Abstract
This work aimed to evaluate the effects of ultrasound pretreatment on the microstructure, antioxidant activity, and carotenoid retention of biofortified Beauregard sweet potato (BBSP). The pretreatment was carried out in an ultrasonic bath at 30 °C for 10 min, and it was evaluated in terms of water loss and solid gain. The drying process was performed at two different temperatures (50 and 70 °C). Six different semi-theoretical mathematical models were examined to characterize the drying curves, and quality analyses were executed. The two-terms exponential model provided the best simulation of the drying curves. Drying time was reduced by performing ultrasound pretreatment and by increasing drying temperature. The ultrasound treatment caused greater agglomeration, breakage, or strangulation of the BBSP structure, increasing porosity, and thus increasing drying rates. Drying caused a diminution of total carotenoids content and influenced antioxidant activity. However, the samples pretreated with ultrasound and dried produced lower total carotenoids loss.
Keywords: Ultrasound, Dehydration, Microstructure, Total carotenoids, Antioxidant activity, Biofortified Beauregard
Introduction
The biofortified Beauregard sweet potato (BBSP) (Ipomoea lam potatoes) is the most consumed and disseminated biofortified vegetable in the world (Santos et al. 2018), mainly in African countries, due to their nutritional characteristics. The orange color of the pulp indicates a strong presence of beta-carotene from provitamin A, which has beneficial effects on human health, preventing eye disorders and skin diseases, aiding in growth and development, and strengthening the immune system. It also has antioxidant properties, acting in the elimination of free radicals, improving iron absorption, and providing anti-mutagenic properties (Vizzotto et al. 2017).
In addition to its nutritional benefits, BBSP shows satisfactory results in several areas related to production. It presents higher root yield and cycle precocity rates when compared to other varieties of the sweet potato. This means a longer harvest period, easier cultivation, and wide adaptation to the low-level technological systems of subsistence farming (Islam et al. 2016).
In this sense, BBSP stands out as a significant player in malnutrition eradication in certain populations, including children, women of childbearing age, and pregnant women (Berni et al. 2015). A viable alternative for the use of the BBSP in the human diet is its processing for flour production by drying method.
Drying is one of the most common methods used to extend shelf life and to maintain the quality of food products stored below ambient temperature (Onal et al. 2019). However, drying also modifies the microstructure of the food. Ultrasound pretreatment is considered an emerging technological alternative which can increase drying rate and improve food quality. Ultrasound application results in the formation of micro-channels, increasing the porosity of the samples, and improving drying rates (Guo et al. 2019; Wang et al. 2019a, b).
Antioxidant compounds, normally found in functional foods, are capable of inhibiting or delaying injuries caused by free radicals (Yan et al. 2016). The reactions caused by free radicals can be compensated by the action of antioxidants obtained through the diet, including carotenoids, abundant in BBSP (Teow et al. 2007). However, regardless of method, carotenoid content and antioxidant activity may be affected by pretreatment, and little data on carotenoid retention in BBSP is currently available.
This work aims to evaluate the effects of ultrasound pretreatment on the microstructure, antioxidant activity, and carotenoid retention of BBSP.
Materials and methods
Preparation of samples
Fresh BBSP samples were acquired at a local market in João Pessoa (Brazil). These samples were harvested in 2018 by conventional culture. They were packed in boxes and transported at room temperature to the Laboratory of Food Engineering (Recife, Brazil). Those free of mechanical injuries, pests, diseases, and deterioration were selected for experimentation. The fresh roots were washed in running water to remove dirt, disinfected with a chlorine solution (150 µL/L) for 15 min, and rinsed under running water to remove excess chlorine adhered to the roots. After the sanitization phase, the fresh roots were peeled manually with the aid of a stainless steel knife. The edible portion (intermediate section of the mesocarp) was cut in 0.5 cm thick and weighed on a semi-analytical digital scale (HAUS Corporation, model AR2140, Brazil).
Ultrasonic pretreatment
The design of the pretreatment step was based on the methods of Azoubel et al. (2010). An ultrasound bath (Unique, model USC-2580A, Brazil) with a frequency of 25 kHz at the intensity of 4870 W/m2 was used to pretreat the potato samples at 30 ± 2 °C. Twelve slices of fresh roots were placed in two 250 mL Erlenmeyer flasks (six per flask) with distilled water. After 10 min, the samples were removed from the conical flasks, dried with absorbent paper to remove excess water, and finally weighed in a semi-analytical balance. The pretreatment measured water loss and solid gain.
Drying
In this study, two drying groups were set, including control samples without ultrasound pretreatment (C) and variable samples with ultrasound pretreatment (US). Drying was performed using an air-circulating oven at two different temperatures (50 and 70 °C) (Azoubel et al. 2010). For each experiment, the twelve slices were spread across the drying grid. Samples were weighed on a semi-analytical scale in 15-min intervals until the condition of equilibrium (constant weight) was reached.
In order to predict the drying kinetics of melon, it is important to accurately model its drying behavior. Therefore, in the present study, semi-theoretical mathematical models were fitted to the experimental data (Table 1), where a, b, c, k, and n, are the empirical constants in drying models and t is the drying time in min. The moisture contents at each time interval were calculated from both weight loss data and the dry solid weights of the samples. The moisture content data was converted to moisture ratio (MR), which is a dimensionless expression and displayed as a function of drying time. The dimensionless MR of melon during the drying experiments was written in the following form (Eq. 1):
| 1 |
where is the average moisture content at time t, kg H2O/kg dry matter; Xe is the equilibrium moisture content, kg H2O/kg dry matter; Xo is the initial moisture content, kg H2O/kg dry matter.
Table 1.
Parameters, coefficients of determination (R2) and mean square deviations (MSD) of the models fitted to the drying curves of BBSP at two drying conditions (50 and 70 °C) for the control samples without ultrasound pre-treatment (C) and samples with ultrasound pretreatment (US)
| Model | Parameters | 50 °C | 70 °C | ||
|---|---|---|---|---|---|
| C | US | C | US | ||
| Page | Equation | RX = exp (− Ktn) | |||
| a | 0.0083 | 0.0379 | 0.0753 | 0.0464 | |
| n | 17.42 | 0.803 | 17.74 | 0.879 | |
| R2 | 0.2483 | 0.9992 | 0.4262 | 0.9999 | |
| P | 93.75 | 25.999 | 90.909 | 61.143 | |
| Henderson-Pabis | Equation | RX = exp (− Kt) | |||
| a | 0.8881 | 0.94 | 0.9681 | 0.9787 | |
| k | 0.021 | 0.015 | 0.033 | 0.029 | |
| R2 | 0.9499 | 0.9886 | 0.9906 | 0.997 | |
| P | 37.482 | 18.314 | 28.606 | 12.916 | |
| Logarithmic | Equation | RX = exp (− Kt) +c | |||
| a | 0.8733 | 0.9204 | 0.9468 | 0.9661 | |
| c | 0.066 | 0.0488 | 0.0369 | 0.0208 | |
| k | 0.03 | 0.018 | 0.037 | 0.031 | |
| R2 | 0.9715 | 0.9952 | 0.9956 | 0.9982 | |
| P | 32.151 | 10.701 | 23.397 | 15.141 | |
| Wang and Singh | RX = 1 + at + bt2 | ||||
| a | 0.0131 | 0.0106 | 0.0192 | 0.0183 | |
| b | 0.00004 | 0.00003 | 0.00009 | 0.00008 | |
| R2 | 0.7288 | 0.9049 | 0.8825 | 0.9306 | |
| Two-terms exponential | RX = aexp(− Kt)+ bexp(− wt) | ||||
| a | 0.4166 | 0.3312 | 0.6257 | 0.8167 | |
| b | 0.5833 | 0.6705 | 0.3745 | 0.1834 | |
| k | 0.149 | 0.048 | 0.023 | 0.025 | |
| w | 0.014 | 0.011 | 0.091 | 0.103 | |
| R2 | 0.9999 | 0.9995 | 0.9999 | 0.9999 | |
| P | 16.503 | 24.092 | 23.265 | 31.611 | |
| Single exponential | RX = exp(− Kt) | ||||
| k | 0.025 | 0.016 | 0.034 | 0.029 | |
| R2 | 0.9344 | 0.9839 | 0.9893 | 0.9965 | |
| p | 47.159 | 23.144 | 31.32 | 15.089 | |
C control, US ultrasound
The modeling was characterized by the mean relative deviation module P (Eq. 2) and the determination coefficient R2.
| 2 |
where N is the number of experimental data, Ve is the experimental value and Vp is the calculated value. Values of P (%) less than or equal to 10% are considered to satisfy the experimental data (Lomauro et al. 1985).
After drying, the BBSP slices were shredded (model A11B S 32, marca?). The obtained powder was standardized with 20 mesh granulometry, stored in plastic containers hermetically coated with aluminum foil, labeled, and placed in a freezer at − 18 ± 1 °C for further analysis.
Ultrastructural analysis by scanning electronic microscopy (SEM)
Flours obtained at different drying temperatures were characterized by scanning electron microscopy. The flour samples were sprinkled onto pieces of double-sided tape affixed to an aluminum stub, followed by metallization (SANYU ELECTRON, SC-701A) for 3 min, with an amperage of 10 mA, for the ionic deposition of a thin layer of 5 nm of gold. Next, samples were examined under a high vacuum in a scanning electronic microscope, JEOL LV 5600, operating at 25 kV, and images were obtained in magnifications ranging from 300× to 1200×.
The extraction and quantification of the total carotenoids
The total carotenoid content was quantified based on Rodriguez-Amaya’s method (1999). In brief, there was a 40–mL acetone extraction of carotenoids from a 5–g sample, followed by separation and dilution in petroleum ether, resulting in an absorbance of 475 nm. Some precautions against pigment degradation or alteration were taken, such as protection from light and high temperatures, and efficient analyses. Total carotenoids were expressed as μg per g of DM. All analyses were carried out in triplicate.
Antioxidant activity determination: DPPH (2,2-diphenyl-1-picrylhydrazyl)
The extracts were prepared according to Brand-Williams et al. (1995), with some modifications. 10 mL of methanol 80% (v/v) were added to the 1-g sample and stirred for 2 min. After 1 h in the dark at room temperature, the extract was centrifuged (2200 g, 20 °C for 20 min) and the supernatant was stored at − 18 °C until the evaluations. All extractions were carried out in triplicate.
DPPH radical scavenging activity assay
The extracts obtained were used to determine antioxidant activity. The total antioxidant activity of samples was measured by the DPPH (2,2-diphenyl-1-picrylhydrazyl) radical scavenging method (Rufino et al. 2007). Each extract (0.1 mL) was mixed with 3.9 mL of DPPH methanol solution in cuvettes. The reaction mixture was shaken properly and kept for 1 h at room temperature in the dark. Methanol was used as the blank, and the control sample was prepared without adding any extract. Absorbance was measured at 515 nm in a spectrophotometer (SH-1000, Hitachi, Japan). The DPPH radical scavenging activity percentage was calculated using the following equation:
where AC: the absorbance of the control (DPPH solution without the sample), AS: the absorbance of the sample.
Statistical analysis
The results were examined using a one-way analysis of variance (ANOVA). The difference between samples was considered significant if it fell within the 95% confidence interval calculated using IMB SPSS 20 (SPSS Inc., Chicago, IL). All the experiments were conducted in triplicate.
Results and discussion
Drying temperature
Figure 1 shows the drying kinetics of BBSP at two temperatures (50 and 70 °C) for the control samples without ultrasound pretreatment (C) and for the samples with ultrasound pretreatment (US). The dehydration times for BBSP to reach equilibrium were 450 and 630 min at 50 °C, and 360 and 330 min at 70 °C for C and US, respectively. The application of pretreatment with ultrasound did not have a positive effect on BBSP drying time at 50 °C. Conversely, the untreated sample’s drying time was reduced by 30 min at 70 °C. Nowacka et al. (2012) reported that ultrasound time significantly affects drying intensity. The authors verified a considerable reduction when the US was performed for 30 min. In the present study, the US pretreatment duration was 10 min, a fact that may have influenced the failure to register a significant reduction in drying time with its application.
Fig. 1.
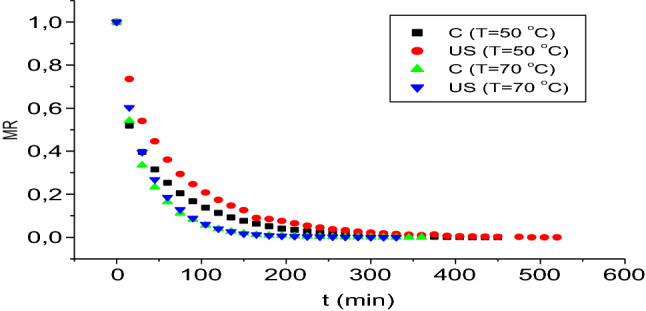
BBSP at two drying conditions (50 and 70 °C) for control samples without ultrasound pretreatment (C) and samples with ultrasound pretreatment (US)
It is also possible to note that increased air temperature reduced drying time. Similar observations have been reported for potato slices (Onwude et al. 2018). This phenomenon is due to the higher water removal rate of the product resulting from a higher moisture gradient between the product and the air caused by the increase in air temperature. In this process, drying kinetics are directly related to temperature. Increased temperature results in increased moisture transfer (Santos et al. 2017).
Drying mathematical modeling
Modeling is important to the analysis and understanding of drying. Empirical models facilitate estimations of drying times and drying curves, allowing one to estimate conditions resulting in desired final moisture content. Adjustments to the mathematical models were tested under various conditions. The parameters of the six mathematical models contrasted with the experimental data of BBSP drying kinetics are presented in Table 1. The two-term exponential model produced solid R2 values and calculated lower mean relative deviation (P). This shows the suitability of this model. A similar result was obtained by Medeiros et al. (2016) for mango and Silva et al. (2019) for mango.
In the present study, parameter k presented higher values in the control group, which implies a prolonged drying time. The application of ultrasound pretreatment resulted in lower parameter values, reinforcing the importance of ultrasound in drying time reduction. Similar results have been reported by Santos et al. (2017) in the drying of pitaya shells (H. undatus) at temperatures of 50, 60, and 70° C.
Microstructure analysis
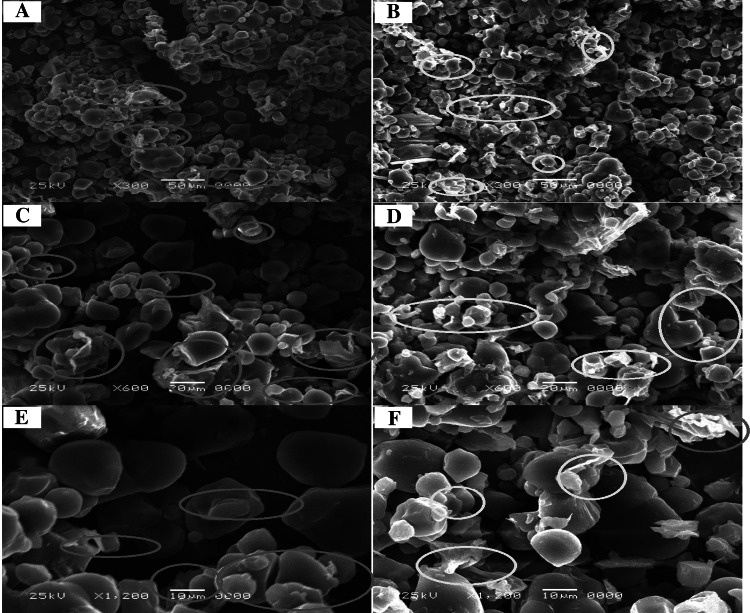
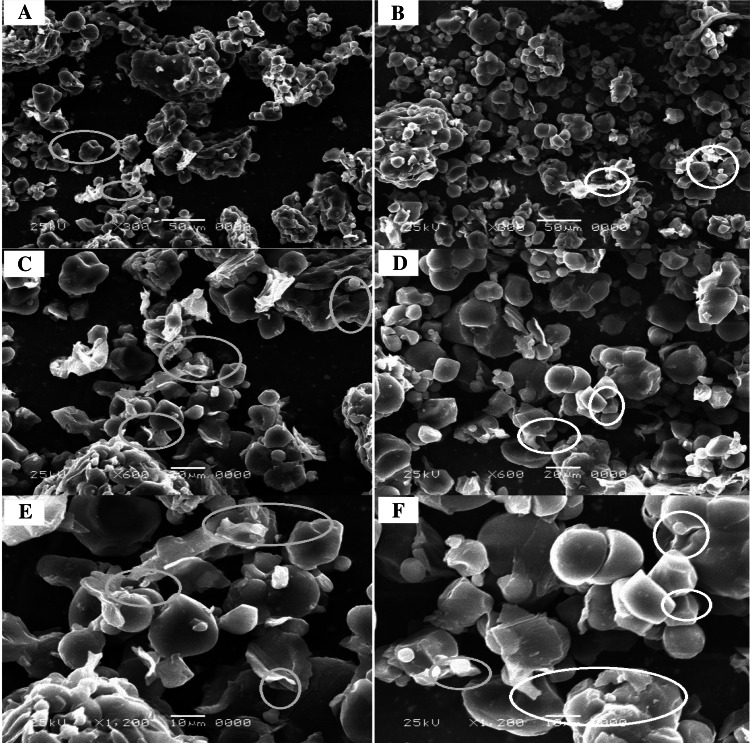
To better understand the effect of ultrasound pre-treatment at the cellular level, scanning electron microscopy (SEM) was used to observe structural changes. Typical SEM images of BBSP at two drying conditions (50 and 70 °C) for the control and ultrasound (C and US) were reported in Figs. 2 and 3.
Fig. 2.
Typical SEM images of BBSP at 50° C. Control (a, c, e) and ultrasound (b, d, f)
Fig. 3.
Typical SEM images of BBSP at 70° C. Control (a, c, e) and ultrasound (b, d, f)
Dehydrated samples at 50 °C showed greater agglomeration and signs of breakage or strangulation of cellular structures (Fig. 2). Structures with some cell fractures can be attributed to the rapid evaporation of moisture by ultrasonic heating (Su et al. 2018). The application of ultrasound seems to induce a greater degradation of the structure of the sweet potato, causing an increase of porosity and pore diameter and rupture of the cellular structure. However, these effects can also make water movement in the matrix easier, improving drying rate (Onwude et al. 2018).
On the other hand, Fig. 3 at 70 °C, shows a more porous and fissured microstructure of BBSP samples in both groups (C and US). The results are similar to those presented by Lagnika et al. (2018) on the drying of the orange pulp sweet potato.
The BBSP structure porosity is justified by the rapid heating of the samples, which leads to lower water levels through evaporation and internal cellular stress and expansion and contraction caused by ultrasonic waves that dilate the intercellular space, accelerating dehydration and indicating good sample quality (Nowacka and Wedzik 2016). Also, ultrasound pre-treatment does not influence sample thickness, which also warrants rapid dehydration of the pretreated sample at 70 °C (Fig. 1).
Cellular rupture could also be attributed to the shear stresses and cavitation effects caused by extensive high-intensity ultrasound processing (Wang et al. 2019a, b).
Total carotenoid
Using the results obtained for the total carotenoid content achieved for the BBSP, Table 2 graphs the spectrophotometer readings, using wavelength as a function of the absorbance of the samples of acetone/petroleum ether solvents. It was scanned at wavelengths of 450 and 475 nm.
Table 2.
Total carotenoids (μg/g of DM) and antioxidant activity (DPPH radical-scavenging activity (%) of BBSP
| Total carotenoids (μg/g of DM) | ||||
|---|---|---|---|---|
| 50 °C | 70 °C | |||
| C | US | C | US | |
| 470 nm | 11.752 ± 0.176a | 14.00 ± 0.49a | 13. 711 ± 0.077b | 13.115 ± 0.776b |
| 450 nm | 19.195 ± 0.244a | 24.238 ± 0.968a | 23.071 ± 0.3535b | 22.187 ± 0.783b |
| %DPPH | 17.72 ± 0.47a | 13.20 ± 0.44a | 31.06 ± 1.73b | 27.45 ± 0.16b |
For each temperature, the same letters on the same line mean that the US (ultrasound) and C (control) indicated no significant differences (p < 0.05) between the two groups (US and C)
As shown in Table 2, there were no significant differences in the total carotenoid content between the US and C. However, Nowacka and Wedzik (2016) reported that ultrasound pretreatment significantly reduced the total carotenoid content in carrots compared with that of the untreated samples. This may have resulted from a long processing duration (20–30 min) in their studies.
Results show that ultrasound pretreatment retained total carotenoid content. This can be attributed to the mechanical rupture of the cell walls of the food matrix, which may increase the content of free carotenoids and the preservation of carotenoid-protecting structures during ultrasound processing (Abid et al. 2014). According to Rodrigues-Amaya (1999), ultrasound preserves hydrophobic compounds (carotenoid compounds), probably due to its increased extraction due to ultrasonic waves that reduce available oxygen for free radical formation, since the mechanism of action of carotenoids is to chelate singlet oxygen and its formation. Degradation is influenced by oxygen at high temperatures. Also, retention of carotenoids during ultrasound application may show that microchannels formed during ultrasonic waves do not allow the passage of compounds from the sample to the solvent, thereby retaining carotenoid contents.
Antioxidant activity
The results of the antioxidant activity of BBSP extracts evaluated by the DPPH free radical sequestration method are presented in Table 2.
Antioxidant activity observed according to the DPPH method (Table 2) showed a positive correlation with temperature. This fact can be attributed to several chemical reactions, such as the Maillard reaction, induced by high thermal intensity, which may lead to the emergence of new compounds with high antioxidant activities (Tian et al. 2016).
Ultrasound pretreatment at 70 °C showed no significant difference (p > 0.05) from the control group. According to Wang et al. (2017), these results suggest that ultrasonic wave changes that lead to increased solubility do not always lead to improved antioxidant activity. Sledz et al. (2015) investigated the influence of ultrasound on drying kinetics, antioxidant activity, and microstructure of basil leaves and concluded that pretreatment with ultrasound does not alter antioxidant activity.
On the other hand, according to Table 2, the application of ultrasound did not influence the antioxidant potential determined by the DPPH method, indicating that the antioxidant compounds that perform via free radical capture mechanism were retained by using ultrasound.
Conclusion
The application of ultrasound pretreatment did not compromise the dehydration of BBSP. Among the mathematical models used in this study, two-terms exponential presented the most accurate predictions. Ultrasound pretreatment and increased temperature reduced drying time. The ultrasound treatment effected greater agglomeration, breakage, or strangulation of the BBSP structure, increasing porosity, and thus shortening drying rates. Drying caused a diminution of total carotenoid content and influenced antioxidant activity. However, the samples pretreated with ultrasound and then dried obtained lower total carotenoid loss.
Acknowledgements
The authors acknowledge, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-CAPES (process nº CAPES/PROEX 1734/2015) and Pró-reitoria de Pesquisa e Pós-graduação da Universidade Federal de Pernambuco-PROPESQ/UFPE (process nº 23076.049914/2017-47) for financial support and research grant. The Laboratório de Experimentação e Análises de Alimentos do Departamento de Nutrição da Universidade Federal de Pernambuco-LEAAL/UFPE, the Centro de Tecnologias Estratégicas do Nordeste-CETENE for the availability of infrastructure. The authors were also grateful to Nucleus of Research in Environmental Sciences and Biotechnology, Catholic University of Pernambuco, by the SEM analysis.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abid M, Jabbar S, Wu T, Hashim MM, Hu B, Lei S, Zeng X. Sonication enhances polyphenolic compounds, sugars, carotenoids and mineral elements of apple juice. Ultrason Sonochem. 2014;21:93–97. doi: 10.1016/j.ultsonch.2013.06.002. [DOI] [PubMed] [Google Scholar]
- Azoubel PM, Baima MAM, Amorim MR, Oliveira SSB. Effect of ultrasound on banana cv Pacovan drying kinetics. J Food Eng. 2010;97:194–198. doi: 10.1016/j.jfoodeng.2009.10.009. [DOI] [Google Scholar]
- Berni P, Chitchumroonchokchai C, Canniatti-brazaca SG, de Moura FF, Failla ML. Comparison of content and in vitro bioaccessibility of provitamin A carotenoids in home cooked and commercially processed orange fleshed sweet potato (Ipomea batatas Lam) Plant Foods Hum Nutr. 2015;70:1–8. doi: 10.1007/s11130-014-0458-1. [DOI] [PubMed] [Google Scholar]
- Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. Lebensm-Wiss u-Technol. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- Guo Z, Zhao B, Huang LH, Miao S, Zheng B. Optimization of ultrasound-microwave synergistic extraction of prebiotic oligosaccharides from sweet potatoes (Ipomoea batatas L.) Innov Food Sci Emerg Technol. 2019;1:5. doi: 10.1016/j.ifset.2019.03.009. [DOI] [Google Scholar]
- Islam SN, Nusrat T, Begum P, Ahsan M. Carotenoids and b-carotene in orange fleshed sweet potato: a possible solution to vitamin A deficiency. Food Chem. 2016;15:628–631. doi: 10.1016/j.foodchem.2015.12.057. [DOI] [PubMed] [Google Scholar]
- Lagnika C, Huang J, Ning J, Li D, Liu C, Song J, Wei Q, Zhang M. Ultrasound-assisted osmotic process on quality of microwave vacuum drying sweet potato. Drying Technol Int J. 2018 doi: 10.1080/07373937.2017.1402786. [DOI] [Google Scholar]
- Lomauro CJ, Bakshi AS, Labuza TP. Evaluation of food moisture sorption isotherm equations. Part I: fruit, vegetable and meat products. Lebensmittel-Wissenschaft and Technol. 1985;18:112–122. [Google Scholar]
- Medeiros RAB, Barros ZMP, Carvalho CBO, Fraga Neta EG, Maciel MIS, Azoubel PM. Influence of dual-stage sugar substitution pretreatment on drying kinetics and quality parameters of mango. LWT Food Sci Technol. 2016;67:167–173. doi: 10.1016/j.lwt.2015.11.049. [DOI] [Google Scholar]
- Nowacka M, Wedzik M. Effect of ultrasound treatment on microstructure, colour and carotenoid content in fresh and dried carrot tissue. Appl Acoust. 2016;103:163–171. doi: 10.1016/j.apacoust.2015.06.011. [DOI] [Google Scholar]
- Nowacka M, Wiktor A, Sledz M, Jurek N, Witrowa-rajchert D. Drying of ultrasound pretreated apple and its selected physical properties. J Food Eng. 2012;113:427–433. doi: 10.1016/j.jfoodeng.2012.06.013. [DOI] [Google Scholar]
- Onal B, Adiletta G, Crescitelli A, Di Matteo M, Russo P. Optimization of hot air drying temperature combined with pre-treatment to improve physico-chemical and nutritional quality of ‘Annurca’ apple. Food Bioprod Process. 2019;115:87–99. doi: 10.1016/j.fbp.2019.03.002. [DOI] [Google Scholar]
- Onwude DI, Norhashila H, Khalina A, Rimfiel J, Guangnan C. Investigating the influence of novel drying methods on sweet potato (Ipomoea batatas L.): kinetics, energy consumption, color, and microstructure. J Food Process Eng. 2018;41:1–12. doi: 10.1111/jfpe.12686. [DOI] [Google Scholar]
- Rodriguez-Amaya DB. A guide to carotenoid analysis in foods. ILSI Press: Scott, J., Rebeille, F., Fletcher, J. Folic acid an folates—the feseability for nutricional enhancement in plant foods. J Sci Food Agric. 1999;6:56–67. [Google Scholar]
- Rufino MSM, Alves RE, Brito ES, Morais SM, Sampaio CG, Jiménez JP, Saura-Calixto FD (2007) Metodologia Científica: Determinação da Atividade Antioxidante Total em Frutas pela Captura do Radical Livre DPPH e pela Captura do Radical Livre ABTS +. Comunicado técnico 128, ISSN 1679-6535. Embrapa Agroindústria Tropical
- Santos FS, de Figueirêdo RMF, Alexandre J, Santos D. Drying kinetics and physical and chemical characterization of white-fleshed ‘pitaya’ peels. R Bras Eng Agríc Ambiental. 2017;21:872–877. doi: 10.1590/1807-1929/agriambi.v21n12p872-877. [DOI] [Google Scholar]
- Santos EA, de Andrade Júnior VC, de Sousa Júnior AS, Okumura F, Simeone MLF, dos Santos JB, Azevedo AM. Mistico selectivity of pre-emergent herbicides in sweet potato genotypes. Rev Bras Cienc Agrar. 2018;13:5511. [Google Scholar]
- Silva HW, Vale LSR, Silva CF, Souza RC, Soares RS. Drying kinetics and physiological quality of ‘Cabacinha’ pepper seeds during storage. R Bras Eng Agríc Ambiental. 2019;22:292–297. doi: 10.1590/1807-1929/agriambi.v22n4p292-297. [DOI] [Google Scholar]
- Sledz M, Wiktor A, Nowacka M, Witrowa-Rajchert D. Drying kinetics, microstructure and antioxidant properties of basil treated by ultrasound. J Food Process Eng. 2015;1–13:1745–4530. doi: 10.1111/jfpe.12271. [DOI] [Google Scholar]
- Su Y, Zhanga M, Bhandarid B, Zhange W. Enhancement of water removing and the quality of fried purple-fleshed sweet potato in the vacuum frying by combined power ultrasound and microwave technology. Ultrason Sonochem. 2018;44:368–379. doi: 10.1016/j.ultsonch.2018.02.049. [DOI] [PubMed] [Google Scholar]
- Teow CC, Truong VD, Mcfeeters RF, Thompson RL, Pecota KV, Yencho GC. Antioxidant activities, phenolic and β-carotene contents of sweet potato genotypes with varying flesh colours. Food Chem. 2007;103:829–838. doi: 10.1016/j.foodchem.2006.09.033. [DOI] [Google Scholar]
- Tian J, Chen J, Chen S, Chen J, Liu D, Ye X. Domestic cooking methods affect the phytochemical composition and antioxidant activity of purple-fleshed potatoes. Food Chem. 2016;97:1264–1270. doi: 10.1016/j.foodchem.2015.11.049. [DOI] [PubMed] [Google Scholar]
- Vizzotto M, Pereira ES, Vinholes JR, Munhoz PC, Ferri NML, Castro LAS, Krolow ACR. Physicochemical and antioxidant capacity analysis of colored sweet potato genotypes: in natura and thermally processed. Ciência Rural. 2017;47:1–8. doi: 10.1590/0103-8478cr20151385. [DOI] [Google Scholar]
- Wang Q, Sheng X, Shi A, Hu H, Yang Y, Liu L, Fei L, Liu H. β-Glucans: relationships between modification, conformation and functional activities. Molecules. 2017;22:1–12. doi: 10.3390/molecules22020257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Xiao HW, Ye JH, Wang J, Raghavan V. Ultrasound pretreatment to enhance drying kinetics of kiwifruit (Actinidia deliciosa) slices: pros and cons. Food Bioprocess Technol. 2019;12(5):865–876. doi: 10.1007/s11947-019-02256-4. [DOI] [Google Scholar]
- Wang J, Wang J, Ye J, Vanga SK, Raghavan V. Influence of high-intensity ultrasound on bioactive compounds of strawberry juice: profiles of ascorbic acid, phenolics, antioxidant activity and microstructure. Food Control. 2019;96:128–136. doi: 10.1016/j.foodcont.2018.09.007. [DOI] [Google Scholar]
- Yan B, Lu MS, Wang L, Mo XF, Luo WP, Du YF, Zhang CX. Specific serum carotenoids are inversely associated with breast cancer risk among Chinese women: a case–control study. Br J Nutr. 2016;115:129–137. doi: 10.1017/S000711451500416X. [DOI] [PubMed] [Google Scholar]