Abstract
Coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused a global pandemic. The virus primarily affects the lungs where it induces respiratory distress syndrome ranging from mild to acute, however, there is a growing body of evidence supporting its negative effects on other system organs that also carry the ACE2 receptor, such as the placenta. The majority of newborns delivered from SARS-CoV-2 positive mothers test negative following delivery, suggesting that there are protective mechanisms within the placenta. There appears to be a higher incidence of pregnancy-related complications in SARS-CoV-2 positive mothers, such as miscarriage, restricted fetal growth, or still-birth. In this review, we discuss the pathobiology of COVID-19 maternal infection and the potential adverse effects associated with viral infection, and the possibility of transplacental transmission.
Keywords: COVID-19, placenta, SARS-CoV-2, transplacental infection, pregnancy
Introduction
The World Health Organization (WHO) declared a global pandemic of coronavirus disease 2019 (COVID-19) in March 2020, caused by the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) (1). As of August 2021, the number of total cases surpassed 200 million and resulted in more than 4 million deaths. There is an ongoing effort to understand transmission, incidence, disease pathogenesis and the short- and long-term impacts following infection. In particular, the impact of SARS-CoV-2 infection on mothers and their babies (2). Evidence suggests that pregnant women with COVID-19 are more susceptible to severe disease with a higher risk of preterm birth (3–5), as well as higher risk of maternal and/or fetal death (6, 7). These findings are reminiscent of the dire outcomes from other similar respiratory viral infections, such as influenza A/H1N1 (8–11), severe acute respiratory syndrome (SARS) (12), and Middle East Respiratory Syndrome (MERS) (13, 14), where infected pregnant women are at increased risk of severe morbidity and mortality to both themselves and their infants (2). While most neonates born to SARS-CoV-2 positive mothers test negative and do not present with virus-induced disease, there have been some cases of newborns testing positive and presenting with early-onset symptoms (15). Whether this is due to the trans-placental transmission of SARS-CoV-2, or infection following delivery is still not well understood (16–18). Examination of the placentas from SARS-CoV-2 positive mothers have mixed reports on viral positivity, and not all neonates born from mothers with a SARS-CoV-2 positive placenta test positive for the virus (19). This suggests that there is a protective mechanism/barrier within the placenta, where its success may rely on the presence or absence of certain receptors/pathways. Fortunately, SARS-CoV-2 positive neonates are yet to present with any congenital defects (20). In this review, we provide an overview of the literature of SARS-CoV-2 infection during pregnancy, as well as the pathobiology of the placenta which may protect the growing fetus.
Immune System Alterations During SARS-CoV-2 Infection
The immune system changes during pregnancy in such a way that it adapts to the growth of a semi-allogeneic fetus in the body of the mother, resulting in a distinct immune response to different infections during pregnancy (21–23). It has been well documented that in patients with COVID-19, particularly those with severe disease, have profound immune dysregulation (24). Studies have revealed an increase in blood leukocytes (leukocytosis), which was characterized by a decrease in lymphocytes (lymphopenia) and an increase in neutrophil-to-lymphocyte ratio (NLR) (25, 26). Using immunophenotyping analyses, researchers discovered that patients with severe COVID-19 had fewer natural killer (NK), CD3+, CD4+, and CD8+ T cells than those with the non-severe disease (27). NK cells were also found to be functionally exhausted during SARS-CoV-2 infection (28–30). Moreover, a reduction in circulating NK cell population has been reported during gestation (31). NK cells have key roles in the innate immune response by killing transformed cells, as consequence of viral infections or oncogenesis; NK cells are also major sources of pro-inflammatory cytokines such as granulocyte-macrophage colony-stimulating factor (GM-CSF) and interferon gamma (IFN-γ), which can restore or activate the antiviral property of the myeloid compartment; thus, any decrease in these cell populations may alter the ability to clear viruses (32). Evidence has shown that lymphopenia and enhanced NLR can be further amplified by COVID-19 disease severity (33). Compared to patients with moderate COVID-19, individuals with severe disease had lower numbers of cytotoxic T lymphocytes (CTLs) (33). Studies investigating COVID-19 patients’ lung tissue and bronchoalveolar lavage fluid (BALF) samples found T cell hyperactivation and/or upregulation of pro-apoptotic factors, including first apoptosis signal receptor (FAS), TNF‐related apoptosis‐inducing ligand (TRAIL), and caspase 3, as the main causes of T cell depletion (34–36). Alterations in CD4+ T cell population toward T helper-2 (Th)-2 phenotypes rather than Th1 phenotypes have been found during pregnancy, which contributes to the promotion of humoral immune responses over cellular immune responses (37). There is also a balance between regulatory T cell (Treg) and Th17 cells during pregnancy; with a shift towards Tregs to ensure fetal-maternal immune tolerance and to prevent fetal allograft rejection (38). In terms of innate immune cells, evidence suggests that, while absolute peripheral blood monocyte counts are not significantly different between patients with severe COVID-19 and those with moderate disease, the activation status of the monocyte/macrophage system is significantly altered (39). It was shown that monocyte/macrophage alterations caused by SARS-CoV-2 infection are similar to a condition known as familial hemophagocytic lymphohistiocytosis (HLH), a systemic inflammatory disorder involving cytokine production and cytopenia (40–42). HLH can be triggered either by abnormalities in genes regulating NK and cytotoxic CD8+ T cell degranulation or by conditions such as autoimmune disease, malignancy, and viral infection (40, 41). It was found that patients with H1N1 influenza who experienced the ‘cytokine storm,’ characterized by the extreme and excessive immune and inflammatory response (43), had mutations in genes associated with HLH (44). Many studies, however, do not support the link between HLH and COVID-19 (45–47). Wood et al., found that only three of 40 COVID-19 patients had Hscores >169, the cut-off used to identify HLH (47). Several studies have reported widespread infiltration of monocytes/macrophages in the lung tissue samples taken from COVID-19 patients (35, 48, 49). Single-cell studies revealed that monocyte-derived FCN1+ macrophages were the most abundant macrophage subset found in BALF samples from severe COVID-19 patients (35). Furthermore, it was discovered that peripheral monocyte trafficking and subsequent differentiation into macrophages in the lungs of COVID-19 patients contributes to pro-inflammatory responses and further activation of innate immune cells (49). Changes in the innate immune system during pregnancy, also, involve the pattern recognition receptors Toll-like receptors (TLRs), in particular TLR4 (50, 51). There are three different levels of TLR4 activation during pregnancy. First, TLR4 activation and the inflammatory response rise during the first trimester, allowing blastocyst implantation. Following that, a decrease in TLR4 activation happens during the second trimester in order to create an anti-inflammatory response for fetal growth. Eventually, TLR4 activation and the inflammatory response increase again in the third trimester to support labor and delivery (52). Infection with COVID-19 leads to pyroptosis of host cells and the release of danger associated molecular patterns (DAMPs) that can act as ligands for TLR molecules and trigger a greater inflammatory response (31). Studies are needed to determine whether such changes in the immune system result in higher susceptibility or are protective against COVID-19 during pregnancy (31).
Expression of ACE2 and TMPRSS2 in Placental and Fetal Cells
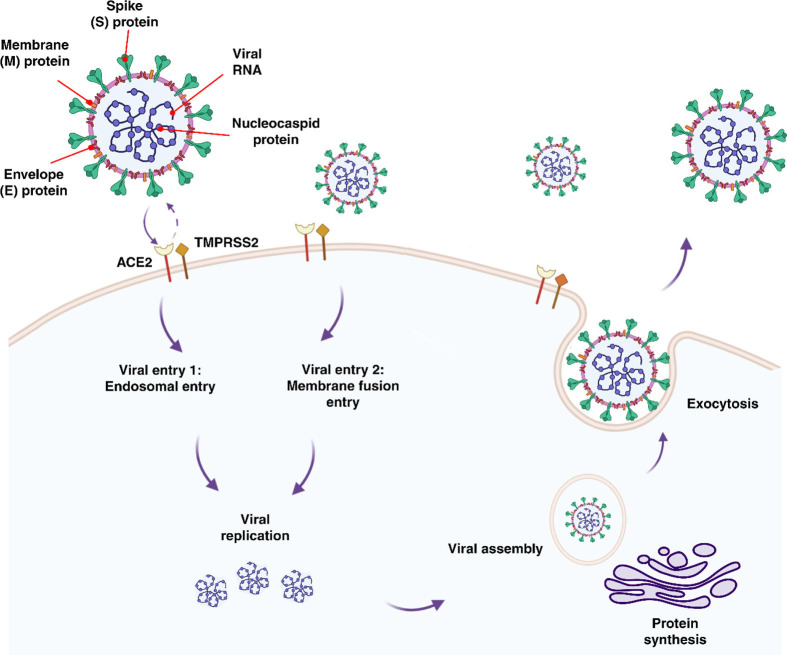
SARS-CoV-2 enters the body through the nasal passage and infects pulmonary cells by binding to the receptor angiotensin-converting enzyme 2 (ACE2) (31, 53–55). It has been found that ACE2 expresses in respiratory and intestinal track, placenta, ovaries, vagina, and uterus (56). Cell entry is further facilitated by viral spike (S) protein priming induced by trans-membrane serine protease 2 (TMPRSS2) (53–55). Cells co-expressing both ACE2 and TMPRSS2 have been found to have a higher susceptibility to SARS-CoV-2 entry (57) (Figure 1). In addition, furin, trypsin, and cathepsins B and L have been reported to be capable of cleaving the spike glycoprotein binding at the S1/S2 site, allowing the virus to enter (53, 58, 59). ACE2 has been shown to be expressed by fetal kidney, ilium, and rectal cells from as early as 15 weeks, barely detectable at 15 weeks in the lungs with undetected expression thereafter, and undetectable in the cerebral ependymal, parenchymal and cardiac cells (60). It has been found that only a proportion of cells which are located in the fetal adrenal gland and the kidney co-expressed ACE2 and TMPRSS2. It was discovered that placental cytotrophoblasts and syncytiotrophoblasts (STBs) express ACE2 from 7 weeks onward, suggesting that SARS-CoV-2 could cross into the placenta at any gestational age (60). Investigation of ACE2 and TMPRSS2 co-expression in the developing embryo up to day 14 (from surplus IVF human embryos) has revealed the co-localization of these genes, raising concern to increased susceptibility to SARS-CoV-2 fetal infection in the early stages of embryonic development (61). To date, cohort studies of SARS-CoV-2 positive mothers with mild symptoms or asymptomatic, have reported no adverse effects to the mother or neonate regardless of the timing of the infection (i.e. first versus third trimester) (62, 63). However, women with severe SARS-CoV-2 infection that required critical care had higher odds of complications, particularly a higher incidence of iatrogenic pre-term delivery mostly due to fear of sudden maternal decompensation (64).
Figure 1.
Features, entry methods, and replication of SARS-CoV-2.
Transplacental Viral Transmission
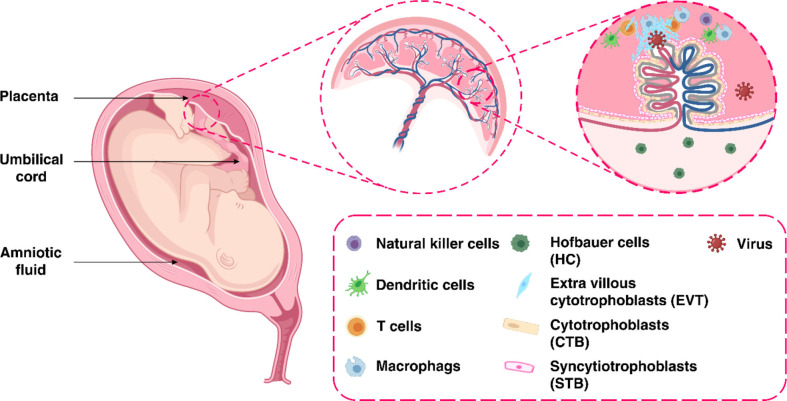
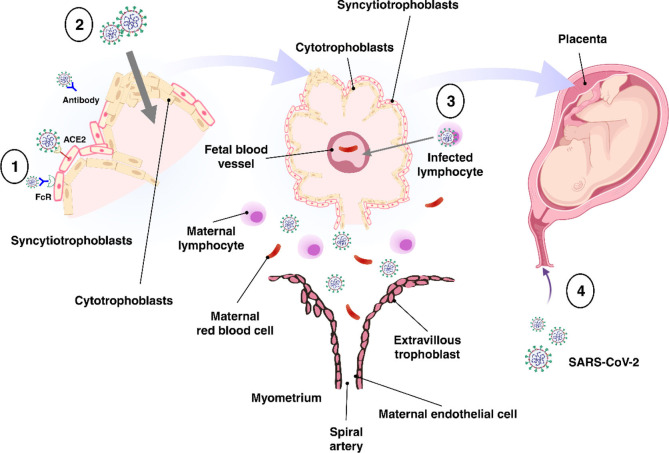
The placenta offers a protective barrier that does not allow the fetus to become exposed to maternal infections (31). The human placenta primarily consists of a number of specific fetal-derived cells called trophoblasts, of which there are three main types. These include terminally differentiated multinuclear syncytiotrophoblast cells, which are in direct contact with the maternal blood and line the villus tree, progenitor villous cytotrophoblast cells, which underlie the syncytiotrophoblast, and invasive extravillous trophoblast (EVT) cells, which anchor the chorionic villi to the uterus and modify its vasculature (Figure 2) (31). Various potential causes may play a role in the vertical transmission of the virus from the mother to the fetus. These include direct damage to the villous tree with a break in the protective syncytiotrophoblast layer, which could be caused by virus-induced apoptosis and vascular damage in the placenta, spread through the virus-infected maternal endothelium to the extravillous trophoblast, trafficking of infected maternal immune cells throughout the syncytiotrophoblast, paracellular or transcellular transport (for example, immunoglobulin-mediated transcytosis) into fetal capillaries, transmission via swallowed or aspirated amniotic fluid (65, 66), as well as ascending infection from the vagina (Figure 3) (31). To define the possibility of vertical transmission of SARS-CoV-2 infection in different studies, a classification system has been proposed by a multidisciplinary team of the WHO (68). Given the timing of vertical transmission, in utero, intrapartum, and early postnatal period, four possibilities exist: confirmed, possible, unlikely, and indeterminate (68). Vertical transmission is considered “possible” if evidence suggests it but cannot confirm infection. However, if there is poor support of diagnosis, but vertical transmission cannot be completely ruled out, this is considered as “unlikely”. The “indeterminate” possibility is when the tests required to define the classification have not been performed (68). Recent findings confirming the presence of SARS-CoV-2 mRNA or virions in syncytiotrophoblasts have strongly suggested transplacental infection caused by the SARS-CoV-2 (69, 70). Nonetheless, given that the presence of SARS-CoV-2 in the blood sample of COVID-19 patients is reported to be around 1%, therefore the likelihood of SARS-CoV-2 being able to directly infect syncytiotrophoblasts is low (71). Another alternative way of transmitting SARS-CoV-2 infection to the neonate is through the vagina during childbirth (72, 73).
Figure 2.
The maternal-fetal interface.
Figure 3.
Possible mechanisms of transplacental transmission. There are several potential mechanisms involved in the virus’s vertical transmission from mother to fetus. (1) Infection caused by direct villous tree damage. (2) Infection through the maternal endothelium to the extravillous trophoblast. (3) Infection caused by maternal immune cell trafficking and transcellular transport. (4) Infection through the vagina. Adapted from (67).
Whilst the possibility of transmitting SARS-CoV-2 from mother to fetus during pregnancy is suggested, the role of the placenta in infection with the virus has not yet been fully understood. However, evidence suggests that pathogens can overcome this barrier, infect the fetus, and even cause serious complications in newborns, such as microcephaly and ocular abnormalities (74). Such pathogens include Cytomegalovirus (CMV), herpes simplex virus (HSV), varicella-zoster virus, and Zika virus (ZIKV) (20, 75–77). It is currently unclear whether neonates who tested positive for SARS-CoV-2 have been infected with the virus from their mothers during pregnancy or have been infected during labor or after birth. (Table 1). Evidence based on infant antibody tests suggests vertical transmission of the virus may be possible. It was discovered that infants born to women infected with SARS-CoV-2 had higher immunoglobulin (Ig)G and IgM levels for SARS-CoV-2 (31, 89, 90). The presence of IgG in the fetus may indicate the transfer of this immunoglobulin from the mother to the fetus during pregnancy, but the presence of IgM indicates that the fetus has produced and secreted this immunoglobulin in response to viral infection because in contrast to IgG, IgM is unable to cross the placenta due to its higher molecular weight (89, 90).
Table 1.
Systematic review and meta-analysis studies on COVID-19 infection during pregnancy.
| Publication name | Number of pregnant women with COVID-19 | Findings | Conclusion |
|---|---|---|---|
| Vertical transmission of coronavirus disease 2019: a systematic review and meta-analysis (78) | NM |
|
|
| Clinical outcomes of 201 neonates born to mothers with COVID-19: a systematic review (79) | 223 |
|
|
| Maternal clinical characteristics and perinatal outcomes among pregnant women with coronavirus disease 2019. A systematic review (80) | 322 |
|
|
| Clinical characteristics and outcomes of pregnant women with COVID-19 and the risk of vertical transmission: a systematic review (81) | 230 |
|
|
| Clinical characteristics and outcomes of pregnant women with COVID-19 and comparison with control patients: A systematic review and meta-analysis (82) | 10,000 |
|
|
| Clinical Characteristics and Neonatal Outcomes of Pregnant Patients With COVID-19: A Systematic Review (83) | 235 |
|
|
| Pregnancy and Breastfeeding During COVID-19 Pandemic: A Systematic Review of Published Pregnancy Cases (84) | 3,985 |
|
|
| COVID-19 (SARS-CoV-2) Infection in Pregnancy: A Systematic Review (85) | 156 |
|
|
| Maternal and perinatal outcomes with COVID-19: A systematic review of 108 pregnancies (17) | 108 |
|
|
| COVID-19 in Pregnant Women and Neonates: A Systematic Review of the Literature with Quality Assessment of the Studies (86) | 275 |
|
|
| Maternal Coronavirus Infections and Neonates Born to Mothers with SARS-CoV-2: A Systematic Review (87) | 1457 |
|
|
| Vertical transmission of SARS CoV-2: a systematic review (88) | 714 |
|
|
NM, not mentioned; SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus 2; COVID-19, coronavirus disease 2019.
Biomarkers of SARS-CoV-2 Infection
Several studies have employed single cell RNA sequencing (scRNA-seq) to gain an understanding of the molecular features of SARS-CoV-2 infection (91–95). In a study by Lu et al., which compared ACE2 and TMPRSS2 gene expression between fetal, placental tissues and adult tissues, a small proportion of trophoblast cells, as well as various fetal organs such as the heart, kidney, stomach, and adrenal glands, had ACE2 expression. The study showed that only the kidney and adrenal gland expressed TMPRSS2 (96). Pique-Regi et al. discovered that very few cells during any of the three trimesters expressed both ACE2 and TMPRSS2. Using single-nuclear RNAseq (snRNA-seq), it has been shown that the placenta is unlikely to express ACE2 and TMPRSS2, and thus be infected by SARS-CoV-2 (59). Using scRNA-seq data, Ashary et al., identified only a small proportion of STB in the first trimester and EVT in the second trimester had ACE2 and TMPRSS2 expression. The ACE2+TMPRSS2+STBs were highly differentiated and expressed genes engaged in mitochondrial metabolism and glucose transport. In addition, the ACE2+TMPRSS2+EVTs were found to have endovascular trophoblast markers. The researchers found that these cells could be the targets of SARS-CoV-2 entry (97). Moreover, robust immune responses at the maternal-fetal interface of SARS-CoV-2-infected women was discovered (98). Researchers found overexpression of interferon-related genes, and increased activation of NK cells and T cells (98–100). Also, it was found that there was an association between SARS-CoV-2 infection and local immune responses at the maternal-fetal interface (98). in a study by Nagy et al, the impact of mutations in SARS-CoV-2 viral genes on clinical outcomes was explored. The study found that mutations in the nucleocapsid phosphoprotein-N, nonstructural proteins-4 (NSP4), NSP6, Open Reading Frame-3a (ORF3a), and ORF8 were associated with mild outcome, while mutations in NSP7 were linked to severe disease (101).
The identification of new biomarkers and prevention strategies requires the fundamental understanding and control of how SARS-CoV-2 spreads to the lungs and elicits a multi-organ inflammatory response. (Table 2). These infection processes rely on their location and spatial context: which cells in which tissue locations are most susceptible to infection (105), infected cell-to-uninfected cell associations, and biochemical factor release of different cell types in response to infection (106). These spatiotemporal relationships in the inflammatory cascade give rise to positive or negative prognoses, and their understanding can triage patients at greater or lesser risks of infection, of response to infection, and inform new therapeutics and treatment regimens (107). Spatial immunoprofiling is rapidly advancing due to several recent technologies: advanced instrumentation, molecular barcoding and immunolabelling, providing a much richer portrait of the immune landscape (108), and recent approaches in biostatics and theoretical biology are incorporating imaging data to deconstruct the relationships between cells and disease within their tissue context (109–111). Spatial resolved transcriptomics are changing the ways in which we interrogate complex tissues and were voted the ‘Method of the Year 2020’ by the journal Nature Methods (112). These technologies combine the benefits in advancements in microscopy and advanced imaging, with simultaneous read out of transcript and proteomic data, thereby alleviating the challenges associated with single cell or bulk profiling. The maintenance of spatial context is key in understanding the underlying cellular profiles, biology, specialization and tissue organization and has begun shedding light into consortia studies such as the Human Cell Atlas. A number of technologies currently exist for RNA applications: Nanostring GeoMX Digital Spatial Profiler (DSP), 10x Genomics Visium, MERFISH and proteomic: Nanostring GeoMX DSP, Akoya Biosciences CODEX, Imaging Mass spectrometry (IMC) (113). Recent application of these methodologies to COVID-19 autopsy tissue studies from lungs, kidney, liver and heart tissue has provided deep insights into cell types and genes implicated with severe COVID-19 disease severity (114).
Table 2.
Potential biomarkers of disease severity in COVID-19.
| Analytes | Changes | Role | Ref |
|---|---|---|---|
| IL-1, IL-2, IL-6, TNF-a, G-CSF, GM-CSF, IFN-γ | Increase | Cytokine storm biomarker | (102) |
| CD3+, CD4+, CD8+, B cells, NK cells | Decrease | Clinical Hematological biomarker | (103) |
| CK, CK-MB, CRP, Ferritin, LDH, BUN, Creatinine, cTnI, AST, ALT, Total bilirubin | Increase | Clinical Biochemical biomarker | (104) |
IL, interleukin; TNF-a, tumor necrosis factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; G-CSF, granulocyte colony-stimulating factor; IFN-γ, interferon gamma; NK, natural killer; CK, creatine kinase; LDH, lactate dehydrogenase; BUN, blood urea nitrogen; cTnI, cardiac troponin I; AST, aspartate aminotransferase; ALT, alanine aminotransferase.
Once region- or cell-specific spatial information is derived from histology sections, statistical relationships between cells and tissues and mathematical predictions of their future behavior with or without treatment are often sought. There exist numerous tools to detect and segment single cell locations from this spatial information. While open-source ImageJ, developed in 1987, remains popular for microscopic image analysis (115–117), more recent software such as CellProfiler, Icy, ilastik, and QuPath provide user-friendly interfaces for the development of bioimage analysis macroscripts (118, 119). Once single-cell data can be derived, spatial relationships can be determined. The most common of which is intercellular clustering or associations, often calculated as cell density within concentric circles away from each cell’s center and averaged across all imaged cells (120). For instance, to characterize the distribution of SARS-CoV-2 bodies from macrophages or monocytes or tissue structures to estimate inflammatory progression (121).
Concluding Remarks and Future Perspectives
Taking into account the changing physiology and immune responses during gestation, pregnant women are more susceptible to developing severe COVID-19, which can lead to pregnancy-related complications. There is limited information for the association of COVID-19 and its direct complications to the growing fetus during pregnancy. These may include preterm birth, stillbirth, or long-term complications for the newborn (122). A study conducted on 827 pregnant women, who have been given the COVID-19 mRNA vaccine, found that the proportion of adverse pregnancy and neonatal outcomes were similar to incidence reported in similar studies conducted prior to the pandemic (123). Furthermore, vaccination of pregnant women has been shown to result in maternal IgG production 5 days after the first dose of vaccination, as well as the transplacental transfer of IgG 16 days after the first dose of vaccination (124). However, longitudinal follow-up is needed to monitor those who are vaccinated, especially during the first trimester, in order to be informed about maternal, pregnancy, and neonatal outcomes. Another important consideration with COVID-19 infection during pregnancy is that current diagnostic tests such as X-ray and CT scans cannot be performed in pregnant women due to potential risks to the growing fetus (125). These factors may therefore delay the diagnosis and treatment of pregnant women, particularly those with more severe symptoms.
These factors may therefore delay the diagnosis and treatment of pregnant women, particularly those with more severe symptoms. Screening tests may be helpful in this respect because of the possibility of transmitting the virus from the mother to the fetus. Understanding the disease progression and its relationship to manifestation severity is necessary to therapeutically intervene and reduce the associated morbidity.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
Funding
This project is supported by the Queensland University of Technology (QUT) ECR grant to AK, MA, NS, and JR. NS is supported by a US Department of Defense Prostate Cancer Early Investigator Award Fellowship (PC190533). MA is supported by an Advance Queensland Fellowship (AQIRF1312018). FSFG is funded by a UQ Diamantina Institute laboratory start-up package, Australian and New Zealand Sarcoma Association – Sarcoma Research Grant, a priority-driven collaborative cancer research scheme grant co-funded by Cancer Australia and Cure Cancer (#1158085), and a US Department of Defense – Breast Cancer Research Program – breakthrough award level 1 (#BC200025). AK is supported by an NHMRC Fellowship (APP1157741) and Cure Cancer (APP1182179).
Conflict of Interest
FSFG is a consultant and has a funded research agreement with Biotheus Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Senthil R, Kunchithapathan B, Ramalingam S, Manivannan P. COVID-19 Awareness and Its Impact in Rural and Urban Puducherry–a Community Based Cross Sectional Study. J Evol Med Dent Sci (2020) 9(51):3862–8. doi: 10.14260/jemds/2020/847 [DOI] [Google Scholar]
- 2. Knight M, Bunch K, Vousden N, Morris E, Simpson N, Gale C, et al. Characteristics and Outcomes of Pregnant Women Admitted to Hospital With Confirmed SARS-Cov-2 Infection in UK: National Population Based Cohort Study. BMJ (2020) 8:369. doi: 10.1136/bmj.m2107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zambrano LD, Ellington S, Strid P, Galang RR, Oduyebo T, Tong VT, et al. Update: Characteristics of Symptomatic Women of Reproductive Age With Laboratory-Confirmed SARS-Cov-2 Infection by Pregnancy Status—United States, January 22–October 3, 2020. MMWR Morb Mortal Wkly Rep 69(44):1641–7. doi: 10.15585/mmwr.mm6944e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Delahoy MJ, Whitaker M, O'Halloran A, Chai SJ, Kirley PD, Alden N, et al. Characteristics and Maternal and Birth Outcomes of Hospitalized Pregnant Women With Laboratory-Confirmed COVID-19—COVID-NET, 13 States, March 1–August 22, 2020. MMWR Morb Mortal Wkly Rep (2020) 69(38)1347–54. doi: 10.15585/mmwr.mm6938e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Panagiotakopoulos L, Myers TR, Gee J, Lipkind HS, Kharbanda EO, Ryan DS, et al. SARS-Cov-2 Infection Among Hospitalized Pregnant Women: Reasons for Admission and Pregnancy Characteristics—Eight US Health Care Centers, March 1–May 30, 2020. MMWR Morb Mortal Wkly Rep 69. (2020) 69(38):1355–9. doi: 10.15585/mmwr.mm6938e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Khalil A, von Dadelszen P, Draycott T, Ugwumadu A, O'Brien P, Magee L, et al. Change in the Incidence of Stillbirth and Preterm Delivery During the COVID-19 Pandemic. (2020) 324(7):705–6. doi: 10.1001/jama.2020.12746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Takemoto ML, Menezes MO, Andreucci CB, Nakamura-Pereira M, Amorim MMR, Katz L, et al. The Tragedy of COVID-19 in Brazil: 124 Maternal Deaths and Counting. (2020) 151(1):154–6. doi: 10.1002/ijgo.13300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. BMJ AIIJ. ANZIC Influenza Investigators and Australasian Maternity Outcomes Surveillance System Critical Illness Due to 2009 a/H1N1 Influenza in Pregnant and Postpartum Women: Population Based Cohort Study. BMJ (2010) 340:c1279. doi: 10.1136/bmj.c1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jamiesan D, Honein M, Rasmussen S, Williams JL, Swerdlow DL, Biggerstaff MS, et al. Novel Influenza A (H1N1) Pregnancy Working Group. H1N1 2009 Influenza Virus Infection During Pregnancy in the USA. Lancet (2009) 374(9688):451–8. doi: 10.1016/S0140-6736(09)61304-0 [DOI] [PubMed] [Google Scholar]
- 10. Louie J, Acosta M, Jamieson DJ, Honein MA. California Pandemic (H1n1) Working Group. N Engl J Med (2010) 362(1):27–35. doi: 10.1056/NEJMoa0910444 [DOI] [PubMed] [Google Scholar]
- 11. Siston AM, Rasmussen SA, Honein MA, Fry AM, Seib K, Callaghan WM, et al. Pandemic 2009 Influenza a (H1N1) Virus Illness Among Pregnant Women in the United States. JAMA (2010) 303(15):1517–25. doi: 10.1001/jama.2010.479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yamashita M, Yamate M, Li GM, Ikuta K. Susceptibility of Human and Rat Neural Cell Lines to Infection by SARS-Coronavirus. Biochem Biophys Res Commun (2005) 334(1):79–85. doi: 10.1016/j.bbrc.2005.06.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mullins E, Evans D, Viner RM, O'Brien P, Morris E. Coronavirus in Pregnancy and Delivery: Rapid Review. Ultrasound Obstet Gynecol (2020) 55(5):586–92. doi: 10.1101/2020.03.06.20032144 [DOI] [PubMed] [Google Scholar]
- 14. Alfaraj SH, Al-Tawfiq JA, Memish ZA. Middle East Respiratory Syndrome Coronavirus (MERS-CoV) Infection During Pregnancy: Report of Two Cases & Review of the Literature. J Microbiol Immunol Infect (2019) 52(3):501–3. doi: 10.1007/978-3-319-74365-3_49-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gajbhiye RK, Modi DN, Mahale SD. Pregnancy Outcomes, Newborn Complications and Maternal-Fetal Transmission of SARS-CoV-2 in Women With COVID-19: A Systematic Review of 441 Cases. MedRxiv (2020). doi: 10.1101/2020.04.11.20062356 [DOI] [Google Scholar]
- 16. Schwartz DA, Dhaliwal A. Infections in Pregnancy With COVID-19 and Other Respiratory RNA Virus Diseases are Rarely, If Ever, Transmitted to the Fetus: Experiences With Coronaviruses, Parainfluenza, Metapneumovirus Respiratory Syncytial Virus, and Influenza. Arch Pathol Lab Med (2020) 144(8):920–8. doi: 10.5858/arpa.2020-0211-SA [DOI] [PubMed] [Google Scholar]
- 17. Zaigham M, Andersson O. Maternal and Perinatal Outcomes With COVID-19: A Systematic Review of 108 Pregnancies. Acta obstetricia gynecologica Scandinavica (2020) 99(7):823–9. doi: 10.1111/aogs.13867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Alzamora MC, Paredes T, Caceres D, Webb CM, Valdez LM, La Rosa M. Severe COVID-19 During Pregnancy and Possible Vertical Transmission. (2020) 37(8):861–865. doi: 10.1055/s-0040-1710050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Robbins JR, Bakardjiev AI. Pathogens and the Placental Fortress. Curr Opin Microbiol (2012) 15(1):36–43. doi: 10.1016/j.mib.2011.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Auriti C, De Rose DU, Santisi A, Martini L, Piersigilli F, Bersani I, et al. Pregnancy and Viral Infections: Mechanisms of Fetal Damage, Diagnosis and Prevention of Neonatal Adverse Outcomes From Cytomegalovirus to SARS-CoV-2 and Zika Virus. Biochim Biophys Acta (BBA)-Molecular Basis Dis (2021) 1867(10):166198. doi: 10.1016/j.bbadis.2021.166198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jamieson DJ, Theiler RN, Rasmussen SA. Emerging Infections and Pregnancy. Emerg Infect Dis (2006) 12(11):1638. doi: 10.3201/eid1211.060152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schjenken JE, Tolosa JM, Paul JW, Clifton VL, Smith R. Mechanisms of Maternal Immune Tolerance During Pregnancy. (2012) 11:211–42. [Google Scholar]
- 23. Silasi M, Cardenas I, Kwon JY, Racicot K, Aldo P, Mor G. Viral Infections During Pregnancy. (2015) 73(3):199–213. doi: 10.1111/aji.12355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Khosroshahi LM, Rokni M, Mokhtari T, Noorbakhsh F. Immunology, Immunopathogenesis and Immunotherapeutics of COVID-19; an Overview. Int Immunopharmacol (2021) 93:107364. doi: 10.1016/j.intimp.2020.107364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical Features of Patients Infected With 2019 Novel Coronavirus in Wuhan, China. Lancet (2020) 395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu J, Li S, Liu J, Liang B, Wang X, Wang H, et al. Longitudinal Characteristics of Lymphocyte Responses and Cytokine Profiles in the Peripheral Blood of SARS-CoV-2 Infected Patients. EBioMedicine (2020) 55:102763. doi: 10.1016/j.ebiom.2020.102763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen G, Wu D, Guo W, Cao y, Huang D, Wang H, et al. Clinical and Immunological Features of Severe and Moderate Coronavirus Disease 2019. J Clin Invest (2020) 130(5):2620–9. doi: 10.1172/JCI137244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zheng M, Gao Y, Wang G, Song G, Liu S, Sun D, et al. Functional Exhaustion of Antiviral Lymphocytes in COVID-19 Patients. Cell Mol Immunol (2020) 17(5):533–5. doi: 10.1038/s41423-020-0402-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Giamarellos-Bourboulis EJ, Netea MG, Rovina N, Akinosoglou K, Antoniadou A, Antonakos N, et al. Complex Immune Dysregulation in COVID-19 Patients With Severe Respiratory Failure. Cell Host Microbe (2020) 27(6):992–1000. e3. doi: 10.1016/j.chom.2020.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Khosroshahi LM, Rezaei N. Dysregulation of the Immune Response in Coronavirus Disease 2019. Cell Biol Int (2021) 45(4):702–7. doi: 10.1002/cbin.11517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wastnedge EAN, Reynolds RM, van Boeckel SR, Stock SJ, Denison FC, Maybin JA, et al. Pregnancy and COVID-19. Physiol Rev (2021) 101(1):303–18. doi: 10.1152/physrev.00024.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Björkström NK, Strunz B, Ljunggren H-G. Natural Killer Cells in Antiviral Immunity. Nat Rev Immunol (2021) p:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chua RL, Lukassen S, Trump S, Hennig BP, Wendisch D, Pott F, et al. COVID-19 Severity Correlates With Airway Epithelium–Immune Cell Interactions Identified by Single-Cell Analysis. Nat Biotechnol (2020) 38(8):970–9. doi: 10.1038/s41587-020-0602-4 [DOI] [PubMed] [Google Scholar]
- 34. Mathew D, Giles JR, Baxter AE, Oldridge DA, Greenplate AR, Wu JE, et al. Deep Immune Profiling of COVID-19 Patients Reveals Distinct Immunotypes With Therapeutic Implications. Science (2020) 369(6508). doi: 10.1126/science.abc8511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liao M, Liu Y, Yuan J, Wen Y, Xu G, Zhao J, et al. Single-Cell Landscape of Bronchoalveolar Immune Cells in Patients With COVID-19. Nat Med (2020) 26(6):842–4. doi: 10.1038/s41591-020-0901-9 [DOI] [PubMed] [Google Scholar]
- 36. Wichmann D, Sperhake JP, Lütgehetmann M, Steurer S, Edler C, Heinemann A, et al. Autopsy Findings and Venous Thromboembolism in Patients With COVID-19: A Prospective Cohort Study. Ann Internal Med (2020) 173(4):268–77. doi: 10.7326/L20-1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Piccinni M-P, Romagnani S. Regulation of Fetal Allograft Survival by Hormone-Controlled Th1-and Th2-Type Cytokines. Immunol Res (1996) 15(2):141–50. doi: 10.1007/BF02918503 [DOI] [PubMed] [Google Scholar]
- 38. Muyayalo KP, Huang DH, Zhao SJ, Xie T, Mor G, Liao AH. COVID-19 and Treg/Th17 Imbalance: Potential Relationship to Pregnancy Outcomes. Am J Reprod Immunol (2020) 84(5):e13304. doi: 10.1111/aji.13304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, et al. Dysregulation of Immune Response in Patients With COVID-19 in Wuhan, China; Clinical Infectious Diseases; Oxford Academic. Clin Infect Dis (2020) 71(15):762–768. doi: 10.1093/cid/ciaa248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Halyabar O, Chang MH, Schoettler ML, Schwartz MA, Baris EH, Benson LA, et al. Calm in the Midst of Cytokine Storm: A Collaborative Approach to the Diagnosis and Treatment of Hemophagocytic Lymphohistiocytosis and Macrophage Activation Syndrome. Pediatr Rheumatol (2019) 17(1):1–12. doi: 10.1186/s12969-019-0309-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Henderson LA, Canna SW, Schulert GS, Volpi S, Lee PY, Kernan KF, et al. On the Alert for Cytokine Storm: Immunopathology in COVID-19. Arthritis Rheumatol (2020) 72(7):1059–63. doi: 10.1002/art.41285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Soy M, Atagündüz P, Atagündüz I, Sucak GT. Hemophagocytic Lymphohistiocytosis: A Review Inspired by the COVID-19 Pandemic. Rheumatol Int (2021) 41(1):7–18. doi: 10.1007/s00296-020-04636-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tisoncik JR, Korth MJ, Simmons CP, Farrar J, Martin TR, Katze MG. Into the Eye of the Cytokine Storm. Microbiol Mol Biol Rev (2012) 76(1):16–32. doi: 10.1128/MMBR.05015-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schulert GS, Zhang M, Fall N, Husami A, Kissell D, Hanosh A, et al. Whole-Exome Sequencing Reveals Mutations in Genes Linked to Hemophagocytic Lymphohistiocytosis and Macrophage Activation Syndrome in Fatal Cases of H1N1 Influenza. J Infect Dis (2016) 213(7):1180–8. doi: 10.1093/infdis/jiv550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Clark KEN, Nevin WD, Mahungu T, Lachmann H, Singh A. Assessment of the Hemophagocytic Lymphohistiocytosis Hscore in Patients With Coronavirus Disease 2019. Clin Infect Dis (2020) 28:ciaa1463. doi: 10.1093/cid/ciaa1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ruscitti P, Bruno F, Berardicurti O, Acanfora C, Pavlych V, Palumbo P, et al. Lung Involvement in Macrophage Activation Syndrome and Severe COVID-19: Results From a Cross-Sectional Study to Assess Clinical, Laboratory and Artificial Intelligence–Radiological Differences. Ann rheumatic Dis (2020) 79(9):1152–5. doi: 10.1136/annrheumdis-2020-218048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wood H, Jones JR, Hui K, Mare T, Pirani T, Galloway J, et al. Secondary HLH is Uncommon in Severe COVID-19. Br J Haematol (2020) 190(5):e283–e285. doi: 10.1111/bjh.16934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yao X, Li TY, He ZC, Ping YF, Liu HW, Yu SC, et al. A Pathological Report of Three COVID-19 Cases by Minimally Invasive Autopsies. Zhonghua bing li xue za zhi= Chin J Pathol (2020) 49:E009–9. doi: 10.3760/cma.j.cn112151-20200312-00193 [DOI] [PubMed] [Google Scholar]
- 49. Liao M, Liu Y, Yuan J, Wen Y, Xu G, Zhao J. The Landscape of Lung Bronchoalveolar Immune Cells in COVID-19 Revealed by Single-Cell RNA Sequencing. MedRxiv (2020). doi: 10.1101/2020.02.23.20026690 [DOI] [Google Scholar]
- 50. Amirchaghmaghi E, Taghavi SA, Shapouri F, Saeidi S, Rezaei A, Aflatoonian R. The Role of Toll Like Receptors in Pregnancy. (2013) 7(3):147. [PMC free article] [PubMed] [Google Scholar]
- 51. Young BC, Stanic AK, Panda B, Rueda BR, Panda A. Longitudinal Expression of Toll-Like Receptors on Dendritic Cells in Uncomplicated Pregnancy and Postpartum. (2014) 210(5):445.e1–445.e6. doi: 10.1016/j.ajog.2013.11.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Firmal P, Shah VK, Chattopadhyay S. Insight Into TLR4-Mediated Immunomodulation in Normal Pregnancy and Related Disorders. Front Immunol (2020) 11:807. doi: 10.3389/fimmu.2020.00807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. (2020) 181(2):271–80.e8. doi: 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cascella M, Rajnik M, Aleem A, Dulebohn SC, Di Napoli R. Features, Evaluation and Treatment Coronavirus (COVID-19). In: StatPearls Treasure Island (FL):StatPearls Publishing. (2021). [PubMed] [Google Scholar]
- 55. Tay MZ, Poh CM, Ré nia L, MacAry PA, Ng LFP. The Trinity of COVID-19: Immunity, Inflammation and Intervention. Nat Rev Immunol (2020) 20 (6):363–74. doi: 10.1038/s41577-020-0311-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Auriti C, De Rose DU, Tzialla C, Caforio L, Ciccia M, Manzoni P, et al. Vertical Transmission of SARS-CoV-2 (COVID-19): Are Hypotheses More Than Evidences? Am J Perinatol (2020) 37(S02):S31–8. doi: 10.1055/s-0040-1714346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rabi FA, Al Zoubi MS, Kasasbeh GA, Salameh DM, Al-Nasser AD. SARS-CoV-2 and Coronavirus Disease 2019: What We Know So Far. Pathogens (2020) 9(3):231. doi: 10.3390/pathogens9030231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Jaimes JA, Millet JK, Whittaker GRJI. Proteolytic Cleavage of the SARS-CoV-2 Spike Protein and the Role of the Novel S1/S2 Site. iScience (2020) 23 (6):101212. doi: 10.1016/j.isci.2020.101212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Pique-Regi R, Romero R, Tarca AL, Luca F, Xu Y, Alazizi A, et al. Does the Human Placenta Express the Canonical Cell Entry Mediators for SARS-CoV-2? Elife (2020) 9:e58716. doi: 10.7554/eLife.58716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Faure-Bardon V, Isnard P, Roux N, Leruez-Ville M, Molina T, Bessieres B, et al. Protein Expression of Angiotensin-Converting Enzyme 2, a SARS-CoV-2-Specific Receptor, in Fetal and Placental Tissues Throughout Gestation: New Insight for Perinatal Counseling. Ultrasound Obstetrics Gynecol (2021) 57(2):242–7. doi: 10.1002/uog.22178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Weatherbee BA, Glover DM, Zernicka-Goetz M. Expression of SARS-CoV-2 Receptor ACE2 and the Protease TMPRSS2 Suggests Susceptibility of the Human Embryo in the First Trimester. Open Biol (2020) 10(8):200162. doi: 10.1098/rsob.200162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cosma S, Carosso AR, Cusato J, Borella F, Carosso M, Bovetti M, et al. Coronavirus Disease 2019 and First-Trimester Spontaneous Abortion: A Case-Control Study of 225 Pregnant Patients. Am J Obstetrics Gynecol (2021) 224(4):391.e1–e7. doi: 10.1016/j.ajog.2020.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. la Cour Freiesleben N, Egerup P, Hviid KVR, Severinsen ER, Kolte AM, Westergaard D, et al. SARS-CoV-2 in First Trimester Pregnancy: A Cohort Study. Hum Reprod (2021) 36(1):40–7. doi: 10.1093/humrep/deaa311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Khalil A, Kalafat E, Benlioglu C, O'Brien P, Morris E, Draycott T, et al. SARS-CoV-2 Infection in Pregnancy: A Systematic Review and Meta-Analysis of Clinical Features and Pregnancy Outcomes. EClinicalMedicine (2020) 25:100446. doi: 10.1016/j.eclinm.2020.100446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Blumberg DA, Underwood MA, Hedriana HL, Lakshminrusimha S. Vertical Transmission of SARS-CoV-2: What is the Optimal Definition? Am J Perinatol (2020) 37(08):769–72. doi: 10.1055/s-0040-1712457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zamaniyan M, Ebadi A, Aghajanpoor S, Rahmani Z, Haghshenas M, Azizi S. Preterm Delivery in Pregnant Woman With Critical COVID-19 Pneumonia and Vertical Transmission. Prenat Diagn (2020) 40(13):1759–1761. doi: 10.1002/pd.5713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Komine-Aizawa S, Takada K, Hayakawa S. Placental barrier against COVID-19. Placenta (2020) 99:45–9. doi: 10.1016/j.placenta.2020.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Auriti C, De Rose DU, Mondì V, Stolfi I, Tzialla C. Neonatal SARS-CoV-2 Infection: Practical Tips. Pathogens (2021) 10(5):611. doi: 10.3390/pathogens10050611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Patanè L, Morotti D, Giunta MR, Sigismondi C, Piccoli MG, Frigerio L, et al. Vertical Transmission of COVID-19: SARS-CoV-2 RNA on the Fetal Side of the Placenta in Pregnancies With COVID-19 Positive Mothers and Neonates at Birth. Am J Obstet Gynecol (2020) 2(3):100145. doi: 10.1016/j.ajogmf.2020.100145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Algarroba G, Rekawek P, Vahanian SA. Visualization of SARS-CoV-2 Virus Invading the Human Placenta Using Electron Microscopy. Am J Obstet Gynecol (2020) 223(2):275–78. doi: 10.1016/j.ajog.2020.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, et al. Detection of SARS-CoV-2 in Different Types of Clinical Specimens. JAMA (2020) 323(18):1843–4. doi: 10.1001/jama.2020.3786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Juan J, Gil MM, Rong Z, Zhang Y, Yang H, Poon LC. Effect of Coronavirus Disease 2019 (COVID-19) on Maternal, Perinatal and Neonatal Outcome: Systematic Review. J Obstet Gynecol (2020) 56(1):15–27. doi: 10.1002/uog.22088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Yang Z, Liu Y. Vertical Transmission of Severe Acute Respiratory Syndrome Coronavirus 2: A Systematic Review. Am J Perinatol (2020) 37(10):1055. doi: 10.1055/s-0040-1712161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Coyne CB, Lazear HM. Zika Virus—Reigniting the TORCH. Nat Rev Microbio (2016) 14(11):707–15. doi: 10.1038/nrmicro.2016.125 [DOI] [PubMed] [Google Scholar]
- 75. Stegmann B, Carey JC. TORCH Infections. Toxoplasmosis, Other (Syphilis, Varicella-Zoster, Parvovirus B19), Rubella, Cytomegalovirus (CMV), and Herpes Infections. Curr Womens Health Rep (2002) 2(4):253–8. [PubMed] [Google Scholar]
- 76. Arvin AM, Moffat JF, Sommer M, Oliver S, Che X, Vleck S, et al. Varicella-Zoster Virus T Cell Tropism and the Pathogenesis of Skin Infection. Curr Top Microbiol Immunol (2010) 342:189–209. doi: 10.1007/82_2010_29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Pereira L. Congenital Viral Infection: Traversing the Uterine-Placental Interface. Annu Rev Virol (2018) 5(1):273–99. doi: 10.1146/annurev-virology-092917-043236 [DOI] [PubMed] [Google Scholar]
- 78. Kotlyar AM, Grechukhina O, Chen A, Popkhadze S, Grimshaw A, Tal O, et al. Vertical Transmission of Coronavirus Disease 2019: A Systematic Review and Meta-Analysis. Am J Obstet Gynecol (2021) 224(1):35–53.e3. doi: 10.1016/j.ajog.2020.07.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Yoon SH, Kang JM, Ahn JG. Clinical Outcomes of 201 Neonates Born to Mothers With COVID-19: A Systematic Review. Eur Rev Med Pharmacol Sci (2020) 24(14):7804–15. doi: 10.26355/eurrev_202007_22285 [DOI] [PubMed] [Google Scholar]
- 80. Novoa RH, Quintana W, Llancarí P, Urbina-Quispe K, Guevara-Ríos E, Ventura W. Maternal Clinical Characteristics and Perinatal Outcomes Among Pregnant Women With Coronavirus Disease 2019. A Systematic Review. Travel Med Infect Dis (2021) 39:101919. doi: 10.1016/j.tmaid.2020.101919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Chi J, Gong W, Gao Q. Clinical Characteristics and Outcomes of Pregnant Women With COVID-19 and the Risk of Vertical Transmission: A Systematic Review. Arch Gynecol Obstetrics (2020) p:1–9. doi: 10.1007/s00404-020-05889-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Jafari M, Pormohammad A, Sheikh Neshin SA, Ghorbani S, Bose D, Alimohammadi S, et al. Clinical Characteristics and Outcomes of Pregnant Women With COVID-19 and Comparison With Control Patients: A Systematic Review and Meta-Analysis. Rev Med Virol (2021) p:e2208. doi: 10.1002/rmv.2208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Islam M, Poly TN, Walther BA, Yang HC, Wang CW, Hsieh WS, et al. Clinical Characteristics and Neonatal Outcomes of Pregnant Patients With COVID-19: A Systematic Review. Front Med (2020) 7:909. doi: 10.3389/fmed.2020.573468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Rodrigues C, Baía I, Domingues R, Barros H. Pregnancy and Breastfeeding During COVID-19 Pandemic: A Systematic Review of Published Pregnancy Cases. Front Public Health (2020) 8:806. doi: 10.3389/fpubh.2020.558144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Akhtar H, Patel C, Abuelgasim E, Harky A. COVID-19 (SARS-CoV-2) Infection in Pregnancy: A Systematic Review. Gynecol Obstetric Invest (2020) 85(4):295–306. doi: 10.1159/000509290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Trippella G, Ciarcià M, Ferrari M, Buzzatti C, Maccora I, Azzari C, et al. COVID-19 in Pregnant Women and Neonates: A Systematic Review of the Literature With Quality Assessment of the Studies. Pathogens (2020) 9(6):485. doi: 10.3390/pathogens9060485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Amaral WN, Moraes CL, Rodrigues APDS, Noll M, Arruda JT, Mendonça CR. Maternal Coronavirus Infections and Neonates Born to Mothers With SARS-CoV-2: A Systematic Review. Healthcare (2020) 8(4):511. Multidisciplinary Digital Publishing Institute. doi: 10.3390/healthcare8040511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Deniz M, Tezer H. Vertical Transmission of SARS CoV-2: A Systematic Review. J Maternal-Fetal Neonatal Med (2020) 21:1–8. doi: 10.1080/14767058.2020.1793322 [DOI] [PubMed] [Google Scholar]
- 89. Dong L, Tian J, He S, Zhu C, Wang J, Liu C, et al. Possible Vertical Transmission of SARS-CoV-2 From an Infected Mother to Her Newborn. (2020) 323(18):1846–8. doi: 10.1001/jama.2020.4621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Zeng H, Xu C, Fan J, Tang Y, Deng Q, Zhang W, et al. Antibodies in Infants Born to Mothers With COVID-19 Pneumonia. (2020) 323(18):1848–9. doi: 10.1001/jama.2020.4861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Colaco S, Chhabria K, Singh D, Bhide A, Singh N, Singh A, et al. Expression Map of Coronavirus Receptors and Associated Factors in Developing Human Embryos. J Assist Reprod Genet (2021) 38(7):1709–20. doi: 10.1007/s10815-021-02192-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Lukassen S, Chua RL, Trefzer T, Kahn NC, Schneider MA, Muley T, et al. SARS-CoV-2 Receptor ACE2 and TMPRSS2 Are Primarily Expressed in Bronchial Transient Secretory Cells. EMBO J (2020) 39(10):e105114. doi: 10.15252/embj.20105114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Qi J, Zhou Y, Hua J, Zhang L, Bian J, Liu B, et al. The ScRNA-Seq Expression Profiling of the Receptor ACE2 and the Cellular Protease TMPRSS2 Reveals Human Organs Susceptible to SARS-CoV-2 Infection. Int J Environ Res Public Health (2021) 18(1):284. doi: 10.3390/ijerph18010284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Seow JJW, Pai R, Mishra A, Shepherdson E, Lim TKH, Goh BKP, et al. ScRNA-Seq Reveals ACE2 and TMPRSS2 Expression in TROP2+ Liver Progenitor Cells: Implications in COVID-19 Associated Liver Dysfunction. Front Med (Lausanne) (2021) 8:603374. doi: 10.3389/fmed.2021.603374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Singh M, Bansal V, Feschotte C. A Single-Cell RNA Expression Map of Human Coronavirus Entry Factors. Cell Rep (2020) 32(12):108175. doi: 10.1016/j.celrep.2020.108175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Lü M, Qiu L, Jia G, Guo R, Leng Q. Single-Cell Expression Profiles of ACE2 and TMPRSS2 Reveals Potential Vertical Transmission and Fetus Infection of SARS-CoV-2. Aging (Albany NY) (2020) 12(20):19880. doi: 10.18632/aging.104015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Ashary N, Bhide A, Chakraborty P, Colaco S, Mishra A, Chhabria K, et al. Single-Cell RNA-Seq Identifies Cell Subsets in Human Placenta That Highly Expresses Factors Driving Pathogenesis of SARS-CoV-2. Front Cell Dev Biol (2020) 8:783. doi: 10.3389/fcell.2020.00783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Lu-Culligan A, Chavan AR, Vijayakumar P, Irshaid L, Courchaine EM, Milano KM, et al. Maternal Respiratory SARS-CoV-2 Infection in Pregnancy is Associated With a Robust Inflammatory Response at the Maternal-Fetal Interface. Med (2021) 2(5):591–610. e10. doi: 10.1016/j.medj.2021.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Maucourant C, Filipovic I, Ponzetta A, Aleman S, Cornillet M, Hertwig L, et al. Natural Killer Cell Immunotypes Related to COVID-19 Disease Severity. Sci Immunol (2020) 5(50):eabd6832. doi: 10.1126/sciimmunol.abd6832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Björkström NK, Ponzetta A. Natural Killer Cells and Unconventional T Cells in COVID-19. Curr Opin Virol (2021) 49:176–82. doi: 10.1016/j.coviro.2021.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Nagy Á., Pongor S, Győrffy B. Different Mutations in SARS-CoV-2 Associate With Severe and Mild Outcome. Int J Antimicrobial Agents (2021) 57(2):106272. doi: 10.1016/j.ijantimicag.2020.106272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Mariappan V, Manoharan PS, R P, Shanmugam L, Rao SR, Pillai AB. Potential Biomarkers for the Early Prediction of SARS-COV-2 Disease Outcome. Microbial Pathogen (2021) p:105057. doi: 10.1016/j.micpath.2021.105057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Xiong TY, Redwood S, Prendergast B, Chen M. Coronaviruses and the Cardiovascular System: Acute and Long-Term Implications. Eur Heart J (2020) 41(19):1798–800. doi: 10.1093/eurheartj/ehaa231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Ponti G, Maccaferri M, Ruini C, Tomasi A, Ozben T. Biomarkers Associated With COVID-19 Disease Progression. Crit Rev Clin Lab Sci (2020) 57(6):389–99. doi: 10.1080/10408363.2020.1770685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Gavriatopoulou M, Korompoki E, Fotiou D, Ntanasis-Stathopoulos I, Psaltopoulou T, Kastritis E, et al. Organ-Specific Manifestations of COVID-19 Infection. Clin Exp Med (2020) p:1–14. doi: 10.1007/s10238-020-00648-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Chen LY, Quach TT. COVID-19 Cytokine Storm Syndrome: A Threshold Concept. Lancet Microbe (2021) 2(2):e49. doi: 10.1016/S2666-5247(20)30223-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Rendeiro AF, Ravichandran H, Bram Y, Chandar V, Kim J, Meydan C, et al. The Spatial Landscape of Lung Pathology During COVID-19 Progression. Nature (2021) p:1–6. doi: 10.1038/s41586-021-03475-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Kulasinghe A, Tan CW, dos Santos Miggiolaro AF, Monkman J, Bhuva D, Junior JD, et al. Spatial Profiling of Lung SARS-CoV-2 and Influenza Virus Infection Dissects Virus-Specific Host Responses and Gene Signatures. medRxiv (2020). doi: 10.1158/1557-3265.COVID-19-21-S03-04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. King J, Eroumé KS, Truckenmüller R, Giselbrecht S, Cowan AE, Loew L, et al. Ten Steps to Investigate a Cellular System With Mathematical Modeling. PloS Comput Biol (2021) 17(5):e1008921. doi: 10.1371/journal.pcbi.1008921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Fletcher A, Osborne J. Seven Challenges in the Multiscale Modelling of Multicellular Tissues. WIREs Mechanisms of Disease (2020) 3:e1527. doi: 10.20944/preprints202007.0022.v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Bravo RR, Baratchart E, West J, Schenck RO, Miller AK, Gallaher J, et al. Hybrid Automata Library: A Flexible Platform for Hybrid Modeling With Real-Time Visualization. PloS Comput Biol (2020) 16(3):e1007635. doi: 10.1371/journal.pcbi.1007635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Method of the Year 2020: Spatially Resolved Transcriptomics. Nat Methods (2021) 18(1):1–1. doi: 10.1038/s41592-020-01042-x [DOI] [PubMed] [Google Scholar]
- 113. Sadeghi Rad H, Bazaz SR, Monkman J, Ebrahimi M, Warkiani N, Rezaei K, et al. The Evolving Landscape of Predictive Biomarkers in Immuno-Oncology With a Focus on Spatial Technologies. (2020) 9(11):e1215. doi: 10.1002/cti2.1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Delorey TM, Ziegler CGK, Heimberg G, Normand R, Yang Y, Segerstolpe Å, et al. COVID-19 Tissue Atlases Reveal SARS-CoV-2 Pathology and Cellular Targets. Nature (2021) 595(7865):107–13. doi: 10.1038/s41586-021-03570-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Schneider CA, Rasband WS, Eliceiri KW. NIH Image to Imagej: 25 Years of Image Analysis. Nat Methods (2012) 9(7):671–5. doi: 10.1038/nmeth.2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Rueden CT, Schindelin J, Hiner MC, DeZonia BE, Walter AE, Arena ET, et al. Imagej2: Imagej for the Next Generation of Scientific Image Data. BMC Bioinf (2017) 18(1):1–26. doi: 10.1186/s12859-017-1934-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Rueden CT, Eliceiri KW. The Imagej Ecosystem: An Open and Extensible Platform for Biomedical Image Analysis. Microscopy Microanalysis (2017) 23(S1):226–7. doi: 10.1017/S1431927617001817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. McQuin C, Goodman A, Chernyshev V, Kamentsky L, Cimini BA, Karhohs KW, et al. Cellprofiler 3.0: Next-Generation Image Processing for Biology. PloS Biol (2018) 16(7):e2005970. doi: 10.1371/journal.pbio.2005970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Bankhead P, Loughrey MB, Fernández JA, Dombrowski Y, McArt DG, Dunne PD, et al. Qupath: Open Source Software for Digital Pathology Image Analysis. Sci Rep (2017) 7(1):1–7. doi: 10.1038/s41598-017-17204-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Allenby MC, Misener R, Panoskaltsis N, Mantalaris A. A Quantitative Three-Dimensional Image Analysis Tool for Maximal Acquisition of Spatial Heterogeneity Data. Tissue Eng Part C: Methods (2017) 23(2):108–17. doi: 10.1089/ten.tec.2016.0413 [DOI] [PubMed] [Google Scholar]
- 121. Ferrando-Martinez S, Moysi E, Pegu A, Andrews S, Nganou Makamdop K, Ambrozak D, et al. Accumulation of Follicular CD8+ T Cells in Pathogenic SIV Infection. J Clin Invest (2018) 128(5):2089–103. doi: 10.1172/JCI96207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. De Rose DU, Piersigilli F, Ronchetti MP, Santisi A, Bersani I, Dotta A, et al. Novel Coronavirus Disease (COVID-19) in Newborns and Infants: What We Know So Far. Ital J Pediatr (2020) 46(1):1–8. doi: 10.1186/s13052-020-0820-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Shimabukuro TT, Kim SY, Myers TR, Moro PL, Oduyebo T, Panagiotakopoulos L, et al. Preliminary Findings of mRNA Covid-19 Vaccine Safety in Pregnant Persons. N Engl J Med (2021) 384(24):2273–82. doi: 10.1056/NEJMoa2104983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Prabhu M, Murphy EA, Sukhu AC, Yee J, Singh S, Eng D, et al. Antibody Response to Coronavirus Disease 2019 (COVID-19) Messenger RNA Vaccination in Pregnant Women and Transplacental Passage Into Cord Blood. Obstet Gynecol (2021) 138(2):278–80. doi: 10.1097/AOG.0000000000004438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Atwell TD, Lteif AN, Brown DL, McCann M, Townsend JE, Leroy AJ. Neonatal Thyroid Function After Administration of IV Iodinated Contrast Agent to 21 Pregnant Patients. Am J Roentgenol (2008) 191(1):268–71. doi: 10.2214/AJR.07.3336 [DOI] [PubMed] [Google Scholar]