Abstract
Innovations in computer technology and implant design have paved the way for the development of smart instruments and intelligent implants in trauma and orthopaedics to improve patient-related functional outcomes. Sensor technology uses embedded devices that detect physical, chemical and biological signals and provide a way for these signals to be measured and recorded. Sensor technology applications have been introduced in various fields of medicine in the diagnosis, treatment and monitoring of diseases. Intelligent ‘Smart’ implants are devices that can provide diagnostic capabilities along with therapeutic benefits. In trauma and orthopaedics, applications of sensors is increasing because of the advances in microchip technologies for implant devices and research designs. It offers real-time monitoring from the signals transmitted by the embedded sensors and thus provides early management solutions. Smart orthopaedic implants have applications in total knee arthroplasty, hip arthroplasty, spine surgery, fracture healing, early detection of infection and implant loosening. Here we have explored the role of Smart sensor implant technology in total knee arthroplasty. Smart sensor assisted can be used intraoperatively to provide objective assessment of ligament and soft tissue balancing whilst maintaining the sagittal and coronal alignment to achieve desired kinematic targets following total knee arthroplasty. It can also provide post-implantation data to monitor implant performance in natural conditions and patient's clinical recovery during rehabilitation. The use of Smart Sensor implant technology in total knee arthroplasty appears to provide superior patient satisfaction rates and improved functional outcomes.
Keywords: Sensor, Smart implant, Total knee arthroplasty, Orthopaedic surgery, Orthopaedics
1. Introduction
A sensor is an electronic device that measures physical properties such as temperature, pressure, distance, speed, torque, acceleration, force, flow, etc., and sends the information to an electronic processor.1 In the medical field, smart sensor technology has been used to diagnose diseases, monitor conditions (e.g., Asthma O2 concentration or glucose concentration in diabetes mellitus), deliver anaesthesia, operate insulin pumps, run kidney dialysis, intravenous fluid management systems, undertake oncology screening and provide automatic drug delivery.2, 3, 4, 5 The ability of the sensors to measure multifarious data on patient's biological activities allows sensor technology to be used in the management of patient care. The focus of this technology is to improve patient care and increase health care efficiency.
Advances in Smart sensor technology have allowed it to develop a niche in trauma and orthopaedics with various applications.6,7 [Table 1]. The goal of newer technologies such as Artificial Intelligence (AI) is to improve patient-related health care outcomes and enhance the quality of life.8 This has been made possible with research and development in the arena of commonly used implants, prosthetics, devices, and instrumentation. Technology innovation thus can create intelligent implants and smart instruments in the broad field of orthopaedics. Big data analytics, computer-aided orthopaedic surgery (CAOS), Deep learning, Intelligent orthopaedics, Machine learning, Medical robotics and Smart instrumentation are revolutionising the art of providing patient-centred care in trauma and orthopaedics.9,10 Smart Sensor Technology has found applications in the treatment of hip and knee osteoarthritis, spinal instrumentation, and fracture healing.11, 12, 13
Table 1.
Smart sensor (SS) technology applications in Trauma and orthopaedics.
| No. | Field | Applications/Description/Examples/ |
|---|---|---|
| 1 | Orthopaedic Surgery- |
|
| 2 | Trauma surgery |
|
| 3 | Post-operative |
|
| 4 | Monitoring |
|
| 5 | Surveillance |
|
| 6 | Research and Development |
|
| 7 | Training |
|
| 8 | Future applications |
|
Abbreviations: MHRA = Medical health Regulatory Authority; TKA = Total knee arthroplasty; THR = Total hip replacement.
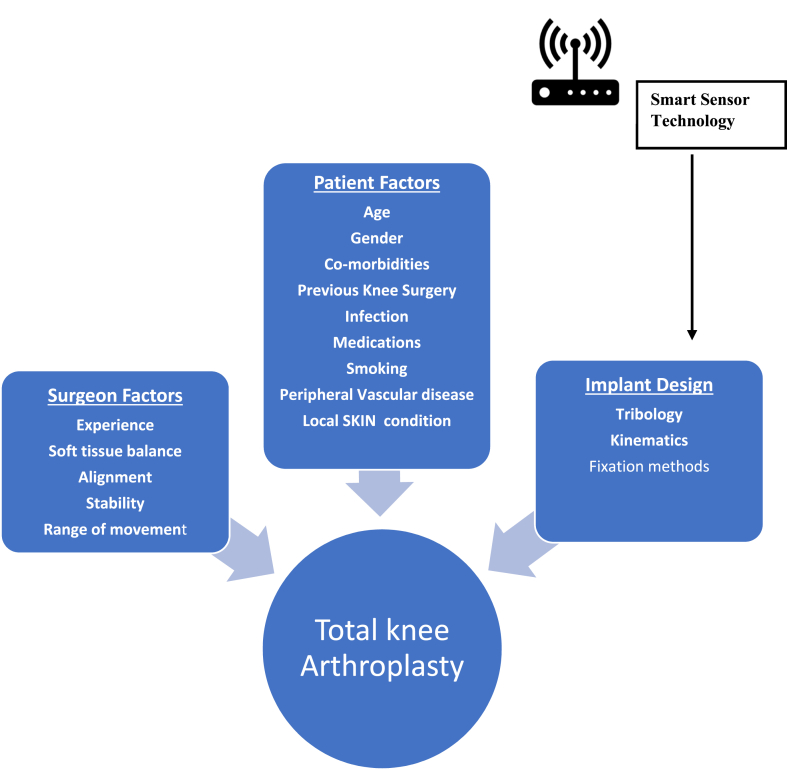
Osteoarthritis of the knee is one of the common musculoskeletal pathologies that can lead to pain, disability, and affect activities of daily living (ADL). Total knee arthroplasty (TKA) is one of the most regularly performed procedures to treat severe degenerative and inflammatory arthropathy of the knee.14 However, in this widely practiced procedure with low complication rates, about 1 in 20 patients (nearly 20%) of patients who undergo TKA are dissatisfied with the results.15 The cause of disappointment includes patients' expectations, ongoing pain, functional impairment or inability to undertake activities that are critical elements in their daily life, such as kneeling, squatting, and stair climbing.16,17 A successful outcome in TKA is broadly dependent on three factors: patient dependant factors, surgical technique, and implant design [Fig. 1]. Patient dependant factors such as obesity can be optimized and surgical precision improved. Poor outcomes due to aseptic loosening, implant wear, osteolysis, etc., can be mitigated by improved implant designs with advances in tribology. Maximising goals in TKA by increasing longevity and design of implants will enhance functional patient-related outcomes and consequently, health-related quality of life (HRQOL).18,19
Fig. 1.
Factors affecting outcome in Total Knee Arthroplasty and influence site of Smart Sensor technology application.
To influence the results of TKA, smart sensor technology can be applied to the design of implants at the intra-operative, post-operative, and rehabilitation phases of TKA pathway. In this article, we evaluate the current concepts and future applications of Smart sensor implant technology in improving TKA outcomes.
1.1. Search strategy
We carried out a comprehensive review of the literature using suitable keywords such as ‘Sensor’, ‘smart implant’, ‘Smart sensor technology’ and ‘total knee arthroplasty’ on the search engines of PubMed, SCOPUS and Google Scholar databases in between 1999 and 2021. Relevant articles were chosen to write this narrative review.
2. The need for innovative technologies in TKA
TKA is a widely undertaken procedure for managing a range of orthopaedic conditions affecting the knee joint, predominantly arthritis of the knee associated with pain and disability secondary to degenerative and inflammatory or post-traumatic pathologies. However, as with other joint arthroplasty procedures, some of them fail. Failure mechanisms in TKA include infection and implant-associated factors, including polythene wear, loosening, mal-alignment, and instability.20 With improvements in polyethylene manufacture, polyethylene wear is no longer a leading cause of failure. Nevertheless, there is a role in improving implant designs and surgical techniques to further enhance longevity and avoid technical errors. A typical TKA basic construct consists of a metallic femoral component, a tibial base plate component, ultra-high molecular-weight polyethylene (UHMWPE) plastic spacer, and a patellar component.
2.1. Smart implants can be developed in broadly two ways
-
(i)
Additive manufacturing (AM): is a new manufacturing technique that develops custom implants and patient-specific instrumentations (PSI) using a computer-aided design (CAD) model, layer-by-layer from computed tomography (CT) data.21 3-D printing and other technologies assist this process.
-
(ii)
Smart sensor technology: Smart sensor (SS) devices are incorporated into implants to provide real-time or post-implantation data to monitor implant performance and patient clinical parameters.1,6,7
3. Smart sensor technology in TKA and its theory behind the practical application
SS devices are incorporated in the polyethylene spacer to provide real-time intra-operative monitoring or integrated into the tibial baseplate component for patient monitoring. The implanted SS device in the TKA has undergone significant evolution. The earliest SS implants utilized percutaneous lead wires that extended from the instrumented implant directly to an external data logging device and hence were not of practical use in patients. Advances in microchip technologies, molecular chemistry, research, and design have made it possible to develop miniature size SS devices applied to trial implants intra-operatively or used later for patient monitoring. OrthoSensor® [Stryker (NYSE:SYK)], a SS company, is leading the development of SS technology applications in TKA. OrthoSensor® has developed VERASENSE™ Sensor-Assisted Total Knee Arthroplasty. In addition, it has teamed with Zimmer Biomet® to produce next-generation TKA implant technology with the incorporation of wireless SS devices in a range of TKA implant designs.22,23
The SS device can be used in two ways:
-
(i)
Intra-operative: as a disposable, wireless chip in the trial implants during the TKA procedure. (Fig. 2). This can provide real-time analysis to allow ideal ligament and soft tissue balancing during TKA. Sensors facilitate the replication of natural joint stability and improves the rate of achieving a balanced knee.24 A balanced knee permits appropriate implant positioning and thus helps in better alignment leading to improved outcomes.25
-
(ii)
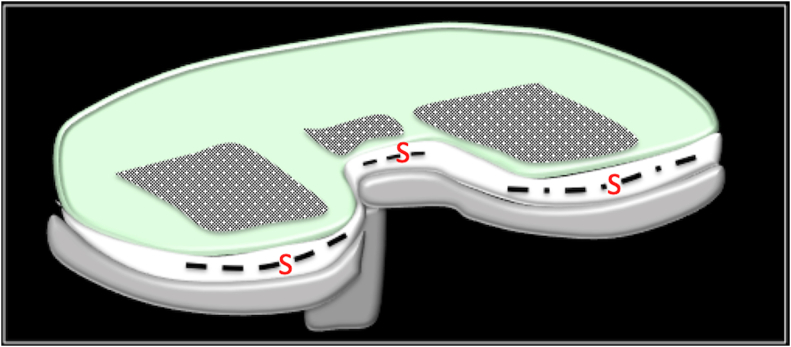
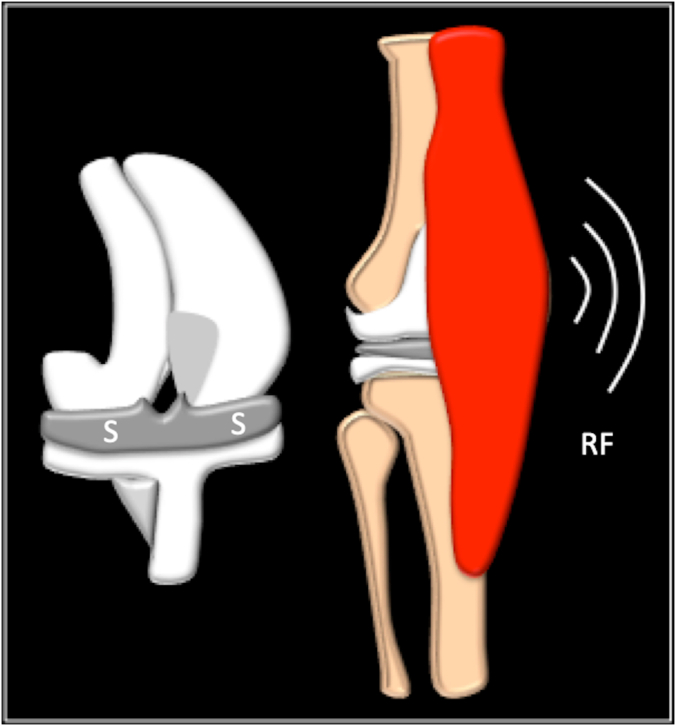
Monitoring: A similar SS implant could be incorporated for long-term monitoring of the TKA implant and the patient. This would allow the clinician to monitor physical, chemical, and biological signals. For example, physical attributes such as temperature, pressure, speed, torque can be measured to identify early changes, implant wear, and infection. The signals from the SS device in the implant are transmitted using Radio Frequency Identification (RFID) technique to appropriate data servers for computer-based analysis and action (Fig. 3-).
Fig. 2.
Diagrammatic representation of a Smart Sensor device in a TKA component. S= Sensor chip.
Fig. 3.
Diagrammatic representation Smart Sensor implant technology in TKA. S= Sensor chip; RF = Radiofrequency wave technique for data transfer.
4. Advantages of SS based TKA technology
4.1. Intra-operative application
TKA is considered by some as a soft-tissue procedure with bone cuts. Consequently, ‘soft-tissue and ligament balancing’ is a crucial step in maximising goals to achieve a successful outcome in TKA.26 Traditionally, this is undertaken by subjective feel and judgment to determine adequate ‘soft-tissue’ balancing of the TKA. However, the subjective assessment may not allow critical analysis of such balancing, especially during dynamic phases of the knee range of movement, particularly mid-flexion. Flexion instability is one of the common causes of errors in a symptomatic TKA and needs addressing with revision surgery. SS based TKA technology thus provides real-time intra-operative assessment and quantitative feedback of the TKA to achieve optimum ‘soft-tissue and ligament balancing’. Thus, surgeon-defined assessment can be improved with the use of sensor based ‘Gap balancing’.27 It has been reported patients undergoing TKA with quantifiably balanced soft tissue achieve significantly better clinical outcomes, sooner than unbalanced patients.28 This leads to superior satisfaction rates and improved knee outcomes.25
4.2. Post-operative monitoring
The SS implant will relay diagnostic data from inside the patient to the computer. The attending surgeon and/or physiotherapist can analyse this data about implant positioning, load bearing and pressure. Range-of-motion, gait analysis, joint stability, particle count around an implant (connecting to osteolysis) can be measured. Other sensor properties such as temperature, pH, and local biochemical changes of lactate, glucose levels can be monitored for early signs of infection.
4.3. Implant survival rates and surveillance
The unique Implant ID and Patient ID data accessed by an RFID tag can be recorded with existing and new National Joint Registry (NJR) to evaluate long-term outcomes, implant survival (e.g. Kaplan-Meir Survival analysis). This subsequently will provide information to support Research and development or modification of future implant designs.
4.4. Rehabilitation
Post-operative supervised physiotherapy and accelerated rehabilitation programmes have enhanced patient-related outcome measures (PROMs) in patients undergoing TKA. This is currently face-to-face or with the use of telemedicine-based platforms. SS technology can be expanded to provide remote performance, load bearing, range of movement, and gait analysis data collection. Compliance with rehabilitation or modification of a physiotherapy regime can be initiated depending on the progress data.
4.5. Cost benefits
Advancement in implant designs, Patient-specific implants are projected to provide cost-benefit to the patients and broader health care by reducing the incidence of revision TKA surgery. This would be possible by improved longevity of implants and early detection of potential complications or mechanisms of failure such as aseptic loosening or infection.
4.6. Operating theatre (OT) time
There is no extra-time required to use SS based technology for objectively balancing soft tissue while performing TKA. It has been observed that sensor assisted TKA cases required similar OT time and matched with manually balanced TKA cases.29
4.7. Role in complex TKA
Role of TKA on SS based technology could be particularly valuable in undertaking complex knee surgeries such as patients with severe varus or valgus deformity, associated distal femur or proximal femoral deformities, and severe bone loss.24 However, this application is yet to be assessed in large clinical trials.
4.8. Research and development
SS technology from the above applications will enable researchers and scientists to modify or develop new implant designs to improve outcomes further.
5. Future advancements of SS based TKA technology
-
•
Future Diagnostics: Current available SS implant devices provide real-time data for intra-operative assessment and post-operative monitoring. Future applications in SS technology will allow long-term surveillance of TKA. For e.g., diagnostic SS chips may remain dormant till the device detects an abnormality. The SS device will send diagnostic data through the wireless network to the cloud-based computers to detect abnormal signals (abnormal pressure/load pattern suggestive of instability) or increase biochemical markers to highlight sub-clinical or clinical suspicion of infection.
-
•
Future ‘Self-Treatment’ options: It is anticipated with the advancement in diagnostic, monitoring and surveillance capabilities of the SS devices, it may provide innovative implants to both diagnose and treat the patient, without surgical intervention but with clinical supervision. Nanotechnology and drug delivery systems may help the local, early release of antibiotics.30
6. Challenges in implementation of SS based TKA technology
-
•
Cost: Currently, in-vivo applications can be incorporated into custom implants. Hence the SS-based TKA technology implants are costlier than conventional implants. This may restrict its use in public-funded health care systems such as the National Health Service, England however, increased availability, mass production, and improved results with SS-based designs may make SS-based TKA technology cost-neutral.
-
•
Safety Data: All newer implant designs and technology are monitored by national and international institutions for performance. Approval by regulatory authorities based on clinical trials and patient outcomes will be essential for widespread acceptance of the SS- based TKA technology. NJR data collection and distribution of results will reinforce the evidence-based application of this technology.
-
•
Data Security: Since patient data is collected for analysis, patient consent, confidentiality, data protection will have to adhere to the principles of information governance and digital security laws.
7. Conclusion
TKA outcomes can be improved with the application of SS-based technology in the newer design of implants. Real-time SS implants will assist the operating surgeon in achieving the essential ‘soft-tissue and ligament balancing’ – a crucial step in accomplishing successful outcome. SS- supported TKA can provide significant benefits of monitoring post-operative rehabilitation goals and wear. Challenges in regular implementation of SS technology-based TKA in clinical practice will require evaluation of cost-benefit analysis compared to traditional methods, performance data, and evidence from trials. Adaptation of new technology, surgeons experience, training and patient involvement in the decision-making process will be key in the success of this technology. However, future research and designs with SS technology will allow early detection of standard modes of failure associated with TKA and prevent the need for revision surgery. Improving implant survival rates and ability to apply SS- based technology in complex knee deformities will lead to better patient satisfaction rates. The development of future ‘Self-diagnostic’ and ‘Self-treatment capabilities’ coupled with Nanotechnology applications will advance the effectiveness of TKA. As the SS-technology gets more refined, it has the potential of transforming the current implant design of TKA.
Statement of ethics
The current submitted article is not a clinical study and does not involve any patients.
Funding statement
The authors have not declared a specific grant for this research from any funding agency in public, commercial or not-for-profit sectors.
Authors statements
Author's Contributions: KPI involved in Conceptualization, literature search, manuscript writing, and editing. BG/VKJ/RA/RB/in Literature search, methodology, Data curation, manuscript review, and editing. RV supervised overall submission and approved the final draft. All authors read and agreed on the final draft submitted.
Source of funding
None.
Declaration of competing interest
None.
Contributor Information
Karthikeyan. P. Iyengar, Email: kartikp31@hotmail.com.
Benjamin Thomas Vincent Gowers, Email: benjamin.gowers@doctors.org.uk.
Vijay Kumar Jain, Email: drvijayortho@gmail.com.
Raju. S. Ahluwalia, Email: r.ahluwalia1@nhs.net.
Rajesh Botchu, Email: Drbrajesh@yahoo.com.
Raju Vaishya, Email: raju.vaishya@gmail.com.
References
- 1.Ledet E.H., D'Lima D., Westerhoff P., Szivek J.A., Wachs R.A., Bergmann G. Implantable sensor technology: from research to clinical practice. J Am Acad Orthop Surg. 2012 Jun;20(6):383–392. doi: 10.5435/JAAOS-20-06-383. [DOI] [PubMed] [Google Scholar]
- 2.Hunter G.W., Xu J.C., Biaggi-Labiosa A.M. Smart sensor systems for human health breath monitoring applications. J Breath Res. 2011 Sep;5(3) doi: 10.1088/1752-7155/5/3/037111. Epub 2011 Sep. 6. [DOI] [PubMed] [Google Scholar]
- 3.Wilson C.B. Sensors 2010. BMJ. 1999 Nov 13;319(7220):1288. doi: 10.1136/bmj.319.7220.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dunn J., Runge R., Snyder M. Wearables and the medical revolution. Per Med. 2018 Sep;15(5):429–448. doi: 10.2217/pme-2018-0044. Epub 2018 Sep. 27. [DOI] [PubMed] [Google Scholar]
- 5.Teymourian H., Barfidokht A., Wang J. Electrochemical glucose sensors in diabetes management: an updated review (2010-2020) Chem Soc Rev. 2020 Nov 7;49(21):7671–7709. doi: 10.1039/d0cs00304b. [DOI] [PubMed] [Google Scholar]
- 6.Ledet E.H., Liddle B., Kradinova K., Harper S. Smart implants in orthopedic surgery, improving patient outcomes: a review. Innovat Enterpren Health. 2018;5:41–51. doi: 10.2147/IEH.S133518. Epub 2018 Aug 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haleem A., Javaid M., Haleem K.I. Smart sensors applications in Orthopaedics. Current Medicine Research and Practice. 2019;9:246e–248. doi: 10.1016/j.cmrp.2019.10.002. Available at : [DOI] [Google Scholar]
- 8.Haleem A., Vaishya R., Javaid M., Khan I.H. Artificial Intelligence (AI) applications in orthopaedics: an innovative technology to embrace. J Clin Orthop Trauma. 2020 Feb;11(Suppl 1):S80–S81. doi: 10.1016/j.jcot.2019.06.012. Epub 2019 Jun 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kubicek J., Tomanec F., Cerny M., Vilimek D., Kalova M., Oczka D. Recent trends, technical concepts and components of computer-assisted orthopedic surgery systems: a comprehensive review. Sensors. 2019 Nov 27;19(23):5199. doi: 10.3390/s19235199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng G., Nolte L.P. Computer-aided orthopaedic surgery: state-of-the-art and future perspectives. Adv Exp Med Biol. 2018;1093:1–20. doi: 10.1007/978-981-13-1396-7_1. [DOI] [PubMed] [Google Scholar]
- 11.Jeong J.B., Kim H., Yoo J.I. Triboelectric touch sensor for position mapping during total hip arthroplasty. BMC Res Notes. 2020 Aug 26;13(1):395. doi: 10.1186/s13104-020-05238-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramakrishna V.A.S., Chamoli U., Rajan G., Mukhopadhyay S.C., Prusty B.G., Diwan A.D. Smart orthopaedic implants: a targeted approach for continuous postoperative evaluation in the spine. J Biomech. 2020 May 7;104:109690. doi: 10.1016/j.jbiomech.2020.109690. Epub 2020 Feb 25. [DOI] [PubMed] [Google Scholar]
- 13.Pelham H., Benza D., Millhouse P.W. Implantable strain sensor to monitor fracture healing with standard radiography. Sci Rep. 2017 May 4;7(1):1489. doi: 10.1038/s41598-017-01009-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Irmola T., Kangas J., Eskelinen A. Functional outcome of total knee arthroplasty: a study protocol for a prospective, double-blinded, parallel-group randomized, clinical controlled trial of novel, personalized and conventional implants. BMC Muscoskel Disord. 2019 Oct 12;20(1):443. doi: 10.1186/s12891-019-2830-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grosu I., Lavand'homme P., Thienpont E. Pain after knee arthroplasty: an unresolved issue. Knee Surg Sports Traumatol Arthrosc. 2014 Aug;22(8):1744–1758. doi: 10.1007/s00167-013-2750-2. Epub 2013 Nov 8. [DOI] [PubMed] [Google Scholar]
- 16.Choi Y.J., Ra H.J. Patient satisfaction after total knee arthroplasty. Knee Surg Relat Res. 2016 Mar;28(1):1–15. doi: 10.5792/ksrr.2016.28.1.1. Epub 2016 Feb 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scott C.E., Bugler K.E., Clement N.D., MacDonald D., Howie C.R., Biant L.C. Patient expectations of arthroplasty of the hip and knee. J Bone Joint Surg Br. 2012 Jul;94(7):974–981. doi: 10.1302/0301-620X.94B7.28219. [DOI] [PubMed] [Google Scholar]
- 18.Hamilton D.F., Burnett R., Patton J.T. Implant design influences patient outcome after total knee arthroplasty: a prospective double-blind randomised controlled trial. Bone Joint Lett J. 2015 Jan;97-B(1):64–70. doi: 10.1302/0301-620X.97B1.34254. [DOI] [PubMed] [Google Scholar]
- 19.Haanstra T.M., van den Berg T., Ostelo R.W. Systematic review: do patient expectations influence treatment outcomes in total knee and total hip arthroplasty? Health Qual Life Outcome. 2012 Dec 18;10:152. doi: 10.1186/1477-7525-10-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lombardi A.V., Jr., Berend K.R., Adams J.B. Why knee arthroplastys fail in 2013: patient, surgeon, or implant? Bone Joint Lett J. 2014 Nov;96-B(11 Supple A):101–104. doi: 10.1302/0301-620X.96B11.34350. [DOI] [PubMed] [Google Scholar]
- 21.Narra S.P., Mittwede P.N., DeVincent Wolf S., Urish K.L. Additive manufacturing in total joint arthroplasty. Orthop Clin N Am. 2019 Jan;50(1):13–20. doi: 10.1016/j.ocl.2018.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.VERASENSE Multicenter Randomized Controlled Trial. Pending Publication. Data on file at OrthoSensor, Inc. Available at : https://www.orthosensor.com/wp-content/uploads/2018/02/2017-Clinical-Research-Summary.pdf ( Accessed 24 July 2021).
- 23.Gustke K.A., Golladay G.J., Roche M.W., Elson L.C., Anderson C.R. A new method for defining balance: promising short-term clinical outcomes of sensor guided TKA. J Arthroplasty. 2014 May;29(5):955–960. doi: 10.1016/j.arth.2013.10.020. Epub 2013 Oct 24. [DOI] [PubMed] [Google Scholar]
- 24.Batailler C., Swan J., Marinier E.S., Servien E., Lustig S. Current role of intraoperative sensing technology in total knee arthroplasty. Arch Orthop Trauma Surg. 2021 Aug 24 doi: 10.1007/s00402-021-04130-5. [DOI] [PubMed] [Google Scholar]
- 25.Gustke K.A., Golladay G.J., Roche M.W., Jerry G.J., Elson L.C., Anderson C.R. Increased satisfaction after total knee arthroplasty using sensor-guided technology. Bone Joint Lett J. 2014 Oct;96-B(10):1333–1338. doi: 10.1302/0301-620X.96B10.34068. [DOI] [PubMed] [Google Scholar]
- 26.Peters C.L. Soft tissue balancing in primary total knee arthroplasty. Instr Course Lect. 2006;55:413–417. [PubMed] [Google Scholar]
- 27.Zhao R., Liu Y., Tian H. Accuracy of soft tissue balancing in total knee arthroplasty using surgeon-defined assessment versus a gap-balancer or electronic sensor. J Orthop Surg Res. 2021 May 8;16(1):305. doi: 10.1186/s13018-021-02439-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gustke K.A., Golladay G.J., Roche M.W., Elson L.C., Anderson C.R. Primary TKA patients with quantifiably balanced soft-tissue achieve significant clinical gains sooner than unbalanced patients. Adv Orthop. 2014. 2014:628695. doi: 10.1155/2014/628695. Epub 2014 Aug 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lakra A., Sarpong N.O., Jennings E.L. The learning curve by operative time for soft tissue balancing in total knee arthroplasty using electronic sensor technology. J Arthroplasty. 2019 Mar;34(3):483–487. doi: 10.1016/j.arth.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 30.Parvizi J., Antoci V., Jr., Hickok N.J., Shapiro I.M. Self-protective smart orthopedic implants. Expet Rev Med Dev. 2007 Jan;4(1):55–64. doi: 10.1586/17434440.4.1.55. [DOI] [PubMed] [Google Scholar]