Abstract
A 45-year-old man with allergic bronchopulmonary aspergillosis (ABPA) was treated with oral prednisolone (PSL) (30 mg/day), inhaled corticosteroids, and long-acting beta2-agonists. After confirmation of a PSL-dependent status (8 mg/day), subcutaneous injection with anti-interleukin (IL)-5 antibody (mepolizumab, 100 mg/month) was performed, and the PSL dose was tapered to 5 mg/day. However, ABPA recurred and proved refractory to oral itraconazole (200 mg/day). Alternative subcutaneous injection therapy with dupilumab (induction dose of 600 mg followed by a maintenance dose of 300 mg/2 weeks) enabled the successful withdrawal of oral PSL without clinical deterioration. This case demonstrates the potential utility of dupilumab for steroid-dependent ABPA via the synergistic suppression of IL-4 and IL-13 compared to monotherapy with anti-IL-5 antibody.
Keywords: allergic bronchopulmonary aspergillosis, dupilumab, mepolizumab, prednisolone
Introduction
Recurrence of mucus plugs in allergic bronchopulmonary aspergillosis (ABPA) can cause bronchiectasis and lung fibrosis, which may lead to lung destruction. Approximately 22% of patients with ABPA experience exacerbation 2 years after the cessation of systemic prednisolone treatment, a so-called glucocorticoid-dependent status. Furthermore, the long-term use of systemic prednisolone has been associated with nontuberculous mycobacterial infection, especially in patients with comorbid cystic fibrosis and ABPA (1).
Recently, dupilumab, a fully human anti-IL-4 receptor α (IL-4Rα) monoclonal antibody, has been used to treat severe asthma to block both IL-4 and IL-13 signaling. Dupilumab can decrease the rate of asthma attacks compared to placebo under a glucocorticoid-dependent asthmatic status (2). However, there are few reported cases regarding the use of dupilumab for ABPA (3).
We herein report a case of ABPA refractory to mepolizumab plus prednisolone therapy that responded well to dupilumab without the use of prednisolone.
Case Report
A 45-year-old man with bronchial asthma from childhood, treated with a high dose of inhaled salmeterol/fluticasone, presented to our hospital with brownish phlegm that had persisted for the last month.
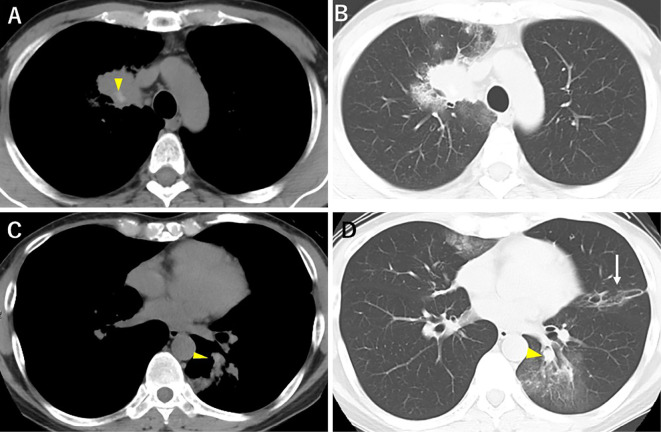
His vital signs were as follows: body temperature, 36.5℃; blood pressure, 104/62 mmHg; heart rate, 82 bpm; SpO2, 95% with ambient air; and respiratory rate, 16 breaths per minute. On a physical examination, Johnson Grade 1 wheezing was recognized in all lung fields. Chest radiography (Fig. 1) on admission revealed dense infiltration in the right upper and left lower lung fields. Non-enhanced thoracic computed tomography (CT) demonstrated the soft tissue in right S1 with high attenuation mucus in the central proximal portion of right B1 (Fig. 2A, arrowhead) and left B6 (Fig. 2C, arrowhead). The bronchiectasis of the proximal portion of the left B4 (Fig. 2D, arrow) and multiple patchy ground glass opacities (GGO) were also noted (Fig. 2B, D). Serum data on admission demonstrated elevations in the white blood cell count (12,400/μL) with eosinophilia (1,914/μL), and total IgE (2,306 IU/μL). Furthermore, Aspergillus fumigatus-specific-IgE was also elevated (14 UA/mL, reference range 0-0.34).
Figure 1.
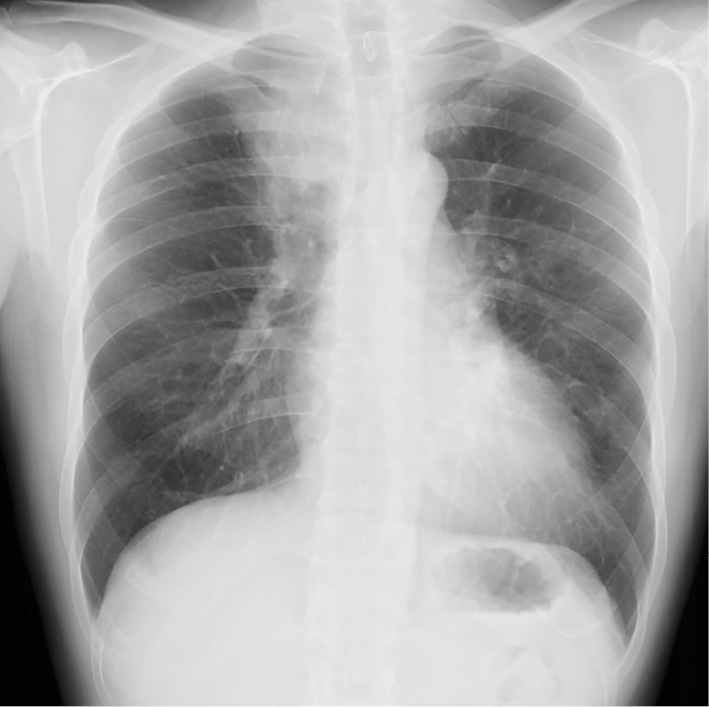
Chest X-ray showing dense consolidation at the right upper and left lower lung fields.
Figure 2.
Non-enhanced thoracic CT reveals high-attenuation mucus in right B1 (A, arrowhead) with atelectasis surrounded by GGOs (B). Central bronchiectasis is noted in both the left B6 (C, D, arrowhead) and B4 (D, arrow) with mucoid impaction (D, arrowhead). Multiple GGOs can be seen at the right middle lobe, left S4, and left S6. CT: computed tomography, GGO: ground glass opacity
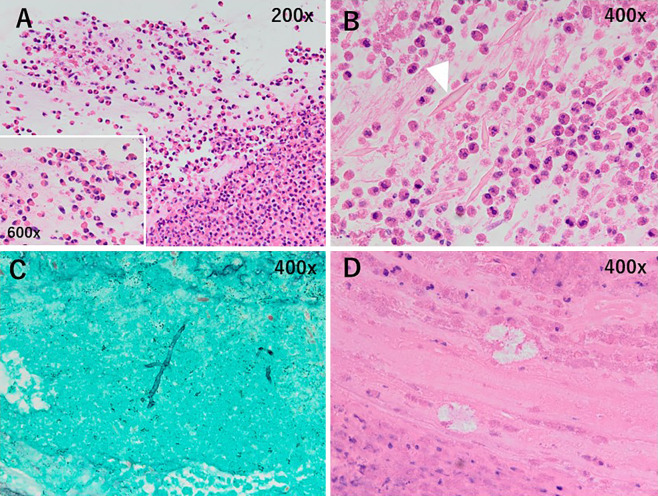
Based on the tentative diagnosis of ABPA, bronchoscopy was performed. It revealed that the mucosa of the orifice of the right upper lobe bronchus was edematous and reddened with mucoid impaction (Fig. 3). Mucoid impaction occluded the tracheal lumen of the right B1 (Fig. 3, asterisk), and the biopsied specimen from the lesion was pathologically proven to be mucoid impaction, containing abundant eosinophils (Fig. 4A, 200×, inset 600×), Charcot-Leyden crystals (Fig. 4B, 400×), filamentous fungal hyphae (Fig. 4C, 400×), and calcium oxalate crystals (Fig. 4D, 400×). He was diagnosed with ABPA according to the criteria of the International Society for Human and Animal Mycology (4).
Figure 3.
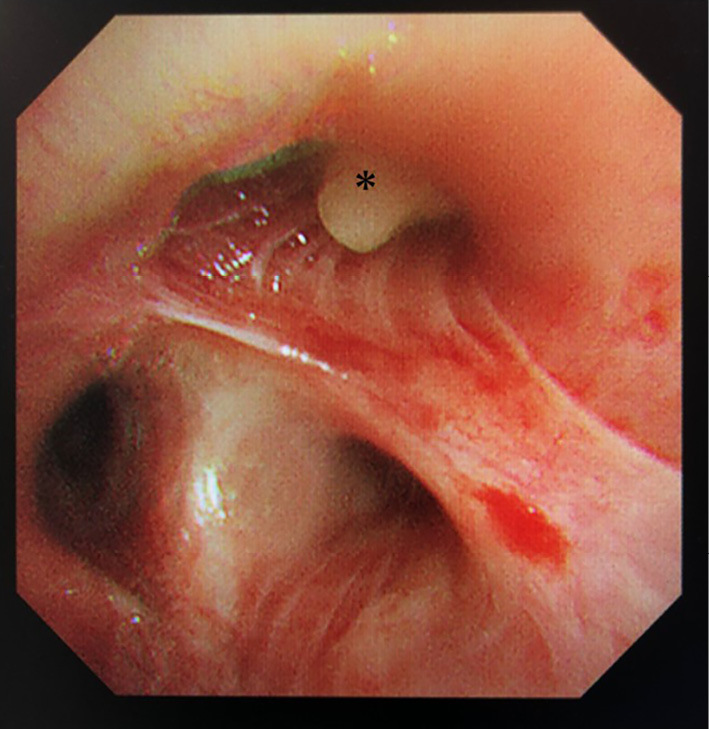
Bronchoscopy reveals that the orifice of the right upper lobe bronchus was occluded by mucoid impaction (asterisk) accompanied by mucosal edema and redness.
Figure 4.
Hematoxylin and Eosin staining shows that the material biopsied from the mucoid impaction contains abundant eosinophils (A, 200×, inset 600×), Charcot-Leyden crystals (B, 400×, arrowhead), and calcium oxalate crystals (D, 400×). Filamentous fungal hyphae are noted on the Papanicolaou-stained specimen (C, 400×).
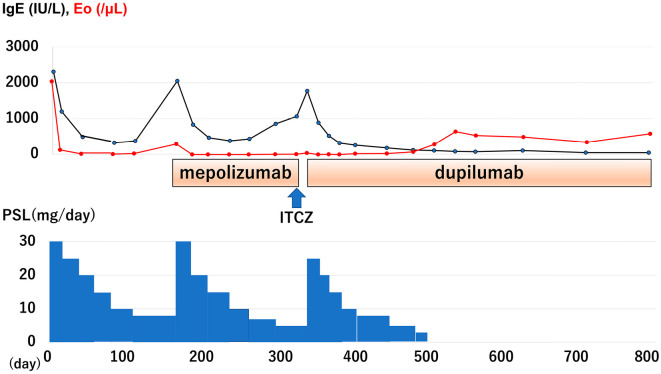
After the initiation of oral therapy with PSL of 30 mg/day, his symptoms disappeared, and his serum total IgE levels dramatically decreased (321 IU/mL) (Fig. 5) along with the complete resolution of abnormal lung lesions. However, when the maintenance dose of PSL was tapered to 8 mg/day, asthma attacks recurred with a marked increase in IgE (2,049 IU/μL), which was considered the first recurrence of ABPA (Fig. 5). He was treated again with an intensive dose of PSL (30 mg/day) together with subcutaneous induction of mepolizumab (100 mg/month) due to his PSL-dependent status. However, at 150 days after the initiation of mepolizumab with oral PSL (5 mg/day), a second recurrence occurred. Additional therapy with oral itraconazole (ITCZ) (200 mg/day) did not improve the symptoms. Therefore, mepolizumab with ITCZ and PSL (5 mg/day) was replaced by PSL (25 mg/day) and dupilumab with a subcutaneous induction dose of 600 mg followed by a maintenance dose of 300 mg/2 weeks. One month after the induction of dupilumab, oral PSL was decreased to 10 mg/day with the complete resolution of radiological abnormal lesions. Four months later (507 days after initiation of the first oral PSL), PSL was able to be safely withdrawn with the ABPA in remission. One year later, the patient remained in complete remission of ABPA with low serum total IgE levels (44 IU/mL).
Figure 5.
Clinical course of the present case.
Discussion
This case demonstrated the clinical utility of dupilumab for severe ABPA compared to combination therapies with mepolizumab, PSL, and ITCZ. At present, approved biologic agents, such as IL-5 drugs (mepolizumab, benralizumab) and IL-4Rα (dupilumab), have shown oral glucocorticoid-sparing effects in patients with severe asthma (5,6). However, the effect of biologic agents on ABPA is unclear. Jian-Xiong et al. reported a systematic review of omalizumab for steroid-dependent ABPA, in which 28.43% withdrew from PSL, and 65.68% reduced their PSL dose (7). However, these data seemed to have selection bias, and no randomized trial concerning the steroid-sparing effect of biologic agents for steroid-dependent ABPA has been reported. In this regard, the present case clearly demonstrated the clinical utility of dupilumab for cases that are refractory to combination therapy with mepolizumab, ITCZ, and PSL.
Although the difference in the steroid-sparing effect between mepolizumab and dupilumab has not been elucidated, our results suggest the significance of IL-4 and IL-13 in the pathogenesis of ABPA. Backer et al. revealed a Th2 response in peripheral blood mononuclear cells derived from patients with ABPA (8). Furthermore, patients with ABPA comorbid with cystic fibrosis (CF) have increased sensitivity to IL-4 compared to those with non-ABPA CF, as confirmed by the upregulation of the CD23 expression on B-cells (9). Intratracheal mucus plugs are hallmarks of the pathogenesis of ABPA, and IL-13 appears to play a crucial role in ABPA by triggering or prolonging airway hyperreactivity, mucus secretion, and remodeling (10).
Furthermore, Gargi et al. indicated the role of group 2 innate lymphoid cells (ILC2), which mediate airway inflammation and hyper-responsiveness through the production of IL-5, IL-13, and vascular endothelial growth factor. They showed that the blockade of IL4Rα by dupilumab effectively suppressed the ILC2 response, subsequently reducing the serum levels of IL-5 and IL-13 in patients with asthma (11). These results suggest that dupilumab suppresses not only the IL-4R receptor pathway but also the IL-5 pathway in patients with ABPA.
Biological agents may have a potential role in treating steroid-dependent severe ABPA; however, Ramonell et al. reported an asthma attack associated with dupilumab-induced hypereosinophilia without the concomitant use of oral PSL (3). This indicates that the concurrent use of oral PSL may be preferable at the induction phase of dupilumab therapy, as in the present case.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Mussaffi H, Rivlin J, Shalit I, Ephros M, Blau H. Nontuberculous mycobacteria in cystic fibrosis associated with allergic bronchopulmonary aspergillosis and steroid therapy. Eur Respir J 25: 324, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Rabe KF, Nair P, Brusselle G, et al. Efficacy and safety of dupilumab in glucocorticoid-dependent severe asthma. N Engl J Med 378: 2475, 2018. [DOI] [PubMed] [Google Scholar]
- 3.Ramonell RP, Lee FE, Swenson C, Kuruvilla M. Dupilumab treatment for allergic bronchopulmonary aspergillosis: a case series. J Allergy Clin Immunol Pract 8: 742, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agarwal R, Chakrabarti A, Shah A, et al. Allergic bronchopulmonary aspergillosis: review of literature and proposal of new diagnostic and classification criteria. Clin Exp Allergy 43: 850-873, 2013. [DOI] [PubMed] [Google Scholar]
- 5.Nair P, Wenzel S, Rabe KF, et al. Oral glucocorticoid-sparing effect of benralizumab in severe asthma. N Engl J Med 376: 2448, 2017. [DOI] [PubMed] [Google Scholar]
- 6.Nair P, Pizzichini MM, Kjarsgaard M, et al. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. N Engl J Med 360: 985, 2009. [DOI] [PubMed] [Google Scholar]
- 7.Li JX, Fan LC, Li MH, Cao WJ, Xu JF. Beneficial effects of omalizumab therapy in allergic bronchopulmonary aspergillosis: a synthesis review of published literature. Respir Med 122: 33, 2017. [DOI] [PubMed] [Google Scholar]
- 8.Becker KL, Gresnigt MS, Smeekens SP, et al. Pattern recognition pathways leading to a Th2 cytokine bias in allergic bronchopulmonary aspergillosis patients. Clin Exp Allergy 45: 42, 2015. [DOI] [PubMed] [Google Scholar]
- 9.Knutsen AP, Hutchinson PS, Albers GM, Consolino J, Smick J, Kurup VP. Increased sensitivity to IL-4 in cystic fibrosis patients with allergic bronchopulmonary aspergillosis. Allergy 59: 81, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Moss RB. The use of biological agents for the treatment of fungal asthma and allergic bronchopulmonary aspergillosis. Ann NY Acad Sci 1272: 49, 2012. [DOI] [PubMed] [Google Scholar]
- 11.Patel G, Pan J, Ye L, et al. Blockade of IL-4Rα inhibits group 2 innate lymphoid cell responses in asthma patients. Clin Exp Allergy 50: 267, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]