Abstract
Background
In accordance with expert guidance, patients have typically continued to receive treatment with subcutaneous interferon beta-1a (sc IFN β-1a) for relapsing multiple sclerosis (MS) during the COVID-19 pandemic.
Methods
We provide a summary of outcomes among sc IFN β-1a-treated patients with adverse events related to confirmed or suspected COVID-19, as reported to the Merck Global Patient Safety Database (as of 2 February 2021). Serious COVID-19-related adverse events (as classified by the reporting clinician) included those leading to hospitalization, admission to intensive care, or death. Outcomes were classified per usual pharmacovigilance practice.
Results
The evaluable cohort comprised 603 patients of median age 43 (range, 13–84) years and 75.1% were female. COVID-19 was experienced at a median of 33.0 (range, 0–321.8) months after start of treatment with sc IFN β-1a. A total of 136 (22.6%) patients experienced serious COVID-19 events, including 59 hospitalizations (4 patients admitted to intensive care) and 5 deaths (fatality rate, 0.8%). Regarding non-fatal outcomes, 47.8% of patients (289/603) with COVID-19 adverse events were recovered or recovering at time of analysis; similar findings were apparent for the serious and hospitalized cohorts.
Conclusion
Findings of this analysis from the Merck Global Patient Safety Database suggest that, compared with available statistics for the general population and those with MS, patients receiving sc IFN β-1a for treatment of relapsing MS have relatively low rates of serious disease and/or severe outcomes with COVID-19.
Keywords: COVID-19, Multiple sclerosis, Subcutaneous interferon beta-1a
Interferon-beta (IFN β) comprises a range of well-established treatment options for patients with relapsing multiple sclerosis (MS) (Goldschmidt and Hua, 2020). These agents were initially characterized for their antiviral activity and effects on cells of the innate immune system. More recently, they have been shown to affect almost all immune cell types, but the anti-inflammatory and immunomodulatory mechanisms underlying the effectiveness of IFN β therapy are still not fully understood (Dhib-Jalbut and Marks, 2010). Among available agents, subcutaneous (sc) IFN β-1a (Rebif®) has cumulative exposure of 1,809,458 patient-years since launch (to 8 January 2021; data on file, Merck). Many patients have continued to receive such therapy during the COVID-19 pandemic, in accordance with guidance from the MS International Federation (http://www.emsp.org/news-messages/coronavirus-disease-covid-19-and-multiple-sclerosis/) and expert opinion (Reyes et al., 2021). Against this background, numerous authors have published on patients with MS who acquired COVID-19 while on disease-modifying therapy (DMT), including interferons (Adamczyk-Sowa et al., 2021; Gemcioglua et al., 2020; Louapre et al., 2020; Simpson-Yap, 2021; Sormani et al., 2021).
In the current analysis, we report on outcomes among sc IFN β-1a-treated patients with adverse events related to confirmed or suspected COVID-19, as reported to the Merck Global Patient Safety Database (as of 2 February 2021). Serious COVID-19-related adverse events (as classified by the reporting clinician) included those leading to hospitalization, admission to intensive care, or death. Outcomes were classified as per usual pharmacovigilance practice, i.e. recovered/recovering, not recovered, or not reported (including missing data).
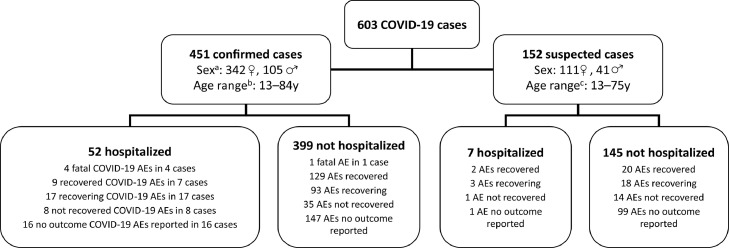
The evaluable cohort comprised 603 patients aged 13–84 (median, 43) years who were predominantly female (n = 453, 75.1%) (Fig. 1 ). A small proportion of patients were aged ≥60 years (n = 86, 14.3%). For 451 patients (74.8%), infection with SARS-CoV-2 virus was confirmed by diagnostic testing. The median time from start of treatment with sc IFN β-1a to onset of COVID-19 adverse events was 33.0 (range, 0–321.8) months. One hundred and thirty-six patients (22.6%) experienced serious COVID-19 events; there was a tendency for such patients to be older and more likely to be male compared to the total study population. Among serious cases there were 59 hospitalizations (four patients admitted to intensive care) and five deaths. Regarding nine fatalities among those with confirmed SARS-CoV-2 infection, ‘COVID-19 infection’ was noted as the cause of death in three cases, ‘COVID-19 pneumonia’ as the cause of death in the fourth case, and ‘COVID-19, sepsis, and bilateral pneumonia’ as the causes of death in the fifth case (cause of death was unknown in the remainder). Patient demographics for these subgroups, along with the total cohort, are summarized in Table 1 .
Fig. 1.
Overview of COVID-19 cases and outcomes in patients treated with subcutaneous interferon beta-1a, as of 2 February 2021.
In some instances, the number of AEs does not correspond to the number of cases because multiple events were reported.
Nine fatal cases were reported among patients with confirmed COVID-19: 5 fatal COVID-19 events in 5 cases (COVID-19 infection as the cause of death in 3 cases; COVID-19 pneumonia as the cause of death in the 4th case; and COVID-19, sepsis, and bilateral pneumonia as the causes of death in the 5th case) and 4 unknown causes of death in 4 cases. Among those with suspected COVID-19, there was 1 fatal case for which the case of death was not confirmed.
aUnknown sex for 4 patients; bUnknown age for 28 patients; cUnknown age for 14 patients. AEs, adverse events.
Table 1.
Demographics and patient characteristics for suspected or confirmed COVID-19 cases among patients treated with subcutaneous interferon beta-1a (as of 2 February 2021).
| All patients (n = 603) | |
|---|---|
| Median age, years (range) | 43 (13–84) |
| Aged ≥60 years, n (%) | 86 (14.3) |
| Not reported, n (%) | 26 (4.3) |
| Sex, n (%) Male Female Not reported |
146 (24.2) 453 (75.1) 4 (0.7) |
| Median time to COVID-19 from start of treatment, months (range) | 33.0 (0–321.8) |
| Serious COVID-19 cases (n = 136)a | |
| Median age, years (range) | 46.5 (13–84) |
| Aged ≥60 years, n (%) | 27 (19.9) |
| Not reported, n (%) | 7 (5.1) |
| Sex, n (%) Male Female Not reported |
39 (28.7) 96 (70.6) 1 (0.7) |
| Median time to COVID-19 from start of treatment, months (range) | 29.7 (0.1–203.4) |
| Hospitalized cases (n = 59) | |
| Median age, years (range) | 55.5 (13–84) |
| Aged ≥60 years, n (%) | 21 (35.6) |
| Not reported, n (%) | 3 (5.1) |
| Sex, n (%) Male Female |
21 (35.6) 38 (64.4) |
| Median time to COVID-19 from start of treatment, months (range) | 34.5 (1.3–198.4) |
| Admitted to intensive care (n = 4) | |
| Median age, years (range) | 57.0 (56–69) |
| Aged ≥60 years, n (%) | 1 (25.0) |
| Sex, n (%) Male Female |
1 (25.0) 3 (75.0) |
| Median time to COVID-19 from start of treatment, months (range) | 76.8 (29.7–194.8) |
| Fatal cases (n = 5) | |
| Median age, years (range) | 64.5 (50–84) |
| Aged ≥60 years, n (%) | 2 (40.0) |
| Not reported, n (%) | 1 (20.0) |
| Sex, n (%) Male Female |
3 (60.0) 2 (40.0) |
| Median time to COVID-19 from start of treatment, months (range) | 7.1 (4.0–10.3) |
Includes hospitalized patients (and those admitted to intensive care) and fatal cases.
Outcomes are summarized in Fig. 1 and Table 2 . For the evaluable cohort of 603 patients, 47.9% were recovered or recovering at the time of the analysis; the corresponding value was 44.8% (61/136) for patients categorized as having serious COVID-19.
Table 2.
Summary of outcomes (as of 2 February 2021).
| Outcome, n (%) | All patients (n = 603) | Serious COVID-19 cases | ||
|---|---|---|---|---|
| All cases (n = 136) | Hospitalized cases (n = 59) | Admitted to intensive care (n = 4) | ||
| Recovered/recovering | 289 (47.9) | 61 (44.8) | 29 (49.1) | 1 (25.0) |
| Not recovered/not resolved | 58 (9.6) | 20 (14.7) | 9 (15.2) | 1 (25.0) |
| Died | 5 (0.8) | 5 (3.7) | 4 (6.8) | – |
| Not reported/missing | 251 (41.6) | 50 (36.8) | 17 (28.8) | 2 (50.0) |
Previous reports have alluded to the fact that sc IFN β-1a-treated patients who acquire COVID-19 are generally not at greater risk of serious disease and/or severe outcomes compared with other DMTs in patients with MS (Louapre et al., 2020; Sharifian-Dorche et al., 2021; Sormani et al., 2021), and our findings are in general agreement. The COViMS Registry also noted that, compared with those not taking any DMT, patients receiving interferons had a non-significant decreased odds of hospitalization for COVID-19 and/or requirement for admission to intensive care and need of ventilator support (COViMS Registry, 2021).
The case fatality rate in our study – 0.8% for the total cohort – compares favorably with reported COVID-19-related fatality rates for MS patients globally (3.5%) (Simpson-Yap, 2021) and in the US (2.7%) (COViMS Registry, 2021). In this regard, the cumulative COVID-19 fatality rate for the worldwide general population at the time of our database lock was 2.2% (2 February 2021; https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/). However, results should be caveated by the fact that the underlying characteristics of our MS population – typically younger and female – is different to the population of patients who tend to have worse outcomes with COVID-19 (i.e. older, male, co-morbidities). Moreover, our dataset is limited by the absence of findings for disability (i.e. Expanded Disability Status Scale score), which is expected to be higher in those with serious COVID-19 based on other reports (Arrambide et al., 2021; Chaudhry et al., 2021).
Given that our analysis is drawn from a safety database of voluntary pharmacovigilance reports, certain limitations need to be considered. For example, data can be incomplete. This is highlighted by outcomes not being reported or missing for up to 50% of patients across the cohorts at the time that the data were extracted from the database. The follow-up time is also variable, so patients reported at the beginning of the pandemic have had more time for data to be completed compared to patients reported close to the time of data extraction. Information on presenting symptoms and co-morbidities/confounding factors (including disability, as outlined above) that could have influenced outcomes is also scant. These limitations aside, and accepting that results of our analysis are potentially influenced by data that are not known, findings are in general agreement with those reported from international and national registries of COVID-19 among patients receiving interferons for treatment of MS (Louapre et al., 2020; Sormani et al., 2020).
With the advent of vaccines for COVID-19, there has been considerable debate concerning their utility, safety, and effectiveness among the MS community. If at all possible, such vaccination is recommended for patients with MS (Centonze, 2021). It was therefore of interest to review the safety database for case reports of concurrent use of sc IFN β-1a and COVID-19 vaccines. A total of 30 reports were identified (detailing 124 adverse events), to 2 March 2021. While the precise type of COVID-19 vaccine was rarely reported, and data are not available regarding any measure of efficacy of chosen vaccines (e.g. antibody formation), there were no fatal or life-threatening adverse events. Moreover, no new emerging safety issue has been identified relative to the known safety profiles of either sc IFN β-1a or approved COVID-19 vaccines.
In summary, findings of this analysis from the Merck Global Patient Safety Database are consistent with existing reports and suggest that patients receiving sc IFN β-1a for treatment of relapsing MS have relatively low rates of serious disease and/or severe outcomes with COVID-19 compared with available statistics for the general population and those with MS.
Author contributions
MSF: data interpretation. DJ and MT: Study design, data analysis, and interpretation. ZM: Study design, data curation, analysis, and interpretation. AS: Data analysis and interpretation. All authors contributed to writing of the article and in the decision to submit for publication.
Data availability statement
Any requests for data by qualified scientific and medical researchers for legitimate research purposes will be subject Data Sharing Policy of Merck. All requests should be submitted in writing to the data sharing portal of Merck https://www.merckgroup.com/en/research/our-approach-to-research-and-development/healthcare/clinical-trials/commitment-responsible-data-sharing.html. When Merck has a co-research, co-development, or co-marketing or co-promotion agreement, or when the product has been out-licensed, the responsibility for disclosure might be dependent on the agreement between parties. Under these circumstances, Merck will endeavor to gain agreement to share data in response to requests.
Funding
This study was funded by Merck (CrossRef Funder ID: 10.13039/100009945). As noted under author contributions, the sponsor was involved with study design and in the curation, analysis, and interpretation of data.
Declaration of Competing Interest
MSF has received honoraria or consultation fees from Actelion (Janssen/J&J), Alexion, Biogen, Celgene (BMS), EMD Inc., Canada (an affiliate of Merck KGaA), EMD Serono, Inc., USA (an affiliate of Merck KGaA), Merck, Novartis, Sanofi-Genzyme, Roche, and Teva Canada Innovation; has received research support unrelated to this study from EMD Inc., Canada (an affiliate of Merck KGaA), Roche, and Sanofi-Genzyme Canada; was a member of a company advisory board, board of directors, or other similar group for Actelion (Janssen/J&J), Alexion, Atara Biotherapeutics, Bayer, Biogen, Celgene (BMS), Clene Nanomedicine, GRI Bio, Magenta Therapeutics, Merck, Novartis, Roche, Sanofi-Genzyme, and Teva Canada Innovation; and has been a participant in a company sponsored speaker's bureau for EMD Serono, Inc., USA (an affiliate of Merck KGaA), and Sanofi-Genzyme.
DJ is an employee of Merck Serono Ltd, Feltham, UK (an affiliate of Merck KGaA).
ZM is an employee of Merck spol. s r.o., Bratislava, Slovakia (an affiliate of Merck KGaA).
MT and AS are employees of Merck Healthcare KGaA, Darmstadt, Germany.
Acknowledgments
Medical writing assistance was provided by Steve Winter of inScience Communications, Springer Healthcare Ltd, UK, and was funded by Merck Healthcare KGaA, Darmstadt, Germany.
References
- Adamczyk-Sowa M., et al. SARS-CoV-2/COVID-19 in multiple sclerosis patients receiving disease-modifying therapy. Clin. Neurol. Neurosurg. 2021;201 doi: 10.1016/j.clineuro.2020.106451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrambide G., et al. SARS-CoV-2 infection in multiple sclerosis: results of the Spanish Neurology Society Registry. Neurol. Neuroimmunol. Neuroinflamm. 2021;8:e1024. doi: 10.1212/NXI.0000000000001024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centonze D., et al. Disease-modifying therapies and SARS-CoV-2 vaccination in multiple sclerosis: an expert consensus. J. Neurol. 2021 doi: 10.1007/s00415-021-10545-2. ; Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry F., et al. Review of the COVID-19 risk in multiple sclerosis. J. Clin. Immunol. 2021;3:68–77. doi: 10.33696/immunology.3.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COViMS Registry . 2021. The COViMS Database Public Data Update. Available at: https://www.covims.org/current-data (accessed 12 May 2021) [Google Scholar]
- Dhib-Jalbut S., Marks S. Interferon-beta mechanisms of action in multiple sclerosis. Neurology. 2010;74(Suppl. 1):S17–S24. doi: 10.1212/WNL.0b013e3181c97d99. [DOI] [PubMed] [Google Scholar]
- Gemcioglua E., et al. Are type 1 interferons treatment in multiple sclerosis as a potential therapy against COVID-19? Mult. Scler. Relat. Disord. 2020;42 doi: 10.1016/j.msard.2020.102196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt C.H., Hua L.H. Re-evaluating the use of IFN-β and relapsing multiple sclerosis: safety, efficacy and place in therapy. Degener. Neurol. Neuromuscul. Dis. 2020;10:29–38. doi: 10.2147/DNND.S224912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louapre C., et al. Clinical characteristics and outcomes in patients with coronavirus disease 2019 and multiple sclerosis. JAMA Neurol. 2020;77:1079–1088. doi: 10.1001/jamaneurol.2020.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes S., et al. Update on the management of multiple sclerosis during the COVID-19 pandemic and post pandemic: an international consensus statement. J. Neuroimmunol. 2021;357 doi: 10.1016/j.jneuroim.2021.577627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifian-Dorche M., et al. COVID-19 and disease-modifying therapies in patients with demyelinating diseases of the central nervous system: a systematic review. Mult. Scler. Relat. Disord. 2021;50 doi: 10.1016/j.msard.2021.102800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson-Yap S., et al. medRxiv preprint; 2021. Associations of DMT therapies with COVID-19 severity in multiple sclerosis. doi:(accessed 12 May 2021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sormani M.P. An Italian programme for COVID-19 infection in multiple sclerosis. Lancet Neurol. 2020;19:481–482. doi: 10.1016/S1474-4422(20)30147-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sormani M.P., et al. Disease-modifying therapies and coronavirus disease 2019 severity in multiple sclerosis. Ann. Neurol. 2021;21:780–789. doi: 10.1002/ana.26028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Any requests for data by qualified scientific and medical researchers for legitimate research purposes will be subject Data Sharing Policy of Merck. All requests should be submitted in writing to the data sharing portal of Merck https://www.merckgroup.com/en/research/our-approach-to-research-and-development/healthcare/clinical-trials/commitment-responsible-data-sharing.html. When Merck has a co-research, co-development, or co-marketing or co-promotion agreement, or when the product has been out-licensed, the responsibility for disclosure might be dependent on the agreement between parties. Under these circumstances, Merck will endeavor to gain agreement to share data in response to requests.