Abstract
Diagnostic methods based on SARS-CoV-2 antigen detection are a promising alternative to SARS-CoV-2 RNA amplification. We evaluated the automated chemiluminescence-based Lumipulse® G SARS-CoV-2 Ag assay as compared to real time assays (combined results from RT-PCR Allplex™ SARS-CoV-2 assay and Easy SARS-CoV-2 WE kit) on 513 nasopharyngeal swabs (NPS). Among these, 53.6% resulted positive to RT-PCR, considered as the reference test. Compared to the reference test, overall sensitivity and specificity of Lumipulse® G SARS-CoV-2 Ag assay were 84.0%, and 89.1%, respectively, and overall agreement between the antigen and molecular assays was substantial (κ = 0.727). When stratifying samples into groups based on ranges of RT-PCR cycle threshold (Ct), the antigen test sensitivity was >95% for samples with Ct <30.
Linear regression analysis showed strong and highly significant correlation between the Lumipulse Ag concentrations and the RT-PCR Ct values (RdRp gene), irrespective of whether the Ct values from molecular test were combined in a unique regression analysis or analysed separately. Overall, chemiluminescence-based antigen assay may be reliably applied to NPS samples to identify individuals with high viral loads, more likely to transmit the virus.
Keywords: SARS-CoV-2, Antigen assay, RT-PCR, COVID-19, Lumipulse
Introduction
As COVID-19 continues to strain public health systems and vaccination programmes race against new variants that might be more transmissible or capable of evading immune responses, the urgent need for simple, accessible, and frequent testing remains [Tan SH et al 2021]. Despite the fact that molecular assays are considered the gold standard for SARS-CoV-2 diagnosis, antigen detection assays currently deserve great attention, since they are intrinsically less laborious, require a shorter time to receive results and have the potential to satisfy the pressing demand for early SARS-CoV-2 infection diagnosis, thus allowing timely adoption of prevention measures against infection spread [Porte L et al 2020; Lambert-Niclot S et al 2020; Kobayashi R et al 2021]. In this study, we compared the performance of Lumipulse® G SARS-CoV-2 Ag assay with two widely used molecular assays (Allplex™ SARS-CoV-2 Assay and Easy SARS-CoV-2 WE kit) using nasopharyngeal swab (NPS) samples consecutively collected for routine diagnosis of SARS-CoV-2 infection.
Methods
A total of 513 NPS from individuals seeking SARS-CoV-2 infection diagnosis [median age: 47 years (range 1-98); 254 males and 259 females] collected during either the admission visit at the San Camillo-Forlanini Hospital or at a screening visit at peripheral testing points surrounding this hospital were tested in parallel at the hospital Laboratory, using both an automated chemiluninescence-based antigen (Lumipulse® G SARS-CoV-2 Ag assay), and a molecular test (either Allplex™ SARS-CoV-2 Assay, Seegene Inc., Seoul, Republic of Korea or Easy SARS-CoV-2 WE kit, Diatech Pharmacogenetics srl, Jesi, Italy), according to the manufacturer‘s instructions.
Data management and analyses were performed using GraphPad Prism version 8.00 (GraphPad Software, La Jolla, CA, USA). The evaluation of the qualitative concordance between results was performed using the weighted Cohen's kappa statistics and its 95%CI. Correlation analyses were performed using a linear regression analysis. All results were anonymized prior to the analysis here reported. Clinical status information was not available for the Laboratory, and could not be reconstructed after anonymization.
The assay comparison based on anonymized samples has been approved and waived for informed consent signature by the ethical board of the National Institute of Infectious Diseases.
Results
Considering RT-PCR as reference, samples from 275/513 individuals resulted SARS-CoV-2-positive (53.6% positivity rate). Among these, 231 samples resulted positive and 212 resulted negative to both Ag and RT-PCR, showing 86.4% inter-assay concordance, with substantial agreement based on weighted Cohen's kappa statistics (κ = 0.727; 95% CI = 0.668–0.786). Overall antigen test sensitivity of 84.0% (CI: 79.1%-88.1%) and specificity of 89.1% (CI: 84.4%-92.7%) (Table 1 ).
Table 1.
Concordance of Lumipulse G SARS-CoV-2 Ag vs. RT-PCR test results
| Lumipulse G SARS-CoV-2 Ag |
||||
|---|---|---|---|---|
| Positive | Negative | Total | ||
| RT-PCR Test* | Positive | 231 | 44 | 275 |
| Negative | 26 | 212 | 238 | |
| Total | 257 | 256 | 513 | |
Combined RT-PCR results from Allplex™ SARS-CoV-2 Assay (n = 387) and Easy SARS-CoV-2 WE kit (n = 126).
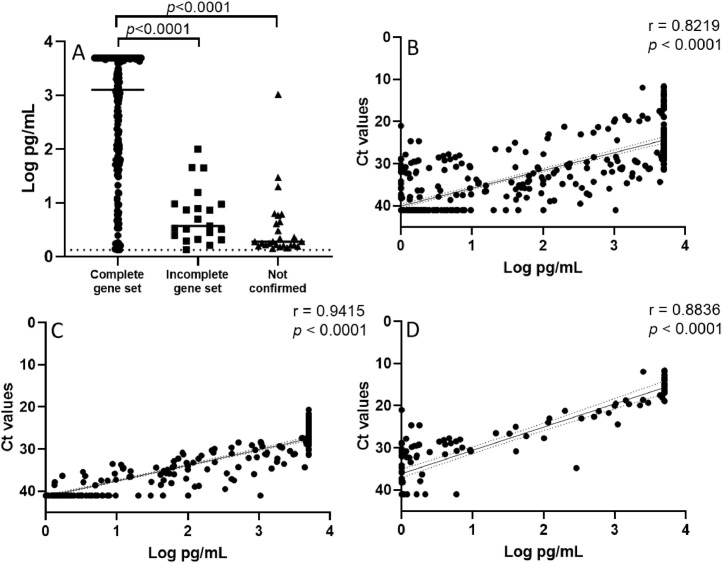
When stratifying the Ag test results into groups based on ranges of RT-PCR cycle threshold (Ct), considered an inverse proxy of viral load, the Ag concentration increased in parallel with the extent of viral load, i.e. higher Ag concentration observed in the groups with lower Ct values. Overall, the Ag test sensitivity was >95% for samples with Ct <30; consistently, the median Ag concentration in samples not confirmed by RT-PCR or confirmed with an incomplete set of target genes was significantly lower than the median Ag value in samples showing RT-PCR positivity to the complete set of target genes (Figure 1 A). Linear regression analysis showed a highly significant (r=0.8219, p<0.0001) correlation between the Lumipulse Ag concentrations (Log pg/mL) and Ct values of RdRp gene from the two RT-PCR assays (considered together) (Figure 1B). When separately analysed, Allplex™ SARS-CoV-2 Assay showed a slightly higher correlation (r=0.9415, p<0.0001) with Lumipulse Ag concentration (Figure 1C) as compared to Easy SARS-CoV-2 WE kit (r=0.8836, p<0.0001; Figure 1D).
Figure 1.
(A) SARS-CoV-2 N antigen concentration in 257 samples positive to the Lumipulse® G SARS-CoV-2 Ag assay, grouped according to the number of genes resulted positive by RT-PCR: confirmed with complete set of genes, i.e. to 3 genes with the Allplex™ SARS-CoV-2 assay and to 2 genes with the Easy SARS-CoV-2 WE assay (median: 3.11 Log pg/mL); confirmed with incomplete set of genes, i.e. to 2 genes with the Allplex™ SARS-CoV-2 assay and to 1 gene with the Easy SARS-CoV-2 WE assay (median: 0.57 Log pg/mL); not confirmed (median: 0.28 Log pg/mL). Statistically significant differences in Student´s T test are indicated in the figure. (B) Correlation between Lumipulse Ag concentrations (Log pg/mL) and Ct values of RdRp gene from the RT-PCR combined results of Allplex™ SARS-CoV-2 Assay and Easy SARS-CoV-2 WE kit (n=513). (C and D) Correlation between Lumipulse Ag concentrations (Log pg/mL) and Ct values of RdRp gene split for from the Allplex™ SARS-CoV-2 Assay (n=387, C) and for the Easy SARS-CoV-2 WE kit (n=126, D). Samples with Ct <40 or pg/mL ≥1.34pg/mL were considered positive. For statistical calculations, an arbitrary value of 41 Ct was assigned to all RT-PCR negative samples and an arbitrary value of 1.0 pg/mL was assigned to all samples with Ag concentration <1.0 pg/mL.
Discussion
Our results are in agreement with previous studies [Menichelli G. et al 2021; Hirotsu Y et al. 2021a; Amendola et al., 2021; Bordi L et al. 2021; Kobayashi R et al. 2021] and with the recently published article by Hirotsu et al. [Hirotsu et al. 2021b], showing a satisfactory performance of the Lumipulse G® SARS-CoV-2 Ag assay for quantitatively measuring the SARS-CoV-2 N antigen concentration in diagnostic samples from infected persons. In the present study the Lumipulse G® SARS-CoV-2 Ag assay showed 86.4% concordance and substantial agreement with molecular tests, including two broadly used molecular assays (Allplex™ SARS-CoV-2 Assay and Easy SARS-CoV-2 WE kit), with 84.0% overall sensitivity and 89.1% specificity. Notably, when stratifying samples into groups based on ranges of Ct from RT-PCR, the sensitivity of Lumipulse G® SARS-CoV-2 Ag assay was very high (>95%) for samples with low Ct values (<30). It is commonly assumed that these samples most likely derive from persons with active SARS-CoV-2 infection, and are associated with high contagiousness. Linear regression analysis showed highly significant correlation between the Lumipulse Ag concentrations and the Ct values of RdRp gene from both RT-PCR assays.
Overall, the present results support the concept that the chemiluminescence-based antigen assay may be reliably applied to NPS samples to identify individuals with high viral loads, who are more likely to transmit the virus.
Funding
This research was supported by funds to National Institute for Infectious Diseases ‘Lazzaro Spallanzani’ IRCCS from Ministero della Salute (Ricerca Corrente, linea 1; COVID-2020-12371817), the European Commission – Horizon 2020 (EU project 101003544 – CoNVat; EU project 101003551 – EXSCALATE4CoV; EU project 101005111 - DECISION; EU project 101005075 - KRONO) and the European Virus Archive – GLOBAL (grants no. 653316 and no. 871029).
Author Contributions
G.S., L.B.: conceptualization, analysed of results, writing; F.B., M.L.G.: laboratory testing, methodology; M.R.C., G.P.: conceptualization, methodology, review and editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Amendola A, Sberna G, Lalle E, Colavita F, Castilletti C, Menchinelli G, et al. Saliva is a valid alternative to Nasopharyngeal Swab in Chemiluminescence-based assay for detection of SARS-CoV-2 Antigen. J Clin Med. 2021 Apr 2;10(7):1471. doi: 10.3390/jcm10071471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordi L, Parisi G, Sberna G, Amendola A, Mariani B, Meoni G, et al. Effective screening strategy against SARS-CoV-2 on self-collected saliva samples in primary school setting: A pilot project. J Infect. 2021;83(1):e8–e10. doi: 10.1016/j.jinf.2021.05.013. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirotsu Y, Maejima M, Shibusawa M, Amemiya K, Nagakubo Y, Hosaka K, et al. Prospective study of 1308 nasopharyngeal swabs from 1033 patients using the LUMIPULSE SARS-CoV-2 antigen test: Comparison with RT-qPCR. Int J Infect Dis. Apr 2021;105:7–14. doi: 10.1016/j.ijid.2021.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirotsu Y, Sugiura H, Maejima M, Hayakawa M, Mochizuki H, Tsutsui T, et al. Comparison of Roche and Lumipulse quantitative SARS-CoV-2 antigen test performance using automated systems for the diagnosis of COVID-19. Int J Infect Dis. Jun 1 2021;108:263–269. doi: 10.1016/j.ijid.2021.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi R, Murai R, Asanuma K, Fujiya Y, Takahashi S. Evaluating a novel, highly sensitive, and quantitative reagent for detecting SARS-CoV-2 antigen. J Infect Chemother. Jun 27 2021;(6):800–807. doi: 10.1016/j.jiac.2021.01.007. Epub 2021 Jan 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert-Niclot S, Cuffel A, Le Pape S, Vauloup-Fellous C, Morand-Joubert L, Roque-Afonso AM, et al. Evaluation of a rapid diagnostic assay for detection of SARS-CoV- 2 antigen in nasopharyngeal swabs. J Clin Microbiol. 2020;58(8):e00977–e001020. doi: 10.1128/JCM.00977-2058:e00977-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menchinelli G, Bordi L, Liotti FM, Palucci I, Capobianchi MR, Sberna G, et al. Lumipulse G SARS-CoV-2 Ag assay evaluation using clinical samples from different testing groups. Clin Chem Lab Med. 2021 Apr 7;59(8):1468–1476. doi: 10.1515/cclm-2021-0182. [DOI] [PubMed] [Google Scholar]
- Porte L, Legarraga P, Vollrath V, Aguilera X, Munita JM, Araos R, et al. Evaluation of novel antigen-based rapid detection test for the diagnosis of SARS-CoV-2 in respiratory samples. Int J Infect Dis. 2020;99:328–333. doi: 10.1016/j.ijid.2020.05.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan SH, Allicock O, Armstrong-Hough M, Wyllie AL. Saliva as a gold-standard sample for SARS-CoV-2 detection. The Lancet Respiratory Medicine. 2021;9(12):562–564. doi: 10.1016/S2213-2600(21)00178-8. ISSN 2213-2600. [DOI] [PMC free article] [PubMed] [Google Scholar]