Abstract
Introduction
The objective of this study is to investigate the effect of gelatin microspheres incorporating growth factors on the therapeutic efficacy in cell transplantation. The strength of this study is to combine gelatin hydrogel microspheres incorporating basic fibroblast growth factor and platelet growth factor mixture (GM/GF) with bioabsorbable injectable hydrogels (iGel) for transplantation of adipose-derived stem cells (ASCs).
Methods
The rats ASCs suspended in various solutions were transplanted in masseter muscle. Rats were euthanized 2, 7, 14 days after injection for measurement of the number of ASCs retention in the muscle and morphological evaluation of muscle fibers and the inflammation of the injected tissue by histologic and immunofluorescent stain.
Results
Following the injection into the skeletal muscle, the GM/GF allowed the growth factors to release at the injection site over one week. When ASCs were transplanted into skeletal muscle using iGel incorporating GM/GF (iGel+GM/GF), the number of cells grafted was significantly high compared with other control groups. Moreover, for the groups to which GM/GF was added, the cells transplanted survived, and the Myo-D expression of a myoblast marker was observed at the region of cells transplanted.
Conclusions
The growth factors released for a long time likely enhance the proliferative and differentiative capacity of cells. The simple combination with iGel and GM/GF allowed ASCs to enhance their survival at the injected site and consequently achieve improved therapeutic efficacy in cell transplantation.
Keywords: Stem cell transplantation, Injectable hydrogel, Drug delivery system, Adipose-derived stem cells
Abbreviations: ASCs, adipose-derived stem cells; bFGF, basic fibroblast growth factor; DMEM, Dulbecco modified Eagle medium; ELISA, Enzyme-Linked ImmunoSorbent Assay; HGF, hepatocyte growth factor; GM, gelatin hydrogel microspheres; GM/GF, GM containing bFGF and PGFM; iGel, bioabsorbable injectable hydrogels; iGel+GM/GF, iGel incorporating GM/GF; PBS, phosphate-buffered saline solution; PGFM, platelet growth factor mixture; VEGF, vascular endothelial growth factor
Highlights
-
•
The rats adipose-derived stem cells (ASCs) suspended in various solutions were transplanted in masseter muscle.
-
•
The number of cells transplanted using this study's technology was significantly high compared with other control groups.
-
•
For the groups with growth factors, the Myo-D (myoblast marker) expression was observed at the region of cells transplanted.
1. Introduction
Stem cell transplantation is expected to regenerate lost tissue and improve surrounding tissue function [1,2]. Stem cells can be isolated from various tissues such as bone marrow and adipose [3,4]. Among them, adipose-derived stem cells (ASCs) are easy to collect and have high immunological tolerance, and thus they are expected as a source of allogeneic transplantation [[5], [6], [7]]. Stem cell transplantation includes a method of locally injecting a cell suspension, placing a cell sheet in a tissue, culturing cells in a scaffold and embedding in a tissue, and systemic administration by drip [[8], [9], [10], [11], [12], [13]]. However, a method of systemic administration is inefficient because the transplanted cells disperse beyond the intended site.
On the other hand, a locally injecting cell suspension method can be used for fragile or thin tissues because of the low risk of exposing materials [14,15]. In addition, it is attractive that the method is a minimally invasive treatment since surgical operations are not required, unlike placing cell sheets or embedding scaffolds. However, it is well recognized that the retention of cells transplanted at the injection site is inferior [[16], [17], [18]].
We used bioabsorbable injectable hydrogels (iGel) based on the physico–chemical interaction among gelatin, alginate, and ferric ion to overcome this problem. A unique feature of iGel is that they are not only capable of retaining the cell transplanted at the site of injection, but also do not interfere with the cell survival and proliferation due to this rapid degradation [19]. Providing growth factors to mesenchymal stem cells can be expected to promote proliferation, differentiation, and secretion of cytokines, leading to improved therapeutic efficiency in cell transplantation [[20], [21], [22]]. Platelet growth factor mixture (PGFM) and basic fibroblast growth factor (bFGF) are well-known growth factors. They have been used clinically in various surgical treatments for soft and hard tissues, such as cosmetic surgery, orthopedics, and maxillofacial surgery [[23], [24], [25]]. However, the free form of these growth factors simply added to iGel will disappear immediately. To overcome this problem, we used gelatin hydrogel microspheres (GM). The GM have been designed and prepared to control the growth factors [[26], [27], [28]]. Among them, the GM prepared using gelatin with an isoelectric point of 5.0 have been revealed to control the release of bFGF and PGFM [27].
In this study, iGel incorporating GM containing bFGF and PGFM (iGel+GM/GF) were prepared. The time profile of bFGF and PGFM from the iGel+GM/GF was evaluated. Following stem cell transplantation, combined with the growth factors released material into rat skeletal muscle, the cell retention and the therapeutic efficacy were investigated. In addition, we examined the effect on the surrounding tissues histologically.
2. Materials and methods
2.1. Isolation of rat ASCs from adipose tissue
Under general anesthesia with 3 mixed anesthetics (midazolam 5 mg/kg, medetomidine 0.375 mg/kg, butorphanol 2.5 mg/kg), ASCs were isolated from the inguinal fat pads of Sprague–Dawley rat (8 weeks old; Japan SLC Inc.), according to the method previously reported [29]. In brief, subcutaneous adipose tissue was digested with 0.25 w/v% collagenase I (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan) under gentle agitation for 45 min at 37 °C and added Dulbecco modified Eagle medium (DMEM, Sigma Aldrich, Missouri, United States) containing 10 v/v% fetal bovine serum (FBS, Sigma Aldrich) and centrifuged at 200 g for 5 min. After aspirating to remove cellular debris and supernatant, the pellet was suspended in the control medium (DMEM supplemented with 10 v/v% FBS and 1 v/v% antibiotic/antimycotic). Next, the cell suspension was filtered through a 40 μm nylon sieve to remove cellular debris and centrifuged at 200 g for 5 min. Then, the pellet was resuspended in a control medium and cultured in 100 mm tissue culture dishes at a density of 1 × 106 cells per plate. These cells were cultured in the control medium at 37 °C and in 5% CO2. ASCs at passage 3 isolated from rats were used for this study.
2.2. Characterization of ASCs
Characterization of ASCs were carried out by confirmation of surface marker and pluripotency. Surface markers of ASCs were confirmed by using antibodies of CD29, 34, 45, 73, 90, 105, according to the method previously reported [5]. The pluripotency of ASCs was confirmed by culturing at adipogenic, osteogenic, and chondrogenic differentiation medium. Adipogenic differentiation was induced by culturing ASCs in 6 well dish for 3 weeks in an adipogenic medium prepared by adding dexamethasone (1 μM), isobutyl-methylxanthine (500 μM), insulin (10 μM), and indomethacin (200 μM) to the control medium and assessed using an Oil red O staining. Osteogenic differentiation was induced by culturing ASCs in 6 well dish for 3 weeks in an osteogenic medium prepared by adding dexamethasone (0.1 μM), L-ascorbic diphosphate (100 μM), and β-glycerophosphate (10 mM) to the control medium and assessed using an Alizarin red staining. Chondrogenic differentiation was induced by culturing ASCs at pellet culture for 3 weeks in a chondrogenic medium prepared by adding dexamethasone (0.1 μM), TGF-β3 (10 ng/ml), L-ascorbic diphosphate (200 μM), ITS+premix (BD biosciences, New Jersey, United States) (1 v/v%), and pyruvate (2 mM) to the control medium and assessed using Alcian blue staining.
2.3. Preparation of platelet growth factor mixture (PGFM)
Platelet-rich plasma was prepared by double spin methods according to the method previously reported [27]. In brief, Sprague–Dawley rat (8 weeks old; Japan SLC Inc., Shizuoka, Japan) was anesthetized by the intraperitoneal injection of 3 mixed anesthetics (midazolam 5 mg/kg, medetomidine 0.375 mg/kg, butorphanol 2.5 mg/kg). First, 10 ml of Animal blood was collected into a tube containing 1 ml of acid-citrate-dextrose solution and centrifuged for 10 min at 800 g and 4 °C. Next, the yellow plasma with the buffy coat was carefully transferred into another tube and centrifuged for 10 min at 2100 g and 4 °C. Then, the platelet pellet was collected, and the thrombolytic pellet in 1.4 ml of plasma was used as platelet-rich plasma. Finally, platelet rich plasma prepared was mixed with CaCl2 solution at concentrations of 2 wt% at a ratio of 7:1 by volume, and then left for 1 h at 37 °C. After incubation, platelet-rich plasma was activated, and PGFM was collected.
2.4. Preparation of bFGF solution
The human recombinant bFGF (Kaken Pharmaceutical co. Ltd, Tokyo, Japan) solution was diluted with double distilled water (Otsuka Pharmaceutical co. Ltd, Tokyo, Japan) to give a solution concentration of 1 μg/μl.
2.5. Evaluation of the effect of GF addition on ASCs proliferation and secretion of cytokine
First, ASCs at passage 3 isolated from rats were plated in 96-well tissue culture plates at a density of 3.0 × 103 cells per plate and maintained in the control medium at 37 °C and in 5% CO2 at 2hr. Next, the control medium was changed into conditioned media: group A, control medium only; group B, control medium added 1 μl of bFGF; group C, control medium added 1 μl of bFGF solution and 1 μl of PGFM; group D, control medium added 1 μl of bFGF solution and 1 μl of PGFM without ASCs (n = 16). Conditioned media were collected at days 2 to measure the levels of growth factors, including hepatocyte growth factor (HGF), vascular endothelial growth factor (VEGF). The concentration of each growth factor was measured by the Quantikine® enzyme-linked immunosorbent assay (ELISA) kit (B&D Systems, MN, United States). Then, MTT assay was performed to evaluate the number of ASCs remaining on the plate after collecting the supernatant according to the method previously reported [30].
2.6. Preparation of injectable hydrogels (iGel)
iGel were prepared by simply mixing the mixed alginate/gelatin solution and the FeCl3 solution according to the method previously reported [19]. In brief, 250 μl of FeCl3 solution (10 mM) was added to 250 μl of phosphate-buffered saline solution (PBS, pH7.4) containing 0.2 wt% sodium alginate (weight-averaged molecular weight of 2,300,000; KIMICA Inc., Tokyo, Japan) and 0.8 wt% gelatin (isoelectric point 5.0 and the weight-averaged molecular weight of 100,000, Nitta Gelatin, Inc., Osaka, Japan).
2.7. Preparation of gelatin hydrogel microspheres (GM)
GM were prepared according to the method previously reported [27]. In brief, an aqueous solution (20 ml) of 10wt% gelatin (isoelectric point 5.0) was preheated at 40 °C, followed by stirring at 300 rpm for 10 min to prepare the water-in-oil emulsion. Next, the emulsion temperature was decreased at 4 °C to obtain non-crosslinked hydrogel microspheres. The resulting microspheres were washed three times with cold acetone combined with centrifugation (5000 rpm, 4 °C, 5 min) to exclude the residual oil completely. Then, microspheres were fractionated by size using sieves with apertures of 32 and 53 μm (Iida Seisakusho Ltd., Osaka, Japan) and air dried at 4 °C. Then, microspheres were treated in a vacuum oven at 160 °C to allow to dehydrothermally crosslink for 72 h, and GM were collected. After thermal crosslink, GM were sterilized with ethylene oxide gas.
2.8. Preparation of iGel incorporating GM/GF (iGel+GM/GF)
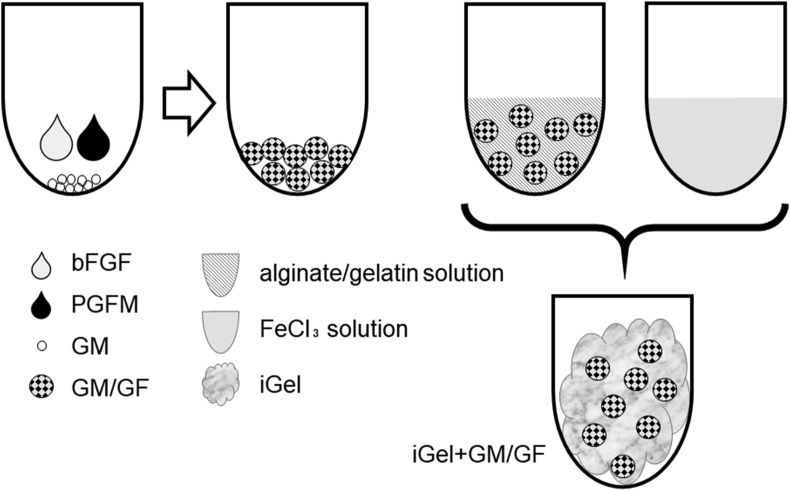
Fig. 1 shows a schematic illustration of the preparation of iGel incorporating GM/GF. GM/GF were prepared according to the method previously reported [27]. In brief, the aqueous solution containing 25 μl of bFGF and 25 μl of PGFM was dropped onto 5 mg of GM to impregnate bFGF and PGFM into a granule. The bFGF and PGFM were completely sorbed into the hydrogel granule by swelling at 4 °C for 24 hbecause the solution volume was less than theoretically required for the equilibrated swelling of hydrogels. After that, 250 μl of FeCl3 solution (10 mM) was added to 250 μl of alginate/gelatin solution containing 5 mg GM/GF to give a final polymer (iGel+GM/GF).
Fig. 1.
Preparation of iGel incorporating GM/GF (iGel+GM/GF). The bFGF and PGFM solutions were completely sorbed into GM by swelling at 4 °C for 24 h (GM/GF). After that, FeCl3 solution was added to alginate/gelatin solution containing GM/GF to give a final polymer.
2.9. Degradation test of iGel+GM/GF and release test of bFGF and PGFM
iGel+GM/GF were incubated in PBS. At each time point, the buffer was removed and replaced with fresh PBS. After 24 h, PBS was replaced with 10 μg/ml of collagenase at 37 °C. At different time intervals, the solution supernatant was removed and replaced with the same volume of fresh PBS with collagenase. The supernatant was sampled at scheduled times, and amounts of protein in each sample were measured using BCA Protein Assay Kit (Thermo, Inc., Waltham) to evaluate the degradation speed of iGel+GM/GF. Amounts of bFGF and PDGF-BB (one of the PGFM) in each sample were determined by the Quantikine® enzyme-linked immunosorbent assay (ELISA) kit (B&D Systems, MN, United States).
2.10. Cell transplantation in masseter muscle by injection
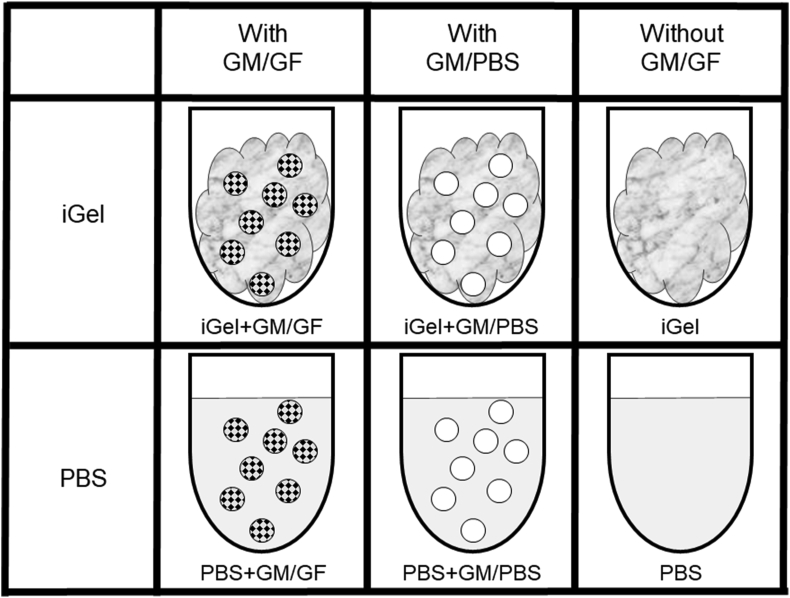
Animal experiments were approved by the Osaka University Committee for Animal Experimentation, and the Guidelines for Animal Experimentation (Japanese Association for Laboratory Animal Science, 1987) were observed. Under general anesthesia with 3 mixed anesthetics (midazolam 5 mg/kg, medetomidine 0.375 mg/kg, butorphanol 2.5 mg/kg), rats were transplanted in masseter muscle with ASCs suspended in various solutions. The rats were divided into the 6 groups according to their treatment: the solution was roughly divided into 2 types, iGel and PBS, and then each solution was divided into 3 groups; with GM/GF, with GM/PBS, and without GM. Fig. 2 shows the illustration of experimental samples. Using a syringe designed to mix the two solutions and a 25 G needle, 500 μl was injected into the rat's left and right masseter muscles. To analyze the effect of cell transplantation, cells were on the right, and no cells were on the left. ASCs were suspended in alginate/gelatin solution or PBS to a final concentration of 1.0 × 106 cells/ml after stained with PKH26 Red Fluorescent Cell Linker Mini Kit for Cell Membrane Labeling for Phanos Technologies (Sigma Aldrich Inc., St. Louis, United States) according to the manufacturer's protocol. Rats were euthanized 2, 7, 14 days after injection, and the masseter muscles were collected and fixed with 4% paraformaldehyde. The masseter muscles were divided into 3 mm intervals to transverse the muscle fiber. Four blocks near the injection point were embedded in O.C.T. compound (Sakura Finetek Japan Co., Ltd., Tokyo, Japan) to prepare frozen blocks. 5 frozen sections (8 μm thick at a 200 μm interval) were prepared from each block, making a total of 20 frozen sections.
Fig. 2.
Preparation of 6 groups: the solution was divided into 2 types, iGel and PBS, and then each solution was divided into 3 groups; with GM/GF, with GM/PBS, and without GM.
2.11. Measurement of the number of ASCs retention in the muscle
The number of PKH stained ASCs was observed using fluorescence microscopy BZ-X700 (KEYENCE Co., Ltd., Osaka, Japan) at 40× magnification. Fluorescence images of 20 frozen sections were taken, and the number of PKH stained ASCs were counted using ImageJ software (National Institutes of Health, Bethesda, Md). All fluorescence images were processed and measured in a unified standard, specifically converted to 8-bits grayscale images and then counted only for particles above the threshold of 30.
2.12. Histologic and immunofluorescent staining analysis
Each section was stained with hematoxylin-eosin for morphological evaluation of muscle fibers around retention GM and the inflammation of the injected tissue. In addition, immunofluorescent analyses were performed using a rabbit anti-rat Myo-D polyclonal antibody (1:100 dilution; Bioss Antibodies) and a goat anti-Rabbit IgG secondary antibody Alexa Fluor 488 (1:1000 dilution; Thermo Fisher Scientific).
2.13. Statistical analysis
All the statistical data were expressed as the mean ± standard deviations. The data were analyzed for variance by Bartlett test and Single factor ANOVA and then compared between groups by the Tukey–Kramer method. All statistical analyses were performed with Statcel 4 (OMS publishing, Tokyo, Japan). The statistical significance was accepted at p < 0.05.
3. Results
3.1. Characterization of ASCs
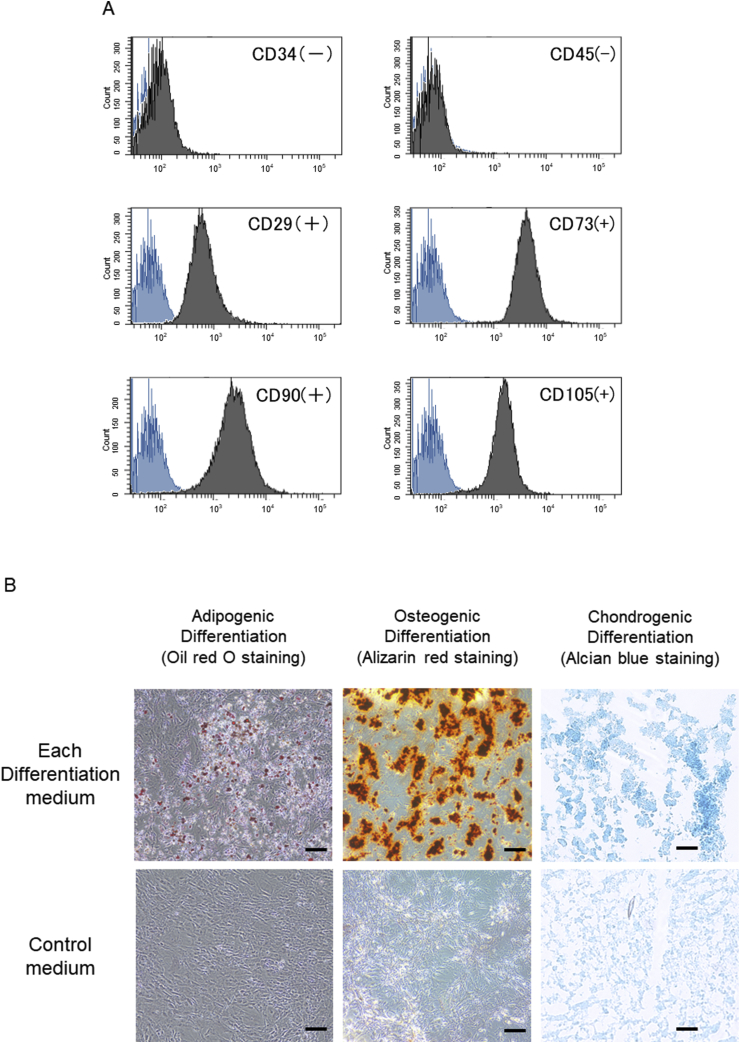
Fig. 3A shows that ASCs at passage 3 isolated from rats are positive for CD29, 73, 90, 105 and negative for CD34, 45. Fig. 3B shows that ASCs have pluripotency into adipogenic, osteogenic, and chondrogenic differentiation.
Fig. 3.
(A) ASCs at passage 3 isolated from rats are positive for CD29, 73, 90, 105 and negative for CD34, 45. Blue histogram shows negative control. (B) After culturing passage 3 ASCs in each differentiation medium for 3 weeks, it was confirmed that the cells cultured in each medium had pluripotency into adipogenic, osteogenic, and chondrogenic differentiation by oil red O staining, alizarin red staining, and alcian blue staining. On the other hand, in control medium, ASCs did not have differentiation. The scale bar indicates 100 μm.
3.2. Evaluation of the effect of GF addition on ASCs proliferation and secretion of cytokine
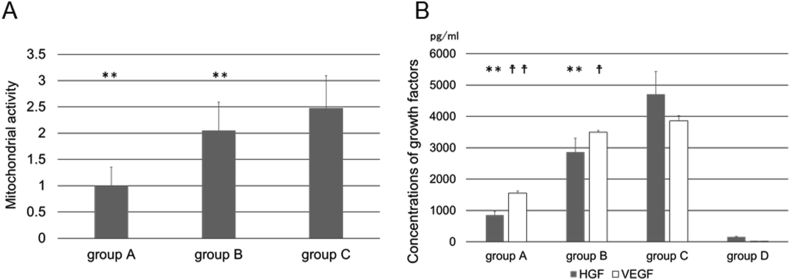
Fig. 4A shows the proliferation of ASCs at each conditioned media. The proliferation at group C was significantly higher than the other groups. Fig. 4B shows the concentrations of HGF and VEGF contained in the supernatant at each conditioned media. The secretion of cytokine at group C was significantly higher than the other groups. The amount of cytokine originally contained in the conditioned media was extremely low.
Fig. 4.
(A) The proliferation of ASCs at each conditioned media evaluated by MTT assay. ∗∗P < 0.01; significantly different against mitochondrial activity for group C at the same time. (B) The secretion of (□) HGF and (□) VEGF from ASCs at each conditioned media. ∗∗p < 0.01; significantly different against secretion of HGF for group C at the same time. †p < 0.05; significantly different against secretion of VEGF for group C at the same time. ††p < 0.01; significantly different against secretion of VEGF for group C at the same time. Group A, control medium only; group B, control medium added 1 μl of bFGF; group C, control medium added 1 μl of bFGF solution and 1 μl of PGFM; group D, control medium added 1 μl of bFGF solution and 1 μl of PGFM without ASCs.
3.3. Degradation test of iGel+GM/GF and release test of bFGF and PGFM
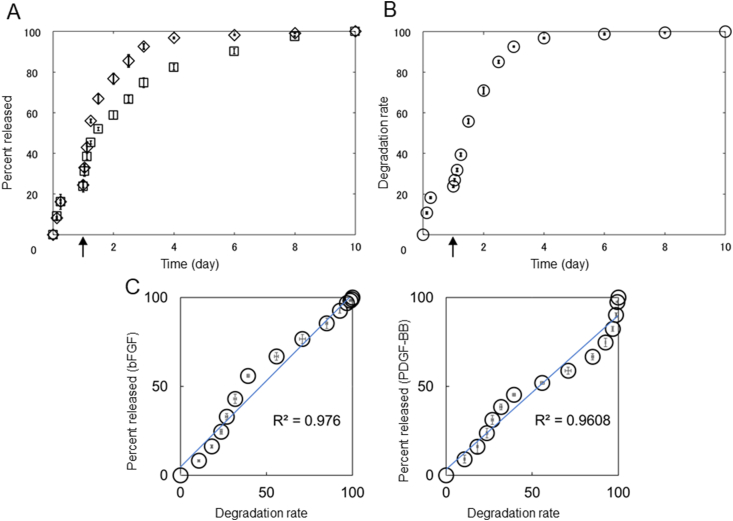
Fig. 5A shows the bFGF and PGFM release profiles from iGel+GM/GF in PBS with or without collagenase. When iGel+GM/GF was incubated into PBS, a slow release of bFGF and PGFM was observed. By the addition of collagenase, bFGF and PGFM were rapidly released. Fig. 5B shows the degradation profile of iGel+GM/GF in PBS with or without collagenase. There was a good correlation between the GF release profile and the GM degradation profile (Fig. 5C).
Fig. 5.
(A) In vitro time profile of (◇) bFGF and (□) PDGF-BB from iGel+GM/GF in PBS. Collagenase was added into PBS at 24 h indicated by the arrow. (B) Degradation profile of iGel+GM/GF in PBS and collagenase. (C) Relationship between GF release and iGel+GM/GF degradation profile.
3.4. Measurement of the number of ASCs retention in the muscle
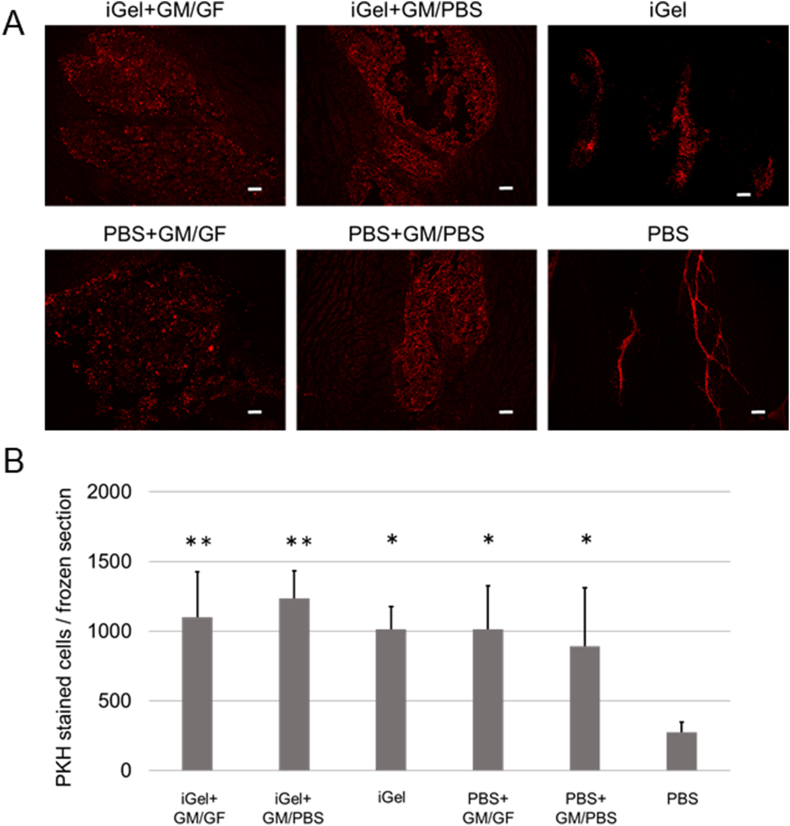
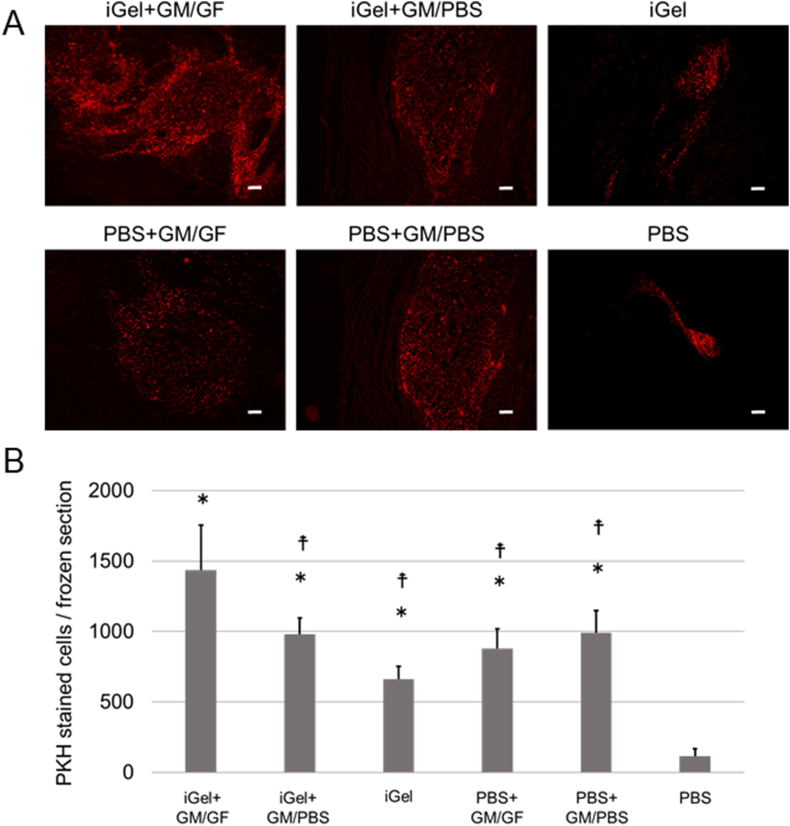
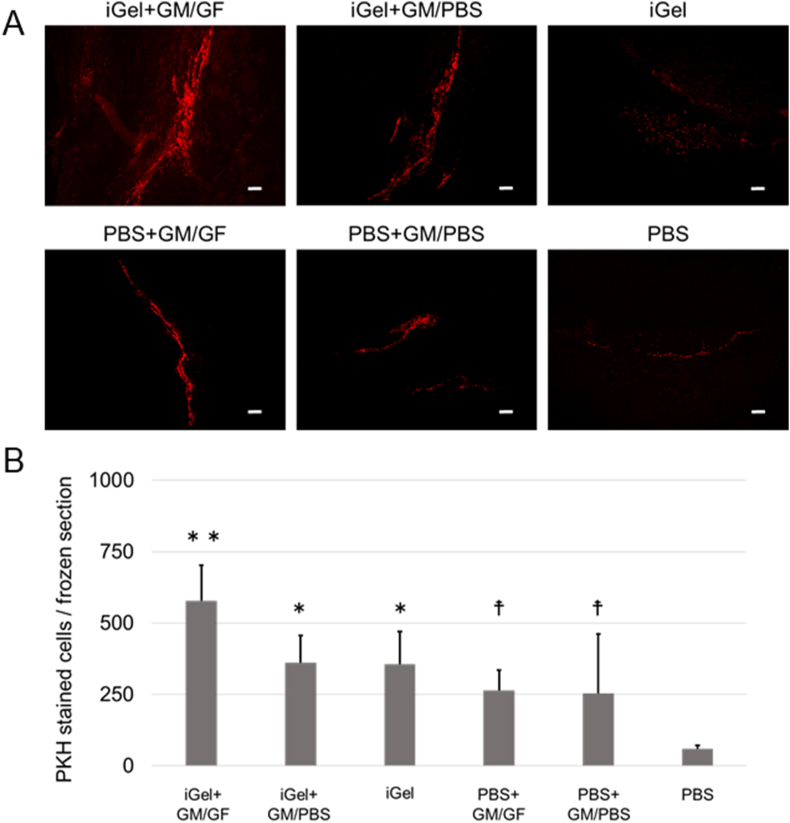
Fig. 6A shows the picture of fluorescence images of each frozen section 2 days after injection. PKH-stained ASCs remained in the scaffolding formed by the injectable gel and particles. Fig. 5B shows the cell number on the section 2 days after injection. The cell number in the PBS group was significantly lower than in the other groups. No significant difference was observed between the other groups. Fig. 7A shows the picture of fluorescence images of each frozen section 7 days after injection. Fig. 7B shows the cell number on the section 7 days after injection. The cell number in the iGel+GM/GF group was significantly higher than the other groups. In addition, the PBS group was significantly lower than the other groups. No significant difference was observed between the other groups. Fig. 8A shows the cell number on the section 14 days after injection, and Fig. 8B shows the cell number on the section 14 days after injection. The tendency of the retention number of cells was similar to the result 7 days after injection.
Fig. 6.
(A) The picture of fluorescence images of each frozen section at 2 days after injection. Red: PKH-stained ASCs. The scale bar indicates 200 μm. (B) The average cell number on each frozen section at 2 days after injection. ∗p < 0.05; significantly different against cell number for PBS at the same time. ∗∗p < 0.01; significantly different against cell number for PBS at the same time.
Fig. 7.
(A) The picture of fluorescence images of each frozen section at 7 days after injection. Red: PKH-stained ASCs. The scale bar indicates 200 μm. (B) The average cell number on each frozen section at 7 days after injection. ∗p < 0.01; significantly different against cell number for PBS at the same time. †p < 0.05; significantly different against cell number for iGel+GM/GF at the same time.
Fig. 8.
(A) The picture of fluorescence images of each frozen section at 14 days after injection. Red: PKH-stained ASCs. The scale bar indicates 200 μm. (B) The average cell number on each frozen section at 14 days after injection. ∗p < 0.05; significantly different against cell number for PBS at the same time. ∗∗p < 0.01; significantly different against cell number for PBS at the same time. †p < 0.05; significantly different against cell number for iGel+GM/GF at the same time.
3.5. Histologic and immunofluorescent staining analysis
Fig. 9A shows the histological sections of the masseter muscle around the cell transplantation site 2 days after injection. In all groups, neutrophils were infiltrated, and inflammatory findings were observed. In addition, in the group to which GM was added, the particles remained in the muscle tissue. Fig. 9B shows the histological sections 7 days after injection. Again, GM remained in the tissue. The PBS group showed normal muscle tissue, while the other groups had inflammatory cells infiltrated into the muscle tissue. In the group to which GM/GF was added, cell proliferation was observed around GM. Fig. 8C shows the histological sections 14 days after injection. Again, GM was absorbed and disappeared in the muscle tissue. Except for the PBS group, there were no differences in inflammatory findings or muscle fiber repair tendencies between the groups.
Fig. 9.
(A) The histological sections of the masseter muscle around cell transplantation site at 2 days after injection. The scale bar indicates 200 μm. The red arrowhead indicates GM. (B) The histological sections at 7 days after injection. The scale bar indicates 200 μm. The red arrowhead indicates GM. (C) The histological sections at 14 days after injection. The scale bar indicates 200 μm.
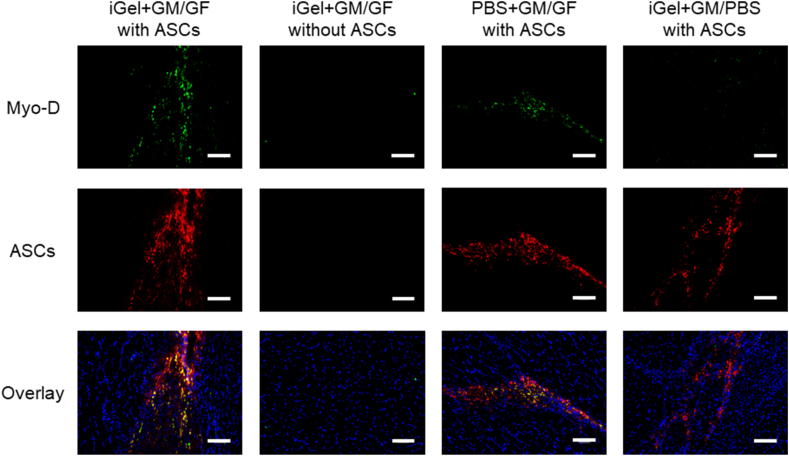
Fig. 10 shows the immunofluorescent staining section 14 days after injection. The expression of Myo-D, a marker of immature myoblasts, consistent with the ASCs retained sites of the iGel+GM/GF and PBS+GM/GF groups 14 days after injection. On the other hand, in the site where cell transplantation was not performed or in the group to which GF was not added, expression of Myo-D was not observed in the injected site.
Fig. 10.
The immunofluorescent staining section at 14 days after injection. Green: Myo-D were stained by Alexa Fluor 488. Red: PKH-stained ASCs. Blue: DAPI. The scale bar indicates 100 μm.
4. Discussion
The purpose of this study is to clarify the effect of adding GF to iGel. The previous study has shown that the injectable hydrogels formed based on physico–chemical interaction of biocompatible polymers with ferric ions are promising for cell transplantation [19]. However, it is not a perfect transplant condition because it does not contain the growth factors to retain the survival cells. Therefore, in this study, we decided to add GM, capable of the controlled release of GF to the iGel. The reason for using bFGF and PGFM as GF is that the transplanted stem cells require these factors in proliferation, secretion of cytokine, and differentiation. Compared with the control medium only or condition media added bFGF, the cell proliferation and secretion of cytokine were improved in condition media added both bFGF and PGFM (Fig. 4A and B). In addition, the previous study has shown that GM impregnates both bFGF and PGFM and has the sustained-release capacity [27]. About 25% of iGel+GM/GF was degraded 24 h after incubation in PBS without collagenase, and almost all iGel+GM/GF was degraded 72 hafter incubation in PBS with collagenase (Fig. 5A). This is because the gelatin involved in the gelation of iGel and the dehydrothermal crosslinked gelatin constituting GM were degraded by collagenase. In addition, there is a good correlation between the GF release profile and iGel+GM/GF degradation profile (Fig. 5C), showing similar trends to previous studies [31]. The correlation (steep-plateau-steep) means that initial burst and the drug diffusion with degradation. Therefore, with the exception of a little initial burst, it is suggested that the time profile of drug release that the bFGF and PGFM are released from GM/GF are associated with not the drug diffusion but the degradation of iGel+GM/GF. Furthermore, it was found that even if GM/GF is embedded in iGel, it gradually releases the GF that has been impregnated in the process of degradation.
In the experiment of cell transplantation with iGel+GM/GF, 2 days after injection, the number of cells retained in the masseter muscle was significantly smaller only in the PBS alone group, and no significant difference was observed between the other groups. This indicates that iGel improved cell retention, similar to previous studies [19]. In addition, the group in which GM were added to PBS also had a large number of cells retained. It was suggested that the GM might have functioned as a drug delivery system and as a scaffold for cells in the tissue. This can be explained by reports that heat-crosslinked gelatin-based materials can provide a scaffold for cells and improve retention [32,33]. Looking at the fluorescence microscopic images, in the 4 groups to which GM was added, the transplanted cells remained wide around the injection site. Still, in the iGel group, the cells were retained in small and cohesive areas, and in the PBS group, the cells were retained in the narrow space between the muscle bellies. This image suggests that GM may play an important role in ensuring space that does not interfere with cell survival or tissue proliferation. 7 days after injection, the iGel+GM/GF group's cells retained significantly more than the other groups. This suggests that it improved the retention of cells transplanted by iGel and GM and that growth factors released from GM may have promoted cell proliferation. Interestingly, on the other hand, the PBS+GM/GF group showed a different tendency from the iGel+GM/GF group, although it was able to continuously release growth factors like the iGel+GM/GF group. The number of cells retained was similar to that of the other GM-added groups. In the PBS+GM/GF case, it is not surrounded by iGel; the transplanted cells could not get the sufficient growth factor in GM. We considered the other various cells penetrated in the injection site and got the growth factor in GM. The results 14 days after injection also showed that the number of cells retained in the iGel+GM/GF group was significantly higher than that in the other groups, suggesting that it is a useful transplant medium for improving the retention of transplanted cells. Cell transplantation using the GM, capable of the controlled release of GF has been reported to improve angiogenesis and differentiate cells transplanted into vascular endothelial cells [26,34,35]. In this study, by combining iGel, it was possible to enhance the retention rate of cells transplanted, and it is considered that more efficient cell transplantation could be established.
Morphological evaluation of muscle fibers by hematoxylin-eosin staining revealed that GM remained in the tissue 7 days after injection, suggesting that the masseter muscle decomposition rate was lower than in the in vitro experiment. In addition, no GM were observed in the tissue 14 days after injection, suggesting that all GM were finally degraded. It is considered that the reason why the retention of transplanted cells was improved even in the group in which GM was simply added to PBS was that the transplanted cells were provided with a scaffold and sufficient colonization time at the injection site because of the degradation speed of GM delayed. In the iGel+GM/GF group, 7 days after injection, the muscle fibers were partially blanked, suggesting that iGel+GM/GF injection may have caused some damage to the muscle fibers. However, 14 days after injection, the muscle fibers tended to be repaired and were reversibly damaged, and since they did not affect the survival of transplanted cells or surrounding tissues, it was considered that there was no problem in safety in a short period after injection.
Immunofluorescent staining revealed the expression of Myo-D, a marker of immature myoblasts, consistent with the ASCs retained sites of the iGel+GM/GF and PBS+GM/GF groups 14 days after injection. In addition, in the site where cell transplantation was not performed or in the group to which GF was not added, expression of Myo-D was not observed in the injected site. This result suggests that the transplanted ASCs differentiated into myoblasts in the tissue and that GF is required for the differentiation. In addition, by using iGel to increase the retention number of cells transplanted into muscle, it is thought that the number of cells that differentiate into myoblasts will also increase. However, in this study, we could not analyze the expression level of Myo-D in iGel+GM/GF groups and PBS+GM/GF groups because the all tissue was used for cell counting and histological analysis. It is considered that the future task is to analyze the gene expression in the muscle to verify the expression level of Myo-D. Contact of platelet-rich plasma with gelatin molecules can trigger the release of PGFM, but since it is not a strong trigger, PGFM may not be released sufficiently, and the effect of platelet-rich plasma may be insufficient [27]. In this experiment, by activating with 2% CaCl2 solution and collecting as much PGFM as possible contained in platelet-rich plasma in advance, it was possible to impregnate PGFM to GM and control release for an extended period. As a result, it was considered that the effect of platelet-rich plasma could be maximized.
Various growth factors are mixed in PGFM, and it has been reported that PDGF-BB, TGF-β1, and HGF contained in PGFM promote the myogenic differentiation of mesenchymal stem cells [[36], [37], [38]]. On the other hand, there are reports that GF contained in PGFM promotes further tissue regeneration, such as PDGF-BB inducing bone differentiation [39] and TGF-β1 inducing cartilage differentiation [40], so it is difficult to determine how PGFM will work. Furthermore, it has been reported that co-culturing mesenchymal stem cells cultured in a muscle differentiation medium with myoblasts further promoted the differentiation of stem cells into myoblasts, so the types of cells around the transplanted site may be involved in the differentiation of stem cells [38,41,42]. In this experiment, it is suggested that ASCs may have been promoted to differentiate into myoblasts because of the presence of myoblasts in the transplanted tissue and the function of GF involved in the differentiation into myoblasts such as PDGF-BB, TGF-β1, and HGF contained in the control-release PGFM. For immature myoblasts to differentiate into mature, oriented myofibers via myotube cells and perform their functions, the tension in the same direction must be applied intermittently [[43], [44], [45]]. With the continuous application of muscle load, many cells are grouped as oriented muscle fibers, leading to the acquisition of contractile force. In animal experiments, ASCs were transplanted into the masseter muscle, hoping that tension would be applied to the muscle in which the transplanted cells retained due to feeding behavior, but differentiation into mature myofibers and muscle regeneration could not be confirmed.
It is considered that this is because the muscle load of the feeding behavior was not sufficient to promote the differentiation into myofibers. Therefore, stem cell transplantation into the masseter muscle cannot be expected to enhance muscle strength. A future improvement is to perform cell transplantation in the skeletal muscles of the lower limbs. Since the muscle load can be applied using a device such as a treadmill, it is also possible to promote differentiation into mature myofibers of cells transplanted and evaluate the function of the muscle by electromyography and tension measurement [46,47].
The strength of this study is to combine iGel for stem cell retention and the GM for the controlled release of GF. This system enables highly efficient cell transplantation and differentiation into target cells at the region transplanted. This is considered to be the basis for regeneration therapy by stem cell transplantation. Our final goal is to achieve the functional regeneration of muscles. We will integrate rehabilitation with stem cell transplantation that combines tissue engineering for efficient regenerative therapy as a future direction.
5. Conclusion
The iGel+GM/GF were degraded in a few days in the PBS containing collagenase and had a control released growth factor. As a result, the number of retained cells was significantly high compared with other control groups. In addition, it was suggested that the controlled release of bFGF and PGFM promote the differentiation of transplanted stem cells in muscle into myoblasts. Therefore, it is concluded that the encapsulation of stem cells by iGel+GM/GF was a promising and straightforward method for efficient cell transplantation.
Acknowledgements
We would like to acknowledge the help from the member of Laboratory of Biomaterials, Department of Regeneration Science and Engineering, Institute for Frontier Life and Medical Sciences, Kyoto University for the help with in vitro experiments and histological analysis. This work was supported by the Cooperative Research Program (Joint Usage/Research Center program) of Institute for Frontier Life and Medical Sciences, Kyoto University and JSPS KAKENHI Grant Number JP19K24089.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Contributor Information
Ryo Mitsui, Email: cheminton0804@gmail.com.
Makoto Matsukawa, Email: pineriver6@gmail.com.
Kiyoko Nakagawa, Email: k.happy.go.lucky.6.15@gmail.com.
Emiko Isomura, Email: tanaemi@dent.osaka-u.ac.jp.
Toshie Kuwahara, Email: tse0722@gmail.com.
Teruki Nii, Email: nii.teruki.204@m.kyushu-u.ac.jp.
Susumu Tanaka, Email: stanaka@dent.osaka-u.ac.jp.
Yasuhiko Tabata, Email: yasuhiko@infront.kyoto-u.ac.jp.
References
- 1.Bajada S., Mazakova I., Richardson J.B., Ashammakhi N. Updates on stem cells and their applications in regenerative medicine. J Tissue Eng Regen Med. 2008;2:169–183. doi: 10.1002/term.83. [DOI] [PubMed] [Google Scholar]
- 2.Mahla R.S. Stem cells applications in regenerative medicine and disease therapeutics. Int J Cell Biol. 2016;2016:6940283. doi: 10.1155/2016/6940283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zuk P.A., Zhu M., Mizuno H., Huang J., Futrell J.W., Katz A.J. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 4.Pittenger M.F., Mackay A.M., Beck S.C., Jaiswal R.K., Douglas R., Mosca J.D. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 5.Rivera-Izquierdo M., Cabeza L., Lainez-Ramos-Bossini A., Quesada R., Perazzoli G., Alvarez P. An updated review of adipose derived-mesenchymal stem cells and their applications in musculoskeletal disorders. Expert Opin Biol Ther. 2019;19:233–248. doi: 10.1080/14712598.2019.1563069. [DOI] [PubMed] [Google Scholar]
- 6.Qazi T.H., Duda G.N., Ort M.J., Perka C., Geissler S., Winkler T. Cell therapy to improve regeneration of skeletal muscle injuries. J Cachexia Sarcopenia Muscle. 2019;10:501–516. doi: 10.1002/jcsm.12416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao L., Johnson T., Liu D. Therapeutic angiogenesis of adipose-derived stem cells for ischemic diseases. Stem Cell Res Ther. 2017;8:125. doi: 10.1186/s13287-017-0578-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pers Y.M., Rackwitz L., Ferreira R., Pullig O., Delfour C., Barry F. Adipose mesenchymal stromal cell-based therapy for severe osteoarthritis of the knee: a phase I dose-escalation trial. Stem Cells Transl Med. 2016;5:847–856. doi: 10.5966/sctm.2015-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miyahara Y., Nagaya N., Kataoka M., Yanagawa B., Tanaka K., Hao H. Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat Med. 2006;12:459–465. doi: 10.1038/nm1391. [DOI] [PubMed] [Google Scholar]
- 10.Takemura S., Shimizu T., Oka M., Sekiya S., Babazono T. Transplantation of adipose-derived mesenchymal stem cell sheets directly into the kidney suppresses the progression of renal injury in a diabetic nephropathy rat model. J Diabetes Investig. 2019;11:545–553. doi: 10.1111/jdi.13164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lipner J., Shen H., Cavinatto L., Liu W., Havlioglu N., Xia Y. In Vivo evaluation of adipose-derived stromal cells delivered with a nanofiber scaffold for tendon-to-bone repair. Tissue Eng Part A. 2015;21:2766–2774. doi: 10.1089/ten.tea.2015.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tateno A., Asano M., Akita D., Toriumi T., Tsurumachi-Iwasaki N., Kazama T. Transplantation of dedifferentiated fat cells combined with a biodegradable type I collagen-recombinant peptide scaffold for critical-size bone defects in rats. J Oral Sci. 2019;61:534–538. doi: 10.2334/josnusd.18-0458. [DOI] [PubMed] [Google Scholar]
- 13.Perlee D., van Vught L.A., Scicluna B.P., Maag A., Lutter R., Kemper E.M. Intravenous infusion of human adipose mesenchymal stem cells modifies the host response to lipopolysaccharide in humans: a randomized, single-blind, parallel group, placebo controlled trial. Stem Cells. 2018;36:1778–1788. doi: 10.1002/stem.2891. [DOI] [PubMed] [Google Scholar]
- 14.Watanabe T., Maruyama S., Yamamoto T., Kamo I., Yasuda K., Saka Y. Increased urethral resistance by periurethral injection of low serum cultured adipose-derived mesenchymal stromal cells in rats. Int J Urol. 2011;18:659–666. doi: 10.1111/j.1442-2042.2011.02795.x. [DOI] [PubMed] [Google Scholar]
- 15.Zhou X., Ning K., Ling B., Chen X., Cheng H., Lu B. Multiple injections of autologous adipose-derived stem cells accelerate the burn wound healing process and promote blood vessel regeneration in a rat model. Stem Cell Dev. 2019;28:1463–1472. doi: 10.1089/scd.2019.0113. [DOI] [PubMed] [Google Scholar]
- 16.Young S.A., Sherman S.E., Cooper T.T., Brown C., Anjum F., Hess D.A. Mechanically resilient injectable scaffolds for intramuscular stem cell delivery and cytokine release. Biomaterials. 2018;159:146–160. doi: 10.1016/j.biomaterials.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 17.Laurila J.P., Laatikainen L., Castellone M.D., Trivedi P., Heikkila J., Hinkkanen A. Human embryonic stem cell-derived mesenchymal stromal cell transplantation in a rat hind limb injury model. Cytotherapy. 2009;11:726–737. doi: 10.3109/14653240903067299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Y., Wu T., Huang S., Suen C.W., Cheng X., Li J. Sustained release SDF-1alpha/TGF-beta1-loaded silk fibroin-porous gelatin scaffold promotes cartilage repair. ACS Appl Mater Interfaces. 2019;11:14608–14618. doi: 10.1021/acsami.9b01532. [DOI] [PubMed] [Google Scholar]
- 19.Anamizu M., Tabata Y. Design of injectable hydrogels of gelatin and alginate with ferric ions for cell transplantation. Acta Biomater. 2019;100:184–190. doi: 10.1016/j.actbio.2019.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Chignon-Sicard B., Kouidhi M., Yao X., Delerue-Audegond A., Villageois P., Peraldi P. Platelet-rich plasma respectively reduces and promotes adipogenic and myofibroblastic differentiation of human adipose-derived stromal cells via the TGFbeta signalling pathway. Sci Rep. 2017;7:2954. doi: 10.1038/s41598-017-03113-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lai F., Kakudo N., Morimoto N., Taketani S., Hara T., Ogawa T. Platelet-rich plasma enhances the proliferation of human adipose stem cells through multiple signaling pathways. Stem Cell Res Ther. 2018;9:107. doi: 10.1186/s13287-018-0851-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fujimoto Y., Yokozeki T., Yokoyama A., Tabata Y. Basic fibroblast growth factor enhances proliferation and hepatocyte growth factor expression of feline mesenchymal stem cells. Regen Ther. 2020;15:10–17. doi: 10.1016/j.reth.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawazoe T., Kim H.H. Tissue augmentation by white blood cell-containing platelet-rich plasma. Cell Transplant. 2012;21:601–607. doi: 10.3727/096368911X605538. [DOI] [PubMed] [Google Scholar]
- 24.Zhang X.L., Wang Y.M., Chu K., Wang Z.H., Liu Y.H., Jiang L.H. The application of PRP combined with TCP in repairing avascular necrosis of the femoral head after femoral neck fracture in rabbit. Eur Rev Med Pharmacol Sci. 2018;22:903–909. doi: 10.26355/eurrev_201802_14368. [DOI] [PubMed] [Google Scholar]
- 25.Imada M., Yagyuu T., Ueyama Y., Maeda M., Yamamoto K., Kurokawa S. Prevention of tooth extraction-triggered bisphosphonate-related osteonecrosis of the jaws with basic fibroblast growth factor: an experimental study in rats. PLos One. 2019;14 doi: 10.1371/journal.pone.0211928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horikoshi-Ishihara H., Tobita M., Tajima S., Tanaka R., Oshita T., Tabata Y. Coadministration of adipose-derived stem cells and control-released basic fibroblast growth factor facilitates angiogenesis in a murine ischemic hind limb model. J Vasc Surg. 2016;64:1825–1834. doi: 10.1016/j.jvs.2015.09.054. e1821. [DOI] [PubMed] [Google Scholar]
- 27.Matsui M., Tabata Y. Enhanced angiogenesis by multiple release of platelet-rich plasma contents and basic fibroblast growth factor from gelatin hydrogels. Acta Biomater. 2012;8:1792–1801. doi: 10.1016/j.actbio.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 28.Tabata Y., Nagano A., Ikada Y. Biodegradation of hydrogel carrier incorporating fibroblast growth factor. Tissue Eng. 1999;5:127–138. doi: 10.1089/ten.1999.5.127. [DOI] [PubMed] [Google Scholar]
- 29.Yoshida Y., Matsubara H., Fang X., Hayashi K., Nomura I., Ugaji S. Adipose-derived stem cell sheets accelerate bone healing in rat femoral defects. PLos One. 2019;14 doi: 10.1371/journal.pone.0214488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nii T., Makino K., Tabata Y. Influence of shaking culture on the biological functions of cell aggregates incorporating gelatin hydrogel microspheres. J Biosci Bioeng. 2019;128:606–612. doi: 10.1016/j.jbiosc.2019.04.013. [DOI] [PubMed] [Google Scholar]
- 31.Nii T., Makino K., Tabata Y. A cancer invasion model combined with cancer-associated fibroblasts aggregates incorporating gelatin hydrogel microspheres containing a p53 inhibitor. Tissue Eng C Methods. 2019;25:711–720. doi: 10.1089/ten.TEC.2019.0189. [DOI] [PubMed] [Google Scholar]
- 32.Matsuno K., Saotome T., Shimada N., Nakamura K., Tabata Y. Effect of cell seeding methods on the distribution of cells into the gelatin hydrogel nonwoven fabric. Regen Ther. 2020;14:160–164. doi: 10.1016/j.reth.2020.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakamura K., Saotome T., Shimada N., Matsuno K., Tabata Y. A gelatin hydrogel nonwoven fabric facilitates metabolic activity of multilayered cell sheets. Tissue Eng C Methods. 2019;25:344–352. doi: 10.1089/ten.TEC.2019.0061. [DOI] [PubMed] [Google Scholar]
- 34.Kimura Y., Ozeki M., Inamoto T., Tabata Y. Adipose tissue engineering based on human preadipocytes combined with gelatin microspheres containing basic fibroblast growth factor. Biomaterials. 2003;24:2513–2521. doi: 10.1016/s0142-9612(03)00049-8. [DOI] [PubMed] [Google Scholar]
- 35.Nii T., Makino K., Tabata Y. Three-dimensional culture system of cancer cells combined with biomaterials for drug screening. Cancers (Basel) 2020;12 doi: 10.3390/cancers12102754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tian H., Bharadwaj S., Liu Y., Ma P.X., Atala A., Zhang Y. Differentiation of human bone marrow mesenchymal stem cells into bladder cells: potential for urological tissue engineering. Tissue Eng Part A. 2010;16:1769–1779. doi: 10.1089/ten.tea.2009.0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu R., Liu G., Bharadwaj S., Zhang Y. Isolation and myogenic differentiation of mesenchymal stem cells for urologic tissue engineering. Methods Mol Biol. 2013;1001:65–80. doi: 10.1007/978-1-62703-363-3_7. [DOI] [PubMed] [Google Scholar]
- 38.Sassoli C., Vallone L., Tani A., Chellini F., Nosi D., Zecchi-Orlandini S. Combined use of bone marrow-derived mesenchymal stromal cells (BM-MSCs) and platelet rich plasma (PRP) stimulates proliferation and differentiation of myoblasts in vitro: new therapeutic perspectives for skeletal muscle repair/regeneration. Cell Tissue Res. 2018;372:549–570. doi: 10.1007/s00441-018-2792-3. [DOI] [PubMed] [Google Scholar]
- 39.Ma Q., Jiang N., Liang S., Chen F., Fang L., Wang X. Functionalization of a clustered TiO2 nanotubular surface with platelet derived growth factor-BB covalent modification enhances osteogenic differentiation of bone marrow mesenchymal stem cells. Biomaterials. 2020;230:119650. doi: 10.1016/j.biomaterials.2019.119650. [DOI] [PubMed] [Google Scholar]
- 40.Askari M., Bonakdar S., Anbouhi M.H., Shahsavarani H., Kargozar S., Khalaj V. Sustained release of TGF-beta1 via genetically-modified cells induces the chondrogenic differentiation of mesenchymal stem cells encapsulated in alginate sulfate hydrogels. J Mater Sci Mater Med. 2018;30:7. doi: 10.1007/s10856-018-6203-9. [DOI] [PubMed] [Google Scholar]
- 41.Choi J.S., Yoon H.I., Lee K.S., Choi Y.C., Yang S.H., Kim I.S. Exosomes from differentiating human skeletal muscle cells trigger myogenesis of stem cells and provide biochemical cues for skeletal muscle regeneration. J Control Release. 2016;222:107–115. doi: 10.1016/j.jconrel.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 42.Milner D.J., Bionaz M., Monaco E., Cameron J.A., Wheeler M.B. Myogenic potential of mesenchymal stem cells isolated from porcine adipose tissue. Cell Tissue Res. 2018;372:507–522. doi: 10.1007/s00441-017-2764-z. [DOI] [PubMed] [Google Scholar]
- 43.Andersen J.I., Juhl M., Nielsen T., Emmersen J., Fink T., Zachar V. Uniaxial cyclic strain enhances adipose-derived stem cell fusion with skeletal myocytes. Biochem Biophys Res Commun. 2014;450:1083–1088. doi: 10.1016/j.bbrc.2014.06.124. [DOI] [PubMed] [Google Scholar]
- 44.Bayati V., Sadeghi Y., Shokrgozar M.A., Haghighipour N., Azadmanesh K., Amanzadeh A. The evaluation of cyclic uniaxial strain on myogenic differentiation of adipose-derived stem cells. Tissue Cell. 2011;43:359–366. doi: 10.1016/j.tice.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 45.Helms F., Lau S., Klingenberg M., Aper T., Haverich A., Wilhelmi M. Complete myogenic differentiation of adipogenic stem cells requires both biochemical and mechanical stimulation. Ann Biomed Eng. 2019;30:319–324. doi: 10.1007/s10439-019-02234-z. [DOI] [PubMed] [Google Scholar]
- 46.Tsai S.W., Chen C.J., Chen H.L., Chen C.M., Chang Y.Y. Effects of treadmill running on rat gastrocnemius function following botulinum toxin A injection. J Orthop Res. 2012;30:319–324. doi: 10.1002/jor.21509. [DOI] [PubMed] [Google Scholar]
- 47.Hahn S.A., Ferreira L.F., Williams J.B., Jansson K.P., Behnke B.J., Musch T.I. Downhill treadmill running trains the rat spinotrapezius muscle. J Appl Physiol. 2007;102:412–416. doi: 10.1152/japplphysiol.00581.2006. (1985) [DOI] [PubMed] [Google Scholar]