Abstract
The ongoing vaccination program in the control of novel coronavirus (COVID-19)is providing an opportunity to public health authorities to emerge from the current pandemic crisis.Covid vaccination which protects from getting Covid may have some minor side effects and in rare cases was accompanied by severe or immediate reactions. The development of hyaluronic acid soft tissue filler delayed inflammatory reactions following COVID-19 vaccination was reported. Treatments based on hyaluronic acid represent one of the most largely used practice of aesthetic medicine. In this we present a case of a 38-year-old female patient with a confirmed hypersensitivity reaction after having the BNT162b2 vaccine (Pfizer, USA). This case indicates that an adequate patient knowledge prior to hyaluronic acid injections to avoid adverse effect of a delayed-type hypersensitivity response after COVID-19 vaccination is desirable.
KEYWORDS: SARS-CoV-2, COVID-19, Vaccination, Dermal filler, Hyaluronic acid, Hypersensitivity response
1. Introduction
The novel coronovirus (2019-nCoV), named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)has infected approximately 250 million people worldwide killed more than 3.7 million individuals (n.d.). The rapid spreading of the pandemia has stimulated the implementation of numerous measures to combat the spread of the virus, such as closures of geographical areas, lockdowns, restrictions on the circulation of citizens and closure of various types of activities and businesses (Galanakis, 2020). The development of several effective vaccines against the virus has given new energy to the fight against Covid. The availability of vaccines has allowed the launch of vaccination campaigns that are reducing the spread of infection and countering the complications caused by the disease among the most vulnerable sections of the population (Shrotri et al., 2021). From the starting of vaccination campaigns by December 2020, it is estimated that 49.7% of the world population has received at least one dose of a COVID-19 vaccine, 7.13 billion doses have been administered globally, and 27.81 million are now administered each day. Unfortunately, only 3.9% of people in low-income countries have received at least one dose of vaccine. Despite the undoubted advantages of vaccination against Covid, the vaccine may induce adverse reactions or even serious side effects in particular patients. The practices of aesthetic medicine are to be considered with some attention in subjects that must be vaccinated or have been vaccinated against the Covid close to medical-aesthetic treatment. The possible interactions and adverse reactions caused by Covid-19 vaccines in aesthetic medicine practice have been discussed. Some scientific societies have issued guidelines or recommendations for aesthetic medicine treatments. The main attention was concentrated to aesthetic fillers in conjunction with administration of Covid vaccines (Arora et al., 2020, Gotkin et al., 2021). In this case report, we present a hypersensitivity reaction to hyaluronic acid dermal filler in a subject being vaccinated against the SARS-CoV-2 virus.
2. Case presentation
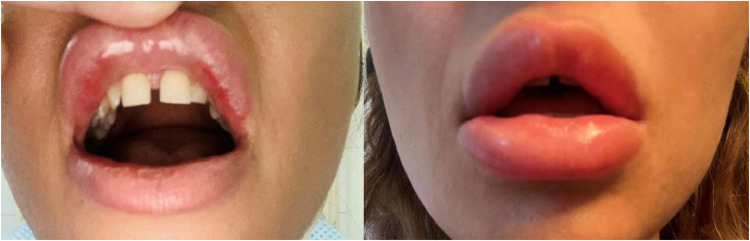
A 38-year-old female patient with no known allergies, who had previously been treated with hyaluronic acid fillers in the lips, had an adverse reaction following a booster shot vaccination with the COVID-19 Pfizer-BioNTech vaccine. The hyaluronic acid injections had been done on the December 11, 2020. Using a 0.4 ml solution of hyaluronic acid 25 mg/ml using a 27G needle.The needle was placed according to a linear technique on the vermilion border of the upper and lower lip. A 0.6 ml amount of hyalournic acid was used for a volumizing effect. The patient under went her first vaccine dose on January 8, 2021. After vaccination a mild reaction to the dermal filler that subsided by itself occured. Small erythematous nodules were visible on both the upper and lower lip with mild pain were visible from day 2 post-vaccine. These nodules disappeared completely in 7 days with no medical intervention. On February 11, 2021 the patient proceeded with her second dose of the Pfizer vaccine. On April 13, 2021 the patient complained a mild tenderness on her upper lip and two days later a painful erythematous oedema was developed on both the upper and lower lip (Figure 1 ).
Figure 1.
Small erythematous nodules after the first vaccine dose (left) and painful erythematous oedema after the second vaccination (right).
After the development of these lesions, the patient was carefully checked and her medical history established that she did not suffered from any infection or trauma in the area, no dental incident occured, no new medicines were adminsitrated , and no medical procedures took place between the filler injection and the local swelling. No extra-site symptoms were noted, except the localized pain and erythematous oedema on her lips. A diagnosis of a delayed-type hypersensitivity reaction to hyaluronic acid dermal fillers after SARS-CoV-2 vaccination was conequently made. The patient was treated with methylprednisolone tablets (Day 1&2: 6mg, Day 3-5: 4mg, Day 6-8: 2mg), In 5 days an obvious improvement characterized by a reductions in the swelling and the pain was noticeable (Figure 2 ).
Figure 2.
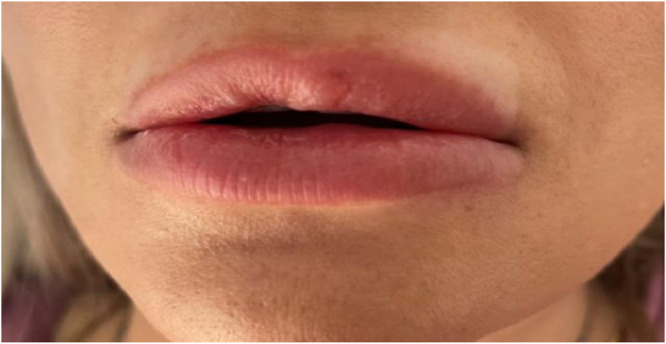
Result after 5 days using methylprednisolone [Au?4].
3. Discussion
The use of dermal fillers has an increasing demand and popularity in the last few years in aesthetic medicine. Hyaluronic acid fillers are preferencing for people seeking a quick, temporary, and non-invasive procedure with minimal side effects. Dermal fillers have a very low risk profile, with side effects that include local and transient bruising and oedema. They are non-allergenic, non-carcinogenic, and generally represent a stable and cost-effective choice for the patient.
Hypersensitivity reactions can be easily classified into acute and delayed. Acute or type I hypersensitivity reactions have an onset within minutes to hours from injection time and they are immunoglobulin-E, mediated. They resulted in an anaphylactic reaction often presented as angioedema (Arron and Neuhaus, 2007). The mechanism of action of the delayed-type hypersensitivity reaction to dermal fillers is not yet clear, but is more likely to be multifactorial. Various aetiological factors have been suggested, including filler properties (cross-linking, rheology, etc.), injection technique (quantity, repeated injections, needle placement instead of cannula, intramuscular placement, etc.), and previous trauma or infection in the area of injection (Polack et al., 2020).
Delayed or type IV hypersensitivity reactions can take place from 24 hours to months after allergen contact. Delayed-type hypersensitivity reaction to dermal fillers is a very rare complication that usually presents as a tender, erythematous swelling. It is a cell-mediated hypersensitivity reaction caused by T-lymphocytes (Marshall et al., 2018). Delayed hypersensitivity reactions of type IV with dermal fillers aetiology is not yet fully understood . Various factors including filler properties (cross-linking, rheology, etc.), injection technique (quantity, repeated injections, needle placement instead of cannula, intramuscular placement, etc.), and previous trauma or infection in the area of injection have shown a correlation with this type of hypersensitivity reaction post-injection (Marshall et al., 2018; Turkmani et al., 2019a) . It is worth mentioning that various case reports of delayed hypersensitivity reactions to dermal fillers after influenza-type illnesses have been published (Rowland-Warmann, 2021; (Turkmani et al., 2019b)).
Five cases of a delayed-type hypersensitivity reaction to dermal fillers, with influenza-like symptoms occurring a couple of days before the onset of inflammationare reported (Bhojani-Lynch, 2017). Oral corticosteroids were used to treat the inflammation and hyaluronidase to suppress the persistent areas. Another 14 cases of delayed-type hypersensitivity reactions to fillers have been reported following an influenza-like illness (Turkmani et al., 2019a). Almost all cases were treated with oral corticosteroids, whereas a few persistent nodules needed intralesional hyaluronidase injections to be dissolved completely. There is no evidence of delayed hypersensitivity with hyaluronic acid fillers following influenza vaccination.
Clinical trials on COVID vaccines (Pfizer-BioNTech, Moderna, and AstraZeneca) have reported some cutaneous adverse reactions (Baden et al., 2021; Jackson et al., 2020; Mulligan et al., 2020; Ramasamy et al., 2020; Walsh et al., 2020). Rosacea and pruritus occurred in the AstraZeneca vaccine trials, wheareas transient urticaria and a case of angioedema were noted in the Moderna vaccine trial. It is important to note that no cases of delayed type hypersensitivity reactions are noted for the AstraZeneca or Pfizer vaccine. We can concluded that they either did not take place or the number of cases did not meet the minimum threshold incident value for the protocol for reporting adverse effects. Various cases of delayed-type hypersensitivity reactions related to viral vaccines and illnesses are reported.
The exact mechanism of triggering a delayed-type hypersensitivity reaction in our case needs be clarified, but the most probable hypothesis is the occuracne of an immunological reaction between the hyaluronic acid filler and the SARS-CoV-2 vaccine. Generally, hyaluronic acid fillers are very safe to use and only minimal side effects can occur. The aesthetic practitioner must be aware of those potential side effects, and precautions before and after use of these fillers as well the contraindications of using hyaluronic acid fillers should be taken into account. In view of the possibility of being more prone to possible filelr side affects in concomitance with a Covid vaccination, informed consents should include specific information about the possible side effects after influenza-like illnesses/vaccination and nowadays after Covid-19 infection and/ or after Covid 19 vaccination. The American Society of Dermatologic Surgery (ASDS) has issued a guidance report on SARS-CoV-2 vaccine-related adverse effects, and in particular on delayed dermal filler inflammatory reactions (American Society for Dermatological Surgery, 2021). An adequate patient’s information about these rare but possible adverse reactions before proceeding with the treatment is therefore necessary. Another important point is to inform patients treated with hyaluronic acid filler injections before their vaccination, about the rare possibility of a delayed-type hypersensitivity reaction, as part of the post-vaccination adverse effects.
Funding
This work did not receive any source of funding.
Ethical approval
Written informed consent for publication of the clinical details and images was obtained from the patient.
Conflict of interest
The authors declare no financial or other competing interests associated with this work [Au?2].
References
- American Society for Dermatological Surgery, 2021; retrived fromhttps://www.plasticsurgery.org/for-medical-professionals/covid19-member-resources/covid19-vaccine-dermal-fillers
- Arora G, Arora S, Talathi A, Kandhari R, Joshi V, Langar S, et al. Practice of Aesthetic Dermatology during the COVID-19 Pandemic: Recommendations by SIG Aesthetics (IADVL Academy) Indian Dermatol Online J. 2020;2011(4):534–539. doi: 10.4103/idoj.IDOJ_328_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arron S.T., Neuhaus I.M. Persistent delayed-type hypersensitivity reaction to injectable non-animal-stabilized hyaluronic acid. Journal of Cosmetic Dermatology. 2007;6(3):167–171. doi: 10.1111/j.1473-2165.2007.00331.x. [DOI] [PubMed] [Google Scholar]
- Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., Diemert D., Spector S.A., Rouphael N., Creech C.B., McGettigan J., Khetan S., Segall N., Solis J., Brosz A., Fierro C., Schwartz H., Neuzil K., Corey L., …, Zaks T. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. New England Journal of Medicine. 2021;384(5):403–416. doi: 10.1056/nejmoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhojani-Lynch T. Late-onset inflammatory response to hyaluronic acid dermal fillers. Plastic and Reconstructive Surgery - Global Open. 2017;5(12) doi: 10.1097/GOX.0000000000001532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanakis C.M. MDPI Multidisciplinary Digital Publishing Institute; 2020. The food systems in the era of the coronavirus (CoVID-19) pandemic crisis. In Foods (Vol. 9, Issue 4, p. 523) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Home - Johns Hopkins Coronavirus Resource Center. (n.d.). 2021 Retrieved November 4, 2021, from https://coronavirus.jhu.edu/
- Jackson L.A., Anderson E.J., Rouphael N.G., Roberts P.C., Makhene M., Coler R.N., McCullough M.P., Chappell J.D., Denison M.R., Stevens L.J., Pruijssers A.J., McDermott A., Flach B., Doria-Rose N.A., Corbett K.S., Morabito K.M., O'Dell S., Schmidt S.D., Swanson P.A., …, Beigel J.H. An mRNA Vaccine against SARS-CoV-2 — Preliminary Report. New England Journal of Medicine. 2020;383(20):1920–1931. doi: 10.1056/nejmoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J.S., Warrington R., Watson W., Kim H.L. An introduction to immunology and immunopathology. In Allergy, Asthma and Clinical Immunology (Vol. 2018;14 doi: 10.1186/s13223-018-0278-1. Issue Suppl 2)BioMed Central Ltd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan M.J., Lyke K.E., Kitchin N., Absalon J., Gurtman A., Lockhart S., Neuzil K., Raabe V., Bailey R., Swanson K.A., Li P., Koury K., Kalina W., Cooper D., Fontes-Garfias C., Shi P.Y., Türeci Ö., Tompkins K.R., Walsh E.E., …, Jansen K.U. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature. 2020;586(7830):589–593. doi: 10.1038/s41586-020-2639-4. [DOI] [PubMed] [Google Scholar]
- Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Marc Pérez, Moreira G., E D., Zerbini C., Bailey R., Swanson K.A., Roychoudhury S., Koury K., Li P., Kalina W.V., Cooper D., Frenck R.W., Hammitt L.L., Gruber …, W C. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. New England Journal of Medicine. 2020;383(27):2603–2615. doi: 10.1056/nejmoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasamy M.N., Minassian A.M., Ewer K.J., Flaxman A.L., Folegatti P.M., Owens D.R., Voysey M., Aley P.K., Angus B., Babbage G., Belij-Rammerstorfer S., Berry L., Bibi S., Bittaye M., Cathie K., Chappell H., Charlton S., Cicconi P., Clutterbuck E.A., …, Zizi D. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. The Lancet. 2020;396(10267):1979–1993. doi: 10.1016/S0140-6736(20)32466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland-Warmann M. Hypersensitivity reaction to Hyaluronic Acid Dermal filler following novel Coronavirus infection – a case report. Journal of Cosmetic Dermatology. 2021;20(5):1557–1562. doi: 10.1111/JOCD.14074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrotri M., Swinnen T., Kampmann B., Parker E.P.K. Vol. 9. Elsevier Ltd; 2021. An interactive website tracking COVID-19 vaccine development; pp. e590–e592. (The Lancet Global Health). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkmani M.G., Boulle K.De, Philipp-Dormston W.G. Delayed hypersensitivity reaction to hyaluronic acid dermal filler following influenza-like illness. Clinical, Cosmetic and Investigational Dermatology, 2019;12:277–283. doi: 10.2147/CCID.S198081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkmani M.G., Boulle K.De, Philipp-Dormston W.G. Delayed hypersensitivity reaction to hyaluronic acid dermal filler following influenza-like illness. Clinical, Cosmetic and Investigational Dermatology, 2019;12:277. doi: 10.2147/CCID.S198081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh E.E., Frenck R.W., Falsey A.R., Kitchin N., Absalon J., Gurtman A., Lockhart S., Neuzil K., Mulligan M.J., Bailey R., Swanson K.A., Li P., Koury K., Kalina W., Cooper D., Fontes-Garfias C., Shi P.-Y., Türeci Ö., Tompkins K.R., …, Gruber W.C. Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates. New England Journal of Medicine. 2020;383(25):2439–2450. doi: 10.1056/nejmoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotkin RH, Gout U, Sattler S, Piansay-Soriano ME, Wanitphakdeedecha R, Ghannam S, et al. Global Recommendations on COVID-19 Vaccines and Soft Tissue Filler Reactions: A Survey-Based Investigation in Cooperation With the International Society for Dermatologic and Aesthetic Surgery (ISDS) J Drugs Dermatol. 2021;120(4):374–378. doi: 10.36849/JDD.2021.6041. [DOI] [PubMed] [Google Scholar]