Abstract
Objective
Little is known about the time from developing a first cancer to confirming the presence of a mismatch repair (MMR) gene mutation for Lynch syndrome (LS) probands.
Methods
This was a retrospective single center study. LS probands, who have an MMR gene mutation that was confirmed first in a pedigree and thereafter developed at least one cancer, were included in this study.
Results
There were 21 LS probands who had developed at least one cancer; 6 with MLH1 mutations, 9 with MSH2 mutations, 4 with MSH6 mutations, and 2 with EPCAM deletions. The median ages at the first cancer and the genetic diagnosis were 47 (34-71) and 62 (38-84) years old, respectively. The mean interval between the first cancer and the genetic diagnosis was 11.0 (0-25) years, and 20 years or longer interval was required for the 5 probands. Six (28.6%) probands were older than 70 years, and 3 (14.3%) were in their 80s when they were diagnosed to have LS. The genetic diagnosis was confirmed at the first, second, third, and fourth cancer or later in 5, 5, 6, and 5 probands, respectively. Of the 16 cancers examined, 2 (12.5%) were microsatellite stable (MSS), both of whom had germline MSH6 mutations. All 17 LS probands who developed colorectal cancer met the revised Bethesda guidelines at the genetic diagnosis, but only 7 of 11 (63.6%) met them at the first cancer. Twelve out of 21 (57.1%) met the revised Amsterdam criteria.
Conclusion
It took 11 years for the LS probands from the first cancer to the diagnostic confirmation by genetic tests. A quarter of the probands were in their 70s or 80s at genetic diagnosis.
Keywords: Lynch syndrome, probands, colorectal cancer, mismatch repair genes, microsatellite instability
Introduction
Lynch syndrome (LS) is a hereditary cancer syndrome caused by a germline mutation of the mismatch repair (MMR) genes. MMR gene mutation carriers tend to develop colorectal cancer (CRC), endometrial cancer (EMC), and other LS-associated cancers earlier than the general population. Past studies have demonstrated LS patients' age at cancer diagnosis (1-4), whereas little is known about patient's age at the genetic diagnosis, i.e., confirmation of the pathogenic germline MMR gene mutation. A genetic analysis is necessary to confirm the LS diagnosis, but genetic tests for LS are often hampered by such factors as the fact that the cost is not covered by Japanese national health insurance and genetic counseling is not available. In addition, LS patients are not always young, and often do not have any remarkable family history. These atypical LS patients can be missed by gastroenterologists, surgeons and gynecologists.
For probands, a genetic term referring to the first person in a pedigree who was diagnosed to have a hereditary disorder, it usually takes a long time from developing symptoms to genetic diagnosis confirmation than the second, third, or other family members. If there is a patient with a confirmed genetic mutation in a pedigree, then diagnosing other family members tends to be quick and easy. Probands also have other difficulties. For example, past studies (5-7) and major guidelines (8,9) recommend subtotal colectomy as an option for colon cancer in LS cases. However, it is hard to confirm the presence of an MMR mutation before surgery for LS probands who developed CRC as the first cancer because it takes about a month to obtain the results of a mutation analysis.
We have experienced LS probands for whom genetic diagnosis was confirmed in their 80s, and it took longer than 20 years from the initial cancer diagnosis. In this study, we focused on the interval between the first cancer and the genetic diagnosis in LS probands.
Materials and Methods
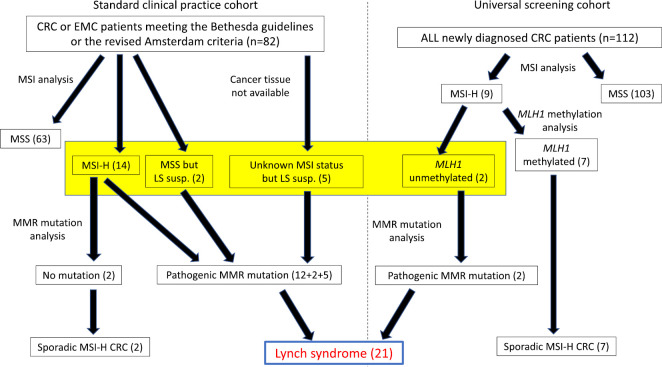
This was a single center retrospective observational study. Eligible patients were LS probands, the first person in a pedigree whom we have diagnosed as LS by confirming the pathogenic germline MMR gene mutation, and who have developed at least one cancer. Germline MMR gene mutations were confirmed during the period from May 2010 to March 2020. LS patients were identified from 2 cohorts in this study, and Fig. 1 shows how we identified the LS patients. Firstly, we recommended an MSI analysis to CRC and EMC patients meeting the revised Bethesda guidelines or the revised Amsterdam criteria (standard clinical practice cohort). If a cancer was found to be MSI-H, then we recommended a germline MMR gene mutation analysis after genetic counseling. We also recommended the MMR gene mutation analysis to MSS cancer patients or patients with an unknown MSI status (cancer tissue specimens were not available), if their clinical features strongly suggested LS (early onset, significant past or family history compatible with LS).
Figure 1.
A study flow chart. The yellow box indicates patients who underwent a germline MMR gene mutation analysis.
In addition, we also conducted universal screening by the MSI analysis for all newly diagnosed CRC patients at our hospital during the period from 2016 to 2020. If a CRC was MSI-H, then the methylation of the MLH1 promoter was analyzed by pyrosequencing. MSI-H CRC without MLH1 methylation was subjected to a germline MMR mutation analysis after genetic counseling.
MSI was analyzed by fluorescent PCR using 5 microsatellite markers (BAT-26, BAT-25, BAT-40, D2S123, D17S250). A mutation of 2 or more markers was judged to be MSI-H. A germline mutation of MMR genes (MLH1, MSH2, MSH6, and PMS2) was analyzed by Sanger sequencing, and a large deletion was analyzed by the multiplex ligation-dependent probe amplification (MLPA) method. Written informed consent was obtained, and all probands underwent genetic counseling before and after the MMR gene mutation analysis.
Results
The standard clinical cohort included 82 patients, whereas 112 CRC patients participated in the universal screening (Fig. 1). MMR mutations were analyzed in 21 patients of the standard clinical cohort (14 MSI-H, 2 MSS and 5 unknown MSI status but LS was strongly suspected), and 2 of 9 MSI-H CRC patients from the universal screening cohort (yellow box in Fig. 1). A pathogenic MMR germline mutation was identified in 19 from the standard clinical practice cohort and 2 from the universal screening cohort. As a result, a total of 21 probands, who developed at least one cancer, were thus diagnosed as having LS. Male: female ratio was 4:17; this female predominance was probably due to the fact that a significant number of cases were referred from gynecologists after surgery for endometrial or ovarian cancer. The mutated MMR genes were as follows; 6 probands with MLH1, 9 with MSH2, 4 with MSH6, and 2 with EPCAM deletion (Fig. 2). Regarding type of mutation, large (1-several exons) deletion and splice site mutation were most common (n=8, each), followed by small (2-5 bases) deletions (n=3), and nonsense mutations (n=2). Neither any insertion or missense mutations were observed (Fig. 3). The first cancer was CRC in 11 probands, two of whom had synchronous multiple CRCs (Table). Eight probands developed gynecological malignancies as the first cancer; 5 EMC, 2 EMC and ovarian, and 1 ovarian. The other first cancers were skin cancer (n=1, diagnosed as Muir-Torre syndrome), and urothelial cancer (n=1). Sixteen (76%) probands developed at least 2 metachronous cancers, while 5 (24%) developed 4 or more metachronous malignancies (Table).
Figure 2.
Mutated mismatch repair genes in the Lynch syndrome probands.
Figure 3.
The type of mutation.
Table.
Ages at Genetic Diagnosis, Ages at Cancer Diagnosis, Family History and Genetic Profile of 21 Lynch Syndrome Probands.
| # | Sex | Age at LS dx | 1st cancer | Age | Interval | 2nd cancer | Age | 3rd cancer | Age | 4th cancer | Age | 5th cancer | Age | 6th cancer | Age | Amsterdam | FDR with LSaM | Bethesda | MSI | MMR gene mutated | Mutation site | Mutation detail | Mutation type | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| at 1st ca. | at LS dx | |||||||||||||||||||||||
| 1 | F | 47 | EMC/OV | 47 | 0 | O | O | NA | NA | NA | MLH1 | Exon5 | c.381-415_453+733del (p.Ala128Trpfs*8) | Large deletion (1.2kb) | ||||||||||
| 2 | F | 38 | CRC | 38 | 0 | O | O | O | O | MSI-H | EPCAM | Exon8-9 | del | Large deletion | ||||||||||
| 3 | F | 46 | CRC | 46 | 0 | O | O | O | O | MSI-H | EPCAM | Exon8-9 | c.859-691_*1991del | Large deletion (4.1kb) | ||||||||||
| 4 | F | 49 | CRC | 47 | 2 | O | O | O | O | MSI-H | MSH2 | Exon4-6 | del | Large deletion | ||||||||||
| 5 | F | 48 | EMC | 45 | 3 | CRC | 48 | X | X | NA | O | MSS | MSH6 | Exon5 | c.3311_3312delTT | Small deletion | ||||||||
| 6 | F | 45 | EMC | 41 | 4 | X | O | NA | NA | NA | MLH1 | Intron6 | IVS6+3G>A | Splice site | ||||||||||
| 7 | F | 44 | EMC | 40 | 4 | CRC | 44 | X | O | NA | O | MSI-H | MSH2 | Intron13 | c.2211-2A>G | Splice site | ||||||||
| 8 | F | 49 | OV | 45 | 4 | UR | 49 | O | O | NA | NA | MSI-H | MSH2 | Exon13 | c.2038C>T (p.Arg680*) | Nonsense | ||||||||
| 9 | F | 39 | EMC/OV | 34 | 5 | CRC | 39 | O | O | NA | O | MSI-H | MSH2 | Intron7 | IVS7+1G>C | Splice site | ||||||||
| 10 | M | 62 | CRC x4 | 56 | 6 | CRC | 61 | CRC | 62 | X | NI | O | O | MSI-H | MSH2 | Exon4 | c.715C>T (p.Gln239*) | Nonsense | ||||||
| 11 | F | 81 | CRC x2 | 71 | 10 | EMC | 72 | SBC x2 | 81 | O | O | O | O | MSI-H | MSH6 | Intron9 | c.4001+1delG (p.Ala1268Glyfs*6) | Splice site | ||||||
| 12 | F | 48 | CRC | 36 | 12 | CRC | 45 | CRC | 48 | O | O | O | O | MSI-H | MLH1 | Exon15 | c.1731G>A (p.Ser556Argfs*14) | Splice site | ||||||
| 13 | F | 73 | CRC | 60 | 13 | EMC | 72 | CRC | 73 | NI | NI | X | O | MSI-H | MSH2 | Exon7 | del | Large deletion | ||||||
| 14 | F | 65 | CRC | 53 | 13 | Panc | 57 | EMC | 60 | UR | 61 |
Gast/
Brea |
65 | X | O | X | O | NA | MSH2 | Exon1-6 | c.-11917_1076+4711del | Large deletion (30kb) | ||
| 15 | M | 66 | CRC | 52 | 14 | Gast | 61 | CRC | 66 | X | O | X | O | MSI-H | MSH6 | Exon4 | c.3037_3041 del AAGAA | Small deletion | ||||||
| 16 | F | 80 | EMC | 62 | 18 | CRC | 65 | O | O | NA | O | MSS | MSH6 | Intron9 | c.4001+1delG (p.Ala1268Glyfs*6) | Splice site | ||||||||
| 17 | F | 68 | EMC | 47 | 21 | CRC | 50 | CRC | 54 | CRC | 64 | CRC | 65 | CRC | 68 | O | O | NA | O | MSI-H | MSH2 | Exon2 | c. 254_255delTT | Small deletion |
| 18 | F | 84 | UR | 61 | 23 | EMC | 73 | Skin | 78 | Eso | 84 | O | O | NA | NA | NA | MSH2 | Exon10 | c.1511-1360_1662-1063del (p.Gly504Alafs*3) | Large deletion (4.6kb) | ||||
| 19 | F | 68 | CRC | 45 | 23 | EMC | 50 | RCC | 62 | SBC/CRC | 62 | RCC | 67 | SBC | 68 | NI | NI | O | O | MSI-H | MLH1 | Intron15 | IVS15-1G>A | Splice site |
| 20 | M | 76 | CRC | 51 | 25 | CRC | 54 | CRC x2 | 76 | X | O | X | O | MSI-H | MLH1 | Intron6 | c.545+4_545+5delCA (p.Glu153Phefs*8) | Splice site | ||||||
| 21 | M | 77 | Skin | 52 | 25 | Skin | 71 | Skin | 76 | Skin | 77 | CRC | 77 | O | O | NA | O | NA | MLH1 | Exon5 | del | Large deletion (1.2kb) | ||
Probands were sorted by the interval (between the first cancer and genetic diagnosis).
Bold and Italic fonts in cancer and age from 1st to 6th are those led to the genetic diagnosis of Lynch syndrome in each probands.
EMC: endometrial cancer, OV: ovarian cancer, CRC: colorectal cancer, UR: urothelial cancer, Panc: pancreatic cancer, Gast: gastric cancer, SBC: small bowel cancer, RCC: renal cell cancer, Eso: esophageal cancer, Brea: breast cancer, FDR: first degree relative, LSaM: Lynch syndrome associated malignancy, NI: not informative, NA (in Bethesda): not applicable (because they do not have history of CRC), MSI-H: microsatellite instability-high, MSS: microsatellite stable, NA (in MSI): not analyzed, del: deletion
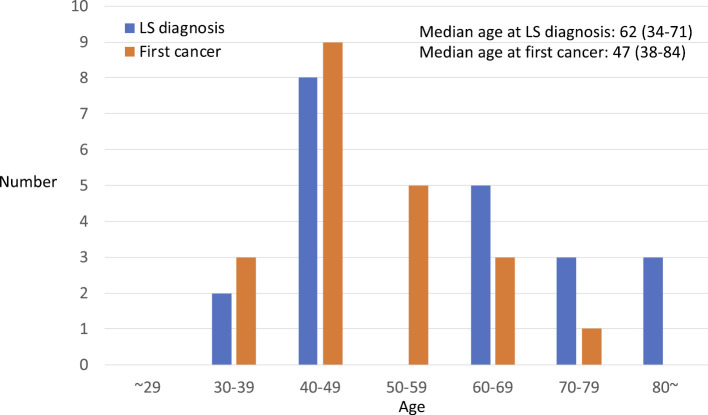
The median age at first cancer was 47 years (range 34 to 71), whereas the median age at the genetic diagnosis (confirmation of germline MMR mutation) was 62 years (range 38 to 84) (Fig. 4). There was no proband who developed a first cancer before 30 years of age, whereas 4 (19%) of 21 probands developed the first cancer at 60 years of age or later (Fig. 4, Table). The median and mean interval between the development of the first cancer and genetic confirmation were 11 and 10.95 years (range 0 to 25), respectively. The interval was 0 (i.e. MMR gene mutation was confirmed within a year of the first cancer diagnosis) in 3 (14%) probands, whereas 20 years or longer interval was required for the 5 (24%) probands. Six (28.6%) probands were older than 70 years of age, and 3 (14.3%) were in their 80s when they were diagnosed to have LS. The genetic diagnosis was confirmed at the first, second, third, and fourth cancer or later in 5, 5, 6, and 5 probands, respectively.
Figure 4.
The ages at the genetic diagnosis and first cancer in the Lynch syndrome probands.
Fourteen (87.5%) of the 16 cancers examined were MSI-H, whereas 2 (12.5%) were MSS. Both the 2 MSS cancer patients had MSH6 mutations (Table). Although there was no proband who had an MMR gene mutation confirmed before the first colectomy, MSI-H was confirmed before the first colectomy in 2 probands. One proband with transverse colon cancer chose total colectomy, whereas another with cecal cancer underwent a standard ileocecal resection. Of the 17 LS probands we are currently following up, 16 probands are alive without cancer. One patient (#18 in Table) died of metastatic brain tumors of an unknown primary cancer.
All 17 (100%) and 7 of 11 (63.6%) probands met the revised Bethesda guidelines at the genetic diagnosis and at the first cancer, respectively, while 12 of 21 (57.1%) met the revised Amsterdam criteria (Table). Of the 21 probands, 17 (81%) had at least one first degree relative with LS associated malignancies. Therefore, 4 (19%) probands either did not have any cancer family history or their family history was not informative (Table). Probands with longer than a 20 year-interval (long interval group, n=5) were compared with those with less than a 5 year-interval (short interval group, n=8). The first cancer age was younger in the short interval group than in the long one (median 45 years vs. 51). Although the number is small, other profiles including the Amsterdam criteria, MSI, and mutated genes, were similar between the two groups.
Discussion
There is no past study focusing on the probands' age at the genetic diagnosis for LS. Our study for the first time demonstrated that it took more than a decade from developing the first cancer to confirming the genetic diagnosis for LS probands. The 11 year-interval was longer than we expected, so we would like to speculate on some of the plausible reasons for this time gap.
First, a substantial number of LS patients do not show a typical clinical presentation as hereditary cancer syndrome; namely, they are either not so young or have no particular family history. Our study also demonstrated that 19% of the LS probands developed the first cancer at 60 years of age or older, and 19% did not have a cancer family history. Such atypical LS patients can be missed by gastroenterologists, surgeons, or gynecologists. We also tend to have problems in identifying LS patients in our clinical practice. Our interview of cancer patients regarding their past and family history is often insufficient. For example, we tend to check only the type of cancer family member suffered, without asking the age at cancer diagnosis. In addition, when a colon cancer patient says his (her) mother has a history of “uterine cancer”, we often fail to confirm whether it was cervical or endometrial. It can also be an obstacle to obtain accurate past history that LS patients often had surgery for previous cancer at other hospital many years previously.
Second, there are hurdles in the genetic diagnosis of Lynch syndrome. For example, genetic tests are expensive and not covered by the Japanese national medical insurance system at present. A MMR gene mutation analysis costs approximately ¥100,000-150,000 ($1,000-$1,500) for probands in Japan. In addition, genetic counseling is necessary for LS diagnostic confirmation, but the number of hospitals providing genetic counseling is still insufficient in Japan.
MSH6 and PMS2 mutation carriers generally show an attenuated phenotype, such as a later onset and a lower penetrance than MLH1 and MSH2 carriers (3,4). LS patients with MSH6 and PMS2 mutations thus might be missed more frequently than those with MLH1 or MSH2 mutations. In our study, MSH6 mutation carriers account for 19% (4/21) of all probands, and there were no PMS2 mutation carriers. Although the number is small, the mean interval between the first cancer and the genetic diagnosis for LS probands according to mutated gene was 14.8 years for MLH1, 10.1 years for MSH2, 11.3 years for MSH6, and 0 years for EPCAM in this study. It is thus unlikely that the study results were influenced by a mutated MMR gene.
In general practice, we should make an effort to shorten the 11-year interval as well as not to miss LS patients. Traditional methods to identify LS patients are based on the Amsterdam criteria (10) and the Bethesda guidelines (11). However, the sensitivity of the revised Amsterdam criteria is as low as 20-30%. The revised Bethesda guidelines have an improved sensitivity of 80-90%, but the Bethesda guidelines can be applicable only for patients who developed CRC. In our study, all LS probands who developed CRC met the revised Bethesda criteria at genetic diagnosis, but it dropped to 63.6% at the first cancer diagnosis. Therefore it might be difficult to identify all LS probands at the first cancer based on the Bethesda guidelines. Several prediction models for LS are also available (12-14), but they are only useful when we suspect LS and obtain a precise past and family history. Recently, the American gastroenterological association recommended universal screening, in which ALL newly diagnosed CRC patients should be tested mismatch repair status either by a MSI analysis or immunohistochemistry (15). Although the cost-efficiency of this strategy has not yet been fully estimated, such universal screening might be helpful to shorten the interval between the first cancer and the genetic diagnosis of LS probands.
One limitation associated with this study is the retrospective study design and the small number of patients. It is difficult to conclude from our results that an early diagnosis leads to a better survival for LS probands and their family members. However, the benefits of an early diagnosis by surveillance colonoscopy in LS has been demonstrated in past studies (16-19). We therefore believe that an early diagnosis is beneficial both to the probands and family members of LS patients.
We herein demonstrated that it takes more than a decade from the first cancer to genetic diagnosis confirmation for LS probands in current general practice. We should therefore increase our efforts, such as by conducting careful interviews regarding the patients' past and family history, obtaining pathology reports and surgical specimens (for MSI analysis or IHC) from patients at other hospitals who have undergone surgery for previous cancers. Finally, universal screening also might be useful for shortening the interval.
The authors state that they have no Conflict of Interest (COI).
Financial Support
This study was partly supported by the Japan agency for medical research and development (AMED) under grant JP 18kk025004, JSPS KAKENHI grant number JP18K07339, and the National Cancer Center research and development fund grant number 31-A-2, all to Akagi K.
References
- 1.Bonadona V, Bonaiti B, Olschwang S, et al. Cancer risks associated with germline mutations in MLH1, MSH2, and MSH6 genes in Lynch syndrome. JAMA 305: 2304-2310, 2011. [DOI] [PubMed] [Google Scholar]
- 2.Dowty JG, Win AK, Buchanan DD, et al. Cancer risks for MLH1 and MSH2 mutation carriers. Hum Mutat 34: 490-497, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baglietto L, Lindor NM, Dowty JG, et al. Risks of Lynch syndrome cancers for MSH6 mutation carriers. J Natl Cancer Inst 102: 193-201, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.ten Broeke SW, Brohet RM, Tops CM, et al. Lynch syndrome caused by germline PMS2 mutations: delineating the cancer risk. J Clin Oncol 33: 319-325, 2015. [DOI] [PubMed] [Google Scholar]
- 5.Kalady MF, McGannon E, Vogel JD, Manilich E, Fazio VW, Church JM. Risk of colorectal adenoma and carcinoma after colectomy for colorectal cancer in patients meeting Amsterdam criteria. Ann Surg 252: 507-511, 2010. [DOI] [PubMed] [Google Scholar]
- 6.Parry S, Win AK, Parry B, et al. Metachronous colorectal cancer risk for mismatch repair gene mutation carriers: the advantage of more extensive colon surgery. Gut 60: 950-957, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Renkonen-Sinisalo L, Seppala TT, Jarvinen HJ, Mecklin JP. Subtotal colectomy for colon cancer reduces the need for subsequent surgery in Lynch syndrome. Dis Colon Rectum 60: 792-799, 2017. [DOI] [PubMed] [Google Scholar]
- 8.Syngal S, Brand RE, Church JM, Giardiello FM, Hampel HL, Burt RW. ACG clinical guideline: genetic testing and management of hereditary gastrointestinal cancer syndromes. Am J Gastroenterol 110: 223-262, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herzig DO, Buie WD, Weiser MR, et al. Clinical practice guidelines for the surgical treatment of patients with Lynch syndrome. Dis Colon Rectum 60: 137-143, 2017. [DOI] [PubMed] [Google Scholar]
- 10.Vasen HF, Watson P, Mecklin JP, Lynch HT. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the international collaborative group on HNPCC. Gastroenterology 116: 1453-1456, 1999. [DOI] [PubMed] [Google Scholar]
- 11.Umar A, Boland CR, Terdiman JP, et al. Revised Bethesda guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst 96: 261-268, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barnetson RA, Tenesa A, Farrington SM, et al. Identification and survival of carriers of mutation in DNA mismatch-repair genes in colon cancer. N Engl J Med 354: 2751-2763, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Chen S, Wang W, Lee S, et al. Prediction of germline mutations and cancer risk in the Lynch syndrome. JAMA 296: 1479-1487, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kastrinos F, Steyerberg EW, Mercado R, et al. The PREMM(1,2,6) model predicts risk of MLH1, MSH2, and MSH6 germline mutations based on cancer history. Gastroenterology 140: 73-81, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rubenstein JH, Enns R, Heidelbaugh J, Barkun A; Clinical Guidelines Committee. American gastroenterological association institute guideline on the diagnosis and management of Lynch syndrome. Gastroenterology 149: 777-782, 2015. [DOI] [PubMed] [Google Scholar]
- 16.Engel C, Rahner N, Schulmann K, et al. Efficacy of annual colonoscopic surveillance in individuals with hereditary nonpolyposis colorectal cancer. Clin Gastroenterol Hepatol 8: 174-182, 2010. [DOI] [PubMed] [Google Scholar]
- 17.de Jong AE, Hendriks YM, Kleibeuker JH, et al. Decrease in mortality in Lynch syndrome families because of surveillance. Gastroenterology 130: 665-671, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Järvinen HJ, Aarino M, Mustonen H, et al. Controlled 15-year trial on screening for colorectal cancer in families with hereditary nonpolyposis colorectal cancer. Gastroenterology 118: 829-834, 2000. [DOI] [PubMed] [Google Scholar]
- 19.Syngal S, Weeks JC, Schrag D, Garber JE, Kuntz KM. Benefits of colonoscopic surveillance and prophylactic colectomy in patients with hereditary nonpolyposis colorectal cancer mutations. Ann Intern Med 129: 787-796, 1998. [DOI] [PubMed] [Google Scholar]