Abstract
5-methylcytosine (m5C) post-transcriptional modifications affect the maturation, stability, and translation of the mRNA molecule. These modifications play an important role in many physiological and pathological processes, including stress response, tumorigenesis, tumor cell migration, embryogenesis, and viral replication. Recently, there has been a better understanding of the biological implications of m5C modification owing to the rapid development and optimization of detection technologies, including liquid chromatography-tandem mass spectrometry (LC-MS/MS) and RNA-BisSeq. Further, predictive models (such as PEA-m5C, m5C-PseDNC, and DeepMRMP) for the identification of potential m5C modification sites have also emerged. In this review, we summarize the current experimental detection methods and predictive models for mRNA m5C modifications, focusing on their advantages and limitations. We systematically surveyed the latest research on the effectors related to mRNA m5C modifications and their biological functions in multiple species. Finally, we discuss the physiological effects and pathological significance of m5C modifications in multiple diseases, as well as their therapeutic potential, thereby providing new perspectives for disease treatment and prognosis.
Keywords: 5-methylcytosine, post-transcriptional modification, mRNA, prediction, RNA epigenetics, disease
Graphical abstract
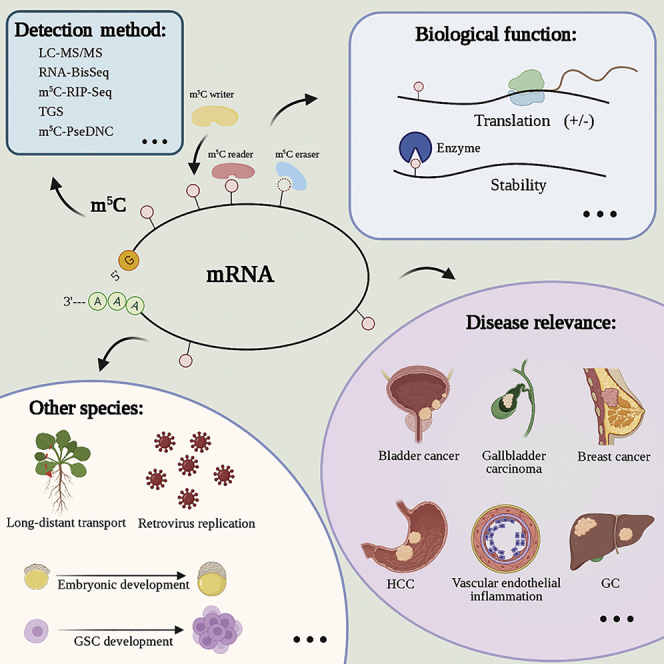
In this review, we discuss the detection methods and predictive models of mRNA 5-methylcytosine (m5C) modifications. We also surveyed the latest research progress on m5C-related effectors and their biological functions. Finally, we discuss the role of m5C modification in human disease pathology and its therapeutic potential.
Introduction
Since the discovery of the first post-transcriptional RNA modification, pseudouridine (ψ), in the 1950s,1 researchers have explored the mechanisms underlying gene regulation at the RNA level. This emerging field has been designated as “RNA epigenetics” or “epitranscriptomics.”2, 3, 4 Compared with the chemical modifications of DNA, RNA modifications are more abundant, with a total of 170 identified to date,5 including N6-methyladenosine (m6A), 5-methylcytosine (m5C), N4-acetylcytidine (ac4C), N1-methyladenosine (m1A), ψ, and 7-methylguanylate (m7G). These modifications constitute a relatively unexplored gene-expression regulatory layer, highlighting the complexity and diversity of RNA.4 The structural diversity of modified nucleosides plays a key role in maintaining RNA stability,6 translation,7 and splicing.8 Therefore, these modifications have critical roles in regulating the development and pathophysiology of several diseases.9, 10, 11
In m5C modifications, a methyl group attached to the fifth carbon of the cytosine ring in DNA and RNA molecules. This modification was first identified on DNA12 and later on RNA in the 1970s.13 It is an important post-transcriptional modification (PTCM) that has significant roles in many biological processes.14 Hence, m5C modifications have recently garnered increased attention and have been reported in many species, including yeast, adenovirus, Phaseolus vulgaris, starfish, Tetrahymena thermophila, rat, Drosophila melanogaster, and wheat germ.15, 16, 17, 18, 19, 20, 21, 22, 23, 24
Studies on m5C modifications were initially focused on tRNA and rRNA.25 In tRNA, m5C has been shown to participate in optimizing codon–anticodon pairing, maintaining homeostasis, regulating stress response, and controlling translation efficiency and accuracy.26, 27, 28, 29, 30, 31 Meanwhile, m5C modifications in rRNA play an important role in conferring bacterial drug resistance,32,33 glioma sensitivity to bioactive substrates of the stress-related enzyme NQO1,34 thermal adaptability (e.g., m5C modifications mediated by ribosomal RNA small subunit methyltransferase F (RsmF) in Thermus thermophilus reduce its thermal adaptability),35 and structural stability of the tertiary rRNA–tRNA–mRNA complex under stress (i.e., promotion of adaptive translation).34 However, although there is a large amount of data on m5C in tRNA and rRNA, little is known about its role in mRNA owing to the low abundance of mRNA and the lack of effective separation and purification technologies.36 However, with recent advances in detection methods, studies on mRNA m5C modifications have gradually increased.
In mRNA, m5C modifications, along with multiple effector enzymes, such as NOP2/Sun RNA methyltransferase 2 (NSUN2),37 NSUN6,38 tRNA aspartic acid methyltransferase 1 (TRDMT1),39 and Aly/REF export factor (ALYREF),40 perform numerous functions, including the promotion of mRNA nuclear–cytoplasmic trafficking;40 viral protein expression;41 DNA damage repair;42 mRNA stability;43 cell tolerance,44 proliferation and migration;39 development, differentiation, and reprogramming of stem cells;45,46 and regulation of mRNA splicing.47 In addition, the distribution of m5C varies among cell types.24 m5C modifications at specific positions of mRNAs exhibit different regulatory activities:48 they can either promote or inhibit translation.49,50 Therefore, abnormal mRNA m5C modifications have been associated with the development and progression of multiple diseases, including cancer,51 autoimmune diseases,52 and arteriosclerosis.53
To date, no systematic review exists on the related effectors, specific biological functions in different species, or the clinical relevance of mRNA m5C modifications. Therefore, in this review, we comprehensively summarize the current detection methods, predictive models, related effectors, and roles of mRNA m5C modifications in normal physiology and diseased states, which may serve as a guide for researchers as well as new insights into the development of novel diagnostic methods, treatments, and prognostic biomarkers.
Detection methods
The identification of modified target genes is essential to investigating the biological functions of mRNA m5C modifications. With recent advances in sequencing technology and experimental methods, methylation modifications of mRNA can be qualitatively and quantitatively analyzed at the single-nucleoside level, providing novel insights into its biological functions. Currently, the primary detection methods for m5C modifications include: (1) physicochemical methods, such as chromatography, mass spectrometry (MS), high-performance liquid chromatography (HPLC; Figure 1), and liquid chromatography-tandem MS (LC-MS/MS; Figure 1); (2) chemical conversion, which combines next-generation sequencing (NGS) techniques, such as RNA bisulfite sequencing (RNA-BisSeq), with ten-eleven translocation (Tet)-assisted peroxotungstate oxidation sequencing (TAWO-seq; Figure 2); (3) immunoprecipitation combined with NGS techniques, such as aza-immunoprecipitation (5-Aza-seq; Figure 2) with m5C individual-nucleotide-resolution cross-linking and immunoprecipitation (miCLIP; Figure 2); (4) third-generation sequencing (TGS), based on the differences in electrical signals (Nanopore-seq; Figure 2); (5) predictive models, such as a machine learning-based m5C predictor trained with features extracted from the flanking sequence of m5C modifications (PEA-m5C), 5-methylcytosine sites via pseudo nucleotide compositions (m5C-PseDNC), Pm5CS-Comp-minimum redundancy maximum relevance (mRMR), identifying RNA 5-methylcytosine sites via pseudo nucleotide compositions (iRNA-m5C-PseDNC), and m5C-heuristic nucleotide physical-chemical property reduction (m5C-HPCR). These methods are commonly used in the detection of m5C modifications in target genes.
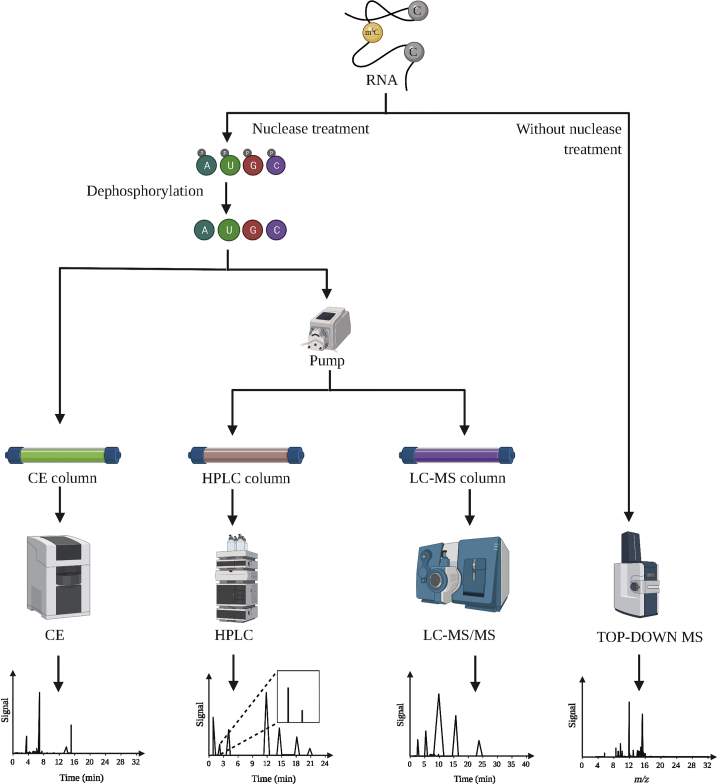
Figure 1.
5-methylcytosine (m5C) detection methods based on chromatography and mass spectrometry (MS)
Nuclease is used to digest the RNA molecule into its constituting nucleotides, which are dephosphorylated. These nucleosides are prepared as input for capillary electrophoresis (CE), high-performance liquid chromatography (HPLC), and liquid chromatography tandem MS (LC-MS/MS). CE: in high-voltage electric field drive, nucleoside samples are separated using a quartz capillary column as a separation channel. LC-MS/MS: the LC system is connected to the mass spectrometer. TOP-DOWN MS: without nuclease treatment, using a high-resolution mass spectrometer and various fragmentation patterns of ribonucleotides, RNA can be directly analyzed using MS to obtain information, including the identification of post-transcriptional modifications, relative quantification, and positional information.
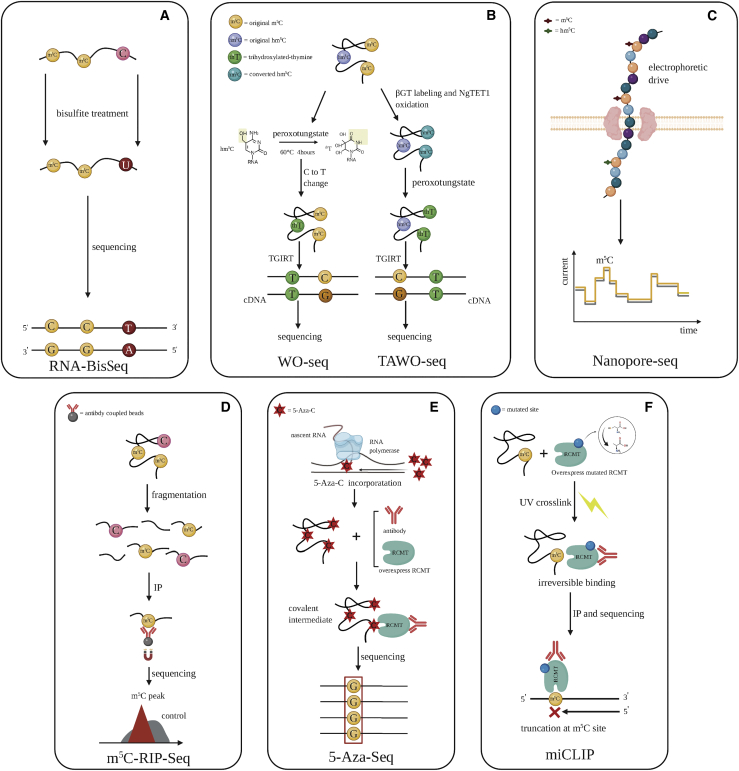
Figure 2.
Transcriptome sequencing methods after m5C modification
(A) RNA-BisSeq: unmethylated cytosines of RNA fragments could be transformed into uridines when treated with bisulfite, while bisulfite does not affect m5C. (B) Peroxotungstate oxidation sequencing (WO-seq) and Tet-assisted WO-seq (TAWO-seq): for WO-seq, hm5C can be transformed into trihydroxylated thymine (thT) by peroxotungstate, after which, the C-to-T mutation site was identified by sequencing. For TAWO-seq, m5C was converted to hm5C with NgTET1 oxidation, and hm5C was converted to thT according to the principle of WO-seq. However, the original hm5C of RNA was protected from being altered to thT by labeling with β-glucosyltransferase (βGT). The mutation of C to T was detected and identified as the original m5C but not the original hm5C. TGIRT, thermostable group II intron reverse transcriptase. (C) Nanopore-seq: a unique, scalable technology that enables direct, real-time analysis of long RNA fragments. The electrical current was monitored and recorded when nucleic acids passed through a protein nanopore. The modification signals were then decoded and identified along RNA fragments. (D) m5C-RIP-seq: RNA fragments containing m5C could be pulled down specifically by the anti-m5C antibody and then used to construct a sequencing library. The m5C peaks were identified against the input as background. (E) 5-Aza-seq: 5-azacytidine (5-Aza-C), a cytidine analog, is incorporated into the RNA molecule by RNA polymerase. RNA (cytosine-5)-methyltransferases (RCMT) can form a temporary intermediate with potential m5C residues; however, 5-Aza-C can inhibit the complex separation. The RCMT-RNA complex was pulled down with a specific anti-RCMT antibody, and the pulled-down RNA was used for library construction and sequencing to identify the m5C sites as C-to-G transversion (red underlined frame). (F) m5C individual-nucleotide-resolution cross-linking and immunoprecipitation (miCLIP)-seq: mutating cysteine (C271) into alanine (C271A) for RCMT inhibited the separation of the enzyme-RNA complex with strong covalent bonding, which was achieved by UV cross-linking, resulting in stop position during PCR. The RCMT-RNA complex was pulled down with anti-RCMT antibody, the RNA was washed to construct the library, and the significant truncation site was considered as the m5C site.
Physicochemical methods
In early studies, the detection of modified nucleosides was based on techniques, such as thin-layer chromatography (TLC) and capillary electrophoresis (CE).54,55 Although these methods are primarily used for the detection of m5C in DNA, they are also suitable for RNA.56,57 The advantages of these techniques include that they are well established, are easy to perform, and can be applied quantitatively.58 Radioactive labeling has also been used to improve the low sensitivity associated with these early methods;59 they remain unable to achieve single-base resolution, making quantitative assessment of methylation patterns in the natural sequence background difficult.
HPLC
The sensitivity of HPLC detection can reach the nanogram level; moreover, radioactive auxiliary compounds are not required for chromatographic separation, thus avoiding the effects of radioactive labeling on the nucleoside structure.60 However, it remains necessary to develop HPLC for the simultaneous detection of multiple RNA modifications and to determine the appropriate HPLC conditions for tandem use with MS.
MS
MS is a physicochemical method that is used for detecting RNA modifications and has been used for more than 40 years.61 It does not require the conversion of RNA into complementary DNA (cDNA) by polymerase chain reaction (PCR); rather, RNA sequences are directly treated with ribonucleases to catalyze their degradation into nucleosides, which are separated and identified using MS.62,63 Modified nucleosides are identified based on the increases in mass and comparison with the mass-to-charge ratio of normal nucleosides.64 Thus, the stripping of all edited bases and epigenetic information from molecules65 and the occasional introduction of substantial artifacts66 for reverse transcription can be avoided. MS can also be used for direct RNA sequencing (RNA-seq) and transcriptomic analysis (modification type/level). In theory, MS is suitable for all modification types, including m5C and m6A.67,68 However, it has certain limitations: (1) it requires relatively high-target RNA purity and concentration; (2) its sensitivity must be improved compared with other amplification-based detection methods;68 (3) considering that MS is primarily used in a serial mode (one RNA sequence at a time), it is unable to meet the required upper limit of the RNA sequence number for analysis, even with multi-channel technology.69 In spite of these limitations, MS remains a powerful tool for studying RNA modifications.
LC-MS/MS
Owing to its superior qualitative ability, MS is often used in tandem with LC, e.g., LC-MS/MS. In fact, Cui et al.70 used this method to identify dynamic m5C mRNA modification in Arabidopsis thaliana. In LC-MS/MS, RNA is digested into oligonucleosides of different lengths by RNases, which are subsequently isolated by LC. Methylation modifications are identified according to the quality difference compared to standard nucleosides, and then modified residues are assigned to sequence sites in oligonucleosides according to gene sequence data.71,72 Moreover, its sensitivity is at the femtomole level,73 making it suitable for the detection of low-abundance modifications (e.g., mRNA m5C modifications). Besides screening for known modified nucleotides, it can also detect those that are unknown, such as the 3-(3-amino-3-carboxypropyl)-5,6-dihydrouridine in tRNALys (tRNA lysine, UUU) from Trypanosoma brucei.74,75 In principle, LC-MS/MS requires at least tens to hundreds of nanograms of purified molecules, which is not feasible for heterogeneous and low-abundance mRNA. However, as this method involves processing RNA into nucleosides using enzymes, RNA samples cannot exist in their full-length form, which can mask the detection of modified sites, cause loss of base sequence context information, and increase the signal/noise in low-quality areas.76
Top-down MS
Unlike LC-MS/MS, top-down MS avoids the complications of enzymatic digestion and the need for oligoribonucleotide separation.58 Thus, it provides the additional application in the detection of unknown modifications of RNAs. This method can also reveal site cytosine with altered mass and its modification type without labeling.77 Although it uses a denaturing solution, reports have shown little effect on methylated bases.78 Top-down MS has a unique potential for the detection, localization, and relative quantification, even though it relies heavily on specialized high-end mass equipment.
Nucleic acid isotope labeling-coupled MS (NAIL-MS)
Notably, the extent of m5C modifications differs during the course of development; thus, the fate of methylated RNA during growth should be tracked to provide data on dynamically changing modified nucleosides. NAIL-MS was developed by Matthias Heiss et al.79 and can be used to assess the influence of the external environment on the apparent transcriptome. However, due to the difficulty associated with adding internal isotope standards to cultured human cells, its utility is limited for these applications.
Nanoscale liquid chromatography coupled to tandem mass spectrometry (Nano-LC-MS/MS/MS)
With the exception of early TLC and CE, these methods are suitable for the detection of single RNA modification types; however, they cannot simultaneously detect multiple RNA modifications. In 2015, Fu et al.80 established a nano-flow LC-MS/MS coupled with a stable isotope-dilution method capable of simultaneously detecting four modified nucleosides (m5C, 2′-O-methylcytidine, m6A, and 2′-O-methyladenosine). Compared with LC-MS/MS, the new method has a clear chemical specificity as well as superior sensitivity for the identification of analytes.80 Moreover, the addition of stable isotope markers in the nucleoside mixture produces more stable and accurate results; thus, it is applicable to studies on RNA modification-related effectors (writer, reader, and eraser).80
2-dimensional mass-retention time hydrophobic end-labeling strategy into MS-based sequencing (2D-HELS MS Seq)
Another method for detecting multiple modification types is the 2D-HELS MS Seq. This method introduces 2D HEL into traditional MS, thereby improving the use efficiency of samples, while also broadening the application scope of RNA direct sequencing.76,81
Chemical conversion methods
RNA-BisSeq
As the gold standard for the detection of m5C modifications,82 RNA-BisSeq can recognize cytosine methylation sites at single-base resolution.83,84 Bisulfite treatment chemically deaminates unmethylated cytosine in single-stranded RNA into uracil; however, methylated cytosine remains unchanged. The m5C methylation sites on RNA can then be identified by constructing a library and sequencing85,86 (Figure 2A). Currently, this method is widely used in the study of mRNA m5C modification in the tissues and cells of multiple animals and plants, including humans, mice, A. thaliana, and Oryza sativa.40,44,87
Although RNA-BisSeq is a robust method for detecting m5C modification sites in high-abundance tRNA and rRNA, it is associated with high rates of errors for low-abundance mRNAs.25 One cause of this is the permanent loss of base-modification information, as the modified bases are less abundant than unmodified bases. Additionally, RNA-BisSeq converts bisulfite-treated RNA into cDNA, requiring NGS after PCR amplification.65,88,89 Moreover, the common generation of artifacts can cause the data to become biased.90 RNA-BisSeq also cannot sufficiently distinguish m5C modifications from other cytosine modifications, such as hm5C (5-hydroxymethylcytidine), which interferes with accuracy and validation.70 Further, experimental pH conditions damage RNA and even lead to its degradation, thus affecting subsequent experiments and limiting the enrichment of RNA containing m5C modifications.91 Lastly, incomplete deamination occurs in unmodified cytidine treated with bisulfite, which is often present in the RNA stem and double-stranded RNA regions, resulting in false positives.87 Therefore, the application of RNA-BisSeq to detect m5C modification sites in mRNA requires further improvement.
TAWO-seq
WO-seq is a method for hm5C detection.92 Yuan et al.86 used Tet enzymes to oxidize m5C to hm5C to establish a new detection method for m5C, termed TAWO-seq (Figure 2B). Here, m5C is first converted to hm5C by peroxotungstate oxidation with Naeglaria Tet-like oxygenase (NgTET1) oxidation, while the original hm5C is protected from oxidation by β-glucosyltransferase.86 TAWO-seq causes less damage to RNA than does RNA-BisSeq. Furthermore, TAWO-seq does not alter unmodified cytosine but rather directly detects the transformation of modified cytosine, avoiding false-positive results caused by the incomplete transformation of unmodified cytosines in RNA-BisSeq. However, as it relies on the transformation of m5C, its incomplete transformation presents a new limitation. Therefore, its transformation efficiency requires further optimization to improve its applicability for mRNA.86
Immunoprecipitation
To address the issue of RNA damage associated with chemical transformation methods, immunoprecipitation, primarily using anti-m5C antibodies that bind to modified RNA and form noncovalent complexes to locate RNA with m5C modifications, has been employed.93
m5C RNA immunoprecipitation sequencing (m5C-RIP-seq)
Combining chromatin immunoprecipitation sequencing (ChIP-seq) and RNA-seq, this method can enrich for and identify m5C modifications in the transcriptome without altering RNA sequence information or requiring RNA-modifying enzymes (Figure 2D). It also avoids the harsh chemical and temperature conditions required for other methods (e.g., the temperature for PCR amplification and acid-base conditions for RNA-BisSeq).94 Therefore, m5C-RIP-seq is theoretically suitable for detecting the distribution of multiple RNA modifications in the transcriptome95 and has been applied to the analysis of bacterial, archaeal, yeast, and plant transcriptomes.70 The limitations of m5C-RIP-seq include its high dependence on specific antibodies and the risk of nonspecific binding to RNA.93 To address these issues, Weichmann et al.96 developed an experimental toolbox capable of verifying antibody performance, while improving the accuracy and credibility of m5C-RIP-seq data. However, m5C-RIP-seq cannot accurately identify the location of single nucleoside sites, as the length of the sequence reads it produces is generally 100–150 nt and is hindered by the RNA secondary structure.70,94
5-Aza-seq and miCLIP
These new derivatization methods (Figures 2E and 2F) have been developed based on immunoprecipitation. Compared with m5C-RIP-seq, 5-Aza-seq can recognize specific targets of enzymes and specific catalytic sites of RNA (cytosine-5)-methyltransferases (RCMTs) at single-base pair resolution. Specifically, 5-azacytidine (5-Aza-C) is incorporated into RNA to prevent the decomposition of the methyltransferase-RNA complex, transforming the target site of methyltransferase from C to G. The m5C modification site can then be identified.36,37,97,98 This method can identify specific enzyme targets and the specific catalytic sites of RCMTs at the resolution of single bases, thus addressing the issue of nonspecific binding with MeRIP-seq, as well as the inability to accurately locate single nucleosides. Accordingly, Schaefer et al.28 used 5-Aza-seq to characterize DNA methyltransferase (DNMT)2 function in the methylation of tRNA. However, 5-Aza-C destroys RNA integrity;99 miCLIP forms irreversible covalent cross-linkages between the enzyme and RNA substrate by mutating the cysteine (Cys) in methyltransferase to alanine. Consequently, reverse transcription during amplification terminates at −1 of the methylation site, and the m5C site is mapped at the +1 of the read sequence data.100, 101, 102 Recently, Selmi et al.103 mapped the m5C modifications in the human transcriptome using miCLIP.
Both 5-Aza-seq and miCLIP depend on the formation of covalent bonds between RNA methylase and its substrate; however, the processes for the formation of this bond differ. Unlike 5-Aza-seq, miCLIP does not affect the methyltransferase step or the structure or stability of RNA.37 However, miCLIP is time consuming and costly and depends on the mutation rate of methyltransferase, with a high rate altering the methylation pattern.104 Nevertheless, both 5-Aza-seq and miCLIP can be used to identify targets of m5C methylase. For example, in 2013, Khoddami and Cairns97 discovered the target of methylase NSUN2 in noncoding RNAs, such as tRNA, using 5-Aza-seq. In the same year, Hussain et al.102 analyzed and confirmed using miCLIP that NSUN2 can act on non-coding RNAs. Thus, both of these techniques can locate targets of the m5C methylase NSUN2, thereby expanding the current knowledge regarding its scope of action and function.105 Although these immunoprecipitation-based methods do not require PCR amplification, their application in trace-volume samples is limited. In addition, their reproducibility is poor due to batch-to-batch differences in antibodies and changes in their affinity.106 Hence, it is necessary to develop more effective antibody-independent detection techniques in the future.
TGS
Although NGS can accurately localize modified nucleosides within the transcriptome,107 sequence-by-synthesis technologies can only sequence short RNA reads,65 limiting their ability to analyze long, repetitive sequences108 and the subsequent plotting of genome-wide modification maps. Recently, third-generation, single-molecule long reads (TGS) generating long-read information have been developed, including Nanopore-seq technology as well as single-molecule real-time (SMRT) sequencing technology. These methods do not require RNA recombination or qPCR amplification when sequencing transcriptomes,109 addressing the limitations of NGS and effectively improving continuity and integrity.110
Nanopore-seq technology offers unique advantages in the analysis of splice variants,111 which provide more abundant biological information for RNA research. This technique is based on the principle that when RNA passes through nanopores, the bases cause characteristic changes in the current, which is unique to each normal and modified base. The corresponding bases can then be inferred from the signal-spatial data112 (Figure 2C). In addition, SMRT technology uses reverse transcription to locate m6A modification sites on RNA113 and has been proposed to be capable of detecting other types of modifications in natural RNA.114 However, TGS faces certain challenges. For example, RNA molecules readily degrade and fold, causing inaccurate results when sequencing longer RNA molecules. Although TGS has a high error rate, it can obtain complete genome sequences and transcriptional landscapes,115 while addressing the issues associated with crosstalk and dependence among multiple RNA modifications in the same RNA molecule.65 These advantages provide a solid foundation for further studies on biological etiologies and disease pathologies.
Predictive models
Although the above-mentioned experimental detection methods can obtain relatively accurate information on mRNA modifications, they are difficult to perform, time consuming, and costly; they are, therefore, often inappropriate for the in-depth analysis of m5C-related mechanisms and functions.116 In the post-genome era, computational tools for predicting m5C sites have become both important and feasible. Studies on the optimization of these tools primarily focus on the construction of improved datasets by optimizing mixed datasets and determining the gold standard for negative groups to eliminate the influence of false negatives (unmodified samples). However, studies also focus on determining optimal composite feature extraction methods and classifiers.14
Currently, 12 models have been developed to predict mRNA m5C modifications: PEA-m5C,117 a hybrid model for quickly and accurately identifying m5C sites from non- m5C sites in Homo sapiens RNA (Pm5CS-Comp-mRMR), 118 m5C-PseDNC, 119 iRNA-m5C-PseDNC,120 m5C-HPCR,116 prediction of RNA 5-methylcytosine sites based on three different kinds of nucleotide composition (RNA-m5C-Pred),121 identifying the occurrence sites of different RNA modifications by incorporating collective effects of nucleotides into PseKNC (iRNA-PseColl),122 RNA-m5C-finder,123 iRNA-m5C,119 a novel method for predicting m5C sites of RNA (m5C-Pred-SVM),124 a new predictor for multiple types of RNA modification sites using deep learning (DeepMRMP),125 and a platform for simultaneously identifying multiple kinds of RNA modifications (iMRM) (Table S1).126 With the exception of PEA-m5C, which specifically predicts m5C methylation sites in A. thaliana,104,117 all of these models can predict human m5C modifications. Compared with the m5C-PseDNC model, Pm5CS-Comp-mRMR is not affected by large or unbalanced datasets and adopts a mRMR algorithm with higher sensitivity;118,119 however, these two models are not available on web servers.
In the early datasets used to construct predictive models, the numbers of positive and negative sequences were unbalanced, with the former far outnumbering the latter. In addition, these datasets contained minimal information on modifications and faced issues related to data redundancy. For example, iRNA-m5C-PseDNC, created in 2017, retains highly similar sequences, leading to higher predictive results, whereas data redundancy in training datasets leads to a decline in generalizability.120 In 2018, Zhang et al.116 established a new calculation model based on Met1900 and Met1320. Their m5C-HPCR server employs a HPCR algorithm to optimize encoding;116 moreover, its area under the receiver operating characteristic curve, as well as the associated Matthews correlation coefficient, is superior to that of iRNA-m5C-PseDNC.116 In 2019, Fang et al.121 selected three different features, K-nucleotide frequencies (Kmer) + K-spaced nucleotide pair frequencies (KSNPF) + pseudo-dinucleotide composition (PseDNC), and established a new model, RNA-m5C-Pred, based on the Met935 database, with higher specificity and sensitivity than the models developed before 2019.
A total of seven site-prediction models have been developed to date: iRNA-m5C-PseDNC, iRNA-PseColl, RNA-m5C-finder, iRNA-m5C, m5C-Pred-SVM, DeepMRMP, and iMRM. Of the models capable of predicting m5C modifications in humans and mice, iRNA-m5C-PseDNC has the highest overall accuracy,120 particularly when users want to detect more m5C sites in human samples; it is preferred for its high sensitivity. However, this method also has poor specificity.36 For instance, although iRNA-PseColl can identify the methylation sites of three different RNA modifications (m1A, m6A, and m5C), it is accompanied by a high false-positive detection rate.122
Unlike iRNA-PseColl, the RNA-m5C-finder model can recognize m5C modification sites in eight tissues and cell types (mouse_embryonic stem cell, mouse_heart, mouse_kidney, mouse_liver, mouse_muscle, mouse_small intestine, mouse_brain, and human_HeLa);123 however, it cannot predict methylation in fungi.119 Meanwhile, the iRNA-m5C model can detect m5C modification sites in four different species (Homo sapiens, Mus musculus, Saccharomyces cerevisiae, and A. thaliana) and has a higher predictive capacity than RNA-m5C-finder in terms of precision and accuracy.119 The m5C-Pred-SVM model can detect m5C modification sites in H. sapiens, M. musculus, and A. thaliana by introducing position-specific propensity-related features; its performance, including sensitivity, specificity, overall accuracy, and Matthews correlation coefficient, is superior to that of other existing methods124. Similarly, DeepMRMP can predict m1A, ϕ, and m5C modification sites in H. sapiens, M. musculus, and S. cerevisiae RNA,125 while integrating multiple modification types in different species, making it both time and cost effective. In addition, iMRM, a recently reported prediction tool based on eXtreme gradient boosting (XGboost), can predict m1A, m6A, m5C, ψ, and A to I in the RNAs of H. sapiens, M. musculus, and S. cerevisiae.126
Predictive models for a single-modification type or species cannot provide information on the deep features or semantic information of such modification sites. Although these seven predictive models have effectively solved this problem, there remains much room for improvement in the use of these models in the prediction of the types of modification sites that may be involved. There is also a need to improve the predictive abilities of modification sites across different species. Therefore, additional experimental data are needed to improve the quality of datasets that can be used to establish more accurate prediction models.36 However, this does not negate the importance of species-specific prediction models. Considering the high species specificity of eukaryotic m5C modifications,119 it is also necessary to optimize and develop more tailored models with higher performance for specific species.119 Currently, the predictive technology of traditional computational models is highly dependent on the effectiveness of relevant feature extraction, whereas it remains challenging to determine the most relevant species-specific feature combinations.125 Therefore, it is necessary to compare and verify the predictive performance of models that integrate different feature extraction methods and avoid the impact of noise features on performance.
In summary, the establishment of computational models for predicting potential modification sites provides an excellent supplement to the experimental methods for identifying m5C sites. The continued development and optimization of bioinformatic tools will help researchers to better understand experimental data. In the future, predictive and analytical tools will be effectively applied to increasingly complex samples and experimental conditions.
Writers, readers, and erasers related to m5C modifications of mRNA
The PTCM of RNA primarily involves three types of effectors:65,127,128 (1) writers that “write” specific chemical groups into mRNA, which subsequently mediates mRNA modifications; (2) readers that “read” the information contained in these mRNA modifications to maintain mRNA stability and participate in RNA translation and splicing; and (3) erasers that “erase” mRNA modification signals, mediate mRNA modifications, and convert them back into unmodified nucleosides. Currently, identified writers of mRNA m5C include NSUN2,37 NSUN6,38 TRDMT1,39 the tRNA-specific methyltransferase 4B (TRM4B),70 and Osnsun2,44 and readers include ALYREF,40 Y box binding protein 1 (YBX1),43 and DNA repair protein RAD52 homolog (RAD52)42 (Figure 3). To date, there have been no reported erasers.
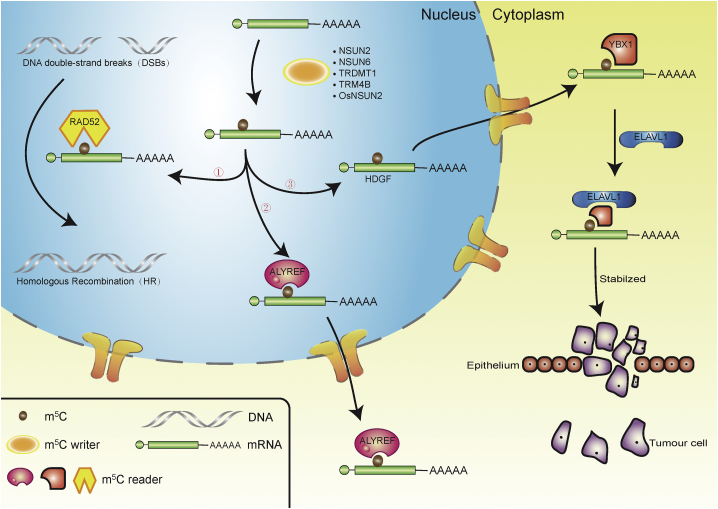
Figure 3.
Overview of the effectors (writers, readers, and erasers) related to m5C modifications of mRNA
NSUN2, NSUN6, TRDMT1, TRM4B, and OsNSUN2 are RNA methyltransferases responsible for m5C modification. (1) m5C reader RAD52 recognizes m5C-methylated RNA to promote reactive oxygen species (ROS)-induced atypical HR repair of DSBs through the TRDMT1-m5C-RAD52-RAD51 axis. (2) m5C reader ALYREF recognizes m5C-methylated mRNA and transfers mRNA from the nucleus to the cytoplasm. (3) In bladder cancer, m5C reader YBX1 recognizes methylated heparin-binding growth factor (HDGF) mRNA and recruits ELAV-like protein 1 (ELAVL1) to stabilize HDGF, finally promoting the proliferation and metastasis of bladder cancer cells.
Writers
Writing of m5C into RNA is primarily accomplished by methyltransferases from two families, namely the DNMT family, i.e., TRDMT1, and the nucleolar protein 1 (NOL1)/NSUN protein family, which has seven members in humans (NSUN1 to NSUN7).129,130
NSUN2 is an enzyme with a wide target spectrum and was first discovered in association with a tRNA modification131,132 and mRNA methylation.133,134 It can catalyze the attachment of methyl to the 5th carbon atom of cytosine in mRNA.89 The RNA-recognition motif of NSUN2, as well as the Rossmann-fold catalytic core containing S-adenosylmethionine (SAM) cofactor, plays major roles in catalytic modification. They use the covalent connection between two Cys and the cytosine in mRNA to activate the electron-deficient pyrimidine heterocycle. The carbon 5 then nucleophilically attacks the methyl group of SAM, completing methylation.135,136 One of the main biological functions of NSUN2-mediated mRNA m5C modification is its influence on protein translation.137 Modifications at different mRNA sites can promote or inhibit translation. For instance, Xing et al.134 reported that NSUN2 methylates the 3ʹ UTR (C1733) of cyclin-dependent kinase 1 (CDK1) mRNA, enhancing its translation by increasing the assembly of ribosomes on CDK1 mRNA. Similarly, Wang et al.138 found that NSUN2 mediates the methylation of cytosine at site 466 of interleukin 17A (IL-17A) mRNA to promote its translation in T lymphocytes, mediating homocysteine-induced upregulation of IL-17A expression. Additionally, correlations between increased expression of src homology 2 domain containing (SHC) proteins (p66SHC, p52SHC, and p46SHC), p21, and ICAM-1 (intercellular adhesion molecule 1) and mRNA m5C modifications mediated by NSUN2 have been reported.50,53,139 Tang et al.140 reported that NSUN2 inhibits p27 translation by modifying C64 methylation (m5C) in the 5′ UTR of p27 mRNA. Similarly, Mei et al.141 found that m5C modifications in mRNA 3′ UTR mediated by NSUN2 inhibit the expression of p57Kip2. Schumann et al.137 showed that the abundance of m5C modifications in the coding sequence (CDS) region negatively correlates, whereas methylation enhancement in the 3′ UTR positively correlates, with mRNA translation efficiency. Therefore, the function of NSUN2 in m5C modifications may be closely related to the position of the methylation or the specific modified target gene. Fang et al.38 discovered NSUN6, a new mRNA m5C methyltransferase, using CRISPR integrated gRNA and reporter sequencing (CIGAR-seq), a CRISPR-Cas9-based method for the unbiased screening of novel mRNA modification effectors. Using mRNA bisulfite sequencing in NSUN6- and/or NSUN2-knockout a near-haploid human leukemia cell line (HAP1) cells, they reported that the mRNA m5C sites targeted by the two methylases do not overlap and that these enzymes are responsible for nearly all of the m5C modifications in the mRNA.38
TRDMT1, another type of RCMT that regulates mRNA methylation and inhibits the proliferation and migration of HEK293 cells,39 can catalyze m5C modifications in tRNA.28,130 This indicates that TRDMT1 has a wide target spectrum, similar to NSUN2, and that m5C methylase may modify many types of RNAs. Therefore, further investigation is required to determine which methylase mediates m5C methylation in different spatiotemporal states and RNA species. Unlike NSUN2, methylation by TRDMT1 only uses Cys at a single site.136 Chen et al.42 found that TRDMT1 is also a writer of RNA m5C at a DNA damage site; its loss hampers homologous recombination (HR) and increases sensitivity to DNA double-strand breaks (DSBs). In addition, TRM4B in A. thaliana has mRNA m5C methyltransferase activity.70 Another study identified Osnsun2 as an m5C methyltransferase targeting mRNA in O. sativa.44
As m6A is the most common modification type in eukaryotic mRNA,127,142 many methylases have been reported to catalyze its installation.143, 144, 145, 146, 147, 148, 149, 150 Huang et al.23 found that certain m5C sites maintain high methylation levels in NSUN2 knockdown or knockout cell lines. Furthermore, these m5C sites have different sequences and structural characteristics from those dependent on NSUN2, suggesting the involvement of unknown methyltransferases in the catalysis of m5C sites, independent of NSUN2 in human mRNA.23 Collectively, the diversity of m6A writers suggests that numerous m5C methylases remain to be discovered.
Interestingly, some studies have linked m5C to m6A. For example, m5C mediated by NSUN2 and m6A by METTL3/METTL14 have been shown to reciprocally promote each other and enhance p21 expression synergistically at the translation level during oxidative stress-induced cell aging.50 Zhang et al.133 found that NSUN2 acts on the 988th adenine of p16 mRNA to produce m6A modification, suggesting that NSUN2 is an m6A methyltransferase of mRNA. In addition, crosstalk between m6A and m5C regulators in human cancers has been proposed.151 However, their precise interactions that are associated with the regulation of mRNA function remain unknown. It is also important to determine whether other types of modifications, apart from m6A and m5C, also interact. We speculate that these topics may become future research hotspots.
Readers
Reader proteins for m6A modification in mRNA have been extensively studied and found to play roles in mRNA splicing, output, stabilization, and translation.152, 153, 154 Relative to studies on mRNA m6A readers, those on mRNA m5C readers are in the early stages. For instance, ALYREF plays a role in the nucleoplasmic export of mRNA;155 as a reader, it binds specifically to m5C-modified mRNA via K171 (lysine at the 171st site) to regulate mRNA export.40 Meanwhile, YBX1 participates in maintaining the stability of m5C-modified mRNA.43,51 In addition, RAD52 has a higher affinity for hybrid strands containing m5C-modified RNA and DNA (complementary pair of RNA), indicating that it is an m5C reader of DNA damage sites. Finally, RAD52 promotes the atypical HR repair of DSBs induced by reactive oxygen species through the TRDMT1-m5C-RAD52-RAD51 axis.42
Erasers
The reversibility of mRNA m6A modifications has been confirmed, with the mediation of RNA demethylases, such as fat mass and obesity-associated protein (FTO)156 and AlkB homolog 5 (ALKBH5).157 Early studies identified a demethylation pathway associated with m5C modifications of DNA; that is, during DNA modification, m5C is oxidized to 5-carboxycystine (5caC) by Tet and then cleaved by thymine-DNA glycosylase to complete the process.158 The m5C modifications can be hydroxylated to form hm5C by the modification of mitochondrial (mt) tRNA methionine, with hm5C further oxidized to 5-formylcytosine (F5C).159,160 However, the erasers of mRNA m5C remain unknown. Shen et al.161 reported that Tet2 mediates the oxidation of mRNA m5C. However, in mouse cells, the level of mRNA hm5C is lower than that of m5C, and the presence of Tet2 protein does not increase the mRNA hm5C content. Therefore, unknown proteins may reconvert hm5C to m5C,161 suggesting that m5C modifications may have a reversible erasure pathway. Further research is needed to determine whether mRNA m5C modifications are reversible.
mRNA m5C modification in disease
Cancer
Multiple mRNA m5C effectors, which regulate m5C modifications, have been identified as participants in the development and progression of cancer (Figure 4). Specifically, m5C plays an important role in cancer cell proliferation and metastasis, as well as cancer stem cell development, by regulating mRNA stability, expression, and translation (Table 1).51,141,166,167 Chen et al.51 showed that YBX1 recognizes m5C-modified mRNA via the W65 indole ring in the cold shock domain (CSD) and recruits ELAVL1 (ELAV-like protein 1) to stabilize HDGF (heparin-binding growth factor) mRNA, ultimately promoting the proliferation and metastasis of bladder cancer cells. Zhang et al.168 found that the number of mRNA m5C methylation peaks in liver cancer tissues was significantly higher than that in adjacent normal tissues, with significant differences in the distribution of genes in different positions. These results suggest that the development and progression of liver cancer correlate with mRNA m5C modifications. High expression of m5C-related effectors NSUN4 and ALYREF is correlated with poor prognosis of patients with hepatocellular carcinoma (HCC).167 Although the specific underlying mechanisms remain unclear, these results suggest that m5C modifications are closely related to the development, progression, and prognosis of HCC. Moreover, NSUN2 is reportedly significantly upregulated in gastric cancer tissues compared with levels in adjacent normal tissues and may promote the proliferation of gastric cancer cells in an m5C-dependent manner by inhibiting the expression of p57Kip2.141 Collectively, these studies indicate that mRNA m5C modifications play a role in certain cancers; however, whether m5C is involved in the development and progression of other cancers needs to be investigated.
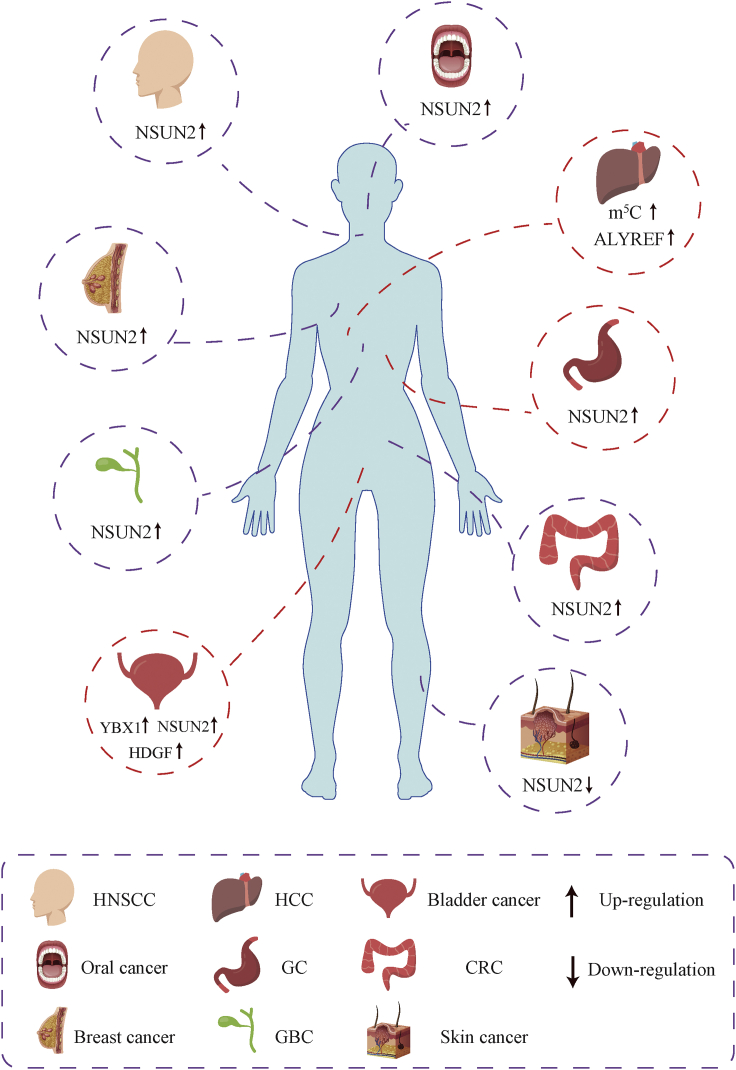
Figure 4.
The mRNA m5C modification associations with tumorigenesis and metastasis in multiple cancers
The red dotted line indicates that tumorigenesis and metastasis in multiple cancers are related to m5C modifications, whereas the purple dotted line indicates that the disease correlation with m5C modifications is uncertain.
Table 1.
Clinical relevance of aberrant m5C modifications
| Disease | Effectors | Function | Regulation | Mechanism | Refs. |
|---|---|---|---|---|---|
| Bladder cancer | NSUN2 | writer | upregulated | NSUN2 targets HDGF m5C modification | 51 |
| Gastric cancer | NSUN2 | writer | upregulated | NSUN2 promotes cell proliferation by repressing p57Kip2 expression in an m5C-dependent manner | 141 |
| Gallbladder carcinoma | NSUN2 | − | upregulated | NSUN2 promotes the proliferation and tumorigenesis cells by close cooperation with ribosomal protein L6 (RPL6) | [162] |
| Breast cancer | NSUN2 | − | upregulated | hypomethylation of NSUN2 leads to its overexpression | 163 |
| Head and neck squamous carcinoma | NSUN2 | − | upregulated | − | 164 |
| Oral cancer and colorectal cancer | NSUN2 | − | upregulated | − | 165 |
| Skin cancer | NSUN2 | − | downregulation | − | 166 |
| Systemic lupus erythematosus | NSUN2 | − | downregulation | − | 52 |
| Bladder cancer | YBX1 | reader | upregulated | YBX1 recognizes m5C-modified mRNA and recruits ELAVL1 to stabilize HDGF mRNA, finally promoting the proliferation and metastasis of bladder cancer cells | 51 |
| HCC | ALYREF | − | upregulated | high levels of ALYREF expression are associated with cell-cycle regulation and mitosis | 167 |
| Vascular endothelial inflammation | NSUN2 | writer | − | NSUN2 promotes the expression of ICAM-1 by upregulating the methylation of ICAM-1 mRNA, thus promoting the development of vascular endothelial inflammation | 53 |
−, unknown function.
It should be noted that only a few studies have reported associations between mRNA m5C modifications and cancer, most of which are focused on NSUN2, whereas no evidence has been presented to implicate NSUN2 in cancer development or progression by regulating m5C modification (Figure 4). Gao et al.162 found that a high expression of NSUN2 can promote the proliferation and tumorigenesis of gallbladder carcinoma cells both in vitro and in vivo by closely cooperating with ribosomal protein L6. Using immunohistochemistry, Yi et al.163 reported that the expression of NSUN2 correlates with the clinical stage, tumor classification, and pathological differentiation of breast cancer. Furthermore, the hypomethylation of NSUN2 led to its overexpression, thus promoting the proliferation, migration, and invasion of breast cancer cells. The upregulation of NSUN2 has also been correlated with poor prognosis in head and neck squamous carcinoma, oral cancer, and colorectal cancer.164,165 In contrast, other studies have reported that its deletion in mice enhances the self-renewal potential of tumor-initiating cells, thus promoting the occurrence of skin cancer. Furthermore, the overexpression of NSUN2 in human skin cancer negatively correlates with disease malignancy.166 Hence, certain questions remain and require further investigation. It is important to determine whether NSNU2 promotes or inhibits cancer development and progression by regulating specific cancer-causing or cancer-inhibiting mRNAs in different cancers. Additionally, validating whether NSUN2 plays a role in cancer development and progression via the regulation of m5C modifications is necessary.
The m5C modification and its effectors, such as NSUN2 and YBX1, are involved in the development and progression of multiple cancers, suggesting new targets for disease treatment. For example, inhibition of sphingosine kinase (SPHK) in breast cancer can reduce the expression of NSUN2, suggesting that SPHK1 is a potential breast cancer marker.169,170 The YBX1 phosphorylation inhibitors TAS0612 (multikinase inhibitor) and everolimus (rapamycin complex 1 inhibitor), as readers, can overcome the resistance to estrogen in progressive breast cancer cells.171 However, whether the role of YBX1 inhibitors in the treatment of breast cancer is related to the regulation of m5C modifications remains to be elucidated. In particular, whether mRNA m5C-related effectors can be used as markers to predict cancer prognosis and whether their inhibitors, combined with conventional chemotherapy drugs, represent effective anti-cancer strategies, remain to be confirmed.65,166 Meanwhile, DNA modifications play an important role in regulating the characteristics of cancer stem cells. For instance, inhibitors of regulatory enzymes, such as those of histone deacetylase, e.g., vorinostat, and those of DNMT, e.g., azacitidine, decitabine, and SGI-110, are undergoing drug clinical trials for the treatment of multiple cancers.172 Therefore, increasing our understanding of mRNA m5C modifications may lead to improving the diagnosis, treatment, and prognosis of cancer.
Autoimmune diseases
Previously, we found that the 5-methylcytosine/cytosine (m5C/C) ratio of CD4+ T cells in patients with systemic lupus erythematosus (SLE) was lower than that in healthy controls. The distribution of m5C modifications in the mRNA of SLE patients and healthy individuals is highly conservative and primarily concentrated near the mRNA translation initiation site. Hypermethylated and upregulated genes in SLE function in the immune system, including cytokine and interferon signaling pathways.52 In addition, we observed the downregulation of NSUN2 and m5C modifications in SLE patients; however, many genes were hypermethylated in SLE patients, suggesting the presence of other m5C methylases.52 For instance, IL-17A is an important mediator in many autoimmune diseases.173 In rat T lymphocytes, NSUN2 promotes the translation of IL-17A by methylating its C466 site;138 however, it is unclear whether m5C modifications of IL-17A mRNA are involved in immune diseases. The specific mechanisms underlying mRNA m5C modifications in the remission and recurrence of SLE are also not well understood. Further research is needed to address these questions.
Vascular endothelial inflammation
Luo et al.53 found that NSUN2 promotes the expression of ICAM-1 by upregulating the methylation of ICAM-1 mRNA, thus improving the adhesion between leukocytes and endothelial cells and promoting vascular endothelial inflammation, a key factor in the pathogenesis of multiple vascular diseases, including atherosclerosis, hypertension, restenosis, and ischemia/reperfusion damage.53,174 Moreover, the lack of donor NSUN2 hindered the formation of allograft arteriosclerosis in a rat model of aortic allograft,53 suggesting that the NSUN2-ICAM-1 regulatory axis is involved in vascular endothelial inflammation. Besides ICAM-1 mRNA, NSUN2 can also catalyze the methylation of other mRNAs and non-coding RNAs.102,133 Therefore, the mechanism by which NSUN2 regulates the development and progression of vascular inflammation and atherosclerosis requires further investigation.
Clinical relevance and future directions
The clinical relevance of RNA methylation may potentially become a research hotspot in the coming years. However, current studies on the clinical effects of mRNA m5C modification tend to focus on neoplastic diseases, with few focusing on non-neoplastic diseases (Table 1).52 Hence, broadening the types of diseases in future studies will advance our understanding of the clinical consequences of m5C modifications. Additionally, mRNA methylation may be a potential biomarker in the progression of specific diseases. Thus, it is essential to develop novel techniques for the rapid identification of modifications that give rise to diseased states and the quantification of the protein levels associated with these modifications.175 Further exploration of the role of m5C modifications in immune responses will provide a broader prospect for tumor immunotherapy176 and tumor drug resistance.177 There have been several companies targeting RNA apparent modifications.178 The investigation of small-molecule inhibitors that are capable of targeting m5C modification-related effector proteins in the context of disease treatment may be a promising research direction.179 As the preclinical efficacy of these targeted drugs is revealed, the development of RNA epigenetic drugs will enter a new era.
mRNA m5C modifications in other species
Plants
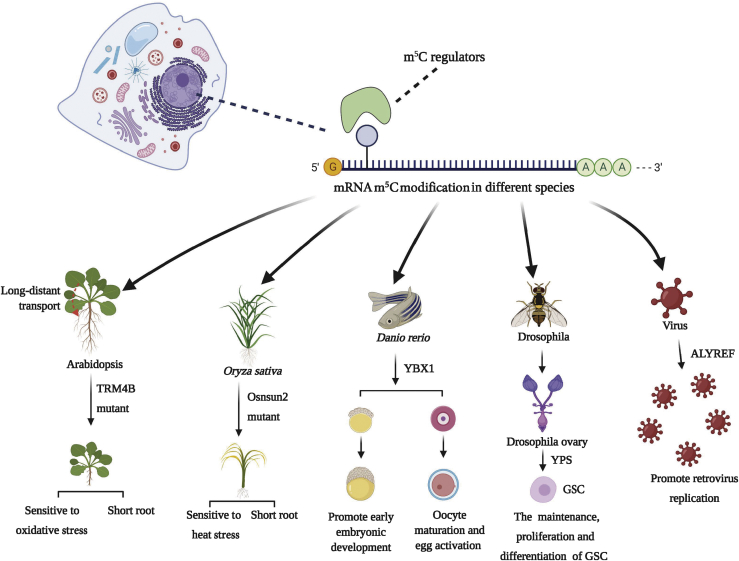
There is evidence for widespread mRNA modifications in plants.70 Although the PTCMs of mRNA have been reported in A. thaliana, O. sativa, Helianthus annuus, Triticum aestivum, Glycine max, and Solanum tuberosum,180 most studies on mRNA m5C modifications have been conducted on A. thaliana87 and O. sativa44 (Figure 5).
Figure 5.
Physiological effects of m5C modifications in different species
A. thaliana
In A. thaliana, mRNA m5C modification sites differ between tissue types, including seeding shoots, roots, siliques, and rosette leaves.87,181 The low overlap between these sites indicates that mRNA performs tissue-specific functions through specific gene methylation patterns. Notably, the homologous protein TRM4B of human NSUN2 in A. thaliana and the homologous protein Osnsun2 in O. sativa exhibit high sequence conservation. These homologous proteins exist widely in green algae and flowering plants and contain a conserved catalytic domain.40,44 In addition, a TRM4B mutant has been reported to suppress division in root tip meristem cells, resulting in the short root phenotype of A. thaliana.87 The TRM4B mutation is speculated to affect mRNA stability; i.e., decreased levels of m5C modifications lead to decreased half-lives of SHY2 and IAA16, which are critical for root development.70 Furthermore, m5C regulates the transport of TCTP1 (translationally controlled tumor protein 1) mRNA between the rootstock and scion in grafted seedlings, thus regulating root development.181 Not only does m5C influence plant development, but it also plays an important role in regulating environmental adaptation. In fact, its biological function is similar to that of m5C in mammals.40,166 Compared with wild-type A. thaliana, the TRM4B mutant is more sensitive to oxidative stress,87 the mechanism of which may be related to the loss of tRNA methylation, reducing its stability. However, considering that TRM4B can act on multiple RNAs, whether methylation modifications of mRNA and other types of RNA participate in the oxidative stress response of A. thaliana, needs to be confirmed.
O. sativa
Tang et al.44 reported that the conservation of m5C sites between O. sativa and A. thaliana is relatively low, indicating that m5C modifications in plants may not be as conserved as those in mammals. However, the distributions of m5C in mRNAs of A. thaliana and O. sativa are similar, both primarily enriched in the coding sequence CDS located near the initiation and termination codons.44,70,87 The mutant Osnsun2 presents with short roots;44 however, the underlying mechanism remains unclear. Osnsun2 can also exhibit a heat-sensitive phenotype, as Osnsun2 primarily plays a role in O. sativa resistance to heat damage by selectively methylating mRNAs involved in photosynthesis and detoxification-related processes.44 Although heat-adaptation mechanisms of Solanum lycopersicum,182 Citrullus Schrad,182 and Daucus carota183 have been reported, it remains to be determined whether mRNA m5C modifications participate in them. Investigating the role of m5C modifications in the environmental tolerance of crops can help to increase yields.
Danio rerio
Recently, Yang et al.43 reported that the reader YBX1 recognizes m5C-modified mRNA via a π-π interaction between the key residue Trp45 of the CSD and m5C. YBX1 then recruits the poly(A)-binding protein Pabpc1a to maintain the stability of maternal mRNA, thus ensuring maternal-to-zygotic transition and promoting early embryonic development. However, the deletion of YBX1 or Pabpc1a leads to stagnation in the blastula and gastrula stages43 (Figure 5). Another study in D. rerio revealed that YBX1 plays a role in oocyte maturation and egg activation by binding to individual mRNAs to inhibit the translation of related proteins184 (Figure 5), suggesting that the biological function of YBX1 is far more complicated than simply recognizing and stabilizing m5C-modified mRNA. Moreover, these roles of m5C modifications in the embryonic development of D. rerio suggest that they may also be involved in coordinating embryonic development in other species.
D. melanogaster
Ypsilon Schachtel (YPS), a human YBX1 homolog, reportedly promotes the maintenance, proliferation, and differentiation of D. melanogaster ovarian germline stem cells (GSCs) by preferentially binding to m5C-modified mRNA (Figure 5), whereas human YBX1 can functionally replace YPS to promote GSC development in the D. melanogaster ovary.45 Considering that the function of YPS/YBX family proteins is highly conserved in D. melanogaster and humans, the D. melanogaster ovary is an attractive model to analyze the function of these proteins and the role of m5C modifications in germ-cell development.
Viruses
As a post-transcriptional regulator of HIV-1 mRNA splicing and functions, m5C has a direct impact on gene expression.41 Meanwhile, m5C modifications of murine leukemia virus (MLV) transcripts promote the replication and expression of viral genes.185 m5C is more common in the genomic RNA of MLV than in the mRNA of uninfected cells, promoting virus replication in an ALYREF reader-dependent manner186 (Figure 5). Therefore, since m5C regulates viral gene expression, viruses can activate the writers and readers of infected cells (hosts) to increase the expression and replication of their own genes. However, whether epitranscriptomic modifications have a beneficial role in viral life cycles remains controversial, as some studies have demonstrated that the methylation m6A inhibits the replication of HIV-1187 and Zika virus.188 Therefore, in-depth investigations are needed to reveal the roles of these RNA modification types. The regulation of m5C modifications during virus replication provides new insights into viral pathology and suggests the potential of m5C methylase inhibitors as antiviral drugs.189
Future prospects and conclusion
In conclusion, m5C modifications have been detected in various organisms and exert unique biological functions in humans and plants,44,87,89 with certain common features.190 It is most abundant in tRNA, rRNA, and mRNA where it affects the RNA stability,28 translational fidelity,191 cell differentiation,192 nervous system regulation, reproductive system development,193,194 and viral viability.195 Therefore, m5C modifications are important to understand a variety of physiological and pathological processes.
It is necessary to further improve and develop techniques for m5C detection to clarify whether m5C modifications actually exist in eukaryotic mRNA or are merely the result of experimental artifacts, as has been suggested by some studies.196 We expect that TGS methods will improve the direct detection of RNA methylation. It is necessary to determine whether RNA m5C modifications can be converted into hm5C and F5C,159,160 whether m5C is only a transient intermediate, and whether the dynamic balance between m5C and its oxidation products have any functional relevance. Lastly, it is necessary to determine whether additional m5C effector proteins exist and to investigate their potential mechanisms in disease to advance the current understanding. In summary, research related to the many mRNA m5C modification-related enzymes and their biological functions is in its infancy, with countless significant discoveries left to be made.
Acknowledgments
This study was supported by the National Nature Science Foundation of China (grant numbers [nos.] 82001705 and 81672707), Key R&D Program of Zhejiang Province (grant no. 2020C03029), Zhejiang Provincial Natural Science Foundation of China (grant no. LY20H100003), Medical Health Science and Technology Project of Zhejiang Provincial Health Commission (grant no. 2019KY453), and Zhejiang Province Science and Technology Plan Research and Xinmiao Talent Program (grant no. 2019R413001).
Author contributions
G.G., X.X., and H.Z. conceived the manuscript. G.G., K.P., S.F., and L.Y. drafted the manuscript. G.G., K.P., S.F., L.Y., X.T., Z.W., H.Z., and X.X. participated in the literature search, discussions, and manuscript revisions. K.P. and S.F. created figures. All authors read and approved the final manuscript for publication.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.omtn.2021.08.020.
Contributor Information
Gangqiang Guo, Email: gangqiangg@yeah.net.
Xiangyang Xue, Email: wzxxy001@163.com.
Huidi Zhang, Email: hd_zhang@163.com.
Supplemental information
References
- 1.Cohn W.E. Pseudouridine, a carbon-carbon linked ribonucleoside in ribonucleic acids: isolation, structure, and chemical characteristics. J. Biol. Chem. 1960;235:1488–1498. [PubMed] [Google Scholar]
- 2.Saletore Y., Meyer K., Korlach J., Vilfan I.D., Jaffrey S., Mason C.E. The birth of the Epitranscriptome: deciphering the function of RNA modifications. Genome Biol. 2012;13:175. doi: 10.1186/gb-2012-13-10-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blanco S., Frye M. Role of RNA methyltransferases in tissue renewal and pathology. Curr. Opin. Cell Biol. 2014;31:1–7. doi: 10.1016/j.ceb.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He C. Grand challenge commentary: RNA epigenetics? Nat. Chem. Biol. 2010;6:863–865. doi: 10.1038/nchembio.482. [DOI] [PubMed] [Google Scholar]
- 5.Boccaletto P., Machnicka M.A., Purta E., Piatkowski P., Baginski B., Wirecki T.K., de Crécy-Lagard V., Ross R., Limbach P.A., Kotter A. MODOMICS: a database of RNA modification pathways. 2017 update. Nucleic Acids Res. 2018;46(D1):D303–D307. doi: 10.1093/nar/gkx1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang X., Lu Z., Gomez A., Hon G.C., Yue Y., Han D., Fu Y., Parisien M., Dai Q., Jia G. N6-methyladenosine-dependent regulation of messenger RNA stability. Nature. 2014;505:117–120. doi: 10.1038/nature12730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y., Wang X., Zhang X., Wang J., Ma Y., Zhang L., Cao X. RNA-binding protein YTHDF3 suppresses interferon-dependent antiviral responses by promoting FOXO3 translation. Proc. Natl. Acad. Sci. USA. 2019;116:976–981. doi: 10.1073/pnas.1812536116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adhikari S., Xiao W., Zhao Y.L., Yang Y.G. m(6)A: Signaling for mRNA splicing. RNA Biol. 2016;13:756–759. doi: 10.1080/15476286.2016.1201628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roundtree I.A., Evans M.E., Pan T., He C. Dynamic RNA Modifications in Gene Expression Regulation. Cell. 2017;169:1187–1200. doi: 10.1016/j.cell.2017.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frye M., Harada B.T., Behm M., He C. RNA modifications modulate gene expression during development. Science. 2018;361:1346–1349. doi: 10.1126/science.aau1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsu P.J., Shi H., He C. Epitranscriptomic influences on development and disease. Genome Biol. 2017;18:197. doi: 10.1186/s13059-017-1336-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zin’kovskaia G.G., Berdyshev G.D., Vaniushin B.F. [Tissue-specific decrease and change in the character of DNA methylation in cattle with aging] Biokhimiia. 1978;43:1883–1892. [PubMed] [Google Scholar]
- 13.Dubin D.T., Stollar V. Methylation of Sindbis virus “26S” messenger RNA. Biochem. Biophys. Res. Commun. 1975;66:1373–1379. doi: 10.1016/0006-291x(75)90511-2. [DOI] [PubMed] [Google Scholar]
- 14.Dou L., Li X., Ding H., Xu L., Xiang H. Prediction of m5C Modifications in RNA Sequences by Combining Multiple Sequence Features. Mol. Ther. Nucleic Acids. 2020;21:332–342. doi: 10.1016/j.omtn.2020.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barciszewska M., Dirheimer G., Keith G. The nucleotide sequence of methionine elongator tRNA from wheat germ. Biochem. Biophys. Res. Commun. 1983;114:1161–1168. doi: 10.1016/0006-291x(83)90684-8. [DOI] [PubMed] [Google Scholar]
- 16.Addison W.R., Gillam I.C., Tener G.M. The nucleotide sequence of tRNA4Val of Drosophila melanogaster. Chloroacetaldehyde modification as an aid to RNA sequencing. J. Biol. Chem. 1982;257:674–677. [PubMed] [Google Scholar]
- 17.Kuchino Y., Shindo-Okada N., Ando N., Watanabe S., Nishimura S. Nucleotide sequences of two aspartic acid tRNAs from rat liver and rat ascites hepatoma. J. Biol. Chem. 1981;256:9059–9062. [PubMed] [Google Scholar]
- 18.Kuchino Y., Mita T., Nishimura S. Nucleotide sequence of cytoplasmic initiator tRNA from Tetrahymena thermophila. Nucleic Acids Res. 1981;9:4557–4562. doi: 10.1093/nar/9.18.4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuchino Y., Kato M., Sugisaki H., Nishimura S. Nucleotide sequence of starfish initiator tRNA. Nucleic Acids Res. 1979;6:3459–3469. doi: 10.1093/nar/6.11.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Montasser Kouhsari S., Keith G., Weil J.H. Methylation of yeast tRNAPhe by enzymes from cytoplasm, chloroplasts and mitochondria of Phaseolus vulgaris. Biochim. Biophys. Acta. 1978;521:576–583. doi: 10.1016/0005-2787(78)90299-x. [DOI] [PubMed] [Google Scholar]
- 21.Sommer S., Salditt-Georgieff M., Bachenheimer S., Darnell J.E., Furuichi Y., Morgan M., Shatkin A.J. The methylation of adenovirus-specific nuclear and cytoplasmic RNA. Nucleic Acids Res. 1976;3:749–765. doi: 10.1093/nar/3.3.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoon K., Turner D.H., Tinoco I., Jr., Haar F., Cramer F. The kinetics of binding of U-U-C-A to a dodecanucleotide anticodon fragment from yeast tRNA-Phe. Nucleic Acids Res. 1976;3:2233–2241. doi: 10.1093/nar/3.9.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang T., Chen W., Liu J., Gu N., Zhang R. Genome-wide identification of mRNA 5-methylcytosine in mammals. Nat. Struct. Mol. Biol. 2019;26:380–388. doi: 10.1038/s41594-019-0218-x. [DOI] [PubMed] [Google Scholar]
- 24.Amort T., Rieder D., Wille A., Khokhlova-Cubberley D., Riml C., Trixl L., Jia X.Y., Micura R., Lusser A. Distinct 5-methylcytosine profiles in poly(A) RNA from mouse embryonic stem cells and brain. Genome Biol. 2017;18:1. doi: 10.1186/s13059-016-1139-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Helm M., Motorin Y. Detecting RNA modifications in the epitranscriptome: predict and validate. Nat. Rev. Genet. 2017;18:275–291. doi: 10.1038/nrg.2016.169. [DOI] [PubMed] [Google Scholar]
- 26.Strobel M.C., Abelson J. Effect of intron mutations on processing and function of Saccharomyces cerevisiae SUP53 tRNA in vitro and in vivo. Mol. Cell. Biol. 1986;6:2663–2673. doi: 10.1128/mcb.6.7.2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen Y., Sierzputowska-Gracz H., Guenther R., Everett K., Agris P.F. 5-Methylcytidine is required for cooperative binding of Mg2+ and a conformational transition at the anticodon stem-loop of yeast phenylalanine tRNA. Biochemistry. 1993;32:10249–10253. doi: 10.1021/bi00089a047. [DOI] [PubMed] [Google Scholar]
- 28.Schaefer M., Pollex T., Hanna K., Tuorto F., Meusburger M., Helm M., Lyko F. RNA methylation by Dnmt2 protects transfer RNAs against stress-induced cleavage. Genes Dev. 2010;24:1590–1595. doi: 10.1101/gad.586710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tuorto F., Liebers R., Musch T., Schaefer M., Hofmann S., Kellner S., Frye M., Helm M., Stoecklin G., Lyko F. RNA cytosine methylation by Dnmt2 and NSun2 promotes tRNA stability and protein synthesis. Nat. Struct. Mol. Biol. 2012;19:900–905. doi: 10.1038/nsmb.2357. [DOI] [PubMed] [Google Scholar]
- 30.Chan C.T., Pang Y.L., Deng W., Babu I.R., Dyavaiah M., Begley T.J., Dedon P.C. Reprogramming of tRNA modifications controls the oxidative stress response by codon-biased translation of proteins. Nat. Commun. 2012;3:937. doi: 10.1038/ncomms1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shanmugam R., Fierer J., Kaiser S., Helm M., Jurkowski T.P., Jeltsch A. Cytosine methylation of tRNA-Asp by DNMT2 has a role in translation of proteins containing poly-Asp sequences. Cell Discov. 2015;1:15010. doi: 10.1038/celldisc.2015.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Galimand M., Schmitt E., Panvert M., Desmolaize B., Douthwaite S., Mechulam Y., Courvalin P. Intrinsic resistance to aminoglycosides in Enterococcus faecium is conferred by the 16S rRNA m5C1404-specific methyltransferase EfmM. RNA. 2011;17:251–262. doi: 10.1261/rna.2233511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Doi Y., Wachino J.I., Arakawa Y. Aminoglycoside Resistance: The Emergence of Acquired 16S Ribosomal RNA Methyltransferases. Infect. Dis. Clin. North Am. 2016;30:523–537. doi: 10.1016/j.idc.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Janin M., Ortiz-Barahona V., de Moura M.C., Martínez-Cardús A., Llinàs-Arias P., Soler M., Nachmani D., Pelletier J., Schumann U., Calleja-Cervantes M.E. Epigenetic loss of RNA-methyltransferase NSUN5 in glioma targets ribosomes to drive a stress adaptive translational program. Acta Neuropathol. 2019;138:1053–1074. doi: 10.1007/s00401-019-02062-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Demirci H., Larsen L.H., Hansen T., Rasmussen A., Cadambi A., Gregory S.T., Kirpekar F., Jogl G. Multi-site-specific 16S rRNA methyltransferase RsmF from Thermus thermophilus. RNA. 2010;16:1584–1596. doi: 10.1261/rna.2088310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li J., Huang Y., Zhou Y. A Mini-review of the Computational Methods Used in Identifying RNA 5-Methylcytosine Sites. Curr. Genomics. 2020;21:3–10. doi: 10.2174/2213346107666200219124951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hussain S., Aleksic J., Blanco S., Dietmann S., Frye M. Characterizing 5-methylcytosine in the mammalian epitranscriptome. Genome Biol. 2013;14:215. doi: 10.1186/gb4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fang L., Wang W., Li G., Zhang L., Li J., Gan D., Yang J., Tang Y., Ding Z., Zhang M. CIGAR-seq, a CRISPR/Cas-based method for unbiased screening of novel mRNA modification regulators. Mol. Syst. Biol. 2020;16:e10025. doi: 10.15252/msb.202010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xue S., Xu H., Sun Z., Shen H., Chen S., Ouyang J., Zhou Q., Hu X., Cui H. Depletion of TRDMT1 affects 5-methylcytosine modification of mRNA and inhibits HEK293 cell proliferation and migration. Biochem. Biophys. Res. Commun. 2019;520:60–66. doi: 10.1016/j.bbrc.2019.09.098. [DOI] [PubMed] [Google Scholar]
- 40.Yang X., Yang Y., Sun B.F., Chen Y.S., Xu J.W., Lai W.Y., Li A., Wang X., Bhattarai D.P., Xiao W. 5-methylcytosine promotes mRNA export - NSUN2 as the methyltransferase and ALYREF as an m5C reader. Cell Res. 2017;27:606–625. doi: 10.1038/cr.2017.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Courtney D.G., Tsai K., Bogerd H.P., Kennedy E.M., Law B.A., Emery A., Swanstrom R., Holley C.L., Cullen B.R. Epitranscriptomic Addition of m5C to HIV-1 Transcripts Regulates Viral Gene Expression. Cell Host Microbe. 2019;26:217–227.e6. doi: 10.1016/j.chom.2019.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen H., Yang H., Zhu X., Yadav T., Ouyang J., Truesdell S.S., Tan J., Wang Y., Duan M., Wei L. m5C modification of mRNA serves a DNA damage code to promote homologous recombination. Nat. Commun. 2020;11:2834. doi: 10.1038/s41467-020-16722-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang Y., Wang L., Han X., Yang W.L., Zhang M., Ma H.L., Sun B.F., Li A., Xia J., Chen J. RNA 5-Methylcytosine Facilitates the Maternal-to-Zygotic Transition by Preventing Maternal mRNA Decay. Mol. Cell. 2019;75:1188–1202.e11. doi: 10.1016/j.molcel.2019.06.033. [DOI] [PubMed] [Google Scholar]
- 44.Tang Y., Gao C.C., Gao Y., Yang Y., Shi B., Yu J.L., Lyu C., Sun B.F., Wang H.L., Xu Y. OsNSUN2-Mediated 5-Methylcytosine mRNA Modification Enhances Rice Adaptation to High Temperature. Dev. Cell. 2020;53:272–286.e7. doi: 10.1016/j.devcel.2020.03.009. [DOI] [PubMed] [Google Scholar]
- 45.Zou F., Tu R., Duan B., Yang Z., Ping Z., Song X., Chen S., Price A., Li H., Scott A. Drosophila YBX1 homolog YPS promotes ovarian germ line stem cell development by preferentially recognizing 5-methylcytosine RNAs. Proc. Natl. Acad. Sci. USA. 2020;117:3603–3609. doi: 10.1073/pnas.1910862117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Warren L., Manos P.D., Ahfeldt T., Loh Y.H., Li H., Lau F., Ebina W., Mandal P.K., Smith Z.D., Meissner A. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010;7:618–630. doi: 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Young J.I., Hong E.P., Castle J.C., Crespo-Barreto J., Bowman A.B., Rose M.F., Kang D., Richman R., Johnson J.M., Berget S., Zoghbi H.Y. Regulation of RNA splicing by the methylation-dependent transcriptional repressor methyl-CpG binding protein 2. Proc. Natl. Acad. Sci. USA. 2005;102:17551–17558. doi: 10.1073/pnas.0507856102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shi H., Chai P., Jia R., Fan X. Novel insight into the regulatory roles of diverse RNA modifications: Re-defining the bridge between transcription and translation. Mol. Cancer. 2020;19:78. doi: 10.1186/s12943-020-01194-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoernes T.P., Clementi N., Faserl K., Glasner H., Breuker K., Lindner H., Hüttenhofer A., Erlacher M.D. Nucleotide modifications within bacterial messenger RNAs regulate their translation and are able to rewire the genetic code. Nucleic Acids Res. 2016;44:852–862. doi: 10.1093/nar/gkv1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li Q., Li X., Tang H., Jiang B., Dou Y., Gorospe M., Wang W. NSUN2-Mediated m5C Methylation and METTL3/METTL14-Mediated m6A Methylation Cooperatively Enhance p21 Translation. J. Cell. Biochem. 2017;118:2587–2598. doi: 10.1002/jcb.25957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen X., Li A., Sun B.F., Yang Y., Han Y.N., Yuan X., Chen R.X., Wei W.S., Liu Y., Gao C.C. 5-methylcytosine promotes pathogenesis of bladder cancer through stabilizing mRNAs. Nat. Cell Biol. 2019;21:978–990. doi: 10.1038/s41556-019-0361-y. [DOI] [PubMed] [Google Scholar]
- 52.Guo G., Wang H., Shi X., Ye L., Yan K., Chen Z., Zhang H., Jin Z., Xue X. Disease Activity-Associated Alteration of mRNA m5 C Methylation in CD4+ T Cells of Systemic Lupus Erythematosus. Front. Cell Dev. Biol. 2020;8:430. doi: 10.3389/fcell.2020.00430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Luo Y., Feng J., Xu Q., Wang W., Wang X. NSun2 Deficiency Protects Endothelium From Inflammation via mRNA Methylation of ICAM-1. Circ. Res. 2016;118:944–956. doi: 10.1161/CIRCRESAHA.115.307674. [DOI] [PubMed] [Google Scholar]
- 54.Schwartz I., Ofengand J. E coli tRNAPhe modified at the 3-(3-amino-3-carboxypropyl) uridine with a photoaffinity label is fully functional for aminoacylation and for ribosomal interaction. Biochim. Biophys. Acta. 1982;697:330–335. doi: 10.1016/0167-4781(82)90096-3. [DOI] [PubMed] [Google Scholar]
- 55.Xue H., Glasser A.L., Desgres J., Grosjean H. Modified nucleotides in Bacillus subtilis tRNA(Trp) hyperexpressed in Escherichia coli. Nucleic Acids Res. 1993;21:2479–2486. doi: 10.1093/nar/21.10.2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Adachi H., DeZoysa M.D., Yu Y.T. Detection and Quantification of Pseudouridine in RNA. Methods Mol. Biol. 2019;1870:219–235. doi: 10.1007/978-1-4939-8808-2_17. [DOI] [PubMed] [Google Scholar]
- 57.Bodi Z., Fray R.G. Detection and Quantification of N 6-Methyladenosine in Messenger RNA by TLC. Methods Mol. Biol. 2017;1562:79–87. doi: 10.1007/978-1-4939-6807-7_6. [DOI] [PubMed] [Google Scholar]
- 58.You X., Yuan B., Feng Y. Research Advances in Analytical Methods of RNA Modifications. Fenxi Ceshi Xuebao. 2018;37:1104–1118. [Google Scholar]
- 59.Keith G. Mobilities of modified ribonucleotides on two-dimensional cellulose thin-layer chromatography. Biochimie. 1995;77:142–144. doi: 10.1016/0300-9084(96)88118-1. [DOI] [PubMed] [Google Scholar]
- 60.Buck M., Connick M., Ames B.N. Complete analysis of tRNA-modified nucleosides by high-performance liquid chromatography: the 29 modified nucleosides of Salmonella typhimurium and Escherichia coli tRNA. Anal. Biochem. 1983;129:1–13. doi: 10.1016/0003-2697(83)90044-1. [DOI] [PubMed] [Google Scholar]
- 61.Jebanathirajah J.A., Pittman J.L., Thomson B.A., Budnik B.A., Kaur P., Rape M., Kirschner M., Costello C.E., O’Connor P.B. Characterization of a new qQq-FTICR mass spectrometer for post-translational modification analysis and top-down tandem mass spectrometry of whole proteins. J. Am. Soc. Mass Spectrom. 2005;16:1985–1999. doi: 10.1016/j.jasms.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 62.Kellner S., Burhenne J., Helm M. Detection of RNA modifications. RNA Biol. 2010;7:237–247. doi: 10.4161/rna.7.2.11468. [DOI] [PubMed] [Google Scholar]
- 63.Mishima E., Jinno D., Akiyama Y., Itoh K., Nankumo S., Shima H., Kikuchi K., Takeuchi Y., Elkordy A., Suzuki T. Immuno-Northern Blotting: Detection of RNA Modifications by Using Antibodies against Modified Nucleosides. PLoS ONE. 2015;10:e0143756. doi: 10.1371/journal.pone.0143756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yuan B.-F., Feng Y.-Q. Recent advances in the analysis of 5-methylcytosine and its oxidation products. Trends Analyt. Chem. 2014;54:24–35. [Google Scholar]
- 65.Jonkhout N., Tran J., Smith M.A., Schonrock N., Mattick J.S., Novoa E.M. The RNA modification landscape in human disease. RNA. 2017;23:1754–1769. doi: 10.1261/rna.063503.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen D., Patton J.T. Reverse transcriptase adds nontemplated nucleotides to cDNAs during 5′-RACE and primer extension. Biotechniques. 2001;30:574–580, 582. doi: 10.2144/01303rr02. [DOI] [PubMed] [Google Scholar]
- 67.Païs de Barros J.P., Keith G., El Adlouni C., Glasser A.L., Mack G., Dirheimer G., Desgrès J. 2′-O-methyl-5-formylcytidine (f5Cm), a new modified nucleotide at the ‘wobble’ of two cytoplasmic tRNAs Leu (NAA) from bovine liver. Nucleic Acids Res. 1996;24:1489–1496. doi: 10.1093/nar/24.8.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thomas B., Akoulitchev A.V. Mass spectrometry of RNA. Trends Biochem. Sci. 2006;31:173–181. doi: 10.1016/j.tibs.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 69.Gaston K.W., Limbach P.A. The identification and characterization of non-coding and coding RNAs and their modified nucleosides by mass spectrometry. RNA Biol. 2014;11:1568–1585. doi: 10.4161/15476286.2014.992280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cui X., Liang Z., Shen L., Zhang Q., Bao S., Geng Y., Zhang B., Leo V., Vardy L.A., Lu T. 5-Methylcytosine RNA Methylation in Arabidopsis Thaliana. Mol. Plant. 2017;10:1387–1399. doi: 10.1016/j.molp.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 71.Thüring K., Schmid K., Keller P., Helm M. LC-MS Analysis of Methylated RNA. Methods Mol. Biol. 2017;1562:3–18. doi: 10.1007/978-1-4939-6807-7_1. [DOI] [PubMed] [Google Scholar]
- 72.Kowalak J.A., Pomerantz S.C., Crain P.F., McCloskey J.A. A novel method for the determination of post-transcriptional modification in RNA by mass spectrometry. Nucleic Acids Res. 1993;21:4577–4585. doi: 10.1093/nar/21.19.4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nikcevic I., Wyrzykiewicz T.K., Limbach P.A. Detecting Low-Level Synthesis Impurities in Modified Phosphorothioate Oligonucleotides Using Liquid Chromatography - High Resolution Mass Spectrometry. Int. J. Mass Spectrom. 2011;304:98–104. doi: 10.1016/j.ijms.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thüring K., Schmid K., Keller P., Helm M. Analysis of RNA modifications by liquid chromatography-tandem mass spectrometry. Methods. 2016;107:48–56. doi: 10.1016/j.ymeth.2016.03.019. [DOI] [PubMed] [Google Scholar]
- 75.Krog J.S., Español Y., Giessing A.M., Dziergowska A., Malkiewicz A., Ribas de Pouplana L., Kirpekar F. 3-(3-amino-3-carboxypropyl)-5,6-dihydrouridine is one of two novel post-transcriptional modifications in tRNALys(UUU) from Trypanosoma brucei. FEBS J. 2011;278:4782–4796. doi: 10.1111/j.1742-4658.2011.08379.x. [DOI] [PubMed] [Google Scholar]
- 76.Zhang N., Shi S., Jia T.Z., Ziegler A., Yoo B., Yuan X., Li W., Zhang S. A general LC-MS-based RNA sequencing method for direct analysis of multiple-base modifications in RNA mixtures. Nucleic Acids Res. 2019;47:e125. doi: 10.1093/nar/gkz731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Taucher M., Breuker K. Characterization of modified RNA by top-down mass spectrometry. Angew. Chem. Int. Ed. Engl. 2012;51:11289–11292. doi: 10.1002/anie.201206232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Glasner H., Riml C., Micura R., Breuker K. Label-free, direct localization and relative quantitation of the RNA nucleobase methylations m6A, m5C, m3U, and m5U by top-down mass spectrometry. Nucleic Acids Res. 2017;45:8014–8025. doi: 10.1093/nar/gkx470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Heiss M., Reichle V.F., Kellner S. Observing the fate of tRNA and its modifications by nucleic acid isotope labeling mass spectrometry: NAIL-MS. RNA Biol. 2017;14:1260–1268. doi: 10.1080/15476286.2017.1325063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fu L., Amato N.J., Wang P., McGowan S.J., Niedernhofer L.J., Wang Y. Simultaneous Quantification of Methylated Cytidine and Adenosine in Cellular and Tissue RNA by Nano-Flow Liquid Chromatography-Tandem Mass Spectrometry Coupled with the Stable Isotope-Dilution Method. Anal. Chem. 2015;87:7653–7659. doi: 10.1021/acs.analchem.5b00951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang N., Shi S., Yoo B., Yuan X., Li W., Zhang S. 2D-HELS MS Seq: A General LC-MS-Based Method for Direct and de novo Sequencing of RNA Mixtures with Different Nucleotide Modifications. J. Vis. Exp. 2020:e71281. doi: 10.3791/61281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wreczycka K., Gosdschan A., Yusuf D., Grüning B., Assenov Y., Akalin A. Strategies for analyzing bisulfite sequencing data. J. Biotechnol. 2017;261:105–115. doi: 10.1016/j.jbiotec.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 83.Trixl L., Rieder D., Amort T., Lusser A. Bisulfite Sequencing of RNA for Transcriptome-Wide Detection of 5-Methylcytosine. Methods Mol. Biol. 2019;1870:1–21. doi: 10.1007/978-1-4939-8808-2_1. [DOI] [PubMed] [Google Scholar]
- 84.Blanco S., Dietmann S., Flores J.V., Hussain S., Kutter C., Humphreys P., Lukk M., Lombard P., Treps L., Popis M. Aberrant methylation of tRNAs links cellular stress to neuro-developmental disorders. EMBO J. 2014;33:2020–2039. doi: 10.15252/embj.201489282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jacob R., Zander S., Gutschner T. The Dark Side of the Epitranscriptome: Chemical Modifications in Long Non-Coding RNAs. Int. J. Mol. Sci. 2017;18:2387. doi: 10.3390/ijms18112387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yuan F., Bi Y., Siejka-Zielinska P., Zhou Y.L., Zhang X.X., Song C.X. Bisulfite-free and base-resolution analysis of 5-methylcytidine and 5-hydroxymethylcytidine in RNA with peroxotungstate. Chem. Commun. (Camb.) 2019;55:2328–2331. doi: 10.1039/c9cc00274j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.David R., Burgess A., Parker B., Li J., Pulsford K., Sibbritt T., Preiss T., Searle I.R. Transcriptome-Wide Mapping of RNA 5-Methylcytosine in Arabidopsis mRNAs and Noncoding RNAs. Plant Cell. 2017;29:445–460. doi: 10.1105/tpc.16.00751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Trixl L., Lusser A. The dynamic RNA modification 5-methylcytosine and its emerging role as an epitranscriptomic mark. Wiley Interdiscip. Rev. RNA. 2019;10:e1510. doi: 10.1002/wrna.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Squires J.E., Patel H.R., Nousch M., Sibbritt T., Humphreys D.T., Parker B.J., Suter C.M., Preiss T. Widespread occurrence of 5-methylcytosine in human coding and non-coding RNA. Nucleic Acids Res. 2012;40:5023–5033. doi: 10.1093/nar/gks144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mortazavi A., Williams B.A., McCue K., Schaeffer L., Wold B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods. 2008;5:621–628. doi: 10.1038/nmeth.1226. [DOI] [PubMed] [Google Scholar]
- 91.Frommer M., McDonald L.E., Millar D.S., Collis C.M., Watt F., Grigg G.W., Molloy P.L., Paul C.L. A genomic sequencing protocol that yields a positive display of 5-methylcytosine residues in individual DNA strands. Proc. Natl. Acad. Sci. USA. 1992;89:1827–1831. doi: 10.1073/pnas.89.5.1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hayashi G., Koyama K., Shiota H., Kamio A., Umeda T., Nagae G., Aburatani H., Okamoto A. Base-Resolution Analysis of 5-Hydroxymethylcytosine by One-Pot Bisulfite-Free Chemical Conversion with Peroxotungstate. J. Am. Chem. Soc. 2016;138:14178–14181. doi: 10.1021/jacs.6b06428. [DOI] [PubMed] [Google Scholar]
- 93.Gu X., Liang Z. Transcriptome-Wide Mapping 5-Methylcytosine by m5C RNA Immunoprecipitation Followed by Deep Sequencing in Plant. Methods Mol. Biol. 2019;1933:389–394. doi: 10.1007/978-1-4939-9045-0_24. [DOI] [PubMed] [Google Scholar]
- 94.Saplaoura E., Perrera V., Colot V., Kragler F. Methylated RNA Immunoprecipitation Assay to Study m5C Modification in Arabidopsis. J. Vis. Exp. 2020:e61231. doi: 10.3791/61231. [DOI] [PubMed] [Google Scholar]
- 95.Meng J., Lu Z., Liu H., Zhang L., Zhang S., Chen Y., Rao M.K., Huang Y. A protocol for RNA methylation differential analysis with MeRIP-Seq data and exomePeak R/Bioconductor package. Methods. 2014;69:274–281. doi: 10.1016/j.ymeth.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Weichmann F., Hett R., Schepers A., Ito-Kureha T., Flatley A., Slama K., Hastert F.D., Angstman N.B., Cardoso M.C., König J. Validation strategies for antibodies targeting modified ribonucleotides. RNA. 2020;26:1489–1506. doi: 10.1261/rna.076026.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Khoddami V., Cairns B.R. Identification of direct targets and modified bases of RNA cytosine methyltransferases. Nat. Biotechnol. 2013;31:458–464. doi: 10.1038/nbt.2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jones P.A., Taylor S.M., Wilson V.L. Inhibition of DNA methylation by 5-azacytidine. Recent Results Cancer Res. 1983;84:202–211. doi: 10.1007/978-3-642-81947-6_15. [DOI] [PubMed] [Google Scholar]
- 99.Khoddami V., Cairns B.R. Transcriptome-wide target profiling of RNA cytosine methyltransferases using the mechanism-based enrichment procedure Aza-IP. Nat. Protoc. 2014;9:337–361. doi: 10.1038/nprot.2014.014. [DOI] [PubMed] [Google Scholar]
- 100.Stojković V., Chu T., Therizols G., Weinberg D.E., Fujimori D.G. miCLIP-MaPseq, a Substrate Identification Approach for Radical SAM RNA Methylating Enzymes. J. Am. Chem. Soc. 2018;140:7135–7143. doi: 10.1021/jacs.8b02618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang T., Chen B., Kim M., Xie Y., Xiao G. A model-based approach to identify binding sites in CLIP-Seq data. PLoS ONE. 2014;9:e93248. doi: 10.1371/journal.pone.0093248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hussain S., Sajini A.A., Blanco S., Dietmann S., Lombard P., Sugimoto Y., Paramor M., Gleeson J.G., Odom D.T., Ule J., Frye M. NSun2-mediated cytosine-5 methylation of vault noncoding RNA determines its processing into regulatory small RNAs. Cell Rep. 2013;4:255–261. doi: 10.1016/j.celrep.2013.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Selmi T., Hussain S., Dietmann S., Heiß M., Borland K., Flad S., Carter J.M., Dennison R., Huang Y.L., Kellner S. Sequence- and structure-specific cytosine-5 mRNA methylation by NSUN6. Nucleic Acids Res. 2021;49:1006–1022. doi: 10.1093/nar/gkaa1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.George H., Ule J., Hussain S. Illustrating the Epitranscriptome at Nucleotide Resolution Using Methylation-iCLIP (miCLIP) Methods Mol. Biol. 2017;1562:91–106. doi: 10.1007/978-1-4939-6807-7_7. [DOI] [PubMed] [Google Scholar]
- 105.Liu J., Jia G. Methylation modifications in eukaryotic messenger RNA. J. Genet. Genomics. 2014;41:21–33. doi: 10.1016/j.jgg.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 106.Zhou B., Yang H., Yang C., Bao Y.L., Yang S.M., Liu J., Xiao Y.F. Translation of noncoding RNAs and cancer. Cancer Lett. 2021;497:89–99. doi: 10.1016/j.canlet.2020.10.002. [DOI] [PubMed] [Google Scholar]
- 107.Su D., Chan C.T., Gu C., Lim K.S., Chionh Y.H., McBee M.E., Russell B.S., Babu I.R., Begley T.J., Dedon P.C. Quantitative analysis of ribonucleoside modifications in tRNA by HPLC-coupled mass spectrometry. Nat. Protoc. 2014;9:828–841. doi: 10.1038/nprot.2014.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lu H., Giordano F., Ning Z. Oxford Nanopore MinION Sequencing and Genome Assembly. Genomics Proteomics Bioinformatics. 2016;14:265–279. doi: 10.1016/j.gpb.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.van Dijk E.L., Jaszczyszyn Y., Naquin D., Thermes C. The Third Revolution in Sequencing Technology. Trends Genet. 2018;34:666–681. doi: 10.1016/j.tig.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 110.Xu M., Guo L., Gu S., Wang O., Zhang R., Peters B.A., Fan G., Liu X., Xu X., Deng L., Zhang Y. TGS-GapCloser: A fast and accurate gap closer for large genomes with low coverage of error-prone long reads. Gigascience. 2020;9:giaa094. doi: 10.1093/gigascience/giaa094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Garalde D.R., Snell E.A., Jachimowicz D., Sipos B., Lloyd J.H., Bruce M., Pantic N., Admassu T., James P., Warland A. Highly parallel direct RNA sequencing on an array of nanopores. Nat. Methods. 2018;15:201–206. doi: 10.1038/nmeth.4577. [DOI] [PubMed] [Google Scholar]
- 112.Zhao L., Zhang H., Kohnen M.V., Prasad K.V.S.K., Gu L., Reddy A.S.N. Analysis of Transcriptome and Epitranscriptome in Plants Using PacBio Iso-Seq and Nanopore-Based Direct RNA Sequencing. Front. Genet. 2019;10:253. doi: 10.3389/fgene.2019.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Vilfan I.D., Tsai Y.C., Clark T.A., Wegener J., Dai Q., Yi C., Pan T., Turner S.W., Korlach J. Analysis of RNA base modification and structural rearrangement by single-molecule real-time detection of reverse transcription. J. Nanobiotechnology. 2013;11:8. doi: 10.1186/1477-3155-11-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Murray I.A., Clark T.A., Morgan R.D., Boitano M., Anton B.P., Luong K., Fomenkov A., Turner S.W., Korlach J., Roberts R.J. The methylomes of six bacteria. Nucleic Acids Res. 2012;40:11450–11462. doi: 10.1093/nar/gks891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jenjaroenpun P., Wongsurawat T., Pereira R., Patumcharoenpol P., Ussery D.W., Nielsen J., Nookaew I. Complete genomic and transcriptional landscape analysis using third-generation sequencing: a case study of Saccharomyces cerevisiae CEN.PK113-7D. Nucleic Acids Res. 2018;46:e38. doi: 10.1093/nar/gky014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang M., Xu Y., Li L., Liu Z., Yang X., Yu D.J. Accurate RNA 5-methylcytosine site prediction based on heuristic physical-chemical properties reduction and classifier ensemble. Anal. Biochem. 2018;550:41–48. doi: 10.1016/j.ab.2018.03.027. [DOI] [PubMed] [Google Scholar]
- 117.Song J., Zhai J., Bian E., Song Y., Yu J., Ma C. Transcriptome-Wide Annotation of m5C RNA Modifications Using Machine Learning. Front. Plant Sci. 2018;9:519. doi: 10.3389/fpls.2018.00519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sabooh M.F., Iqbal N., Khan M., Khan M., Maqbool H.F. Identifying 5-methylcytosine sites in RNA sequence using composite encoding feature into Chou’s PseKNC. J. Theor. Biol. 2018;452:1–9. doi: 10.1016/j.jtbi.2018.04.037. [DOI] [PubMed] [Google Scholar]
- 119.Lv H., Zhang Z.M., Li S.H., Tan J.X., Chen W., Lin H. Evaluation of different computational methods on 5-methylcytosine sites identification. Brief. Bioinform. 2020;21:982–995. doi: 10.1093/bib/bbz048. [DOI] [PubMed] [Google Scholar]
- 120.Qiu W.R., Jiang S.Y., Xu Z.C., Xiao X., Chou K.C. iRNAm5C-PseDNC: identifying RNA 5-methylcytosine sites by incorporating physical-chemical properties into pseudo dinucleotide composition. Oncotarget. 2017;8:41178–41188. doi: 10.18632/oncotarget.17104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fang T., Zhang Z., Sun R., Zhu L., He J., Huang B., Xiong Y., Zhu X. RNAm5CPred: Prediction of RNA 5-Methylcytosine Sites Based on Three Different Kinds of Nucleotide Composition. Mol. Ther. Nucleic Acids. 2019;18:739–747. doi: 10.1016/j.omtn.2019.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Feng P., Ding H., Yang H., Chen W., Lin H., Chou K.C. iRNA-PseColl: Identifying the Occurrence Sites of Different RNA Modifications by Incorporating Collective Effects of Nucleotides into PseKNC. Mol. Ther. Nucleic Acids. 2017;7:155–163. doi: 10.1016/j.omtn.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Li J., Huang Y., Yang X., Zhou Y., Zhou Y. RNAm5Cfinder: A Web-server for Predicting RNA 5-methylcytosine (m5C) Sites Based on Random Forest. Sci. Rep. 2018;8:17299. doi: 10.1038/s41598-018-35502-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chen X., Xiong Y., Liu Y., Chen Y., Bi S., Zhu X. m5CPred-SVM: a novel method for predicting m5C sites of RNA. BMC Bioinformatics. 2020;21:489. doi: 10.1186/s12859-020-03828-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sun P.P., Chen Y.B., Liu B., Gao Y.X., Han Y., He F., Ji J.C. DeepMRMP: A new predictor for multiple types of RNA modification sites using deep learning. Math. Biosci. Eng. 2019;16:6231–6241. doi: 10.3934/mbe.2019310. [DOI] [PubMed] [Google Scholar]
- 126.Liu K., Chen W. iMRM: a platform for simultaneously identifying multiple kinds of RNA modifications. Bioinformatics. 2020;36:3336–3342. doi: 10.1093/bioinformatics/btaa155. [DOI] [PubMed] [Google Scholar]
- 127.Shi H., Wei J., He C. Where, When, and How: Context-Dependent Functions of RNA Methylation Writers, Readers, and Erasers. Mol. Cell. 2019;74:640–650. doi: 10.1016/j.molcel.2019.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Delaunay S., Frye M. RNA modifications regulating cell fate in cancer. Nat. Cell Biol. 2019;21:552–559. doi: 10.1038/s41556-019-0319-0. [DOI] [PubMed] [Google Scholar]
- 129.Reid R., Greene P.J., Santi D.V. Exposition of a family of RNA m(5)C methyltransferases from searching genomic and proteomic sequences. Nucleic Acids Res. 1999;27:3138–3145. doi: 10.1093/nar/27.15.3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Goll M.G., Kirpekar F., Maggert K.A., Yoder J.A., Hsieh C.L., Zhang X., Golic K.G., Jacobsen S.E., Bestor T.H. Methylation of tRNAAsp by the DNA methyltransferase homolog Dnmt2. Science. 2006;311:395–398. doi: 10.1126/science.1120976. [DOI] [PubMed] [Google Scholar]
- 131.Brzezicha B., Schmidt M., Makalowska I., Jarmolowski A., Pienkowska J., Szweykowska-Kulinska Z. Identification of human tRNA:m5C methyltransferase catalysing intron-dependent m5C formation in the first position of the anticodon of the pre-tRNA Leu (CAA) Nucleic Acids Res. 2006;34:6034–6043. doi: 10.1093/nar/gkl765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Frye M., Watt F.M. The RNA methyltransferase Misu (NSun2) mediates Myc-induced proliferation and is upregulated in tumors. Curr. Biol. 2006;16:971–981. doi: 10.1016/j.cub.2006.04.027. [DOI] [PubMed] [Google Scholar]
- 133.Zhang X., Liu Z., Yi J., Tang H., Xing J., Yu M., Tong T., Shang Y., Gorospe M., Wang W. The tRNA methyltransferase NSun2 stabilizes p16INK4 mRNA by methylating the 3′-untranslated region of p16. Nat. Commun. 2012;3:712. doi: 10.1038/ncomms1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Xing J., Yi J., Cai X., Tang H., Liu Z., Zhang X., Martindale J.L., Yang X., Jiang B., Gorospe M., Wang W. NSun2 Promotes Cell Growth via Elevating Cyclin-Dependent Kinase 1 Translation. Mol. Cell. Biol. 2015;35:4043–4052. doi: 10.1128/MCB.00742-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.King M.Y., Redman K.L. RNA methyltransferases utilize two cysteine residues in the formation of 5-methylcytosine. Biochemistry. 2002;41:11218–11225. doi: 10.1021/bi026055q. [DOI] [PubMed] [Google Scholar]
- 136.Liu Y., Santi D.V. m5C RNA and m5C DNA methyl transferases use different cysteine residues as catalysts. Proc. Natl. Acad. Sci. USA. 2000;97:8263–8265. doi: 10.1073/pnas.97.15.8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Schumann U., Zhang H.N., Sibbritt T., Pan A., Horvath A., Gross S., Clark S.J., Yang L., Preiss T. Multiple links between 5-methylcytosine content of mRNA and translation. BMC Biol. 2020;18:40. doi: 10.1186/s12915-020-00769-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang N., Tang H., Wang X., Wang W., Feng J. Homocysteine upregulates interleukin-17A expression via NSun2-mediated RNA methylation in T lymphocytes. Biochem. Biophys. Res. Commun. 2017;493:94–99. doi: 10.1016/j.bbrc.2017.09.069. [DOI] [PubMed] [Google Scholar]
- 139.Cai X., Hu Y., Tang H., Hu H., Pang L., Xing J., Liu Z., Luo Y., Jiang B., Liu T. RNA methyltransferase NSUN2 promotes stress-induced HUVEC senescence. Oncotarget. 2016;7:19099–19110. doi: 10.18632/oncotarget.8087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tang H., Fan X., Xing J., Liu Z., Jiang B., Dou Y., Gorospe M., Wang W. NSun2 delays replicative senescence by repressing p27 (KIP1) translation and elevating CDK1 translation. Aging (Albany NY) 2015;7:1143–1158. doi: 10.18632/aging.100860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mei L., Shen C., Miao R., Wang J.Z., Cao M.D., Zhang Y.S., Shi L.H., Zhao G.H., Wang M.H., Wu L.S., Wei J.F. RNA methyltransferase NSUN2 promotes gastric cancer cell proliferation by repressing p57Kip2 by an m5C-dependent manner. Cell Death Dis. 2020;11:270. doi: 10.1038/s41419-020-2487-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Desrosiers R., Friderici K., Rottman F. Identification of methylated nucleosides in messenger RNA from Novikoff hepatoma cells. Proc. Natl. Acad. Sci. USA. 1974;71:3971–3975. doi: 10.1073/pnas.71.10.3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bokar J.A., Shambaugh M.E., Polayes D., Matera A.G., Rottman F.M. Purification and cDNA cloning of the AdoMet-binding subunit of the human mRNA (N6-adenosine)-methyltransferase. RNA. 1997;3:1233–1247. [PMC free article] [PubMed] [Google Scholar]
- 144.Liu J., Yue Y., Han D., Wang X., Fu Y., Zhang L., Jia G., Yu M., Lu Z., Deng X. A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat. Chem. Biol. 2014;10:93–95. doi: 10.1038/nchembio.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ping X.L., Sun B.F., Wang L., Xiao W., Yang X., Wang W.J., Adhikari S., Shi Y., Lv Y., Chen Y.S. Mammalian WTAP is a regulatory subunit of the RNA N6-methyladenosine methyltransferase. Cell Res. 2014;24:177–189. doi: 10.1038/cr.2014.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wang X., Feng J., Xue Y., Guan Z., Zhang D., Liu Z., Gong Z., Wang Q., Huang J., Tang C. Structural basis of N(6)-adenosine methylation by the METTL3-METTL14 complex. Nature. 2016;534:575–578. doi: 10.1038/nature18298. [DOI] [PubMed] [Google Scholar]
- 147.Yue Y., Liu J., Cui X., Cao J., Luo G., Zhang Z., Cheng T., Gao M., Shu X., Ma H. VIRMA mediates preferential m6A mRNA methylation in 3’UTR and near stop codon and associates with alternative polyadenylation. Cell Discov. 2018;4:10. doi: 10.1038/s41421-018-0019-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Patil D.P., Chen C.K., Pickering B.F., Chow A., Jackson C., Guttman M., Jaffrey S.R. m(6)A RNA methylation promotes XIST-mediated transcriptional repression. Nature. 2016;537:369–373. doi: 10.1038/nature19342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wen J., Lv R., Ma H., Shen H., He C., Wang J., Jiao F., Liu H., Yang P., Tan L. Zc3h13 Regulates Nuclear RNA m6A Methylation and Mouse Embryonic Stem Cell Self-Renewal. Mol. Cell. 2018;69:1028–1038.e6. doi: 10.1016/j.molcel.2018.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Warda A.S., Kretschmer J., Hackert P., Lenz C., Urlaub H., Höbartner C., Sloan K.E., Bohnsack M.T. Human METTL16 is a N6-methyladenosine (m6A) methyltransferase that targets pre-mRNAs and various non-coding RNAs. EMBO Rep. 2017;18:2004–2014. doi: 10.15252/embr.201744940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chen Y.T., Shen J.Y., Chen D.P., Wu C.F., Guo R., Zhang P.P., Lv J.W., Li W.F., Wang Z.X., Chen Y.P. Identification of cross-talk between m6A and 5mC regulators associated with onco-immunogenic features and prognosis across 33 cancer types. J. Hematol. Oncol. 2020;13:22. doi: 10.1186/s13045-020-00854-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Xiao W., Adhikari S., Dahal U., Chen Y.S., Hao Y.J., Sun B.F., Sun H.Y., Li A., Ping X.L., Lai W.Y. Nuclear m(6)A Reader YTHDC1 Regulates mRNA Splicing. Mol. Cell. 2016;61:507–519. doi: 10.1016/j.molcel.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 153.Roundtree I.A., Luo G.Z., Zhang Z., Wang X., Zhou T., Cui Y., Sha J., Huang X., Guerrero L., Xie P. YTHDC1 mediates nuclear export of N6-methyladenosine methylated mRNAs. eLife. 2017;6:e31311. doi: 10.7554/eLife.31311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Shi H., Wang X., Lu Z., Zhao B.S., Ma H., Hsu P.J., Liu C., He C. YTHDF3 facilitates translation and decay of N6-methyladenosine-modified RNA. Cell Res. 2017;27:315–328. doi: 10.1038/cr.2017.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhou Z., Luo M.J., Straesser K., Katahira J., Hurt E., Reed R. The protein Aly links pre-messenger-RNA splicing to nuclear export in metazoans. Nature. 2000;407:401–405. doi: 10.1038/35030160. [DOI] [PubMed] [Google Scholar]
- 156.Fu Y., Jia G., Pang X., Wang R.N., Wang X., Li C.J., Smemo S., Dai Q., Bailey K.A., Nobrega M.A. FTO-mediated formation of N6-hydroxymethyladenosine and N6-formyladenosine in mammalian RNA. Nat. Commun. 2013;4:1798. doi: 10.1038/ncomms2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zheng G., Dahl J.A., Niu Y., Fedorcsak P., Huang C.M., Li C.J., Vågbø C.B., Shi Y., Wang W.L., Song S.H. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol. Cell. 2013;49:18–29. doi: 10.1016/j.molcel.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.He Y.F., Li B.Z., Li Z., Liu P., Wang Y., Tang Q., Ding J., Jia Y., Chen Z., Li L. Tet-mediated formation of 5-carboxylcytosine and its excision by TDG in mammalian DNA. Science. 2011;333:1303–1307. doi: 10.1126/science.1210944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Haag S., Sloan K.E., Ranjan N., Warda A.S., Kretschmer J., Blessing C., Hübner B., Seikowski J., Dennerlein S., Rehling P. NSUN3 and ABH1 modify the wobble position of mt-tRNAMet to expand codon recognition in mitochondrial translation. EMBO J. 2016;35:2104–2119. doi: 10.15252/embj.201694885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kawarada L., Suzuki T., Ohira T., Hirata S., Miyauchi K., Suzuki T. ALKBH1 is an RNA dioxygenase responsible for cytoplasmic and mitochondrial tRNA modifications. Nucleic Acids Res. 2017;45:7401–7415. doi: 10.1093/nar/gkx354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Shen Q., Zhang Q., Shi Y., Shi Q., Jiang Y., Gu Y., Li Z., Li X., Zhao K., Wang C. Tet2 promotes pathogen infection-induced myelopoiesis through mRNA oxidation. Nature. 2018;554:123–127. doi: 10.1038/nature25434. [DOI] [PubMed] [Google Scholar]
- 162.Gao Y., Wang Z., Zhu Y., Zhu Q., Yang Y., Jin Y., Zhang F., Jiang L., Ye Y., Li H. NOP2/Sun RNA methyltransferase 2 promotes tumor progression via its interacting partner RPL6 in gallbladder carcinoma. Cancer Sci. 2019;110:3510–3519. doi: 10.1111/cas.14190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Yi J., Gao R., Chen Y., Yang Z., Han P., Zhang H., Dou Y., Liu W., Wang W., Du G. Overexpression of NSUN2 by DNA hypomethylation is associated with metastatic progression in human breast cancer. Oncotarget. 2017;8:20751–20765. doi: 10.18632/oncotarget.10612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lu L., Zhu G., Zeng H., Xu Q., Holzmann K. High tRNA Transferase NSUN2 Gene Expression is Associated with Poor Prognosis in Head and Neck Squamous Carcinoma. Cancer Invest. 2018;36:246–253. doi: 10.1080/07357907.2018.1466896. [DOI] [PubMed] [Google Scholar]
- 165.Okamoto M., Hirata S., Sato S., Koga S., Fujii M., Qi G., Ogawa I., Takata T., Shimamoto F., Tatsuka M. Frequent increased gene copy number and high protein expression of tRNA (cytosine-5-)-methyltransferase (NSUN2) in human cancers. DNA Cell Biol. 2012;31:660–671. doi: 10.1089/dna.2011.1446. [DOI] [PubMed] [Google Scholar]
- 166.Blanco S., Bandiera R., Popis M., Hussain S., Lombard P., Aleksic J., Sajini A., Tanna H., Cortés-Garrido R., Gkatza N. Stem cell function and stress response are controlled by protein synthesis. Nature. 2016;534:335–340. doi: 10.1038/nature18282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.He Y., Yu X., Li J., Zhang Q., Zheng Q., Guo W. Role of m5C-related regulatory genes in the diagnosis and prognosis of hepatocellular carcinoma. Am. J. Transl. Res. 2020;12:912–922. [PMC free article] [PubMed] [Google Scholar]
- 168.Zhang Q., Zheng Q., Yu X., He Y., Guo W. Overview of distinct 5-methylcytosine profiles of messenger RNA in human hepatocellular carcinoma and paired adjacent non-tumor tissues. J. Transl. Med. 2020;18:245. doi: 10.1186/s12967-020-02417-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Alshaker H., Wang Q., Brewer D., Pchejetski D. Transcriptome-Wide Effects of Sphingosine Kinases Knockdown in Metastatic Prostate and Breast Cancer Cells: Implications for Therapeutic Targeting. Front. Pharmacol. 2019;10:303. doi: 10.3389/fphar.2019.00303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zheng X., Li W., Ren L., Liu J., Pang X., Chen X., Kang D., Wang J., Du G. The sphingosine kinase-1/sphingosine-1-phosphate axis in cancer: Potential target for anticancer therapy. Pharmacol. Ther. 2019;195:85–99. doi: 10.1016/j.pharmthera.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 171.Shibata T., Watari K., Kawahara A., Sudo T., Hattori S., Murakami Y., Izumi H., Itou J., Toi M., Akiba J. Targeting Phosphorylation of Y-Box-Binding Protein YBX1 by TAS0612 and Everolimus in Overcoming Antiestrogen Resistance. Mol. Cancer Ther. 2020;19:882–894. doi: 10.1158/1535-7163.MCT-19-0690. [DOI] [PubMed] [Google Scholar]
- 172.Toh T.B., Lim J.J., Chow E.K. Epigenetics in cancer stem cells. Mol. Cancer. 2017;16:29. doi: 10.1186/s12943-017-0596-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Patel D.D., Lee D.M., Kolbinger F., Antoni C. Effect of IL-17A blockade with secukinumab in autoimmune diseases. Ann. Rheum. Dis. 2013;72(Suppl 2):ii116–ii123. doi: 10.1136/annrheumdis-2012-202371. [DOI] [PubMed] [Google Scholar]
- 174.Isobe M., Yagita H., Okumura K., Ihara A. Specific acceptance of cardiac allograft after treatment with antibodies to ICAM-1 and LFA-1. Science. 1992;255:1125–1127. doi: 10.1126/science.1347662. [DOI] [PubMed] [Google Scholar]
- 175.Mongan N.P., Emes R.D., Archer N. Detection and analysis of RNA methylation. F1000Res. 2019;8:559. doi: 10.12688/f1000research.17956.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zhang M., Song J., Yuan W., Zhang W., Sun Z. Roles of RNA Methylation on Tumor Immunity and Clinical Implications. Front. Immunol. 2021;12:641507. doi: 10.3389/fimmu.2021.641507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Wood S., Willbanks A., Cheng J.X. The Role of RNA Modifications and RNA-modifying Proteins in Cancer Therapy and Drug Resistance. Curr. Cancer Drug Targets. 2021;21:326–352. doi: 10.2174/1568009621666210127092828. [DOI] [PubMed] [Google Scholar]
- 178.Cully M. Chemical inhibitors make their RNA epigenetic mark. Nat. Rev. Drug Discov. 2019;18:892–894. doi: 10.1038/d41573-019-00179-5. [DOI] [PubMed] [Google Scholar]
- 179.Nombela P., Miguel-López B., Blanco S. The role of m6A, m5C and Ψ RNA modifications in cancer: Novel therapeutic opportunities. Mol. Cancer. 2021;20:18. doi: 10.1186/s12943-020-01263-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Chmielowska-Bąk J., Arasimowicz-Jelonek M., Deckert J. In search of the mRNA modification landscape in plants. BMC Plant Biol. 2019;19:421. doi: 10.1186/s12870-019-2033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Yang L., Perrera V., Saplaoura E., Apelt F., Bahin M., Kramdi A., Olas J., Mueller-Roeber B., Sokolowska E., Zhang W. m5C Methylation Guides Systemic Transport of Messenger RNA over Graft Junctions in Plants. Curr. Biol. 2019;29:2465–2476.e5. doi: 10.1016/j.cub.2019.06.042. [DOI] [PubMed] [Google Scholar]
- 182.Rivero R.M., Ruiz J.M., García P.C., López-Lefebre L.R., Sánchez E., Romero L. Resistance to cold and heat stress: accumulation of phenolic compounds in tomato and watermelon plants. Plant Sci. 2001;160:315–321. doi: 10.1016/s0168-9452(00)00395-2. [DOI] [PubMed] [Google Scholar]
- 183.Commisso M., Toffali K., Strazzer P., Stocchero M., Ceoldo S., Baldan B., Levi M., Guzzo F. Impact of Phenylpropanoid Compounds on Heat Stress Tolerance in Carrot Cell Cultures. Front. Plant Sci. 2016;7:1439. doi: 10.3389/fpls.2016.01439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Sun J., Yan L., Shen W., Meng A. Maternal Ybx1 safeguards zebrafish oocyte maturation and maternal-to-zygotic transition by repressing global translation. Development. 2018;145:dev166587. doi: 10.1242/dev.166587. [DOI] [PubMed] [Google Scholar]
- 185.Courtney D.G., Chalem A., Bogerd H.P., Law B.A., Kennedy E.M., Holley C.L., Cullen B.R. Extensive Epitranscriptomic Methylation of A and C Residues on Murine Leukemia Virus Transcripts Enhances Viral Gene Expression. MBio. 2019;10:e01209–e01219. doi: 10.1128/mBio.01209-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Eckwahl M., Xu R., Michalkiewicz J., Zhang W., Patel P., Cai Z., Pan T. 5-Methylcytosine RNA Modifications Promote Retrovirus Replication in an ALYREF Reader Protein-Dependent Manner. J. Virol. 2020;94:e00544-20. doi: 10.1128/JVI.00544-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Tirumuru N., Zhao B.S., Lu W., Lu Z., He C., Wu L. N(6)-methyladenosine of HIV-1 RNA regulates viral infection and HIV-1 Gag protein expression. eLife. 2016;5:e15528. doi: 10.7554/eLife.15528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Lichinchi G., Zhao B.S., Wu Y., Lu Z., Qin Y., He C., Rana T.M. Dynamics of Human and Viral RNA Methylation during Zika Virus Infection. Cell Host Microbe. 2016;20:666–673. doi: 10.1016/j.chom.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wnuk M., Slipek P., Dziedzic M., Lewinska A. The Roles of Host 5-Methylcytosine RNA Methyltransferases during Viral Infections. Int. J. Mol. Sci. 2020;21:8176. doi: 10.3390/ijms21218176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zhang P., Wang Y., Gu X. RNA 5-Methylcytosine Controls Plant Development and Environmental Adaptation. Trends Plant Sci. 2020;25:954–958. doi: 10.1016/j.tplants.2020.07.004. [DOI] [PubMed] [Google Scholar]
- 191.Tuorto F., Herbst F., Alerasool N., Bender S., Popp O., Federico G., Reitter S., Liebers R., Stoecklin G., Gröne H.J. The tRNA methyltransferase Dnmt2 is required for accurate polypeptide synthesis during haematopoiesis. EMBO J. 2015;34:2350–2362. doi: 10.15252/embj.201591382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Van Haute L., Lee S.Y., McCann B.J., Powell C.A., Bansal D., Vasiliauskaitė L., Garone C., Shin S., Kim J.S., Frye M. NSUN2 introduces 5-methylcytosines in mammalian mitochondrial tRNAs. Nucleic Acids Res. 2019;47:8720–8733. doi: 10.1093/nar/gkz559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Abbasi-Moheb L., Mertel S., Gonsior M., Nouri-Vahid L., Kahrizi K., Cirak S., Wieczorek D., Motazacker M.M., Esmaeeli-Nieh S., Cremer K. Mutations in NSUN2 cause autosomal-recessive intellectual disability. Am. J. Hum. Genet. 2012;90:847–855. doi: 10.1016/j.ajhg.2012.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Hussain S., Tuorto F., Menon S., Blanco S., Cox C., Flores J.V., Watt S., Kudo N.R., Lyko F., Frye M. The mouse cytosine-5 RNA methyltransferase NSun2 is a component of the chromatoid body and required for testis differentiation. Mol. Cell. Biol. 2013;33:1561–1570. doi: 10.1128/MCB.01523-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Dev R.R., Ganji R., Singh S.P., Mahalingam S., Banerjee S., Khosla S. Cytosine methylation by DNMT2 facilitates stability and survival of HIV-1 RNA in the host cell during infection. Biochem. J. 2017;474:2009–2026. doi: 10.1042/BCJ20170258. [DOI] [PubMed] [Google Scholar]
- 196.Legrand C., Tuorto F., Hartmann M., Liebers R., Jacob D., Helm M., Lyko F. Statistically robust methylation calling for whole-transcriptome bisulfite sequencing reveals distinct methylation patterns for mouse RNAs. Genome Res. 2017;27:1589–1596. doi: 10.1101/gr.210666.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.