMSV-p57Kip2 at EcoRI-SmaI sites of green fluorescent protein (GFP) expression plasmid pEGFP-CI (Clontech). The resultant plasmid was used as an intermediate to generate an EcoRI-MluI (filled) fragment, inserted into pEMSV at the EcoRI site filled in with Klenow polymerase. pEMSV-p57ΔCKI was generated by inserting in frame the PvuII-HindIII fragment from p57Kip2 at the PvuII-HindIII sites of pRSET-C (InVitrogen). This plasmid was further used as an intermediate to generate an NdeI-HindIII fragment filled in with Klenow polymerase and inserted into pEMSV at the EcoRI site filled in with Klenow polymerase. pEMSV-p57CKI was generated by inserting the EcoRI-PvuII fragment from p57Kip2 at the EcoRI-Sma sites of pEGFP-C1. This plasmid was used as an intermediate to generate an EcoRI-MluI fragment filled in with Klenow polymerase and inserted into pEMSV at the EcoRI site filled in with Klenow polymerase. All constructions were tested by in vitro translation using the T3 polymerase site of pEMSV.
pGEX-2TKP-p57Kip2 was obtained by inserting in frame the NcoI-HindIII fragment from pEX10X-p57Kip2 into the NcoI-HindIII sites of expression plasmid pGEX-2 TKP (a generous gift of L. Kouzarides). pGEX-2T-p57CKI was constructed by inserting in frame the SmaI-PvuII fragment from pGEX-2TKP-p57Kip2 at the SmaI site of expression plasmid pGEX-2T (Pharmacia). pGEX-2TK-p57ΔCKI was constructed by deleting an NcoI-BglII fragment of pGEX-2TKP-p57Kip2. pGEX-2TK-p57ΔQT was generated by inserting in frame the BlpI (filled)-HindIII fragment obtained from pEX10X-p57Kip2 at the SmaI (filled) site of pGEX-2TK.
Cell cultures, DNA transfections, and CAT assays.
The mouse skeletal muscle cell line C2C12 and the fibroblast cell line C3H10T1/2 were maintained in growth medium (GM) supplemented with antibiotics (a mixture of penicillin and streptomycin [Life Technologies, Inc.]) and with 20 and 15% of fetal calf serum (FCS) in Dulbecco’s modified Eagle’s medium (DMEM), respectively. C2C12 cells were transfected by the calcium phosphate procedure as previously described (28). C3H10T1/2 fibroblasts were transfected by using polyethyleneimine essentially as described previously (3). Briefly, 4 × 104 cells per well were plated onto 24-well plates. On the following day, cells were transfected with various combinations of plasmids as indicated in the figure legends. The total amount of DNA used for each plate was normalized with the relevant empty expression vehicle. CAT activity was determined with aliquots of cell extracts from harvested cells 48 h after transfection in GM as previously described (28). Five hundred nanograms of plasmid pCH110 (Pharmacia) was included in transfections as an internal control for transfection efficiency. All CAT activities were determined with equivalent quantities of proteins in triplicate, and assays were repeated at least twice. A phosphorimager system was used to determine the amount of 14C-labeled reaction products and substrate from thin-layer chromatographic plates.
Metabolic labeling.
Cell cultures were incubated for 1 h in methionine-free medium supplemented with 5% FCS followed by incubation for 30 min in the same medium with 300 μCi of [35S]methionine (Trans35S-label; ICN) per ml. In cold-chase experiments, the [35S]methionine-labeled cultures were rinsed with fresh medium supplemented with 10 mM nonradioactive methionine and 15% FCS and harvested at appropriate times. For immunoprecipitation of HA-MyoD, cells were lysed as described as follows.
Kinase assay.
Cyclin E-Cdk2 complexes were mixed with purified glutathione S-transferase (GST) or GST-p57 fusion proteins in 50 mM HEPES (pH 8.0) and incubated for 1 h at 4°C. The kinase reaction was carried out at 30°C for 30 min in a 30-μl reaction mixture containing 50 mM HEPES (pH 8.0), 10 mM MgCl2, 2.5 mM EGTA, 1 mM dithiothreitol, 10 mM β-glycerophosphate, 1 mM NaF, 0.1 mM Na3VO4, 0.1 mM phenylmethylsulfonyl fluoride, 10 μM ATP, and 150 kBq of [γ-32P]ATP (5,000 Ci/mmol; Amersham). One microgram of GST-MyoD or MyoD (29) was used as the substrate. The reaction products were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and phosphorylated proteins were detected by autoradiography and quantitated with a Fuji BAS-1000 imaging analyzer.
Antibodies, immunoprecipitation, and Western blot analyses.
For immunoprecipitation, precleared cell lysates in immunoprecipitation buffer (50 mM Tris [pH 7.4], 150 mM NaCl, 10% glycerol, 0.5% NP-40, 0.5 mM sodium orthovanadate, 50 mM NaF, 80 μM β-glycerophosphate, 10 mM sodium pyrophosphate, 1 mM dithiothreitol, 1 mM EGTA, 10 μg each of leupeptin, pepstatin, and aprotinin per ml) were incubated with the indicated antibody for 2 to 3 h at 4°C with gentle agitation. Immunocomplexes bound to protein G-Sepharose were collected by centrifugation and washed several times in immunoprecipitation buffer. Immunoprecipitated proteins were resolved by SDS-PAGE (10% gel) followed by fluorography (35S-labeled proteins) and autoradiography.
For immunoblot analyses, total-cell extracts or immunoprecipitates were solubilized in radioimmunoprecipitation assay-EGTA buffer and processed as previously described (27). Analyses were performed on 10% polyacrylamide gels with a 5% polyacrylamide stacking gel. After electrophoretic transfer of proteins from SDS-polyacrylamide gels to nitrocellulose membranes, the membranes were blocked with 50 mM Tris-HCl (pH 7.4)–150 mM NaCl–0.05% Tween 20 containing 5% skimmed milk and incubated overnight at 4°C with primary antibodies: polyclonal anti-MyoD antibody C-20 diluted 1/500, polyclonal anti-mouse p57Kip2 antibody E-17 diluted 1/250, anti-monoclonal anti-p27Kip1 antibody F8 diluted 1/250, monoclonal anti-p21Cip1 antibody F5 diluted 1/250, and polyclonal anti-p16 antibody M-156 diluted 1/250 (provided by Santa Cruz Biotechnology, Santa Cruz, Calif.); monoclonal anti-HA antibody 12CA5 (provided by Boehringer Mannheim); and monoclonal anti-troponin T antibody JTL-12 (supplied by Sigma). Membranes were washed and incubated 1 h with a peroxidase-conjugated secondary antibody (Sigma) at a dilution of 1/10,000 with polyclonal antibodies or 1/4,000 with monoclonal antibodies. After several washes, membranes were incubated with an enhanced chemiluminescence (ECL) system (Amersham) according to the manufacturer’s instructions. Exposure was done with Agfa Curix RP2 films and intensifying screens.
Phosphatase treatment.
Extracts normalized to protein content were immunoprecipitated with the mouse monoclonal anti-MyoD antibody 5.8A (Pharmingen) and protein G-Sepharose beads. After immunoprecipitation, beads were washed twice with ECB buffer without phosphatase inhibitors and resuspended in 50 μl of calf intestine phosphatase (CIP) buffer (10 mM Tris-HCl [pH 8], 1 mM MgCl2, 1 mM phenylmethylsulfonyl fluoride) for phosphatase treatment with 50 U of CIP (New England Biolabs) for 60 min at 50°C. Proteins were separated by SDS-PAGE, immunoblotted with anti-MyoD polyclonal antibody C-20 (Santa Cruz), and analyzed by ECL.
RESULTS
Increase in p57Kip2 protein levels during the differentiation of C2C12 myoblasts to myotubes.
Little is known about the role of p57Kip2 in myogenic differentiation; we initially characterized p57Kip2 expression during early myogenic differentiation by Western blot analysis using the C2C12 cell line, a well-defined model for ex vivo differentiation. These cells proliferate as myoblasts in high serum concentrations and can be induced to differentiate by reducing the serum concentration from 20% to 2%. Under our conditions, C2C12 myoblasts fuse into myotubes within 48 to 60 h with an efficiency of 75 to 80%. As shown in Fig. 1A, C2C12 myoblasts express p57Kip2 that migrates as a doublet around 57 kDa, as previously observed in SAOS-2 cells (33). The presence of p57Kip2 protein in our C2C12 cell line has been confirmed by using four antibodies directed against four different epitopes of p57Kip2 protein (data not shown). p57Kip2 is detectable in myoblasts as MyoD and accumulates at high levels (about 10-fold) in C2C12 myotubes. In contrast, troponin T, a skeletal muscle marker, is observed only in differentiated cells, and Cdk4 is equally expressed in both myoblasts and myotubes (51, 63). As Ckis such as p21Cip1, p27Kip1, and p16Ink4a have been implicated in differentiation and cell cycle arrest, we also analyzed their protein expression in C2C12 myogenic differentiation by Western blot analyses (Fig. 1A). p21Cip1 expression was stimulated by threefold in myoblasts cultured in differentiation medium (DM), in agreement with previous observations (17). p27Kip1 was induced in C2C12 cells following culture in DM, but this enhancement was only twofold between proliferating myoblasts and differentiated myotubes. In contrast to the Cip/Kip protein family, p16Ink4a protein was not observed in proliferating myoblasts and was barely detectable during myogenic differentiation. As shown in Fig. 1B, p57Kip2 expression levels increase as soon as 4 h after cells are placed in DM and peak after 24 h of differentiation. By this time, MyoD is also up-regulated and then slightly decreases during differentiation. Altogether, these results suggest that p57Kip2 may play a particular role during early differentiation.
FIG. 1.
Protein expression of Ckis during muscle differentiation. C2C12 myoblasts were cultured in high-mitogen medium (20% FCS-containing GM (A, lane 1) or in low-mitogen medium (2% FCS DM) for 48 h (A, lane 2) or for increasing various times as indicated (B, lanes 2 to 6). For each stage, total-cell extracts corresponding to 150 μg of proteins were resolved by SDS-PAGE on a 10% gel, and MyoD, troponin T, p16Ink4a, p21Cip1, p27Kip1, and p57Kip2 were detected by Western blot analysis using specific antibodies as described in Materials and Methods. Exposure times were 5 min for the Cip/Kip protein family, 1 min for MyoD, 1 min for troponin T, and 60 min for p16Ink4a.
p57Kip2 enhances the effect of MyoD transcription on MCK expression.
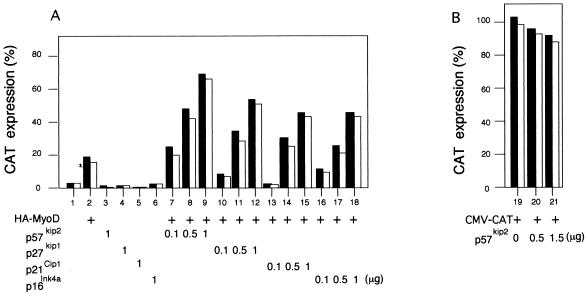
Transient transfection assays were performed to determine the effect of overexpression of p57Kip2 on MyoD-mediated transcription of muscle-specific genes. C3H10T1/2 cells were transiently transfected with expression vectors encoding MyoD and/or either p16Ink4a, p21Cip1, p27Kip1, or p57Kip2 Ckis along with a skeletal muscle reporter construct containing 1,256 bp from the MCK promoter driving expression of CAT (MCK-CAT) (6). MCK-CAT is not expressed in C3H10T1/2 cells when transfected alone, but it was activated efficiently by cotransfection with a MyoD expression vector (Fig. 2A, lanes 1 and 2, respectively). In a dose-dependent manner, expression of p16Ink4a, p21Cip1, p27Kip1, or p57Kip2 increased the transactivation of MCK-CAT by MyoD, with the highest increase (about 3- to 3.5-fold) observed in the presence of p57Kip2 (lanes 7 to 9). The vector alone (Fig. 2A, lane 1) or the Ckis alone (lanes 3 to 6) had no effect on MCK promoter activity. In contrast, transactivation of a non-muscle-specific reporter (CMV-CAT) was not significantly altered (reduced by 10 to 15%) when cotransfected with p57Kip2 compared to control cells, indicating that p57Kip2 has no significant effect on the CMV promoter (Fig. 2B, lanes 19 to 21). These results indicate that transactivation of MCK-CAT expression by MyoD is increased by the addition of a Cki expression vector and suggest a functional interaction between Ckis and MyoD during myogenesis.
FIG. 2.
Effect of ectopically expressed Ckis on MyoD-dependent transcriptional transactivation of the MCK enhancer-promoter. C3H10T1/2 cells were cotransfected with 0.5 μg of MCK-CAT reporter plasmid (A, lanes 1 to 18) or a non-muscle-specific reporter (CMV-CAT) (B, lanes 19 to 21) together with 1 μg of an expression vector encoding HA-MyoD (lane 2), pCMV-p57Kip2 (lane 3), pCMV-p27Kip1 (lane 4), pCMV-p21Cip1 (lane 5), or pCMV-p16Ink4a (lane 6) or with 0.5 μg of pCMV-HA-MyoD (lanes 7 to 18) and increasing amounts of p57Kip2 (lanes 7 to 9), p27Kip1 (lanes 10 to 12), p21Cip1 (lanes 13 to 15), or p16Ink4a (lanes 16 to 18). Expression vector pCMV without insert was included to normalize DNA in all transfections. CAT levels were determined 48 h after transfection in high-serum (20% FCS) medium. Protein concentration was equalized by the Bradford method. Typically, 15 μg of total-cell extract was used for the reaction. CAT activities in duplicate plates (black and white bars) from a representative experiment are expressed as percentages of acetylated forms of chloramphenicol versus the nonacetylated substrate.
p57Kip2 increases the level of MyoD in cotransfected cells.
We next examined the influence of ectopic expression of p16Ink4a and p57Kip2 on the levels of coexpressed MyoD in transiently transfected C3H10T1/2 fibroblasts. Immunoblotting analyses revealed that p57Kip2 increased the steady-state level of coexpressed MyoD (Fig. 3) in a dose-dependent manner. This much higher level of MyoD was specific for the Cip/Kip proteins because coexpression of p27Kip1 (Fig. 3, lanes 9 to 11) also increased MyoD expression whereas the p16Ink4a expression vector (lanes 6 to 8) or an empty vector (lanes 3 to 5) had no effect.
FIG. 3.
Differential accumulation of MyoD protein by ectopic expression of Ckis. (A) Immunoblot showing exogenous MyoD and Ckis in transiently transfected C3H10T1/2 cells. As for Fig. 2, C3H10T1/2 cells were transiently transfected with 0.5 μg of expression vector encoding HA-MyoD (lanes 2 to 14) plus 0.5, 1, or 1.5 μg of empty expression vector (lanes 3 to 5) or 0.5, 1, or 1.5 μg of pCMV-p16Ink4a (lanes 6 to 8), pCMV-p27Kip1 (lanes 9 to 11), or pCMV-p57Kip2 (lanes 12 to 14). Whole-cell lysates (10 μg) were separated by SDS-PAGE. Proteins were transferred to nitrocellulose and immunoblotted with monoclonal antibody 12CA5 (Boehringer Mannheim) and visualized by ECL (Amersham). (B) Fifty-microgram aliquots of the same lysates from transfected cells were analyzed for expression of exogenous p16Ink4a, p27Kip1, and p57Kip2 by Western blotting with an anti-p16Ink4a (M-156; Santa Cruz), anti-p27Kip1 (F8; Santa Cruz), or anti-p57Kip2 (E-17; Santa Cruz) polyclonal antibody.
p57Kip2 increases the stability of MyoD.
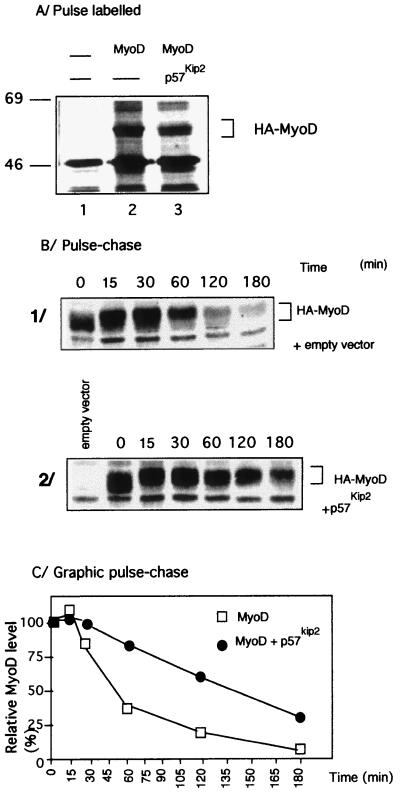
To elucidate the mechanism by which the levels of coexpressed MyoD is enhanced by the Cip/Kip protein family and especially by p57Kip2, we first tested whether p57Kip2 affected transcription or translation of the cotransfected MyoD. Northern blot analyses showed that the level of MyoD mRNA did not differ significantly between expression of MyoD alone and coexpression of MyoD and p57Kip2 (data not shown). Furthermore, the amount of newly synthesized MyoD was measured by immunoprecipitation analysis with the anti-HA monoclonal antibody in cells pulse-labeled for 30 min with [35S]methionine. The amounts of MyoD did not differ significantly between the single or pairwise transfections of MyoD (Fig. 4A, lanes 2 and 3). The metabolic stability of MyoD was investigated by a pulse-chase experiment. Immunoprecipitation analyses revealed that MyoD has a half-life of 40 to 50 min (Fig. 4B and C), in agreement with previously reported results (1, 23, 52, 55). Surprisingly, when coexpressed with p57Kip2, MyoD showed a half-life extended to 140 min (Fig. 4B and C). This result suggests that the increased level of MyoD observed upon coexpression with p57Kip2 is due to the stabilization of the protein by p57Kip2.
FIG. 4.
Stabilization of MyoD protein by p57Kip2 coexpression. (A) Pulse-labeling experiment showing the initial translation rate of MyoD with or without p57Kip2 coexpression. C3H10T1/2 fibroblasts transfected with either control vector alone (lane 1), HA-MyoD expression vector alone (lane 2), or HA-MyoD plus pCMV-p57Kip2 vector (lane 3) were labeled for 30 min with [35S]methionine. Cell lysates having the same radioactive counts were subjected to immunoprecipitation with specific monoclonal antibody 12CA5 and analyzed by SDS-PAGE and autofluorography. (B) Pulse-chase of 35S-labeled MyoD. C3H10T1/2 fibroblasts were cotransfected by pCMV-HA-MyoD and empty expression vector (1) or cotransfected by pCMV-HA-MyoD and pCMV-p57Kip2 expression vectors (2). Cells were cultured in methionine-free medium for 1 h and then pulsed with 300 μCi of [35S]methionine per ml for 30 min. Following incubation, cells were washed with medium containing an excess of unlabeled methionine (10 mM) and chased for the indicated times. Cell extracts were immunoprecipitated with specific monoclonal antibody 12CA5. One 60-mm-diameter dish of transfected cells was used for each time point. This experiment was performed three times. (C) Graphic display of intensities of MyoD shown in panel B. Results are representative of three independent experiments. For quantitation, the autoradiograms were scanned with a phosphorimager (Fuji).
MyoD is a substrate for phosphorylation by cyclin E-Cdk2 complexes.
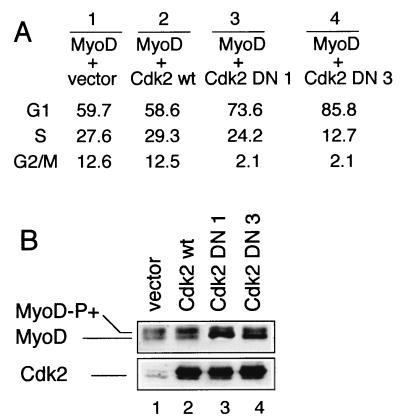
Cdk-mediated phosphorylation is known to be often involved in proteolytic degradation; we next examined whether p57Kip2 induces an increased stability of MyoD by blocking Cdk2-dependent phosphorylation of MyoD. As shown in Fig. 5A, MyoD is detected as a doublet following transfection of wild-type MyoD (MyoDwt). These two bands contain rapidly and slowly migrating forms of MyoD (Fig. 5A, lane 1). The slowly migrating form is shown to be the hyperphosphorylated species and was resolved into a single fast-migrating species after treatment with the CIP (Fig. 5A, lanes 1 and 2). Because p57Kip2 is known as a potent inhibitor of G1- and S-phase Cdks (25), we tested its ability to inhibit and/or to reduce hyperphosphorylation of MyoD. For this purpose, a p57Kip2 expression vector was transiently transfected into C2C12 myoblasts, transfected cells were grown for 48 h in GM, and MyoD expression was monitored by Western blotting of whole-cell extracts. As shown in Fig. 5B, in C2C12 myoblasts, MyoD is detected as two bands with a major slowly migrating hyperphosphorylated form following transfections with the empty vector. In contrast, the fast-migrating band corresponding to the hypophosphorylated form of MyoD accumulates in p57Kip2-transfected myoblasts compared with control cells transfected with vector alone (Fig. 5B and C). These results show that in vivo, Cdk-dependent phosphorylation of MyoD can be repressed by p57Kip2. Recently Cdk1 and Cdk2 have been shown to phosphorylate MyoD in vitro (23). To verify that Cdk2 phosphorylates MyoD in vivo, Cdk2 DN clones 1 and 3 were cotransfected with MyoD in C2C12 proliferating myoblasts. A shown in Fig. 6A, expression of Cdk2 DN clones 1 and 3 (lanes 3 and 4) caused a large increase in the G1 population, whereas wild-type Cdk2 (Cdk2 wt; lane 2) did not affect the cell type distribution. Expression of Cdk2 DN but not Cdk2 wt had noticeable effect on the phosphorylation of MyoD (Fig. 6B, lanes 2 to 4) with respect to MyoD phosphorylation in cells cotransfected with the empty vector (Fig. 6B, lane 1). Expression of Cdk2 DN induces hypophosphorylation of MyoD, as evidenced by the accumulation of the fast-migrating form of MyoD. Altogether, these data strongly suggest that in vivo p57Kip2 inhibition of Cdk2 activity plays a role in MyoD-mediated myoblast differentiation.
FIG. 5.
Forced expression of p57Kip2 reduces MyoD phosphorylation in growing myoblasts. (A) Total cellular proteins from C3H10T1/2 fibroblasts transfected by pEMSV-MyoDwt were split in two, immunoprecipitated with a MyoD monoclonal antibody (5.8A; Pharmingen), and resuspended in SDS-containing sample medium (lane 1) or treated with CIP (lane 2) as described in Materials and Methods. The immunoprecipitates were then subjected to SDS-PAGE and transferred to a nitrocellulose membrane. MyoD was detected by Western blot analysis using anti-MyoD polyclonal antibody C-20 (Santa Cruz). (B) C2C12 myoblasts were transfected with either the empty vector (lane 3) or pCMV-p57Kip2 (lane 4). Transfected cells were grown in GM (DMEM containing 20% FCS) for 48 h. Cells were collected, and 50 μg of whole-cell extract was analyzed by Western blot for MyoD and p57Kip2 expression. (C) The signals were quantitated with a Gel Scan (Pharmacia).
FIG. 6.
Cdk2 DN causes the accumulation of C2C12 myoblasts in G1 and reduces hyperphosphorylation of MyoD. (A) C2C12 proliferating myoblasts were transiently cotransfected with 0.5 μg of CMV-GFP plasmid in combination with 7.5 μg of the CMV vector (lane 1), 7.5 μg of the CMV-Cdk2 wt (lane 2), or 7.5 μg of Cdk2 DN clone 1 (lane 3) or 3 (lane 4). The cells were harvested 48 h after transfection, visualized for GFP fluorescence, stained for DNA content, and analyzed by flow cytometry. (B) Fifty-microgram aliquots of total-cell lysates were used for the expression pattern of MyoD and analyzed by protein immunoblotting with anti-MyoD antibody C-20 (Santa Cruz). The same blot was also analyzed by protein immunoblotting with polyclonal anti-Cdk2 antibody M2 (Santa Cruz).
Phosphorylation of MyoD by cyclin E-Cdk2 is inhibited by p57Kip2.
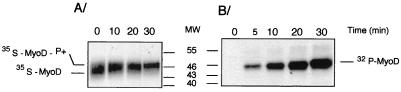
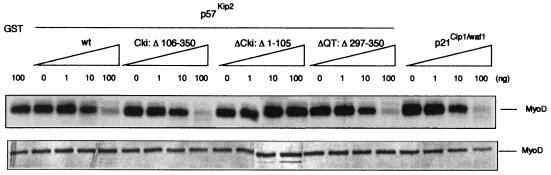
To assess the consequence of inhibition of Cdk2 activity by p57Kip2 upon MyoD phosphorylation, MyoDwt was translated in the presence of [35S]methionine in rabbit reticulocyte lysate and subjected to phosphorylation by purified cyclin E-Cdk2. Phosphorylated proteins were separated by SDS-PAGE and visualized by autoradiography. As shown in Fig. 7A, phosphorylation of MyoDwt by cyclin E-Cdk2 resulted in a decrease of its electrophoretic mobility. This slower-migrating form corresponded to phosphorylation, as shown by 32P incorporation in Fig. 7B. We next examined whether p57Kip2 was sufficient for the inhibition of cyclin E-Cdk2 activity upon MyoD phosphorylation. We generated GST-fused wild-type p57Kip2 and mutants defective in either cyclin and Cdk binding domains (p57ΔCKI) or deleted of the COOH-terminal region (p57ΔQT) or GST-fused CKI domain (p57CKI) and tested their abilities to inhibit phosphorylation of MyoD by the cyclin E-Cdk2 complexes. As shown in Fig. 8A, wild-type p57Kip2, p57CKI, and p57ΔQT inhibited cyclin E-Cdk2 kinase activity, whereas p57ΔCKI was defective in kinase inhibitory activities. Thus, both cyclin and Cdk binding domains in the NH2-terminal region of p57Kip2 seems to be required for inhibiting phosphorylation of MyoD by cyclin E-Cdk2. It has recently been reported that CKI domain in the NH2-terminal region appears to be well conserved among members of the Cip/Kip family (Fig. 8B) (20, 46). We found that GST-fused wild-type p21Cip1 was also able to inhibit phosphorylation of MyoD by cyclin E-Cdk2 complexes (Fig. 8A). Based on these findings, it appears that the NH2 region of p57Kip2 is necessary and sufficient to inhibit phosphorylation of MyoD by cyclin E-Cdk2.
FIG. 7.
MyoD is a substrate of cyclin E-Cdk2-dependent phosphorylation. (A) In vitro-translated 35S-labeled MyoDwt was incubated with purified cyclin E-Cdk2 complexes for various periods of time. Phosphorylation shifts were visualized after SDS-PAGE. Shown is the autoradiogram of the SDS-PAGE analysis. (B) Bacterially produced MyoD protein was phosphorylated by purified cyclin E-Cdk2 complex for the time indicated. Shown is the autoradiogram for 32P phosphorylation reactions. Unphosphorylated MyoD protein migrates as 45-kDa band that progressively shifts to 47 kDa in the course of phosphorylation by cyclin E-Cdk2 kinase reaction. Positions of molecular weight markers (MW) are shown in thousands.
FIG. 8.
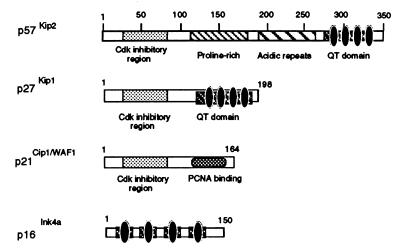
The NH2-terminal domain of p57Kip2 containing the cyclin-Cdk binding sites is necessary and sufficient to inhibit phosphorylation of MyoD by cyclin E-Cdk2. (A) Cyclin E-Cdk2 complexes expressed in Sf9 insect cells were incubated with the indicated amounts of wild-type p57Kip2 (wt), the indicated mutants, or p21Cip1/Waf1. Kinase activity was measured by using GST fused MyoDwt as a substrate. Coomassie blue staining of the protein substrate GST-MyoDwt is also shown. (B) Schematic representation of p57Kip2, p27Kip1, p21Cip1, and p16Ink4a protein domain structures. The Cip/Kip protein family contains a region of similarity that corresponds to the CKI domain (dotted box).
MyoD accumulation and transactivation of the MCK promoter by MyoD are enhanced by the NH2 domain of p57Kip2.
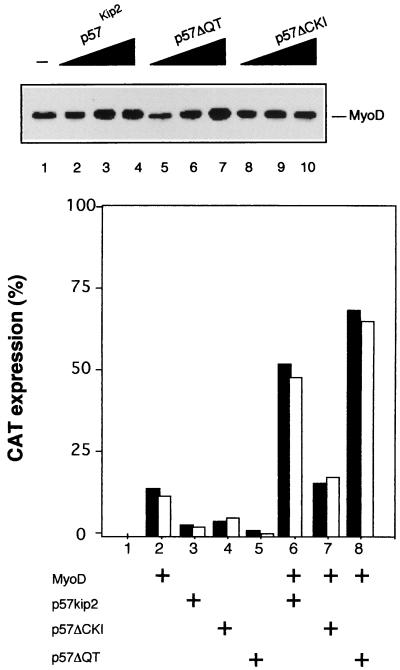
To determine whether the NH2 domain of p57Kip2 is also sufficient for the stabilization of MyoD in C2C12 cells, we transfected C2C12 myoblasts with either wild-type or mutant p57 expression vectors. MyoD protein was monitored by Western blot analysis. Figure 9A shows that wild-type p57Kip2 or p57ΔQT induced MyoD to accumulate at higher levels (lanes 2 to 7). In contrast, p57ΔCKI failed to increase MyoD protein abundance (lanes 8 to 10).
FIG. 9.
(A) Differential accumulation of endogenous MyoD protein by ectopically expressed p57Kip2 mutants. C2C12 myoblasts (lane 1) were transiently transfected with 0.5, 1, or 1.5 μg of a pEMSV expression vehicle encoding p57Kip2 (lanes 2 to 4), p57ΔQT (lacking amino acids 297 to 350) (lanes 5 to 7), or p57ΔCKI (lacking amino acids 1 to 105) (lanes 8 to 10) and were cultured in high-serum (20% FCS) medium. After 48 h, 50 μg of total-cell extract was analyzed by Western blotting with anti-MyoD antibodies (Santa Cruz). (B) Differential activation of MyoD function by ectopically expressed p57Kip2 mutants. Bars represent CAT activities from whole-cell extracts of C3H10T1/2 fibroblasts which were cotransfected with plasmids encoding the MCK-CAT reporter (0.5 μg) (lanes 1 to 8), MyoD (1 μg), wild-type p57 (p57Kip2), p57ΔCKI, and p57ΔQT (1 μg). The corresponding empty expression vector was added to normalize DNA content to 3 μg. CAT levels were determined 48 h after transfection. Protein concentration was equalized by the Bradford method. Typically, 15-μg aliquots of total cell extracts were used for the reactions. CAT activities in duplicate plates (black and white bars) are from a representative experiment.
MCK transactivation by MyoD is enhanced by coexpression of p57Kip2 (Fig. 2). To investigate if the NH2 domain of p57Kip2 was also sufficient for this activation, C3H10T1/2 cells were transiently transfected with MCK-CAT reporter plasmid in the presence of MyoD and of various mutants of p57Kip2. MCK transcriptional activity was analyzed in GM. As shown in Fig. 9B, in high-mitogen GM, MCK promoter activity was enhanced by ectopic expression of wild-type p57Kip2 or p57ΔQT but not by p57ΔCKI. Altogether, these data show that the NH2 region of p57Kip2, common to the other Cip/Kip family members, stabilizes MyoD and enhances the transcriptional activity of MyoD.
DISCUSSION
Control of myogenesis is achieved by a network of various factors interacting with each other in positive and negative regulatory mechanisms. MyoD has been implicated as a master regulatory gene in the process of muscle differentiation. Its activity is highly controlled in particular by growth factors, oncogenes, and negative HLH proteins such as Id (2, 39, 40). Phosphorylation of MyoD is one of the crucial mechanisms that control its activity in eukaryotic cells, and recent reports show that phosphorylation of MyoD Ser200 in proliferating myoblasts appears to play a major role in modulating MyoD half-life and myogenic activity (23, 52). The apparent antagonism between proliferation and differentiation implies that signaling pathways driving proliferation must be suppressed to allow induction of differentiation. Indeed, recent works demonstrated the role of G1 cyclins and their partners (Cdk and Ckis) in the cell cycle arrest and muscular differentiation program (15–17, 51).
In this study, we show that p57Kip2 levels are up-regulated during myogenesis (Fig. 1). This raises the possibility that p57Kip2 participates in growth arrest of myoblasts through inhibition of Cdks and thereby in the initiation of differentiation. We show that overexpression of Ckis in proliferating myoblasts reverses mitogen-mediated repression of MyoD function. We found that p57Kip2 and p27Kip1 but not p16Ink4a lead to accumulation of MyoD. We demonstrate that accumulation of MyoD is due to an increased half-life of MyoD protein by coexpressed p57Kip2 (Fig. 4C). Such an increased stability of MyoD was also observed recently when Ser200, the major site of Cdk-dependent phosphorylation, was changed to nonphosphorylatable alanine (23, 52). The fact that the resultant protein, MyoDAla200, was more stable than MyoDwt supports the view that a p57Kip2-induced increase in MyoD half-life occurs via the repression of cyclin-Cdk activities implicated in the phosphorylation of MyoD at Ser200. This specific phosphorylation is a prerequisite for MyoD degradation by the ubiquitin pathway (21). Interestingly, in C2C12 myoblasts, MyoD is expressed as two bands, a major slow-migrating hyperphosphorylated form and a fast-migrating band corresponding to the hypophosphorylated form which accumulates in p57Kip2-transfected myoblasts (Fig. 5). This raises the possibility that MyoD is a substrate of Cdc2, Cdk2, or Cdk4 complexes that control progression through the cell cycle and are inhibited by p57Kip2. It has been shown that overexpression of cyclin D1 results in an increase of the hyperphosphorylated form of MyoD and an inhibition of MyoD-mediated MCK-CAT transactivation (41, 51). However, cyclin D1-Cdk4 complexes fail to phosphorylate MyoD (23). Recently, a mechanism has been described to explain this finding. The cyclin-mediated inhibition of myogenesis by cyclin D1 involves nuclear translocation of Cdk4 by cyclin D1 and the subsequent formation of a MyoD-Cdk4 complex that specifically inhibits the transactivation functions of MyoD in the absence of Cdk4 kinase activity (63). This implies that p16Ink4a, which interacts exclusively with Cdk4 and Cdk6, does not increase the stability of MyoD. p16Ink4a binds to and inhibits Cdk4, leading to the sequestration of Cdk4, and promotes the transactivation functions of MyoD (63). In contrast, Cdc2 and Cdk2 have recently been shown to be involved in the direct phosphorylation of MyoD Ser200 in proliferative myoblasts (23). In agreement with these data, we show that ectopic expression of a Cdk2 DN mutant induces hypophosphorylation of MyoD (Fig. 6B) and cyclin E-Cdk2 efficiently phosphorylates MyoD in vitro (Fig. 7). Furthermore, when purified as a recombinant protein from bacteria and added to assays of recombinant cyclin E-Cdk2 preparations, p57Kip2 inhibits MyoD phosphorylation. p57Kip2 is known to inhibit Cdk2, Cdk4, and Cdk6 but is much less effective toward Cdc2-cyclin B and does not associate with CAK (Cdk7-cyclin H) (25). Altogether, these data suggest that p57Kip2 preferentially inhibits cyclin A/E-Cdk2 rather than cyclin B-Cdc2 activities in proliferating myoblasts.
Extensive structure-function studies of the Cip/Kip molecules have suggested that binding of both cyclin and Cdk is not only necessary but also sufficient for the inhibition of cyclin-Cdk activities (20, 25). In p57Kip2, the amino- and carboxy-terminal domains are well conserved between species, but the internal region contains proline-alanine repeats in human p57Kip2 (33) and a proline-rich region followed by an acidic repeat region in mouse p57Kip2 (25). This absence of sequence conservation in the internal region could be attributable to the lack of functional conservation. On the other hand, the carboxy-terminal domain (also termed the QT box) is a structural motif conserved with p27Kip1. The QT box is likely to function in protein-protein interactions. We observed that the NH2 domain of p57Kip2, which contains the cyclin-Cdk binding sites, is necessary and sufficient for inhibiting phosphorylation of MyoD by cyclin E-Cdk2. This result is supported by the fact that the carboxy-terminal domain of p57Kip2 is required neither for cell cycle arrest in SAOS2 cells (33) nor for a positive effect on transactivation of the MCK promoter by MyoD (Fig. 9B). Thus, these data indicate that p57Kip2, independently of its QT box, can function to arrest cell cycle in G1 and to stabilize MyoD by preventing its phosphorylation by cyclin-Cdk complexes. It is interesting that the cyclin and Cdk binding domains of p57Kip2 are highly conserved in the Cip/Kip protein family, suggesting that p21 and p27 could also be involved in vivo as p57Kip2. High-level ectopic expression of MyoD into nonmuscle cells is known to stop cell cycle progression and allow cells to undergo myogenic differentiation (53, 58). Controlled degradation of cyclin E-Cdk2-mediated phosphorylation of MyoD by the ubiquitin pathway (21) may be one of the control mechanism necessary to prevent MyoD from reaching a threshold that could interfere with normal cell cycle progression before triggering myogenic differentiation. Furthermore, phosphorylation of MyoD may result in a change in MyoD-associated protein by reducing its association with a partner such as pRB (14), MEF-2 (35), or p300/CBP (11). Cdk-dependent phosphorylation has been shown to modify the interaction between pRB and E2F and to change the interaction specificity of the HLH factor Id3 (9, 19, 22). Finally, degradation of MyoD by the ubiquitin pathway may be regulated by specific DNA binding (21). Altogether, these data indicate that MyoD is subjected to a variety of specific regulatory mechanisms and strongly suggest that MyoD plays a crucial role in a terminal cell cycle withdrawal decision that clearly involves the control of the phosphorylation of MyoD by Cip/Kip inhibitors such as p57Kip2.
ACKNOWLEDGMENTS
We are grateful to Anne Fernandez and Ned Lamb for critically reading the manuscript.
E. Reynaud and K. Pelpel are fellows of Ministère de la Recherche et de la Technologie. This work was supported by the Institut National de la Santé et de la Recherche Médicale, The Centre National de la Recherche Scientifique, and grants from Association Française contre les Myopathies, Ligue Nationale contre le Cancer, Association pour la Recherche sur le Cancer (grant 6829), and the Institut Gustave Roussy.
REFERENCES
- 1.Benayoun B, Pelpel K, Solhonne B, Guillier M, Leibovitch S A. Overexpression of Mosrat proto-oncogene product enhances the positive autoregulatory loop of MyoD. FEBS Lett. 1998;437:39–43. doi: 10.1016/s0014-5793(98)01192-2. [DOI] [PubMed] [Google Scholar]
- 2.Benezra R, Davis R, Lockson D, Turner D, Weintraub H. The protein Id: a negative regulator of helix-loop-helix DNA binding proteins. Cell. 1990;61:49–69. doi: 10.1016/0092-8674(90)90214-y. [DOI] [PubMed] [Google Scholar]
- 3.Boussif O, Lezoualc’h F, Zanta M A, Mergy M D, Scherman D, Demeneix B, Behr J P. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci USA. 1995;92:7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braun T E, Buschhausen-Denker G, Bober E, Tannich E, Arnold H. A novel human muscle factor related to but distinct from MyoD1 induces myogenic conversion in 10T1/2 fibroblasts. EMBO J. 1989;8:701–709. doi: 10.1002/j.1460-2075.1989.tb03429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braun T E, Bober G, Buschhausen-Denker G, Kotz S K, Grzeschik H, Arnold H. Differential expression of myogenic determination genes in muscle cells: possible autoactivation by the Myf gene products. EMBO J. 1989;8:3617–3625. doi: 10.1002/j.1460-2075.1989.tb08535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buskin J N, Hauschka S D. Identification of a myocyte nuclear factor which binds to the muscle-specific enhancer of the mouse muscle creatine kinase gene. Mol Cell Biol. 1989;9:2627–2640. doi: 10.1128/mcb.9.6.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis R L, Weintraub H, Lassar A B. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987;51:987–1000. doi: 10.1016/0092-8674(87)90585-x. [DOI] [PubMed] [Google Scholar]
- 8.Davis R L, Cheng P, Lassar A B, Weintraub H. The MyoD DNA binding domain contains a recognition code for muscle specific gene activation. Cell. 1990;60:733–746. doi: 10.1016/0092-8674(90)90088-v. [DOI] [PubMed] [Google Scholar]
- 9.Deed R W, Hara E, Atherton G T, Peters G, Norton J D. Regulation of Id3 cell cycle function by Cdk2-dependent phosphorylation. Mol Cell Biol. 1997;17:6815–6821. doi: 10.1128/mcb.17.12.6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deng C, Zhang P, Harper J W, Elledge S J, Leder P. Mice lacking p21Cip1/WAF1 undergo normal development but are defective in G1 checkpoint control. Cell. 1995;82:675–684. doi: 10.1016/0092-8674(95)90039-x. [DOI] [PubMed] [Google Scholar]
- 11.Eckner R, Yao T P, Odlread E, Livingston D M. Interaction and functional collaboration of p300/CBP and bHLH proteins in muscle and B-cell differentiation. Genes Dev. 1996;10:2478–2490. doi: 10.1101/gad.10.19.2478. [DOI] [PubMed] [Google Scholar]
- 12.Edmondson D G, Olson E N. A gene with homology to the myc similarity region of MyoD1 is expressed during myogenesis and is sufficient to activate the muscle differentiation program. Genes Dev. 1989;3:628–640. doi: 10.1101/gad.3.5.628. [DOI] [PubMed] [Google Scholar]
- 13.Fero M I, Rivkin M, Tasch M, Porter P, Carow C E, Firpo E, Polyak K, Tsai L, Broudy V, Perlmutter R M, Kaushansky K, Roberts J M. A syndrome of multiorgan hyperplasia with features of gigantism, tumorigenesis and female sterility in p27Kip1-deficient mice. Cell. 1996;85:733–744. doi: 10.1016/s0092-8674(00)81239-8. [DOI] [PubMed] [Google Scholar]
- 14.Gu W, Schneider J, Condorelli G, Kaushal S, Mahdavi V, Nadal-Ginard B. Interaction of myogenic factors and the retinoblastoma protein mediates muscle cell commitment and differentiation. Cell. 1993;72:309–324. doi: 10.1016/0092-8674(93)90110-c. [DOI] [PubMed] [Google Scholar]
- 15.Guo K, Wang J, Andres V, Smith R C, Walsh K. MyoD-induced expression of p21 inhibits cyclin-dependent kinase activity upon myocyte terminal differentiation. Mol Cell Biol. 1995;15:3823–3829. doi: 10.1128/mcb.15.7.3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo K, Walsh K. Inhibition of myogenesis by multiple cyclin-cdk complexes. J Biol Chem. 1997;272:791–797. doi: 10.1074/jbc.272.2.791. [DOI] [PubMed] [Google Scholar]
- 17.Halevy O, Novitch B G, Spicer D B, Skapek S X, Rhee J, Hannon G J, Beach D, Lassar A B. Correlation of terminal cell cycle arrest of skeletal muscle with induction of p21 by MyoD. Science. 1995;267:1018–1021. doi: 10.1126/science.7863327. [DOI] [PubMed] [Google Scholar]
- 18.Hardy S, Kong Y, Konieczny S F. Fibroblast growth factor inhibits MRF4 activity independently of the phosphorylation status of a conserved threonine residue within the DNA-binding domain. Mol Cell Biol. 1993;13:5943–5956. doi: 10.1128/mcb.13.10.5943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harper J W, Elledge S J. Cdk inhibitors in development and cancer. Curr Opin Genet Dev. 1996;6:56–64. doi: 10.1016/s0959-437x(96)90011-8. [DOI] [PubMed] [Google Scholar]
- 20.Hashimoto Y, Kohri K, Kaneko Y, Morisaki H, Kato T, Ikeda K, Nakanishi M. Critical role of the 310 helix region of p57Kip2 in cyclin-dependent kinase2 inhibition and growth suppression. J Biol Chem. 1998;273:16544–16550. doi: 10.1074/jbc.273.26.16544. [DOI] [PubMed] [Google Scholar]
- 21.Hatoum O A, Gross-Mesilaty S, Breitschopf K, Hoffman A, Gonen H, Ciechanover A, Bengal E. Degradation of myogenic transcription factor MyoD by the ubiquitin pathway in vivo and in vitro: regulation by specific DNA binding. Mol Cell Biol. 1998;18:5670–5677. doi: 10.1128/mcb.18.10.5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Helin K. Regulation of cell proliferation by the E2F transcription factors. Curr Opin Genet. 1998;8:28–35. doi: 10.1016/s0959-437x(98)80058-0. [DOI] [PubMed] [Google Scholar]
- 23.Kitzmann M, Vandromme M, Schaeffer V, Carnac G, Labbé J C, Lamb N, Fernandez A. cdk1/2-mediated phosphorylation of MyoD Ser200 in growing C2 myoblasts: role in modulating MyoD half-life and myogenic activity. Mol Cell Biol. 1999;19:3167–3176. doi: 10.1128/mcb.19.4.3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kiyokawa H, Kineman R D, Manova-Todorova K O, Soares V C, Hoffman E S, Ono M, Khanam D, Hayday A C, Frohman L A, Koff A. Enhanced growth of mice lacking the cyclin-dependent kinase inhibitor function of p27Kip1. Cell. 1996;85:721–713. doi: 10.1016/s0092-8674(00)81238-6. [DOI] [PubMed] [Google Scholar]
- 25.Lee M H, Reynisdottir I, Massagué J. Cloning of p57Kip2, a cyclin-dependent kinase inhibitor with unique domain structure and tissue distribution. Genes Dev. 1995;9:639–649. doi: 10.1101/gad.9.6.639. [DOI] [PubMed] [Google Scholar]
- 26.Lee M H, Nicolic M, Baptista C A, Lai E, Tsai L H, Massagué J. The brain specific activator p35 allows Cdk5 to escape inhibition by p27Kip1 in neurons. Proc Natl Acad Sci USA. 1996;93:3259–3263. doi: 10.1073/pnas.93.8.3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leibovitch S A, Guillier M, Lenormand J L, Leibovitch M P. Accumulation of the c-mos protein is correlated with post-natal development of skeletal muscle. Oncogene. 1991;6:1617–1622. [PubMed] [Google Scholar]
- 28.Lenormand J L, Guillier M, Leibovitch S A. Identification of a cis acting element responsible for muscle specific expression of the c-mos proto-oncogene. Nucleic Acids Res. 1993;21:695–702. doi: 10.1093/nar/21.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lenormand J L, Benayoun B, Guillier M, Vandromme M, Leibovitch M P, Leibovitch S A. Mos activates myogenic differentiation by promoting heterodimerization of MyoD and E12 proteins. Mol Cell Biol. 1997;17:583–593. doi: 10.1128/mcb.17.2.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li L, Zhou J, James G, Heller-Harrison R, Czech M P, Olson E N. FGF inactivates myogenic helix-loop-helix proteins through phosphorylation of a conserved protein kinase C site in their DNA-binding domains. Cell. 1992;71:1181–1194. doi: 10.1016/s0092-8674(05)80066-2. [DOI] [PubMed] [Google Scholar]
- 31.Li L, Heller-Harrison R, Czech M P, Olson E N. Cyclic AMP-dependent protein kinase inhibits the activity of myogenic helix-loop-helix proteins. Mol Cell Biol. 1992;12:4478–4485. doi: 10.1128/mcb.12.10.4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu M, Lee M H, Cohen M, Bommakanti M, Freedman L P. Transcriptional activation of the Cdk inhibitor p21 by vitamin D3 leads to the induced differentiation of the myelomonocytic cell line U937. Genes Dev. 1996;9:650–662. doi: 10.1101/gad.10.2.142. [DOI] [PubMed] [Google Scholar]
- 33.Matsuoka S, Edwards M C, Bai C, Parker S, Zhang P, Baldini A, Harper J W, Elledge S J. p57Kip2, a structurally distinct member of the p21Cip1 CDK inhibitor family, is a candidate tumor suppressor gene. Genes Dev. 1995;9:650–662. doi: 10.1101/gad.9.6.650. [DOI] [PubMed] [Google Scholar]
- 34.Miner J H, Wold B. Herculin, a fourth member of the MyoD family of myogenic regulatory genes. Proc Natl Acad Sci USA. 1990;87:1089–1093. doi: 10.1073/pnas.87.3.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Molkentin J D, Black B L, Martin J F, Olson E N. Cooperative interaction of muscle expression by MEF2 and myogenic bHLH proteins. Cell. 1995;83:1125–1136. doi: 10.1016/0092-8674(95)90139-6. [DOI] [PubMed] [Google Scholar]
- 36.Murre C, McCaw P S, Baltimore D. A new DNA binding and dimerization motif in immunoglobulin enhancer binding, daughterless, MyoD and myc proteins. Cell. 1989;56:777–783. doi: 10.1016/0092-8674(89)90682-x. [DOI] [PubMed] [Google Scholar]
- 37.Murre C, McCaw P S, Vaessin H, Caudy M, Jan L Y, Jan Y N, Cabrera C V, Buskin J N, Hauschka S D, Lassar A B. Interactions between heterologous helix-loop-helix proteins generate complexes that bind specifically to a common DNA sequence. Cell. 1989;58:537–544. doi: 10.1016/0092-8674(89)90434-0. [DOI] [PubMed] [Google Scholar]
- 38.Nakayama K, Ishida N, Shirane M, Inomata A, Inoue T, Shishido N, Horii I, Loh D Y, Nakayama K I. Mice lacking p27Kip1 display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell. 1996;85:707–720. doi: 10.1016/s0092-8674(00)81237-4. [DOI] [PubMed] [Google Scholar]
- 39.Olson E N. Interplay between proliferation and differentiation within the myogenic lineage. Genes Dev. 1992;4:1454–1461. doi: 10.1016/0012-1606(92)90066-p. [DOI] [PubMed] [Google Scholar]
- 40.Olson E N. Proto-oncogenes in the regulatory circuit for myogenesis. Semin Cell Biol. 1992;3:127–136. doi: 10.1016/s1043-4682(10)80022-4. [DOI] [PubMed] [Google Scholar]
- 41.Parker S B, Eichele G, Zhang P, Rawls A, Sands A T, Bradley A, Olson E N, Harper J W, Elledege S J. p53-independent expression of p21Cip1 in muscle and other terminally differentiating cells. Science. 1995;267:1024–1027. doi: 10.1126/science.7863329. [DOI] [PubMed] [Google Scholar]
- 42.Polyak K, Lee M H, Erdjument-Bromage H, Koff A, Tempst P, Robert J M, Massagué J. Cloning of p27Kip1 a cyclin-Cdk inhibitor and a potential mediator of extracellular antimitotic signal. Cell. 1994;78:59–66. doi: 10.1016/0092-8674(94)90572-x. [DOI] [PubMed] [Google Scholar]
- 43.Rao S S, Chu C, Kohtz D S. Ectopic expression of cyclin D1 prevents activation of gene transcription by myogenic basic helix-loop-helix regulators. Mol Cell Biol. 1994;14:5259–5267. doi: 10.1128/mcb.14.8.5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Resnisdottir I, Polyak K, Iavaronne A, Massagué J. Kip/Cip and Ink4 Cdk inhibitors cooperate to induce cell cycle arrest in response to TGF-β. Genes Dev. 1995;9:1831–1845. doi: 10.1101/gad.9.15.1831. [DOI] [PubMed] [Google Scholar]
- 45.Rhodes S J, Konieczny S F. Identification of MRF4: a new member of the muscle regulatory factor gene family. Genes Dev. 1989;3:2050–2061. doi: 10.1101/gad.3.12b.2050. [DOI] [PubMed] [Google Scholar]
- 46.Russo A A, Jeffrey P D, Patten A K, Massagué J, Pavletich N P. Crystal structure of the p27Kip1 cyclin-dependent-kinase inhibitor bound to the cyclinA-Cdk2 complex. Nature. 1996;382:325–331. doi: 10.1038/382325a0. [DOI] [PubMed] [Google Scholar]
- 47.Serrano M, Lee H W, Cordon-Cardo C, Beach D, Delinho R. Role of the INK4a locus in tumor suppression and cell mortality. Cell. 1996;85:27–37. doi: 10.1016/s0092-8674(00)81079-x. [DOI] [PubMed] [Google Scholar]
- 48.Sherr C J. G1 phase progression: cycling on cue. Cell. 1994;79:551–555. doi: 10.1016/0092-8674(94)90540-1. [DOI] [PubMed] [Google Scholar]
- 49.Sherr C J. Cancer cell cycles. Science. 1996;274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- 50.Sherr C J, Roberts J M. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 1995;9:1149–1163. doi: 10.1101/gad.9.10.1149. [DOI] [PubMed] [Google Scholar]
- 51.Skapek S X, Rhee J, Spicer D B, Lassar A B. Inhibition of myogenic differentiation in proliferating myoblasts by cyclin-D1-dependent kinase. Science. 1995;267:1022–1024. doi: 10.1126/science.7863328. [DOI] [PubMed] [Google Scholar]
- 52.Song A, Wang Q, Goebl M G, Harrington M A. Phosphorylation of nuclear MyoD is required for its rapid degradation. Mol Cell Biol. 1998;18:4994–4999. doi: 10.1128/mcb.18.9.4994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sorrentino V, Pepperkok R, Davis R L, Ansorge W, Philipson L. Cell proliferation inhibited by MyoD1 independently of myogenic differentiation. Nature. 1990;345:813–815. doi: 10.1038/345813a0. [DOI] [PubMed] [Google Scholar]
- 54.Tapscott S J, Davis R L, Thayer M J, Cheng P, Weintraub H, Lassar A B. MyoD1: a nuclear phosphoprotein requiring a myc homology region to convert fibroblasts to myoblasts. Science. 1988;242:405–411. doi: 10.1126/science.3175662. [DOI] [PubMed] [Google Scholar]
- 55.Thayer M J, Tapscott S J, Davis R L, Wright W E, Lassar A B, Weintraub H. Positive autoregulation of the myogenic determination gene MyoD1. Cell. 1989;58:241–248. doi: 10.1016/0092-8674(89)90838-6. [DOI] [PubMed] [Google Scholar]
- 56.Toyoshima H, Hunter T. p27, a novel inhibitor of G1 cyclin-cdk protein kinase activity, is related to p21. Cell. 1994;78:67–74. doi: 10.1016/0092-8674(94)90573-8. [DOI] [PubMed] [Google Scholar]
- 57.Weinberg R A. The retinoblastoma protein and the cell cycle control. Cell. 1995;81:323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- 58.Weintraub H, Tapscott S J, Davis R L, Thayer M J, Adam M A, Lassar A B, Miller A D. Activation of muscle specific genes in pigment, nerve, fat, liver and fibroblast cell lines by forced expression of MyoD. Proc Natl Acad Sci USA. 1989;86:5434–5438. doi: 10.1073/pnas.86.14.5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wright W E, Sassoon D A, Lin V K. Myogenin, a factor regulating myogenesis, has a domain homologous to MyoD. Cell. 1989;56:607–617. doi: 10.1016/0092-8674(89)90583-7. [DOI] [PubMed] [Google Scholar]
- 60.Xiong Y. Why are there so many CDK inhibitors? Biochim Biophys Acta. 1996;1288:1–5. doi: 10.1016/0304-419x(96)00012-1. [DOI] [PubMed] [Google Scholar]
- 61.Yan Y, Frisén J, Lee M-H, Massagué J, Barbacid M. Ablation of the Cdk inhibitor p57Kip2 results in increased apoptosis and delayed differentiation during mouse development. Gene Dev. 1997;11:973–983. doi: 10.1101/gad.11.8.973. [DOI] [PubMed] [Google Scholar]
- 62.Zhang P, Liegois N J, Wong C, Finegold M, Hou H, Thompson J C, Silverman A, Harper J W, DePinho R A, Elledge S J. Altered cell differentiation and proliferation in mice lacking p57Kip2 indicates a role in Beckwith-Wiedeman syndrome. Nature. 1997;387:151–158. doi: 10.1038/387151a0. [DOI] [PubMed] [Google Scholar]
- 63.Zhang J-M, Wei Q, Zhao X, Paterson B M. Coupling of the cell cycle and myogenesis through the cyclin D1-dependent interaction of MyoD with Cdk4. EMBO J. 1999;18:926–933. doi: 10.1093/emboj/18.4.926. [DOI] [PMC free article] [PubMed] [Google Scholar]