Abstract
Background
Gastrointestinal (GI) events are the most frequent treatment-emergent adverse events (TEAEs) reported for glucagon-like peptide-1 receptor agonist therapies. This post hoc analysis of the AWARD-11 phase 3 trial assessed the GI tolerability of dulaglutide at once-weekly doses of 1.5, 3.0, and 4.5 mg.
Methods
The AWARD-11 trial randomized patients to once-weekly dulaglutide 1.5 mg (n = 612), 3.0 mg (n = 616), or 4.5 mg (n = 614) for 52 weeks. Patients started on dulaglutide 0.75 mg for 4 weeks before escalating stepwise every 4 weeks until the final randomized dose was reached. This study analyzes the onsets, incidences, prevalences, and severities of nausea, vomiting, and diarrhea events reported through 52 weeks.
Results
The highest incidences of nausea (≤ 8%), vomiting (≤ 2%), and diarrhea (≤ 4%) were primarily observed soon after the initiation of dulaglutide treatment at 0.75 mg. Incidence then declined throughout the remainder of the study, even with dose escalation to 1.5, 3.0, and 4.5 mg. Most of these GI TEAEs were mild to moderate in severity, with severe nausea, vomiting, or diarrhea events occurring in ≤ 0.6% of patients. Treatment discontinuation due to nausea was low across treatment groups (≤ 1.5%).
Conclusions
The tolerability profiles of dulaglutide 3.0 mg and 4.5 mg were consistent with that of the 1.5-mg dose. Patients experiencing GI events were most likely to do so within 2 weeks of treatment initiation, and few patients experienced a new GI event after escalating to the 3.0-mg or 4.5-mg dose. Severe events were infrequent, and when they did occur, no relationship with dose at time of event was observed.
Supplementary file1 (MP4 33880 kb)
Supplementary Information
The online version contains supplementary material available at 10.1007/s13300-021-01140-9.
Keywords: Dulaglutide, Gastrointestinal, Glucagon like peptide-1 (GLP-1) agonist, Nausea, Tolerability, Type 2 diabetes
Plain Language Summary
Dulaglutide is a glucagon-like peptide 1 receptor agonist (GLP-1 RA) prescribed for the treatment of type 2 diabetes (T2D). The most frequently reported side effects of GLP-1 RAs are nausea, vomiting, or diarrhea. This analysis of a 52-week study in adult patients with T2D details the tolerability of dulaglutide injected once weekly at a dose of 1.5 mg, 3 mg, or 4.5 mg, as assessed by looking at the nausea, vomiting, and diarrhea events reported during the study. All patients started dulaglutide at 0.75 mg before escalating to 1.5 mg after 4 weeks. Depending on the group they were randomly assigned to, the patients then either remained on the 1.5-mg dose, escalated to 3 mg after another 4 weeks and remained on this dose, or escalated further to 4.5 mg after another 4 weeks. The minority of patients who experienced nausea, vomiting, or diarrhea events (less than 16% of patients in each case) generally did so at the beginning of treatment, when all groups were taking the same dose (0.75 mg). Episodes of nausea, vomiting, or diarrhea then became less frequent, even as patients escalated to each of the higher doses. Most of these events were mild to moderate in severity, and most did not cause patients to stop taking the treatment. In general, this analysis shows that, for the minority of patients who experienced nausea, vomiting, or diarrhea, these events were most likely to happen shortly after starting treatment and lessened over time, even as patients escalated to higher dulaglutide doses.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13300-021-01140-9.
Key Summary Points
| Why carry out this study? |
| Gastrointestinal (GI) events are the most frequent treatment-emergent adverse events reported for glucagon-like peptide-1 receptor agonist therapies. |
| Providing tolerability information can inform both clinicians’ and patients’ expectations for dose escalation and may improve adherence. |
| This post hoc analysis of the AWARD-11 phase 3 trial assessed the GI tolerability of dulaglutide at once-weekly doses of 1.5 mg, 3.0 mg, and 4.5 mg. |
| What was learned from the study? |
| The gastrointestinal tolerability profiles of dulaglutide at doses of 3.0 mg and 4.5 mg were consistent with that established for the 1.5-mg dose. |
| The incidences of nausea, vomiting, and diarrhea peaked soon after treatment initiation and declined thereafter, even with dose escalation. |
| Most events were mild to moderate in severity and led to few treatment discontinuations. |
Digital Feature
This article is published with digital features, including plain language summary and video abstract, to facilitate understanding of the article. To view digital features for this article go to 10.6084/m9.figshare.15125622.
Introduction
Medication adherence is a significant problem in attempts to attain adequate glycemic control and prevent complications in diabetes [1]. Although multifactorial in cause, treatment discontinuation due to adverse events may be a contributing factor to the failure to achieve optimal glycemic control [2]. Dulaglutide is a long-acting glucagon-like peptide-1 receptor agonist (GLP-1 RA) approved for glycemic control in patients with type 2 diabetes (T2D) and is associated with a low inherent risk of hypoglycemia [3–14]. The most frequent treatment-emergent adverse events (TEAEs) associated with dulaglutide, and other GLP-1 RA therapies [15], are nausea, vomiting, and diarrhea [16]. These events are typically mild and tend to peak soon after treatment initiation before decreasing in the following weeks [15, 17]. While the frequency of gastrointestinal (GI) TEAEs can be dose dependent [18, 19], previous studies involving GLP-1 RA therapies have shown that escalating the dose in a measured, stepwise manner can lessen the occurrence and severity of GI events [20, 21]. The AWARD-11 trial compared the efficacy and safety of dulaglutide at once-weekly doses of 3.0 mg and 4.5 mg with its efficacy and safety at the previously approved 1.5-mg dose [22], with dose escalation occurring every 4 weeks from the 0.75-mg starting dose to mitigate potential tolerability issues at higher doses.
This post hoc analysis of AWARD-11 provides a detailed evaluation of the most frequent GI-related TEAEs of nausea, vomiting, and diarrhea for dulaglutide at doses of 1.5, 3.0, and 4.5 mg, including a time course of these events through to week 52 of the trial.
Methods
Study Design
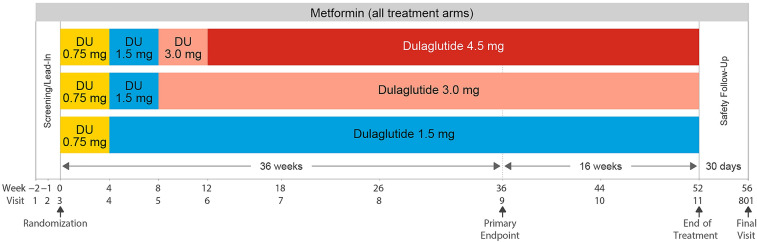
Participants in the 52-week randomized, double-blind, parallel-arm AWARD-11 phase 3 trial who received at least one dose of the study drug were included in this analysis. The AWARD-11 study design and results have previously been published [22]. Briefly, eligible adults who had T2D, had HbA1c (glycated hemoglobin) ≥ 7.5% (58 mmol/mol) and ≤ 11.0% (97 mmol/mol), were on stable doses of metformin ≥ 1500 mg/day, were GLP-1 RA and insulin naïve (except for short-term [≤ 14 consecutive days] use of insulin for acute conditions), and had a body mass index (BMI) ≥ 25 kg/m2 were randomized 1:1:1 to dulaglutide 1.5, 3.0, or 4.5 mg administered once weekly via subcutaneous injection (Fig. 1; full inclusion and exclusion criteria are shown in Supplementary Table S1). All patients initiated treatment on dulaglutide 0.75 mg for 4 weeks before escalating to 1.5 mg. Patients in the 1.5-mg group remained on this dose for the duration of the study, while patients in the 3.0-mg and 4.5-mg groups continued dose escalation in 1.5-mg dose increments every 4 weeks to reach the final randomized dose of 3.0 or 4.5 mg.
Fig. 1.
AWARD-11 study design. Eligible patients were randomized 1:1:1 to dulaglutide 1.5 mg, 3.0 mg, or 4.5 mg, administered once weekly via subcutaneous injection. For all patients, treatment was initiated with once-weekly dulaglutide 0.75 mg before stepwise dose escalation to the randomized dose of 1.5 mg, 3.0 mg, or 4.5 mg. DU dulaglutide
Patients unable to tolerate dulaglutide during dose escalation could temporarily interrupt and then restart dulaglutide once. Patients were not permitted to lower the dose. If intolerable symptoms returned, the study drug was discontinued. Guidance was provided to study sites regarding the treatment of patients with GI symptoms, including advice on dietary behaviors to mitigate nausea and vomiting, and the use of oral antiemetic or antidiarrheal medication was considered on an as-needed basis.
The AWARD-11 study was conducted in accordance with the International Conference on Harmonisation Guidelines for Good Clinical Practice and the Declaration of Helsinki. Patients provided a signed informed consent, and all protocols were approved by local ethics review boards.
Study Assessments and Statistical Analyses
In this post hoc analysis of AWARD-11, the GI tolerability of dulaglutide at doses of 1.5, 3.0, or 4.5 mg once weekly, based on events of nausea, vomiting, and diarrhea, was assessed. Adverse events were classified according to the Medical Dictionary for Regulatory Activities (MedDRA) version 22.0 and summarized by treatment arm. Adverse event severity was categorized as either mild, moderate, or severe by investigators who used their medical judgment to compare the reported event to similar events or conditions observed in clinical practice. The time course of GI events was assessed using 2-week intervals over the 52-week treatment period. Onset (the first occurrence for each participant), incidence (any new event occurrence within the 2-week interval), and prevalence (a new or ongoing event during the 2-week interval) are reported regardless of whether an event end date was captured. A sensitivity analysis that re-estimated prevalence using only events for which both start and stop dates were reported was also performed. Kaplan–Meier curves were used to summarize both time to onset and treatment discontinuation due to a GI event. The curves depict the cumulative percentage of patients experiencing onset or discontinuation as a function of time. GI TEAE severity and a treatment-by-subgroup analysis of GI TEAEs based on intrinsic factors (age, race, sex, duration of diabetes, baseline BMI, and baseline estimated glomerular filtration rate [eGFR]) were also assessed.
Results
Patient Disposition and Baseline Characteristics
A total of 1,842 patients with T2D were randomized to dulaglutide 1.5 mg (n = 612), 3.0 mg (n = 616), or 4.5 mg (n = 614). Patients had a mean BMI of 34.2 kg/m2 and a mean duration of diabetes of 7.6 years. Baseline characteristics were consistent across treatment groups (Supplementary Table S2). As previously reported, there was no significant difference between dose groups in the proportion of patients completing the study through 52 weeks (1.5 mg: 90.8%; 3.0 mg: 89.1%; 4.5 mg: 91.2%; p = 0.427) or completing the study on the study drug (1.5 mg: 87.1%; 3.0 mg: 84.9%; 4.5 mg: 84.7%; p = 0.416) [22].
The primary objective of the study (change from baseline to week 36 in HbA1c) has been reported previously [22]: in the on-treatment analysis excluding data after rescue, all dulaglutide doses were efficacious at lowering HbA1c (least-square mean [LSM] change from baseline: − 1.53% for 1.5 mg, − 1.71% for 3.0 mg, and − 1.87% for 4.5 mg), with both the dulaglutide 3.0 mg (p = 0.003) and 4.5 mg (p < 0.001) doses demonstrating superiority to the 1.5-mg dose [22].
Incidence and Prevalence of Gastrointestinal Adverse Events Over Time
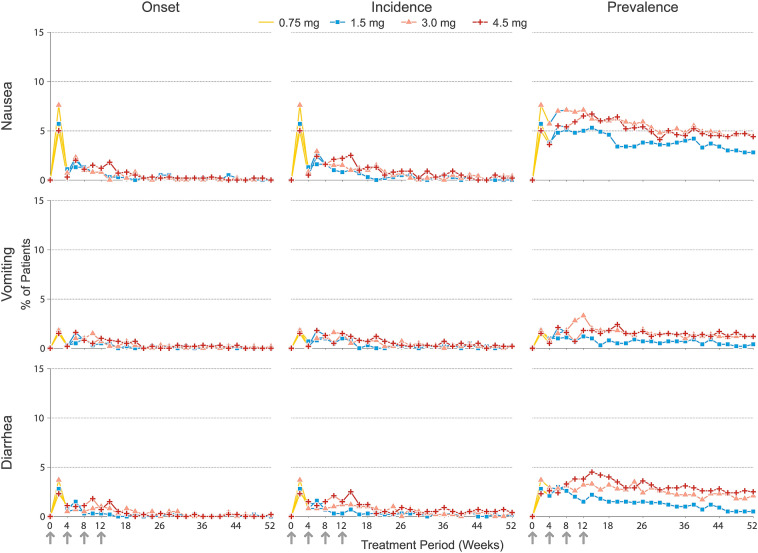
As detailed previously [22], the most frequently reported TEAEs for all treatment groups through 52 weeks were GI-related, led by nausea (1.5 mg: 14.2%; 3 mg: 16.1%; 4.5 mg: 17.3%; p = 0.336), diarrhea (1.5 mg: 7.7%; 3 mg: 12.0%; 4.5 mg: 11.6%; p = 0.021), and vomiting (1.5 mg: 6.4%; 3 mg: 9.1%; 4.5 mg: 10.1%; p = 0.048). The highest proportions of patients with new-onset nausea, vomiting, or diarrhea occurred during the first 2-week study interval after initiating the 0.75-mg dose (5.0–7.6% for nausea, 1.5–1.8% for vomiting, and 2.3–3.7% for diarrhea) within each treatment group (Fig. 2). New-onset incidence rapidly declined by more than 50% thereafter, with slight variations between dose groups. New-onset incidence of GI AEs was lower at each subsequent 2-week interval, even after escalation to the 1.5-mg dose (with the exception of vomiting in the 4.5-mg group) and further escalation to the assigned maintenance dose of 3.0 mg or 4.5 mg. The incidences of nausea, vomiting, and diarrhea in 2-week intervals, including both first and subsequent events, followed a pattern very similar to that of new onsets (Fig. 2). As expected, based on the overall incidence through 52 weeks, small numerical differences (≤ 1.8%) between treatment groups in the incidence of GI events were evident during the study intervals following escalation to 3.0 mg (after week 8) or 4.5 mg (after week 12). However, after week 26, the new-onset incidence and the incidences of nausea, vomiting, and diarrhea were low and similar across dose groups within each 2-week interval (≤ 0.5% and ≤ 0.9% for new onsets and the event incidences, respectively; Fig. 2).
Fig. 2.
New onsets, incidences, and prevalences of nausea, vomiting, and diarrhea events from initiation of treatment to week 52, measured at 2-week intervals. Arrows denote weeks at which dose escalation steps occurred. Line marker color indicates the planned final dose (i.e., treatment arm), while line color denotes the actual dose administered at a specific point in time. All patients began treatment with a once-weekly dose of 0.75 mg for a period of 4 weeks before gradually escalating to the target dose of 1.5, 3.0, or 4.5 mg at 4-week intervals
The prevalences of nausea (5.7–7.6%) and diarrhea (3.0–4.5%) tended to be highest during the dose escalation period through to the final assigned dose, before decreasing steadily through to week 52 (Fig. 2). The prevalence of vomiting was low in all time intervals, affecting at most 1.8–3.3% of the patients in any dose group, and it declined after the maintenance dose was reached, remaining within the range 0.5–1.5% after week 20. The prevalence of each type of event tended to decrease at a slower rate compared to new onsets and incidence, as prevalence is based on the occurrence of all events, including ongoing events, within the respective study interval. Importantly, duration of adverse events was not systematically collected in the study and was dependent upon the reporting of the event stop date.
A total of 113 (9.8%) GI AEs (nausea n = 68, vomiting n = 16, diarrhea n = 29) had no recorded stop date and were included in the analysis of prevalence. A sensitivity analysis was performed to include only those events for which both start and stop dates were reported (Supplementary Fig. S1). Both analyses yielded similar general prevalence patterns for GI events over time, with the highest prevalence generally observed during dose escalation, followed by a declining prevalence of each event type over time.
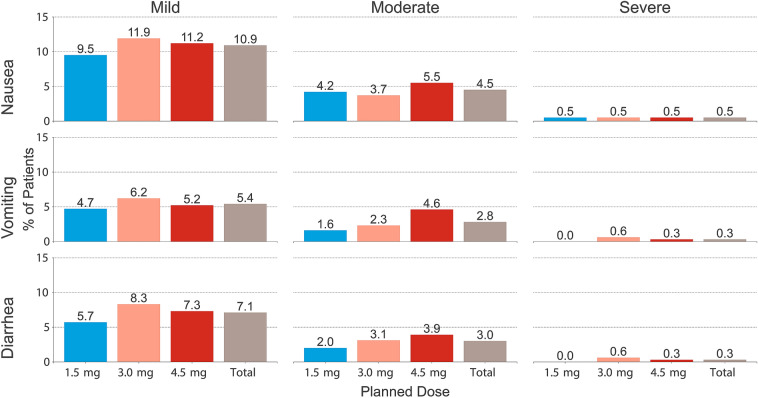
Severity of Gastrointestinal TEAEs
The majority of the reported GI TEAEs (69% for nausea, 68% for diarrhea, and 63% for vomiting) were mild in severity (Fig. 3). The proportion of participants who experienced at least one severe event of nausea, vomiting, or diarrhea was below 1% for each event type, and was not significantly different between treatment groups (Fig. 3, Supplementary Table S3).
Fig. 3.
Summary of the severities of the reported nausea, vomiting, and diarrhea events. Total n = 1842; 1.5 mg n = 612; 3.0 mg n = 616; 4.5 mg n = 614. Bars represent the proportion of patients (%) for each category and treatment. Patients who experienced > 1 adverse event can be present in multiple severity categories
Importantly, although all patients reporting a severe diarrhea or vomiting event were in the 3.0-mg or 4.5-mg dose groups, some of those severe events occurred while patients were taking a lower dulaglutide dose during the escalation phase: in the 3.0-mg treatment group, half of the severe vomiting (2 out of 4) and diarrhea (2 out of 4) events were reported before reaching the 3.0-mg dose (Supplementary Table S3).
In patients who reported a severe GI TEAE, there was no discernible trend of a preceding event of lesser severity. For nausea, most patients with a severe event (7 out of 9) had not reported a prior nausea event of milder severity. For vomiting and diarrhea, half of the severe events were preceded by one or more events of milder severity (3 out of 6 patients for both severe vomiting and severe diarrhea events). One participant who experienced a severe GI TEAE had a subsequent event of milder severity; all other participants who experienced a severe GI TEAE had no subsequent events. The onset study day for severe events ranged from day 2 to day 310, with no clear trend toward early or late onset (data not shown).
Time to Onset of and Treatment Discontinuation Due to Gastrointestinal Adverse Events
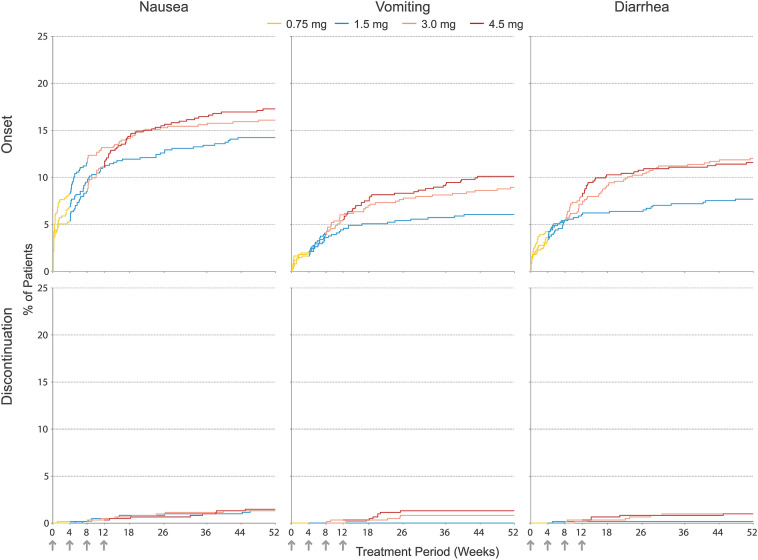
Kaplan–Meier analysis of the cumulative incidence of onset for nausea, vomiting, and diarrhea is presented in Fig. 4. During the first 8 weeks of the study, when all patients were receiving the same dose of dulaglutide, the cumulative onset incidences of these events were similar between treatment groups, although some variability was evident for nausea. However, as patients escalated from the 1.5-mg to the 3.0-mg or from the 3.0-mg to the 4.5-mg dose, there were small differences in the cumulative incidence of onset between treatment groups. Consistent with the new-onset and overall incidences at discrete study intervals (Fig. 2), the cumulative incidence of onset tended to plateau for all dose groups after week 18, with few new events reported afterwards.
Fig. 4.
Kaplan–Meier curves for cumulative incidence of the onset of nausea, vomiting, or diarrhea adverse events (top) and discontinuation due to nausea, vomiting, or diarrhea adverse events (bottom) by treatment group through week 52. Arrows denote weeks at which dose escalation steps occurred. Line color denotes the actual dose administered at a specific point in time. All patients began treatment with a once-weekly dose of 0.75 mg (yellow) for a period of 4 weeks and then escalated to the target dose of 1.5, 3.0, or 4.5 mg at 4-week intervals
Relative to the overall incidence of patients experiencing an event of nausea, vomiting, or diarrhea, few patients permanently discontinued the study drug due to one of these events (Fig. 4): 1.4% of the total discontinued due to nausea (1.5 mg: 1.3%, 3.0 mg: 1.3%, 4.5 mg: 1.5%), 0.7% due to vomiting (1.5 mg: 0%, 3.0 mg: 0.8%, 4.5 mg: 1.3%), and 0.7% due to diarrhea (1.5 mg: 0.2%, 3.0 mg: 1.0%, 4.5 mg: 1.0%). The cumulative incidence of study drug discontinuation due to nausea was similar across dose groups (Fig. 4). Most patients who discontinued the study drug in the 3.0-mg or 4.5-mg treatment groups due to vomiting or diarrhea did so after escalating to the final assigned dose.
Subgroup Analyses
No significant treatment-by-subgroup interactions for incidence of nausea, vomiting, or diarrhea were observed for age (above and below 65 years and 75 years), BMI (above and below median BMI), race, duration of diabetes, or baseline eGFR. Significant (p < 0.10) treatment-by-subgroup interactions were observed for female versus male subgroups for nausea (p = 0.030) and diarrhea (p = 0.022). The significant treatment-by-sex interactions for nausea and diarrhea were the result of differences in patterns across the dose groups between females and males. Females experienced a higher incidence of nausea than males in each dulaglutide dose group, with similar incidences of nausea observed across the dulaglutide dose groups in females. In males, the overall incidences of nausea and diarrhea were lower, but there were higher incidences of these events in the 3.0-mg and 4.5-mg groups compared to the 1.5-mg group (Supplementary Table S4).
Discussion
GI-related TEAEs are among the most frequent reasons provided for treatment discontinuation of GLP-1 RAs [23], and are an important determinant of tolerability. The time courses of GI-related TEAEs and other tolerability indicators for dulaglutide 1.5, 3.0, and 4.5 mg are important factors for patients and health-care providers to consider when initiating or escalating dulaglutide doses. In the AWARD-11 trial, GI TEAEs were the most frequently reported adverse events (8.5–15.9%), but they resulted in few patients discontinuing dulaglutide treatment (1.4% for nausea, 0.7% each for vomiting and diarrhea). The incidences of nausea, vomiting, and diarrhea were generally highest across all dose groups early after the initiation of dulaglutide, before lowering during each subsequent study interval, even after escalating to the 3.0-mg or 4.5-mg dose. The results did not suggest a greater risk of more severe GI events with escalation to higher doses. Overall, these findings indicate that incremental escalation of the dulaglutide dose from 1.5 to 3.0 mg and then to 4.5 mg once weekly has an acceptable GI tolerability profile which is generally consistent with those of lower doses of dulaglutide.
Several findings from these analyses could help inform patients on what to expect with dulaglutide treatment. This study shows that most patients who experience GI adverse events with dulaglutide will do so within the first 2 weeks, and symptoms are not likely to persist. Patients escalating the dose after at least 4 weeks on 1.5 mg have a low likelihood of increasing the incidence or severity of GI adverse events. If GI adverse events are going to occur with dulaglutide treatment, they are most likely to occur soon after initiating treatment. Analysis of the first-onset incidences over time and the similarity between new-onset incidence and overall incidence by study interval support the conclusion that few patients experience new or repeat GI events when escalating the dulaglutide dose. While the absence of GI events after first initiating dulaglutide cannot completely exclude the possibility of experiencing new GI events with later dose escalation, the low incidence of treatment discontinuation due to these events after escalation suggests they will usually be manageable.
Precise measurement of the duration of GI TEAEs has proven difficult in previous studies [24–26]. Similarly, in this analysis, missing stop dates (an unlikely clinical event) and inconsistent reporting methods for intermittent events made the precise determination of event duration difficult. However, where an end date was available, the duration of the GI adverse event was generally found to be less than a week. While excluding events with no stop date may underestimate the overall incidence, a sensitivity analysis that only includes events for which a stop date was recorded may better reflect the change in prevalence over time, and the sensitivity analysis provided a trend consistent with that seen in the main analysis.
Treatment-by-subgroup analysis of GI TEAEs yielded significant treatment-by-sex interactions for nausea and diarrhea due to different patterns of incidence across dose groups in males and females. While the higher incidence rates in females were not observed in previous dulaglutide trials, higher rates of nausea in females have been documented in other studies involving GLP-1 RA therapies [19, 27, 28]. Indeed, for reasons not well understood, gastrointestinal symptoms are consistently reported more frequently in females than in males, with or without diabetes [29].
In this study, the tolerability of dulaglutide is described in the context of the AWARD-11 dose escalation scheme, with 4-week increments for escalation from dulaglutide 0.75 to 1.5 mg and subsequent 1.5-mg escalation steps to 3.0 mg and 4.5 mg. This clinical trial dose escalation scheme may not necessarily reflect routine clinical care situations, which may be influenced by a variety of considerations. However, this safety and efficacy study informed the recommended minimum 4-weekly dose escalation to 3.0 mg and 4.5 mg [14]. In addition, modeling results using either a 4-, 8-, or 12-week increment for escalation from 1.5 to 3.0 mg and a subsequent 4-week increment to 4.5-mg dulaglutide indicated a tolerability profile consistent with that observed in this post hoc analysis [30].
Conclusions
When treatment intensification to either dulaglutide 3.0 mg or 4.5 mg for additional glycemic control is required, patients can expect a GI tolerability profile consistent with that of dulaglutide 1.5 mg. The highest incidences of GI TEAEs occurred shortly after initiating dulaglutide, before they rapidly declined over time. Few patients who escalated from 1.5 to 3.0 mg and subsequently to 4.5 mg in 4-week increments experienced new or additional GI TEAEs, and for those patients who did, most were mild in severity. Patients’ reports of tolerability concerns contribute to poor treatment adherence [23, 31]. Providing tolerability information associated with dulaglutide 3.0 mg and 4.5 mg to patients during consultation for dose escalation may improve adherence to a medication regimen.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
This study and the journal’s Rapid Service Fee were funded and supported by Eli Lilly and Company. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Medical Writing and Editorial Assistance
The authors thank Stephen Hayes, PhD, from Eli Lilly and Company for medical writing support, and Dana Schamberger, Syneos Health, for editorial support. Eli Lilly and Company funded writing and editing support.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
S.R. and J.M. were involved in the acquisition of data. J.V., J.P.F., E.B., S.R., J.M., H.J., D.C., M.K., J.P., and M.A.B. were responsible for the analysis and interpretation of data and drafting and critical revision of the manuscript for important intellectual content. All authors had full access to the data related to this study and approved the final version submitted for publication.
Prior Presentation
Part of the data from this analysis were presented at the American Diabetes Association’s 81st Scientific Sessions, held virtually between June 25–29, 2021, and ADCES21 Annual Conference, held virtually between August 12–15, 2021.
Disclosures
JV declares research support from Eli Lilly and Company, Pfizer, and Sanofi. EB declares: honoraria from Eli Lilly and Company, Novo Nordisk, and Sanofi; travel support from Eli Lilly and Company and Servier Laboratories; and has participated on advisory boards for Abbott, AstraZeneca, Bayer, Becton Dickinson, Boehringer Ingelheim, Daiichi-Sanyo, Eli Lilly and Company, MSD, Novo Nordisk, and Sanofi. JPF declares: research support from Akero, AbbVie, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly and Company, Intercept, Janssen, Madrigal, Metacrine, Merck, NorthSea Therapeutics, Novartis, Novo Nordisk, Oramed, Pfizer, Poxil, Sanofi, and Theracos; participation on advisory boards for Akero, Altimmune, Axcella Health, Boehringer Ingelheim, Coherus Therapeutics, Echosens, 89bio, Eli Lilly and Company, Gilead, Intercept, Merck, Novo Nordisk, Pfizer, and Sanofi; honoraria from Echosens, Eli Lilly and Company, Merck, Novo Nordisk, and Sanofi; and meeting/travel support from Eli Lilly and Company, Novo Nordisk, and Sanofi. SR, JM, HJ, DC, MK, JP, and AB are employees and minor shareholders of Eli Lilly and Company.
Compliance with Ethics Guidelines
The AWARD-11 study (ClinicalTrials.gov NCT03495102) protocol was reviewed and approved by institutional ethics committees at each study center (see Supplementary Material 1) and was conducted in accordance with the principles of the Declaration of Helsinki of 1964 and its later amendments, Good Clinical Practice guidelines, and applicable laws and regulations. Written informed consent was obtained from each patient before participation.
Data Availability
The datasets used during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Chatterjee JS. From compliance to concordance in diabetes. J Med Ethics. 2006;32:507–510. doi: 10.1136/jme.2005.012138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guerci B, Chanan N, Kaur S, Jasso-Mosqueda JG, Lew E. Lack of treatment persistence and treatment nonadherence as barriers to glycaemic control in patients with type 2 diabetes. Diabetes Ther. 2019;10:437–449. doi: 10.1007/s13300-019-0590-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wysham C, Blevins T, Arakaki R, et al. Efficacy and safety of dulaglutide added onto pioglitazone and metformin versus exenatide in type 2 diabetes in a randomized controlled trial (AWARD-1) Diabetes Care. 2014;37:2159–2167. doi: 10.2337/dc13-2760. [DOI] [PubMed] [Google Scholar]
- 4.Giorgino F, Benroubi M, Sun J-H, Zimmermann AG, Pechtner V. Efficacy and safety of once-weekly dulaglutide versus insulin glargine in patients with type 2 diabetes on metformin and glimepiride (AWARD-2) Diabetes Care. 2015;38:2241–2249. doi: 10.2337/dc14-1625. [DOI] [PubMed] [Google Scholar]
- 5.Umpierrez G, Povedano ST, Manghi FP, Shurzinske L, Pechtner V. Efficacy and safety of dulaglutide monotherapy versus metformin in type 2 diabetes in a randomized controlled trial (AWARD-3) Diabetes Care. 2014;37:2168–2176. doi: 10.2337/dc13-2759. [DOI] [PubMed] [Google Scholar]
- 6.Blonde L, Jendle J, Gross J, et al. Once-weekly dulaglutide versus bedtime insulin glargine, both in combination with prandial insulin lispro, in patients with type 2 diabetes (AWARD-4): a randomised, open-label, phase 3, non-inferiority study. Lancet. 2015;385:2057–2066. doi: 10.1016/S0140-6736(15)60936-9. [DOI] [PubMed] [Google Scholar]
- 7.Skrivanek Z, Gaydos B, Chien J, et al. Dose-finding results in an adaptive, seamless, randomized trial of once-weekly dulaglutide combined with metformin in type 2 diabetes patients (AWARD-5) Diabetes Obes Metab. 2014;16:748–756. doi: 10.1111/dom.12305. [DOI] [PubMed] [Google Scholar]
- 8.Dungan KM, Povedano ST, Forst T, et al. Once-weekly dulaglutide versus once-daily liraglutide in metformin-treated patients with type 2 diabetes (AWARD-6): a randomised, open-label, phase 3, non-inferiority trial. Lancet. 2014;384:1349–1357. doi: 10.1016/S0140-6736(14)60976-4. [DOI] [PubMed] [Google Scholar]
- 9.Tuttle KR, Lakshmanan MC, Rayner B, et al. Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7): a multicentre, open-label, randomised trial. Lancet Diabetes Endocrinol. 2018;6:605–617. doi: 10.1016/S2213-8587(18)30104-9. [DOI] [PubMed] [Google Scholar]
- 10.Dungan K, Weitgasser R, Perez Manghi F, et al. A 24-week study to evaluate the efficacy and safety of once-weekly dulaglutide added on to glimepiride in type 2 diabetes (AWARD-8) Diabetes Obes Metab. 2016;18:475–482. doi: 10.1111/dom.12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pozzilli P, Norwood P, Jódar E, et al. Placebo-controlled, randomized trial of the addition of once-weekly glucagon-like peptide-1 receptor agonist dulaglutide to titrated daily insulin glargine in patients with type 2 diabetes (AWARD-9) Diabetes Obes Metab. 2017;19:1024–1031. doi: 10.1111/dom.12937. [DOI] [PubMed] [Google Scholar]
- 12.Ludvik B, Frías JP, Tinahones FJ, et al. Dulaglutide as add-on therapy to SGLT2 inhibitors in patients with inadequately controlled type 2 diabetes (AWARD-10): a 24-week, randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2018;6:370–381. doi: 10.1016/S2213-8587(18)30023-8. [DOI] [PubMed] [Google Scholar]
- 13.Nauck M, Weinstock RS, Umpierrez GE, Guerci B, Skrivanek Z, Milicevic Z. Efficacy and safety of dulaglutide versus sitagliptin after 52 weeks in type 2 diabetes in a randomized controlled trial (AWARD-5) Diabetes Care. 2014;37:2149–2158. doi: 10.2337/dc13-2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trulicity: Highlights of prescribing information. Indianapolis, IN: Eli Lilly and Company; 2014. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/125469s007s008lbl.pdf.
- 15.Aroda VR. A review of GLP-1 receptor agonists: evolution and advancement, through the lens of randomised controlled trials. Diabetes Obes Metab. 2018;20:22–33. doi: 10.1111/dom.13162. [DOI] [PubMed] [Google Scholar]
- 16.Trujillo JM, Nuffer W, Ellis SL. GLP-1 receptor agonists: a review of head-to-head clinical studies. Ther Adv Endocrinol Metab. 2015;6:19–28. doi: 10.1177/2042018814559725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jendle J, Grunberger G, Blevins T, et al. Efficacy and safety of dulaglutide in the treatment of type 2 diabetes: a comprehensive review of the dulaglutide clinical data focusing on the AWARD phase 3 clinical trial program. Diabetes Metab Res Rev. 2016;32:776–790. doi: 10.1002/dmrr.2810. [DOI] [PubMed] [Google Scholar]
- 18.Bettge K, Kahle M, Abd El-Aziz MS, Meier JJ, Nauck MA. Occurrence of nausea, vomiting and diarrhoea reported as adverse events in clinical trials studying glucagon-like peptide-1 receptor agonists: a systematic analysis of published clinical trials. Diabetes Obes Metab. 2017;19:336–347. doi: 10.1111/dom.12824. [DOI] [PubMed] [Google Scholar]
- 19.Shiomi M, Takada T, Tanaka Y, et al. Clinical factors associated with the occurrence of nausea and vomiting in type 2 diabetes patients treated with glucagon-like peptide-1 receptor agonists. J Diabetes Investig. 2019;10:408–417. doi: 10.1111/jdi.12900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nauck MA, Petrie JR, Sesti G, et al. A phase 2, randomized, dose-finding study of the novel once-weekly human GLP-1 analog, semaglutide, compared with placebo and open-label liraglutide in patients with type 2 diabetes. Diabetes Care. 2016;39:231–241. doi: 10.2337/dc15-2479. [DOI] [PubMed] [Google Scholar]
- 21.Frias JP, Wynne AG, Matyjaszek-Matuszek B, et al. Efficacy and safety of an expanded dulaglutide dose range: a phase 2, placebo-controlled trial in patients with type 2 diabetes using metformin. Diabetes Obes Metab. 2019;21:2048–2057. doi: 10.1111/dom.13764. [DOI] [PubMed] [Google Scholar]
- 22.Frias JP, Bonora E, Ruiz LN, et al. Efficacy and safety of dulaglutide 3.0 mg and 4.5 mg versus dulaglutide 1.5 mg in metformin-treated patients with type 2 diabetes in a randomized controlled trial (AWARD-11) Diabetes Care. 2021;44:765–773. doi: 10.2337/dc20-1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Climens AR, Pain E, Boss A, Shaunik A. Understanding reasons for treatment discontinuation, attitudes and education needs among people who discontinue type 2 diabetes treatment: results from an online patient survey in the USA and UK. Diabetes Ther. 2020;11:1873–1881. doi: 10.1007/s13300-020-00843-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lean M, Carraro R, Finer N, et al. Tolerability of nausea and vomiting and associations with weight loss in a randomized trial of liraglutide in obese, non-diabetic adults. Int J Obes (Lond) 2014;38:689–697. doi: 10.1038/ijo.2013.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joelson S, Joelson IB, Wallander MA. Geographical variation in adverse event reporting rates in clinical trials. Pharmacoepidemiol Drug Saf. 1997;6:S31–35. doi: 10.1002/(SICI)1099-1557(199710)6:3+<S31::AID-PDS288>3.3.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 26.Raisch DW, Troutman WG, Sather MR, Fudala PJ. Variability in the assessment of adverse events in a multicenter clinical trial. Clin Ther. 2001;23:2011–2020. doi: 10.1016/S0149-2918(01)80153-3. [DOI] [PubMed] [Google Scholar]
- 27.Knop F, Harring S, Holst I, et al. Gastrointestinal adverse events with once-weekly semaglutide: risk predictors and effect on semaglutide response. Diabetologia. 2020;63:S290–S291. [Google Scholar]
- 28.Horowitz M, Aroda VR, Han J, Hardy E, Rayner CK. Upper and/or lower gastrointestinal adverse events with glucagon-like peptide-1 receptor agonists: incidence and consequences. Diabetes Obes Metab. 2017;19:672–81. [DOI] [PMC free article] [PubMed]
- 29.Bytzer P, Talley NJ, Leemon M, Young LJ, Jones MP, Horowitz M. Prevalence of gastrointestinal symptoms associated with diabetes mellitus: a population-based survey of 15000 adults. Arch Intern Med. 2001;161:1989–1996. doi: 10.1001/archinte.161.16.1989. [DOI] [PubMed] [Google Scholar]
- 30.Lai San Tham CCT, Sioh Keow L, Heike J, Manige K, Li Shen L. 642-P: A model-based evaluation of nausea and vomiting events for additional doses of dulaglutide in existing and dulaglutide-naïve patients with type 2 diabetes. Diabetes 2021;70(Supplement 1).
- 31.Sikirica MV, Martin AA, Wood R, et al. Reasons for discontinuation of GLP1 receptor agonists: data from a real-world cross-sectional survey of physicians and their patients with type 2 diabetes. Diabetes Metab Syndr Obes. 2017;10:403. doi: 10.2147/DMSO.S141235. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used during and/or analyzed during the current study are available from the corresponding author on reasonable request.