Abstract
Objective
Although lowering the low-density lipoprotein cholesterol (LDL-C) levels using statins can reduce cardiovascular risk, 70% of the cardiovascular risk remains despite treatment with statins. Several studies have shown that elevated triglyceride (TG)-rich lipoprotein is the primary therapeutic target for reducing the residual risk. However, conventional treatment with fibrates is frequently associated with adverse drug reactions, especially in patients with chronic kidney disease (CKD), and even with a reduction in TG. Pemafibrate is a novel selective peroxisome proliferator-activated receptor α modulator (SPPARMα) with fewer side effects and greater effectiveness that can overcome these challenges. We aimed to investigate the safety and efficacy of pemafibrate in patients with CKD and herein present a real-world profile of pemafibrate.
Methods
Between January 2019 and January 2020, 126 consecutive patients with hyperglyceridemia from two institutions (54 patients with CKD; 43%) who received pemafibrate were enrolled in this retrospective observational study. Blood samples were collected before (baseline) and at 24 weeks after commencing pemafibrate therapy. The primary endpoint was a decrease in the serum lipid levels. The secondary endpoints were the incidence of rhabdomyolysis, hepatargy, and an exacerbation of CKD.
Results
All patients, including 51% of patients who were concurrently taking statins, reported significantly reduced total cholesterol, non-high-density lipoprotein-cholesterol (non-HDL-C), LDL-C, and TG, and increased HDL-C (p<0.05). The subgroup of patients with CKD showed similar results without increased HDL-C. No adverse events were observed in any patients.
Conclusion
Pemafibrate has a good safety profile and efficacy for treating patients with serum lipid abnormalities, including those with CKD.
Keywords: pemafibrate, triglyceride, chronic kidney disease
Introduction
Low-density lipoprotein cholesterol (LDL-C) is an established cardiovascular disease (CVD) risk factor. However, even after statin treatment to lower LDL-C levels, a 70% CVD risk remains (1). Hyperglyceridemia is another known risk factor for CVD and may be a potential therapeutic target for further risk reduction (2).
Fibrates, which activate peroxisome proliferator-activated receptor α (PPARα), are effective for the treatment of elevated triglyceride (TG) and for lowereing the high-density lipoprotein cholesterol (HDL-C) levels (3). Meta-analyses revealed that fibrates could reduce the CVD risk, with the largest effect occurring in trials with higher TG, but the mortality was unaffected (4,5). This lack of an improvement in mortality may occur because due to the use of fibrates which is frequently associated with adverse drug reactions such as increased serum creatinine, liver enzymes, and homocysteine (6). Moreover, a meta-analysis of patients with chronic kidney disease (CKD) demonstrated that fibrate therapy reduced the estimated glomerular filtration rate (eGFR) (7). A reduced renal function is associated with an elevated risk of CVD and death (8,9). Additionally, combination therapy with fibrate and statins increases the risk of developing myopathy and rhabdomyolysis, which is greater in patients with CKD (10,11). Therefore, the concomitant use of conventional fibrates and statins should be avoided in patients with CKD, whereas the risks vary with different fibrates and statins used in combination for patients without CKD (12).
Conventional fibrates are termed pan PPARα agonists because of their low selectivity for PPARα. Pemafibrate is a novel selective PPARα modulator (SPPARMα) that was designed to have a higher selectivity as a PPARα agonist (13). Compared to conventional fibrates, pemafibrate does not significantly increase creatinine, alanine aminotransferase (ALT), or γ-glutamyl transferase (γGT) levels (14-16). In patients receiving statin therapy after acute coronary syndrome (ACS), during treatment, TG <150 mg/dL was associated with a lower risk of recurrent CVD, independent of the LDL-C level (17). Therefore, pemafibrate could address an unmet medical need for the treatment of residual CVD risk. However, the real-world data for pemafibrate are currently insufficient.
In the present study, we aimed to confirm the efficacy and safety of pemafibrate in a real-world setting, especially for the treatment of hyperglyceridemia in patients with CKD.
Materials and Methods
Study design and patient population
This retrospective observational study enrolled 126 consecutive patients with hyperglyceridemia from two institutions who received pemafibrate between January 2019 and January 2020. Patients who had a fasting serum TG ≥150 mg/dL and/or HDL-C <40 mg/dL during the screening evaluation were enrolled. The exclusion criteria were as follows: patients who required additional drug treatment for dyslipidemia during the study period, patients who were taking fibrates, serum creatinine ≥2.5 mg/dL or creatinine clearance <40 mL/min, and those with poor adherence. This study was approved by the institutional review board, and the requirement for written informed consent was waived because of the retrospective study design. This study was conducted in accordance with the principles of the Declaration of Helsinki. During the study period, the patients received 0.2 mg pemafibrate.
Blood samples for the diagnosis of hyperglyceridemia were collected in the fasting state for clinical laboratory tests. Blood samples were collected before (baseline) and at 24 weeks after commencing pemafibrate therapy. The blood samples collected at 24 weeks after therapy were not restricted to being collected in a fasting state.
The patients' demographic data were collected, including information on age, sex, body mass index, past medical history, and usage history of therapeutic agents affecting lipid metabolism. The clinical laboratory data collected included total cholesterol (TC), LDL-C, non-HDL-C, TG, HDL-C, blood urea nitrogen (BUN), creatinine (Cre), estimated glomerular filtration rate (eGFR), alanine aminotransferase (ALT), γGT, and creatine kinase (CK).
Clinical laboratory
The cholesterol levels were measured by a homogeneous assay, Cholestest LDL (SEKISUI Medical, Tokyo, Japan) for LDL-C, CholestestN HDL (SEKISUI Medical) for HDL-C and Cholestest CHO (SEKISUI Medical) for TC. Non-HDL-C was calculated by subtracting HDL-C from TC.
Endpoints
The primary efficacy endpoint was the percent change in serum TG at week 24 compared to baseline. The primary safety endpoints were the incidence of rhabdomyolysis, hepatargy, and exacerbation of CKD. The secondary efficacy endpoints included changes or percent changes at week 24, compared to baseline, regarding such parameters as TC, LDL-C, non-HDL-C, and HDL-C.
Definitions
We extracted the patient's diagnoses from the medical records. The disease was classified according to the International Statistical Classification of Diseases and Related Health Problems, 10th revision (ICD-10). ACS was defined as unstable angina pectoris (UAP) or acute myocardial infarction. Stable ischemic heart disease (SIHD) was defined as proven ischemia by myocardial perfusion scintigraphy and/or invasive ischemia detection. Peripheral artery disease (PAD) was defined as ankle-brachial index is ≤0.90 and claudication by limb artery stenosis. Hypertension was defined as a clinical room blood pressure ≥140/90 mmHg or home blood pressure ≥135/85 mmHg and/or receiving oral antihypertensive drugs. Heart failure (HF) was defined as medical treatment for HF. Diabetes mellitus (DM) was defined as hemoglobin A1c ≥6.5% [National Glycohemoglobin Standardization Program (NGSP) value], medical treatment for DM, or a history of DM. Cerebral infarction (CI) and malignancy were defined as medical treatment for CI/malignancy and/or a history of CI/malignancy before admission. CKD was defined as eGFR <60 mL/min/1.73 m2. We calculated eGFR from the serum creatinine level, age, weight, and sex using the following formula: eGFR=194×Cr1.094×age-0.287 (male), eGFR=194×Cr1.094×age-0.287×0.739 (female).
Statistical analysis
The data are expressed as the median (25%, 75%) or percentage. Categorical variables are presented as the number (percentage) and were compared using the Mann-Whitney U test. p values <0.05 indicate statistical significance. All analyses were performed using the SPSS 24.0 software program (Chicago, USA).
Results
Study patients
A total of 126 consecutive patients with hyperglyceridemia were enrolled, of which 54 had concurrent CKD. The median follow-up period was 196 days (interquartile range, 162-264). The clinical characteristics of all patients are shown in Table 1. The therapeutic agents combined with pemafibrate for the treatment of dyslipidemia were statins (51%), ezetimibe (21%), proprotein convertase subtilisin/kexin type9 (PCSK9) inhibitor (1%), and n-3 (omega-3) fatty acid sequestrants (FAS) (13%).
Table 1.
Clinical Characteristics of All Patients.
| n=126 | ||
|---|---|---|
| Age (years) | 65.0 [53.8, 73.0] | |
| Female | 45 (36) | |
| BMI (kg/m2) | 25.3 [22.6, 29.1] | |
| History of ACS | 31 (25) | |
| History of AMI | 23 (18) | |
| History of UAP | 8 (6) | |
| History of SIHD | 12 (10) | |
| History of PCI | 41 (33) | |
| History of CABG | 2 (2) | |
| History of PAD | 9 (7) | |
| History of HF | 7 (6) | |
| Hypertension | 71 (56) | |
| Diabetes mellitus | 74 (59) | |
| Chronic kidney disease | 54 (43) | |
| Cerebral infarction | 15 (12) | |
| Malignancy | 13 (10) | |
| Current smoker | 31 (25) | |
| Medical therapy on admission | ||
| Statin | 64 (51) | |
| Ezetimibe | 27 (21) | |
| PCSK9I | 1 (1) | |
| n-3 fatty acid sequestrants | 17 (13) |
Data are presented as the median [25%, 75%] or n (%).
BMI: body mass index, ACS: acute coronary syndrome, AMI: acute myocardial infarction, UAP: unstable angina pectoris, SIHD: stable ischemic heart disease, PCI: percutaneous coronary intervention, CABG: coronary artery bypass grafting, PAD: peripheral artery disease, HF: heart failure, PCSK9I: proprotein convertase subtilisin/kexin type9 inhibitor
The efficacy analysis for all patients demonstrated significant reductions in TC, LDL-C, non-HDL-C, TG, and a significant increase in HDL-C (Table 2). The safety analysis for all patients demonstrated no significant change in renal function, liver function, or CK (Table 2).
Table 2.
Efficacy and Safety Analyses for All Patients.
| All patients (n=126) | Baseline | Week 24 | p value | |||
|---|---|---|---|---|---|---|
| Efficacy parameters | ||||||
| TC (mg/dL) | 192.0 [163.5, 222.0] | 173.0 [144.3, 204.0] | <0.001 | |||
| LDL-C (mg/dL) | 105.0 [82.8, 129.5] | 95.0 [72.5, 119.0] | 0.025 | |||
| non-HDL-C (mg/dL) | 146.0 [119.0, 178.0] | 121.0 [94.0, 148.0] | <0.001 | |||
| TG (mg/dL) | 269.0 [180.0, 423.8] | 161.0 [107.0, 227.3] | <0.001 | |||
| HDL-C (mg/dL) | 44.0 [36.8, 53.0] | 51.0 [43.0, 59.0] | 0.001 | |||
| Safety parameters | ||||||
| BUN (mg/dL) | 16.0 [13.0, 20.0] | 16.6 [13.0, 20.0] | 0.821 | |||
| Cre (mg/dL) | 0.87 [0.69, 1.04] | 0.84 [0.70, 1.10] | 0.996 | |||
| eGFR (mL/min/1.73m2) | 62.1 [50.9, 74.9] | 64.2 [49.5, 78.5] | 0.965 | |||
| ALT (IU/L) | 26.0 [17.8, 37.0] | 17.5 [14.0, 27.0] | 0.001 | |||
| γGT (IU/L) | 40.5 [26.3, 68.8] | 29.5 [18.0, 50.8] | 0.07 | |||
| CK (U/L) | 81.0 [66.0, 112.5] | 76.0 [56.0, 102.0] | 0.088 |
Data are presented as the median [25%, 75%]. p<0.05 vs. baseline value by Mann-Whitney U test.
TC: total cholesterol, LDL-C: low-density lipoprotein cholesterol, non-HDL-C: non-high-density lipoprotein cholesterol, TG: triglycerides, HDL-C: high-density lipoprotein cholesterol, BUN: blood urea nitrogen, Cre: creatinine, eGFR: estimated glomerular filtration rate, ALT: alanine aminotransferase, γGT: γ-glutamyl transferase, CK: creatine kinase
Comparisons between the CKD and non-CKD groups
Table 3 shows a comparison of the clinical characteristics between the CKD and non-CKD groups. The CKD group had a significantly higher median age (70 vs. 61 years of age) and a significantly higher percentage of patients with UAP, SIHD, PAD, and HT (p<0.05). The CKD group had a significantly greater number of patients taking statins, owing to the higher percentage of CVD (p<0.05).
Table 3.
Comparison of Patient Clinical Characteristics between the CKD and Non-CKD Groups.
| CKD (n=54) | non-CKD (n=72) | p value | ||||
|---|---|---|---|---|---|---|
| Age (years) | 70.0 [60.0, 75.0] | 61.0 [50.0, 71.0] | <0.001 | |||
| Female | 17 (31) | 28 (39) | 0.39 | |||
| BMI (kg/m2) | 25.1 [22.9, 29.5] | 25.3 [22.5, 28.5] | 0.919 | |||
| History of ACS | 17 (31) | 14 (19) | 0.121 | |||
| History of AMI | 10 (19) | 13 (18) | 0.947 | |||
| History of UAP | 7 (13) | 1 (1) | 0.008 | |||
| History of SIHD | 9 (17) | 3 (4) | 0.018 | |||
| History of PCI | 24 (44) | 17 (24) | 0.014 | |||
| History of CABG | 1 (2) | 1 (1) | 0.837 | |||
| History of PAD | 7 (13) | 2 (3) | 0.028 | |||
| History of HF | 4 (7) | 3 (4) | 0.432 | |||
| Hypertension | 37 (69) | 34 (47) | 0.017 | |||
| Diabetes mellitus | 29 (54) | 41 (57) | 0.638 | |||
| Cerebral infarction | 8 (15) | 7 (10) | 0.382 | |||
| Malignancy | 7 (13) | 6 (8) | 0.398 | |||
| Current smoker | 15 (28) | 16 (22) | 0.474 | |||
| Medical therapy on admission | ||||||
| Statin | 34 (63) | 30 (42) | 0.018 | |||
| Ezetimibe | 14 (26) | 13 (18) | 0.287 | |||
| PCSK9I | 1 (2) | 0 (0) | 0.246 | |||
| N-3 fatty acid sequestrants | 6 (11) | 11 (15) | 0.643 |
Data are presented as the median [25%, 75%] or n (%). p<0.05 vs. non-CKD by Mann-Whitney U test.
CKD: chronic kidney disease, BMI: body mass index, ACS: acute coronary syndrome, AMI: acute myocardial infarction, UAP: unstable angina pectoris, SIHD: stable ischemic heart disease, PCI: percutaneous coronary intervention, CABG: coronary artery bypass grafting, PAD: peripheral artery disease, HF: heart failure, PCSK9I: proprotein convertase subtilisin/kexin type-9 inhibitor
Table 4 shows a comparison of the baseline laboratory data between the CKD and non-CKD groups. There were no significant differences in the baseline lipid parameters between the two groups.
Table 4.
Comparison of Baseline Laboratory Data between the CKD and Non-CKD Groups
| CKD (n=54) | non-CKD (n=72) | p value | ||||
|---|---|---|---|---|---|---|
| Efficacy parameters | ||||||
| TC (mg/dL) | 184.5 [161.5, 221.5] | 199.5 [164.3, 222.0] | 0.415 | |||
| LDL-C (mg/dL) | 96.5 [74.5, 129.0] | 109.5 [86.3, 131.0] | 0.441 | |||
| non-HDL-C (mg/dL) | 144.5 [116.0, 175.8] | 152.5 [121.0, 178.8] | 0.751 | |||
| TG (mg/dL) | 260.0 [200.8, 383.0] | 273.0 [155.5, 525.5] | 0.745 | |||
| HDL-C (mg/dL) | 42.5 [35.0, 48.0] | 46.0 [37.3, 53.0] | 0.079 | |||
| Safety parameters | ||||||
| BUN (mg/dL) | 19.0 [16.0, 23.6] | 14.4 [11.0, 18.0] | <0.001 | |||
| Cre (mg/dL) | 1.07 [0.94, 1.29] | 0.74 [0.62, 0.89] | <0.001 | |||
| eGFR (mL/min/1.73m2) | 47.6 [41.7, 55.8] | 73.5 [66.3, 86.3] | <0.001 | |||
| ALT (IU/L) | 23.0 [17.0, 35.3] | 27.0 [18.3, 40.5] | 0.272 | |||
| γGT (IU/L) | 41.5 [24.3, 52.5] | 41.0 [29.0, 82.0] | 0.909 | |||
| CK (U/L) | 79.0 [59.5, 101.0] | 85.5 [68.0, 114.0] | 0.936 |
Data are presented as the median [25%, 75%]. p<0.05 vs. non-CKD by Mann-Whitney U test.
CKD: chronic kidney disease, TC: total cholesterol, LDL-C: low-density lipoprotein cholesterol, non-HDL-C: non-high-density lipoprotein cholesterol, TG: triglycerides, HDL-C: high-density lipoprotein cholesterol, BUN: blood urea nitrogen, Cre: creatinine, eGFR: estimated glomerular filtration rate, ALT: alanine aminotransferase, γGT: γ-glutamyl transferase, CK: creatine kinase
Analysis of the CKD group
Table 5 shows the efficacy and safety analyses for the CKD group. Regarding the lipid parameters in this group, an efficacy analysis demonstrated significant reductions in TC, non-HDL-C, and TG (p<0.05). No significant differences in LDL-C and HDL-C were observed. A safety analysis for the CKD group demonstrated no significant changes in the renal function, liver function, or CK.
Table 5.
Efficacy and Safety Analyses for the Group of Patients with Chronic Kidney Disease (CKD Group).
| CKD group (n=54) | Baseline | Week 24 | p value | |||
|---|---|---|---|---|---|---|
| Efficacy parameters | ||||||
| TC (mg/dL) | 184.5 [161.5, 221.5] | 165.0 [138.8, 187.5] | <0.001 | |||
| LDL-C (mg/dL) | 96.5 [74.5, 129.0] | 94.0 [71.0, 108.0] | 0.055 | |||
| non-HDL-C (mg/dL) | 144.5 [116.0, 175.8] | 115.0 [93.0, 137.5] | <0.001 | |||
| TG (mg/dL) | 260.0 [200.8, 383.0] | 167.0 [112.0, 221.0] | 0.002 | |||
| HDL-C (mg/dL) | 42.5 [35.0, 48.0] | 48.0 [39.3, 53.8] | 0.26 | |||
| Safety parameters | ||||||
| BUN (mg/dL) | 19.0 [16.0, 23.6] | 18.4 [15.2, 22.1] | 0.761 | |||
| Cre (mg/dL) | 1.07 [0.94, 1.29] | 1.11 [0.86, 1.33] | 0.853 | |||
| eGFR (mL/min/1.73m2) | 47.6 [41.7, 55.8] | 48.0 [40.4, 59.1] | 0.6 | |||
| ALT (IU/L) | 23.0 [17.0, 35.3] | 18.0 [14.0, 27.0] | 0.098 | |||
| γGT (IU/L) | 41.5 [24.3, 52.5] | 25.0 [17.8, 42.3] | 0.698 | |||
| CK (U/L) | 79.0 [59.5, 101.0] | 76.0 [56.0, 98.0] | 0.486 |
Data are presented as the median [25%, 75%]. p<0.05 vs. baseline value by Mann-Whitney U test.
TC: total cholesterol, LDL-C: low-density lipoprotein cholesterol, non-HDL-C: non-high-density lipoprotein cholesterol, TG: triglycerides, HDL-C: high-density lipoprotein cholesterol, BUN: blood urea nitrogen, Cre: creatinine, eGFR: estimated glomerular filtration rate, ALT: alanine aminotransferase, γGT: γ-glutamyl transferase, CK: creatine kinase
Analysis of the non-CKD group
Table 6 shows the efficacy and safety analyses for the non-CKD group. For the lipid parameters, the efficacy analysis demonstrated significant reductions in TC, non-HDL-C, and TG, and a significant increase in HDL-C (p<0.05). LDL-C showed no significant difference from baseline. The safety analysis for the non-CKD group demonstrated no significant changes in renal function, liver function, and CK.
Table 6.
Efficacy and Safety Analyses for the Group of Patients without Chronic Kidney Disease (non-CKD Group).
| non-CKD group (n=72) | Baseline | Week 24 | p value | |||
|---|---|---|---|---|---|---|
| Efficacy parameters | ||||||
| TC (mg/dL) | 199.5 [164.3, 222.0] | 179.0 [147.0, 209.5] | 0.013 | |||
| LDL-C (mg/dL) | 109.5 [86.3, 131.0] | 96.0 [75.3, 123.8] | 0.174 | |||
| non-HDL-C (mg/dL) | 152.5 [121.0, 178.8] | 128.0 [94.8, 152.8] | 0.001 | |||
| TG (mg/dL) | 273.0 [155.5, 525.5] | 154.0 [87.3, 242.3] | 0.001 | |||
| HDL-C (mg/dL) | 46.0 [37.3, 53.0] | 52.0 [44.3, 62.0] | 0.008 | |||
| Safety parameters | ||||||
| BUN (mg/dL) | 14.4 [11.0, 18.0] | 15.0 [12.0, 18.0] | 0.396 | |||
| Cre (mg/dL) | 0.74 [0.62, 0.89] | 0.75 [0.65, 0.86] | 0.648 | |||
| eGFR (mL/min/1.73m2) | 73.5 [66.3, 86.3] | 76.9 [65.1, 81.3] | 0.591 | |||
| ALT (IU/L) | 27.0 [18.3, 40.5] | 17.0 [13.0, 26.8] | 0.003 | |||
| γGT (IU/L) | 41.0 [29.0, 82.0] | 32.0 [18.0, 52.0] | 0.01 | |||
| CK (U/L) | 85.5 [68.0, 114.0] | 78.5 [56.0, 104.5] | 0.071 |
Data are presented as the median [25%, 75%]. p<0.05 vs. baseline value by Mann-Whitney U test.
TC: total cholesterol, LDL-C: low-density lipoprotein cholesterol, non-HDL-C: non-high-density lipoprotein cholesterol, TG: triglycerides, HDL-C: high-density lipoprotein cholesterol, BUN: blood urea nitrogen, Cre: creatinine, eGFR: estimated glomerular filtration rate, ALT: alanine aminotransferase, γGT: γ-glutamyl transferase, CK: creatine kinase
Comparisons between baseline and week 24
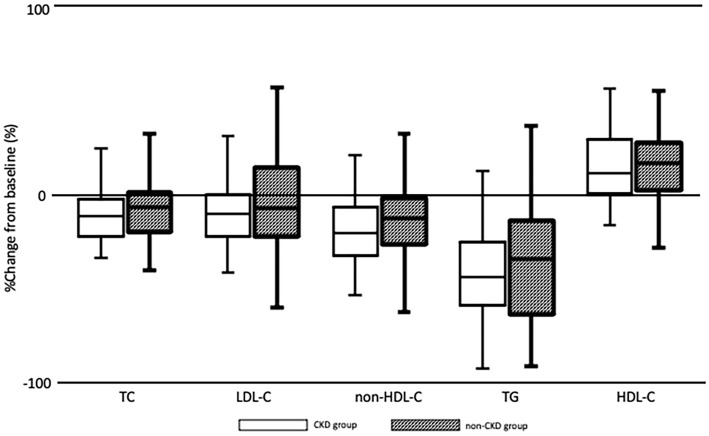
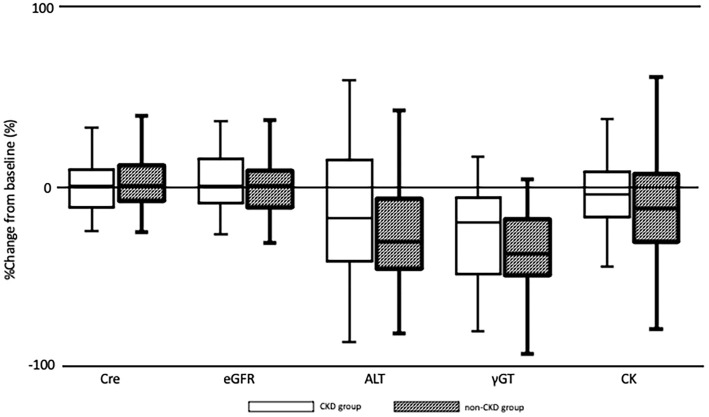
Fig. 1 shows the percent change from baseline to week 24 in serum lipid levels for the CKD and non-CKD groups. Fig. 2 shows the percent change from baseline to week 24 in renal function, liver function, and CK for the CKD and non-CKD groups. There were no significant differences between the two groups.
Figure 1.
Box plots showing the percent change from baseline to week 24 regarding the serum lipid levels as demonstrated by the Mann-Whitney U test. A p value of 0.05 was considered to be significant. Boxes show the medians and interquartile ranges with the lowest and highest values being shown above and below each box. This figure illustrates the findings of efficacy analyses, which found no significant differences between the CKD and non-CKD groups. CKD: chronic kidney disease, TC: total cholesterol, LDL-C: low-density lipoprotein cholesterol, non-HDL-C: non-high-density lipoprotein cholesterol, TG: triglycerides, HDL-C: high-density lipoprotein cholesterol
Figure 2.
Box plots showing the percent change from baseline to week 24 regarding the renal function, liver function, and creatine kinase demonstrated by the Mann-Whitney U test. A p value of 0.05 was considered to be significant. Boxes show the medians and interquartile ranges with the lowest and highest values being shown above and below each box. The figure illustrates the findings of safety analyses, which found no significant differences between the CKD and non-CKD groups. Cre: creatinine, eGFR: estimated glomerular filtration rate, ALT: alanine aminotransferase, γGT: γ-glutamyl transferase, CK: creatine kinase
Discussion
In the present study, we confirmed a significant reduction in TG and an acceptable safety profile following pemafibrate treatment in a real-world sample of patients with hyperglyceridemia and CKD. Previous studies on pemafibrate were highly controlled with very selective inclusion criteria and therefore may not be representative of a typical clinical experience. This study used less stringent exclusion criteria, and therefore, the results may be more generalizable to real world clinical experiences.
All patient data showed that pemafibrate significantly reduced TC, LDL-C, non-HDL-C, and TG, and increased HDL-C. No studies have previously identified any effect of pemafibrate administration alone on the LDL-C levels. Therefore, the significant reduction in LDL-C was considered a result of the combination therapy with statins (Table 1).
Previous studies have reported that pemafibrate significantly reduced ALT (14). Basic research has shown that pemafibrate can improve the pathogenesis of nonalcoholic fatty liver disease (NAFLD)/nonalcoholic steatohepatitis (NASH) by modulating lipid turnover and the energy metabolism in the liver, and it is expected to show this same effect in patients with hyperglyceridemia (18).
The adverse events of concern associated with fibrate administration are rhabdomyolysis and a decline in the renal and/or liver function. The risk of adverse events may increase when fibrates are administered to patients with CKD and in combination therapy with statins. However, no adverse events were observed in this study, even though the CKD group had significantly more patients using combination therapy with statins and it was significantly older than the non-CKD group. This result is consistent with an earlier study reporting that pemafibrate treatment had lower rates of adverse events compared to fenofibrate, especially those related to the kidney function, which were very low with pemafibrate (15). The reason that the renal function is not reduced by pemafibrate is thought to be because the structure of pemafibrate is selective to PPARα (19,20). As reported in a previous study, the non-CKD group experienced significantly decreased TC, non-HDL-C, and TG, and increased HDL-C. However, the CKD group did not show a significantly increased HDL-C level. Patients with CKD often have atherogenic dyslipidemia, including high TG and low HDL-C, which occurs as a result of an impaired TG-rich lipoprotein catabolism associated with a decreased lipoprotein lipase activity or impaired HDL maturation caused by a reduced lecithin-cholesterol acyltransferase activity (21-25). It is necessary to investigate whether the HDL-C levels in patients with CKD may increase by an add-on dosage of pemafibrate.
There were no cardiovascular events observed in this study. However, this retrospective study cannot address whether pemafibrate is associated with an improved prognosis because of the short observation period and the small number of patients. The results of the PROMINENT study are expected to address these limitations (26).
Study limitations
The present study is associated with several limitations. First, the patient sample may not be representative because it was derived from only two hospitals and the study was retrospective in nature. Second, many patients were already receiving treatments for dyslipidemia, including statin treatment. Third, the duration of the treatment period was approximately 24 weeks, which is relatively short for the evaluation of a chronic disease. A longer study period is therefore needed to investigate the risk-to-benefit balance. Finally, blood samples obtained 24 weeks after therapy were not restricted to being collected in a fasting state, which may have affected the laboratory data, especially the lipid parameters. Therefore, it is considered that these data did not show a normal distribution.
Conclusion
In conclusion, pemafibrate improved the TG and HDL-C levels in a real-world sample of patients. Moreover, pemafibrate improved the TG levels without any adverse events, even in patients with CKD. Pemafibrate could therefore potentially address the unmet medical need for the treatment of residual CV risk.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Fruchart JC, Sacks F, Hermans MP, et al. The residual risk reduction initiative: a call to action to reduce residual vascular risk in patients with dyslipidemia. Am J Cardiol 102: 1K-34K, 2008. [DOI] [PubMed] [Google Scholar]
- 2.Iso H, Imano H, Yamagishi K, et al. Fasting and non-fasting triglycerides and risk of ischemic cardiovascular disease in Japanese men and women: the Circulatory Risk in Communities Study (CIRCS). Atherosclerosis 237: 361-368, 2014. [DOI] [PubMed] [Google Scholar]
- 3.Chapman MJ, Redfern JS, McGovern ME, Giral P. Niacin and fibrates in atherogenic dyslipidemia: pharmacotherapy to reduce cardiovascular risk. Pharmacol Ther 126: 314-345, 2010. [DOI] [PubMed] [Google Scholar]
- 4.Jun M, Foote C, Lv J, et al. Effects of fibrates on cardiovascular outcomes: a systematic review and meta-analysis. Lancet 375: 1875-1884, 2010. [DOI] [PubMed] [Google Scholar]
- 5.Wang D, Liu B, Tao W, Hao Z, Liu M. Fibrates for secondary prevention of cardiovascular disease and stroke. Cochrane Database Syst Rev 2015: CD009580, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davidson MH, Armani A, McKenney JM, Jacobson TA. Safety considerations with fibrate therapy. Am J Cardiol 99: 3C-18C, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Jun M, Zhu B, Tonelli M, et al. Effects of fibrates in kidney disease: a systematic review and meta-analysis. J Am Coll Cardiol 60: 2061-2071, 2012. [DOI] [PubMed] [Google Scholar]
- 8.Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet 375: 2073-2081, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levey AS, de Jong PE, Coresh J, et al. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int 80: 17-28, 2011. [DOI] [PubMed] [Google Scholar]
- 10.Catapano AL, Graham I, De Backer G, et al. 2016 ESC/EAS guidelines for the management of dyslipidaemias: the Task Force for the Management of Dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) developed with the special contribution of the European Assocciation for Cardiovascular Prevention & Rehabilitation (EACPR). Atherosclerosis 253: 281-344, 2016. [DOI] [PubMed] [Google Scholar]
- 11.Catapano AL, Graham I, De Backer G, et al. 2016 ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J 37: 2999-3058, 2016. [DOI] [PubMed] [Google Scholar]
- 12.Graham DJ, Staffa JA, Shatin D, et al. Incidence of hospitalized rhabdomyolysis in patients treated with lipid-lowering drugs. JAMA 292: 2585-2590, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto Y, Takei K, Arulmozhiraja S, et al. Molecular association model of PPARα and its new specific and efficient ligand, pemafibrate: structural basis for SPPARMα. Biochem Biophys Res Commun 499: 239-245, 2018. [DOI] [PubMed] [Google Scholar]
- 14.Ishibashi S, Yamashita S, Arai H, et al. Effects of K-877, a novel selective PPARα modulator (SPPARMα), in dyslipidaemic patients: a randomized, double blind, active- and placebo-controlled, phase 2 trial. Atherosclerosis 249: 36-43, 2016. [DOI] [PubMed] [Google Scholar]
- 15.Ishibashi S, Arai H, Yokote K, Araki E, Suganami H, Yamashita S. Efficacy and safety of pemafibrate (K-877), a selective peroxisome proliferator-activated receptor α modulator, in patients with dyslipidemia: results from a 24-week, randomized, double blind, active-controlled, phase 3 trial. J Clin Lipidol 12: 173-184, 2018. [DOI] [PubMed] [Google Scholar]
- 16.Arai H, Yamashita S, Yokote K, Araki E, Suganami H, Ishibashi S. Efficacy and safety of pemafibrate versus fenofibrate in patients with high triglyceride and low HDL cholesterol levels: a multicenter, placebo-controlled, double-blind, randomized trial. J Atheroscler Thromb 25: 521-538, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller M, Cannon CP, Murphy SA, Qin J, Ray KK, Braunwald E. Impact of triglyceride levels beyond low-density lipoprotein cholesterol after acute coronary syndrome in the PROVE IT-TIMI 22 trial. J Am Coll Cardiol 51: 724-730, 2008. [DOI] [PubMed] [Google Scholar]
- 18.Honda Y, Kessoku T, Ogawa Y, et al. Pemafibrate, a novel selective peroxisome proliferator-activated receptor alpha modulator, improves the pathogenesis in a rodent model of nonalcoholic steatohepatitis. Sci Rep 7: 42477, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fruchart JC. Selective peroxisome proliferator-activated receptor α modulators (SPPARMα): the next generation of peroxisome proliferator-activated receptor α-agonists. Cardiovasc Diabetol 12: 82, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamamoto Y, Takei K, Arulmozhiraja S, et al. Molecular association model of PPARα and its new specific and efficient ligand, pemafibrate: structural basis for SPPARMα. Biochem Biophys Res Commun 499: 239-245, 2018. [DOI] [PubMed] [Google Scholar]
- 21.Yokote K, Yamashita S, Arai H, Araki E, Suganami H, Ishibashi S. Long-term efficacy and safety of pemafibrate, a novel selective peroxisome proliferator-activated receptor-α modulator (SPPARMα), in dyslipidemic patients with renal impairment. Int J Mol Sci 20: 706, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vaziri ND. Dyslipidemia of chronic renal failure: the nature, mechanisms, and potential consequences. Am J Physiol Renal Physiol 290: F262-F272, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Reiss AB, Voloshyna I, De Leon J, Miyawaki N, Mattana J. Cholesterol metabolism in CKD. Am J Kidney Dis 66: 1071-1082, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsimihodimos V, Mitrogianni Z, Elisaf M. Dyslipidemia associated with chronic kidney disease. Open Cardiovasc Med J 5: 41-48, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaysen GA. Lipid-lowering therapy in CKD: should we use it and in which patients. Blood Purif 43: 196-199, 2017. [DOI] [PubMed] [Google Scholar]
- 26.Pradhan AD, Paynter NP, Everett BM, et al. Rationale and design of the Pemafibrate to Reduce Cardiovascular Outcomes by Reducing Triglycerides in Patients with Diabetes (PROMINENT) study. Am Heart J 206: 80-93, 2018. [DOI] [PubMed] [Google Scholar]