Abstract
Mexico is an extensively diverse country with a wide variety of wild species of blackberries (Rubus spp.), which are rich in bioactive compounds, however, these fruits are underutilized. Fermentation is a process that transforms the chemical compounds of fruits and increases nutraceutical properties. This study aimed to determine the physicochemical changes and the bioactive compounds profile that take place during the fermentation of wild blackberries using yeast EC 1118 and to evaluate its relationship with antioxidant activity (AOx). The results indicated that after 96 h of fermentation the content of carbohydrates (56%), total phenolic compounds (37%), and anthocyanins (22%), decreased, respectively. The physicochemical parameters showed statistic differences (p ≤ 0.05) at the endpoint of fermentation. The diversity of fatty acids was increased (55%), compared with unfermented blackberries. The modification of carbohydrates, anthocyanins, catechin, gallic and ellagic acid profiles were also monitored performing chromatographic techniques. The AOx, determined by ORAC and DPPH assays, showed the highest results for ORAC at 96 h increased a 140.2%, while DPPH values enhanced a 36.6% at 48 h of bioprocessing. Strong positive correlations were found between fermentation time and DPPH values (r = 0.8131), between ORAC and gallic acid content (r = 0.8688), and between anthocyanin content and pH (r = 0.9126). The fermentation of wild blackberries with EC 1118 yeast represents an alternative for development and formulation of potential ingredients for functional foods.
Supplementary information
The online version contains supplementary material available at (10.1007/s13197-020-04953-x).
Keywords: Wild blackberry, Yeast fermentation, Physicochemical parameters, Polyphenols, Anthocyanins, Antioxidant capacity
Introduction
Blackberries (Rubus spp.) are excellent nutritious fruits that are rich in vitamins, minerals, carbohydrates, and essential fatty acids (FAs), and also contain a great diversity of phenolic compounds in high concentrations (Cuevas-Rodríguez et al. 2010b; Hidalgo and Almajano 2017). The primary phenolic compounds in blackberries are anthocyanins and tannins, which account for approximately more than 59% of total polyphenols in the different blackberry species that have been studied. In wild species, several investigations reported the presence of considerable variability in the content and profile of polyphenols, which gives wild blackberries a high antioxidant activity (AOx) in comparison with domesticated species (Cuevas-Rodríguez et al. 2010a, 2010b; Hidalgo and Almajano 2017; Nowak et al. 2018; Sánchez-Velázquez et al. 2019). These phytochemicals make wild blackberries a potent nutraceutical source with positive health effects, since the phytochemicals interact as the first line of defense against harmful agents such as free radicals and exert anti-inflammatory, anticancer, antimicrobial, and antioxidant effects in the human body (Cuevas-Rodríguez et al. 2010a; Hidalgo and Almajano 2017). Wild species of blackberries exist at different latitudes around the world. In Mexico, the presence of some wild species has been reported in the south and center of the country; although southern and central species have been shown to be a great source of phytochemicals with antioxidant and anti-inflammatory potential, northern species have been understudied (Cuevas-Rodríguez et al. 2010a, b; Sánchez-Velázquez et al. 2019). Ripened fruits of two wild blackberries species were collected from Northwest of Mexico, identified and recorded as Rubus liebmanni and R. palmeri into distinctive species of this region (Sánchez-Velázquez et al. 2019). Traditionally, blackberries are consumed as fresh fruits, as well as processed into juice or juice concentrate for later use in beverages (Nowak et al. 2018), but Mexican species of wild blackberries are also underutilized by the population. Therefore, it is necessary to study alternatives that will allow the use and consumption of these wild species either through breeding programs or by the development of new food products or ingredients.
In developed countries, blackberries are included in the diet as fresh fruits, pulps, jams, juices or fermented beverages (Hidalgo and Almajano 2017; Nowak et al. 2018). The berries fermentation process biotransforms the chemical compounds present in the anatomical structures of the fruits (epidermis, pulp, seed, and receptacle) by the action of enzymes synthesized and released by the microorganisms (e.g. yeast) that are used in the process (Johnson et al. 2011). Among the yeasts applied in fermentation, Saccharomyces cerevisiae is the quintessential species used for the production of fruit liqueurs, being S. cerevisiae (ex bayanus) strain EC 1118 frequently used for making fruit wines (Arozarena et al. 2012). Yeast strain EC 1118 is known for improving the sensory properties of wines by reducing astringency and maintaining color stability, flavor, and odor, while also modifying the phenolic compound profile and increasing the AOx (Johnson et al. 2013); these characteristics are developed in the product owing to the synthesis of exogenous enzymes such as phenoloxidase (EC 1.14.18.1), β-glucosidase (EC 3.2.1.21), and tannase (EC 3.1.1.20), which catabolize complexes between polyphenols and macromolecules (proteins, carbohydrates, lipids, etc.), acting mainly on the ester and glycosidic bonds of tannins and anthocyanins (Arozarena et al. 2012; Johnson et al. 2013; Ascacio-Valdés et al. 2016). Furthermore, the yeast hydrolyzes carbohydrates from glycosylated polyphenols and incorporates them into its metabolism, along with free sugars. This also increases the concentration of organic acids (e.g. gallic [GA] ellagic acids [EA]) and (+ / −)-epicatechin subunits and decreases and/or changes the carbohydrate and lipid profiles in the matrix, directly affecting the physicochemical, sensorial, and nutraceutical properties of the ferments, including pH, turbidity, and resistance to decomposition by UV light (Cho and Jeon 2013). In addition, the AOx increases during relatively short fermentation times (Yacco et al. 2016; Šeruga et al. 2011).
Most of the available information about the alcoholic fermentation of non-grapefruits is mainly aimed on physicochemical parameters, organoleptic properties, phenolic profiles and AOx, however, macro-compounds, such as fatty acids and carbohydrates, have been sparingly studied, especially on wild species (Šeruga et al. 2011; Yacco et al. 2016). This study aimed to determine the changes that take place during the fermentation of wild blackberries using EC 1118 yeast and to evaluate the impact of the alcoholic fermentation process on the AOx of wild blackberries from northwestern Mexico.
Materials and methods
Biological material
Ripe wild blackberry (Rubus sp.) fruits (2.5 kg) were collected in the pine-oak forest area of the Chara Pinta Reserve, El Palmito, Concordia, Sinaloa (23° 48’ 48.4” N, 105° 50’ 15.1” W, > 1900 m a.s.l.). Immediately, after harvested, all fruits were washed, stored in airtight bags at − 80 °C until use. Dry wine yeast strain Saccharomyces cerevisiae ex bayanus (Lalvin EC1118) was obtained from Lallemand Inc. (Montreal, Quebec, Canada).
Chemicals and reagents
Citric acid (221,275), GA (G7384), EA (E2250), cyanidin (79,457), catechin (43,412), and ( ±)-6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid (Trolox, 238,813) standards, sodium hydroxide (NaOH, S8045), sodium potassium tartrate (KNaC4•H4O6, 217,255), Folin-Ciocalteu reagent (F9252), derivatizing N,O-bis(trimethylsilyl)trifluoroacetamide (BSTFA, B-023), pyridine (270,970), sucrose (S7903), and 2,2′-azobis(2-methylpropionamidine) dihydrochloride (AAPH, 440,914) were purchased from Sigma-Aldrich (Steinheim, Germany).
Fermentation of wild Mexican blackberries
The bioprocessing started with activation of 0.217 g of the yeast strain EC 1118 with 50 mL of sterile distilled water at 40 °C. Then, 360 g/L of crushed blackberries (T 18 Ultra-Turrax; IKA, Germany) was mixed with 100 g/L of sucrose and 217 mg/L of activated EC 1118. The mixture was adjusted to 1 L with sterile water. The blackberry must was fermented in an incubator (LOM-150-Series; MRC, Haifa, Israel) for 96 h under agitation at 100 rpm and at 12–14 °C. Aliquots of 100 mL were recovered every 24 h, pasteurized at 80 °C for 10 min, centrifuged at 4000 rpm for 10 min, and filtered through Whatman No. 1 paper. The aliquots were kept at − 80 °C until later use (Fig. 1).
Fig. 1.
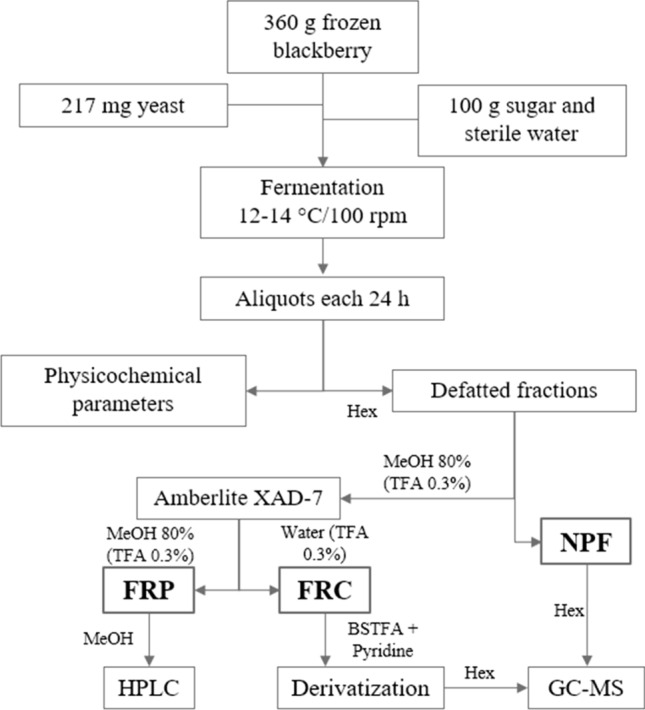
Fermentation process scheme and chemical analysis. Hex: hexane; MeOH: methanol; TFA: trifluoroacetic acid; NPF: non-polar fraction; FRP: fraction rich in polyphenols; FRC: fraction rich in carbohydrates; BSTFA: N,O-bis(trimethylsilyl)trifluoroacetamide; HPLC: high-performance liquid chromatography; GC–MS: gas chromatography–mass spectrometry
Physicochemical parameters
The physicochemical parameters of the aliquots were analyzed according to AOAC methods (AOAC 2016). The pH change was measured with a potentiometer (Hanna Instruments, Woonsocket, RI, USA), and total soluble solids (TSS) was measured with a refractometer (Milton Roy Co., Tokyo, Japan) and expressed as degrees Brix (°Brix). For the determination of titratable acidity (TA), 2.5 mL of sample was mixed with methyl red–methylene blue solution (1:1, v/v) and then with 100 mL of distilled water. To that mixture, 0.1 N NaOH was added until the color changed from pink to brown, and the results were expressed as mg of citric acid equivalents per L (mg CAE/L).
Phenolic and anthocyanin content
The Folin-Ciocalteu colorimetric assay was used to determine the total phenolic content (TPC). Five mL of sample was mixed with 0.5 mL of 1 N Folin–Ciocalteu reagent, the mixture was stirred for 2 to 5 min, 1 mL of 20% Na2CO3 was added, and the solution was left to rest for 10 min, after which the absorbance was measured at 765 nm with a Synergy HT spectrophotometer (BioTek Instruments, Winooski, VT, USA). The TPC was calculated using GA as the standard and was expressed as μg of gallic acid equivalents per L (µg GAE/L). Total anthocyanin content (TAC) was measured by the pH differential method described by Lee et al. (2005) with some modifications, using 1.0 N HCl and NaOH as pH regulators. These results were expressed as mg of cyanidin-3-glucoside equivalents per L (mg C3GE/L).
Fractionation of chemical compounds
The aliquot obtained of each time of fermentation of blackberries were fractionated using a protocol described by Cuevas-Rodríguez et al. (2010b). The non-polar fraction (NPF) was obtained from each aliquot with hexane (1:1, v/v) under stirring. The procedure was performed in duplicate. The solvent was removed from the defatted aqueous fraction, which was then resuspended in acidified methanol (MeOH) (0.3% trifluoroacetic acid [TFA]) and mixed with Amberlite XAD-7 resin at 75 rpm for 12 h and 20 °C. A glass column (25 × 300 mm) was packed, with the resin loaded along with the aqueous fraction. The fraction rich in carbohydrates (FRC) was eluted with acidified water (0.3% TFA). The fraction rich in polyphenols (FRP) was eluted with acidified MeOH. The NPF, FRC, and FRP were concentrated until dryness using a SpeedVac DDA concentrator (Savant SC250EXP; ThermoFisher Scientific, Asheville, NC, USA) to eliminate the solvent.
Phenolic and anthocyanin profiling
The analysis of the phenolic compound profile from the FRP was carried out by high-performance liquid chromatography (HPLC) (PerkinElmer, Waltham, MA, USA) under conditions previously reported by Cuevas-Rodríguez et al. (2010b). The elution column was a YMC Pack ODS AM-303 LC-18 column 250 mm × 4.6 mm × 5 µm (YMC America, Inc., Milford, MA, USA). The dried PRPs were resuspended in HPLC-grade MeOH (5 mg/mL) and passed through 0.22 µm PTFE (polytetrafluoroethylene) filters before injection. The column temperature was set at 25 °C and the sample injection volume at 20 µL. The mobile phase was (A) 100% MeOH and (B) acidified water (1% TFA). The elution gradient was 90%, 80%, 75%, 65%, 35%, 0%, 0%, 90%, and 90% of B, over a time of 0.1, 5, 5, 10, 10, 1, 14, 5, and 5 min and a flow rate of 1.5, 1.5, 0.8, 0.8, 0.8, 1, 1, 1.3, and 1.3 mL/min, respectively. Detection was carried out at 280, 360 and 520 nm. For the processing and analysis of the data, Chromera software (version 4.0; PerkinElmer) was used. External standards of GA, EA, catechin, and cyanidin anthocyanins (previously identified) were used, and the results were expressed as μg of GAE per L (µg GAE/L), μg of ellagic acid equivalents per L (µg EAE/L), μg of catechin equivalents per L (µg CatE/L), and μg of cyanidin-3-glucoside equivalents per L (µg C3GE/L), respectively.
Carbohydrates and fatty acids profiling
Carbohydrates and fatty acids from fermented blackberries were determined by chromatography–mass spectrometry (GC–MS) analysis according to Ahumada-Santos et al. (2013), a mixture of 2 mg of the NPF and 15 mL of hexane was stirred for 1 h and centrifuged at 4000 rpm for 10 min. The supernatant was mixed with 2.5 mL of KCl (0.75%) and then centrifuged at 3000 rpm. Subsequently, the supernatant was sequenced at 50 °C in the dark. Then, 0.1 g of the recovered oil was mixed with 80 µL of KOH (0.14 mg/mL MeOH) and 1.5 mL of MeOH. The mixture was sonicated at 60 MHz for 20 s and then taken to dryness. Afterward, the mixture was resuspended in 2 mL of GC-grade hexane, centrifuged at 5000 rpm for 5 min, and passed through a 0.22-µm nylon filter. Then, 20 µL of sample was injected into a gas chromatograph coupled to a mass spectrometer HP 6890 equipment (GC–MS) (Agilent Technologies, Santa Clara, CA, USA) equipped with an automatic liquid injector (Series 7683), using an Omega Wax 250 capillary column 30 m × 0.25 mm × 0.25 µm and using helium gas as mobile phase at a flow rate of 1 mL/min. The samples were analyzed at initial conditions of 50 °C, after which the temperature increased 5 °C every minute until it reached 270 °C, which was then maintained for 10 min. The results were expressed as g of FAs per L (g/L). The tests were performed in triplicate.
The analysis of carbohydrates was carried out according to Ahumada-Santos et al. (2013). 10 mg of the FRC (adjusted to pH 7.0) was mixed with 50 µL of BSTFA for derivatization and 50 µL of pyridine. Then, the vial was sealed with parafilm, sonicated for 5 min, and placed in an oven at 70 °C for 4 h. After that time, the mixture was resuspended in 1.5 mL of hexane and injected into the GC–MS system under the same conditions as above. The results were expressed as g of carbohydrates per L (g/L).
Antioxidant activity
The AOx was determined by a hydrophilic oxygen radical absorbance capacity (ORAC) assay in accordance with Johnson et al. (2011). In each well, 20 µL of Trolox (12.5–100 µM), sample (0.1 mg/mL), or blank (phosphate buffered saline at pH 7.4 at 75 mM) was mixed with 120 µL of 0.117 mM fluorescein and 60 µL of 40 mM AAPH. The 96-well black microplate was read at 485 and 582 nm every 2 min for 1 h at 37 °C in a microplate reader (Synergy HT, BioTek Instruments). The results were analyzed using Gen5 software (BioTek Instruments) and expressed as mM Trolox equivalents per L (mM TE/L) of fermented sample. The antioxidant activity of 2,2-diphenyl-1-picrylhydrazyl (DPPH) was measured using the assay reported by Nowak et al. (2018). A DPPH solution (150 µM) dissolved in MeOH was prepared. In a 96-well microplate, 22 µL of each aliquot, previously diluted in MeOH, was mixed with 200 µL of the DPPH solution (150 µM). After 30 min of incubation at room temperature in complete darkness, the absorbance at 520 nm was measured using a microplate reader (Synergy HT, Biotek Instruments). A Trolox standard curve (0–200 µM) was prepared. The data were expressed as mM Trolox equivalents per L (mM TE/L) of sample.
Statistical analysis
The data were reported as the means ± standard deviation (SD). Statistical analysis of the data was performed with Statgraphics Centurion XVII statistical package (Statgraphics Technologies, The Plains, VA, USA). One-way analysis of variance (ANOVA) with the least significant difference used to detect the statistical significance, and differences were considered significant when p < 0.05. A Pearson correlation test was conducted to determine the correlation between variables.
Results and discussion
Physicochemical parameters
The physicochemical parameters of the fermented wild blackberries are presented in Table 1. The fermentation process started at a pH of 3.27, and after the first 24 h, the pH decreased to 3.13, remaining constant until 96 h of bioprocessing. The pH is closely related to chemical changes in the profile of organic acids present in the blackberry must (Tyl and Sadler 2017), but also, by the content of ethanol in the matrix, since at time 0 its presence incipient, at 24 h the alcohol increases considerably (data not included) (Akin et al. 2008). Low-pH preservation in winemaking or alcoholic fermentation helps to maintain abiotic conditions, flavor properties, and the structural stability of bioactive molecules such as phenolic compounds (Skrede et al. 2000; Tyl and Sadler 2017).
Table 1.
Chemical parameters of wild Mexican blackberries during fermentation
| Parameter | Fermentation time (FT) | ||||
|---|---|---|---|---|---|
| 0 h | 24 h | 48 h | 72 h | 96 h | |
| pH | 3.27 ± 0.03a | 3.13 ± 0.02b | 3.11 ± 0.01b | 3.14 ± 0.01b | 3.13 ± 0.04b |
| TA (mg CAE/L) | 8.41 ± 0.22d | 8.69 ± 0.27d | 8.79 ± 0.13c | 9.22 ± 0.18b | 10.15 ± 0.21a |
| TSS (°Brix) | 19.90 ± 0.17b | 21.07 ± 0.12a | 20.00 ± 0.00b | 18.00 ± 0.00c | 16.20 ± 0.00d |
FT fermentation time; TA titratable acidity; mg CAE/L: mg of citric acid equivalents/L; TSS total soluble solids. Data are indicated as mean values ± standard deviation (n = 3). Different letters in the same row indicate significant differences (p < 0.05)
TA showed a starting value of 8.41 mg CAE/L (Table 1); after 24 h of fermentation, this increased to 10.15 mg CAE/L, indicating significant differences (p < 0.05) every 24 h after the first day of fermentation. Titratable acidity is an essential organoleptic parameter related to flavor and color in food products, in which the organic acids are partially ionized (Skrede et al. 2000; Tyl and Sadler 2017). Changes in TA are associated with the concentration of organic acids, which are present naturally in blackberries and are transformed during fermentation (Tyl and Sadler 2017).
During the first 24 h of fermentation, TSS increased from 19.73 to 22.07°Brix (Table 1), which could be associated to the degradation of water-soluble macromolecules from the fruit cellular structures (mainly carbohydrates) by the action of enzymes released by yeast, such as pectinases (EC 3.2.1.15) and cellulases (EC 3.2.1.4), which increases the turbidity of the blackberry must during the first hours of fermentation (Ascacio-Valdés et al. 2016). After that, TSS started decreasing until 16.20°Brix when 96 h of fermentation had elapsed, with significant differences (p < 0.05) between 24, 48, 72, and 96 h. As the fermentation process continues, the recent released carbohydrates of high molecular weight are hydrolyzed into simple monosaccharides, which are incorporated through the yeast metabolism by exogenous enzymes such as β-glucosidases (Johnson et al. 2011; Ascacio-Valdés et al. 2016; Tyl and Sadler, 2017).
Chemical composition of fermented blackberriesThe total carbohydrate concentration (TCC) of Mexican wild blackberries decreased (p < 0.05) from 141.79 to 61.12 g/L after fermentation (0–96 h) bioprocess, this decrease to almost 43.1% when compared to non-fermented samples (Table 2). Several studies estimated that the fermentation process produces a reduction in total sugars content of around 48% after the first 48 h of fermentation, and those changes have been attributed to the presence of enzymes such as glucose dehydrogenases (EC 1.1.99.10) and hexokinases (EC 2.7.1.1) that are synthesized by S. cerevisiae (van Djiken et al. 2002; Regelmann et al. 2003). A strong positive correlation was found between TSS and TCC (r = 0.8403) (Table 3), which has been related to the degradation of the chemical compounds (including soluble sugars and pectins) present in fresh fruits (Regelmann et al. 2003).
Table 2.
Carbohydrates and fatty acids profile of wild Mexican blackberries during fermentation
| Parameter | Fermentation time (FT) | ||||
|---|---|---|---|---|---|
| 0 h | 24 h | 48 h | 72 h | 96 h | |
| CHO | |||||
| D-Fructose | 65.1 ± 5.91a | 64.90 ± 0.49a | 46.13 ± 2.48b | 34.12 ± 3.23c | 34.02 ± 2.89c |
| L-Altrose | 8.43 ± 0.70a | 3.47 ± 0.34b | 3.27 ± 0.24b | 1.00 ± 0.01c | 0.62 ± 0.01d |
| β-Galactose | 25.11 ± 3.01a | 23.51 ± 2.30a | 17.68 ± 1.52b | 13.25 ± 1.35c | 11.68 ± 1.00d |
| L-Arabinose | 1.91 ± 0.12a | 1.82 ± 0.22a | 1.30 ± 0.10b | 0.18 ± 0.00c | 0.18 ± 0.00c |
| D-Glucose | 41.24 ± 4.04a | 38.41 ± 1.59a | 30.91 ± 3.14b | 17.28 ± 1.69c | 14.62 ± 0.23d |
| TCC (g/L) | 141.79 ± 7.76a | 132.11 ± 4.94a | 99.29 ± 7.48b | 65.83 ± 1.25c | 61.12 ± 3.11d |
| Fatty acids (g/L) | |||||
| Caprylic (C8:0) | ND | ND | ND | 0.22 ± 0.06a | 0.27 ± 0.09a |
| Capric (C10:0) | ND | 0.83 ± 0.27c | 2.74 ± 0.14a | 1.34 ± 0.02b | 1.42 ± 0.14b |
| Lauric (C12:0) | ND | ND | 1.82 ± 0.14a | 0.88 ± 0.07b | 0.97 ± 0.09b |
| Myristic (C14:0) | ND | ND | 0.84 ± 0.08a | 0.36 ± 0.15b | 0.30 ± 0.01b |
| Palmitic (C 16:0) | 1.85 ± 0.03e | 3.40 ± 0.06c | 5.15 ± 0.41a | 3.08 ± 0.5d | 4.07 ± 0.36b |
| Stearic (C18:0) | ND | ND | ND | 0.58 ± 0.11a | 0.61 ± 0.03a |
| Oleic (C18:1) | 2.68 ± 0.30c | 5.18 ± 0.05a | 5.35 ± 0.35a | 4.98 ± 0.07a | 4.46 ± 0.20b |
| Linoleic (C8:2) | 3.39 ± 0.41c | 5.54 ± 0.11a | 5.34 ± 0.51a | 4.05 ± 0.14b | 4.19 ± 0.21b |
| α-Linoleic (C18:3) | 3.21 ± 0.62 cd | 4.00 ± 0.11b | 4.43 ± 0.41a | 3.48 ± 0.08c | 3.05 ± 0.25d |
| TOC (g/L) | 11.13 ± 0.21d | 18.95 ± 1.01b | 25.67 ± 0.09a | 18.97 ± 0.87b | 19.34 ± 0.89b |
FT Fermentation time, CHO Carbohydrates, TCC Total carbohydrates, TOC Total lipids. Data are indicated as mean values ± standard deviation (n = 3). Different letters in the same row indicate significant differences (p < 0.05, LSD). ND Non-detected
Table 3.
Correlation coefficients (r) between fermentation time and physicochemical parameters, total carbohydrates, lipids, phenolics and anthocyanins, gallic, ellagic acids, catechin and antioxidant activity (ORAC and DPPH)
| FT | pH | TA | TSS | TCC | TOC | TPC | TAC | GA | EA | CAT | ORAC | DPPH | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FT | 1 | − 0.6603 | 0.7841 | − 0.7950 | − 0.9867 | 0.6130 | − 0.7036 | − 0.8866 | 0.5464 | − 0.9472 | − 0.7085 | 0.5489 | 0.8131 |
| pH | 1 | − 0.5248 | 0.0807 | 0.5927 | − 0.8764 | 0.9741 | 0.9126 | − 0.2070 | 0.8453 | − 0.0314 | 0.1245 | − 0.9599 | |
| TA | 1 | − 0.5752 | − 0.6843 | 0.1894 | − 0.6817 | − 0.6733 | 0.1804 | − 0.6658 | − 0.5030 | 0.2074 | 0.5637 | ||
| TSS | 1 | 0.8403 | − 0.1523 | 0.1362 | 0.4329 | − 0.5015 | 0.5856 | 0.9875 | − 0.7835 | − 0.3306 | |||
| TCC | 1 | − 0.6239 | 0.6128 | 0.8470 | − 0.6137 | 0.9300 | 0.7611 | − 0.6369 | − 0.7761 | ||||
| TOC | 1 | − 0.7598 | − 0.8325 | 0.3748 | − 0.8250 | − 0.0595 | 0.0945 | 0.9152 | |||||
| TPC | 1 | 0.9215 | − 0.2160 | 0.8413 | 0.0169 | 0.0852 | − 0.9236 | ||||||
| TAC | 1 | − 0.5177 | 0.9767 | 0.3081 | − 0.2123 | − 0.9606 | |||||||
| GA | 1 | − 0.5276 | − 0.3934 | 0.8911 | 0.3271 | ||||||||
| EA | 1 | 0.4800 | − 0.3741 | − 0.9493 | |||||||||
| CAT | 1 | − 0.7259 | − 0.2279 | ||||||||||
| ORAC | 1 | 0.0569 | |||||||||||
| DPPH | 1 |
FT fermentation time; TA titratable acidity; TSS total soluble solids; TCC total carbohydrate content; TOC total oil content; TPC total phenolic content; TAC total anthocyanin content; GA gallic acid content; EA ellagic acid content; CAT catechin content; ORAC oxygen radical absorbance capacity assay; DPPH 2,2-diphenyl-1-picrylhydrazyl radical assay
The composition of carbohydrates was determined in wild Mexican blackberries extracts, which were identified and quantified through the fermentation process. Four aldohexoses (altrose, arabinose, galactose, and glucose) and one ketohexose (fructose) were detected, and their profiles changed during the fermentation process (Table 2, Fig. 2a). After fermentation results revealed more than 52.3% reduction in the concentration of D-fructose, 35.4% in D-glucose, 46.5% in β-galactose, 7.4% in l-altrose, and, 9.4% l-arabinose, this behavior was expected since aldohexoses are an essential energy source for S. cerevisiae yeast during fermentation (Shompoosang et al. 2014). Saccharomyces yeast and other fermentative microorganisms synthesize enzymes such as aldose isomerases (EC 4.1.2.13), which act on aldohexoses, and fructose-1,6-bisphosphatase (EC 3.1.3.11) for the degradation of ketohexoses (as fructose). However, fructose-1,6-bisphosphatase requires a molecular shift into fructose by one or two catabolic pathways: cytosolic ubiquitin–proteasome machinery and another vacuolar-dependent proteolysis (Rychlik and Russell 2002; van Dijken et al. 2002; Regelmann et al. 2003). Although not a common carbohydrate in nature, altrose is synthesized from aldohexoses by fermentative bacteria present on the surface of fruits, including wild blackberries, during maturation, and this could be associated with the presence of altrose in these fruits (Rychlik and Russell 2002). Galactose and arabinose are not abundant carbohydrates in berry fruits (< 15%) but constitute the arabinogalactan protein (AGP) systems present in cell walls, with signaling functions in fruits. The AGP content increases during the maturation process; however, pectins participate in the production of AGP, and its decrease is also associated with its incorporation into the yeast metabolism, in combination with the reduction in pectin enzymatic activity, since AGP tends to aggregate and precipitate as the alcohol content in the matrix increases (Shompoosang et al. 2014). Glucose and fructose are the principal fruit sugars and are the primary energy source for Saccharomyces yeasts and other fermentative microorganisms (Regelmann et al. 2003).
Fig. 2.
Chromatograms of carbohydrates (a), fatty acids (b), and anthocyanins (c) at different fermentation times. The dark signal and the gray signal indicate 0 and 96 h of the fermentation process, respectively. Cya-3-rut: cyanidin-3-rutinoside; Cya-3-xyl-rut: cyanidin-3-xylosyl-rutinoside; Cya-3-glu: cyanidin-3-glucoside; Cya-3-6-mal-glu: cyanidin-3-(6-malonyl)-glucoside
The predominant feature of winemaking is the conversion of juice sugars into ethanol (EtOH) and microorganism biomass, a task that is carried out almost exclusively by unicellular eukaryotes such as yeast, that is, Saccharomyces (Regelmann et al. 2003; Wang et al. 2004). Wang et al. (2004) reported changes in the carbohydrate profile of apple wine and observed constant decreases in glucose, fructose, and sucrose, and those authors’ correlation analysis showed a strong relationship between the biomass content, EtOH production, and sugar consumption. However, studies on EtOH synthesis from substrates with a higher diversity of carbohydrates are still scarce.
Concerning, the total oil content (TOC) of wild blackberries were significantly increased (p < 0.05) in a time-dependent manner after fermentation time (48 h), TOC values were up 25.67 g/L, which was 2.3 times higher than the initial concentration of unfermented blackberries, but, thereafter, the TOC value decreased until 19.34 g/L at the end of bioprocessing (Table 2). This could be related to the subsequent decrease in TOC content (after 48 h) when the yeast population is decreasing (Gallart et al. 2002; Torija et al. 2003). In red wines, fatty acids (FAs) account for less than 1.5% of the total content of volatile compounds, FAs concentration is variable during fermentation owing to the enzymatic activity of the microbiota, with esterases (EC 3.1.1) and lipases (EC 3.1.1.3) being the main enzymes responsible for the degradation and synthesis of lipid compounds during alcoholic fermentation (Sumby et al. 2010). The diversity of FAs increases during fermentation (> 55%) (Table 2, Fig. 2b.). In blackberry must (0 h of fermentation), the FA profile was made up of long-chain FAs (LCFA, > 14 carbon atoms). Furthermore, a saturated FA (palmitic acid) and three unsaturated FAs (oleic, linoleic, and α-linolenic acids) were detected. After 24 h, these FAs showed significant (p < 0.05) increases (12–45%), and additionally, capric acid was detected at a low concentration (0.83 g/L). At 48 h, two more saturated FAs, lauric and myristic acids, were found, at concentrations of 2.74 and 0.84 g/L, respectively, and α-linolenic acid showed a significant increase (p < 0.05), of more than 9%. At 72 h, different FAs, caprylic and stearic acids, were detected, without significant differences (p < 0.05) between them at 72 and 96 h of fermentation. LCFA accounted for the most significant proportion of the lipid fraction (> 79%). The presence of medium-chain FAs (MCFA) such as caprylic, capric, and lauric acids is associated with the degradation of LCFA, while the low fermentation temperature (12–14 °C) maintains the degree of unsaturation in 18-carbon FAs before 72 h of bioprocessing; after that time, however, saturation may occur in the 18-carbon FAs (Torija et al. 2003). The presence of MCFA gives important aromatic properties to fermented beverages; for example, caprylic and capric acids increase the foaming of fermented beverages, a feature that is highly valued in this type of products (Gallart et al. 2002). According to Torija et al. (2003), and Gallart et al. (2002), the occurrence of MCFA could be related to the degradation of LCFA, which generate essential aromatic properties to fermented beverages.
Phenolic compounds content and profile of fermented blackberries
Our studies have shown that bioprocessing by fermentation had a significant time-dependent reduction of the TPC levels (Table 4). The concentration of TPC varied from 502,371 to 329,633 µg GAE/L, during the entire period fermentation process, a significant decrease in the concentration of TPC around of 34.4% when compared to unfermented blackberries samples. The same trend for berries fermentation was also observed in previous studies (Su and Chien 2007; Wang et al. 2012). In contrast, Johnson et al. (2011) found that fermentation of highbush blueberry for 72 h increases TPC by 75%. These conflicting results on the effect of fermentation might be attributed to differences in the fermentation conditions (Johnson et al. 2011, 2013). There was a strong positive correlation among values of TPC with pH (r = 0.9741) (Table 3), which could indicate that pH affects the chemical structure and degree of polymerization in phenolic compounds and hence their content.
Table 4.
Phenolic profile, anthocyanin profile and antioxidant activity of wild Mexican blackberries during fermentation
| Parameter | Fermentation time (FT) | ||||
|---|---|---|---|---|---|
| 0 h | 24 h | 48 h | 72 h | 96 h | |
| TPC (mg GAE/L)1 | 502.4 ± 8.8a | 351.7 ± 8.9b | 344.7 ± 1.9bc | 357.2 ± 15.7b | 329.6 ± 8.8c |
| GA (µg GAE/L)2 | 0.0031 ± 0.0002e | 0.0053 ± 0.0003d | 0.0412 ± 0.0010c | 0.0928 ± 0.0171a | 0.0596 ± 0.0051b |
| EA (µg EAE/L)3 | 0.0975 ± 0.0021a | 0.0782 ± 0.0048b | 0.0651 ± 0.0035c | 0.0599 ± 0.0049d | 0.0565 ± 0.0025e |
| CAT (µg CatE/L)4 | 0.0026 ± 0.0003c | 0.0034 ± 0.0003a | 0.0031 ± 0.0002b | 0.0022 ± 0.0002d | 0.0009 ± 0.0001e |
| TAC (mg C3GE/L)5 | 188.87 ± 0.93a | 168.63 ± 3.59b | 158.23 ± 0.63c | 156.50 ± 1.51d | 147.23 ± 0.57d |
| Anthocyanins (mg C3GE/L)5 | |||||
| Cya-3-rut | 45.67 ± 4.23a | 26.88 ± 0.11b | 23.09 ± 0.04c | 15.19 ± 11.15d | 9.78 ± 0.33e |
| Cya-3-xyl-rut | 42.08 ± 0.08a | 32.58 ± 3.25b | 26.00 ± 0.02c | 23.46 ± 1.23d | 14.98 ± 0.68e |
| Cya-3-glu | 108.68 ± 2.19a | 88.19 ± 7.36b | 64.51 ± 2.68c | 63.49 ± 2.37c | 40.29 ± 0.28d |
| Cya-3-mal-glu | 5.66 ± 0.68a | 4.31 ± 0.30b | 4.02 ± 0.11c | 2.49 ± 0.04d | 1.91 ± 0.02e |
| Antioxidant activity (mM TE/L)6 | |||||
| ORAC | 35.05 ± 2.07b | 24.06 ± 2.24d | 27.43 ± 2.22c | 50.89 ± 5.10a | 57.79 ± 5.74a |
| DPPH | 4.43 ± 0.24b | 5.55 ± 0.33a | 6.13 ± 0.26a | 5.89 ± 0.73a | 6.05 ± 0.77a |
FT Fermentation Time; TPC total phenolic content GA Gallic acid; EA ellagic acid; CAT catechin; TAC Total anthocyanin content; 1 mg GAE/L, mg of gallic acid equivalents per L; 2 μg GAE/L: μg of gallic acid equivalents per L; 3 μg EAE/L: μg of ellagic acid equivalents per L; 4 μg CatE/L: μg of catechin equivalents per L; 5 mg C3GE/L: mg of cyanidin-3-glucoside equivalents per L; Cya-3-rut: cyaniding-3-rutinoside; Cya-3-xyl-rut: cyaniding-3-xylosil-rutinoside; Cya-3-glu:Cyanidin-3-glucoside; Cya-3-mal-glu:Cyanidin-3malonil-glucoside; ORAC oxygen radical absorbance capacity assay; DPPH 2,2-diphenyl-1-picrylhydrazyl radical assay; 6 mM TE/L, mM of trolox equivalent per L. Data are indicated as mean values ± standard deviation (n = 3). Different letters in the same row indicate significant differences (p < 0.05, LSD)
The gallic acid (GA) content increased from 0.0031 to 0.0928 µg GAE/L (~ 30 times) during 72 h of fermentation time, showing a significant difference (p < 0.05) each 24 h, but after that time, the GA content decreased (0.00596 µg GAE/L). Previous works reported a similar increase in cranberry juice fermented with Serratia vaccinii bacterium at 24 h of fermentation and in black raspberry wine made with commercial Saccharomyces cerevisiae for 45 days (Cho et al. 2013). Increased GA content has been associated with the degradation of polymeric polyphenols present in the cell wall, which releases single phenolic acids into the fermentation matrix as a result of the activity of enzymes released by the yeast. The reduction of GA concentration may be due to the ability of fermentative microorganisms to remove organic compounds that could act as pollutants (Guo et al. 2014). Yeast and other fermentative fungi release exogenous enzymes, such as laccase (EC 1.10.3.2) and lignin peroxidase (EC 1.11.1.14), which degrade excessive GA and other phenolic acids to methyl gallate and pyrogallic acid (Guo et al. 2014).
The ellagic acid (EA) content decreased from 0.0975 to 0.0565 µg EAE/L from 0 to 96 h of fermentation (p < 0.05), owing to the depolymerization and delactonization of EA by yeast tannase (Wang et al. 2012). This enzyme can hydrolyze covalent and glycosidic structural bonds in macromolecular ellagitannins, while on monomeric EA, yeast tannase may exhibit depsidase (EC 3.11.20) and esterase activities to release two monomeric GAs (Ascacio-Valdés et al. 2016). In contrast, Johnson et al. (2011) reported an increase of 24% during the first 7 days of fermentation of blueberry fruits with EC 1118 yeast. In our study, EA showed strong positive correlations with pH (r = 0.8453), TCC (r = 0.9300), and TPC (r = 0.8413) and a strong negative correlation with fermentation time (r = − 0.9472) (Table 3). These values indicate that the relationship between fermentation time and yeast enzymes acts mainly on molecules such as carbohydrates and phenolic compounds (Ascacio-Valdés et al. 2016).
The catechin content increased from 0.0026 to 0.0034 µg CatE/L (> 33%) during the first 24 h of fermentation and decreased to 0.0009 µg CatE/L (> 80%) at 96 h. The increase in catechin content is associated with the interaction of the yeast with the anatomical parts of the fruits, especially seeds and peel, furthermore, catechin content had a strong positive correlation with TSS (r = 0.9875) (Table 3); that may indicate a relationship between proanthocyanidins and the degradation of sugar and molecules present in blackberry must (Liu and Pilone 2000). These fruit structures are rich in proanthocyanidins linked to carbohydrates, which are used by the yeast as nutritive compounds, and hence proanthocyanidin monomers (catechins) are released to the matrix. However, catechins in an acid medium (pH < 4.0) are hydrolyzed to form carbocations or active catechins, which are able to react with anthocyanins and other phenolic compounds through ethyl bonds and produce adducts, maintaining color intensity and reducing astringency (Liu and Pilone 2000).
In berries, hydrolyzable tannins and condensed tannins are present mainly in the epidermis and seeds (Sánchez-Velázquez et al. 2019). Hydrolyzable tannins are polymers of GA esters (gallotannins) or EA esters (ellagitannins), commonly associated with hexoses, which can generate high-molecular-weight heteropolymers (1000–20,000 Da). Both GA and EA are hydroxylated compounds produced by plants as secondary metabolites, which include a wide variety of chemical structures. During fermentation, yeast releases tannase and polyphenol oxidase, which hydrolyzes hydrolyzable tannins and condensed tannins into phenolic compounds of relatively low molecular weight, such as GA, EA, and catechin (Martin and Matar 2005; Johnson et al. 2011; Ascacio-Valdés et al. 2016).
Anthocyanin content and profile
In this study, the total anthocyanin content (TAC) of wild blackberries was significantly (p < 0.05) decreased from 188.87 to 147.23 mg C3GE/L during the fermentation process (Table 4), this decreased to almost 22.1% when compared with unprocessed samples. Wang et al. (2012) reported that TAC decreased around 24.5% in fermented maqui berry after 9 days with EC 1118 yeast at 20 °C, while Nie et al. (2017) reported a 29% loss of TAC after 40 h of fermentation with Lactobacillus species at 37 °C. The correlation analysis showed that TAC was positively correlated with TCC (r = 0.8358), TPC (r = 0.9098), pH (r = 0.9126), and EA content (r = 0.9767) and negatively correlated with fermentation time (r = − 0.8866) (Table 3).
In wild blackberries, four major anthocyanins were identified: three glycosylated cyanidins (cyanidin-3-rutinoside [Cya-3-rut], cya-3-xylosyl-rutinoside [Cya-3-xyl-rut], and cya-3-glucoside [Cya-3-glu]) and one acylated cyanidin [Cya-3-(6-malonyl)-glucoside (Cya-3–6-mal-glu)] (Fig. 2c). The concentration of individual anthocyanins changed during the fermentation process as follows: Cya-3-glu from 108.68 to 40.29 mg C3GE/L, Cya-3-rut from 45.67 to 9.78 mg C3GE/L, Cya-3-xyl-rut from 42.08 to 14.93 mg C3GE/L, and Cya-3–6-mal-glu from 5.66 to 1.91 mg C3GE/L. Cya-3-glu was the most prevalent anthocyanin present in the wild fruits as well as in the fermented products. Cya-3-glu is the most frequent and abundant anthocyanin reported in blackberries (> 50% of anthocyanin content) (Cuevas-Rodríguez et al. 2010b; Ascacio-Valdés et al. 2016) and the main anthocyanin responsible for the organoleptic characteristics of fresh blackberries fruits and their products (Arozarena et al. 2012). Cya-3-rut and Cya-3-xyl-rut are other anthocyanins reported frequently in Rubus fruits, especially in the subgenus Idaeobatus, which includes black and red raspberries (Martin and Matar 2005). Cya-3-6-mal-glu is an anthocyanin reported in Rubus species grown in subtropical areas and does not make up more than 5% of the anthocyanin content (Macierzyński et al. 2014). Cya-3-rut content showed a noticeable decrease in comparison with Cya-3-6-xyl-rut since the xylosyl sugar group provides high chemical stability and resistance to degradation in the matrix against chemical, physical, and biological agents (Martin and Matar 2005).
The decrease in the TAC and the content of individual anthocyanins during fermentation could be associated with non-controlled factors, such as the metabolic activity of yeast, changes in °Brix, and water mobility. Another explanation that has been suggested for the changes in the TAC, is that, during berry fermentation, the TAC decreases since the structure and composition of the yeast cell walls could influence the monomeric anthocyanin content. Saccharomyces cerevisiae yeast cells and anthocyanins have some potential to interact (e.g. absorbing, degrading, and changing the pH in the extracellular matrix), which reduces the anthocyanin content significantly, resulting in a loss of color, and it is known that some S. cerevisiae species and strains can absorb up to twice as many anthocyanins as other fermentative microorganisms (Arozarena et al. 2012; Gómez-Gallego et al. 2012).
On the other hand, anthocyanin degradation reactions have been related to the activities of yeast enzymes, such as polyphenol oxidase, which is not involved in the initial reactions of must fermentation but, after 40 h, generate new pigments such as pyranocyanins (vitisins, pinotins, portisins, and oxovitisins) and flavan-3-ols conjugates (anthocyanin-alkyl-flavan-3-ol, anthocyanin-flavanol, and flavanol–anthocyanin), which have a stable structure and are resistant to modifications in the wine matrix produced by SO2 and pH during fermentation by hyperchromic effects. These new compounds are more resistant to fermentative conditions than anthocyanins and contribute to color stability and other properties (Arozarena et al. 2012; Sumby et al. 2010; Gómez-Gallego et al. 2012; Macierzyński et al. 2014).
Antioxidant capacity
The antioxidant capacity (AOx) determined by the ORAC and DPPH assays is shown in Table 4. For ORAC, the AOx values obtained ranged from 24.06 to 57.79 mM TE/L. During the first 24 h, the AOx decreased from 35.05 to 24.06 mM TE/L and subsequently increased again after 48 and 72 h of the fermentation process, to 27.43 and 50.89 mM TE/L, respectively. Results revealed that the AOx was higher at 72–96 h of bioprocessing in comparison with other berries fermented for short times (< 15 days) (Wang et al. 2012; Johnson et al. 2013). The ORAC assay is based on hydrogen-atom transfer reactions from an antioxidant to a radical generator. This assay measures the effect of an antioxidant for avoiding the loss of fluorescence of an indicator by oxidative degradation of a free radical initiator (Hidalgo and Almajano 2017). The ORAC values showed a strong positive correlation with the presence of GA (r = 0.8688) (Table 3), possibly owing to the fact that, during the fermentation process, the GA concentration increases; OH groups of released recent GA from macromolecular phenolics by action of yeast enzymes, like tannase and phenoloxidase, have been shown to have antioxidant activity due to the capacity of this molecule to donate protons to oxidizing agents (Martin and Matar 2005).
For the DPPH assay, the observed values started at 4.43 mM TE/L and then increased to 5.55 mM TE/L (> 20%) after the first 24 h of fermentation, showing a significant difference (p < 0.05). Subsequently, statistical differences (p < 0.05) in DPPH values were not observed. The DPPH values showed strong positive correlations with fermentation time (r = 0.8131) and TOC (r = 0.9152), while strong negative correlations with TPC (r = − 0.9236), TAC (r = − 0.9606), and EA (r = − 0.9493) were found. This behavior could be associated to the single-electron transfer mechanism of this AOx method. Single-electron transfer measures the AOx required to transfer one electron to reduce any radicals and other compounds. In this assay, one of two reactions (hydrogen-atom transfer and single-electron transfer) is dominant in the system, which is determined by the antioxidant characteristics of the compounds tested (Hidalgo and Almajano 2017). Unsaturated FAs could shed an electron (Bushman et al. 2004). Our results suggest that the AOx obtained by DPPH is not due principally to the presence of polyphenols and polyunsaturated FAs.
The fermentation process changes the phenolic profile associated with the action of yeast enzymes (Su and Chien 2007). Yeast enzymes, such as polyphenol oxidase, β-glucosidase, and tannase, degrade polymeric phenolic compounds, acting on covalent and glycosidic bonds and replacing them with substituent groups (H, OH, COOH, etc.) (Yacco et al. 2016). Previous investigations found a relationship between the AOx and polyphenols in terms of their structure, with a strong correlation between four factors: (1) the structures that appear in the substituent groups (H, OH, COOH, CH3, etc.) are diverse; (2) the planar shape of the phenolics allows electronic dislocation between the adjacent rings; (3) the presence and number of OH groups causes the molecules to yield protons to free radicals and oxidizing agents, which generates resonance states between nearby OH groups; and (4) the hydroxyls could be able to mix the conjugations between the carbon double bonds within the aromatic ring (Cuevas-Rodríguez et al. 2010b; Badhani et al. 2015; Hidalgo and Almajano 2017). Other antioxidant compounds were found, such as FAs, which in biological systems have exhibited scavenging activities against DPPH reagent and the lipophilic ORAC assay; those activities may come from cell membrane structures and contribute to the AOx of fermented beverages (Bushman et al. 2004).
Conclusion
Saccharomyces cerevisiae (ex bayanus) EC 1118 was found to be useful to produce a fermented beverage in a short time using wild blackberries from northwestern Mexico as a fermentable source. The fermentation process caused modifications to physicochemical properties, nutritional (which nutritional compounds) and phenolic compounds. Total and individual carbohydrates and anthocyanins showed a constant decrease, whereas MCFA content increased. FA diversity was improved with fermentation. The AOx measured by ORAC was strongly correlated (r ≥ 0.8) with GA content, whereas DPPH was related to TOC, with the highest values observed at 96 and 48 h of fermentation, respectively. On the basis of the behavior of compound groups and individual phytochemicals during fermentation, it is possible to predict their effects on the AOx and physicochemical parameters. The results obtained in this research showed that the fermentation process enhances the profile of bioactive compounds of wild Mexican blackberries. Further studies are needed to assess in depth its possible application and/or addition as a nutraceutical ingredient for the development of functional foods.
Supplementary information
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Oscar Abel Sánchez-Velazquez, Edith-Oliva Cuevas-Rodríguez, Cuauhtémoc Reyes-Moreno, Erika Yudit Ríos-Iribe, Liliana León-López, Alan Javier Hernández-Álvarez, Jorge Milán-Carrillo. The first draft of the manuscript was written by Oscar Abel Sánchez-Velázquez, and all authors commented and reviewed on previous versions of the manuscript. All authors read and approved the final manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that there are no conflicts of interest.
Human or animal rights
This article does not contain any studies with human or animal subjects.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ahumada-Santos YP, et al. Chemical characterization, antioxidant and antibacterial activities of six Agave species from Sinaloa, Mexico. Ind Crops Prod. 2013;49:143–149. doi: 10.1016/j.indcrop.2013.04.050. [DOI] [Google Scholar]
- Akin H, Brandam C, Meyer X-M, Strehaiano P. A model for pH determination during alcoholic fermentation of a grape must by Saccharomyces cerevisiae. Chem Eng Process. 2008;47(11):1986–1993. doi: 10.1016/j.cep.2007.11.014. [DOI] [Google Scholar]
- AOAC International . Official methods of analysis. 20. Saint Paul, MN, USA: AOAC International; 2016. [Google Scholar]
- Arozarena Í, et al. Color, ellagitannins, anthocyanins, and antioxidant activity of Andean blackberry (Rubus glaucus Benth.) wines. J Agric Food Chem. 2012;60(30):7463–7473. doi: 10.1021/jf300924z. [DOI] [PubMed] [Google Scholar]
- Ascacio-Valdés JA, et al. The complete biodegradation pathway of ellagitannins by Aspergillus niger in solid-state fermentation. J Basic Microbiol. 2016;56:329–336. doi: 10.1002/jobm.201500557. [DOI] [PubMed] [Google Scholar]
- Badhani A, et al. Variation in chemical constituents and antioxidant activity in yellow Himalayan (Rubus ellipticus Smith) and hill raspberry (Rubus niveus Thunb.) J Food Biochem. 2015;39:663–672. doi: 10.1111/jfbc.12172. [DOI] [Google Scholar]
- Bendary E, et al. Antioxidant and structure–activity relationship (SARs) of some phenolic and anilines compounds. Ann Agric Sci. 2013;58(2):173–181. doi: 10.1016/j.aoas.2013.07.002. [DOI] [Google Scholar]
- Bushman BS, et al. Chemical composition of caneberry (Rubus spp.) seeds and oils and their antioxidant potential. J Agric Food Chem. 2004;52(26):7982–7987. doi: 10.1021/jf049149a. [DOI] [PubMed] [Google Scholar]
- Cho J-Y, Jeong JH, et al. Change in the content of phenolic compounds and antioxidant activity during manufacturing of black raspberry (Rubus coreanus Miq.) wine. Food Sci Biotechnol. 2013;22(5):1–8. doi: 10.1007/s10068-013-0207-5. [DOI] [Google Scholar]
- Cuevas-Rodríguez EO, et al. Inhibition of pro-inflammatory responses and antioxidant capacity of Mexican blackberry (Rubus spp.) extracts. J Agric Food Chem. 2010;58(17):9542–9548. doi: 10.1021/jf102590p. [DOI] [PubMed] [Google Scholar]
- Cuevas-Rodríguez EO, et al. Characterization of anthocyanins and proanthocyanins in wild and domesticated Mexican blackberry (Rubus spp.) J Agric Food Chem. 2010;58(12):7458–7464. doi: 10.1021/jf101485r. [DOI] [PubMed] [Google Scholar]
- Gallart M, et al. Influence of fatty acids on wine foaming. J Agric Food Chem. 2002;50(24):7042–7045. doi: 10.1021/jf0204452. [DOI] [PubMed] [Google Scholar]
- Gómez-Gallego MA, et al. Effect of co-winemaking in phenolic composition, color and antioxidant capacity of young red wines from La Mancha region. Eur Food Res Technol. 2012;235(1):155–167. doi: 10.1007/s00217-012-1745-4. [DOI] [Google Scholar]
- Guo D, et al. A comparative study on the degradation of gallic acid by Aspergillus oryzae and Phanerochaete chrysosporium. Water Sci Technol. 2014;70(1):175–181. doi: 10.2166/wst.2014.213. [DOI] [PubMed] [Google Scholar]
- Hidalgo G-I, Almajano MP. Red fruits: extraction of antioxidants, phenolic content, and radical scavenging determination: a review. Antiox. 2017;6(1):1–27. doi: 10.3390/antiox6010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MH, et al. Anthocyanins and proanthocyanidins from blueberry-blackberry fermented beverages inhibit markers of inflammation in macrophages and carbohydrate-utilizing enzymes in vitro. Mol Nutr Food Res. 2013;57:1182–1197. doi: 10.1002/mnfr.201200678. [DOI] [PubMed] [Google Scholar]
- Johnson MH, et al. Cultivar evaluation and effect of fermentation on antioxidant capacity and in vitro inhibition of α-amylase and α-glucosidase by highbush blueberry (Vaccinium corombosum) J Agric Food Chem. 2011;59(16):8923–8930. doi: 10.1021/jf201720z. [DOI] [PubMed] [Google Scholar]
- Lee J, et al. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: collaborative study. J AOAC Int. 2005;88:1269–1278. doi: 10.1093/jaoac/88.5.1269. [DOI] [PubMed] [Google Scholar]
- Liu S-Q, Pilone GJ. An overview of formation and roles of acetaldehyde in winemaking with emphasis on microbiological implications. Int J Food Sci Technol. 2000;35:49–61. doi: 10.1046/j.1365-2621.2000.00341.x. [DOI] [Google Scholar]
- Macierzyński J, et al. Skład polifenolowy owoców jeżyny Rubus fruticosus. Nauka Technol Jakość. 2014;5(96):183–194. doi: 10.15193/ZNTJ/2014/96/183-194. [DOI] [Google Scholar]
- Martin LJ, Matar C. Increase of antioxidant capacity of the lowbush blueberry (Vaccinium angustifolium) during fermentation by a novel bacterium from the fruit microflora. J Sci Food Agric. 2005;85:1477–1484. doi: 10.1002/jsfa.2142. [DOI] [Google Scholar]
- Nie Q, et al. Effect of fermentation and sterilization on anthocyanins in blueberry. J Sci Food Agric. 2017;97:1459–1466. doi: 10.1002/jsfa.7885. [DOI] [PubMed] [Google Scholar]
- Nowak D, et al. The antioxidant properties of exotic fruit juices from acai, maqui berry and noni berries. Eur Food Res Technol. 2018;244(11):1897–1905. doi: 10.1007/s00217-018-3102-8. [DOI] [Google Scholar]
- Regelmann J, et al. Catabolite degradation of fructose-1,6-bisphosphatase in the yeast Saccharomyces cerevisiae: a genome-wide screen identifies eight novel GID genes and indicates the existence of two degradation pathways. Mol Biol Cell. 2003;14(4):1652–1663. doi: 10.1091/mbc.e02-08-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rychlik JL, Russell JB. Bacteriocin-like activity of Butyrivibrio fibrisolvens JL5 and its effect on other ruminal bacteria and ammonia production. Appl Environ Microbiol. 2002;68(3):1040–1046. doi: 10.1128/AEM.68.3.1040-1046.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Velázquez OA, et al. Characterization of tannins from two wild blackberries (Rubus spp) by LC–ESI–MS/MS, NMR and antioxidant capacity. J Food Meas Charact. 2019;13(3):2265–2274. doi: 10.1007/s11694-019-00146-z. [DOI] [Google Scholar]
- Šeruga M, et al. Determination of polyphenols content and antioxidant activity of some red wines by differential pulse voltammetry HPLC and spectrophotometric methods. Food Chem. 2011;124(3):1208–1216. doi: 10.1016/j.foodchem.2010.07.047. [DOI] [Google Scholar]
- Shompoosang S, et al. Enzymatic production of three 6-deoxy-aldohexoses from l-rhamnose. Biosci Biotechnol Biochem. 2014;78(2):317–325. doi: 10.1080/09168451.2014.878217. [DOI] [PubMed] [Google Scholar]
- Skrede G, et al. Changes in anthocyanins and polyphenolics during juice processing of highbush blueberries (Vaccinium corymbosum L.) J Food Sci. 2000;65(2):357–364. doi: 10.1111/j.1365-2621.2000.tb16007.x. [DOI] [Google Scholar]
- Su M-S, Chien P-J. Antioxidant activity, anthocyanins, and phenolics of rabbiteye blueberry (Vaccinium ashei) fluid products as affected by fermentation. Food Chem. 2007;104:182–187. doi: 10.1016/j.foodchem.2006.11.021. [DOI] [Google Scholar]
- Sumby KM, et al. Microbial modulation of aromatic esters in wine: current knowledge and future prospects. Food Chem. 2010;121(1):1–16. doi: 10.1016/j.foodchem.2009.12.004. [DOI] [Google Scholar]
- Torija MJ, et al. Effects of fermentation temperature and Saccharomyces species on the cell fatty acid composition and presence of volatile compounds in wine. Int J Food Microbiol. 2003;85:127–136. doi: 10.1016/S0168-1605(02)00506-8. [DOI] [PubMed] [Google Scholar]
- Tyl C, Sadler GD. pH and titratable acidity. In: Nielsen SS, editor. Food analysis. Food science text series. 5. Cham, Switzerland: Springer International; 2017. [Google Scholar]
- van Dijken JP, et al. Novel pathway for alcoholic fermentation of δ-gluconolactone in the yeast Saccharomyces bulderi. J Bacteriol. 2002;184(3):672–678. doi: 10.1128/JB.184.3.672-678.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, et al. Fermentation kinetics of different sugars by apple wine yeast Saccharomyces cerevisiae. J Inst Brew. 2004;110(4):340–346. doi: 10.1002/j.2050-0416.2004.tb00630.x. [DOI] [Google Scholar]
- Wang JZ et al (2012) Maqui berry (Aristotelia chilensis) juices fermented with yeasts: effects on phenolic composition, antioxidant capacity, and iNOS and COX-2 protein expression. In: Patil BS, Jayaprakasha GK, Chidambara Murthy KN, Seeram NP (eds) Emerging trends in dietary components for preventing and combating disease. ACS Symposium Series. America Chemical Society, Washington, DC, USA. Chapter 6, pp 95–11610.1021/bk-2012-1093.ch006
- Yacco RS, et al. Red wine tannin structure–activity relationships during fermentation and maceration. J Agric Food Chem. 2016;64(4):860–869. doi: 10.1021/acs.jafc.5b05058. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



