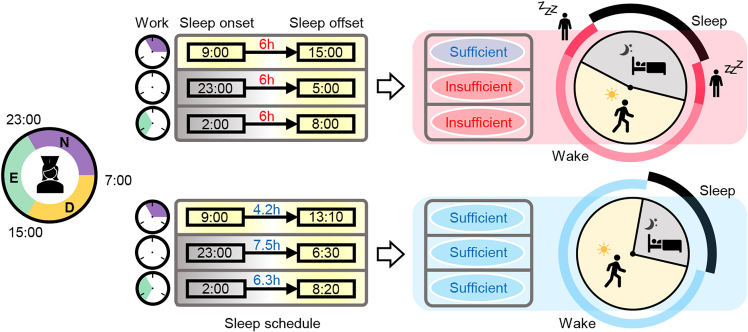
Summary
Shift workers and many other groups experience irregular sleep-wake patterns. This can induce excessive daytime sleepiness that decreases productivity and elevates the risk of accidents. However, the degree of daytime sleepiness is not correlated with standard sleep parameters like total sleep time, suggesting other factors are involved. Here, we analyze real-world sleep-wake patterns of shift workers measured with wearables by developing a computational package that simulates homeostatic sleep pressure – physiological need for sleep – and the circadian rhythm. This reveals that shift workers who align sleep-wake patterns with their circadian rhythm have lower daytime sleepiness, even if they sleep less. The alignment, quantified by the sleep parameter, circadian sleep sufficiency, can be increased by dynamically adjusting daily sleep durations according to varying bedtimes. Our computational package provides flexible and personalized real-time sleep-wake patterns for individuals to reduce their daytime sleepiness and could be used with wearables to develop smart alarms.
Subject areas: Biological sciences, Neuroscience, Behavioral neuroscience, Mathematical biosciences, Systems biology
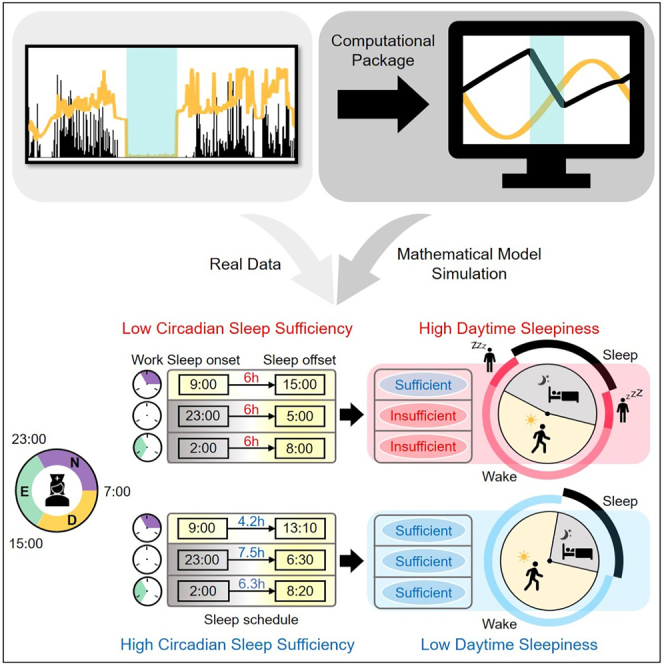
Graphical abstract
Highlights
-
•
Sleep-wake patterns measured by wearables are analyzed with a mathematical model
-
•
A new sleep parameter CSS measures the circadian alignment of sleep-wake patterns
-
•
Sleep-wake patterns more aligned with circadian rhythm decrease daytime sleepiness
-
•
Our computational package calculates the CSS to provide personalized sleep schedules
Biological sciences; Neuroscience; Behavioral neuroscience; Mathematical biosciences; Systems biology.
Introduction
In our modern 24-h society, approximately 20% of the working population is engaged in shift work but more than 80% of the population has a shift work-like lifestyle with artificial light exposure (Sulli et al., 2018). Irregular sleep-wake patterns cause shift work disorder with symptoms including fatigue, sleepiness, insomnia, and poorer mental agility (Drake et al., 2004; Foster, 2020). In particular, excessive daytime sleepiness (EDS) reduces performance efficiency, increases the risk of work-related injuries, and is a significant public health problem (James et al., 2017; Slater and Steier, 2012). One way to reduce EDS could be to increase sleep duration since longer sleep leads to less sleepiness for non-shift workers with regular sleep-wake patterns (Liu et al., 2000; Ohayon, 2012; Queiroz et al., 2020). However, significant correlations between sleep durations or the other standard sleep parameters, including sleep efficiency (SE) and sleep latency (SL), with daytime sleepiness of shift workers have not been identified (Gumenyuk et al., 2015; Kato et al., 2012). Furthermore, there have also been no connections identified between EDS and broader clinical features or features measured by polysomnography, i.e., in-depth sleep studies (Eiseman et al., 2012). This suggests the involvement of other, unknown factors in mediating the effects of irregular sleep-wake patterns.
The effect of irregular sleep-wake patterns on sleepiness has also been investigated with mathematical models (Abel et al., 2020; Van Dongen, 2004). While the details between the models differ, they are mainly based on the two-process model (Borbély, 1982), which simulates sleep-wake patterns according to interactions between homeostatic sleep pressure (the physiological need for sleep, which appears to be mainly determined by the level of somnogens such as cytokines, prostaglandin D2, and adenosine (Shi and Ueda, 2018; Skeldon et al., 2017)) and the circadian (∼24 h) rhythm of the master clock in the suprachiasmatic nucleus. By simulating homeostatic sleep pressure and the circadian rhythm, the models successfully predicted subjective sleepiness and fatigue measured during long-lasting sleep deprivation in laboratory studies (Daan et al., 1984; Postnova et al., 2018; Puckeridge et al., 2011; Van Dongen, 2004), and irregular real-world work schedules (Moore-Ede et al., 2004; Van Dongen, 2004). While these model predictions suggested work schedules avoiding strenuous activities during times of high sleepiness to improve performance and minimize risks (Moore-Ede et al., 2004; Postnova et al., 2014), their widespread application is challenging without continual reinforcement (i.e., forcing a specific schedule) (Czeisler et al., 1982). Importantly, shift workers even with similar work schedules have dramatically different sleep-wake patterns and thus different daytime sleepiness (Vetter et al., 2015). This demonstrates a need for personalized and flexible sleep-wake schedules to prevent EDS.
Here, we analyzed the relationship between daytime sleepiness and real-world sleep-wake patterns of individual shift workers measured by wearable wrist actigraphy. Specifically, to analyze the complex individual sleep-wake patterns by simulating underlying homeostatic sleep pressure and the circadian rhythm, we developed a publicly accessible user-friendly computational package based on a physiologically based mathematical model of sleep-wake cycles (Phillips et al., 2010; Skeldon et al., 2017; Swaminathan et al., 2017). This analysis revealed that as sleep-wake patterns became more aligned with an individual's circadian rhythm, daytime sleepiness decreased, even if total sleep times (TSTs) were similar. To effectively investigate this relationship, we developed a sleep parameter that we call circadian sleep sufficiency (CSS). CSS was strongly correlated with daytime sleepiness, unlike other standard sleep parameters. CSS can be increased by adaptively adjusting daily sleep duration according to the personal choice of bedtime day-by-day rather than by forcing a specific work and sleep-wake pattern, thus providing a flexible and personalized solution to reduce daytime sleepiness. The personalized sleep-wake patterns can be provided in real-time when the user-friendly computational package developed in this study is linked with wearable devices.
Results
Daytime sleepiness is not significantly correlated with standard sleep parameters
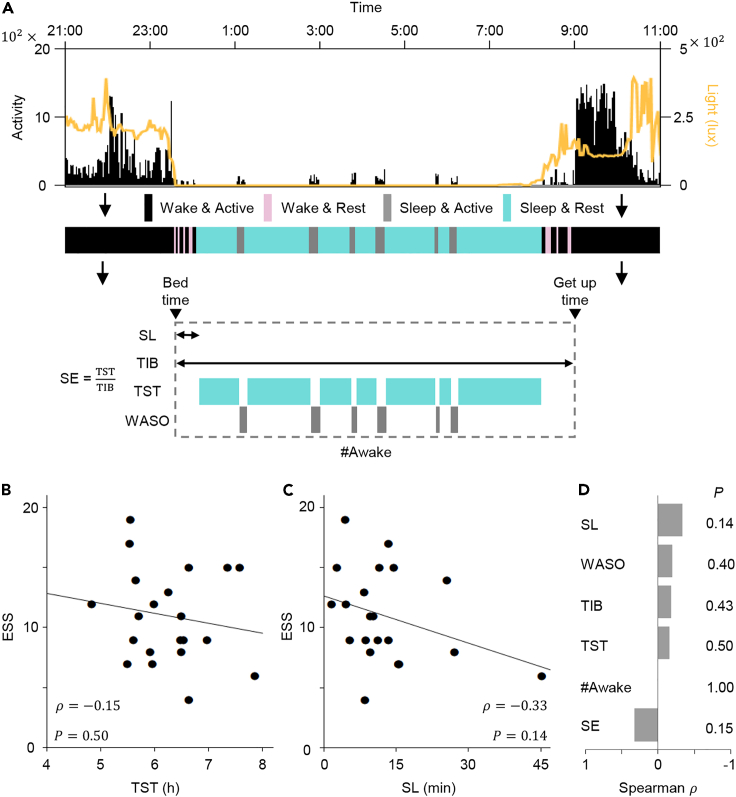
We measured the activity and light exposure of 21 rotating nurses from Samsung Medical Center (SMC) every 2-min over 13 days using wrist activity monitors (Figure 1A and Table S1). Then, in each 2-min epoch, the status of the participants was categorized as either wake and active, wake and rest, sleep and active, or sleep and rest with Actiware-Sleep software whose accuracy has been validated previously (Edinger et al., 2004; Kushida et al., 2001). This allowed us to calculate six major standard sleep parameters: time in bed (TIB), SL, TST, wakefulness after sleep onset (WASO), number of awakenings (#Awak) and SE. We expected that as daily sleep duration (i.e., TST) increased, shift workers would be getting as much sleep as they needed, and this would decrease their daytime sleepiness, which was measured by the Epworth sleepiness scale (ESS). However, TST was not significantly correlated with daytime sleepiness (; Figure 1B). In particular, the daytime sleepiness of shift workers who had similar TST (6-7 h) differed dramatically. The other sleep parameters were also not significantly correlated with daytime sleepiness (Figures 1C and 1D, and S1A-S1D). The partial correlation between the standard sleep parameters and daytime sleepiness controlling for demographics of nurses (e.g., Age, BMI, and the number of shift schedules) is also not significant (Table S1 and Figure S1E). Similarly, a previous study has also reported that the standard sleep parameters may not have a strong correlation with the daytime sleepiness of shift workers (Kato et al., 2012). This indicates the need for a different approach to analyze dynamic and complex sleep-wake patterns of shift workers.
Figure 1.
No significant correlation between standard sleep parameters and daytime sleepiness of shift workers
(A) Using activity (black vertical lines) and light exposure (yellow line) measured by the wrist actigraphy, the status of participants over time was categorized as either wake and active (black shade), wake and rest (pink shade), sleep and active (gray shade), or sleep and rest (blue shade) with Actiware-Sleep software, and then the six standard sleep parameters were calculated. TIB: time in bed; SL: sleep latency; TST: total sleep time; WASO: wakefulness after sleep onset; #Awak: number of awakenings; SE: sleep efficiency.
(B and C) Scatterplots of TST (B) and SL (C) versus ESS of shift workers (). See Figures S1A–S1D for scatterplots of the other sleep parameters. Shift workers with similar TST (e.g., 6-7 h; B) had dramatically different daytime sleepiness. The line represents the least-square fitting line. and denote the Spearman's rank correlation coefficient and p value of Spearman's rank correlation test, respectively.
(D) Correlations between the six standard sleep parameters and daytime sleepiness of shift workers were weak and not significant.
A mathematical model is adopted to analyze dynamic sleep-wake patterns
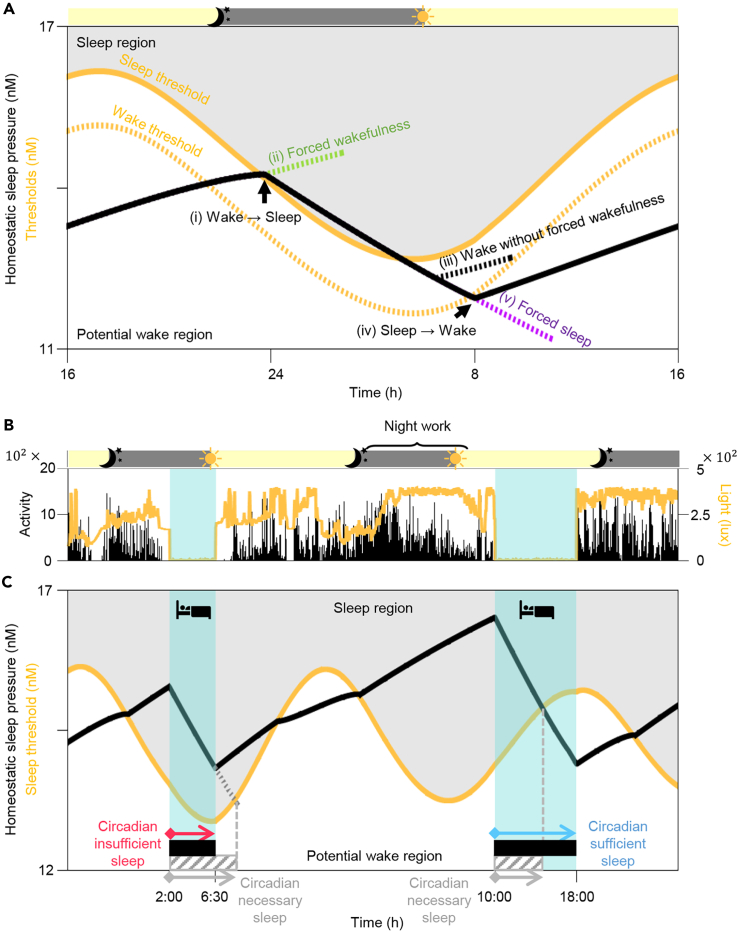
To analyze the sleep-wake patterns of shift workers systematically, we modified a physiologically-based mathematical model of human sleep-wake cycles (Phillips et al., 2010; Skeldon et al., 2017; Swaminathan et al., 2017) (see STAR Methods). In the model, the activity of sleep- and wake-promoting neurons, and thus sleep timing and duration, are determined by the interaction between the homeostatic sleep pressure and the circadian rhythm (Figures S2 and S3). The homeostatic sleep pressure describes the physiological need for sleep, which increases during wake and dissipates during sleep (black line; Figure 2A). The circadian rhythm, entrained to the external day-night cycle, determines the sleep and wake thresholds (yellow lines; Figure 2A). When the homeostatic sleep pressure increases above the circadian sleep threshold, the transition from wake to sleep is triggered (Figure 2A (i)). Thus, the model would naturally fall asleep whenever the homeostatic sleep pressure is higher than the circadian sleep threshold (i.e., in the gray “sleep region”; Figure 2A). To simulate wakefulness in the sleep region, similar to when shift workers work through the night, we modified the model to incorporate a “forced wakefulness” (Figures 2A (ii) and S4) (Phillips and Robinson, 2008; Postnova et al., 2014). When the homeostatic sleep pressure passes below the circadian sleep threshold (i.e., in the white “potential wake region”; Figure 2A), the transition from sleep to wake can occur naturally without forced wakefulness (Figures 2A (iii) and S5). When the homeostatic sleep pressure further decreases below the circadian wake threshold (Figure 2A (iv)), the transition from sleep to wake is actively triggered. To simulate sleep (e.g., oversleeping or nap) in this case when the model would be naturally awake, we modified the model to incorporate a “forced sleep” (Figures 2A (v) and S4) (Phillips and Robinson, 2008; Postnova et al., 2014). Note that due to the lower level of the circadian sleep threshold during the night compared with the day (Figure 2A), falling asleep during the night occurs at a lower level of homeostatic sleep pressure (i.e., easier to sleep).
Figure 2.
Sleep episodes are categorized as either circadian sufficient or circadian insufficient with a physiologically based mathematical model of sleep-wake cycles
(A) In the mathematical model, the homeostatic sleep pressure (black line) dissipates during sleep and increases during wake. When it becomes higher than the circadian sleep threshold (yellow solid line), a transition from wake to sleep occurs (i). When the model would naturally fall asleep in the sleep region (gray region), forced wakefulness is needed to simulate wakefulness (ii). On the other hand, when homeostatic sleep pressure falls below the circadian sleep threshold and thus the model is in the potential wake region (white region), wakefulness can be simulated without forced wakefulness (iii). When the homeostatic sleep pressure falls below the circadian wake threshold (yellow dotted line), a transition from sleep to wake actively occurs (iv). In this case, forced sleep is required to simulate sleep (v). See Figures S2–S5 for details. Gray and yellow shades on top indicate the night (22:00-6:00 h) and the day (6:00-22:00 h), respectively.
(B and C) The computational package based on the mathematical model simulated homeostatic sleep pressure (black line; C) according to sleep-wake patterns (blue shade; B), which were estimated by measured activity (black vertical lines; B). It also simulated the circadian variation of the sleep threshold (yellow line; C) by estimating the light signal reaching the circadian clock based on measured light exposure (yellow line; B). Then, the minimum sleep duration required to wake-up specifically in the potential wake region (i.e., the circadian necessary sleep; gray striped bars; C) was calculated for each sleep episode. Compared with circadian necessary sleep, longer or shorter sleep episodes (black bars; C) are referred to as circadian sufficient or circadian insufficient sleep, respectively. Gray and yellow shades on top of (B) indicate the night (22:00-6:00 h) and the day (6:00-22:00 h), respectively.
See also Figures S2–S5.
A sleep parameter, circadian sleep sufficiency, is strongly correlated with daytime sleepiness
With the modified mathematical model, we developed a publicly accessible user-friendly computational package (Figure S6) that simulates an individual's homeostatic sleep pressure based on real-world sleep-wake patterns (blue shade; Figure 2B) that were mainly estimated by the wrist activity monitor (see STAR Methods). Specifically, for the individual illustrated in Figure 2, the simulated homeostatic sleep pressure increased and decreased during wake and sleep, respectively, as expected (black line; Figure 2C). In particular, the homeostatic sleep pressure became extremely high after night shift work before the second sleep episode. Furthermore, the computational package estimated the light signal transmitted to the circadian clock based on the measured light exposures of the participant over time (yellow line; Figure 2B) and then simulated the circadian variation of the sleep threshold entrained to these light-dark cycles (yellow line; Figure 2C). The overall level of the simulated sleep threshold increased when exposed to light during the day or during the night shift, which inhibits falling asleep.
Based on the homeostatic sleep pressure and the sleep threshold simulated with the computational package, the duration of necessary sleep needed to wake up without effort can be predicted. Specifically, when people fall asleep, the computational package predicted how long they need to sleep so that their homeostatic sleep pressure decreased below their sleep threshold (into the potential wake region; Figure 2C) and thus they could wake up without effort (i.e., without forced wakefulness). We defined “circadian necessary sleep” as the sleep episode with the minimum duration required so that awakening occurs in the potential wake region (gray striped bars; Figure 2C). The duration of circadian necessary sleep depends on when individuals fall asleep and the subsequent intersection between their homeostatic sleep pressure and their sleep threshold, which are linked with their circadian rhythm. In the example in Figure 2C, the duration of the first sleep episode (black bar) is shorter than the duration of the predicted circadian necessary sleep (gray striped bar). This represents a situation when the individual wakes up before his/her homeostatic sleep pressure falls below the sleep threshold – i.e., forced wakefulness. We refer to this sleep episode as “circadian insufficient sleep” throughout the study. In contrast, the duration of the second sleep episode is longer than the duration of the predicted circadian necessary sleep for that cycle, meaning that the individual awakens several hours after their need for sleep has fallen below their sleep threshold. This is referred to as “circadian sufficient sleep” throughout the study.
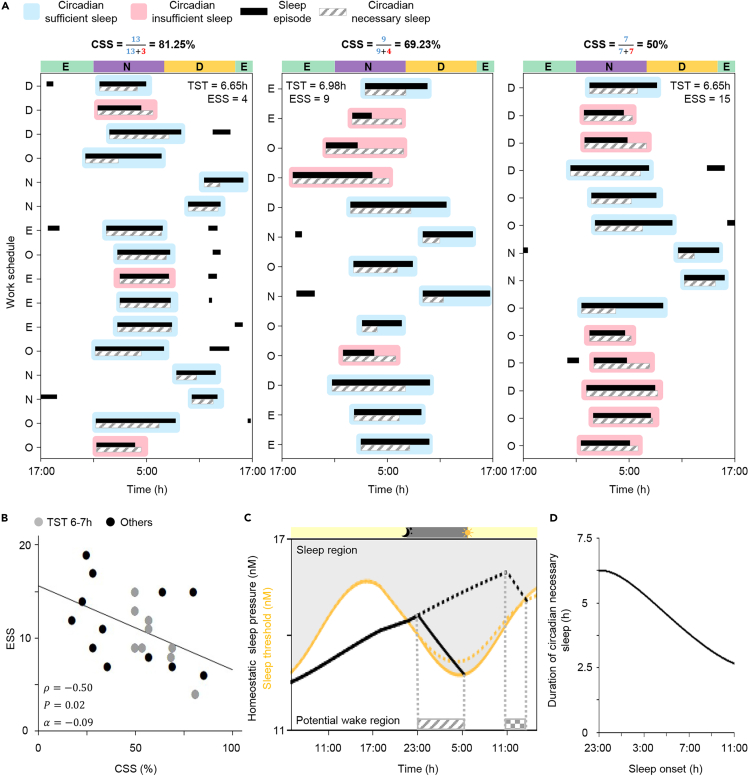
We hypothesized that having circadian insufficient sleep – i.e., sleep accompanied by forced wakefulness– causes EDS. To investigate this, we compared the sleep-wake patterns during the whole monitoring period (13–16 days) of three shift workers who had considerably different daytime sleepiness measured by ESS despite having similar TST (6.65–6.98 h). Specifically, we categorized their daily sleep episodes (black bars; Figure 3A) as either circadian sufficient sleep (blue shade; Figure 3A) or circadian insufficient sleep (pink shade; Figure 3A) after comparing with their predicted circadian necessary sleep (gray striped bars; Figure 3A). The fractions of circadian sufficient sleeps in total sleep episodes were dramatically different between the three shift workers (from 81.25 to 69.23 to 50%) although they all had similar TST. Notably, as the fraction of circadian sufficient sleeps decreased, daytime sleepiness measured by ESS increased from 4 to 9 to 15 (Figure 3A).
Figure 3.
CSS is significantly correlated with the daytime sleepiness of shift workers
(A) Sleep-wake patterns of shift workers with similar TST but different ESS. Daily sleep episodes (black bars) whose duration is longer or shorter than the duration of circadian necessary sleep (gray striped bars) are categorized as either circadian sufficient sleep (blue shade) or circadian insufficient sleep (pink shade), respectively. As the fraction of circadian sufficient sleeps (i.e., CSS) decreased, daytime sleepiness (ESS) increased. D, E, N, and O denote the day shift (7:00-15:30 h), the evening shift (15:00-23:30 h), the night shift (23:00-7:30 h), and days off, respectively.
(B) CSS had a strong and significant correlation with ESS. The line represents the least-square fitting line with the slope of . and denote the Spearman's rank correlation coefficient and p value of Spearman's rank correlation test, respectively.
(C) After regular 7-h sleep-wake patterns between 23:00 h and 6:00 h, simulations of sleep onset occurring at the usual time (23:00 h; solid line) compared with 12-h delayed sleep onset (11:00 h; dotted line). The duration of circadian necessary sleep needed after the delayed sleep (patterned bar) is much shorter than the duration of circadian necessary sleep needed after the regular sleep (striped bar).
(D) As sleep onset is delayed from 23:00 h to 11:00 h, the duration of the predicted circadian necessary sleep gradually decreases by ∼3.6 h.
See also Figure S6 and Tables S2–S5.
To investigate this further in all the data recorded from the SMC participants, we developed a sleep parameter, circadian sleep sufficiency (CSS), defined as the fraction of circadian sufficient sleeps in total sleep days during the study period. Indeed, over all the individuals, although there was some variation, CSS was significantly correlated with daytime sleepiness (; Figure 3B) unlike the other standard sleep parameters (Figures 1B–1D and S1A–S1D). To the best of our knowledge, CSS is the first statistically significant sleep parameter for daytime sleepiness. Furthermore, CSS had a higher correlation with daytime sleepiness () than any other standard sleep parameter previously discussed (Figures 1B–1D and S1A–S1D). In particular, in 9 participants, despite having similar TST (6-7 h), as CSS increased, daytime sleepiness decreased (gray dots; Figure 3B).
Sleep-wake patterns aligned with the circadian rhythm increase circadian sleep sufficiency
The duration of predicted circadian necessary sleep changed dramatically depending on the sleep onset time and previous sleep history (gray striped bars; Figure 3A). Thus, we further investigated how circadian necessary sleep was determined so that we could identify the sleep-wake patterns increasing CSS and thus decreasing daytime sleepiness. After regular 7-h sleep-wake patterns between 23:00 h and 06:00 h, we considered sleep onset occurring at the usual time (23:00 h; solid line; Figure 3C) compared to sleep onset delayed by 12 h (11:00 h; dotted line; Figure 3C). Despite a dramatically increased homeostatic sleep pressure, the mathematical model predicted that the duration of circadian necessary sleep needed after the delayed sleep onset (patterned bar; Figure 3C) is much shorter than the duration of circadian necessary sleep needed after regular sleep onset (striped bar; Figure 3C). This shorter duration of circadian necessary sleep was due to the higher level of the sleep threshold during the day compared to the night, which is determined by the circadian rhythm (yellow lines; Figure 3C). That is, during the day, even after a short sleep, the homeostatic sleep pressure drops lower than the sleep threshold, and thus one can wake up without effort. Indeed, as sleep onset was delayed from 23:00 h to 11:00 h (Figure 3D), the duration of the predicted circadian necessary sleep gradually decreased by ∼3.6 h. This indicates that the circadian rhythm is the key determinant of circadian necessary sleep. The importance of circadian rhythmicity has also been shown in previous studies reporting a decrease in sleep duration after sleep deprivation (Åkerstedt and Gillberg, 1981; Czeisler et al., 1980; Daan et al., 1984; Phillips and Robinson, 2008).
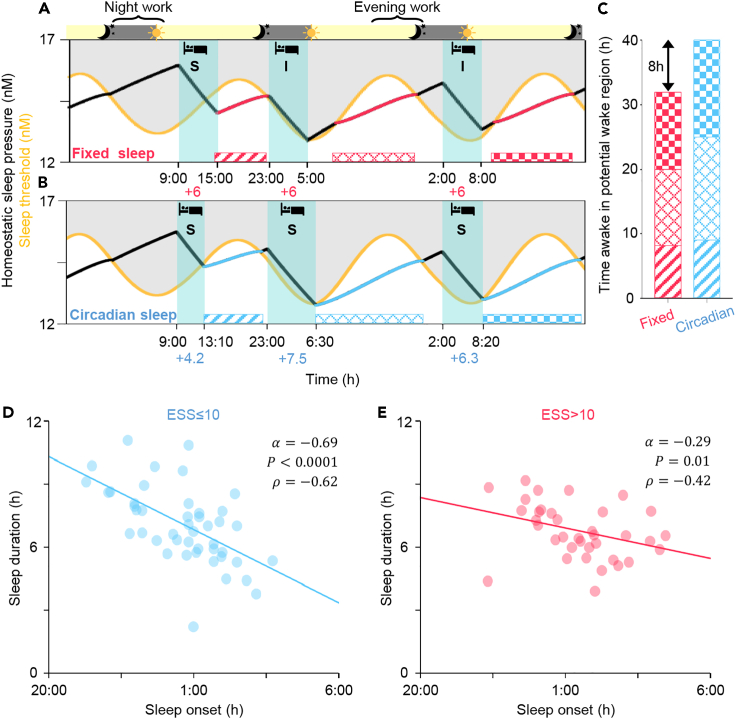
As circadian necessary sleep was mainly determined by the circadian rhythm, we hypothesized that sleep-wake patterns aligned with the circadian rhythm increase CSS and thus reduce daytime sleepiness. To investigate this, we simulated two different 3-day sleep-wake patterns. One follows three 6-h sleep episodes across three days regardless of sleep onset time, which is referred to as fixed sleep (Figure 4A). In the other simulation, sleep duration was adjusted according to the circadian phase of sleep onset, which is referred to as circadian sleep (Figure 4B). Despite having the same average sleep duration and sleep onset times, in the fixed sleep simulation only one sleep episode was categorized as circadian sufficient sleep (Figure 4A), while in the circadian sleep simulation all sleep episodes were circadian sufficient sleeps (Figure 4B). As a result, for the two circadian insufficient sleeps in the fixed sleep simulation, an awakening occurred before the homeostatic sleep pressure decreased below the sleep threshold (i.e., forced wakefulness). In real life, this situation can occur for example when using an alarm clock, or be caused by a disease, or stress (Foster, 2020; James et al., 2017; Skeldon et al., 2017; Van Dongen, 2004). Thus, after awakening from a circadian insufficient sleep, it takes some time for the individual to reach their potential wake region, where awakening would have occurred without effort (patterned bars; Figure 4A), and thus they may feel increased daytime sleepiness. In contrast, an individual awakening from circadian sufficient sleep is already in their potential wake region (patterned bars; Figure 4A). Furthermore, after circadian sufficient sleep, the homeostatic sleep pressure was lower than after circadian insufficient sleep, and thus these individuals could be awake for longer before reaching their sleep threshold (e.g. ∼32 min is longer before the third sleep; Figures 4A and 4B). Thus, the time awake in the potential wake region was ∼8 h longer in the circadian sleep simulation compared to the fixed sleep simulation despite having the same average sleep duration (Figure 4C). Similar results were obtained for different sleep onsets times during the shift schedule and different lengths of the shift schedule (Figure S7). This indicates that when the sleep-wake pattern is aligned with the circadian rhythm, the actual wake time is more likely to be aligned with the time of the potential wake region, and the duration of the potential wake region increases. As a result, there is less need for forced wakefulness, which may reflect daytime sleepiness.
Figure 4.
Sleep-wake patterns aligned with the circadian rhythm increase CSS and reduce daytime sleepiness
(A and B) Model simulations of three 6-h sleep episodes across three days regardless of sleep onset time (fixed sleep; A) and three sleep episodes with durations adjusted according to the circadian phase of sleep onset (circadian sleep; B). Although these two sleep-wake patterns have the same TST, two circadian insufficient sleeps (denoted as I) occur with the fixed sleep (A), but only circadian sufficient sleeps (denoted as S) occur with the circadian sleep (B). As a result, time awake in the potential wake region (patterned bars) is longer in the circadian sleep simulation (B) than in the fixed sleep simulation (A).
(C) Quantification of the time awake in the potential wake region.
(D and E) Alignment of sleep-wake patterns with the circadian rhythm of shift workers having similar TST (6-7 h) from SMC data (D and E; ). The group without EDS (ESS≤10; blue dots; ) show a much stronger negative dependence of sleep duration on the sleep onset time, compared with the group with EDS (ESS>10; red dots; ). The number of analyzed main sleep episodes which were the longest sleep episodes of each day (noon-to-noon) were 45 (D) and 36 (E), respectively. The line represents the least-square fitting line with the slope of . and P denote the Spearman's rank correlation coefficient and p value of Spearman's rank correlation test, respectively.
See also Figure S7.
Sleep-wake patterns aligned with the circadian rhythm reduce daytime sleepiness
To investigate whether the better alignment of sleep with the circadian rhythm was associated with reduced daytime sleepiness, we analyzed the sleep-wake patterns of the shift workers from SMC. Specifically, we investigated whether a negative relationship between sleep onset time and sleep duration, as predicted by the mathematical model (Figure 3D), was stronger in the group without EDS (ESS≤10) compared to the group with EDS (ESS>10). For this comparison, we considered data only from shift workers having similar TST (6-7 h). Furthermore, sleep episodes before a day shift (7:00-15:30 h) whose wake onsets were usually triggered by an alarm clock, were excluded in this analysis to focus on the dependence of sleep duration on the circadian rhythm rather than forced sleep restriction following previous studies (Åkerstedt and Gillberg, 1981). As predicted, in the group without EDS, when sleep onset was delayed, which occurs often in shift workers, sleep duration clearly decreased following the circadian rhythm (; Figure 4D). This relationship was weaker in the group with EDS (; Figure 4E). This indicates that shift workers who aligned their sleep duration with their circadian rhythm had reduced daytime sleepiness (Figure 5). This provides personalized and flexible sleep-wake schedules reducing daytime sleepiness (Figure 5).
Figure 5.
A sleep-wake pattern leading to circadian sufficient sleep reduces daytime sleepiness
Due to the alteration among day, evening and night shifts, sleep onset times of shift workers dramatically change. If they sleep for the same duration regardless of their sleep onset time, they frequently sleep less than the circadian necessary sleep, which is determined by their circadian rhythm and homeostatic sleep pressure, i.e., they have circadian insufficient sleep (top panels). Circadian insufficient sleep can be prevented if they actively change their sleep duration so their sleep-wake patterns match their natural sleep-wake cycle (bottom panels). As a result, they spend more time awake in the potential wake region when they feel less sleepy (bottom right). In contrast, with circadian insufficient sleep, workers are awake in the sleep region, which requires wake effort and increases daytime sleepiness (top right). Note that the circadian insufficient sleep reduces the duration of the potential wake region as well.
Discussion
We developed a user-friendly computational package that simulates the homeostatic sleep pressure and the circadian rhythm according to activity and light exposure, measured by wrist actigraphy (Figures 2 and S6). Using this package, we found that shift workers whose sleep-wake patterns were aligned with their circadian rhythm had lower daytime sleepiness (Figures 3 and 4). Specifically, when they slept according to the computed duration of circadian necessary sleep, which was mainly determined by the circadian phase of bedtime, their sleep-wake patterns matched with their natural sleep-wake patterns (Figure 5). In this way, they awoke in the potential wake region when they would feel less sleepy and thus have lower daytime sleepiness (Figure 5). As these results were based on a retrospective study, it will be important to perform a prospective study investigating whether improving the alignment of sleep-wake patterns with the circadian rhythm reduces daytime sleepiness of individuals. The sleep-wake patterns aligned with the circadian rhythm were highly variable depending on various personal factors including average sleep duration, bedtime, and environmental light exposure (e.g., Figure 3A). Importantly, our computational package can provide personalized and flexible sleep-wake schedules reducing daytime sleepiness.
With the computational package provided in this work, we were able to calculate the sleep parameter CSS. As CSS quantifies the fraction of sleep episodes after which one can wake up without effort, it increases when sleep-wake patterns are better aligned with the circadian rhythm (Figures 4A and 4B). The CSS showed a strong correlation with daytime sleepiness (Figure 3B) unlike standard sleep parameters such as TST and SL (Figures 1B and 1C). Such a different result appears to stem from the fact that the standard sleep parameters capture the average pattern of the entire sleep-wake patterns, but the CSS captures the daily change in the sleep-wake patterns. This highlights that tracking the dynamic changes in the homeostatic sleep pressure and the circadian rhythm is critical to understand the irregular sleep-wake patterns of the shift workers. The importance of the circadian rhythm for understanding daytime sleepiness has also been emphasized in previous studies (Mairesse et al., 2014; Postnova et al., 2018; Puckeridge et al., 2011; Van Dongen, 2004). Importantly, the role of the circadian rhythm to understand complex aspects of sleep can be conveniently investigated with CSS. For instance, CSS can be used to investigate whether the circadian rhythm is a major source of inter-individual variations in sleep qualities and sleepiness depending on work schedules (Czeisler et al., 1982; Dunster et al., 2018; Vetter et al., 2015) and chronotypes (Vetter et al., 2015). Furthermore, the irregular sleep-wake patterns accompanied with the circadian misalignment have been considered as a major risk factor for insomnia, obesity, and cancer (James et al., 2017; Kecklund and Axelsson, 2016). How the risk of getting these diseases depends on sleep-wake patterns can also be effectively investigated with CSS.
Recent advances in wearable technology enable accurate real-time tracking of sleep-wake pattern and the circadian rhythm, which are critical components of our computational package (Cheng et al., 2021; Forger and Walch, 2020; Kim et al., 2020). A plethora of wearables have been developed to track sleep-wake patterns (Perez-Pozuelo et al., 2020). Recently, wearable devices measuring skin temperature and rest-activity successfully track the individual circadian rhythm during daily routine (Komarzynski et al., 2018). Even heart rate (Gao et al., 2014), hormonal changes (Bariya et al., 2018) and core body temperature (Popovic et al., 2014), which are important factors for inferring the circadian rhythm, can also be tracked with wearables. The incorporation of these wearables and recently developed personalized sleep-wake mathematical models (Ramakrishnan et al., 2015) with our computational package can lead to the development of a smart alarm (Perez-Pozuelo et al., 2020). This will provide real-time personalized wake times, which align individual sleep-wake patterns with the circadian rhythm and thus reduce daytime sleepiness for those most suffering from it, including shift workers (Kato et al., 2012), patients of delayed sleep-wake phase disorder (Joo et al., 2017), Parkinson's disease (Videnovic et al., 2017) or cancer (Sun et al., 2011).
Limitations of the study
In this work, we developed the sleep parameter CSS which has a significant correlation with daytime sleepiness of shift workers which was measured by ESS. Although ESS is one of the most widely used metrics to measure daytime sleepiness, it is a subjective metric. Future work will test whether CSS is still significantly correlated with daytime sleepiness measured with objective metrics such as psychomotor vigilance task or multiple SL test. Additionally, when CSS was calculated, we did not consider the degree of circadian sleep sufficiency due to the relatively small size of the data (e.g., 1h and 3h shorter sleep than circadian necessary sleep are considered as the same circadian insufficient sleep). It will be an important future work to identify a function describing the relationship between daytime sleepiness and the degree of circadian sleep sufficiency with large data. For this extension, the amount of the wake effort drive needed to maintain wakefulness can also be used (Fulcher et al., 2010).
To investigate sleep-wake patterns of shift workers with an indoor light profile, we modified the values of three parameters among the original 28 parameters (Skeldon et al., 2017), which were validated under various conditions (Robinson et al., 1997, 2004; Forger et al., 1999; Kronauer et al., 1999; Phillips and Robinson, 2008; Phillips et al., 2010; Swaminathan et al., 2017; Stone et al., 2020; Murray et al., 2021). We modified the parameters to match the simulated sleep phases and dark phases after the entrainment of the model under typical indoor light profiles (Figure S3). This modification appears to be critical for our study because the CSS calculated with the original model was no longer significantly correlated with daytime sleepiness (Table S2). However, the phase of the modified model is considerably advanced compared to the original model (i.e., morning type). It would be interesting in future work to find a way to keep using the original model with validated parameters to investigate daytime sleepiness by modifying the way of calculating the CSS.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Software and algorithms | ||
| MATLAB R2020b | MathWorks | https://mathworks.com; RRID:SCR_001622 |
| Actiware-Sleep software v3.4 | Mini Mitter Co., Inc., (Bend, OR, USA) | https://www.philips.com.au/; RRID:SCR_016440 |
| Deposited data | ||
| Database: CSS | Original Code | https://github.com/Mathbiomed/CSS |
| Other | ||
| Actiwatch Spectrum Pro | Philips Respironics, (Murrysville, PA, USA) | https://www.philips.com.au/ |
| Actiwatch 2 | Mini Mitter Co., Inc., (Bend, OR, USA) | https://www.philips.com.au/ |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Jae Kyoung Kim (jaekkim@kaist.ac.kr).
Materials availability
This study did not generate new unique reagents.
Experimental model and subject details
Human subjects
Activity and light exposure of 21 female rotating nurses who had done shift work for more than a year at a metropolitan hospital (≥2,000 beds; SMC) in Seoul, the Republic of Korea were analyzed. The information of the participants was described in Table S1. This study was approved by the institutional review board ethics committee of the hospital (Approval No. 2017-01-139). The written informed consent forms outlining the details of the study and the confidentiality and privacy of personal information were obtained from all participants before commencing the study.
Methods details
Participants of SMC data
Activity and light exposure of female rotating nurses who had done shift work for more than a year at a metropolitan hospital (SMC) in Seoul, the Republic of Korea were measured. We excluded participants during the recruiting process if they were under the use of hypnotics or central nervous system stimulants, had a history of psychiatric illness or major systemic disease, or didn’t have planned night shift schedules during the study period. As a result, 27 participants were recruited from May 24 to September 27, 2017 whose shift schedule was fast-rotating 8-h three-shift (day shift, 07:00-15:30 h; evening shift, 15:00-23:30 h; night shift, 23:00-07:30 h). During the recruiting period, the activity of each participant was recorded for 13∼19 days. Then, we analyzed sleep-wake patterns during the whole recorded period of 21 participants whose sleep onset and offset data were missing for less than three days during the study period and for less than two days during the off schedules (See Table S1 for detailed information of the participants).
Daytime sleepiness and chronotype
Daytime sleepiness and chronotype of SMC data were measured by using self-reported questionnaires after the activity monitoring over 13 days. Specifically, daytime sleepiness was measured with the Korean version of the ESS (Cho et al., 2010) which was translated from the ESS (Johns, 1991). Subjects with an ESS score>10 are considered as having excessive daytime sleepiness (EDS). Chronotype was measured by using the Korean version of Morningness–Eveningness Questionnaire (MEQ) (Park et al., 1996) which was translated from the MEQ (Horne and Östberg, 1976). The MEQ scores range from 16 to 86. Subjects with scores above 58, below 42, and from 42 to 58 were classified as morning, evening, and intermediate types, respectively (Lee et al., 2014).
Sleep parameters
Six major sleep parameters of SMC data (Figure 1A) were estimated by tracking the sleep-wake patterns of participants through the Actiwatch and sleep diary. Specifically, the activity and light exposure of each participant were measured by using an Actiwatch Spectrum Pro (Philips Respironics, Murrysville, PA, USA; ) or Actiwatch 2 (Mini Mitter Co., Inc., Bend, OR, USA; ) in two-min epochs for over 13 days. Participants were instructed to wear the watch throughout the study period, except while showering or swimming. The recorded activity was analyzed using Actiware-Sleep software (v3.4, Mini Mitter Co., Inc., Bend, OR, USA) to categorize the status of participants as either wake and active, wake and rest, sleep and active, or sleep and rest (Figure 1A). This status was validated by the daily sleep diaries written by the participants about their sleep onset, sleep offset and the time when the watch was removed. The validated status was used to estimate the six major standard sleep parameters for each day (noon-to-noon) and then their average during the study period was used (Figure 1A). When calculating the average, any days without any sleep episode due to missing the actigraphy recording were excluded.
Sleep data processing for SMC data
To track the homeostatic sleep pressure with the computational package, sleep-wake patterns need to be accurately tracked without any missing period. We used the Actiware-Sleep software to identify sleep intervals. For various reasons (e.g., forget wearing the Actiwatch and recharge), missing periods can occur. To determine sleep-wake status during the missing periods, we used the self-reported sleep diaries that participants were instructed to fill out every day. If the sleep record in the sleep diary was also missing, we assumed that participates were in wake during working time. There are 219 short missing periods (30 min27) and four long missing periods (8 h2) whose sleep interval cannot be determined by either sleep diary or work schedule. For the short missing periods, we assumed that the sleep-wake patterns of the first half and second half of the missing period follow the sleep-wake status before and after the missing period, respectively. On the other hand, for the long missing periods, we excluded the whole 24-h sleep-wake data including the long missing periods from our analysis.
Furthermore, to estimate pure sleep duration from the sleep interval, the WASO of every sleep was estimated with the Actiware-Sleep software. When WASO data was missing (2 of 398 sleep episodes, 0.50%), it was determined as the mean WASO of the participant.
If there is more than one sleep episode during the day (noon-to-noon), we need to determine the main sleep episode for calculating CSS. We defined the main sleep episode of each day (noon-to-noon) as the sleep episode having the longest TIB among all sleep episodes in the day after finishing the work schedule.
Furthermore, if there is another sleep episode which is very close to the main sleep episode, we integrated the two sleep episodes as the main sleep to calculate CSS. Specifically, if the period with activity having a non-zero value identified by Actiware-Sleep software during the wake period between the two sleep episodes is less than WASO of normal adults (42 min; 7 cases) (Fekedulegn et al., 2020), we integrated them and treated the wake period with activity having a value of zero, between two sleep episodes as WASO.
Light data processing for SMC data
Based on light exposure, the light signal transmitted to the circadian clock and thus the circadian rhythm entrained to the light-dark cycles were estimated via our computational package. The light exposure was recorded with the “white light” reading supplied by the Actiwatch Spectrum Pro or Actiwatch 2 (Figure 2B). However, we decided not to use the light data from the Actiwatch 2 due to its significant incorrect measurement of light exposure (e.g., only <50 lux was recorded a day after wearing the Actiwatch 2). Thus, the light profiles of shift workers who wore the Actiwatch 2 were assumed as the intensity of 250 lux during wakefulness and 0 lux during sleep because the typical light intensity during working hours in SMC is ∼250 lux (Choi and Joo, 2016). Furthermore, the same assumption was made when the missing period longer than 3 h occurs with the Actiwatch Spectrum Pro. When different levels of light between 100 and 700 lux were used instead of 250 lux, the CSS changes slightly (Table S3), which shows that our results are robust to the choice of the light intensity. When the missing period of the Actiwatch Spectrum Pro is shorter than 3 h, we assumed that the light intensity of the first half and second half of the missing period are the same as the light intensity before and after the missing period, respectively.
Mathematical model description
To simulate homeostatic sleep pressure and the circadian rhythm based on sleep-wake patterns and light exposure, our computational package adopted a physiological based mathematical model of sleep-wake cycles (Phillips et al., 2010; Skeldon et al., 2017; Swaminathan et al., 2017) (Figure S2 and Table S4). We modified the mathematical model to investigate highly irregular sleep-wake patterns of shift workers with an indoor light profile. This included the modification of values for 3 of 28 parameters (Figure S3 and Table S5) and the incorporation of forced wakefulness and forced sleep (Figure S4) and realistic attenuation of light (see Data S1).
Computational package
The computational package developed in this work provides an estimate of homeostatic sleep pressure and the circadian rhythm based on sleep-wake patterns and light exposure (Figure S6). In particular, it calculates the CSS of the provided sleep-wake patterns, which can be used to identify personalized sleep-wake patterns with high CSS. The detailed manual for the computational package is described in Data S2. The MATLAB codes of the computational package are available in the following Database: CSS (https://github.com/Mathbiomed/CSS).
Quantification and statistical analysis
All the simulations were performed using ode15s solver in MATLAB (R2020b, MathWorks, Natick, USA). For this study, Spearman’s rank correlation test and partial Spearman’s correlation test were performed with MATLAB and SPSS, respectively. A p value of <0.05 was considered statistically significant.
Acknowledgments
This work was supported by the LG Yonam Foundation of Korea, the Human Frontiers Science Program Organization (RGY0063/2017), the National Science Foundation (DMS-1853506), the Institute for Basic Science (IBS-R029-C3), and the Samsung Biomedical Research Institute grant (OTC1190671).
Author contributions
E.Y.J. and J.K.K. designed the study. S.J.C. and E.Y.J. collected and processed actiwatch data. J.H. and S.H.P. performed and H.H., V.B., and J.K.K. contributed to computational modeling and simulation. J.H. developed and H.H. contributed to the computational package. All authors analyzed the data. J.K.K. supervised the project. J.H. and J.K.K. wrote the draft of the manuscript; all authors revised the manuscript.
Declaration of interests
The authors declare no competing interests.
Published: October 22, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2021.103129.
Contributor Information
Eun Yeon Joo, Email: ejoo@skku.edu.
Jae Kyoung Kim, Email: jaekkim@kaist.ac.kr.
Supporting Citations
The following references appear in the Supplemental information: Baehr et al., 2000; Hirshkowitz et al., 2015.
Supplemental information
Data and code availability
-
•
SMC data supporting the findings of this study except for private information are available from the Lead Contact upon request.
-
•
The MATLAB codes of the computational package are available in the following Database: CSS (https://github.com/Mathbiomed/CSS).
-
•
Any additional information required to reanalyze the data study except for private information reported in this paper is available from the lead contact upon request.
References
- Abel J.H., Lecamwasam K., St Hilaire M.A., Klerman E.B. Recent advances in modeling sleep: from the clinic to society and disease. Curr. Opin. Physiol. 2020;15:37–46. doi: 10.1016/j.cophys.2019.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Åkerstedt T., Gillberg M. The circadian variation of experimentally displaced sleep. Sleep. 1981;4:159–169. doi: 10.1093/sleep/4.2.159. [DOI] [PubMed] [Google Scholar]
- Baehr E.K., Revelle W., Eastman C.I. Individual differences in the phase and amplitude of the human circadian temperature rhythm: with an emphasis on morningness–eveningness. J. Sleep Res. 2000;9:117–127. doi: 10.1046/j.1365-2869.2000.00196.x. [DOI] [PubMed] [Google Scholar]
- Bariya M., Nyein H.Y.Y., Javey A. Wearable sweat sensors. Nat. Electron. 2018;1:160–171. doi: 10.1038/s41928-018-0043-y. [DOI] [Google Scholar]
- Borbély A.A. A two process model of sleep regulation. Hum. Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- Cheng P., Walch O., Huang Y., Mayer C., Sagong C., Cuamatzi Castelan A., Burgess H.J., Roth T., Forger D.B., Drake C.L. Predicting circadian misalignment with wearable technology: validation of wrist-worn actigraphy and photometry in night shift workers. Sleep. 2021;44:zsaa180. doi: 10.1093/sleep/zsaa180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho Y.W., Lee J.H., Son H.K., Lee S.H., Shin C., Johns M.W. The reliability and validity of the Korean version of the Epworth sleepiness scale. Sleep Breath. 2010;15:377–384. doi: 10.1007/s11325-010-0343-6. [DOI] [PubMed] [Google Scholar]
- Choi S.J., Joo E.Y. Light exposure and sleep-wake pattern in rapidly rotating shift nurses. J. Sleep Med. 2016;13:8–14. doi: 10.13078/jsm.16002. [DOI] [Google Scholar]
- Czeisler C.A., Moore-Ede M.C., Coleman R.H. Rotating shift work schedules that disrupt sleep are improved by applying circadian principles. Science. 1982;217:460–463. doi: 10.1126/science.7089576. [DOI] [PubMed] [Google Scholar]
- Czeisler C.A., Weitzman E., Moore-Ede M.C., Zimmerman J.C., Knauer R.S. Human sleep: its duration and organization depend on its circadian phase. Science. 1980;210:1264–1267. doi: 10.1126/science.7434029. [DOI] [PubMed] [Google Scholar]
- Daan S., Beersma D.G.M., Borbély A.A. Timing of human sleep: recovery process gated by a circadian pacemaker. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1984;246:R161–R178. doi: 10.1152/ajpregu.1984.246.2.R161. [DOI] [PubMed] [Google Scholar]
- Drake C.L., Roehrs T., Richardson G., Walsh J.K., Roth T. Shift work sleep disorder: prevalence and consequences beyond that of symptomatic day workers. Sleep. 2004;27:1453–1462. doi: 10.1093/sleep/27.8.1453. [DOI] [PubMed] [Google Scholar]
- Dunster G.P., de la Iglesia L., Ben-Hamo M., Nave C., Fleischer J.G., Panda S., de la Iglesia H. Sleepmore in Seattle: later school start times are associated with more sleep and better performance in high school students. Sci. Adv. 2018;4:eaau6200. doi: 10.1126/sciadv.aau6200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edinger J.D., Means M.K., Stechuchak K.M., Olsen M.K. A pilot study of inexpensive sleep-assessment devices. Behav. Sleep Med. 2004;2:41–49. doi: 10.1207/s15402010bsm0201_4. [DOI] [PubMed] [Google Scholar]
- Eiseman N.A., Westover M.B., Mietus J.E., Thomas R.J., Bianchi M.T. Classification algorithms for predicting sleepiness and sleep apnea severity. J. Sleep Res. 2012;21:101–112. doi: 10.1111/j.1365-2869.2011.00935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekedulegn D., Andrew M.E., Shi M., Violanti J.M., Knox S., Innes K.E. Actigraphy-based assessment of sleep parameters. Ann. Work Expo. Health. 2020;64:350–367. doi: 10.1093/annweh/wxaa007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forger D.B., Walch O. Wearables have arrived. Let’s make something of it. Curr. Opin. Syst. Biol. 2020 doi: 10.1016/j.coisb.2020.11.002. [DOI] [Google Scholar]
- Forger D.B., Jewett M.E., Kronauer R.E. A simpler model of the human circadian pacemaker. J. Biol. rhythms. 1999;14:533–538. doi: 10.1177/074873099129000867. [DOI] [PubMed] [Google Scholar]
- Foster R.G. Sleep, circadian rhythms and health. Interf. Focus. 2020;10:20190098. doi: 10.1098/rsfs.2019.0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulcher B., Phillips A.J., Robinson P.A. Quantitative physiologically based modeling of subjective fatigue during sleep deprivation. J. Theor. Biol. 2010;264:407–419. doi: 10.1016/j.jtbi.2010.02.028. [DOI] [PubMed] [Google Scholar]
- Gao L., Dong D., He J., Qiao K., Cao F., Li M., Liu H., Cheng Y., Tang J., Song H. Wearable and sensitive heart-rate detectors based on PbS quantum dot and multiwalled carbon nanotube blend film. Appl. Phys. Lett. 2014;105:153702. doi: 10.1063/1.4898680. [DOI] [Google Scholar]
- Gumenyuk V., Belcher R., Drake C.L., Roth T. Differential sleep, sleepiness, and neurophysiology in the insomnia phenotypes of shift work disorder. Sleep. 2015;38:119–126. doi: 10.5665/sleep.4336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshkowitz M., Whiton K., Albert S.M., Alessi C., Bruni O., DonCarlos L., Hazen N., Herman J., Katz E.S., Kheirandish-Gozal L. National Sleep Foundation’s sleep time duration recommendations: methodology and results summary. Sleep Health. 2015;1:40–43. doi: 10.1016/j.sleh.2014.12.010. [DOI] [PubMed] [Google Scholar]
- Horne J.A., Östberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Chronobiol. Int. 1976;4:97–110. [PubMed] [Google Scholar]
- James S.M., Honn K.A., Gaddameedhi S., Van Dongen H.P.A. Shift work: disrupted circadian rhythms and sleep-implications for health and well-being. Curr. Sleep Med. Rep. 2017;3:104–112. doi: 10.1007/s40675-017-0071-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns M.W. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- Joo E.Y., Abbott S.M., Reid K.J., Wu D., Kang J., Wilson J., Zee P.C. Timing of light exposure and activity in adults with delayed sleep-wake phase disorder. Sleep Med. 2017;32:259–265. doi: 10.1016/j.sleep.2016.09.009. [DOI] [PubMed] [Google Scholar]
- Kato C., Shimada J., Hayashi K. Sleepiness during shift work in Japanese nurses: a comparison study using JESS, SSS, and actigraphy. Sleep Biol. Rhythms. 2012;10:109–117. doi: 10.1111/j.1479-8425.2011.00528.x. [DOI] [Google Scholar]
- Kecklund G., Axelsson J. Health consequences of shift work and insufficient sleep. BMJ. 2016;355:i5210. doi: 10.1136/bmj.i5210. [DOI] [PubMed] [Google Scholar]
- Kim D.W., Zavala E., Kim J.K. Wearable technology and systems modeling for personalized chronotherapy. Curr. Opin. Syst. Biol. 2020 doi: 10.1016/j.coisb.2020.07.007. [DOI] [Google Scholar]
- Komarzynski S., Huang Q., Innominato P.F., Maurice M., Arbaud A., Beau J., Bouchahda M., Ulusakarya A., Beaumatin N., Breda G. Relevance of a mobile internet platform for capturing inter-and intrasubject variabilities in circadian coordination during daily routine: pilot study. J. Med. Internet Res. 2018;20:e204. doi: 10.2196/jmir.9779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronauer R.E., Forger D.B., Jewett M.E. Quantifying human circadian pacemaker response to brief, extended, and repeated light stimuli over the phototopic range. J. Biol. Rhythms. 1999;14:500–515. doi: 10.1177/074873049901400609. [DOI] [PubMed] [Google Scholar]
- Kushida C.A., Chang A., Gadkary C., Guilleminault C., Carrillo O., Dement W.C. Comparison of actigraphic, polysomnographic, and subjective assessment of sleep parameters in sleep-disordered patients. Sleep Med. 2001;2:389–396. doi: 10.1016/s1389-9457(00)00098-8. [DOI] [PubMed] [Google Scholar]
- Lee J.H., Kim S.J., Lee S.Y., Jang K.H., Kim I.S., Duffy J.F. Reliability and validity of the Korean version of Morningness–Eveningness Questionnaire in adults aged 20–39 years. Chronobiol. Int. 2014;31:479–486. doi: 10.3109/07420528.2013.867864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Uchiyama M., Kim K., Okawa M., Shibui K., Kudo Y., Doi Y., Minowa M., Ogihara R. Sleep loss and daytime sleepiness in the general adult population of Japan. Psychiatry Res. 2000;93:1–11. doi: 10.1016/S0165-1781(99)00119-5. [DOI] [PubMed] [Google Scholar]
- Mairesse O., De Valck E., Quanten S., Neu D., Cortoos A., Pattyn N., Theuns P., Cluydts R., Hofmans J. Sleepiness phenomics: modeling individual differences in subjective sleepiness profiles. Int. J. Psychophysiol. 2014;93:150–161. doi: 10.1016/j.ijpsycho.2013.03.021. [DOI] [PubMed] [Google Scholar]
- Moore-Ede M., Heitmann A., Guttkuhn R., Trutschel U., Aguirre A., Croke D. Circadian alertness simulator for fatigue risk assessment in transportation: application to reduce frequency and severity of truck accidents. Aviat. Space Environ. Med. 2004;75:A107–A118. [PubMed] [Google Scholar]
- Murray J.M., Magee M., Sletten T.L., Gordon C., Lovato N., Ambani K., Bartlett D.J., Kennaway D.J., Lack L.C., Grunstein R.R. Light-based methods for predicting circadian phase in delayed sleep–wake phase disorder. Sci. Rep. 2021;11:1–12. doi: 10.1038/s41598-021-89924-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohayon M.M. Determining the level of sleepiness in the American population and its correlates. J. Psychiatr. Res. 2012;46:422–427. doi: 10.1016/j.jpsychires.2011.06.008. [DOI] [PubMed] [Google Scholar]
- Park Y.M., Seo Y.J., Matsumoto K. The monrningness-eveningness questionnaire in Korean version and its relations with sleep-wake habits. JESK. 1996;15:37–49. [Google Scholar]
- Perez-Pozuelo I., Zhai B., Palotti J., Mall R., Aupetit M., Garcia-Gomez J.M., Taheri S., Guan Y., Fernandez-Luque L. The future of sleep health: a data-driven revolution in sleep science and medicine. NPJ Digit. Med. 2020;3:1–15. doi: 10.1038/s41746-020-0244-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips A.J.K., Robinson P.A. Sleep deprivation in a quantitative physiologically based model of the ascending arousal system. J. Theor. Biol. 2008;255:413–423. doi: 10.1016/j.jtbi.2008.08.022. [DOI] [PubMed] [Google Scholar]
- Phillips A.J.K., Chen P.Y., Robinson P.A. Probing the mechanisms of chronotype using quantitative modeling. J. Biol. Rhythms. 2010;25:217–227. doi: 10.1177/0748730410369208. [DOI] [PubMed] [Google Scholar]
- Popovic Z., Momenroodaki P., Scheeler R. Toward wearable wireless thermometers for internal body temperature measurements. IEEE Commun. Mag. 2014;52:118–125. doi: 10.1109/MCOM.2014.6917412. [DOI] [Google Scholar]
- Postnova S., Lockley S.W., Robinson P.A. Prediction of cognitive performance and subjective sleepiness using a model of arousal dynamics. J. Biol. Rhythms. 2018;33:203–218. doi: 10.1177/0748730418758454. [DOI] [PubMed] [Google Scholar]
- Postnova S., Postnov D.D., Seneviratne M., Robinson P.A. Effects of rotation interval on sleepiness and circadian dynamics on forward rotating 3-shift systems. J. Biol. Rhythms. 2014;29:60–70. doi: 10.1177/0748730413516837. [DOI] [PubMed] [Google Scholar]
- Puckeridge M., Fulcher B.D., Phillips A.J.K., Robinson P.A. Incorporation of caffeine into a quantitative model of fatigue and sleep. J. Theor. Biol. 2011;273:44–54. doi: 10.1016/j.jtbi.2010.12.018. [DOI] [PubMed] [Google Scholar]
- Queiroz S., Ruiz F., Prado J., Silva A., Frange C., Narciso F., Cruz A., Tufik S., de Mello M.T. The consequences of partial sleep restriction for habitual sleep duration, sleepiness and reaction time in healthy males. Sleep Health. 2020;6:814–821. doi: 10.1016/j.sleh.2020.04.002. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan S., Lu W., Laxminarayan S., Wesensten N.J., Rupp T.L., Balkin T.J., Reifman J. Can a mathematical model predict an individual's trait-like response to both total and partial sleep loss? J. Sleep Res. 2015;24:262–269. doi: 10.1111/jsr.12272. [DOI] [PubMed] [Google Scholar]
- Robinson P.A., Rennie C.J., Wright J.J. Propagation and stability of waves of electrical activity in the cerebral cortex. Phys. Rev. E. 1997;56:826. doi: 10.1103/PhysRevE.56.826. [DOI] [Google Scholar]
- Robinson P.A., Rennie C.J., Rowe D.L., O'connor S. Estimation of multiscale neurophysiologic parameters by electroencephalographic means. Hum. Brain Mapp. 2004;23:53–72. doi: 10.1002/hbm.20032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S., Ueda H.R. Ca2+-Dependent hyperpolarization pathways in sleep homeostasis and mental disorders. Bioessays. 2018;40:1700105. doi: 10.1002/bies.201700105. [DOI] [PubMed] [Google Scholar]
- Skeldon A.C., Phillips A.J.K., Dijk D.J. The effects of self-selected light-dark cycles and social constraints on human sleep and circadian timing: a modeling approach. Sci. Rep. 2017;7:45158. doi: 10.1038/srep45158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater G., Steier J. Excessive daytime sleepiness in sleep disorders. J. Thorac. Dis. 2012;4:608–616. doi: 10.3978/j.issn.2072-1439.2012.10.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone J.E., Postnova S., Sletten T.L., Rajaratnam S.M., Phillips A.J. Computational approaches for individual circadian phase prediction in field settings. Curr. Opin. Syst. Biol. 2020 doi: 10.1016/j.coisb.2020.07.011. [DOI] [Google Scholar]
- Sulli G., Manoogian E.N.C., Taub P.R., Panda S. Training the circadian clock, clocking the drugs, and drugging the clock to prevent, manage, and treat chronic diseases. Trends Pharmacol. Sci. 2018;39:812–827. doi: 10.1016/j.tips.2018.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J.L., Chiou J.F., Lin C.C. Validation of the Taiwanese version of the Athens Insomnia Scale and assessment of insomnia in Taiwanese cancer patients. J. Pain Symptom Manage. 2011;41:904–914. doi: 10.1016/j.jpainsymman.2010.07.021. [DOI] [PubMed] [Google Scholar]
- Swaminathan K., Klerman E.B., Phillips A.J.K. Are individual differences in sleep and circadian timing amplified by use of artificial light sources? J. Biol. Rhythms. 2017;32:165–176. doi: 10.1177/0748730417699310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dongen H.P.A. Comparison of mathematical model predictions to experimental data of fatigue and performance. Aviat. Space Environ. Med. 2004;75:A15–A36. [PubMed] [Google Scholar]
- Vetter C., Fischer D., Matera J.L., Roenneberg T. Aligning work and circadian time in shift workers improves sleep and reduces circadian disruption. Curr. Biol. 2015;25:907–911. doi: 10.1016/j.cub.2015.01.064. [DOI] [PubMed] [Google Scholar]
- Videnovic A., Klerman E.B., Wang W., Marconi A., Kuhta T., Zee P.C. Timed light therapy for sleep and daytime sleepiness associated with Parkinson disease: a randomized clinical trial. JAMA Neurol. 2017;74:411–418. doi: 10.1001/jamaneurol.2016.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
SMC data supporting the findings of this study except for private information are available from the Lead Contact upon request.
-
•
The MATLAB codes of the computational package are available in the following Database: CSS (https://github.com/Mathbiomed/CSS).
-
•
Any additional information required to reanalyze the data study except for private information reported in this paper is available from the lead contact upon request.