Abstract
A 46-year-old woman with exacerbating hemoptysis and dyspnea was diagnosed with diffuse alveolar hemorrhage (DAH). High doses of glucocorticoids were initiated, but afterward, paroxysmal hypertension (210/140 mmHg) with headache and abdominal pain appeared. A 50-mm left adrenal tumor with an intense uptake by iodine-123 metaiodobenzylguanidine scintigraphy and catecholamine hypersecretion revealed complication with pheochromocytoma. Because high doses of glucocorticoids, sometimes required for DAH, can provoke life-threatening paroxysmal hypertension in pheochromocytoma and paraganglioma (PPGL), our case suggests that PPGL needs to be recognized as the cause of DAH and should be detected with whole-body imaging before starting glucocorticoids.
Keywords: pheochromocytoma, diffuse alveolar hemorrhage, high-dose glucocorticoids, catecholamine crisis
Introduction
Pheochromocytoma and paraganglioma (PPGL) are neoplastic diseases derived from chromaffin cells in adrenal medulla and extra-adrenal sympathetic paravertebral gangliasympathetic paravertebral ganglia, respectively (1). PPGL is usually characterized by hypersecretion of catecholamines, including adrenaline and noradrenaline (also known as epinephrine and norepinephrine, respectively) and dopamine (1), which cause various symptoms, such as headaches, palpitations, or anxiety (2). PPGL also causes paroxysmal or refractory hypertension and cardiovascular diseases (1). High-dose glucocorticoids can provoke paroxysmal hypertension in PPGL that can lead to a life-threatening condition called catecholamine crisis (3,4).
Diffuse alveolar hemorrhage (DAH) is a syndrome characterized by the leakage of red blood cells into the alveolar space (5). Clinical features of DAH vary in severity, but it can be life-threatening (5). The causes of DAH are multiple, including autoimmune and nonautoimmune diseases (5), and high-dose glucocorticoids are sometimes used as an immunosuppressive treatment, especially for DAH due to autoimmune diseases (5-7).
We herein report a case of pheochromocytoma that was diagnosed during the treatment of DAH, and paroxysmal hypertension was evoked after starting high-dose glucocorticoids for DAH. There have been reports of simultaneously diagnosed DAH and PPGL, and the hemodynamic effects of PPGL have been considered to be the cause of DAH. Therefore, our case suggests the importance of identifying PPGL as a cause of DAH with whole-body imaging before treatment with high-dose glucocorticoids to avoid the risk of catecholamine crisis.
Case Report
A 46-year-old woman was transported by ambulance with a chief complaint of dyspnea and hemoptysis. She had been treated for cough variant asthma and hypertension with medical histories of frequent hyperventilation attacks and conjunctival bleeding but had no history of surgery for pituitary, adrenal, or parathyroid diseases. Two days before her visit, she experienced sore throat and cough and was diagnosed with upper respiratory tract inflammation at a nearby clinic and treated with antibiotics and symptomatic drugs. On the day of hospitalization, dyspnea and hemoptysis appeared and worsened at midnight, at which point she was brought to our hospital.
The patient was 154.7 cm tall, weighed 62.8 kg, and had a blood pressure of 80/41 mmHg, a pulse rate of 96 beats/min, a body temperature of 36.6℃, a respiratory rate of 40 breaths/min, and pulse oximetry (SpO2) of 91% on room air. Because an electrocardiogram and transthoracic echocardiography revealed no remarkable abnormalities and the N-terminal pro-B-type natriuretic peptide levels were not increasing (67 pg/mL, reference range; <125 pg/mL), heart failure was excluded. Based on the results of chest X-ray and contrast-enhanced computed tomography (CT) (Fig. 1), DAH was diagnosed, and the patient was hospitalized.
Figure 1.
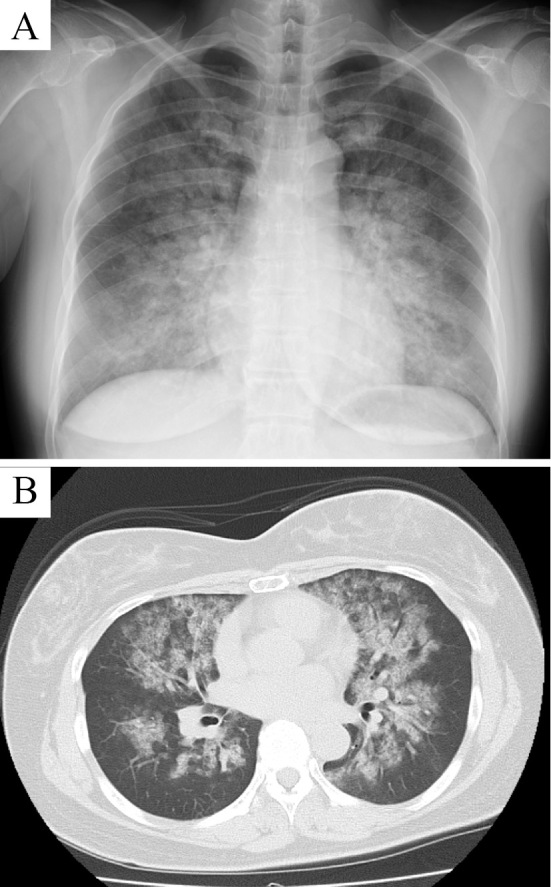
Image showing diffuse alveolar hemorrhage (DAH). (A) Chest X-ray and (B) CT scan showing bilateral diffuse infiltrates.
She had been taking 20 mg/day of telmisartan and 15 mg/day of clotiazepam. The antibiotics and symptomatic drugs from a nearby clinic two days before were not the types of medications to induce catecholamine crisis. Considering the potential involvement of autoimmune diseases, immunosuppressive therapy with glucocorticoid (1,000 mg/day of methylprednisolone) and 500 mg/day of cyclophosphamide were initiated. Plasma exchange was also performed until the fifth day of hospitalization. At midnight, after the dosage of glucocorticoids had been reduced to 60 mg/day of prednisolone a day, paroxysmal hypertension (210/140 mmHg) with headache and abdominal pain occurred, but the administration of acetaminophen alone ameliorated both symptoms and blood pressure. Whole-body CT showed a left adrenal tumor over 50 mm in size with unenhanced attenuation at 20-60 Hounsfield units (Fig. 2A), suggesting pheochromocytoma. Iodine-123 metaiodobenzylguanidine (123I-MIBG) scintigraphy and single-photon emission CT (SPECT)-CT were performed, and a strong uptake at the left adrenal tumor was confirmed (Fig. 2B, C).
Figure 2.
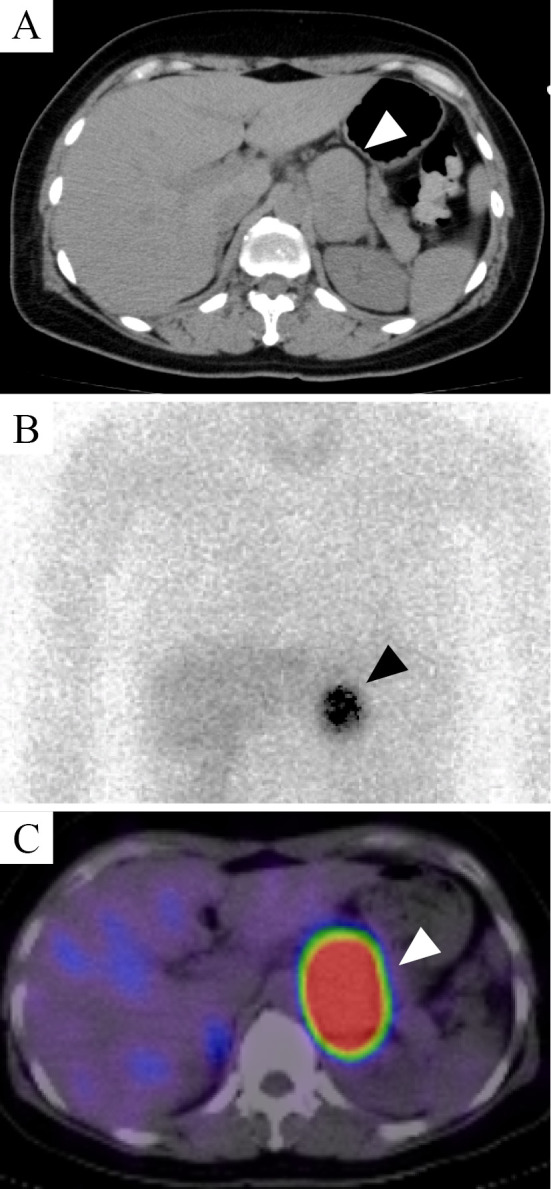
Image showing a left adrenal tumor. (A) Unenhanced CT scan showing a tumor of >50 mm (white arrowhead) in which the attenuation value was 20–60 Hounsfield units. (B) Iodine-123 metaiodobenzylguanidine (123I-MIBG) scintigraphy and (C) single-photon emission CT demonstrated an intense uptake in the adrenal tumor (white and black arrowheads).
Because no findings suggestive of immunological abnormalities (Table 1) in blood sampling tests were present and the patient's general condition improved, glucocorticoid treatment was gradually tapered and terminated until discharge due to concerns about catecholamine crisis caused by high-dose glucocorticoids. After DAH was cured, she was discharged on the 14th day of hospitalization and readmitted for a detailed diagnostic examination for pheochromocytoma 1 month later. Laboratory data from the blood and 24-hour urine collection samples showed higher levels of adrenaline, noradrenaline, metanephrine, and normetanephrine, demonstrating catecholamine hypersecretion (Table 2), whereas the plasma renin activity, plasma aldosterone concentration, adrenocorticotrophic hormone, cortisol, dehydroepiandrosterone sulfate, and estradiol were in the normal ranges (Table 2). The midnight cortisol level was 2.25 μg/dL, and the morning cortisol level after taking 1 mg of dexamethasone at midnight was 0.71 μg/dL. These data indicated that there was no complication with primary aldosteronism, Cushing's syndrome, or adrenocortical carcinoma.
Table 1.
Immunology Tests.
| Immunology tests | ||||||
|---|---|---|---|---|---|---|
| Antinuclear antibody | Negative | Anti-Sm antibody | Negative | |||
| Anti-CCP antibody | Negative | Anti-Ro (SS-A) antibody | Negative | |||
| MPO-ANCA | Negative | Anti-La (SS-B) antibody | Negative | |||
| PR3-ANCA | Negative | Anti-Scl-70 antibody | Negative | |||
| Anti-GBM antibody | Negative | Anti-ARS antibody | Negative | |||
| Anti-RNP antibody | Negative |
ANA: antinuclear antibody, CCP: cyclic citrullinated peptide, MPO: myeroperoxidase, ANCA: anti-neutrophil cytoplasmic antibody, PR3: proteinase3, GBM: glomerular basement membrane, RNP: ribonucleoprotein, Sm: Smith, Scl-70: scleroderma-70, ARS: aminoacyl tRNA synthetase
Table 2.
Endocrinology Tests.
| Blood (Before surgery) | Reference range | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AD | (pg/mL) | 963 | (<100) | ||||||||
| NAD | (pg/mL) | 540 | (100 - 450) | ||||||||
| DA | (pg/mL) | 19 | (<20) | ||||||||
| PRA | (ng/mL/hr) | 1.1 | (0.3 - 2.9) | ||||||||
| PAC | (pg/mL) | 130 | (29.9 - 158.8) | ||||||||
| ACTH | (pg/mL) | 11.9 | (7.2 - 63.3) | ||||||||
| Cortisol | (μg/dL) | 8.49 | (6.2 - 18.0) | ||||||||
| DHEAS | (µg/dL) | 33 | (19 - 231) | ||||||||
| E2 | (pg/mL) | 5.8 | (25 - 550) | ||||||||
| Whole PTH | (pg/mL) | 34.3 | (8.3 - 38.7) | ||||||||
| 24h-urine collection | Before surgery (2 times) | After surgery | Reference range | ||||||||
| AD | (μg/day) | 481.9 | 553.8 | 51.8 | (3.4 - 26.9) | ||||||
| NAD | (μg/day) | 384.0 | 484.2 | 111.0 | (48.6 - 168.4) | ||||||
| DA | (μg/day) | 2,126 | 3,046 | 746.8 | (365.0 - 961.5) | ||||||
| VMA | (mg/day) | 25.7 | 27.3 | 3.5 | (1.5 - 4.3) | ||||||
| HVA | (mg/day) | 6.0 | 6.3 | 3.6 | (2.1 - 4.3) | ||||||
| MN | (mg/day) | 9.31 | 10.01 | 0.62 | (0.0 - 0.2) | ||||||
| NMN | (mg/day) | 2.50 | 2.77 | 0.44 | (0.1 - 0.3) | ||||||
| Free cortisol | (µg/day) | 31.4 | 33.9 | (11.2 - 80.3) | |||||||
| ALD | (µg/day) | 15 | 14 | (<10.0) | |||||||
AD: adrenaline, NAD: noradrenaline, DA: dopamine, PRA: plasma renin activity, PAC: plasma aldosterone concentration, ACTH: adrenocorticotrophic hormone, DHEAS: dehydroepiandrosterone sulfate, E2: estradiol, PTH: parathyroid hormone, VMA: vanillylmandelic acid, HVA: homovanillic acid, MN: metanephrine, NMN: normetanephrine, ALD: aldosterone
A contrast-enhanced systemic scan showed no findings suggestive of thyroid, parathyroid, or pancreatic tumors, or other metastatic lesions. Based on these results, the patient was diagnosed with left adrenal pheochromocytoma. Although the patient's blood pressure was controlled with a small amount of doxazosin, sufficient α-blocking was considered important to prevent catecholamine hypersecretion due to intraoperative surgical procedures and postoperative vascular collapse. Therefore, we did not use other anti-hypertensive drugs, including calcium channel blocker, and administered increasing doses of doxazosin during hospitalization, exceeding the maximal dose.
After adjusting the drug dose, laparoscopic adrenalectomy was performed with 32 mg/day of doxazosin and 2.5 mg/day of bisoprolol. No serious adverse events occurred during or after the procedure. The histopathological tissue was a yellowish tumor with a diameter of 70 mm×55 mm. Histopathologically, cytoplasmic tumor cells proliferated in alveolar form via blood vessels, and immunohistologically, the tumor was positive for CD56, synaptophysin, and chromogranin A. The grading system for adrenal pheochromocytoma and paraganglioma (GAPP) score (8) was 2/10 points (positive for cellularity and Ki-67 labeling index), suggesting well-differentiated pheochromocytoma.
The clinical course after surgery is being monitored, and no recurrence or metastasis has been observed. She occasionally complained of sudden dyspnea even after the surgery, but the symptom was mild and resolved spontaneously each time.
Discussion
We herein report a case of pheochromocytoma that was diagnosed through the treatment of DAH. There have been reports describing PPGL diagnosed during DAH treatment (9-13). Table 3 shows an overview of each case, including our present case. The age at the diagnosis ranged from the teens to 60s, and PPGL has been observed in both men and women. At the time of the diagnosis, cases with a high blood pressure as well as a low blood pressure were observed, and many cases seemed to have a rapid heart rate, suggesting that there were large fluctuations in blood pressure. In addition, left adrenal tumors were observed in many cases, but extra-adrenal and bilateral adrenal tumors were also observed. It should be noted that the tumor sizes were ≥50 mm in all 4 cases for which the tumor size were measured, including our own case. Tumors of such sizes can be clearly identified by whole-body imaging.
Table 3.
Case Series of PPGL with DAH or Hemoptysis.
| Case | Reference 9 | Reference 10 | Reference 11 | Reference 12 | Reference 13 | Our case |
|---|---|---|---|---|---|---|
| Age (years old) | 33 | 40 | 68 | 21 | 14 | 46 |
| Gender | M | M | M | M | F | F |
| Blood pressure (mmHg) | 150/100 | 85/59 | >180/100 | 140/80 | 72/50 | 80/41 |
| Heart rate (bpm) | 160 | 116 | 120 | 90 | 126 | 96 |
| Tumor localization | Left adrenal | Left adrenal | Left adrenal | Extraadrenal | Bilateral adrenal | Left adrenal |
| Tumor size (mm) | 80×80×60 | NA | 46×60 | NA | Left 40×50×30 | 70×50 |
| Right 60×40×30 | ||||||
| Glucocorticoid use | No | No | No | No | Yes* | Yes |
| Paroxysmal hypertension | Yes | Yes | Yes | NA | Yes | Yes |
| Drugs for PPGL | NA | Phenoxybenzamine | Phenoxybenzamine | NA | Phenoxybenzamine | Doxazosin |
| Methyrosine | Propranorol | Bisoprorol | ||||
| 24h-urine AD (µg/day) | 1,500 | 180 | 534 | 8.0 | 2.46** | 553.8 |
| 24h-urine NAD (µg/day) | 485 | 75 | 1,208.4 | 1,750 | 2,254** | 484.2 |
| 24h-urine MN (mg/day) | NA | NA | 12.3 | NA | NA | 10.01 |
| 24h-urine NMN (mg/day) | NA | NA | 5.7 | NA | NA | 2.77 |
| Other manifestations | Nausea | Nausa | Nausea | Chest pain | Nausea, Vomiting | Cough |
| Vomiting | Vomiting | Sweating | Sweating, Pallor | |||
| Headache | Headache | Pallor | Palpitation | |||
| Chest pain | Chest pain, Cough |
PPGL: pheochromocytoma and paraganglioma, DAH: diffuse alveolar hemorrhage, AD: adrenaline, NAD: noradrenaline, MN: metanephrine, NMN: normetanephrine, NA: Data not available. *In case from reference 13, the patient was treated with methylprednisolone under initial diagnosis of pneumonia, septic shock and multiple organ dysfunction syndrome. **In the original article, the unit of concentration of 24h-urine AD and NAD was described in ng/day, but the actual unit was considered to be µg/day from the contents of the manuscript. Therefore they are described in µg/day in Table 3. Reference range, 24h-urine AD; 3.4 - 26.9 µg/day, 24h-urine NAD; 48.6 - 168.4 µg/day, 24h-urine MN mg/day; 0.0 - 0.2, 24h-urine NMN; 0.1 - 0.3 mg/day
Glucocorticoids were used in only one case other than our present case (13), but it seemed to have been administered for a disease other than DAH. Conversely, paroxysmal hypertension was observed in most cases. Regarding hormone secretion, both the adrenaline-secreting type and noradrenaline-secreting type were observed. In addition, manifestations other than hemoptysis and dyspnea, such as nausea and vomiting and classic symptoms of pheochromocytoma, including headache, sweating, and pallor, were also observed. However, in emergency care, these were not considered specific symptoms based on which PPGL could be positively suspected.
Although a direct pathophysiological relationship between DAH and PPGL may be difficult to prove, the possible relationship between blood pressure fluctuation and the appearance of alveolar hemorrhage was discussed in these studies (9-13). The rapid increase in pulmonary vein pressure due to paroxysmal hypertension is considered to cause the rupture of capillaries, resulting in alveolar hemorrhage in patients with PPGL. In our case, we concluded that the DAH may not have been due to an autoimmune disease or drugs because of the patient's negative immunology tests and medication history. Considering her pathophysiology, blood pressure fluctuation may have occurred, as hypotension was present at the time of admission despite her having been treated for hypertension. Furthermore, the patient may have had a predisposition to mental stress because of her medical history of coexisting anxiety and frequent hyperventilation. Emotional stress has been reported to enhance catecholamine secretion (14), especially from PPGL (15). The frequent coughing observed until her transportation to our facility may have caused an increase in abdominal pressure, which would induce additional catecholamine secretion by the pheochromocytoma (16). Therefore, it is possible that these manifestations induced and worsened her blood pressure fluctuations, thereby resulting in increased pulmonary venous pressure and the provocation of alveolar hemorrhage.
In addition, in this case, immunosuppressive therapy including methylprednisolone was started because autoimmune diseases as causes of DAH could not be excluded at the initial hospitalization. High doses of glucocorticoids can provoke catecholamine crisis in PPGL (1,3). According to one literature review, in most cases of catecholamine crisis caused by glucocorticoids, the tumor diameters were reported to be ≥30 mm, while the glucocorticoid dosages were equivalent to ≥60 mg/day of hydrocortisone (3). Furthermore, catecholamine crisis does not develop at 1 mg/day of dexamethasone, which is equivalent to 24-30 mg/day of hydrocortisone (3). In our case, the tumor diameter was ≥50 mm, and the dose of prednisolone at the time of the paroxysmal hypertension was 60 mg/day, which was equivalent to hydrocortisone 300 mg/day. This situation may therefore have constituted a risk factor for catecholamine crisis.
Both DAH and PPGL-induced catecholamine crisis are life-threatening conditions, and urgent glucocorticoid treatment may be required during detection of the etiologies of DAH, as in our patient. Thus, PPGL should be considered a background disease of DAH, and detection efforts should be made whenever possible. Because paraganglioma develops from the extra-adrenal paraganglia from the neck to the pelvis (1), whole-body imaging is required, not merely imaging of the abdomen, to confirm the presence or absence of PPGL when DAH is suspected on chest X-ray. Although it should be noted that head and neck paraganglioma normally does not secrete catecholamine (1), when a tumor suspected of PPGL is found, therapeutic agents for catecholamine crisis, such as intravenous alpha- and beta-blockers (4), may be promptly prepared, even if high-dose glucocorticoids have to be used urgently.
In conclusion, we experienced a case of pheochromocytoma after the diagnosis and treatment of DAH. When the possibility of DAH due to autoimmune disease cannot be excluded, high-dose glucocorticoid treatment should be started. However, because high-dose glucocorticoids may induce catecholamine crisis and exacerbate the disease condition, PPGL should be considered as a cause of DAH, and the presence or absence of the tumor should be immediately confirmed by a systemic search with whole-body imaging, including CT.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Lenders JW, Duh QY, Eisenhofer G, et al. Pheochromocytoma and paraganglioma: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 99: 1915-1942, 2014. [DOI] [PubMed] [Google Scholar]
- 2.Pacak K. Preoperative management of the pheochromocytoma patient. J Clin Endocrinol Metab 92: 4069-4079, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Barrett C, van Uum SH, Lenders JW. Risk of catecholaminergic crisis following glucocorticoid administration in patients with an adrenal mass: a literature review. Clin Endocrinol (Oxf) 83: 622-628, 2015. [DOI] [PubMed] [Google Scholar]
- 4.Whitelaw BC, Prague JK, Mustafa OG, et al. Phaeochromocytoma [corrected] crisis. Clin Endocrinol (Oxf) 80: 13-22, 2014. [DOI] [PubMed] [Google Scholar]
- 5.Lara AR, Schwarz MI. Diffuse alveolar hemorrhage. Chest 137: 1164-1171, 2010. [DOI] [PubMed] [Google Scholar]
- 6.Krause ML, Cartin-Ceba R, Specks U, Peikert T. Update on diffuse alveolar hemorrhage and pulmonary vasculitis. Immunol Allergy Clin North Am 32: 587-600, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martinez-Martinez MU, Oostdam DAH, Abud-Mendoza C. Diffuse alveolar hemorrhage in autoimmune diseases. Curr Rheumatol Rep 19: 27, 2017. [DOI] [PubMed] [Google Scholar]
- 8.Kimura N, Takayanagi R, Takizawa N, et al. Pathological grading for predicting metastasis in phaeochromocytoma and paraganglioma. Endocr Relat Cancer 21: 405-414, 2014. [DOI] [PubMed] [Google Scholar]
- 9.Yoshida T, Ishihara H. Pheochromocytoma presenting as massive hemoptysis and acute respiratory failure. Am J Emerg Med 27: 626 e3-e4, 2009. [DOI] [PubMed] [Google Scholar]
- 10.Park M, Hryniewicz K, Setaro JF. Pheochromocytoma presenting with myocardial infarction, cardiomyopathy, renal failure, pulmonary hemorrhage, and cyclic hypotension: case report and review of unusual presentations of pheochromocytoma. J Clin Hypertens (Greenwich) 11: 74-80, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Querol Ripoll R, del Olmo Garcia MI, Camara Gomez R, Merino-Torres JF. Diffuse alveolar hemorrhage as first manifestation of a pheochromocytoma. Arch Bronconeumol 50: 412-413, 2014. [DOI] [PubMed] [Google Scholar]
- 12.Makuuchi Y, Wada M, Kawashima A, et al. Paraganglioma-induced alveolar hemorrhage. Intern Med 54: 487-489, 2015. [DOI] [PubMed] [Google Scholar]
- 13.Wu R, Tong N, Chen X, et al. Pheochromocytoma crisis presenting with hypotension, hemoptysis, and abnormal liver function: a case report. Medicine (Baltimore) 97: e11054, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurita A, Takase B, Ishizuka T. Disaster and cardiac disease. Anadolu Kardiyol Derg 1: 101-106, 2001. [PubMed] [Google Scholar]
- 15.Young WF, Jr. Pheochromocytoma: 1926-1993. Trends Endocrinol Metab 4: 122-127, 1993. [DOI] [PubMed] [Google Scholar]
- 16.Zuber SM, Kantorovich V, Pacak K. Hypertension in pheochromocytoma: characteristics and treatment. Endocrinol Metab Clin North Am 40: 295-311, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]


