Abstract
Aims
Accumulated evidence indicates that local cell origins may ingrain differences in the phenotypic activity of human osteoblasts. We hypothesized that these differences may also exist in osteoblasts harvested from the same bone type at periarticular sites, including those adjacent to the fixation sites for total joint implant components.
Methods
Human osteoblasts were obtained from the acetabulum and femoral neck of seven patients undergoing total hip arthroplasty (THA) and from the femoral and tibial cuts of six patients undergoing total knee arthroplasty (TKA). Osteoblasts were extracted from the usually discarded bone via enzyme digestion, characterized by flow cytometry, and cultured to passage three before measurement of metabolic activity, collagen production, alkaline phosphatase (ALP) expression, and mineralization.
Results
Osteoblasts from the acetabulum showed lower proliferation (p = 0.034), cumulative collagen release (p < 0.001), and ALP expression (p = 0.009), and produced less mineral (p = 0.006) than those from the femoral neck. Osteoblasts from the tibia produced significantly less collagen (p = 0.021) and showed lower ALP expression than those from the distal femur.
Conclusion
We have demonstrated for the first time an anatomical regional variation in the biological behaviours of osteoblasts on either side of the hip and knee joint. The lower osteoblast proliferation, matrix production, and mineralization from the acetabulum compared to those from the proximal femur may be reflected in differences in bone formation and implant fixation at these sites.
Cite this article: Bone Joint Res 2021;10(9):611–618.
Keywords: Osteoblast, Arthroplasty, Aseptic loosening, osteoblasts, acetabulum, Femoral neck, Collagen, hip, distal femoral, tibia, alkaline phosphatase, total knee arthroplasty (TKA), Flow cytometry
Article focus
The aim of this study was to establish if different anatomical sites and hence environments could account for differences in osteoblast function at periarticular sites.
We hypothesized that bone-forming human osteoblasts differ in their functional capabilities depending on their anatomical source.
Key messages
Human osteoblasts demonstrated different functional outcomes in the assays conducted in this study.
Our study found coincidentally that human osteoblasts harvested from sites more readily associated with earlier failure with regards to arthroplasty, and performed less favourably in the functional assays performed in human osteoblasts isolated from native hip and knee joints.
Strengths and limitations
The study showed consistent anatomical variations in human osteoblast proliferation, cumulative collagen release, alkaline phosphatase expression, and mineral production.
The main limitation of the study is that the number of patient samples could be increased, and we acknowledge that the data, while statistically significant, are preliminary.
Introduction
Earlier work within our laboratory established that an area that has not been evaluated is whether an asymmetric biological difference exists in bone-forming osteoblast function.
Primary hip and knee arthroplasty surgery continues to increase nationally1 and globally2 with revision rates of arthroplasty surgery and their causes being obligatory measures of total joint replacement outcome success.3-7 The current approach to increasing the longevity of implant components is to modify implant designs and materials,8 and the lifetime risk of requiring revision surgery over the age of 70 years is approximately 5%.9 The authors from the same study showed up to a 35% chance of revision for males undergoing primary surgery in their 50s and 20% for females in the same age group.9 They estimated a median time to revision of 4.4 years in those who had surgery under the age of 60 years. It is also estimated that the number of revision total knee arthroplasties (TKAs) will increase by 601% by 2030.10
The clinical and economic impact of revision total hip arthroplasty (THA) and TKA has been estimated and well described.11,12 It poses a significant burden on patients, healthcare professionals, and healthcare systems by way of higher complications and blood transfusion requirement, prolonged operating time, and increased length of stay.13-15 Not only are direct costs increasing, but some have estimated associated costs of up to £75,000 per patient.16,17 Better understanding of the underlying bone biology could direct treatment, reduce the need for surgical intervention of any kind, and thus bring huge benefits.
Evidence exists of a variation in the phenotype of bone-forming osteoblasts depending on their skeletal location in both animal and human models.18-20 Specifically, human osteoblasts isolated from trabecular bone showed lower proliferation than those from cortical and sub-chondral bone, but higher expression of differentiation markers when obtained from human adult humeri. Differences were consistent between cells from patients with osteoarthritis (OA), osteoporosis, and from a single patient without bone pathology.21 One of the limitations of this analysis is that human osteoblasts were acquired from different bone types and obtained via outgrow methods, whereby cells are permitted to grow out of bone specimens without using enzyme digestion.
While these studies provide evidence of osteoblast variability, there have been no studies reporting anatomical variation in osteoblast function specifically from periarticular regions from the hip and knee, and from the same bone type.
We hypothesize that differences in biological behaviour of cells isolated from different sites may exist, and have performed biological assays of human osteoblasts to establish if the underlying cell biology could be a consideration with regards to surgical outcomes.
Methods
Study design
A level II prospective cohort laboratory study was performed.
Patient recruitment and evaluation
Patients were recruited for donation under an ethical agreement (06/Q0108/213) through Institutional Review Board approval. A standardized participant information sheet and consent form were given for completion to those willing to participate in the study.
Radiographs from all patients were evaluated prior to recruitment and subsequent inclusion into the study in order to ensure the absence of further bony pathology. Patients displaying radiological evidence of concomitant osteoporosis with OA were excluded from the study. The degree of cartilage present on the femoral head was also assessed under direct vision at the osteoblast isolation stage. Patients prescribed immunosuppressant medication such as steroids or disease-modifying antirheumatic drugs, who had an underlying diagnosis of rheumatoid arthritis (RA), who had been diagnosed with renal disease, or who had abnormalities of the parathyroid or vitamin D deficiency were also excluded from the study.
Seven THA patients and six TKA patients were recruited into the study. The mean age of those undergoing THA was 79 years (73 to 89; n = 3 males, 4 females) and in those undergoing TKA was 71.2 years (68 to 74; n = 3 males, 3 females).
Cell isolation
Bone samples were obtained from the operating theatre through standardized techniques. Cell isolations were performed on samples from the separate regions studied using a standard enzyme digestion technique.22 When femoral heads were dissected, surface cartilage was re-examined, and samples were excluded and discarded if cartilage was present.
Following thorough washing in phosphate-buffered saline (PBS) (Invitrogen, USA), bone fragments were digested sequentially in 10 ml of each of the following enzymes: trypsin (1 mg/ml; Difco, USA), dispase (2 mg/ml; Roche, Switzerland), and twice in collagenase A (3 mg/ml; Roche). The supernatants from the trypsin and dispase digestions were discarded whereas those from the collagenase digestions were retained and combined.
Collagenase supernatants were centrifuged at 300 g for ten minutes and cell pellets resuspended in McCoy’s 5A medium with stable glutamine without phenol red (BioConcep Amimed, Switzerland) containing 10% foetal calf serum (FCS) (Invitrogen), 100 units/ml penicillin, 100 µg/ml streptomycin, 292 µg/ml glutamine (PSG) (Invitrogen), and 30 µg/ml vitamin C (Vitamin C) (Sigma) (growth medium).
Flow cytometry of cells from the initial cell isolation
Re-suspended cells were counted using a Cell Scepter (MilliporeSigma, USA) and 5 × 104 cells per isolate were suspended in 100 ml of auto-MACS rinsing solution (Miltenyi Biotec, UK) and incubated in 10 µl of the following antibodies and control antibodies for 45 minutes on ice: anti-alkaline phosphatase (ALP) (Cat. no. FAB1448P, IgG1, control cat. no. IC002P, R&D Systems), anti-CD271 (Cat. no. 130-100-019, control cat. no. 130-104-617, Miltenyi Biotec), anti-CD90, CD105, and CD73 (CD90, CD105, and CD73 were used as a positive cocktail cat. no. 51 to 9007663; positive cocktail control cat. no. 51 to 9007664, BD Biosciences, USA). The cell populations were analyzed on the LSR-Fortessa flow cytometer (BD Biosciences; Cambridge Biomedical Research Centre Cell Phenotyping Hub).
Cell culture
Cells from the initial isolates were cultured in growth medium. The medium was changed every two days, cells were used in experiments at passage three, and human osteoblast-specific culture conditions and medium were used.
Human osteoblasts from the acetabulum, femoral neck, and tibial and femoral knee bone were evaluated for phenotype and behaviour by measuring proliferation, cumulative collagen release, ALP expression, and mineralization. Human osteoblasts were cultured in medium specific to their growth with and without the addition of osteogenic supplements, hydrocortisone (200 nM), and β-glycerophosphate (2 mM), to induce cell differentiation and provide phosphate for mineralization. Microscopy was also used to ensure the distinctive human osteoblast morphology was demonstrated and hence cultured.
Cell proliferation
Cells were seeded in six replicate wells of a 96-well plate in 180 µl of growth medium at a concentration of 2 × 104 cells/ml. At days 1, 3, 7, 11, and 14 following seeding 20 µl alamarBlue dye (Bio-Rad AbD Serotec) was added to each well and incubated for four hours.
Following incubation, dye reduction was measured as fluorescence intensity (excitation 544 nm, emission 590 nm) on a FLUOstar Optima microplate reader (BMG Labtech). Plates were then washed with PBS and fresh growth medium added. Total percentage reduction of alamarBlue was calculated for each well at each timepoint and averaged over the six replicates.
Percentage cells expressing ALP by flow cytometry
Cells were seeded in 12-well plates at a concentration of 2.5 × 104 cells/ml using 2 ml in each well with or without beta-glycerophosphate (β-GP) (2 mM) and hydrocortisone (0.2 µM). At day 1 and 7 post-seeding human osteoblasts were assessed for ALP expression.
Cells were pelleted in microcentrifuge tubes (5 × 104/tube) and resuspended in 100 µl autoMACS rinsing solution (Milteyni Biotec). A total of 10 µl of R-Phycoerythrin (PE) conjugated monoclonal anti-ALP antibody (R&D Systems, UK) or 10 µl PE conjugated IgG1 (antibody control) was added and incubated for 45 minutes on ice.
After incubation further antibody binding was stopped by adding 1 ml of MACS buffer. Cells were then centrifuged for five minutes at 300 g, resuspended in 200 µl of PBS, and analyzed on a BD Biosciences FACS CantoII flow cytometer. This process was then repeated at day 7 for untreated and treated human osteoblasts. Results were then expressed as percentage expression of the parent population.
Data were analyzed using FACS DIVA software and Kaluza Flow Cytometry Analysis Software Version 1.2 (Beckman Coulter, Life Sciences, USA).
Mineralization
Mineral nodule formation was determined after 14 days. At day 1, cells were seeded at 4 × 104 cells/ml in 12-well culture plates using 2 ml in each of two wells, one containing osteogenic supplements. On day 14, cells were washed with PBS, fixed in 70% ethanol for 60 minutes on ice, and washed three times thereafter using MilliQ water and 2% Alizarin Red S solution, added for ten minutes at room temperature to stain the nodules.
After washing the cells in deionized water, images of the mineral nodules were captured on a Leitz Dialux 20 microscope (Leitz Group, Germany). Matrix mineralization was quantified by solubilizing the stain in 10% cetylpyridinium and reading the absorbance at 570 nm using a Fluostar OPTIMA microplate spectrophotometer (BMG Labtech, Germany).
Type 1 collagen dot immunoblotting
Cell supernatants corresponding to days 1, 3, 7, 11, and 14 from those seeded to assess mineralization were analyzed by dot-blotting using a BioRad Bio-Dot apparatus. Polyvinylidene difluoride (PVDF) membranes were loaded with 50 µl of supernatant and 50 µl PBS prior to probing with specific type-1 collagen antibodies (Rockland, USA). Membranes were incubated with enhanced chemiluminescence reagents (Amersham ECL Western Blotting Detection Reagents, GE Healthcare Life Sciences, UK) and quantitation of type-1 collagen undertaken by densitometry (GS-800 densitometer, BioRad) using a standard curve of purified human type-1 collagen. Cumulative collagen release was measured as mg/ml of culture medium.
Statistical analysis
An initial multivariate analysis of variance (MANOVA) and subsequent post-hoc analysis with Tukey’s honestly significant difference (HSD) test was used to determine levels of significance. A paired t-test was used to compare data between osteoblasts grown in growth medium and those grown in mineralization medium. A p-value of < 0.05 was considered statistically significant.
Previous pilot data within the laboratory exploring human osteoblast function demonstrated that sample sizes of six or greater were sufficient to produce significant differences in osteoblast mineralization between cells from the femoral neck and the core of the femoral head. Thus, seven patients undergoing THA and six undergoing TKA were considered appropriate to investigate differences using continuous data. Statistical analysis was performed using JMP version 12.0.1 (SAS Institute, USA).
Results
Data gathered from the initial cell isolates
In order to better categorize heterogeneity within the different anatomical areas under investigation, cells were isolated and flow cytometry was performed to establish if mesenchymal stromal cell populations were present and, if so, whether they were present at different concentrations and could hence lead to differences in cell behaviours at the assay stage. Flow cytometry demonstrated low percentage levels of MSC markers; furthermore, no significant differences were seen in the expression of CD271, CD90, CD105, CD73, and ALP between regions when comparing cell populations from the femoral neck with the acetabulum (MANOVA, CD271 p = 0.175; CD90 p = 0.063; CD105 p = 0.900; CD73 p = 0.360; ALP p = 0.243) and the distal femur with proximal tibia (CD271 p = 0.315; CD90 p = 0.060; CD105 p = 0.717; CD73 p = 0.339; ALP p = 0.189, all MANOVA) (Figure 1 and Figure 2).
Fig. 1.
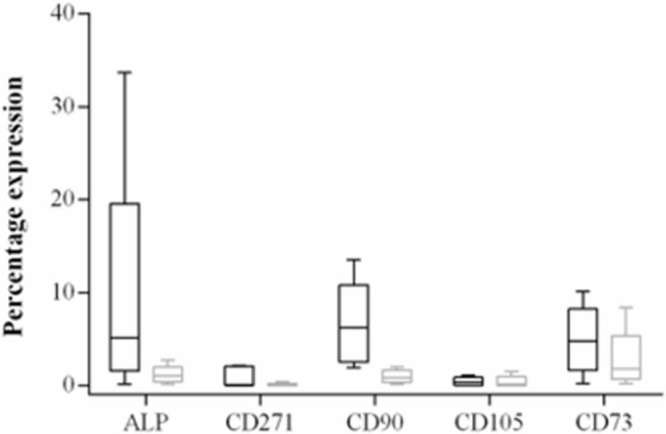
Box plot diagram showing percentage expression of CD271, CD90, CD105, CD73, and alkaline phosphatase (ALP) of primary isolates of acetabular (black box) and femoral neck (grey box) bone. Median, 25% and 75% quartiles, and maximum and minimum values are shown. An outlier for ALP is also demonstrated on the box plot.
Fig. 2.
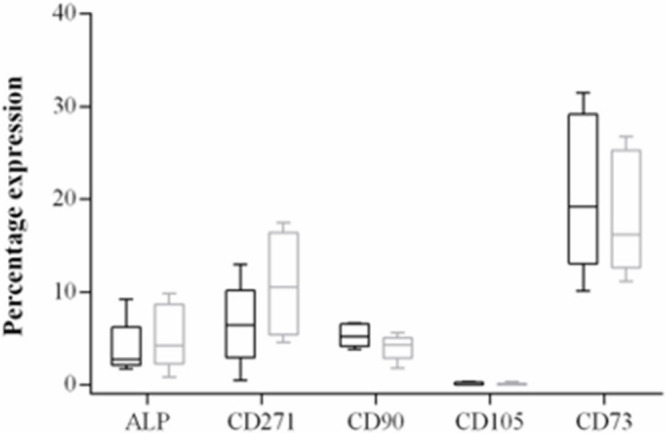
Box plot diagram showing the percentage expression of CD271, CD90, CD105, CD73, and alkaline phosphatase (ALP) of primary isolates of distal femoral (black box) and proximal tibial (grey box) bone. Median, 25% and 75% quartiles, and maximum and minimum values are shown.
Data of cultured human osteoblasts from patients undergoing primary hip arthroplasty
Figure 3 shows the change in metabolic activity of the cultures over the 14-day experimental period assessed with alamarBlue which, during log growth phase, is related to proliferation. The metabolic activity of femoral neck cells (least squares mean 19.7, 95% confidence interval (CI) 17.5 to 22.0) was significantly greater than that of cells from the acetabulum (least squares mean 16.4, 95% CI 14.1 to 18.6) (p = 0.034, Tukey’s HSD) (Figure 3, Table I).
Fig. 3.
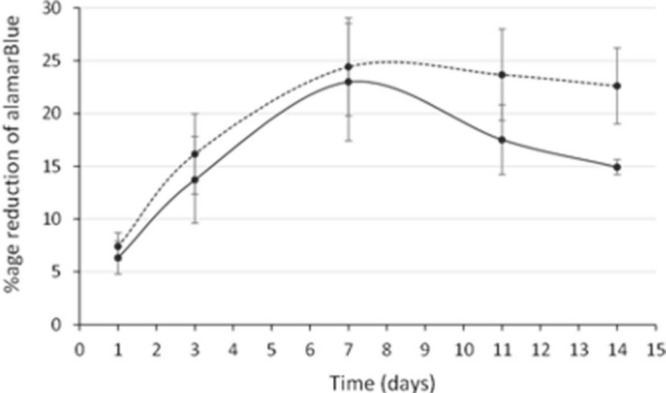
Change in the metabolic activity of human osteoblast cell cultures with time. Acetabular human osteoblasts are represented by the smooth line and neck cells by the dotted line. Each point shows the mean and standard error of the mean.
Table I.
Summary of the data from human osteoblasts obtained from those patients undergoing primary total hip arthroplasty. The columns show which anatomical region the data pertains to with a p-value obtained from comparisons of these data. Least squares mean values are shown.
| Variable | Acetabulum | Femoral neck | p-value* |
|---|---|---|---|
| Percentage reduction of alamarBlue | 16.4 | 19.7 | 0.034 |
| Cumulative collagen release, mg/ml | 5.2 | 8.6 | < 0.001 |
| ALP expression, % of parent population | 15.8 | 62.6 | 0.009 |
| Mineralization, absorbance at 570 nm | 1.3 | 2.2 | 0.006 |
Tukey's honestly significant difference.
ALP, alkaline phosphatase.
Cumulative type-1 collagen release also differed between human osteoblasts from different regions. Femoral neck human osteoblasts produced significantly more type-1 collagen (least squares mean 8.6, 95% CI 7.6 to 9.5) than those from the acetabulum (least squares mean 5.2, 95% CI 4.2 to 6.2) (p < 0.001, Tukey’s HSD) (Figure 4, Table I).
Fig. 4.
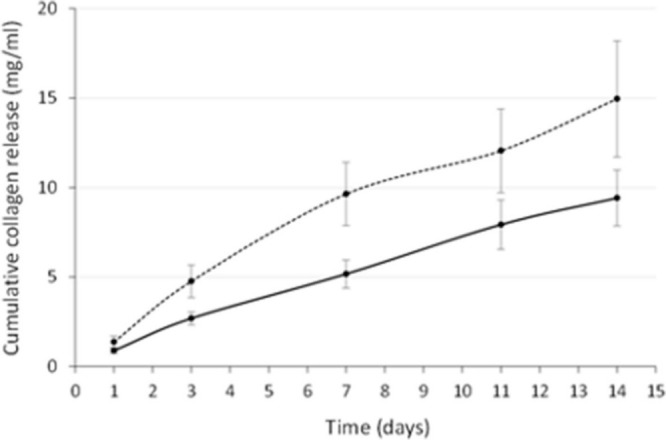
Cumulative collagen release curves over 14 days. Collagen release from acetabular human osteoblasts is represented by the smooth line and release from neck cells by the dotted line. Each point shows the mean and standard error of the mean.
Treatment with β-GP and hydrocortisone increased collagen release significantly in acetabular human osteoblasts only (least squares mean 6.2, 95% CI 4.4 to 8.0) (p = 0.021, paired t-test).
Significantly higher expression of ALP was seen in the neck human osteoblasts (least squares mean 62.6, 95% CI 42.1 to 83.2) when compared to those from the acetabulum (least squares mean 15.8, 95% CI -4.8 to 36.3) as shown in Figure 5 and Table I (p = 0.009, Tukey’s HSD).
Fig. 5.
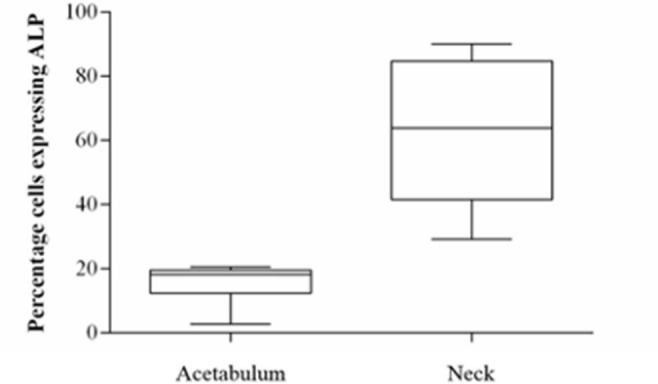
Box plot diagram of alkaline phosphatase expression on day 1 post-seeding of human osteoblasts from the acetabulum and femoral neck. Median and 25% and 75% quartiles are shown.
When treated with β-GP and hydrocortisone, ALP expression significantly increased in human osteoblasts from the neck (p = 0.002, paired t-test) and the acetabulum (p = 0.029, paired t-test).
Greater mineralization was seen in cultures of cells from the femoral neck compared to those from the acetabulum (Figure 6). Absorbance of solubilized Alizarin Red S stain was significantly greater in human osteoblasts from the neck (least squares mean 2.2, 95% CI 1.8 to 2.5) than those from the acetabulum (least squares mean 1.3, 95% CI 0.9 to 1.7) (p = 0.006, Tukey’s HSD) (Table I).
Fig. 6.
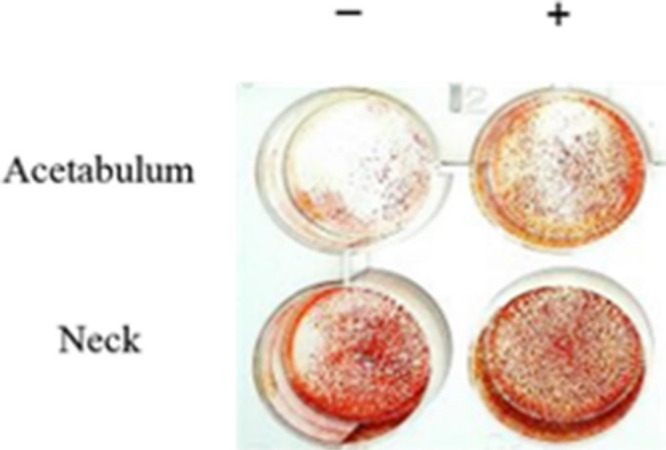
Acetabular and femoral neck human osteoblast cultures at day 14 after staining with Alizarin Red S. Human osteoblasts were cultured in McCoy’s 5A media either with (+) or without (-) hydrocortisone and beta-glycerophosphate.
Treatment with β-GP and hydrocortisone increased mineralization in acetabular human osteoblasts (p = 0.061, paired t-test) but not in cells from the neck (Figure 6).
Data of cultured human osteoblasts from patients undergoing primary knee arthroplasty
The metabolic activity of the cultures was greater in cells from the tibia (least squares mean 32.0, 95% CI 29.2 to 34.6) than the femur (least squares mean 29.6, 95% CI 26.9 to 32.3); however, this difference was not significant (p = 0.228, Tukey’s HSD) (Table II).
Table II.
Summary table of the data from human osteoblasts obtained from those patients undergoing primary total knee arthroplasty. The columns show which anatomical region the data pertains to with a p-value obtained from comparisons of these data. Least squares mean values are shown.
| Variable | Distal femur | Proximal tibia | p-value* |
|---|---|---|---|
| Percentage reduction of alamarBlue | 29.6 | 32.0 | 0.228 |
| Cumulative collagen release, mg/ml | 8.1 | 6.2 | 0.021 |
| ALP expression, % of parent population | 44.4 | 28.8 | 0.075 |
| Mineralization, absorbance at 570 nm | 0.82 | 0.68 | 0.512 |
Tukey's honestly significant difference.
ALP, alkaline phosphatase.
Type-1 collagen production was significantly greater in femoral human osteoblast cultures (least squares mean 8.1, 95% CI 6.9 to 9.2) than those from the tibia (least squares mean 6.2, 95% CI 5.1 to 7.3) (p = 0.021, Tukey’s HSD) (Table II). Culturing femoral or tibial human osteoblasts in mineralization medium did not significantly alter collagen production.
A higher expression of ALP was seen in human osteoblasts from the femur (least squares mean 44.4, 95% CI 31.7 to 57.0) than those from the tibia (least squares mean 28.8, 95% CI 16.1 to 41.4) but this was not significant (p = 0.075, Tukey’s HSD) (Figure 7, Table II)). Treatment with β-GP and hydrocortisone increased ALP expression in tibial human osteoblasts (p = 0.004, paired t-test) but not femoral human osteoblasts (p = 0.151, paired t-test).
Fig. 7.
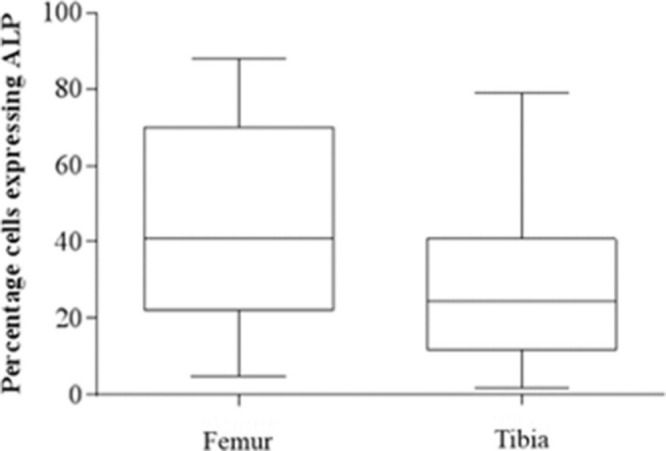
Box plot diagram of alkaline phosphatase (ALP) expression on day 1 post-seeding of human osteoblasts from the femur and tibia. Median and 25% and 75% quartiles are shown.
When comparing mineralization data, human osteoblasts from the femur produced greater amounts of mineral. Absorbance was higher in femoral human osteoblasts (least squares mean 0.82, 95% CI 0.45 to 1.19) than those from the tibia (least squares mean 0.68, 95% CI 0.31 to 1.05), though this was not statistically significant (p = 0.512, Tukey’s HSD) (Table II). Treatment with β-GP and hydrocortisone did not increase mineral production in femoral (p = 0.292, paired t-test) or tibial (p = 0.361, paired t-test) human osteoblasts.
Summaries of the data for comparisons of human osteoblast activity when harvested from different sites of the hip and knee in those undertaking primary THA and TKA are illustrated in Table I and Table II, respectively, to facilitate a comparison of the data.
Discussion
There is a need to study the comparative phenotype and behaviour of bone-forming cells from different anatomical locations. In this investigation, cells were isolated and compared from trabecular bone removed from native joints during primary arthroplasty surgery from proximal femur and acetabulum, and from distal femur and proximal tibia, and investigated as both an initial isolate and as a population of cultured cells.
The way human osteoblasts were harvested in this study needs some explanation as two methods exist for obtaining primary osteoblasts: enzyme digestion,23,24 and primary outgrowth,25 with the latter often used to isolate osteoblasts from non-diseased bone. Both methods have been reported to produce a relatively homogeneous population, containing predominantly early primary human osteoblasts that respond to bone morphogenic proteins and glucocorticoid.26,27 Primary outgrowth from trabecular fragments avoids enzyme cytotoxicity;28 however, this method may select cells with higher proliferation and metabolic activity. Many studies have advocated enzymatic digestion for osteoblast harvest permitting biochemical investigations; this has been considered the most satisfactory method to study cell-biomaterial interactions,29 and hence this method was used in this study. The main effect of adding hydrocortisone and β-GP to osteoblasts was to increase the number of cells showing ALP expression by flow cytometry, which would be expected as the effect of hydrocortisone on the differentiation of these cells is well recognized. There was no statistically significant difference between skeletal sites with the addition of osteogenic medium. This suggests that cells in all cultures were able to respond to hydrocortisone and hence were comparable.
We hypothesized that the initial isolate would contain bone-lining cells and cells from within the bone marrow, and it was important to evaluate the initial cell preparations, as these could contain different proportions of mesenchymal cells at different stages of differentiation, which could account for some of the differences in behaviour seen in this study. Flow cytometry showed that there were no significant differences in the percentage of cells expressing either markers of early mesenchymal cells (CD271, CD73, CD90, and CD105) or ALP, as a marker of a more differentiated phenotype, when comparing acetabulum with the femoral neck or femoral isolates from the knee with those from the tibia. The higher differential expression of CD90 (6.6% in the acetabular and 0.9% in the femoral neck preparations) and CD73 (4.9% and 2.8% in acetabular and femoral neck preparations, respectively; 20.7% distal femur and 18.4% proximal tibia) in the initial isolates suggests the presence of adipogenic cells, as expression of these markers was higher and they have been associated with lipid synthesis and adipocytes.30 Very few cells expressing CD105 (0.4% acetabulum and neck; femur 0.1%, tibia 0.06%) were present in our preparations and CD105-negative cells within subpopulations of MSCs have been shown to possess osteogenic properties.31
The expression of ALP in initial isolates was not significantly different between cells from proximal femur and acetabulum or between the two sites in the knee. By passage three, human osteoblasts used in the experiments were phenotypically comparable showing a spindle shape morphology appropriate for cells of an osteoblastic lineage. In addition, at passage 3, a greater percentage of cells expressed ALP indicating that many were of an osteoblastic lineage and, in the hip, a greater percentage of these cells were seen in those cell populations derived from the femoral neck. This result supports the view that there is functional variation in human osteoblasts derived from these different anatomical areas that was not evident in the phenotype of the initial cell isolate populations. One possible reason for this is that there are fewer bone-lining cells in human osteoblast isolations from the acetabulum, due to a lower trabecular density and reduced trabecular surface area compared to the femoral neck. However, counter to this, no difference was seen in the percentage of cells expressing CD271 between isolates from the acetabulum and the proximal femur, and trabecular bone-lining cells are known to express this marker.32
In the knee, passage three human osteoblasts from the tibia showed lower production of type 1 collagen. However, proliferation, ALP expression, and the ability to mineralize were not significantly different between femur and tibia. This suggests that there is a greater similarity between human osteoblasts from the distal femur and proximal tibia than there is between human osteoblasts from the acetabulum and femoral neck.
Data from this project revealed that human osteoblasts harvested from the acetabulum demonstrated the lowest proliferation rates, produced the lowest amount of type-1 collagen, demonstrated the least expression of ALP, and produced the least amount of mineral as indicated by the amount of Alizarin Red S stain. During any surgical intervention, metalwork, implant fixation, and stability are dependent on the surrounding bone and the human osteoblasts surrounding these implants are, in part, responsible for the biological response of the surrounding tissue leading to effective fixation and subsequent function following implantation.
There are a number of possible reasons for the differences seen between human osteoblasts from the acetabulum and the femoral neck. One putative explanation is that human osteoblasts from the bone below the triradiate cartilage of the acetabulum derive from secondary ossification centres and may have a decreased ability to form functional osteoblasts compared to those from the femoral neck, which are formed from a primary ossification centre. Cells from the two sites in the knee are both from trabecular bone derived from secondary ossification centres below the subchondral bone of the distal femur and tibial plateau, which may explain their relative similarity. The response of the skeleton to mechanical load is well described and plays a role in both physiological and pathophysiological situations, e.g. aseptic loosening. It can be envisaged that the altered mechanical environments of the acetabulum and femoral neck may contribute to the biological differences found in cell populations from these tissues.33 Differences in potential for differentiation and function engendered by these mechanical factors and others provoked by the local environments can be maintained by epigenetic memory,34-36 and could lead to differences in cell function in vitro and, by extrapolation, the ability to respond to metalwork or an implant in vivo. In OA, it is recognized that subchondral sclerosis is one of the first changes to be seen in the acetabulum when compared to the head.37 This links a difference in mechanical performance to an altered cell response. This increase in bone density in the diseased zone is a radiological observation that supports a difference in human osteoblast activity.
The results in this project have suggested that a difference exists in the function and behaviour of human osteoblasts from acetabular bone and those from the femoral neck. The difference between cells from the two periarticular regions in the knee was limited to type-1 collagen production only. The biological difference between cells isolated from these tissues may reflect the underlying biology at these anatomical sites which, in the context of surgical intervention in these areas, could inform the increased propensity of the acetabular component in the hip and the tibial tray in the knee, to loosen.38-41
The main limitation of the study is that the number of patient samples is small and we acknowledge that the data, while statistically significant, are preliminary. Other studies should be carried out aimed at understanding differences in cell function between regions of bone relevant to implant fixation.
In summary, the concept of improving the local cell environment in order to improve longevity of surgical intervention is not new.42,43 Our data demonstrate differences in osteoblast function at periarticular sites in the hip and knee, and the results point to the need for further studies investigating the underlying cell biology to establish if this is an additional aetiology contributing to site-dependent differences in surgical outcome.
Contributor Information
Erden Ali, Email: ea389@cantab.net.
Mark Birch, Email: mab218@cam.ac.uk.
Niina Hopper, Email: niina.hopper@gmail.com.
Neil Rushton, Email: nr10000@cam.ac.uk.
Andrew W. McCaskie, Email: awm41@cam.ac.uk.
Roger A. Brooks, Email: rb10003@cam.ac.uk.
Author contributions
E. Ali: Conceptualization, Investigation, Formal analysis, Writing – original draft.
M. Birch: Writing – original draft.
N. Hopper: Writing – original draft.
N. Rushton: Writing – original draft.
A. W. McCaskie: Writing – review & editing.
R. A. Brooks: Writing – review & editing.
Funding statement
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
ICMJE COI statement
E. Ali and R. A. Brooks were funded by an institutional grant from the British Orthopaedic Association. R. A. Brooks also acknowledges an institutional grant from the National Institute for Health Research Cambridge Biomedical Research Centre for this study.
Open access funding
The authors confirm that the open access funding was provided by the University of Cambridge.
Acknowledgements
The authors acknowledge the help of the NIHR Cambridge BRC Cell Phenotyping Hub and Dr. Stephen Kaptoge - Senior Statistician, Public Health and Primary Care, University of Cambridge.
Ethical review statement
All participants provided written consent prior to enrolment in the study and ethical approval was granted by The Cambridge Research Ethics Committee (REC reference number 06/Q0108/213).
References
- 1.No authors listed . National Joint Registry for England, Wales, Northern Ireland and the Isle of Man: 16th Annual Report. National Joint Registry. 2019. https://reports.njrcentre.org.uk/Portals/0/PDFdownloads/NJR%2016th%20Annual%20Report%202019.pdf (date last accessed 5 August 2021).
- 2.Kärrholm J, Rogmark C, Nauclér E, Vinblad J, Mohaddes M, Rolfson O. The Swedish Hip Arthroplasty Register Annual Report 2018. The Swedish Hip Arthroplasty. 2018. https://registercentrum.blob.core.windows.net/refdocs/10.18158/r1x9rVaoL8.pdf (date last accessed 2 September 2021).
- 3.Mjöberg B. Fixation and loosening of hip prostheses. A review. Acta Orthop Scand. 1991;62(5):500–508. [DOI] [PubMed] [Google Scholar]
- 4.Sumner DR. Long-term implant fixation and stress-shielding in total hip replacement. J Biomech. 2015;48(5):797–800. [DOI] [PubMed] [Google Scholar]
- 5.Galloway F, Kahnt M, Ramm H, et al. . A large scale finite element study of a cementless osseointegrated tibial tray. J Biomech. 2013;46(11):1900–1906. [DOI] [PubMed] [Google Scholar]
- 6.Kolling C, Simmen BR, Labek G, Goldhahn J. Key factors for a successful national arthroplasty register. J Bone Joint Surg Br. 2007;89-B(12):1567–1573. [DOI] [PubMed] [Google Scholar]
- 7.Migliore A, Perrini MR, Romanini E, et al. . Comparison of the performance of hip implants with data from different arthroplasty registers. J Bone Joint Surg Br. 2009;91-B(12):1545–1549. [DOI] [PubMed] [Google Scholar]
- 8.Davis ET, Pagkalos J, Kopjar B. A higher degree of polyethylene irradiation is associated with a reduced risk of revision for aseptic loosening in total hip arthroplasties using cemented acetabular components: an analysis of 290,770 cases from the National Joint Registry of England, Wales, Northern Island and the Isle of Man. Bone Joint Res. 2020;20(9):563–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bayliss LE, Culliford D, Monk AP, et al. . The effect of patient age at intervention on risk of implant revision after total replacement of the hip or knee: a population-based cohort study. Lancet. 2017;389(10077):1424–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89-A(4):780–785. [DOI] [PubMed] [Google Scholar]
- 11.Kurtz SM, Ong KL, Schmier J, et al. . Future clinical and economic impact of revision total hip and knee arthroplasty. J Bone Joint Surg Am. 2007;89 Suppl 3:144–151. [DOI] [PubMed] [Google Scholar]
- 12.Kurtz SM, Ong KL, Lau E, Bozic KJ. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am. 2014;96-A(8):624–630. [DOI] [PubMed] [Google Scholar]
- 13.Iorio R, Healy WL, Richards JA. Comparison of the hospital cost of primary and revision total knee arthroplasty after cost containment. Orthopedics. 1999;22(2):195–199. [DOI] [PubMed] [Google Scholar]
- 14.Barrack RL, Hoffman GJ, Tejeiro WV, Carpenter LJ. Surgeon work input and risk in primary versus revision total joint arthroplasty. J Arthroplasty. 1995;10(3):281–286. [DOI] [PubMed] [Google Scholar]
- 15.Vanhegan IS, Malik AK, Jayakumar P, Ul Islam S, Haddad FS. A financial analysis of revision hip arthroplasty: The economic burden in relation to the national tariff. J Bone Joint Surg Br. 2012;94-B(5):619–623. [DOI] [PubMed] [Google Scholar]
- 16.Briggs T. Getting it right the first time. Improving the quality of orthopaedic care within the National Health Service in England. 2020. https://www.gettingitrightfirsttime.co.uk/surgical-specialty/orthopaedic-surgery/ (date last accessed 2 September 2021).
- 17.Kallala RF, Vanhegan IS, Ibrahim MS, Sarmah S, Haddad FS. Financial analysis of revision knee surgery based on NHS tariffs and hospital costs: does it pay to provide a revision service? Bone Joint J. 2015;97-B(2):197–201. [DOI] [PubMed] [Google Scholar]
- 18.Ioannidis JP, MY N, Sham PC, et al. . Metaanalysis of genome-wide scans provides evidence for sex- and site-specific regulation of bone mass. J Bone Miner Res. 2008;22(2):173–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Varanasi SS, Olstad OK, Swan DC, et al. . Skeletal site-related variation in human trabecular bone transcriptome and signaling. PLoS One. 2010;5(5):1–12:e10692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rawlinson SC, McKay IJ, Ghuman M, et al. . Adult rat bones maintain distinct regionalized expression of markers associated with their development. PLoS One. 2009;4(12):1–11:e8358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shah M, Gburcik V, Reilly P, et al. . Local origins impart conserved bone type-related differences in human osteoblast behaviour. Eur Cell Mater. 2015;4(29):155–175. [DOI] [PubMed] [Google Scholar]
- 22.Meyer F, Wardale J, Best S, et al. . Effects of lactic acid and glycolic acid on human osteoblasts: a way to understand PLGA involvement in PLGA/calcium phosphate composite failure. J Orthop Res. 2012;30(6):864–871. [DOI] [PubMed] [Google Scholar]
- 23.Jackson SF, Smith RH. Studies on the biosynthesis of collagen. I. The growth of fowl osteoblasts and the formation of collagen in tissue culture. J Biophys Biochem Cytol. 1957;3(6):897–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peck WA, Birge SJ, Fedak SA. Bone cells: Biochemical and biological studies after enzymatic isolation. Science. 1964;146(3650):1476–1477. [DOI] [PubMed] [Google Scholar]
- 25.Jones SJ, Boyde A. The migration of osteoblasts. Cell Tissue Res. 1997;184(2):179–193. [DOI] [PubMed] [Google Scholar]
- 26.Beresford JN, Graves SE, Smoothy CA. Formation of mineralized nodules by bone derived cells in vitro: a model of bone formation. Am J Med Genet. 1993;45(2):163–178. [DOI] [PubMed] [Google Scholar]
- 27.Lian JB, Stein GS. Development of the osteoblast phenotype: molecular mechanisms mediating osteoblast growth and differentiation. Iowa Orthop J. 1995;15:118–140. [PMC free article] [PubMed] [Google Scholar]
- 28.Czekanska EM, Stoddart MJ, Richards RG, Hayes JS. In search of an osteoblast cell model for in vitro research. Eur Cell Mater. 2012;24:1–17. [DOI] [PubMed] [Google Scholar]
- 29.Declercq H, Van den Vreken N, De Maeyer E, et al. . Isolation, proliferation and differentiation of osteoblastic cells to study cell/biomaterial interactions: comparison of different isolation techniques and source. Biomaterials. 2004;25(5):757–768. [DOI] [PubMed] [Google Scholar]
- 30.Müller G, Schneider M, Biemer-Daub G, Wied S. Upregulation of lipid synthesis in small rat adipocytes by microvesicle-associated CD73 from large adipocytes. Obesity (Silver Spring). 2011;19(8):1531–1544. [DOI] [PubMed] [Google Scholar]
- 31.Leyva-Leyva M, Barrera L, López-Camarillo C, et al. . Characterization of mesenchymal stem cell subpopulations from human amniotic membrane with dissimilar osteoblastic potential. Stem Cells Dev. 2013;22(8):1275–1287. [DOI] [PubMed] [Google Scholar]
- 32.Jones E, English A, Churchman SM, et al. . Largescale extraction and characterization of CD271+ multipotential stromal cells from trabecular bone in health and osteoarthritis. Arthritis & Rheumatism. 2010;62(7):1944–1954. [DOI] [PubMed] [Google Scholar]
- 33.Van Houcke JV, Khanduja V, Pattyn C, Audenaert E. The history of biomechanics in total hip arthroplasty [published correction appears in. Indian J Orthop. 2017;51(5):359–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen JC, Chua M, Bellon RB, Jacobs CR. Epigenetic changes during mechanically induced osteogenic lineage commitment. J Biomech Eng. 2015;137(2):020902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vrtačnik P, Marc J, Ostanek B. Epigenetic mechanisms in bone. Clin Chem Lab Med. 2014;52(5):589–608. [DOI] [PubMed] [Google Scholar]
- 36.Steinberg J, Brooks RA, Southam L, et al. . Widespread epigenomic, transcriptomic and proteomic differences between hip osteophytic and articular chondrocytes in osteoarthritis. Rheumatology (Oxford). 2018;57(8):1481–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li G, Yin J, Gao J, et al. . Subchondral bone in osteoarthritis: insight into risk factors and microstructural changes. Arthritis Res Ther. 2013;15(6):223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parratte S, Argenson JN, Flecher X, Aubaniac JM. Acetabular revision for aseptic loosening in total hip arthroplasty using cementless cup and impacted morselized allograft. Rev Chir Orthop Reparatrice Appar Mot. 2007;93(3):255–263. [DOI] [PubMed] [Google Scholar]
- 39.Berry DJ, Harmsen WS, Cabanela ME, Morrey BF. Twenty-five-year survivorship of two thousand consecutive primary Charnley total hip replacements: factors affecting survivorship of acetabular and femoral components. J Bone Joint Surg Am. 2002;84-A(2):171–177. [DOI] [PubMed] [Google Scholar]
- 40.Hilding MB, Lanshammar H, Ryd L. Knee joint loading and tibial component loosening. RSA and gait analysis in 45 osteoarthritic patients before and after TKA. J Bone Joint Surg Br. 1996;78-B(1):66–73. [PubMed] [Google Scholar]
- 41.Peters CL, Craig MA, Mohr RA, Bachus KN. Tibial component fixation with cement: full-versus surface-cementation techniques. Clin Orthop Relat Res. 2003;409:158–168. [DOI] [PubMed] [Google Scholar]
- 42.Shanbhag AS. Use of bisphosphonates to improve the durability of total joint replacements. J Am Acad Orthop Surg. 2006;14(4):215–225. [DOI] [PubMed] [Google Scholar]
- 43.Arabmotlagh M, Pilz M, Warzecha J, Rauschmann M. Changes of femoral periprosthetic bone mineral density 6 years after treatment with alendronate following total hip arthroplasty. J Orthop Res. 2009;27(2):183–188. [DOI] [PubMed] [Google Scholar]


