Abstract
Background
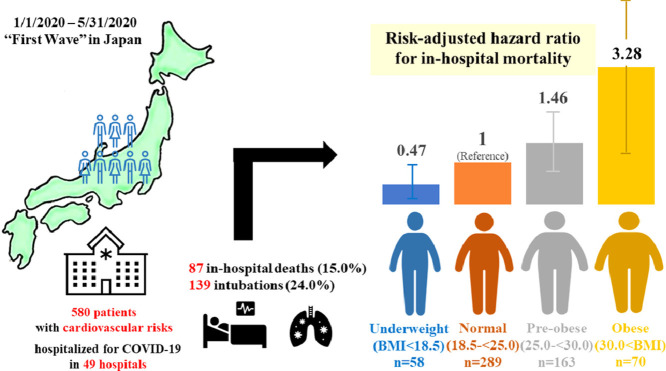
Obesity is reported to be a predictor of adverse clinical events in coronavirus disease 2019 (COVID-19) in Western countries. However, there are limited data reported regarding the prognostic impact of obesity in Asian patients. We investigated the relationship between body mass index (BMI) and in-hospital outcomes in 580 Japanese patients with cardiovascular disease and/or risk factors and who were admitted for COVID-19 infection using data from 49 hospitals in Japan.
Methods
We analyzed data from the Clinical Outcomes of COVID-19 Infection in Hospitalized Patients with Cardiovascular Disease and/or Risk Factors (CLAVIS-COVID) registry. BMI was classified into four groups accordance with the definition of the Japan Society for the Study of Obesity, as follows: underweight, <18.5 kg/m2; normal range, 18.5 to <25 kg/m2; pre-obese, 25 to 30 kg/m2; and obese, ≥30 kg/m2.
Results
In-hospital death occurred in 15.0% (n=87) of the patients and intubation was performed for 139 (24.0%) patients. In a multivariate analysis, we found a significant association between higher BMI and in-hospital mortality [underweight: hazard ratio (HR) 0.47, 95% confidence interval (CI) 0.23-0.97; p=0.041; pre-obese: HR 1.46, 95%CI 0.84-2.55; p=0.18; and obese: HR 3.28, 95%CI 1.34-8.02; p=0.009 vs. normal range]. In contrast, the association between BMI and the intubation rate was not statistically significant.
Conclusions
Obesity was associated with a stepwise increase in the risk of in-hospital mortality in Japanese patients with COVID-19 infection. The threshold BMI for the increased risk of a worse outcome was 30, which was much lower in comparison to Western countries.
Key Words: Body mass index, Cardiovascular disease, Coronavirus disease 2019 (COVID-19), In-hospital mortality, Obesity
Graphical Abstract
Graphical Abstract.
.
Introduction
Severe acute respiratory syndrome coronavirus (SARS-CoV-2) has been pervasive in recent years [1], [2], [3]. Patients who have hypertension, diabetes mellitus, dyslipidemia, and history of cardiovascular diseases are more likely to develop critical illness if infected with coronavirus disease 2019 (COVID-19) [4, 5]. A previous report showed that obesity was also associated with poor outcomes in patients with COVID-19 infection in Western countries [6]. In addition, this association has been seen with other respiratory virus infections, including influenza [7]. Obese patients with influenza have a higher risk of hospitalization and death, a longer duration of hospital stay, and a higher risk of requiring mechanical ventilation in comparison to normal weight individuals with influenza infection [8]. Although there are a few reports regarding the prognostic impact of obesity in Asian patients with COVID-19 infection [9, 10], the results were inconsistent. A previous report from China showed that obesity was linearly associated with severity of COVID-19 only in men but not in women [9]. However, this study was a single centered and the median age was almost 50 years old. In addition, the mortality rate was only 0.8% (3/383). On the contrary, a nationwide epidemiological study in Korea showed a non-linear (U-shaped) relationship between body mass index (BMI) and mortality [10]. However, this study mostly included outpatients and the mortality was only 3.0% (126/4141). Thus, we consider it important to clarify the impact of obesity on outcomes in Japanese populations who were hospitalized with COVID-19 infection because Japanese patients are older than those in other countries and they are generally leaner in comparison to Western populations. In the present analysis, we investigated the relationship between the BMI and in-hospital mortality in Japanese patients with cardiovascular disease and/or risk factors (CVDRF) who were admitted due to COVID-19 infection using the data from a Japanese multicenter registry.
Methods
Study Population
This study was a sub-analysis of the Clinical Outcomes of COVID-19 Infection in Hospitalized Patients with Cardiovascular Disease and/or Risk Factors (CLAVIS-COVID) registry. Detailed information on the CLAVIS-COVID registry has been published elsewhere [11]. Briefly, CLAVIS-COVID was a nationwide multicenter retrospective study that was sponsored by the Japanese Circulation Society. It enrolled Japanese patients with COVID-19 infection who were ≥20 years of age and who were hospitalized at 49 participating hospitals in Japan from January 2020 to May 2020 as the first infection wave in Japan. The diagnosis of COVID-19 was determined by a positive nucleic acid amplification test for SARS-CoV-2. From these registry data, our study used the data of all adults with CVDRF and for whom all BMI data were available. The definition of CVDRF was described in a previous study [11]. We collected the background characteristics, status at admission, treatments, and outcomes of these patients from the database. BMI was classified into four groups, according to the definitions of the Japan Society for the Study of Obesity (JASSO), as follows: underweight, <18.5 kg/m2; normal range, 18.5 to <25 kg/m2; pre-obese, 25 to 30 kg/m2; and obese, ≥30 kg/m2 [12]. Hypertension was defined as a history of antihypertensive medication, systolic blood pressure ≥140 mmHg, or diastolic blood pressure ≥90 mmHg. Dyslipidemia was defined as a history of anti-cholesterol medication, low-density lipoprotein cholesterol ≥140 mg/dl, high-density lipoprotein cholesterol <40 mg/dL, or triglycerides ≥150 mg/dL. Diabetes was defined as history of antidiabetic medication or hemoglobin A1c ≥6.5%. In-hospital mortality was the primary outcome of this study, and the intubation rate and other adverse outcomes were also analyzed.
Ethical consideration
The present study was approved by the Institutional Review Board of the Toranomon Hospital. The requirement for informed consent was waived because of the anonymized nature of the data. All participants were notified through homepages or posters at each hospital of their participation in the study, and they were free to opt out of participation at any time. Our study complies with the Declaration of Helsinki and Japanese Ethical Guidelines for Medical and Health Research Involving Human Subjects.
Statistical analyses
Categorical variables are reported as the frequency (%), whereas continuous variables are reported as the median and interquartile range (IQR). Fisher's exact test was used for the comparison of categorical variables. Student's t-test or the Mann-Whitney test were used for the comparison of continuous variables. A Cox proportional hazards analysis was performed to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) for the association between BMI group and in-hospital mortality and intubation, with adjustment for age, sex, history of myocardial infarction, cerebral infarction, hypertension, heart failure, and diabetes mellitus. The selection of variables for the multivariate analysis was based on previous reports [6] and clinical importance. A receiver operating characteristic (ROC) curve analysis was performed to evaluate the predictive value of this model for in-hospital mortality. We prespecified the normal range group as the reference group for all outcome analyses. Moreover, a Kaplan-Meier survival analysis and log-rank test— with adjustment for covariates—were performed to compare each of the BMI groups. All statistical analyses were performed using the R software program (version 4.0.3, R Foundation for Statistical Computing, Vienna, Austria; ISBN 3- 900051-07-0, http://www.R-project.org). Two-sided p-values of <0.05 were considered statistically significant.
Results
Patient characteristics
Among 693 patients with CVDRF who were enrolled in the CLAVIS-COVID registry, we included 580 (83.7%) patients whose BMI data, in addition to age, sex, and other important background and outcome data were recorded. The baseline characteristics of 580 patients are shown in Table 1 . The numbers of underweight, normal range, pre-obese, and obese patients were 58 (10.0%), 289 (49.8%), 163 (28.1%), and 70 (12.1%), respectively. Lower BMI groups tended to have severity-related variables, such as higher age, lower albumin, lower hemoglobin, higher baseline and maximum brain natriuretic peptide, and higher D-dimer. In addition, the median length of hospitalization was significantly longer in lower BMI groups (Table 1).
Table 1.
Baseline characteristics according to obesity class.
| underweight | normal range | pre-obese | obese | p-value | |
|---|---|---|---|---|---|
| Number | 58 | 289 | 163 | 70 | |
| Body mass index (kg/m2) | 17.30 [16.22 - 17.90] | 22.30 [20.60 - 23.60] | 26.70 [25.70 - 27.65] | 32.05 [31.20 - 35.27] | <0.001 |
| Body weight (kg) | 43.00 [39.25 - 46.75] | 58.00 [52.00 - 65.00] | 74.00 [67.00 - 78.00] | 93.00 [85.00 - 104.50] | <0.001 |
| Height – (cm) | 160.00 [150.25 - 166.50] | 163.40 [155.00 - 170.00] | 166.00 [158.00 - 171.00] | 169.00 [160.00 - 175.93] | <0.001 |
| Age (years) | 80.00 [72.25 - 89.75] | 71.00 [60.00 - 80.00] | 64.00 [54.50 - 73.00] | 55.00 [45.25 - 63.00] | <0.001 |
| Male | 33 (56.9) | 192 (66.4) | 111 (68.1) | 49 (70.0) | 0.397 |
| Length of hospitalization (days) | 22.00 [14.00 - 42.25] | 19.00 [11.00 - 30.00] | 17.00 [12.00 - 24.00] | 15.00 [10.00 - 22.75] | 0.004 |
| Comorbidities | |||||
| Hypertension | 43 (74.1) | 203 (70.2) | 127 (77.9) | 52 (74.3) | 0.361 |
| Diabetes | 19 (32.8) | 104 (36.0) | 72 (44.2) | 35 (50.0) | 0.063 |
| Dyslipidemia | 19 (32.8) | 110 (38.1) | 75 (46.0) | 36 (51.4) | 0.059 |
| Coronary artery disease | 10 (17.2) | 24 (8.3) | 23 (14.1) | 5 (7.1) | 0.064 |
| Old myocardial infarction | 6 (10.3) | 11 (3.8) | 9 (5.5) | 1 (1.4) | 0.085 |
| Heart failure | 9 (15.5) | 25 (8.7) | 12 (7.4) | 6 (8.6) | 0.306 |
| Deep venous thrombosis | 0 (0.0) | 1 (0.3) | 0 (0.0) | 0 (0.0) | 0.799 |
| Aortic dissection | 1 (1.7) | 3 (1.0) | 2 (1.2) | 0 (0.0) | 0.787 |
| Peripheral artery disease | 2 (3.4) | 1 (0.3) | 0 (0.0) | 1 (1.4) | 0.035 |
| Cerebral infarction | 11 (17.2) | 14 (4.5) | 9 (4.9) | 7 (8.6) | 0.002 |
| Data at admission | |||||
| systolic blood pressure (mmHg) | 131.00 [113.00 - 148.00] | 131.00 [118.00 - 145.00] | 131.00 [120.00 - 150.00] | 132.00 [116.00 - 141.00] | 0.463 |
| diastolic blood pressure (mmHg) | 73.00 [59.00 - 82.00] | 77.00 [68.00 - 88.00] | 80.00 [70.00 - 89.75] | 80.00 [71.00 - 90.00] | 0.004 |
| respiratory rate | 20.00 [18.00 - 23.75] | 20.00 [16.00 - 24.00] | 20.00 [16.00 - 24.00] | 20.50 [18.00 - 24.00] | 0.529 |
| Oxygen saturation of peripheral artery (%) | 96.00 [95.00 - 98.00] | 96.00 [94.00 - 98.00] | 96.00 [94.00 - 98.00] | 95.00 [93.00 - 97.00] | 0.244 |
| Hemoglobin (g/dl) | 11.65 [10.30 - 13.00] | 13.30 [11.70 - 14.50] | 13.80 [12.30 - 15.00] | 14.55 [13.28 - 15.70] | <0.001 |
| Albumin (mg/dl) | 3.00 [2.70 - 3.40] | 3.30 [2.90 - 3.70] | 3.30 [2.95 - 3.80] | 3.40 [3.00 - 3.90] | 0.007 |
| Serum creatinine (mg/dl) | 0.88 [0.61 - 1.13] | 0.82 [0.66 - 1.06] | 0.83 [0.64 - 1.04] | 0.82 [0.70 - 1.04] | 0.935 |
| D-dimer (μg/ml) | 2.14 [1.00 - 3.98] | 1.40 [0.80 - 2.80] | 1.33 [0.70 - 2.38] | 1.10 [0.60 - 2.30] | 0.045 |
| Brain natriuretic peptide at admission (pg/ml) | 151.50 [70.35 - 331.47] | 32.90 [12.10 - 134.00] | 29.65 [12.30 - 113.28] | 7.25 [5.80 - 18.03] | <0.001 |
| Max brain natriuretic peptide during hospitalization (pg/ml) | 178.75 [72.97 - 389.58] | 75.80 [18.00 - 188.50] | 47.15 [15.43 - 164.42] | 15.20 [6.60 - 42.10] | <0.001 |
Management
There were no significant differences with respect to the treatments among the groups (Table 2 ). Antibiotics or antiviral treatments were administered to almost half of the patients. Anti-coagulation therapy was administered to almost 20% of the patients.
Table 2.
Treatment by obesity class.
| underweightN=58 | normal rangeN=289 | pre-obeseN=163 | obeseN=70 | p-value | |
|---|---|---|---|---|---|
| Antibiotics | 32 (55.2) | 143 (49.7) | 94 (57.7) | 36 (51.4) | 0.411 |
| Antiviral drugs | 27 (46.6) | 155 (53.6) | 102 (62.6) | 45 (64.3) | 0.058 |
| Catecholamine | 4 (6.9) | 9 (3.1) | 11 (6.7) | 4 (5.7) | 0.282 |
| Nafamostat mesylate | 4 (6.9) | 21 (7.3) | 14 (8.6) | 8 (11.4) | 0.692 |
| Favipiravir | 25 (43.1) | 138 (47.8) | 95 (58.3) | 40 (57.1) | 0.067 |
| Anticoagulation | 8 (13.8) | 65 (22.5) | 38 (23.3) | 17 (24.3) | 0.443 |
| Oseltamivir | 1 (1.7) | 2 (0.7) | 1 (0.6) | 1 (1.4) | 0.805 |
| Intensive care unit | 12 (20.7) | 87 (30.1) | 58 (35.6) | 26 (37.1) | 0.128 |
In-hospital outcomes
The in-hospital outcomes are summarized in Table 3 . We observed 87 in-hospital deaths (15.0%) and 139 (24.0%) intubations in this cohort. There was no significant difference in the in-hospital mortality rate (p=0.385) and intubation rate (p=0.24) among the four groups. Almost half (44/87) of the patients died without intubation. We identified the cause of death in 42 cases. Among these cases, 67% (28/42) died due to respiratory failure. Detailed causes of death in patients who were not intubated are described in Online Table 1. The median age was significantly higher in death without intubation than in death with intubation [86.5 (79.0-91.0) years vs 74.0 (68.0-79.5) years, p<0.001].
Table 3.
Outcomes by obesity class.
| underweightN=58 | normal rangeN=289 | pre-obeseN=163 | obeseN=70 | p-value | |
|---|---|---|---|---|---|
| In-hospital death | 10 (17.2) | 49 (17.0) | 21 (12.9) | 7 (10.0) | 0.385 |
| Intubation | 8 (13.8) | 69 (23.9) | 43 (26.4) | 19 (27.1) | 0.24 |
| Other | |||||
| Coronary artery disease | 1 (1.7) | 1 (0.3) | 0 (0.0) | 0 (0.0) | 0.26 |
| Cerebral infarction | 0 (0.0) | 3 (1.0) | 1 (0.6) | 0 (0.0) | 0.702 |
| Cardiac arrest | 10 (17.2) | 41 (14.2) | 20 (12.3) | 7 (10.0) | 0.623 |
| Heart failure | 1 (1.7) | 1 (0.3) | 2 (1.2) | 0 (0.0) | 0.461 |
| Pulmonary embolism | 1 (1.7) | 7 (2.4) | 1 (0.6) | 1 (1.4) | 0.561 |
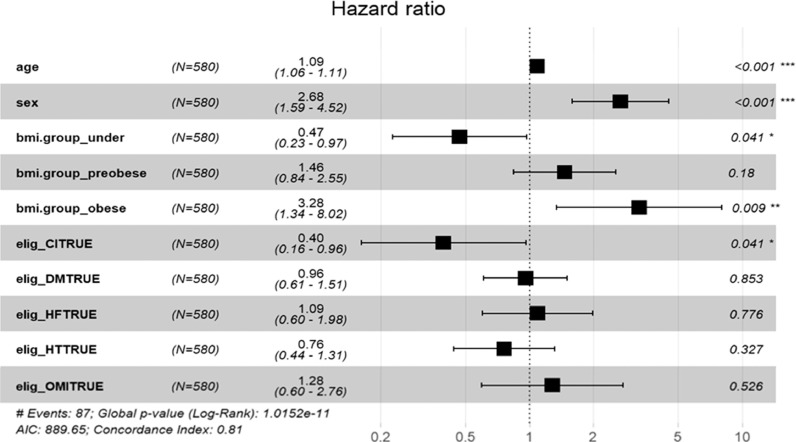
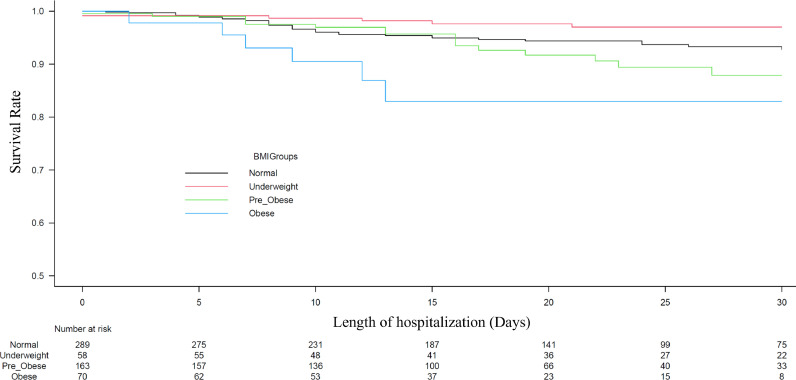
The multivariate Cox regression analysis revealed that higher BMI was significantly associated with higher in-hospital mortality, even after adjustment for covariates [underweight: hazard ratio (HR) 0.47, 95% confidence interval (CI) 0.23–0.97; p=0.041; pre-obese: HR 1.46, 95%CI 0.84–2.55; p=0.18; and obese: HR 3.28, 95%CI 1.34–8.02; p=0.009 vs. normal range] (Fig. 1 ). We found a similar association when BMI was used as a continuous value (HR 1.09, 95%CI 1.03–1.15, p=0.0032). The area under the curve of the selected variables (BMI, age, sex, history of myocardial infarction, cerebral infarction, hypertension, heart failure, and diabetes mellitus) of the ROC curve was 0.845 (95%CI 0.759–0.932). The Kaplan-Meier curve adjusted for these covariates revealed that the obese group showed a significantly higher in-hospital mortality rate in comparison to the other groups (Fig. 2 ). Regarding intubation, multivariate Cox regression analyses showed that higher BMI was not significantly associated with a higher intubation rate (underweight: HR 0.48, 95%CI 0.20–1.13; p=0.09; pre-obese: HR 1.05, 95%CI 0.65–1.68; p=0.85; and obese: HR 1.12, 95%CI 0.58–2.18; p=0.74 vs. normal range).
Fig. 1.
The multivariate Cox regression analysis for in-hospital mortality.
BMI, body mass index; CI, cerebral infarction; DM, diabetes mellitus; HF, heart failure; HT, hypertension; OMI, old myocardial infarction, The BMI groups were defined as follows: underweight, <18.5 kg/m2; normal range, 18.5 to <25 kg/m2; pre-obese, 25 to 30 kg/m2; and obese, ≥30 kg/m2.
Fig. 2.
The in-hospital mortality of four groups stratified by BMI groups. The survival curve was significantly stratified by the BMI groups. The BMI groups were defined as follows: underweight, <18.5 kg/m2; normal range, 18.5 to <25 kg/m2; pre-obese, 25 to 30 kg/m2; and obese, ≥30 kg/m2.
BMI, body mass index.
Discussion
We investigated the clinical impact of obesity on in-hospital outcomes for Japanese patients with CVDRF who were hospitalized with COVID-19 infection. Obesity was associated with a stepwise increase in the risk of in-hospital mortality
Infection with SARS-CoV-2 generally causes mild disease; however, in some individuals it progresses to severe respiratory illness characterized by hyperinflammatory syndrome, multiorgan dysfunction, and death [13,14]. A previous study reported that the risk of COVID-19-related death was strongly associated with higher BMI [15]. In general, obesity is associated with a higher prevalence of cardiometabolic diseases, such as hypertension, hyperlipidemia, and diabetes, which are thought to be linked to worse outcomes [15,16]. Indeed, in-hospital mortality of our study (15.0%) was much higher than those in previous reports from China (0.8%) [9] and Korea (3.0%) [10]. Although there were differences between previous reports and our study (older, hospitalized population in our study), cardiovascular comorbidities may play an important role for poor outcomes.
We found a linear association between increasing BMI and in-hospital mortality, even after adjustment for covariates. This result was consistent with a report from the Western countries [6] but inconsistent with one from Korea that showed non-linear (U-shaped) association [10]. We consider that the difference derives from differences in patients’ backgrounds and mortality rates. Because our study included hospitalized patients with COVID-19, the median age was 67 years old, and the mortality rate was 15.0%. On the contrary, a previous report from Korea included mainly outpatients and mortality was much lower (3.0%) than that in our study.
Our result clarified that the threshold of BMI for increased mortality in Japan was much smaller than that in Western countries. In a previous study [6], the adjusted HR for death was reported to significantly increase with class III obesity (BMI ≥40.0 kg/m2) in Western countries. In contrast, in our database, the obese group according to the Japanese definition (BMI ≥30 kg/m2) only accounted for 12.1% of the population and showed a significantly higher HR for in-hospital mortality.
The risk of requiring invasive mechanical ventilation is reported to increase over the full range of BMIs, possibly due to impairment of the lung function in association with higher BMI values [17]. Obesity has become a well-recognized risk factor for hospitalization and adverse outcomes in patients with COVID-19 [15]. However, our results did not show a statistically significant relationship between higher BMI and a higher intubation rate, contrary to the previous report [6]. One of the possible reasons for this discrepancy is that almost half (44/87) of the patients died without intubation. Among the 42 of these patients for whom the cause of death was known, 67% (28/42) died due to respiratory failure. We hypothesize that these patients were likely to have been amenable to intubation. Indeed, the median age of death without intubation (86.5 years) was significantly higher than death with intubation (74.0 years). Although we did not clarify the reason for non-intubation, high age seems to be associated with non-intubation. In the real-world treatment setting, several factors, such as old age and the patient's wishes, may have affected the intubation rate. For the younger generation, efforts towards achieving a healthy weight would help to reduce the rate of adverse events in COVID-19 infection [18]. In addition, we have to clarify better management practices for this population.
Contrary to the results of the multivariate analysis, the low BMI group showed higher in-hospital mortality in a univariate analysis. We consider that this discrepancy occurred due to the fact that elderly individuals comprised the majority of the low BMI group. The low BMI group showed significantly higher median age (80 years old) than those in other groups (55-71 years old) (Table 1). From the multivariate analysis (Fig. 1), age was a significant predictor for in-hospital mortality in our dataset (it showed a 9% increase in risk of in-hospital mortality as a 1-year increase in age). Thus, we consider that higher in-hospital mortality in the low BMI group could be mainly explained by their old age. In general, the risk of a poor outcome is greater in elderly people with infectious diseases. In fact, in a previous report, old age was associated with a higher mortality rate after COVID-19 infection [11]. In addition, vascular endothelial cell dysfunction, inflammation-associated myocardial depression, stress cardiomyopathy, direct viral infection of the heart and its vessels, or the host response may cause or worsen heart failure, demand-related ischemia, and arrhythmias in this population [19]. These factors may underlie the associations that have been observed between cardiovascular events and COVID-19 infection [20].
Limitations
The present study was associated with some limitations. First, this was a sub-analysis of a retrospective multicenter registry. Thus, a selection bias could not be avoided. In addition, although this study was a large multicenter registry, the sample size of 580 patients was not large and there was a chance of being statistically underpowered. Second, during the first wave in Japan, the Japanese government mandated hospitalization for all patients with COVID-19 infection, regardless of their disease severity. The current management for low-risk patients with COVID-19 is recuperation at their own home or in a hotel. Although there are no data comparing outcomes before and after the policy change, we presume that it is easier for medical staff to cope with a sudden deterioration in respiratory status if the patient is hospitalized. This policy change may have affected the results of our study. Third, our data did not cover the impact of the current variants and the possible effect of vaccination.
CONCLUSIONS
Obesity showed a significant linear association with an increased risk of in-hospital death in Japanese patients with COVID-19 infection. The threshold BMI value for an increased risk of a worse outcome was 30, which was much lower in comparison to Western countries. Further studies are needed to clarify the appropriate management strategy for this population.
Acknowledgements
None
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jjcc.2021.09.013.
Appendix. Supplementary materials
References
- 1.Goyal P, Choi JJ, Pinheiro LC, Schenck EJ, Chen R, Jabri A, et al. Clinical characteristics of Covid-19 in New York city. N Engl J Med. 2020;382:2372–2374. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mai F, Del Pinto R. Ferri C. COVID-19 and cardiovascular diseases. J Cardiol. 2020;76:453–458. doi: 10.1016/j.jjcc.2020.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kadoya Y, Zen K, Wakana N, Yanishi K, Senoo K, Nakanishi N, et al. Knowledge, perception, and level of confidence regarding COVID-19 care among healthcare workers involved in cardiovascular medicine: a web-based cross-sectional survey in Japan. J Cardiol. 2021;77:239–244. doi: 10.1016/j.jjcc.2020.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ikari Y, Matsue Y, Torii S, Hasegawa M, Aihara K, Kuroda S, et al. Association between statin use prior to admission and lower coronavirus disease 2019 (COVID-19) severity in patients with cardiovascular disease or risk factors. Circ J. 2021;85:939–943. doi: 10.1253/circj.CJ-21-0087. [DOI] [PubMed] [Google Scholar]
- 6.Hendren NS, de Lemos JA, Ayers C, Das SR, Rao A, Carter S, et al. Association of body mass index and age with morbidity and mortality in patients hospitalized with COVID-19: Results from the American Heart Association COVID-19 Cardiovascular Disease Registry. Circ. 2021;143:135–144. doi: 10.1161/CIRCULATIONAHA.120.051936. [DOI] [PubMed] [Google Scholar]
- 7.Jain S, Chaves SS. Obesity and influenza. Clin Infect Dis. 2011;53:422–424. doi: 10.1093/cid/cir448. [DOI] [PubMed] [Google Scholar]
- 8.Kwong JC, Campitelli MA, Rosella LC. Obesity and respiratory hospitalizations during influenza seasons in Ontario, Canada: a cohort study. Clin Infect Dis. 2011;53:413–421. doi: 10.1093/cid/cir442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cai Q, Chen F, Wang T, Luo F, Liu X, Wu Q, et al. Obesity and COVID-19 severity in a designated hospital in Shenzhen, China. Diabet Care. 2020;43:1392–1398. doi: 10.2337/dc20-0576. [DOI] [PubMed] [Google Scholar]
- 10.Kang IS, Kong KA. Body mass index and severity/fatality from coronavirus disease 2019: A nationwide epidemiological study in Korea. PLoS One. 2021;16 doi: 10.1371/journal.pone.0253640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsumoto S, Kuroda S, Sano T, Kitai T, Yonetsu T, Kohsaka S, et al. Clinical and biomarker profiles and prognosis of elderly patients with coronavirus disease 2019 (COVID-19) with cardiovascular diseases and/or risk factors. Circ J. 2021;85:921–928. doi: 10.1253/circj.CJ-21-0160. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi H, Mori M. Characteristics and significance of criteria for obesity disease in Japan 2011. Jap J Clin Med. 2013;71:257–261. [PubMed] [Google Scholar]
- 13.Dixon AE, Peters U. The effect of obesity on lung function. Expert Rev Respir Med. 2018;12:755–767. doi: 10.1080/17476348.2018.1506331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siddiqi HK, Mehra MR. COVID-19 illness in native and immunosuppressed states: A clinical-therapeutic staging proposal. J Heart Lung Transplant. 2020;39:405–407. doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao M, Piernas C, Astbury NM, Hippisley-Cox J, O'Rahilly S, Aveyard P, et al. Associations between body-mass index and COVID-19 severity in 6•9 million people in England: a prospective, community-based, cohort study. Lancet Diabet Endocrinol. 2021;9:350–359. doi: 10.1016/S2213-8587(21)00089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ren L, Yu S, Xu W, Overton JL, Chiamvimonvat N, Thai PN. Lack of association of antihypertensive drugs with the risk and severity of COVID-19: A meta-analysis. J Cardiol. 2021;77:482–491. doi: 10.1016/j.jjcc.2020.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289:76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 18.Matsunaga N, Hayakawa K, Terada M, Ohtsu H, Asai Y, Tsuzuki S, et al. Clinical epidemiology of hospitalized patients with COVID-19 in Japan: Report of the COVID-19 registry Japan. Clin Infect Dis. 2020:ciaa1470. doi: 10.1093/cid/ciaa1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mehra MR, Ruschitzka F. COVID-19 illness and heart failure: A missing link? JACC Heart Fail. 2020;8:512–514. doi: 10.1016/j.jchf.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mehra MR, Desai SS, Kuy S, Henry TD, Patel AN. Cardiovascular disease, drug therapy, and mortality in Covid-19. N Engl J Med. 2020;382:e102. doi: 10.1056/NEJMoa2007621. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.