Abstract
Seed oil quality is a function of several attributes which include its bioactive compounds, physicochemical and functional properties. These quality attributes are important in seed oil processing as they determine the oil palatability, nutritional and market value. Besides the health, environmental and economic issues related to seed oil extraction using organic solvents such as hexane, other conventional seed oil extraction techniques such as supercritical fluid extraction, enzyme digestion and cold pressing are associated with low recovery of oil and bioactive compounds. Application of novel seeds pretreatments techniques such as microwaving, enzymatic digestion, pulsed electric field and ultrasonication do not only improve the oil yield and quality attributes, but also reduces seed oil extraction time, solvent and energy consumption. Higher phenolic compounds, carotenoids, tocopherols, phytosterols and antioxidant properties in oil from pretreated seeds offer health benefits related to the prevention of cancer, diabetes, obesity, inflammatory and cardiovascular diseases. Increased consumer interest in functional foods and the potential of seeds pretreatments in enhancing the extractability of bioactive compounds from plant material has increased the application of novel pretreatment techniques on diverse oilseeds. This review describes the commonly studied novel seeds pretreatment techniques and critically discusses their influence on the oil physicochemical attributes, oxidation indices, bioactive compounds and antioxidant properties.
Keywords: Seeds pretreatment, Phenolic compounds, Tocopherols, Phytosterols, Antioxidant properties
Introduction
The consumption of seed oil is important to human health as it provides the body with energy, essential fatty acids and fat-soluble vitamins. Seed oil also contains bioactive compounds with multiple functional properties such as antioxidant, anti-cancer, anti-diabetic, anti-obesity, anti-inflammatory, neuroprotective and nephroprotective (Follegatti-Romero et al. 2009; Boussetta et al. 2014; Koubaa et al. 2016; Talekar et al. 2018). These functional properties have stimulated research interests in seed oils’ potential application in pharmaceuticals, nutraceuticals and foods (Monfalouti et al. 2010; Vermaak et al. 2011). In the food industry, seed oils are consumed directly on fruit and vegetable salad, used for cooking or as a dip for snacks such as bread.
Extraction is one of the key processes in the production of seed oil. Commonly used seed oil extraction techniques include cold pressing, solvent extraction, supercritical fluid extraction and ultrasound-assisted solvent extraction. These extraction techniques have a variety of limitations. In addition to health, environmental and economic related concerns, common seed oil extraction techniques have a low recovery of oil and bioactive compounds. For instance, cold pressing, which requires significant energy input, produces low oil yield (Da Porto et al. 2015; Güneşer and Yilmaz 2017). Concerns such as product, human and environmental safety are attributed to the use of organic solvents such as hexane (Citeau et al. 2018; Fetzer et al. 2018). Supercritical fluid extraction, being a green technology, has thus become a primary alternative to conventional seed oil extraction techniques (Zhang et al. 2010), however, it requires high capital investment. Treatment of the oil-bearing seeds before oil extraction has presented multiple advantages which have solved some of the challenges faced by the oil extraction techniques.
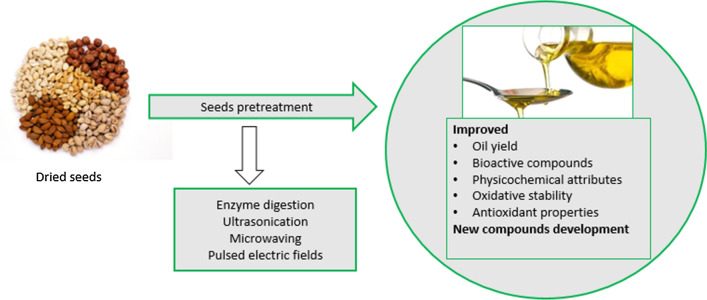
Seeds pretreatment was reported to increase oil yield, oxidative oil stability, bioactive compounds recovery and formation of new functional compounds (Đurđević et al. 2017; McDowell et al. 2017; Fathi-Achachlouei et al. 2019; Mazaheri et al. 2019). Yield is a major factor in seed oil production as maximum oil extraction means that higher volumes of the product can be sold. Enhancing seed oil oxidative stability and functional compounds may improve its shelf life and market value. Commonly reported seeds pretreatment techniques include roasting, microwaving, enzymatic digestion, pulsed electric field and ultrasound exposure (Fig. 1) Increase in oil yield (27%), phenols (796%), tocopherols (14%), carotenoids (47%) and punicic acid (11%) were reported in pretreated camellia, rape, hemp, black cumin and pomegranate seeds, respectively (Zhang and Jin 2011; Tan et al. 2016; Zhou et al. 2016; McDowell et al. 2017; Đurđević et al. 2017). The value presented by seeds pretreatment to seed oil has increased researches on different types of oilseeds. The primary focus of this review is to discuss the effect of novel seeds pretreatment techniques on oil quality attributes and antioxidant properties. This review emphasises on microwaving, enzymatic digestion, pulsed electric field and ultrasound exposure as they present sustainable strategies capable of improving seed oil quality, while significantly reducing the oil extraction time, solvent and energy consumption (Fig. 1). An overview of these novel seeds pretreatment techniques and how they alter the structure of the seeds and improve lipids and bioactive compounds recovery will be presented.
Fig. 1.
Summary of the effect of novel seeds pretreatment techniques on oil quality and antioxidant properties
Novel seeds pretreatment techniques: an overview
Enzymatic digestion
The application of enzymes disintegrates the seeds matrix by rapturing the polysaccharide-protein colloid system. Cellulase, hemicellulase and pectinase are the common enzymes required to degrade the cell wall (cellulose, hemicellulose, pectin) which act as the primary barrier to the oil accessibility. In plant cells, oil exists as oleosomes (0.2–2.0 μm in diameter), which consists of the triglycerides surrounded by phospholipids and proteins (Nikiforidis 2019). Proteases are required to breakdown the peptides bonds of the proteins surrounding the triglycerides. The knowledge of seeds composition is vital for the selection of the right enzymes. The efficiency of enzyme seeds pretreatment is dependent on factors such as temperature, pH, concentration, seed particle size, hydrolysis duration, water to seed ratio and agitation (Ricochon and Muniglia 2010). The application of a minimum amount of heat during enzyme pretreatment produces oil of superior quality compared to thermal seeds pretreatment techniques.
Ultrasonication
The ultrasound treatment technology generates ultrasound waves which produce high energy bubbles when passed through a liquid. The collapse of these cavitation bubbles on a product’s surface induces the disruption of seed cell walls, particle size reduction and enhanced mass transfer of the cell content (Barba et al. 2016). Various mechanisms are involved in the cell wall disintegration and particle size reduction, which include fragmentation, erosion, capillarity, detexturation and sonoporation (Chemat et al. 2017). The efficiency of ultrasound treatment is influenced by factors such as frequency, liquid viscosity, solvent vapour pressure, external pressure, temperature and available gas (Moghimi and Farzaneh 2018). In addition to improved oil and bioactive compounds recovery, ultrasound seeds pretreatment reduces seed oil extraction time, solvent and energy consumption and overall production costs (Barba et al. 2016).
Microwaving
The microwave treatment technique involves the generation of electromagnetic radiation with frequency ranging from 300 MHz to 300 GHz (Barba et al. 2016). These microwaves penetrate the oil-bearing seeds and convert the electromagnetic energy into heat through ionic conduction and dipole rotation (Gaber et al. 2018). The generated heat energy causes a rapid increase in the temperature of the seeds and creates intracellular pressure which ruptures the oilseeds cell walls, facilitating the release of oil. The effectiveness of microwave pretreatment depends on the dielectric properties of the seeds, frequency, power level, and initial seeds temperature (Spigno and De Faveri 2009). The application of seeds microwave pretreatment improves oil yield, reduces oil extraction time, solvent usage and enhances the efficiency of seed oil extraction techniques (Boussetta et al. 2014).
Pulsed electric field
The pulsed electric field treatment technique is a non-thermal technology, which makes it a promising technique for the extraction of highly valued seed oils. Pulsed electric field treatment is applied to a material placed between two electrodes at ambient temperature or marginally higher than the ambient temperature (Barba et al. 2016). Exposing plant cells to a given electric field induces critical electrical potential across the cell membrane, which causes an electrical breakdown. This induces some structural damage to the cell membrane through the creation of pores in a phenomenon called electroporation (Moradi and Rahimi 2018). Depending on the treatment time, pulse form and pulse energy, electric field strength and temperature, the cell membrane damage can be reversible or irreversible (Sharma and Gupta 2006). Resultantly, this increases the mass transfer of cellular material into the extraction medium. Due to its non-thermal nature, pulsed electric field is a novel technique for the recovery of heat-labile bioactive compounds.
Effect of novel seeds pretreatment techniques on oil physicochemical attributes
Physicochemical attributes are important quality determinants in seed oil processing. Several physical attributes (refractive index, density) and chemical attributes (iodine value, saponification value, fatty acids) have been reported to be insignificantly affected by seeds pretreatment (Guderjan et al. 2007; Soto et al. 2007; Uquiche et al. 2008; Latif and Anwar 2009; Zhou et al. 2016; Moradi and Rahimi 2018). Attributes such as oil yield and colour have been reported to be significantly affected by seeds pretreatment (Sharma and Gupta 2006; Passos et al. 2009; Kittiphoom and Sutasinee 2015; Güneşer and Yilmaz 2017). Therefore, in this section, the review focuses on oil yield, colour and fatty acids, which are some of the most important physicochemical quality attributes of seed oil.
Oil yield
Oil yield is an important variable to seed oil processors as it is one of the key profit determinants for the business (McDowell et al. 2017). Therefore, to obtain the maximum value of the oil yield, alteration of the seed structure to enhance mass transfer of lipids from the seed matrix is essential. As shown in Table 1, oilseeds pretreatment significantly improved oil yield.
Table 1.
The effect of novel seeds pretreatment techniques on oil yield
| Type of seed | Pretreatment technique | Oil extraction method | Key finding | References |
|---|---|---|---|---|
| Grape | Enzymatic pretreatment C1: (pectinase = 569, cellulase = 29, xylanase = 21, protease = 1191 U/g sample) C2: (pectinase = 1708, cellulase = 72, xylanase = 55, protease = 2977 U/g, temperature (40 °C ), pH (4.0–7.0), particle size (0.5–1.4 mm) and exposure time of 8–120 h | Solvent extraction with hexane using soxhlet apparatus | Enzymatic pretreatment at concentration: C1, temp: 40 °C , pH: 4 and extraction time of 120 h increased oil yield from 15.3 to 19.5% | Passos et al. (2009) |
| Soybean | Enzymatic pretreatment (α-amylase, glucoamylase, pectinase, hemicellulase, cellulase, neutral protease) at temp (16.4–83.6 °C ), pH (4.45–7.15), time (1.3–14.7 h) | Solvent extraction with hexane using soxhlet apparatus | Higher oil yields (26.78%) and (28.46%) were obtained with enzymatic pretreatment at temp (50 °C ), pH (5.8), time (8 h) for collets and flakes, respectively | Grasso et al. (2012) |
| Pomegranate | Microwave pretreatment (2450 MHz, 2 and 6 min, 100, 250, 600 W and 63–136 °C) | Solvent extraction with hexane using soxhlet apparatus | Microwave pretreatment at 600 W for 6 min increased oil yield from 27.73% (non-pretreated seeds) to 36.34% (microwaved seeds) | Đurđević et al. (2017) |
| Mango | Microwave pretreatment (2450 MHz, 110, 330, 550 W, 0–150 s) | Solvent extraction with hexane (70 °C) using soxhlet apparatus | Microwave pretreatment at 110 W for 150 s exhibited highest oil yield | Kittiphoom and Sutasinee (2015) |
| Milk thistle | Microwave pretreatment (2450 MHz, 800 W for 2 and 4 min) | Solvent extraction using hexane (25 °C) under continuous shaking | Oil yield increased from 29.43 to 32.33 and 35.41% after microwaving for 2 and 4 min, respectively | Fathi-Achachlouei et al. (2019) |
| Black cumin | Microwave pretreatment (2450 MHz, 1100 W, 1–3.5 min) | Cold pressing using a screw press | Black cumin seeds microwave irradiation for 3.5 min increased oil yield by 36.8% | Mazaheri et al. (2019) |
| Hazelnut | Microwave pretreatment (2450 MHz, 400, 600 W, 120, 180, 240 s) | Cold pressing using a hydraulic press | Microwave irradiation of hazelnuts at 400 W for 240 s enhanced oil yield from 6.1 to 45.3% | Uquiche et al. (2008) |
| Watermelon | Ultrasonic pretreatment (100–700 W, 10–50 °C, 5–25 s) | Aqueous enzyme extraction | Ultrasonic pretreatment at 700 W, 40 °C for 25 s showed higher extraction rate of 98% | Liu et al. (2011) |
| Apricot and almond | Ultrasonic pretreatment (42 kHz, 2.5, 10 and 15 min) | Aqueous enzyme extraction | Oil yield for both apricot and almond increased within the range of 19–22% | Sharma and Gupta (2006) |
| Hemp | Ultrasound pretreatment (20 MHz, 200 W, 10, 20 and 40 min) | Supercritical carbon dioxide extraction | Highest oil yield was exhibited by seeds pretreated for 10 min | Da Porto et al. (2015) |
| Cannabis | Pulsed electric fields (voltage: 7 kV; pulse intensity: 0, 3 and 6 kV/cm; pulse duration: 0.5 ms) | Cold pressing using a screw press | Application of pulse intensity of 3 kV/cm gave higher oil yield | Haji-Moradkhami et al. (2018) |
| Sunflower | Pulsed electric field pretreatment (1–7.0 kV/cm, 0.5–15 Hz and 10–50 µs) | Solvent extraction using hexane under continuous shaking | Pretreating seeds at 7.0 kV/cm, 15 Hz for 30 µs improved oil yield from 39.14 to 48.24% | Shorstkii et al. (2017) |
| Black cumin | Pulsed electric field pretreatment (3.24 kV/cm, 20 µs) | Cold pressing using a screw press | Pulsed electric field pretreatment of black cumin seeds increased oil extraction efficiency by approx. 35% | Bakhshabadi et al. (2017) |
| Sesame | Pulsed electric field pretreatment (40 kV, 20 kV/cm for 10 µs) | Cold pressing using a texture analyzer | Oil yield increased by 4.9% after pretreatment | Sarkis et al. (2015) |
| Niger | Pulsed electric field pretreatment (0–5 kV/cm, 20 µs) | Cold pressing using a screw press | Highest oil yield was obtained after pretreating seeds at 1.18 kV/cm | Mohseni et al. (2020a) |
Factors such as enzyme concentration, pH, temperature, substrate particle size and digestion time significantly affect oil yield of enzyme pretreated seeds. In the study of Passos et al. (2009) enzymatic pretreatment of grape seeds at a cocktail concentration (pectinase = 569, cellulase = 29, xylanase = 21, protease = 1191 U/g sample), temperature (40 °C), pH (4.0), particle size (< 0.5 mm) and digestion time of 24 h significantly increased soxhlet hexane extracted oil yield by 192%. In the same study, increasing the treatment time and particle size to 120 h and 1.0–1.4 mm, respectively, reduced the oil yield by 30%. The study emphasised on increasing digestion time since enzymatic hydrolysis is a slow process, although this may be regarded as economically unviable. Grasso et al. (2012) also used a multi-enzyme mixture (α-amylase, glucoamylase, pectinase, hemicellulose, cellulase and protease) to pretreat soya bean prior oil soxhlet extraction using hexane. The authors found out that oil yield significantly increased by 85% and 8% when the soya bean flakes and collects were pretreated at pH 5.4, 38 °C for 9.7 h and at pH 5.8, 43.5 °C for 5.8 h, respectively. Li et al. (2012) reported that enzymatic digestion (enzyme cocktail: cellulase, xylanase, pectinase, protease at 2.0% w/w, 40 °C, pH 4.5 and reaction time 5 h) of silybum marianum seeds before solvent extraction using hexane (soxhlet) improved oil yield by 10.47%, further highlighting the importance of enzyme concentration, pH, treatment time and temperature in seeds pretreatment.
The impact of seeds microwave pretreatment on oil yield was evaluated. Đurđević et al. (2017) reported that microwave pretreatment of ground pomegranate seeds (1 > mm) at 100 W for 2 min significantly enhanced the yield of soxhlet hexane extracted oil by 23%. However, increasing the microwave power (250 and 600 W) and time (6 min) did not significantly increase the pomegranate seed oil yield, indicating that 100 W and 2 min was the optimum condition. In another study, microwaving mango seeds at 110 W for 150 s prior to oil extraction with hexane using the soxhlet apparatus significantly increased oil yield by 80% (Kittiphoom and Sutasinee 2015). It was also observed that increasing the microwave power to 330 and 550 W and time beyond 90 and 30 s, respectively, burnt the mango seeds. In agreement with other studies, Fathi-Achachlouei et al. (2019) reported that microwave pretreatment of milk thistle seeds at 800 W for 2 and 4 min improved the hexane extracted (soxhlet) oil yield by 10 and 20%, accordingly. The significance of both microwave power and time on oil yield was also reported from other studies (Table 1).
According to Liu et al. (2011), ultrasonic pretreatment of water melon seeds at 547 W, 48 °C for 23 s prior to aqueous enzyme extraction increased the oil yield by 21% when compared with the untreated sample. Further increasing the ultrasound power, temperature, and time decreased the oil extraction rate. A similar study by Da Porto et al. (2015) reported that ultrasound pretreatment (20 kHz, 200 W, 10, 20 and 40 min) of hemp seeds for 10 min improved yield of oil extracted using supercritical carbon dioxide extractor by 25% which was the optimum oil yield. When the ultrasound time was increased to 20 and 40 min the oil yield significantly decreased. The authors attributed the significant decrease in oil yield to fatty acids degradation and isomerisation at high temperatures caused by prolonged ultrasonication. In a previous study, ultrasonic pretreatment (42 kHz, 2.5, 10, 15 min) of apricot and almond seeds followed by aqueous enzyme extraction significantly enhanced oil yield between 19 and 22% (Sharma and Gupta 2006).
Due to the fact that pulsed electric field pretreatments do not cause significant changes in the structure of the oilseeds, the oil yield is relatively lower compared to ultrasound, microwave and enzyme pretreatments. Haji-Moradkhani et al. (2019) studied the effect of pulsed electric field pretreatment of cannabis seeds (voltage: 7 kV; pulse intensity: 0, 3 and 6 kV/cm; pulse duration: 0.5 ms) on cold pressed oil and reported that highest increase in oil yield (28%) was achieved when a pulse intensity of 3 kV/cm was applied on the cannabis seeds. In a similar study, pulsed electric field pretreatment of sesame seeds (40 kV, 20 kV/cm and 10 µs) significantly improved the yield of cold pressed oil by 4.9% when compared with the untreated sample (Sarkis et al. 2015). The findings were comparable to the results reported by Shorstkii et al. (2017) on pulsed electric field pretreatment (7.0 kV/cm, 15 Hz and 30 µs) of sunflower seeds and oil extraction with hexane using the soxhlet apparatus as can be seen in Table 1.
Colour
Colour is one of the most important parameters for determining visual acceptance of fresh and processed food materials, including seed oil, and thus influences consumer’s preference (Pathare et al. 2013). Although seed oil colour is attributed to the presence of pigments such as chlorophyll and carotenoids, products of caramelisation and Maillard reaction that are formed during processing also affects seed oil colour. The effect of seeds pretreatment on oil colour is presented in Table 2.
Table 2.
The effect of novel seeds pretreatment techniques on the oil colour
| Type of seed | Pretreatment technique | Key finding | References |
|---|---|---|---|
| Rape | Pulsed electric field (5.0 kV/cm and 60 pulses, 7.0 kV/cm and 120 pulses for 30 µs) | Increasing the pulsed electric field increased the seed oil lightness and yellowness | Guderjan et al. (2007) |
| Cannabis | Pulsed electric fields (voltage: 7 kV; pulse intensity: 0, 3 and 6 kV/cm; pulse duration: 0.5 ms) | Increasing the pulse intensity to 6 kV/cm improved the oil colour index from 119.8 to 167.2 | Haji-Moradkhani et al. (2018) |
| Rape | Pulsed electric fields (5.0 kV/cm and 60 pulses, 7.0 kV/cm and 120 pulses for 30 µs) | Higher lightness and yellowness were exhibited by oil from seeds pretreated at 7 kV/cm and 120 pulses | Guderjan et al. (2007) |
| Walnut | Microwave (2450 MHz, 600 W for 1, 2 and 4 min) | Microwaving seeds for 2 and 4 min produced oil higher in yellowness | Zhou et al. (2016) |
| Orange | Microwave (360 W, 30 min with 3 min pauses after every 3 min) | Microwave pretreatment decreased the oil lightness and yellowness | Güneşer and Yilmaz (2017) |
| Palm | Microwave pretreatment (1000 W, 2450 MHz, 5, 10, 12, 13, 14, 15 min) | Increasing the microwave time decreased the oil yellowness | Tan et al. (2016) |
| Hemp | Enzyme digestion (Protex 7L, Alcalase 2.4L, Viscozyme L, Kemzyme, Natuzyme, 40 °C, 6 h, 45% moisture) | Digestion seeds with Natuzyme and Protex 7L produced seed oil with greater yellowness | Latif and Anwar (2009) |
| Black cumin | Ultrasound pretreatment (30, 60, 90 W, 25 kHz, 30, 45, 60 min) | Higher colour index was exhibited by oil from seed pretreated at 30 W for 30 min | Moghimi and Farzaneh (2018) |
Microwave pretreatment (2450 MHz, 500 W, 5, 10 and 15 min) of sunflower seeds significantly affected the oil colour (Zhou et al. 2016). For instance, increasing the microwave time from 5 to 10 min and then 15 min changed the oil colour from light yellow to yellow and brown, respectively. Similarly, Tan et al. (2016) reported that increasing microwave time from 5 to 15 min changed the palm oil colour from light orange to dark orange. High microwave power and prolonged seeds heating might cause degradation and isomerisation of carotenoids. Alternatively, increased microwave pretreatment at high power levels promotes the formation of browning substances caused by Maillard non-enzymatic reactions, caramelisation and phospholipid degradation. Establishing the appropriate microwave power and time is thus important to avoid the development of unwanted colour substances which deteriorates the seed oil colour.
Guderjan et al. (2007) applied pulsed electric field (5.0 kV/cm and 60 pulses, 7.0 kV/cm and 120 pulses for 30 µs) on rapeseeds. The authors reported that the oil lightness and yellowness significantly increased when compared to the oil from untreated rapeseeds. Higher lightness (approx. 40) and yellowness (approx. 35) were exhibited by oil from seeds pretreated at 7 kV/cm and 120 pulses. Haji-Moradkhani et al. (2018) also reported that cannabis seed oil colour index significantly improved by 1.4 folds after increasing the pulse intensity from 0 to 6 kV/cm pretreatment of the seeds, which was the best oil colour index in the study (Table 2). The authors believed that the improved colour index was due to increased porosity of the cannabis seeds cells by pulsed electric field pretreatment, which led to improved release of oil carotenoids from the seeds matrices.
Moghimi and Farzaneh (2018) studied the impact of ultrasound pretreatment (25 kHz, 30, 60 and 90 W, 30, 45 and 45 min) of black cumin seeds on the extracted oil colour index. The authors reported that increasing the ultrasound power from 30 to 90 W and time from 30 to 60 min significantly increased the oil colour index by 7 and 19%, respectively. The findings indicate that varying ultrasound time had more effect on the oil colour index than varying power. Latif and Anwar (2009) investigated the effect of enzyme pretreatment (Protex 7L, Alcalase 2.4L, Viscozyme L, Kemzyme and Natuzyme, 40 °C, 6 h) on hemp seeds and found out that the oil yellowness significantly increased between 25 and 27% for all the enzymes. The significant increase in oil yellowness was attributed to increased dissociation of carotenoids from the carotenoprotein complexes as a result of enzyme pretreatment (Kha et al. 2013). However, the oil yellowness was not significantly different among the enzymes.
Fatty acids
Maximum recovery of fatty acids during oil extraction is essential to enhance the seed oil nutritional quality. The effect of seeds pretreatment on the oil fatty acid composition has been well investigated. There is a general agreement among authors that seeds pretreatment has an insignificant effect on fatty acids composition, which could be beneficial from a nutritional point of view (Lee et al. 2004; Epaminondas et al. 2011; Wroniak et al. 2016; Moradi and Rahimi, 2018).
Durdevic et al. (2017) studied the effect of pomegranate seeds microwave pretreatment (2450 MHz, 100, 250, 600 W, 2, 6 min) on fatty acids composition. Stearic acid, palmitic acid, oleic acid, linoleic acid and punicic acid the primary fatty acids in pomegranate seeds oil did not significantly change after seeds microwave pretreatment. Similar findings were reported by Güneşer and Yilmaz (2017), Soto et al. (2007) and Moradi and Rahimi (2018) from microwave, enzyme and ultrasound pretreated orange, borage and sunflower seeds, respectively. However, combing the pretreatments techniques was reported to affect the oil fatty acid composition significantly. In their study on microwave-pulsed electric field pretreatment (microwave time: 0–200 s, pulse intensity: 0–5 kV/cm) of niger seeds, Mohseni et al. (2020a) reported that the quantity of oleic and linoleic acids significantly decreased. In contrast, the levels of palmitic acid and stearic acid significantly increased. The authors believed that the decrease in oleic acid and linoleic acid was due to thermal degradation.
Effect of novel seeds pretreatment techniques on oil oxidation indices
Peroxide value, acid value, free fatty acids and conjugated dienes
Peroxide value, acid value, free fatty acids and conjugated dienes are primary products of fatty acids oxidation and essential quality parameters of seed oil quality. There is an inverse relationship between the palatability of seed oil and the level of primary products of oxidation. Low levels of peroxide value, free fatty acids, acid value and conjugated dienes indicate high oil palatability and longer shelf life. Therefore, applying seeds pretreatment conditions that minimise the oxidation of fatty acids and maximise the extraction of antioxidative compounds is essential.
The effect of pretreating moringa seeds with microwaves (100 W, 30, 60, 90 s) on the oil peroxide value and the acid value was studied (Da Porto et al. 2015). The authors found out that microwave pretreatment of moringa seeds for 90 s reduced the acid value and increased peroxide value by 41% and 22%, respectively. The level of conjugated dienes did not significantly change after moringa seeds microwave pretreatment suggesting minimum degradation of the hydroperoxides. Uquiche et al. (2008) also investigated the effect of microwave pretreatment (400 W, 2450 MHz, 240 s) with hazelnuts. They established that acid value significantly increased by 17%, while the level of peroxide value was not significantly affected. Similar observations were reported with microwave pretreated (457 and 607 W, 5 min) rapeseeds (Ramos et al. 2017). Despite the increase in peroxide value and acid value after seeds microwave pretreatment, the levels in all the studies conformed to the Codex Alimentarius commission standard on seed oil, which permits a maximum of 15.0 meqO2/kg oil peroxide value and 4.0 mg KOH/g acid value for unrefined oils (Codex Alimentarius 1999).
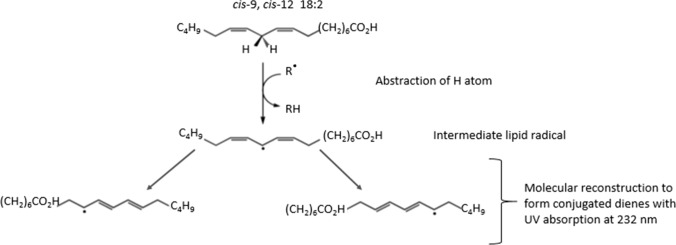
According to the study of Da Porto et al. (2015) on ultrasound pretreatment (20 kHz, 200 W) of hemp seeds, exposing the hemp seeds to ultrasound waves for 10, 20 and 40 min significantly increased the conjugated dienes between 29 and 49%. The significant increase in conjugated dienes was attributed to oxidation and isomerisation of polyunsaturated fatty acids such as linoleic and α-linolenic acids. These fatty acids may undergo free radical oxidation or intramolecular sigmatropic rearrangement of the hydrogen atoms to form conjugated bonds (Fig. 2). Moradi and Rahimi (2018) reported that ultrasound (40 kHz, 100 W) and pulsed electric field pretreatment (pulse intensity: 0.8–1.1 kV/cm, time: 1 ms) of sunflower seeds did not significantly affect the seed oil free fatty acid and peroxide value. Similar observations were reported by Haji-Moradkhani et al. (2019) on pulsed electric field pretreatment (voltage: 7 kV; pulse intensity: 0, 3 and 6 kV/cm; pulse duration: 0.5 ms) of cannabis seeds, indicating that pulsed electric field pretreatment of oilseeds may not degrade the quality of extracted oil, despite the increased porosity of the cell walls and cell membranes.
Fig. 2.
Formation of conjugated dienes through free radical oxidation using linoleic acid as an example
Enzyme pretreatment (Protex 7L, Alcalase 2.4L, Viscozyme L, Kemzyme and Natuzyme, 40 °C, 6 h) of hemp seeds did not significantly change the oil peroxide value and free fatty acids. This phenomenon could be explained by the low temperature employed during seeds pretreatment (Latif and Anwar 2009).
ρ-anisidine value, conjugated trienes and oxidative oil stability
Seed oil hydroperoxides may further decompose to form carbonyl compounds such as aldehydes, alcohols, hydrocarbons and ketones. These secondary products of fatty acids oxidation are responsible for the development of off flavours and odour in the seed oil. The amount of aldehydes (primarily 2‐alkenals and 2,4‐dienals) may be measured using the ρ-anisidine value.
Zhou et al. (2016) reported an increase in oil ρ-anisidine value after walnuts microwave pretreatment (2450 MHz, 600 W, 1, 2 and 4 min). Exposing the walnuts to microwaves for 1, 2, and 4 min significantly increased the ρ-anisidine values by 1.9, 2.6 and 3.1 folds, accordingly. Similar findings were reported with oil from microwave pretreated palm and peanut seeds (Tan et al. 2016; Ali et al. 2017). Higher microwave power and prolonged exposure time may promote peroxides decomposition into secondary oxidation products, further degrading the seed oil quality.
Ultrasound pretreatment (20 kHz, 200 W, 10, 20 and 40 min) of hemp seeds significantly increased the oil conjugated trienes between 1.5 and 1.9 folds due to the creation of hot spots with high temperatures during ultrasound pretreatment (Da Porto et al. 2015). Latif and Anwar (2009) reported that hemp seeds pretreatment (40 °C, 6 h) with Viscozyme L (3%) and Kemzyme (12%) significantly decreased and increased the ρ-anisidine values, respectively. Pretreatment of the hemp seeds with Protex 7L, Feedzyme and Natuzyme did not significantly affect the oil ρ-anisidine value. In the same study, enzyme pretreatment of hemp seeds had no significant effect on the oil conjugated trienes. Increasing enzyme concentration and treatment time may significantly increase the conjugated trienes as indicated in the study of Dandjouma et al. (2008) on enzyme pretreatment (Protamex, Celluclast, 0.1–0.4 g/ 100 substrate, 50 °C, 1–4 h) of bail (Ricinodendron heudelotii) seeds.
The oxidative stability of seed oil is defined as the resistance to oxidation during processing and storage and is influenced by factors such as fatty acid composition and antioxidant compounds (Hu et al. 2019). It is often measured using the rancimat method. Azadmard-Damirchi et al. (2010) study on rapeseeds microwave pretreatment (2450 MHz, 800 W, 2 and 4 min) established that microwave pretreatment for 2 and 4 min significantly increased the oxidative oil stability by 5 and 8 folds, respectively. The significant improvement in oil oxidative stability was attributed to enhanced tocopherols compounds after rapeseeds microwave pretreatment. Hu et al. (2018) also reported significant improvement in peanut oil oxidative stability after microwave irradiation (2450 MHz, 700 W, 1–5 min) of the peanuts. The authors observed that the oil oxidative stability significantly increased between 1.8 and 3 folds when microwave time was increased from 1 to 5 min. The results are in agreement with the findings of Uquiche et al. (2008), Yang et al. (2013) and Wroniak et al. (2016) from microwave pretreated hazelnuts and rapeseeds, respectively. However, Bakhshabadi et al. (2017) reported a significant decrease in the oil oxidative stability when microwave power (180–900 W) and time (1.5–4.5 min) were increased during microwave pretreatment of black cumin seeds, which was attributed to the degradation of the antioxidative compounds at high microwave power and prolonged treatment time.
In another study, Latif and Anwar (2009) established that enzyme pretreatment (Protex 7 L, Alcalase 2.4 L, Viscozyme L, Kemzyme, Natuzyme, 40 °C, 6 h, 45% moisture) of hemp seeds significantly improved the oxidative oil stability by 27% with Kemzyme, while the application of Protex 7 L, Alcalase 2.4 L, Viscozyme L and Natuzyme did not significantly improve the oil oxidative stability.
The authors, in their findings, agreed that pretreatment temperature and duration are critical factors in influencing the seed oil stability to oxidation. Depending with the type of seeds, higher microwave power and prolonged microwave or ultrasound exposure time may result in the degradation of fatty acids and formation of primary and secondary oxidation products.
Effect of novel seeds pretreatment techniques on oil bioactive compounds
Tocopherols
Tocopherols are essential natural antioxidants that are crucial in maintaining seed oil quality during processing and storage through prevention of lipid oxidation which may result in rancidity and off-flavours development. Table 3 illustrates the effect of seeds pretreatment on oil tocopherols.
Table 3.
The effect of novel seeds pretreatment techniques on the oil tocopherols
| Type of seed | Pretreatment technique | Key finding | References |
|---|---|---|---|
| Sunflower | Ultrasound (US) and pulsed electric field (PEF) pretreatment | PEF pretreatment enhanced the recovery of tocopherols | Moradi and Rahimi (2018) |
| Walnut | Microwave pretreatment (600 W, 2450 MHz, 0–4 min) | Microwave pretreatment for 1 min resulted in highest tocopherols | Zhou et al. (2016) |
| Rape | Microwave (800 W, 3, 7 min) | Tocopherols decreased when seeds were microwave heated for 3 min and increased when microwave time was increased to 7 min | Wroniak et al. (2016) |
| Microwaving (2450 MHz, 800 W, 0–7 min) | Seed oil individual and total tocopherols decreased beyond 5 min of microwave heating time | Yang et al. (2013) | |
| Chia | Microwave (2450 MHz, 180, 360, 540, 720, 900 W, 15 min) | Tocopherols decreased with increase in microwave power | Ozcan et al. (2019) |
| Milk thistle | Microwave (2450 MHz, 800 W, 2 and 4 min | Total and individual tocopherols increased with increase in microwave heating time | Fathi-Achachlouei et al. (2019) |
| Niger | Microwave (900 W, 0–200 s) and pulsed electric fields (0–5 kV/cm, 20 µs) | The amount of α-tocopherols and ∆-tocopherols significantly improved | Mohseni et al. (2020a) |
| Rape | Microwave (2450 MHz, 540 W, 0, 100, 200 s) and pulsed electric fields (0–5 kV/cm, 0.5 ms) | The δ-tocopherols increased from 0.00 to 30.07 ppm after seeds pretreatment | Mohseni et al. (2020b) |
| Hemp | Enzyme digestion (Protex 7 L, Alcalase 2.4 L, Viscozyme L, Kemzyme, Natuzyme, 40 °C, 6 h, 45% moisture) | Higher total tocopherols were manifested in oil from seed digested with Natuzyme | Latif and Anwar (2009) |
| Borage | Enzyme pretreatment (Olivex and Celluclast at 0.3% enzyme to substrate ratio, 45 °C for 9 h) | The α-tocopherols varied from 1480 (untreated seeds) to 1494 mg/kg (enzyme treated) | Soto et al. (2008) |
| Goldenberry | Enzyme pretreatment (Cellulase EC, Pektinase L 40 (1:1), 50 °C , pH: 4.3, enzyme concentration: 2% (w/w), 2 h) | The β-tocopherols and γ-tocopherols varied from 2.10–2.11 g/kg and 1.08–1.10 g/kg respectively after seeds enzyme pretreatment | Ramadan et al. (2008) |
| Tiger nut | Enzyme pretreatment (Alcalse, α-amylase and Viscozyme enzymes at pH 8 and 40 °C for 6 h) | The level of α-tocopherols improved from 145.7 to 159.5 µg/g | Ezeh et al. (2016) |
| Rape | Pulsed electric field (5.0 kV/cm and 60 pulses, 7.0 kV/cm and 120 pulses for 30 µs) | Higher α-tocopherols was exhibited by oil from seed pretreated at 7 kV/cm and 120 pulses Higher α-tocopherols was exhibited by oil from seed pretreated at 7 kV/cm and 120 pulses | Guderjan et al. (2007) |
The type of enzyme is one of the most important factors that influence the recovery of tocopherols compounds from the seeds matrices. For instance, Latif and Anwar (2009) evaluated the effect of five different enzymes (Protex 7 L, Alcalase 2.4 L, Viscozyme L, Kemzyme, Natuzyme, 40 °C, 6 h, 45% moisture) on the extractability of tocopherols from hemp seeds. The authors reported that α-tocopherol (23%) and δ-tocopherol (8%) were optimally recovered using Kemzyme. On the other hand, hemp seeds pretreatment with Natuzyme significantly increased the total tocopherols and γ-tocopherol by 14 and 18%, respectively, which represented the optimal recovery of the respective tocopherols. Seeds enzyme digestion results in the breakdown of the bonding forces between the tocopherols and seeds matrices, thereby increasing their release into the oil phase (Uddin et al. 2018). The application of Protex 7L, Viscozyme and Feedzyme did not significantly affect the content of total tocopherols. The use of enzyme mixtures was reported to improve the recovery of bioactive compounds due to their synergistic effect on the seed matrix (Grasso et al. 2012). In this regard, the study of Ezeh et al. (2016) on tiger nuts pretreatment with Alcalase, α-amylase and Viscozyme enzymes at pH 8 and 40 °C for 6 h significantly increased the level of α-tocopherol by 9%. However, the level of β-tocopherol did not significantly improve after enzyme pretreatment an observation which was also reported by Soto et al. (2008) and Ramadan et al. (2008) on borage and goldenberry seeds (Table 3).
Fathi-Achachlouei et al. (2019) investigated the potential of microwave pretreatment (800 W, 2 and 4 min) in improving tocopherols recovery from milk thistle seeds. The α-tocopherol, β-tocopherol, γ-tocopherol, δ-tocopherol and total tocopherols significantly increased by more than 55% after treating the seeds with microwaves for 2 min. Although increasing the microwave time to 4 min reduced the tocopherols, the levels were significantly higher than those from milk thistle seeds not microwaved. Ozcan et al. (2019) pretreated chia seeds with microwaves. The authors observed that increasing microwave power between 180 and 900 W at a constant time of 15 min significantly decreased the tocopherols of pretreated chia seeds. For instance, chia seeds microwave irradiation at 180, 540 and 900 W significantly decreased α-tocopherol by 2, 3 and 7%, respectively. The findings suggest that microwaving chia seeds for 15 min cause significant degradation of the tocopherol compounds. Findings from other authors on the effect of seeds microwave pretreatment on the extracted oil tocopherols are shown in Table 3.
Moradi and Rahimi (2018) reported that ultrasound (40 kHz, 100 W) and pulsed electric field pretreatment (pulse intensity: 0.8–1.1 kV/cm, time: 1 ms) of sunflower seeds slightly but significantly decreased the oil α-tocopherol (1%), whereas the concentration of β-tocopherol, γ-tocopherol and total tocopherol were not significantly affected. In the seed matrix, tocopherols may exist as either free, esterified or glycosylated compounds which may affect their extractability (Uddin et al. 2018). Previous studies by Guderjan et al. (2007) on pulsed electric field pretreatment (5.0 kV/cm and 60 pulses, 7.0 kV/cm and 120 pulses) of rapeseeds concurred with findings of Moradi and Rahimi (2018). Increasing the PEF intensity increased α-tocopherol content but had no significant effect on γ-tocopherol. Integrating pretreatment techniques is a novel and promising technology to optimise the extraction of valuable compounds from plant materials. For example, Mohseni et al. (2020a) established that integrating microwave (900 W, 0–200 s) and pulsed electric field (0–5 kV/cm, 20 µs) and pretreating niger seeds increased the oil α-tocopherol and ∆-tocopherol by 2.8%. Similar observations were reported by Mohseni et al. (2020b) with rapeseeds (Table 3).
Phenols
Seed oil is a good source of phenolic compounds. In addition to antioxidant properties, these bioactive compounds possess biological activities which include anti-inflammatory, anti-microbial, anti-atherosclerosis, and anti-cancer activities. Improved extraction of these bioactive compounds from the seed matrix is therefore vital to enhance the oil functional properties.
In line with this, the potential of rapeseeds microwave pretreatment (800 W, 2 min) in enhancing the oil phenolic compounds was evaluated (McDowell et al. 2017). It was found that total phenolic compounds and sinapic acid significantly improved by 9.23 and 1.98 mg/kg, respectively. Canolol, a derivative of sinapic acid significantly increased from 0.02 to 7.14 mg/kg after rapeseeds microwave irradiation. The authors suggested that canolol was formed from the decarboxylation of sinapic acid through the catalysis of microwave pretreatment. Minor phenolic compounds identified in the rapeseed oil, which include 4-HBA, trans-cinnamic, p-coumaric, syringic acid and vanillic acid, were insignificantly affected by rapeseeds microwave pretreatment. Also, microwave pretreatment (360 W, 30 min) of orange seeds significantly increased the total phenolic compounds by 1535 µg gallic acid equivalent (GAE)/100 g (Güneşer & Yilmaz 2017). These results concurred with findings from microwave pretreatment (800 W, 4 min) of milk thistle seeds, where total phenolic compounds significantly increased by 53.03 mgGAE/100 g (Fathi-Achachlouei et al. 2019).
The effect of pulsed electric field seeds pretreatment on the extracted oil phenolic compounds was investigated. For instance, Guderjan et al. (2007) managed to significantly increase the recovery of phenolic compounds by application of pulsed electric field (5.0 kV/cm and 60 pulses, 7.0 kV/cm and 120 pulses) on rapeseeds. In the same study, the authors established that the recovery of phenols was dependent on the treatment intensity. Applying a pulse intensity of 5.0 kV/cm with 60 pulses and 7.0 kV/cm with 120 pulses significantly enhanced the total phenolic compounds by more than 3 and 4 folds. Similar observations were reported by Haji-Moradkhani et al. (2019) on pulsed electric field pretreatment (voltage: 7 kV; pulse intensity: 0, 3 and 6 kV/cm; pulse duration: 0.5 ms) of cannabis seeds. Moghimi & Farzaneh (2018) studied the influence of ultrasound pretreatment on black cumin seeds. The authors reported that total phenols (121.10 ppm) were optimally recovered when the black cumin seeds were ultrasound pretreated at 90 W for 60 min.
Studies on the impact of seeds enzyme pretreatment on extracted oil phenolic compounds reported varied results. Pretreatment of borage seeds with an equal mixture of Olivex and Celluclast at 0.3% enzyme to substrate ratio, 45 °C for 9 h significantly enhanced the methanol oil extracts total phenolic compounds from 76.71 to 123.42 g Catechin/kg (Soto et al. 2008). In another study, Ezeh et al. (2016) reported that total phenols significantly decreased from 17.9 to 13.2 µgGAE/g oil after pretreating tiger nuts with Alcalse, α-amylase and Viscozyme enzymes at pH 8 and 40 °C for 6 h. In contrast with other studies, Ramadan et al. (2008) observed that total phenolic compounds did not significantly increase after enzyme pretreatment [Cellulase EC, Pektinase L 40 (1:1), 50 °C, pH: 4.3, enzyme concentration: 2% (w/w), 2 h] of goldenberry seeds. The findings from the different studies suggest that the type of seeds, enzymes and treatment conditions have a significant effect on the extractability of the phenolic compounds from the seeds matrices.
Phytosterols
The ability to lower low-density lipoprotein cholesterol and plasma cholesterol absorption, reduce the risk of certain types of cancers and boost immune function has made phytosterols valuable food components (Guderjan et al. 2007).
According to Zhou et al. (2016), microwave pretreatment (2450 MHz, 600 W for 1, 2 and 4 min) of walnuts had a negative impact on the oil phytosterols. Pretreating walnuts with microwaves for 1 min significantly reduced total phytosterols by 40.52 mg/kg, β-sitosterol (21.26 mg/kg), ∆5-avenasterol (1.63 mg/kg), campesterol (8.33 mg/kg), clerosterol (0.22 mg/kg), stigmasterol (7.22 mg/kg) and sitosterol by 1.33 mg/kg. Further increasing the microwave time to 4 min more than doubled the phytosterols losses. Therefore, the optimal microwave time for walnuts for phytosterols was 1–2 min, was marginal losses were observed. Contrarily, Fathi-Achachlouei et al. (2019) reported enrichment of phytosterols with milk thistle seeds microwave pretreatment (800 W, 2, 4 min). It was observed total phytosterols significantly increased from 1816 to 2422 µg/g after treating milk thistle seeds with microwaves for 4 min. Beta-sitosterol the primary phytosterol in milk thistle seed oil significantly increased from 630 to 788 µg/g. Other reported phytosterols, which included cholesterol, campesterol, stigmasterol, clerosterol and ∆7-sterol also significantly improved after seeds microwave pretreatment. Microwave pretreatment of dehulled rapeseeds at 800 W for 6 and 8 min also significantly enhanced brassicasterol, campesterol, stigmasterol, β-sitosterol, ∆-avenasterol and total phytosterols between 5 and 14% (Rekas et al. 2017).
Other authors have studied the effect of applying ultrasound waves (40 kHz, 30 min) on rapeseeds for phytosterols content improvement (Zdanowska et al. 2019). The study reported that rapeseeds ultrasound pretreatment significantly increased the oil brassicasterol, campesterol, β-sitosterol and total sterols by 57.5, 146.20, 209.50 and 412.40 mg/100 g, respectively. Pulsed electric field (5.0 kV/cm and 60 pulses, 7.0 kV/cm and 120 pulses) pretreatment of rapeseeds slightly increased the total phytosterols. Seed oil total phytosterols from non-hulled rapeseeds increased from 619 to 641 mg/100 g after increasing the pulsed electric field to 7.0 kV/cm and 120 pulses (Guderjan et al. 2007). The increase in phytosterols could be attributed to the increased porosity of the seeds cell walls and membranes with increased pulse intensity resulting in the improved release of phytosterols from the fatty acid esters conjugates (Uddin et al. 2018). However, in the study of Ramadan et al. (2008), it was reported that enzyme pretreatment [Cellulase EC, Pektinase L 40 (1:1), 50 °C, pH: 4.3, enzyme concentration: 2% (w/w), 2 h] of goldenberry seeds did not significantly affect the phytosterols content of the extracted oil, indicating that the applied enzymes did not cause a significant breakdown of the bonding forces between the phytosterols and the seeds matrices.
Carotenoids
Carotenoids do not only impart desirable colour to seed oil but also prevent or reduce the risks to be affected by cardiovascular disease, age-related cataract, macular degeneration and cancers (Young and Lowe 2001). Oilseeds are not good sources of dietary carotenoids, and thus maximum recovery of these antioxidant compounds from the seeds matrices is important. Mazaheri et al. (2019) reported that microwave pretreatment (1100 W, 1–3.5 min) of moisture adjusted black cumin seeds significantly improved the seed oil carotenoids concentration between 12 and 62%. The carotenoids bind to seed protein during the thermal pretreatment process to form carotenoids-protein complexes. Protein denaturation due to thermal pretreatment causes fragmentation of the carotenoids-protein complexes releasing the carotenoids in the oil as suggested by Mazaheri et al. (2019). The work reported by Roselló-Soto et al. (2015) on pulsed electric field pretreatment (40 kV, 10 µs) of olive seeds showed that treating olive seeds with pulsed electric field before oil extraction did not significantly affect the total carotenoids.
Effect of novel seeds pretreatment techniques on oil antioxidant activity
Antioxidant activity of seed oil represents the presence of naturally occurring or newly formed antioxidant compounds during the seed pretreatment process. Improving the antioxidant activity of seed oil through seeds pretreatment is essential to enhance the oil suitability for pharmaceutical, cosmetics and food preservation application. The effect of seeds pretreatment on the oil antioxidant properties is summarised in Table 4.
Table 4.
The effect of novel seeds pretreatment techniques on oil antioxidant activity
| Type of seed | Pretreatment technique | Key finding | References |
|---|---|---|---|
| Rape | Microwave (800 W, 2 min) and ultrasound pretreatment (40 kV, 10 µs) | The oil DPPH radical scavenging increased from 60.6 to 215.3 mmol/kg after seeds microwave pretreatment. Ultrasound pretreatment of rapeseeds did not significantly affect the oil DPPH radical scavenging ability | McDowell et al. (2017) |
| Orange | Microwave (360 W, 30 min with 3 min pauses after every 3 min) | Antioxidant capacity increased from 12.43 to 16.51 µm Trolox/100 g | Güneşer and Yilmaz 2017) |
| Rape | Microwave (800 W, 6 and 8 min) | DPPH radical scavenging was highest when dehulled seeds were microwaved for 8 min | Rekas et al. (2017) |
| Apricot | Microwave (360, 540, 760 W, 5 min) | The oil antioxidant capacity improved with increase in microwave power | Juhaimi et al. (2018) |
| Chia | Microwave (180, 350, 540, 720, 900 W, 15 min) | The oil antioxidant activity decreased with increase in microwave power | Ozcan et al. (2019) |
| Rape | Pulsed electric field (5.0 kV/cm and 60 pulses, 7.0 kV/cm and 120 pulses for 30 µs) | DPPH radical scavenging of the oil increased approximately between 7 and 12% | Guderjan et al. (2007) |
| Olive | Ultrasound (amplitude: 20 and 100%, 10 min) | No significant effect on the oil antioxidant activity was observed after seeds ultrasound pretreatment | Roselló-Soto et al. (2015) |
| Goldenberry | Enzyme pretreatment (Cellulase EC, Pektinase L 40 (1:1), 50 °C , pH: 4.3, enzyme concentration: 2% (w/w), 2 h) | The oil DPPH radical scavenging increased after enzyme pretreatment | Ramadan et al. (2008) |
DPPH, 2,2-Diphenyl-1-picryl hydrazyl
McDowell et al. (2017) studied the impact of rapeseeds microwave and established that the oil DPPH radical scavenging significantly increased by four folds after rapeseeds microwave pretreatment (800 W, 2 min). Similarly, significant improvement (33%) in DPPH radical scavenging after orange seeds microwave pretreatment (360 W, 30 min) was reported by Guneser and Yilmaz (2017). Rekas et al. (2017) reported similar findings with microwave pretreated (800 W, 6 and 8 min) rapeseeds (Table 4). Depending on the type of seeds, microwave power and time, treatment of oilseeds with microwaves may reduce the oil antioxidant activities. Juhaimi et al. (2018) reported a 34% decrease in DPPH inhibition of apricot seed oil when the microwave power was increased from 540 to 720 W with 5 min heating time of the apricot seeds. The results are in agreement with findings from oil extracted from microwave pretreated chia seeds that exhibited 89.47% decrease in antioxidant activity after increasing the microwave power from 180 to 900 W (Ozcan et al. 2019). The decrease in the oil antioxidant activity could be related to the degradation of the phenolic compounds, which also significantly decreased with the increase in microwave power.
The potential of the pulsed electric field to improve rapeseed oil antioxidant activity was also evaluated (Guderjan et al. 2007). It was observed that the application of pulsed electric field (5.0 kV/cm and 60 pulses) increased the oil DPPH radical scavenging by approx. 7%. Increasing the pulsed electric field magnitude to 7.0 kV/cm and 120 pulses enhanced DPPH radical scavenging by approx. 12%. In another study, ultrasound pretreatment (20 kHz, 200 W) of hemp seeds significantly decreased the oil antiradical capacity by 57, 25 and 26% after pretreating the seeds with ultrasound waves for 10, 20 and 40 min, respectively (Da Porto et al. (2015). However, ultrasound (amplitude: 20 and 100%, 10 min) and pulsed electric field pretreatments (40 kV, 10 µs) of rape and olive seeds, respectively, did not significantly improve the oil antioxidant activity (Roselló-Soto et al. 2015; McDowell et al. 2017) (Table 4). Other authors have investigated the pretreatment of goldenberry seeds with enzymes on the oil DPPH radical scavenging ability (Ramadan et al. 2008). It was reported that inhibition of DPPH radicals after 30 min was 53.5 and 49.7% for enzyme pretreated (Cellulase EC, Pektinase L 40 (1:1), 50 °C, pH: 4.3, enzyme concentration: 2% (w/w), 2 h) and untreated goldenberry seeds, respectively, indicating a 7% increase in DPPH radical scavenging ability more than the oil from untreated seeds. These findings suggest that the antioxidant activity of seed oil depends on the microwave power, pulse intensity and exposure time.
Conclusion
This review paper examined the effects of novel seeds pretreatment techniques on the oil physicochemical, bioactive compounds and antioxidant properties. Microwave, ultrasound, pulsed electric field and enzyme pretreatment of oilseeds is vital for improved oil quality. However, the quality of the oil is dependent on technical parameters such as microwave power, ultrasound power, pulse intensity and exposure time. Also, the type of enzyme, pH, temperature, enzyme concentration and treatment time influences the oil yield and quality. Application of these novel seeds pretreatment techniques to various oilseeds improved oil yield, colour, tocopherols, phenols, phytosterols, carotenoids and antioxidant activities of the extracted oil with a slight increase in peroxide value, acid value ρ-anisidine value and fatty acids conjugation, indicating that these novel seeds pretreatment techniques may not significantly degrade the oil quality.
Furthermore, new bioactive compounds are formed during seeds pretreatment, for instance, canolol in rapeseed oil. The fatty acid composition and content was insignificantly affected by seeds pretreatments, which is relevant from the nutritional point of view. Improvement of the oil antioxidant properties after seeds pretreatment is a desirable development given the oil application in functional foods and nutraceuticals formulation.
Acknowledgements
This work is based on the research supported wholly or in part by the National Research Foundation of South Africa (Grant Numbers: 64813). The opinions, findings and conclusions or recommendations expressed are those of the author(s) alone, and the NRF accepts no liability whatsoever in this regard. The authors are grateful to Agri Edge, Technology and Human Resources for Industry Programme (THRIP) and Department of Trade and Industry (DTI), South Africa for their partial bursary support.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Umezuruike Linus Opara, Email: opara@sun.ac.za.
Olaniyi Amos Fawole, Email: olaniyif@uj.ac.za.
References
- Ali MA, Islam MA, Hidayu N, Ahmadilfitri O. Effect of heating on oxidation stability and fatty acid composition of microwave roasted groundnut seed oil. J Food Sci Technol. 2017;54:4335–4343. doi: 10.1007/s13197-017-2534-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azadmard-Damirchi S, Habibi-Nodeh F, Hesari J, Nemati J, Achachlouei BF. Effect of pretreeatment with microwaves on oxidative stability and nutraceuticals content of oil from rapeseed. Food Chem. 2010;121:1211–1215. doi: 10.1016/j.foodchem.2010.02.006. [DOI] [Google Scholar]
- Bakhshabadi H, Mirzaei H, Ghodsvali A, Jafari SM, Ziaiifar AM, Farzaneh V. The effect of microwave pretreatment on some physicochemical properties and bioactivity of black cumin. Ind Crop Prod. 2017;97:1–9. doi: 10.1016/j.indcrop.2016.12.005. [DOI] [Google Scholar]
- Barba FJ, Zhu Z, Koubaa M, Sant’Ana AS, Orlien V. Green alternative methods for the extraction of antioxidant bioactivecompounds from winery wastes and by-products: a review. Trends Food Sci Technol. 2016;49:96–109. doi: 10.1016/j.tifs.2016.01.006. [DOI] [Google Scholar]
- Boussetta N, Soichi E, Lanoisellé JL, Vorobiev E. Valorization of oilseed residues: extraction of polyphenols from flaxseed hulls by pulsed electric field. Ind Crop Prod. 2014;52:347–353. doi: 10.1016/j.indcrop.2013.10.048. [DOI] [Google Scholar]
- Chemat F, Rombaut N, Sicaire AG, Meullemiestre A, Fabiano-Tixier AS, Abert-Vian M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason Sonochem. 2017;34:540–560. doi: 10.1016/j.ultsonch.2016.06.035. [DOI] [PubMed] [Google Scholar]
- Citeau M, Slabi SA, Joffre F, Carré P. Improved rapeseed oil extraction yield and quality via cold separation of ethanol miscella. OCL. 2018;25:1–9. doi: 10.1051/ocl/2018012. [DOI] [Google Scholar]
- Codex Alimentarius, Standard for Named Vegetable Oils Codex Stan 210-1999 (1999) Available online: http://www.fao.org/fao-who-codexalimentarius/codex-texts/list-standards/en. Accessed 15 June 2020
- Dandjouma AKA, Tchie C, Kapseu C, Linder M, Parmentier M. Enzyme-assisted hexane extraction of Ricinodendron heudelotii (Bail.) pierre ex pax seeds oil. Int J Food Sci Technol. 2008;43:1169–1175. doi: 10.1111/j.1365-2621.2007.01583.x. [DOI] [Google Scholar]
- Da Porto C, Da Natolino A, Decorti D. Effect of ultrasound pre-treatment of hemp (Cannabis sativa L.) seed on supercritical CO2 extraction of oil. J Food Sci Technol. 2015;52:1748–1753. doi: 10.1007/s13197-013-1143-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Đurđević S, Milovanović S, Šavikin K, Ristić M, Menković N, Pljevljakušić D, Petrovića S, Bogdanović A. Improvement of supercritical CO2 and n-hexane extraction of wild growing pomegranate seed oil by microwave pretreatment. Ind Crop Prod. 2017;104:21–27. doi: 10.1016/j.indcrop.2017.04.024. [DOI] [Google Scholar]
- Epaminondas PS, Araujo KLGV, Nascimento JA, Silva MCD, Rosenhaim R, Soledade LEB, Queiroz N, Souza AL, Santos IMG, Souza AG. Influence of toasting and the seed variety on the physico-chemical and thermo-oxidative characteristics of the flaxseed oil. J Therm Anal Calorim. 2011;106:545–550. doi: 10.1007/s10973-011-1731-2. [DOI] [Google Scholar]
- Ezeh O, Niranjan K, Gordon MH. Effect of enzyme pre-treatments on bioactive compounds in extracted tiger nut oil and sugars in residual meals. J Am Oil Chem Soc. 2016;93:1541–1549. doi: 10.1007/s11746-016-2883-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fathi-Achachlouei B, Azadmard-damirchi S, Zahedi Y, Shaddel R. Microwave pretreatment as a promising strategy for increment of nutraceutical content and extraction yield of oil from milk thistle seed. Ind Crop Prod. 2019;128:527–533. doi: 10.1016/j.indcrop.2018.11.034. [DOI] [Google Scholar]
- Fetzer DL, Cruz PN, Hamerski F, Corazza ML. Extraction of baru (Dipteryx alata vogel) seed oil using compressed solvents technology. J Supercrit Fluid. 2018;137:23–33. doi: 10.1016/j.supflu.2018.03.004. [DOI] [Google Scholar]
- Follegatti-Romero LA, Piantino CR, Grimaldi R, Cabral FA. Supercritical CO2 extraction of omega-3 rich oil from sacha inchi (Plukenetia volubilis L.) seeds. J Supercrit Fluid. 2009;49:323–329. doi: 10.1016/j.supflu.2009.03.010. [DOI] [Google Scholar]
- Gaber MAFM, Tujillo FJ, Mansour MP, Juliano P. Improving oil extraction from canola seeds by conventionaland advanced methods. Food Eng Rev. 2018;10:198–210. doi: 10.1007/s12393-018-9182-1. [DOI] [Google Scholar]
- Grasso FV, Montoya PA, Camusso CC, Maroto BG. Enzymatic pretreatment. Int J Agron. 2012;2012:1–7. doi: 10.1155/2012/543230. [DOI] [Google Scholar]
- Guderjan M, Elez-Martínez P, Knorr D. Application of pulsed electric field at oil yield and content of functional food ingredients at the production of rapeseed oil. Innov Food Sci Emerg Technol. 2007;8:55–62. doi: 10.1016/j.ifset.2006.07.001. [DOI] [Google Scholar]
- Güneşer AB, Yilmaz E. Effects of microwave roasting on the yield and composition of cold pressed orange seed oils. Grasas Aceites. 2017;68:1–10. [Google Scholar]
- Haji-Moradkhani A, Rezaeil R, Moghimi M. Optimization of pulsed electric field-assisted oil extraction from cannabis seeds. J Food Process Eng. 2019;42:1–8. doi: 10.1111/jfpe.13028. [DOI] [Google Scholar]
- Hu H, Liu H, Shi A, Liu LI, Fauconnier ML, Wang Q. The effect of microwave pretreatment on micronutrient contents, oxidative stability and flavor quality of peanut oil. Molecules. 2019;24:1–12. doi: 10.3390/molecules24010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juhaimi FAL, Özcan MM, Ghafoor K, Babiker EE. The effect of microwave roasting on bioactive compounds, antioxidant activity and fatty acid composition of apricot kernel and oils. Food Chem. 2018;243:414–419. doi: 10.1016/j.foodchem.2017.09.100. [DOI] [PubMed] [Google Scholar]
- Kha TC, Nguyen MH, Roach PD, Stathopoulous CE. Effect of galic aril microwave processing conditions on oil extraction efficiency and β-carotene and lycopene content. J Food Eng. 2013;117:486–491. doi: 10.1016/j.jfoodeng.2012.10.021. [DOI] [Google Scholar]
- Kittiphoom S, Sutasinee S. Effect of microwaves pretreatments on extraction yield and quality of mango seed kernel oil. Int Food Res J. 2015;22:960–964. [Google Scholar]
- Koubaa M, Mhemdi H, Barba FJ, Roohinejad S, Greiner R, Vorobiev E. Oilseed treatment by ultrasounds and microwaves to improve oil yield and quality: an overview. Food Res Int. 2016;85:59–66. doi: 10.1016/j.foodres.2016.04.007. [DOI] [PubMed] [Google Scholar]
- Latif S, Anwar F. Physicochemical studies of hemp (Cannabis sativa) seed oil using enzyme-assisted cold-pressing. Eur J Lipid Sci Technol. 2009;111:1042–1048. doi: 10.1002/ejlt.200900008. [DOI] [Google Scholar]
- Lee YC, Oh SW, Chang J, Kim IH. Chemical composition and oxidative stability of safflower oil prepared from safflower seed roasted with different temperatures. Food Chem. 2004;84:1–6. doi: 10.1016/S0308-8146(03)00158-4. [DOI] [Google Scholar]
- Li F, Yang L, Zhao T, Zhao J, Zou Y, Zou Y, Wu X. Optimization of enzymatic pretreatment for n-hexane extraction of oil from Silybum marianum seeds using response surface methodology. Food Bioprod Process. 2012;90:87–94. doi: 10.1016/j.fbp.2011.02.010. [DOI] [Google Scholar]
- Liu S, Jiang L, Li Y. Research of aqueous enzymatic extraction of watermelon seed oil of ultrasonic pretreatment assisted. Procedia Eng. 2011;15:4949–4955. doi: 10.1016/j.proeng.2011.08.921. [DOI] [Google Scholar]
- Mazaheri L, Torbati M, Azadmard-Damirchibi S, Savage GP. Effect of roasting and microwave pre-treatments of Nigella sativa L. seeds on lipase activity and the quality of the oil. Food Chem. 2019;274:480–486. doi: 10.1016/j.foodchem.2018.09.001. [DOI] [PubMed] [Google Scholar]
- Mcdowell D, Elliott CT, Koidis A. Pre-processing effects on cold pressed rapeseed oil quality indicators and phenolic compounds. Eur J Lipid Sci Technol. 2017;119:1–10. [Google Scholar]
- Moghimi M, Farzaneh V. The effect of ultrasound pretreatment on some selected physicochemical properties of black cumin (Nigella Sativa) Nutrire. 2018;43:1–8. doi: 10.1186/s41110-018-0077-y. [DOI] [Google Scholar]
- Mohseni NM, Mirzaei H, Moghimi M. Optimized extraction and quality evaluation of niger seed oil via microwave-pulsed electric field pretreatments. Food Sci Nutr. 2020;8:1383–1393. doi: 10.1002/fsn3.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohseni NM, Mirzaei HO, Moghimi M. Optimization of producing oil and meal from canola seeds using microwave-pulsed electric field pretreatment. OCL. 2020;27:1–12. doi: 10.1051/ocl/2019050. [DOI] [Google Scholar]
- Monfalouti HE, Guillaume D, Denhez C, Charrouf Z. Therapeutic potential of argan oil: a review. J Pharm Pharmacol. 2010;62:1669–1675. doi: 10.1111/j.2042-7158.2010.01190.x. [DOI] [PubMed] [Google Scholar]
- Moradi N, Rahimi M. Effect of simultaneous ultrasound/pulsed electric field pretreatments on the oil extraction from sunflower seeds. Sep Sci Technol. 2018;53:1–12. doi: 10.1080/01496395.2017.1379538. [DOI] [Google Scholar]
- Nikiforidis CV. Structure and function of oleosomes (oil bodies) Adv Colloid Interface Sci. 2019;274:1–6. doi: 10.1016/j.cis.2019.102039. [DOI] [PubMed] [Google Scholar]
- Ozcan MM, Al-Juhaimi EY, Ahmed ISM, Osman MA, Gassem MA. Effect of different microwave power setting on quality of chia seed oil obtained in a cold press. Food Chem. 2019;278:190–196. doi: 10.1016/j.foodchem.2018.11.048. [DOI] [PubMed] [Google Scholar]
- Passos CP, Yilmaz S, Silva CM, Coimbra MA. Enhancement of grape seed oil extraction using a cell wall degrading enzyme cocktail. Food Chem. 2009;115:48–53. doi: 10.1016/j.foodchem.2008.11.064. [DOI] [Google Scholar]
- Pathare PB, Opara UL, Al-Said FA. Colour measurement and analysis in fresh and processed foods: a review. Food Bioprocess Technol. 2013;6:36–60. doi: 10.1007/s11947-012-0867-9. [DOI] [Google Scholar]
- Ramadan MF, Sitohy MZ, Moersel J. Solvent and enzyme-aided aqueous extraction of goldenberry (Physalis peruviana L.) pomace oil: impact of processing on composition and quality of oil and meal. Eur Food Res Technol. 2008;226:1445–1458. doi: 10.1007/s00217-007-0676-y. [DOI] [Google Scholar]
- Ramos LB, Sanchez RJ, De Figueiredo AK, Nolasco SM, Fernandez MB. Optimization of microwave pretreatment variables for canola oil extraction. J Food Process Eng. 2017;40:1–10. doi: 10.1111/jfpe.12431. [DOI] [Google Scholar]
- Rekas A, Scibisz I, Siger A, Wroniak M. The effect of microwave pretreatment of seeds on the stability and degradation kinetics of phenolics compounds in rapeseed oil during long time storage. Food Chem. 2017;222:43–54. doi: 10.1016/j.foodchem.2016.12.003. [DOI] [PubMed] [Google Scholar]
- Ricochon G, Muniglia L. Influence of enzymes on the oil extraction processes in aqueous media. OCL. 2010;17:356–359. doi: 10.1051/ocl.2010.0337. [DOI] [Google Scholar]
- Roselló-Soto E, Barba JF, Parniakov O, Galanakis CM, Lebovka N, Grimi N, Vorobiev E. High voltage electrical discharges, pulsed electric field, and ultrasound assisted extraction of protein and phenolic compounds from olive kernel. Food Bioprocess Technol. 2015;8:885–894. doi: 10.1007/s11947-014-1456-x. [DOI] [Google Scholar]
- Sarkis JR, Boussetta N, Tessaro IC, Marczak LDF, Vorobiev E. Application of pulsed electric fields and high voltage electrical discharges for oil extraction from sesame seeds. J Food Eng. 2015;153:20–27. doi: 10.1016/j.jfoodeng.2014.12.003. [DOI] [Google Scholar]
- Sharma A, Gupta MN. Ultrasonic pre-irradiation effect upon aqueous enzymatic oil extraction from almond and apricot seeds. Ultrason Sonochem. 2006;13:529–534. doi: 10.1016/j.ultsonch.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Shorstkii V, Mirshekarloo MS, Koshevo E. Application of pulsed electric field for oil extraction from sunflower seeds: electrical parameter effects on oil yield. J Food Process Eng. 2017;40:1–8. doi: 10.1111/jfpe.12281. [DOI] [Google Scholar]
- Soto C, Chamy R, Zúñiga ME. Enzymatic hydrolysis and pressing conditions effect on borage oil extraction by cold pressing. Food Chem. 2007;102:834–840. doi: 10.1016/j.foodchem.2006.06.014. [DOI] [Google Scholar]
- Soto C, Concha J, Zuniga ME. Antioxidant content of oil and defatted meal obtained from borage seeds by an enzymatic-aided cold pressing process. Process Biochem. 2008;43:696–699. doi: 10.1016/j.procbio.2008.02.006. [DOI] [Google Scholar]
- Spigno G, De Faveri DM. Microwave-assisted extraction of tea phenols: a phenomenological study. J Food Eng. 2009;93:210–217. doi: 10.1016/j.jfoodeng.2009.01.006. [DOI] [Google Scholar]
- Talekar S, Patti AF, Singh R, Vijayraghavan R, Arora A. From waste to wealth: high recovery of nutraceuticals from pomegranate seed waste using a green extraction process. Ind Crop Prod. 2018;112:790–802. doi: 10.1016/j.indcrop.2017.12.023. [DOI] [Google Scholar]
- Tan JCX, Chuah C, Cheng S. A combined microwave pretreatment/solvent extraction process for the production of oil from palm fruit: optimisation, oil quality and effect of prolonged exposure. J Sci Food Agric. 2016;97:1784–1789. doi: 10.1002/jsfa.7975. [DOI] [PubMed] [Google Scholar]
- Uddin MS, Ferdosh S, Haque Akanda MJ, Ghafoor K, Rukshana AH, Ali ME, Kamaruzzaman BY, Fauzi MB, Hadijah S, Shaarani S, Islam Sarker MZ. Techniques for the extraction of phytosterols and their benefits in human health: a review. Sep Sci Technol. 2018;53:2206–2223. doi: 10.1080/01496395.2018.1454472. [DOI] [Google Scholar]
- Uquiche E, Jeréz M, Ortíz J. Effect of pretreatment with microwaves on mechanical extraction yield and quality of vegetable oil from Chilean hazelnuts (Gevuina avellana Mol) Innov Food Sci Emerg Technol. 2008;9:495–500. doi: 10.1016/j.ifset.2008.05.004. [DOI] [Google Scholar]
- Vermaak I, Kamatou GPP, Komane-mofokeng B, Viljoen AM, Beckett K. African seed oils of commercial importance-Cosmetic applications. S Afr J Bot. 2011;77:920–933. doi: 10.1016/j.sajb.2011.07.003. [DOI] [Google Scholar]
- Wroniak M, Rekas A, Siger A, Janowicz M. Microwave pretreatment effects on the changes in seeds microstructure, chemical composition and oxidative stability of rapeseed oil. LWT: Food Sci Technol. 2016;68:634–641. doi: 10.1016/j.lwt.2016.01.013. [DOI] [Google Scholar]
- Yang M, Huang F, Liu C. Influence of microwave treatment of rapeseed on minor components content and oxidative stability of oil. Food Bioprocess Technol. 2013;6:3206–3216. doi: 10.1007/s11947-012-0987-2. [DOI] [Google Scholar]
- Young AJ, Lowe GM. Antioxidant and prooxidant properties of carotenoids. Arch Biochem Biophys. 2001;385:20–27. doi: 10.1006/abbi.2000.2149. [DOI] [PubMed] [Google Scholar]
- Zdanowska P, Drozdza B, Janakowski S, Derewiaka D. Impact of preliminary ultrasound treatment of rapeseeds on the pressing process and selected oil characteristics. Ind Crop Prod. 2019;138:1–13. doi: 10.1016/j.indcrop.2019.111572. [DOI] [Google Scholar]
- Zhang S, Zu YG, Fu YJ, Luo M, Li WL, Efferth T. Supercritical carbon dioxide extraction of seed oil from yellow horn (Xanthoceras sorbifolia Bunge.) and its anti-oxidant activity. Bioresour Technol. 2010;101:2537–2544. doi: 10.1016/j.biortech.2009.11.082. [DOI] [PubMed] [Google Scholar]
- Zhang W, Jin G. Original article Microwave puffing-pretreated extraction of oil from Camellia oleifera seed and evaluation of its physicochemical characteristics. Int J Food Sci Technol. 2011;46:2544–2549. doi: 10.1111/j.1365-2621.2011.02779.x. [DOI] [Google Scholar]
- Zhou Y, Fan W, Chu F, Pei D. Improvement of the flavor and oxidative stability of walnut oil by microwave pretreatment. J Am Oil Chem Soc. 2016;93:1563–1572. doi: 10.1007/s11746-016-2891-9. [DOI] [Google Scholar]