Highlights
-
•
Patients suffering from both breast and endometrial cancer seem to have unique characteristics.
-
•
The sequence of occurrence of malignancies appears to have significance.
-
•
In patients that endometrial cancer was diagnosed as a second tumor, it tended to present in a more aggressive form.
-
•
In this group, a longer time interval between diagnoses might offer diagnostic and prophylactic opportunities.
-
•
No correlation was found to tamoxifen usage among patients.
Keywords: Breast Cancer, Endometrial Cancer, Uterine serous carcinoma, BRCA, Tamoxifen
Abstract
Objective
To examine whether patients with both breast cancer (BC) and endometrial cancer (EC) have different features of disease, and whether the sequence of appearance of these tumors is correlated with a more aggressive course.
Methods
A retrospective, multi-center observational cohort study of patients treated in two tertiary medical centers between 2014 and 2020. Files of patients who had a co-diagnosis of BC and EC were reviewed and clinical, epidemiological, pathological and genetic characteristics were collected.
Results
67 patients with a co-diagnosis of both malignances were divided into two groups according to primary tumor diagnosis: BC first group (43/67, 64%) and EC first group (24/67, 36%). The time interval between diagnosis of malignancies was significantly longer in the BC first group (mean 144.5 months vs. 67 months, p < 0.05). BRCA mutations were found in higher numbers in the BC first group (27.5% vs. 9.5%, p = 0.18). A significantly higher number of patients in the BC first group had uterine serous carcinoma (USC) histology (44% vs. 12.5%, p < 0.05). This was independent of tamoxifen usage among patients (OR 0.65, 95% CI 0.17–2.49).
Conclusions
In patients suffering from both BC and EC, the sequence of occurrence of malignancies has relevance: When EC presents as a second primary tumor, it tends to present in a more aggressive form, independent of previous tamoxifen use. The time interval between the diagnosis of malignancies was significantly longer in this group, offering an opportunity to improve preventive measures to decrease the likelihood of a subsequent lethal second cancer.
1. Introduction
Endometrial cancer (EC) is the most common malignancy of the female tract and the fourth most common malignancy overall in women, responsible for 6% of new cancer cases in females in the USA in 2019. Breast cancer (BC) is the most common female malignancy overall, accounting for 30% of new cancer cases in females diagnosed in the USA in 2019 (Siegel et al., 2019).
A potential association between BC and EC has been a subject of interest for many years, especially considering shared etiologies, risk factors and a potential genetic predisposition. Some of the notable risk factors that contribute to the occurrence of both cancers include age (Liang, 2011), exposure to exogenous estrogens or endogenous hyper-estrogenic status associated with nulliparity, early age at menarche, late‐onset menopause (Ali, 2014, Kelsey et al., 1993) and obesity (Webb, 2015).
Several investigators have examined patients who have suffered from both malignancies. Mellemkjær et al. showed that patients with BC had a 25% increased risk of developing a second cancer, compared with women without BC, and that EC was one of the most common types to develop (Mellemkjær, 2006). Further studies demonstrated that the occurrence of metachronous tumors was even higher in younger patients (Soliman, 2005). In addition, young patients with a personal history of BC might have a significantly elevated risk of developing the more aggressive sub-type of EC, uterine serous carcinoma (USC) (Liang, 2011). In support of this, Gehrig et al. found that women with BC who subsequently developed EC had a 2.6-fold increased risk of developing USC compared to the less aggressive sub-type of endometrioid carcinoma. Notably, this increased risk was independent of tamoxifen exposure by the women (Gehrig, 2004).
Tamoxifen is a selective estrogen receptor modulator (SERM) which has the potential to reduce risk of breast cancer recurrence and improve survival for women with estrogen receptor (ER) positive disease (Jordan, 1997). However, its’ pro-estrogenic effect on the endometrium has been known to be associated with an increased risk of endometrial cancer as high as two-fold to seven-fold (Magriples et al., 1993). While some studies have implied of an elevated risk of USC and high-grade endometrial carcinoma following tamoxifen exposure (Magriples et al., 1993, Lavie et al., 2008, Bland, 2009, Bergman et al., 2000), others have demonstrated that this risk is equal to developing the less aggressive and low-grade types (Barakat et al., 1994, Fisher, 1994).
Family history of any cancer appears to increase EC risk in women with prior BC, suggesting genetic risk factors for EC. Established genetic factors include high‐risk pathogenic variants in the DNA mismatch repair (MMR) genes causing Lynch Syndrome, and very rarely, germline loss‐of‐function variants in the PTEN tumor suppressor gene causing Cowden Syndrome (Johnatty, 2018, Spurdle et al., 2017, 2017,).
With regard to the BRCA mutations, the evidence is more conflicting: while some studies have not found an elevated risk for EC among BRCA carriers (Levine, 2001), others have pointed to an increased overall risk, especially to the aforementioned subtype of USC (Shu et al., 2016).
As these issues continue to be controversial, we aimed to further examine them and describe this unique patient population and their disease. Our purpose was to divide the patients into groups according to the primary tumor diagnosis (breast cancer first versus endometrial cancer first), and to investigate and compare epidemiological, pathological, clinical and genetic characteristics of both groups.
2. Methods
2.1. Study protocol and Population:
We performed a retrospective, multi-center observational cohort study at Tel-Aviv Sourasky Medical Center (TASMC) and at Sheba Medical center. Data regarding women with a co-diagnosis of breast cancer and endometrial cancer above 18 years treated at TASMC and Sheba between 2014 and 2020 was collected. The computerized files of these patients were analyzed for clinical and pathological characteristics of disease and medical history.
The study was approved by the local Helsinki regulatory ethics committee (Identifier: 0111–18-TLV).
Inclusion criteria included women above 18 years with a diagnosis of breast cancer and a diagnosis of endometrial cancer between 1980 and 2020, who were actively treated in one of the aforementioned medical centers between June 2014 and September 2020.
Exclusion criteria included women treated outside of TASMC and Sheba, women under 18 years and women whose charts were missing significant data. Clinical data collected included demographics, age, gender, family history, date of diagnosis, comorbidities, BMI, genetic alterations, use of tamoxifen and other hormonal therapies, biology, pathology and staging of the tumors and survival data. Hospitalization records and mortality were extracted from computerized patient charts and the population registry bureau.
2.2. Statistical Analysis:
Continuous variables were calculated as mean ± standard deviation and were tested for significance using the Mann-Whitney test. Categorical variables were expressed as frequency and percentages and tested with the Chi-Square test or the Fisher Exact test (as appropriate). All tests were two-tailed and a p-value of < 0.05 was considered significant. The analysis was performed using the R version 4.0.5.
3. Results
A total of 67 women with a co-diagnosis of breast and endometrial cancer were treated at TASMC and Sheba Medical Center between June 2014 and September 2020.
Patients were divided into two groups: Breast cancer (BC) first group (43/67, 64.18%), included patients who received the diagnosis of BC first and endometrial cancer (EC) was diagnosed later. EC first group (24/67, 35.82%), included patients in whom EC was diagnosed first and BC was diagnosed later. The two groups were compared by epidemiological, genetic, pathological and clinical features.
Epidemiological characteristics and comorbidities of the study groups are presented in Table 1.
Table 1.
Epidemiological & comorbidity differences between study groups.
| BC first group n = 43 |
EC first group n = 24 |
P | |
|---|---|---|---|
| Age of diagnosis of first cancer (years), mean ± SD | 54.83 ± 11.84 | 59.27 ± 10.5 | 0.14 |
| Interval between cancers (months), mean ± SD |
144.5 ± 109.2 | 67.08 ± 65.28 | 0.0026 |
| Births, mean ± SD | 2.41 ± 1.18 | 2.2 ± 1.58 | 0.44 |
| BMI (kg/m2), mean ± SD | 28.78 ± 5.86 | 29.28 ± 4.684 | 0.57 |
| Smoking, n (%) | 8 (18.6%) | 3 (12.5%) | 0.73 |
| Ashkenazi Jewish descent, n (%) | 22/26 (84.6%) | 16/20 (80%) | 0.71 |
| Family history of cancer, n (%) | 26/43 (60.4%) | 11/20 (55%) | 0.78 |
| Hypertension, n (%) | 20 (46.5%) | 11 (45.83%) | 1 |
| Diabetes, n (%) | 11 (25.58%) | 10 (41.67%) | 0.27 |
| Hypothyroidism, n (%) | 10/42 (23.8%) | 1/23 (4.35%) | 0.08 |
| Pre-menopause at first cancer diagnosis, n (%) | 11 (25.6%) | 3 (12.5%) | 0.34 |
SD: standard deviation, n: Number, BMI – body mass index.
The mean age of diagnosis of women who received a BC diagnosis first was 54.8 years, while the women who received an EC diagnosis first had a mean age of 59.2 years at their initial cancer diagnosis (p = 0.14). The mean time of diagnosis from BC to diagnosis of EC in the BC first group was 144.5 months, while the mean time of diagnosis from EC to a diagnosis of BC in the EC first group was 67.08 (p = 0.002).The mean births per women was similar between both groups, as was the mean BMI index, smoking rates, the percentage of patients of Ashkenazi Jewish descent and the rates of a history of cancer in the patient’s family.
Regarding comorbidities, hypothyroidism rates (23.8% in the BC first group vs. 4.35% in the EC first group, p = 0.08) and diabetes rates (25.58% in the BC first group vs. 41.67% in the EC first group, p = 0.27) differed between the groups, while hypertension rates were similar. Furthermore, in the BC first group 11 women (25.6%) were pre-menopausal when diagnosed with BC, while in the EC first group 3 women (12.5%) women were pre-menopausal when diagnosed with EC (p = 0.34).
Genetic differences between groups were evaluated, as shown in Table 2. BRCA1/2 gene alterations were more common among the BC first group versus the EC first group in a non-significant trend (27.5% versus 9.52% respectively, p = 0.18). Other genetic alterations were equally common among both groups (PTEN mutation was found in one patient in each group, CHEK2 mutation was found in one patient in the BC group, ATM mutation was found in one patient in the EC first group).
Table 2.
Genetic characteristics of study groups.
| BC first group n = 43 | EC first group n = 24 |
P | |
|---|---|---|---|
| BRCA mutation status, n (%) | 11/40 (27.5%) | 2/21 (9.5%) | 0.18 |
| PTEN | 1 | 1 | n/a |
| CHEK2 | 1 | 0 | n/a |
| ATM | 0 | 1 | n/a |
n: Number.
Fig. 1 depicts the EC pathology of the patients with a BRCA mutation in the BC first group: Out of 11 mutated patients in that group, 8 had a serous type (73%), 2 had an endometroid type (18%) and one patient (9%) had a clear cell type.
Fig. 1.
EC pathology of BRCA mutated patients.
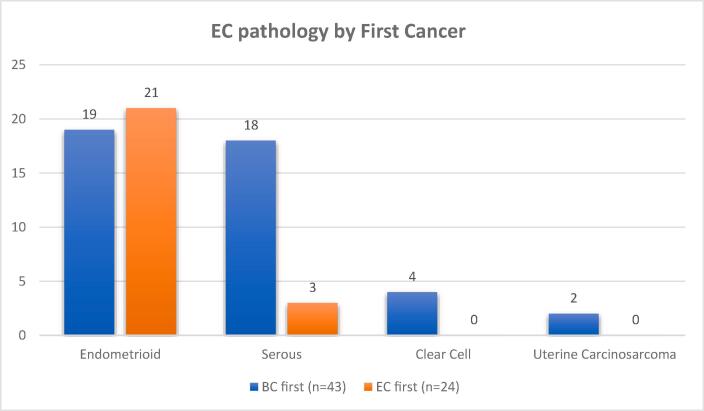
Regarding the pathological type of endometrial cancer diagnosed, there was a difference between the two groups, as shown in Fig. 2: In the BC first group, 19 women had endometrioid type (44.19%), 18 women had serous type (41.86%), 4 women had clear cell type (9.5%) and 2 women had uterine sarcoma (4.25%). In the EC first group, 21 women had endometrioid type (87.5%), 3 women had serous type (12.5%) and no women had clear cell type. The difference between the two groups regarding endometrioid and serous pathology was significant, for a p value of 0.0037.
Fig. 2.
EC pathology by First Cancer.
Out of the 36 patients in the BC first group with available data, 15 patients (41.67%) did not receive tamoxifen therapy at all before their EC diagnosis, while 6 of these patients received therapy only with an aromatase inhibitor. Tamoxifen usage was defined as ever-used among study parcipitants, with a mean time of usage of 5.21 years (range 0.5–10 years).
One of the patients diagnosed with endometrial sarcoma was exposed to tamoxifen, while the other wasn’t. As further shown in Table 3, the use of tamoxifen in this group was not correlated with subsequent development of EC of serous subtype (OR 0.65, 95% CI 0.17–2.49).
Table 3.
Tamoxifen exposure in the BC first group.
| Endometrioid | Serous | total | |
|---|---|---|---|
| Tamoxifen. n (%) | 12 (63.1%) | 9 (52.9%) | 21 |
| No tamoxifen, n (%) | 7 (36.9%) | 8 (47.1%) | 15 |
| Total, n | 19 | 17 | 36 |
n: Number.
Regarding the receptor status types of breast cancer diagnosed, no major differences were observed between the two groups, with similar rates of hormone positive and HER-2 positive diseases in both groups, and slightly higher rates of triple negative type breast cancer seen in the BC first group (5 patients in the BC first groups vs. 1 patient in the EC first group, p = 0.67).
As shown in Table 4, a significantly higher number of patients were diagnosed with advanced stages (stages III-IV) of endometrial cancer in the BC first group than in the EC first group (30.77% vs. 5.6%, p = 0.04). On the contrary, there was no difference between the two groups regarding breast cancer stage at diagnosis.
Table 4.
Cancer stage at diagnosis.
| BC first group n = 43 |
EC first group n = 24 |
P | |
|---|---|---|---|
| EC stage at diagnosis | |||
| Stages I-II | 27/39 (69.2%) | 17/18 (94.4%) | |
| Stages III-IV | 12/39 (30.8%) | 1/18 (5.6%) | 0.044 |
| BC stage at diagnosis | |||
| Stages I-II | 29/34 (85.3%) | 20/24 (83.3%) | |
| Stages III-IV | 5/34 (14.7%) | 4/24 (16.7%) | n/s |
n: Number, n/s = non significant.
4. Discussion
Multiple trials have examined the association between BC and EC and their appearance in the same patient (Liang, 2011, Gehrig, 2004, Magriples et al., 1993, Johnatty, 2018), however to the best of our knowledge this is the first study thus far that stratified this population according to the appearance of the first cancer and compared the two groups regarding epidemiological, pathological, genetic and clinical variables.
Demographic characteristics and comorbidity rates were found to be similar between both groups, apart from hypothyroidism that was higher in the BC first group.
Our study illustrated a higher percentage of patients with a BRCA mutation in the BC first group than in the EC first group, although this difference was not statistically significant. The majority of these patients (73%) had a serous histology, in further support of increasing evidence that uterine serous carcinoma (USC) could be a manifestation of the hereditary breast/ovarian cancer syndrome (Lavie, 2000). This higher percentage of BRCA mutations might partially explain the larger number of USC cases in the BC first group. Other genetic alterations were found in small numbers in both groups.
Most ECs are of endometrioid histology and carry a favorable outcome, with a 5‐year overall survival (OS) rate of more than 85%. USC is a rare subtype, representing less than 10% of all ECs (del Carmen et al., 2012). However, it is important to note that patients with USC have a poor prognosis, with 5-year OS rates of 20–25%, and USC accounts for more than 50% of relapses and deaths attributed to EC. This is thought to be caused by more advanced disease at diagnosis and from high rates of distant recurrences (del Carmen et al., 2012). A link between BC and USC was reported in previous trials: Chan et al. found that the proportional incidence of USC was significantly higher in women with a history of BC (9.4% vs. 6.3%, p < 0.001) (Chan, 2006). In reverse, Geisler et al. reported an increased BC risk in patients with a diagnosis of USC compared to patients with endometroid carcinoma (25% vs. 3.2%, p = 0.001) (Geisler, 2001). In this study, we were able to demonstrate a link between BC and USC and their sequence of appearance, with a significantly higher percentage of USC cases occurring after the diagnosis of BC, compared to vice versa. This was not found to be related to tamoxifen usage among the patients. Moreover, in the group that EC was diagnosed as a second tumor, it presented at a more advanced stage.
On the contrary, besides slightly higher rates of triple negative breast cancer in the BC first group, there was no notable differences between the groups regarding BC pathological features. BC stage also did not seem to differ between the two groups.
We conclude that in patients diagnosed with both BC and EC, the sequence of occurrence of malignancies has clinical significance. In the group of patients that EC presents as a second primary malignancy, it tends to present in a more aggressive form, as it is more likely to be diagnosed as USC and at a more advanced stage. Our study also demonstrated that this was independent of the use of tamoxifen as adjuvant endocrine therapy, in support of previous reports that came to the same conclusion (Gehrig, 2004, Barakat et al., 1994, Fisher, 1994). This study strengthened the notion that women with a prior diagnosis of BC are at increased risk of developing USC as opposed to an endometrioid subtype (Gehrig, 2004). Despite these findings, the time interval between the diagnosis of malignancies was significantly longer in the group that presented first with BC. This possible delay in diagnosis might be attributed to the lack of symptoms of vaginal bleeding in these women, as previous studies have revealed that 98% of women with endometrioid tumor histology present with post-menopausal bleeding compared to only 43% of women with serous tumor histology (Gehrig, 2004). Nonetheless, this time lag may also offer an opportunity to augment diagnostic and prophylactic measures and improve the clinical outcomes of these patients, as USC has a disproportional hazardous effect. It is important to note that while preventive hysterectomy is being raised as an option in BRCA carriers (Shu et al., 2016), a recent large prospective study by Kitston et al. reinforced the notion that there is no significant benefit to preventive hysterectomy in these patients at present time (Kitson, 2020).
Our study is not without limitations. First, we were limited by its retrospective design. Data was not available for all variables of interest, mainly those regarding genetic alterations other than BRCA, such as the genes associated with Lynch syndrome. Incomplete documentation in medical records and immature data prevented successfully expanding the analysis to other variables, such as survival and additional comorbidities. Nonetheless, it is important to note that prospective information on the relationship of two cancers is difficult to ascertain because clinical trials have typically excluded women with a previous history of a malignancy. As such, retrospective data is the only source for studying double-primary malignancies. Furthermore, our study had a small sample size which limited its statistical power. One of the study strengths is that it was conducted at two major tertiary medical centers, treating a large and diverse patient population. However, this fact might also create a potential bias that may be related to patient referral patterns, as patients with tumors of more aggressive histology and stage are often referred to a tertiary center, creating a disproportional case presentation.
To summarize, when comparing patients who suffer both from BC and EC and stratifying them according to sequence of cancer appearance, we found significant differences regarding pathological and clinical characteristics between the groups, and possible genetic differences. More robust evidence is needed to elicit the full relationship between the malignancies and their sequence of appearance, as the ultimate goal is to better inform and counsel woman who already had one malignancy about the likelihood of a subsequent devastating and lethal second cancer diagnosis in her lifetime.
CRediT authorship contribution statement
Tomer Stern: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing - original draft, Writing - review & editing. Shira Peleg Hasson: Conceptualization, Investigation, Methodology, Supervision, Project administration, Writing - review & editing. Akram Saad: Data curation, Investigation. Keren Levanon: Data curation, Investigation. Nadav Michaan: Data curation, Investigation. Ido Laskov: Data curation, Investigation. Ido Wolf: Supervision, Project administration, Validation. Tamar Safra: Conceptualization, Investigation, Methodology, Supervision, Project administration, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2019. CA. Cancer J. Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- Liang S.X. Personal history of breast cancer as a significant risk factor for endometrial serous carcinoma in women aged 55 years old or younger. Int. J. Cancer. 2011;128:763–770. doi: 10.1002/ijc.25395. [DOI] [PubMed] [Google Scholar]
- Ali A.T. Reproductive factors and the risk of endometrial cancer. Int. J. Gynecol. Cancer. 2014;24:384–393. doi: 10.1097/IGC.0000000000000075. [DOI] [PubMed] [Google Scholar]
- Kelsey J.L., Gammon M.D., John E.M. Reproductive factors and breast cancer. Epidemiol Rev. 1993;15:36–47. doi: 10.1093/oxfordjournals.epirev.a036115. [DOI] [PubMed] [Google Scholar]
- Webb P.M. Environmental (nongenetic) factors in gynecological cancers: update and future perspectives. Futur. Oncol. 2015;11(2):295–307. [PubMed] [Google Scholar]
- Mellemkjær L. Risk of second cancer among women with breast cancer. Int. J. Cancer. 2006;118:2285–2292. doi: 10.1002/ijc.21651. [DOI] [PubMed] [Google Scholar]
- Soliman P.T. Risk Factors for Young {Premenopausal} Women {With} Endometrial {Cancer} Obstet. & Gynecol. 2005;105:575–580. doi: 10.1097/01.AOG.0000154151.14516.f7. [DOI] [PubMed] [Google Scholar]
- Gehrig P.A. Association between uterine serous carcinoma and breast cancer. Gynecol. Oncol. 2004;94:208–211. doi: 10.1016/j.ygyno.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Jordan V.C. Tamoxifen: the Herald of a New {Era} of Preventive {Therapeutics} J Natl Cancer Inst. 1997;89(11):747–749. doi: 10.1093/jnci/89.11.747. [DOI] [PubMed] [Google Scholar]
- Magriples U., Naftolin F., Schwartz P.E., Carcangiu M.L. High-grade endometrial carcinoma in tamoxifen-treated breast cancer patients. JCO. 1993;11(3):485–490. doi: 10.1200/JCO.1993.11.3.485. [DOI] [PubMed] [Google Scholar]
- Lavie O., Barnett-Griness O., Narod S.A., Rennert G. The risk of developing uterine sarcoma after tamoxifen use. Int. J. Gynecol. Cancer. 2008;18:352–356. doi: 10.1111/j.1525-1438.2007.01025.x. [DOI] [PubMed] [Google Scholar]
- Bland A.E. Relationship between tamoxifen use and high risk endometrial cancer histologic types. Gynecol. Oncol. 2009;112:150–154. doi: 10.1016/j.ygyno.2008.08.035. [DOI] [PubMed] [Google Scholar]
- Bergman L., Beelen M.LR., Gallee M.PW., Hollema H., Benraadt J., van Leeuwen F.E. Risk and prognosis of endometrial cancer after tamoxifen for breast cancer. Lancet. 2000;356(9233):881–887. doi: 10.1016/s0140-6736(00)02677-5. [DOI] [PubMed] [Google Scholar]
- Barakat R.R., Wong G., Curtin J.P., Vlamis V., Hoskins W.J. Tamoxifen use in breast cancer patients who subsequently develop corpus cancer is not associated with a higher incidence of adverse histologic features. Gynecol. Oncol. 1994;55:164–168. doi: 10.1006/gyno.1994.1271. [DOI] [PubMed] [Google Scholar]
- Fisher B. Endometrial cancer in tamoxifen-treated breast cancer patients: Findings from the national surgical adjuvant breast and bowel project (NSABP) B-14. J. Natl. Cancer Inst. 1994;86:527–537. doi: 10.1093/jnci/86.7.527. [DOI] [PubMed] [Google Scholar]
- Johnatty S.E. Risk and prognostic factors for endometrial carcinoma after diagnosis of breast or Lynch}-associated cancers—{A population-based analysis. Cancer Med. 2018;7:6411–6422. doi: 10.1002/cam4.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurdle A.B., Bowman M.A., Shamsani J., Kirk J. Endometrial cancer gene panels: clinical diagnostic vs research germline DNA testing. Mod. Pathol. 2017, 2017,;308(30):1048–1068. doi: 10.1038/modpathol.2017.20. [DOI] [PubMed] [Google Scholar]
- Levine D.A. Risk of Endometrial {Carcinoma} Associated with BRCA {Mutation} Gynecol. Oncol. 2001;80:395–398. doi: 10.1006/gyno.2000.6082. [DOI] [PubMed] [Google Scholar]
- Shu C.A., Pike M.C., Jotwani A.R., Friebel T.M., Soslow R.A., Levine D.A., Nathanson K.L., Konner J.A., Arnold A.G., Bogomolniy F., Dao F., Olvera N., Bancroft E.K., Goldfrank D.J., Stadler Z.K., Robson M.E., Brown C.L., Leitao M.M., Abu-Rustum N.R., Aghajanian C.A., Blum J.L., Neuhausen S.L., Garber J.E., Daly M.B., Isaacs C., Eeles R.A., Ganz P.A., Barakat R.R., Offit K., Domchek S.M., Rebbeck T.R., Kauff N.D. Uterine Cancer {After} Risk}-{Reducing {Salpingo}-oophorectomy Without {Hysterectomy} in Women {With} BRCA {Mutations} JAMA Oncol. 2016;2(11):1434. doi: 10.1001/jamaoncol.2016.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavie O. BRCA1 germline mutations in women with uterine serous papillary carcinoma. Obstet. Gynecol. 2000;96:28–32. doi: 10.1016/s0029-7844(00)00851-6. [DOI] [PubMed] [Google Scholar]
- del Carmen M.G., Birrer M., Schorge J.O. Uterine papillary serous cancer: A review of the literature. Gynecologic Oncology. 2012;127(3):651–661. doi: 10.1016/j.ygyno.2012.09.012. [DOI] [PubMed] [Google Scholar]
- Chan J.K. Breast cancer followed by corpus cancer: Is there a higher risk for aggressive histologic subtypes? Gynecol. Oncol. 2006;102:508–512. doi: 10.1016/j.ygyno.2006.01.014. [DOI] [PubMed] [Google Scholar]
- Geisler J.P. Papillary serous carcinoma of the uterus: Increased risk of subsequent or concurrent development of breast carcinoma. Gynecol. Oncol. 2001;83:501–503. doi: 10.1006/gyno.2001.6445. [DOI] [PubMed] [Google Scholar]
- Kitson S.J. BRCA1 and BRCA2 pathogenic variant carriers and endometrial cancer risk: A cohort study. Eur. J. Cancer. 2020;136:169–175. doi: 10.1016/j.ejca.2020.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]