Abstract
Objective
The Pfizer BNT162b2 vaccine showed a reassuring safety profile in clinical trials, but real-world data are scarce. Bell's palsy, herpes zoster, Guillain–Barré syndrome (GBS) and other neurological complaints in proximity to vaccination have received special public attention. We compared their rates among vaccinated and unvaccinated individuals.
Methods
Individuals ≥16 years vaccinated with at least one dose of BNT162b2 were eligible for this historical cohort study in a health maintenance organization insuring 1.2 million citizens. Each vaccinee was matched to a non-vaccinated control by sex, age, population sector (general Jewish, Arab, ultra-orthodox Jewish) and comorbidities. Diagnosis of Covid-19 before or after vaccination was an exclusion criterion. The outcome was a diagnosis of Bell's palsy, GBS, herpes zoster or symptoms of numbness or tingling, coded in the visit diagnosis field using ICD-9 codes. Diagnoses of Bell's palsy and GBS were verified by individual file review.
Results
Of 406 148 individuals vaccinated during the study period, 394 609 (97.2%) were eligible (11 539 excluded). A total of 233 159 (59.1%) were matched with unvaccinated controls. Mean follow was 43 ± 15.14 days. In vaccinated and unvaccinated individuals there were 23 versus 24 cases of Bell's palsy (RR 0.96, CI 0.54–1.70), one versus zero cases of GBS, 151 versus 141 cases of herpes zoster (RR 1.07, CI 0.85–1.35) and 605 versus 497 cases of numbness or tingling (RR 1.22, CI 1.08–1.37), respectively.
Discussion
No association was found between vaccination, Bell's palsy, herpes zoster or GBS. Symptoms of numbness or tingling were more common among vaccinees. This study adds reassuring data regarding the safety of the BNT162b2 vaccine.
Keywords: Adverse events, Bell's palsy, COVID-19, mRNA vaccine, SARS-CoV-2
Introduction
The Pfizer–BioNTech and Moderna mRNA vaccines received Food and Drug Administration (FDA) emergency use authorization (EUA) during December 2020, and vaccination started worldwide soon after. In Israel, the vaccination campaign with the Pfizer BNT162b2 vaccine was launched on December 2020. Since then, more than 5 900 000 Israelis have received the first dose [1].
The Pfizer BNT162b2 showed a reassuring safety profile in clinical trials, characterized mainly by short-term mild local reactions. Serious adverse events were rare and had similar frequencies in the vaccine and placebo arms [2,3]. However, adverse events that were not identified in clinical trials might emerge due to the limited number of vaccinees and their specific characteristics. Therefore, continuous monitoring for rare or unexpected side effects in the real world is needed.
The FDA and other authorities have published a list of adverse events of special interest [4] based on historical precedent set by prior vaccines and knowledge acquired during the vaccine development. Several other medical conditions have received attention from healthcare providers and the public, including Bell's palsy (reported also in the clinical trials of Pfizer and Moderna [5,6]), herpes zoster [[7], [8], [9]] and several other neurological adverse events [10,11]. When vaccinating millions of individuals, sporadic clinical events appearing in their usual frequency might be incorrectly associated with vaccination. Therefore, post-marketing comparison of rates of adverse events to the background incidence rates in large populations [12], or comparison of large groups of vaccinated and unvaccinated individuals are indicated.
Materials and methods
Meuhedet Health Maintenance Organization (MHMO) serves approximately 1.2 million citizens in Israel. By mid-March 2021, MHMO had vaccinated more than 560 000 individuals with more than 1 million doses of the BNT162b2 vaccine.
We conducted this observational historical cohort study to assess the possible association between BNT162b2 vaccination and several predefined clinical events in a cohort of vaccinees compared with a matched cohort of unvaccinated individuals.
Data were extracted from the MHMO database, which includes extensive medical and demographic information from all healthcare providers including visit diagnoses, hospital and emergency department diagnoses, dates of vaccination, all Covid-19 PCR and serology tests results, medication dispensed, and laboratory and imaging data. As MHMO enables free access to medical care with same-day availability, we assumed good documentation of significant clinical events in close proximity to vaccination.
Study population and design
Eligibility criteria included insured persons aged ≥16 years who had been vaccinated with the first dose of BNT162b2 vaccine from 19 December 2020 to 12 February 12 2021. We excluded individuals with diagnosis of Covid-19 infection, either by PCR or serology at any time before or after the vaccine (MHMO's physicians could not order serology tests for vaccinated individuals during the study period, therefore positive serology was indicative of past infection). Each vaccinated person was matched with a non-vaccinated control according to sex, age, number of comorbidities (from zero to four comorbidities) and population sector (general Jewish, Arab, ultra-orthodox Jewish), since vaccination rates varied between sectors. For the control group, we applied the same exclusion criteria. The following comorbidities were used for matching: asthma or chronic obstructive pulmonary disease, cardiovascular disease, diabetes mellitus, active cancer. We adjusted for other covariates after matching, including hospitalization during 2020 and socio-economic status (SES), derived from the individual's home address, ranging from 1 to 10. For the purpose of this study, SES levels were grouped into three levels: 1–3 low, 4–7 medium and 8–10 high.
For each pair, the vaccine day of the vaccinated individual was set as the reference day for follow-up for the matched control.
Outcomes
Four outcomes were assessed in this study: Bell's palsy, Guillain–Barré syndrome (GBS), herpes zoster and symptoms of numbness or tingling sensation. Diagnoses were extracted using ICD-9 codes (Table S1) from the visit diagnosis field, which is mandatory in our electronic medical record (EMR). The diagnosis was considered an outcome whether the physician attributed it to the vaccine or not. For each outcome, individuals who had the same diagnosis during the 30 days before vaccination/reference day were excluded (<0.5%). The diagnoses of Bell's palsy and GBS were validated by individual medical record review, performed by a senior physician blinded to the patient vaccine status. Follow-up duration was from vaccine administration or reference day until the end of follow-up period on 1 March 2021. Data collection took place during March 2021.
Statistical analysis
The chi-squared test was used to examine the association between categorical variables and dependent variables. The Student t test was used to assess the difference between continuous variables and dependent variables. Logistic regression was used to ascertain the effect of having the first vaccine on the likelihood of presenting one of the clinical outcomes (OR and CI) [13]. Age was grouped into five levels: <18, 18–35, 35–50, 50–65, 65+ years.
Analyses were conducted with IBM SPSS, Version 27.0. (Armonk, NY). Two-tailed p values are reported, with α = 0.05.
This study was approved by the MHMO Institutional Review Board and was exempted from the requirement for informed consent.
Results
Study population
Of 406 148 MHMO members who were vaccinated during the study period, 394 609 (97.2%) were eligible (11 539 excluded due to past infection). Of 430 395 unvaccinated members 335 242 (77.9%) were eligible (95 133 excluded due to past infection). We were able to match 233 159 individuals vaccinated with at least one dose to unvaccinated controls (59.1% of the eligible vaccinated individuals). A total of 131 033 (56.2%) of the matched vaccinated individuals were vaccinated with two doses.
The baseline characteristics of the matched individuals are shown in Table 1 . Variables used for matching were well balanced between study groups.
Table 1.
Characteristic of participants according to their vaccination status
| Vaccinated with a 1st dose | Unvaccinated | p | Vaccinated with a 2nd dose | Unvaccinated | p | |
|---|---|---|---|---|---|---|
| Overall | 233 159 | 233 159 | 131 033 | 131 033 | ||
| Women† | 118 525 (51) | 111 706 (51) | 1 | 66 453 (51) | 66 453 (51) | 1 |
| Age group† | 1 | 1 | ||||
| <18 | 13 275 (6) | 13 275 (6) | 2288 (2) | 2288 (2) | ||
| 18-35 | 91 320 (39) | 91 320 (39) | 34 666 (26) | 34 666 (26) | ||
| 35-50 | 68 707 (29) | 68 707 (29) | 42 641 (32) | 42 641 (32) | ||
| 50-65 | 38 598 (17) | 38 598 (17) | 31 779 (24) | 31 779 (24) | ||
| 65+ | 21 259 (9) | 21 259 (9) | 19 659 (15) | 19 659 (15) | ||
| Sector† | 1 | 1 | ||||
| General Jewish | 132 980 (57) | 132 980 (57) | 75 848 (58) | 75 848 (58) | ||
| Ultra-orthodox Jewish | 54 929 (24) | 54 929 (24) | 28 029 (21) | 28 029 (21) | ||
| Arab | 45 250 (19) | 45 250 (19) | 27 056 (21) | 27 056 (21) | ||
| Socio-economic status | <0.001 | <0.001 | ||||
| 1-3 | 51 017 (22) | 71 703 (31) | 27 024 (21) | 40 451 (31) | ||
| 4-7 | 130 525 (56) | 128 806 (55) | 75 811 (58) | 75 220 (57) | ||
| 8-10 | 37 973 (16) | 19 670 (8) | 23 692 (18) | 11 211 (9) | ||
| unknown | 13 644 (6) | 12 980 (6) | 4 506 (3) | 4 151 (3) | ||
| No. of comorbidities† | 1 | |||||
| 0 | 181 222 (77) | 181 222 (77) | 1 | 95 523 (73) | 95 523 (73) | |
| 1 | 43 172 (19) | 43 172 (19) | 27 874 (21) | 27 874 (21) | ||
| 2+ | 8765 (4) | 8765 (4) | 7636 (6) | 7636 (6) | ||
| Hospitalization during 2020 | 16 298 (7) | 15 979 (7) | 0.066 | 11 084 (8) | 10 646 (8) | <0.01 |
| Diabetes | 16 095 (7) | 15 799 (7) | 0.086 | 13 469 (10) | 13 001 (10) | <0.01 |
| Lung disease∗ | 31 260 (13) | 32 032 (14) | 0.001 | 18 264 (14) | 19 239 (15) | <0.001 |
| Oncological condition | 5264 (2) | 4283 (2) | <0.001 | 4479 (3) | 3506 (3) | <0.001 |
| Cardiac disease | 9726 (4) | 10 262 (4) | <0.001 | 8380 (6) | 8903 (7) | <0.001 |
Data are presented as n (%).
Asthma or chronic obstructive lung disease.
Variable included in the matching rule.
Overall follow-up was 9 988 509 days for each group, with mean follow-up of 43 ± 15.14 days per participant. Mean gap between doses was 22 ± 1.55 days, and mean follow-up after the second dose was 32 ± 8.28 days (overall follow-up was 4 226 133 days after the second dose).
Clinical outcomes
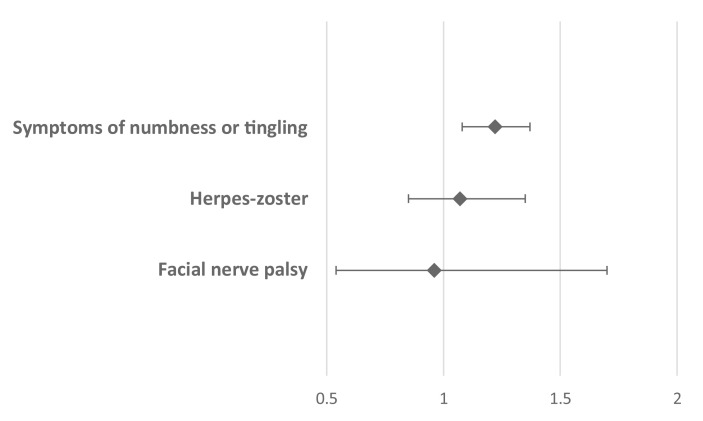
Relative risk (RR) for three of the clinical outcomes (Bell's palsy, herpes zoster and symptoms of numbness and tingling) for vaccinated compared with unvaccinated individuals are summarized in Fig. 1 . Symptoms of numbness or tingling were the only outcome with significantly higher incidence among vaccinated individual compared with unvaccinated ones (RR 1.22, CI 1.08–1.37). Due to the paucity of cases, RR could not be calculated for GBS.
Fig. 1.
Relative risk and 95% confidence interval are shown for three clinical outcomes. RR for GBS could not be calculated due to paucity of cases.
Bell's palsy
A total of 123 cases of Bell's palsy were identified using the appropriate ICD-9 codes of visit diagnosis. Following thorough review of patients' medical records by a senior physician, 76 cases were excluded (mainly cases of past or remote event, or a clinical description not consistent with Bell's palsy).
There were 23 cases of confirmed Bell's palsy following the first dose compared with 24 cases in the control group)84 vs. 88 cases per 100 000 person-years, respectively, RR 0.96, CI 0.54–1.70, non-significant, NS). Eight cases in the vaccinated group and 12 cases in the non-vaccinated group occurred after the second dose (NS). Bell's palsy occurred after an average of 20.4 ± 13.55 days of the first dose compared with 26.8 ± 17.82 days in the control group (NS).
Mean age of vaccinees who developed Bell's palsy was 45.8 compared with 45.3 in the control group (NS), of whom 47.8% (vaccinees) and 29.2% (control) were females (NS).
Guillain–Barré syndrome
We identified one confirmed case of GBS following the first dose (0.4 cases per 10 000 person-years). No cases of GBS were identified in the unvaccinated group.
Herpes zoster
In total, 151 individuals developed herpes zoster following the first dose compared with 141 in the control group (55.2 vs. 51.5 cases per 10 000 person-years respectively, RR 1.07, CI 0.85–1.35, NS). Sixty-seven cases in the vaccinated group and 61 cases in the control group occurred after the second dose (NS). In average, zoster occurred 27.2 ± 18.0 days after the first dose, compared with 24.9 ± 17.22 days in the control group (NS).
Mean age of vaccinees who developed zoster was 51.8 compared with 51.0 in the control group (NS), of whom 57.0% (vaccinees) and 61.0% (control) were females (NS).
Symptoms of numbness or tingling
A total of 605 individuals received the diagnosis of numbness or tingling following the first dose compared with 497 cases in the control group (221.1 vs. 181.6 cases per 10 000 person-years respectively, RR 1.22, CI 1.08–1.37, p 0.001). In average, these complaints occurred 24.3 ± 17.62 days after the first dose compared with 26.9 ± 16.77 days in the control group (p 0.013). 261 cases in the vaccinated group and 239 cases in the control group occurred after the second dose (226.0 vs. 207.0 cases per 10 000 person-years respectively, RR 1.09, CI 0.91–1.30).
The mean age of vaccinees who experienced numbness or tingling was 49.6 compared with 47.0 in the control group (p 0.004), of whom 58.0% (vaccinees) and 59.6% (control) were females (NS). Kaplan–Meier plots for post-vaccination numbness and tingling were constructed for vaccinated and control groups and showed no evidence of clustering over time.
While applying multivariate logistic regression, adjusting for variables associated with numbness and tingling by univariate analysis (sex, age, hospitalization during 2020, diabetes, asthma, cardiac and oncological diseases), symptoms of numbness and tingling were still more common among vaccinees compared with unvaccinated controls (OR 1.21, CI 1.08–1.37, Table 2 ).
Table 2.
Multivariate logistic regression for symptoms of numbness and tingling among individuals who were vaccinated with at least one dose and their controls
| OR | p | 95% CI for OR | |
|---|---|---|---|
| Vaccinated vs. unvaccinated | 1.21 | 0.001 | 1.08-1.37 |
| Women vs. Men | 1.35 | <0.001 | 1.19-1.52 |
| Age, years | |||
| <18 | <0.001 | ||
| 18-35 | 14.96 | <0.001 | 3.72-60.19 |
| 35-50 | 35.87 | <0.001 | 8.94-143.97 |
| 50-65 | 45.01 | <0.001 | 11.20-180.89 |
| 65+ | 28.11 | <0.001 | 6.93-114.04 |
| Sector | |||
| General Jewish | <0.001 | ||
| Ultra-orthodox Jewish | 0.68 | <0.001 | 0.57-0.81 |
| Arab | 1.36 | <0.001 | 1.19-1.56 |
| Hospitalization during 2020 vs. no | 1.88 | <0.001 | 1.59-2.22 |
| Diabetes vs. no diabetes | 1.50 | <0.001 | 1.25-1.79 |
| Lung disease∗ vs. no Lung disease | 1.27 | 0.003 | 1.08-1.49 |
| Oncological condition vs. no oncological condition | 1.50 | 0.005 | 1.13-1.98 |
| Cardiac disease vs. no cardiac disease | 1.29 | 0.03 | 1.03-1.62 |
Asthma or chronic obstructive lung disease.
Discussion
In this study we sought to investigate possible associations between the BNT162b2 vaccine and several medical conditions of concern. We found no association between BNT162b2 vaccination and Bell's palsy, herpes zoster or GBS. Mild, non-specific neurological complaints of numbness and tingling were more frequent among vaccinees, with higher odds for females and older individuals.
Bell's palsy received unusual public attention shortly after the FDA EUA of the vaccine. Reported safety data by Pfizer and Moderna [2,5,6,14] showed seven cases of Bell's palsy compared with one case in the placebo arm, among nearly 40 000 vaccinees, raising safety concerns. While the FDA and other authorities stated that this frequency did not exceed the expected background rate, a commentary published recently highlighted that correcting for the follow-up duration in the studies, the rate of Bell's palsy in these two trials was between 3.5–7 times higher than expected in the general population (105 vs. 15–30 cases per 100 000 person-years) [15], calling for robust surveillance after cases of vaccine-associated Bell's palsy. Our study provides data looking at a large population of vaccinated individuals, approximately six times larger than the population followed in both clinical trials, compared with unvaccinated individuals. The rate of Bell's palsy in our study, 84 and 88 cases per 100 000 person-years among vaccinees and unvaccinated controls respectively (RR 0.96, CI 0.54–1.70, NS), was higher than the reported rate in the general population. This finding may be explained by the mean age of our cohort, as the incidence of Bell's palsy is higher among older persons [16,17], who were the first to get vaccinated, and were over-represented in our cohort (Table 1).
Concerns of association between the vaccine and zoster have been raised by clinicians [[7], [8], [9]] and by the press. Our study found no such association.
As for other new vaccines, concerns regarding possible association with GBS were raised by healthcare providers. In a commentary reviewing the lack of association between COVID itself and GBS, as well with many other vaccines [18], the authors predicted that vaccines encoding the spike protein would not confer additional risk of GBS, and stressed the need for reliable data, as during mass vaccination rare cases of GBS would inevitably occur by coincidence following vaccination. In our cohort we found one case of confirmed GBS among vaccinated individuals, and no cases among the controls. The reported annual incidence of GBS is 1.7 cases per 100 000 population [18,19]; therefore, our study cohort was too small for estimating the incidence of GBS, or its association with the vaccine, and could not exclude an elevated risk.
Mild sensory symptoms were significantly more common among vaccinees. These complaints of numbness and tingling, mainly on one side of the face, might be a manifestation of anxiety, the possible result of reports in popular media of suspected association between the vaccine and Bell's palsy. As previously described in the context of functional neurological disorder appearing in proximity to SARS-CoV-2 vaccination [20], abnormal expectations or beliefs can interact with sensorimotor perceptions. In addition, vaccines can produce nociceptive experiences, such as local pain or systemic myalgias that promote the redirection of attention towards the body. This redirection of attention may be the cause for symptoms, rather than a neurotoxic or immune-mediated processes. Other biological explanations for these sensory complaints have been previously suggested, including a case report of small fibre neuropathy after the second dose of the BNT162b2 vaccine [21]. However, causality cannot be drawn based on a single case.
In addition, individuals recently vaccinated were more likely to seek care than non-vaccinated individuals for mild neurological symptoms, which might have created a bias in our analysis.
The strength of this study is its large cohort and full representation of the Israeli population, ensuring its generalizability for the population in Israel and other countries with similar population characteristics. The large cohort size enabled us to compare the incidence of uncommon clinical events among vaccinated and unvaccinated individuals and to calculate incidence rates, which cannot be calculated using voluntary reporting tools such as the CDC V-safe, smartphone-based reporting tool, or VAERS (Vaccine Adverse Event Reporting System), designated to detect safety signals [22].
The wide vaccine coverage and country-wide computerized data capture in Israel enabled data acquisition within a few weeks from launching of the vaccination campaign. Early assessment was important for ensuring a large, matched control group and long follow-up, as vaccination in Israel proceeded quickly reducing the size of the available controls rapidly.
Secondly, clinical events assessed in our study were routinely reported by healthcare providers in the EMR, and not as part of vaccine safety reports, enabling detection of safety events regardless of physicians' awareness of possible association.
A limitation of our study is its observational design, for which we tried to compensate with a large study cohort and a rigorous matching. While matching for age, sex, population sector, number of comorbidities and length of follow-up reduced possible confounders, it came at a cost of not including 41% of the eligible vaccinated individuals. Multivariate analysis was performed to adjust for further residual confounding.
Outcomes of this study were identified by visit diagnoses, which might be partial or inaccurate. We therefore chose to focus on a few clinical outcomes of concern. Furthermore, we confirmed each case of Bell's palsy and GBS by reviewing the patients' EMR to provide reliable information. Inaccurate or under-reporting of visit diagnoses probably resulted in a non-differential misclassification. In this context it is important to emphasize that GBS is a rare condition and larger cohorts are required for proper monitoring of its association with the vaccine [18]. The relatively short follow-up period further limited the ability of this study to assess the association of GBS with the vaccine. We included data regarding GBS in this report, despite insufficient power, to support future meta-analysis.
In conclusion, we found no association between vaccination and Bell's palsy, GBS or zoster. As fear of possible clinically significant adverse events is one of the main determinants of vaccine hesitancy, our findings should provide reassurance to healthcare providers and vaccine recipients regarding these adverse events and promote confidence in the global vaccination campaign.
Transparency declaration
D.S. reports receiving personal fees from Pfizer, outside the submitted work (advisory board on Trumenba) and consultation fees from GSK and Gilead. The other authors declare no competing interests.
Funding
This study received no funding.
Author contributions
D.S., F.H.S. and G.Z. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Conceptualization and methodology: D.S., G.Z., F.H.S. Formal analysis and interpretation of data: D.S., G.Z., F.H.S., O.G., R.B., A.D.H. Writing of original draft: D.S., G.Z. Writing – review and editing: All authors. Supervision: G.Z.
Editor: A. Huttner
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2021.09.018.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Multimedia component 2
References
- 1.Israel ministry of health covid-19 dashboard. https://datadashboard.health.gov.il/COVID-19/general Available from:
- 2.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walsh E.E., Frenck R.W., Jr., Falsey A.R., Kitchin N., Absalon J., Gurtman A., et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med. 2020;383:2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Center for Biologics Evaluation and Research Office of Biostatistics and Epidemiology CBER surveillance program background rates of adverse events of special interest for COVID-19 vaccine safety monitoring protocol 2021. https://www.bestinitiative.org/wp-content/uploads/2021/02/C19-Vaccine-Safety-AESI-Background-Rate-Protocol-FINAL-2020.pdf Available from:
- 5.mRNA-1273 Sponsor Briefing Document Addendum FDA vaccines and related biological products advisory committee. https://www.fda.gov/media/144453/download Available from:
- 6.FDA Briefing Document Pfizer-BioNTech COVID-19 Vaccine . 2020. Vaccines and related biological products advisory committee meeting December 10, 2020.https://www.fda.gov/media/144245/download Available from: [Google Scholar]
- 7.Bostan E., Yalici-Armagan B. Herpes zoster following inactivated COVID-19 vaccine: a coexistence or coincidence? J Cosmet Dermatol. 2021;20:1566–1567. doi: 10.1111/jocd.14035. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez-Jimenez P., Chicharro P., Cabrera L.M., Segui M., Morales-Caballero A., Llamas-Velasco M., et al. Varicella-zoster virus reactivation after SARS-CoV-2 BNT162b2 mRNA vaccination: report of 5 cases. JAAD Case Rep. 2021;12:58–59. doi: 10.1016/j.jdcr.2021.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Furer V., Zisman D., Kibari A., Rimar D., Paran Y., Elkayam O. Herpes zoster following BNT162b2 mRNA Covid-19 vaccination in patients with autoimmune inflammatory rheumatic diseases: a case series. Rheumatology. 2021;60(SI):S190–S195. doi: 10.1093/rheumatology/keab345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McDonald I., Murray S.M., Reynolds C.J., Altmann D.M., Boyton R.J. Comparative systematic review and meta-analysis of reactogenicity, immunogenicity and efficacy of vaccines against SARS-CoV-2. NPJ Vaccines. 2021;6:74. doi: 10.1038/s41541-021-00336-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bae S., Lee Y.W., Lim S.Y., Lee J.H., Lim J.S., Lee S., et al. Adverse reactions following the first dose of ChAdOx1 nCoV-19 vaccine and BNT162b2 vaccine for healthcare workers in South Korea. J Korean Med Sci. 2021;36:e115. doi: 10.3346/jkms.2021.36.e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li X., Ostropolets A., Makadia R., Shaoibi A., Rao G., Sena A.G., et al. Characterizing the incidence of adverse events of special interest for COVID-19 vaccines across eight countries: a multinational network cohort study. medRxiv. 2021 doi: 10.1136/bmj.n1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pearce N. Analysis of matched case-control studies. BMJ. 2016;352:i969. doi: 10.1136/bmj.i969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ozonoff A., Nanishi E., Levy O. Bell's palsy and SARS-CoV-2 vaccines. Lancet Infect Dis. 2021;21:450–452. doi: 10.1016/S1473-3099(21)00076-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Monini S., Lazzarino A.I., Iacolucci C., Buffoni A., Barbara M. Epidemiology of bell's palsy in an Italian health district: incidence and case-control study. Acta Otorhinolaryngol Ital. 2010;30:198. [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao H., Zhang X., Tang Y.D., Zhu J., Wang X.H., Li S.T. Bell's palsy: clinical analysis of 372 cases and review of related literature. Eur Neurol. 2017;77:168–172. doi: 10.1159/000455073. [DOI] [PubMed] [Google Scholar]
- 18.Lunn M.P., Cornblath D.R., Jacobs B.C., Querol L., van Doorn P.A., Hughes R.A., et al. COVID-19 vaccine and Guillain-Barre syndrome: let's not leap to associations. Brain. 2021;144:357–360. doi: 10.1093/brain/awaa444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sejvar J.J., Baughman A.L., Wise M., Morgan O.W. Population incidence of Guillain-Barre syndrome: a systematic review and meta-analysis. Neuroepidemiology. 2011;36:123–133. doi: 10.1159/000324710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim D.D., Kung C.S., Perez D.L. Helping the public understand adverse events associated with Covid-19 vaccinations: lessons learned from functional neurological disorder. JAMA Neurol. 2021;78:789–790. doi: 10.1001/jamaneurol.2021.1042. [DOI] [PubMed] [Google Scholar]
- 21.Waheed W., Carey M.E., Tandan S.R., Tandan R. Post COVID-19 vaccine small fiber neuropathy. Muscle Nerve. 2021;64:E1–E2. doi: 10.1002/mus.27251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Menni C., Klaser K., May A., Polidori L., Capdevila J., Louca P., et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: a prospective observational study. Lancet Infect Dis. 2021;21:939–949. doi: 10.1016/S1473-3099(21)00224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Multimedia component 2