Abstract
Aims
To investigate whether idiopathic osteonecrosis of the femoral head (ONFH) is related to impaired osteoblast activities.
Methods
We cultured osteoblasts isolated from trabecular bone explants taken from the femoral head and the intertrochanteric region of patients with idiopathic ONFH, or from the intertrochanteric region of patients with osteoarthritis (OA), and compared their viability, mineralization capacity, and secretion of paracrine factors.
Results
Osteoblasts from the intertrochanteric region of patients with ONFH showed lower alkaline phosphatase (ALP) activity and mineralization capacity than osteoblasts from the same skeletal site in age-matched patients with OA, as well as lower messenger RNA (mRNA) levels of genes encoding osteocalcin and bone sialoprotein and higher osteopontin expression. In addition, osteoblasts from patients with ONFH secreted lower osteoprotegerin (OPG) levels than those from patients with OA, resulting in a higher receptor activator of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) ligand (RANKL)-to-OPG ratio. In patients with ONFH, osteoblasts from the femoral head showed reduced viability and mineralized nodule formation compared with osteoblasts from the intertrochanteric region. Notably, the secretion of the pro-resorptive factors interleukin-6 and prostaglandin E2 as well as the RANKL-to-OPG ratio were markedly higher in osteoblast cultures from the femoral head than in those from the intertrochanteric region.
Conclusion
Idiopathic ONFH is associated with a reduced mineralization capacity of osteoblasts and increased secretion of pro-resorptive factors.
Cite this article: Bone Joint Res 2021;10(9):619–628.
Keywords: Osteonecrosis of the femoral head, Idiopathic, Osteoblasts, osteoblasts, Osteonecrosis of the femoral head (ONFH), femoral head, osteoarthritis (OA), secretion, mRNA, RNA, interleukin-6, Alkaline phosphatase, Trabecular bone
Article focus
Alterations in bone tissue distant from the necrotic lesion suggest that non-traumatic osteonecrosis of the femoral head (ONFH) could be related to impaired osteoblast function.
To study the in vitro behaviour of osteoblasts from the intertrochanteric region of patients with idiopathic ONFH compared to that of osteoblasts from the same skeletal site in age-matched patients with osteoarthritis (OA).
To comparatively study the in vitro behaviour of osteoblasts from the femoral head and the intertrochanteric region of patients with ONFH.
Key messages
Osteoblasts from patients with idiopathic ONFH have lower mineralization capacity than those from patients with OA.
Production of pro-resorptive factors is higher in osteoblast cultures from patients with idiopathic ONFH than those from patients with OA.
Strengths and limitations
To our knowledge, this is the first study to show that idiopathic ONFH is related to impaired mineralization capacity of osteoblasts and increased production of pro-resorptive factors.
The limitations of the study include the control group, which consisted of patients with primary OA, and the relatively small sample size.
Introduction
Osteonecrosis of the femoral head (ONFH) is a progressive and disabling condition that often leads to subchondral collapse in late stages, and its diagnosis accounts for approximately 3% to 12% of total hip arthroplasties (THAs).1 ONFH is more frequently the diagnosis for young patients undergoing THA. Previous trauma and massive corticosteroid administration are the most common risk factors for developing ONFH; however, a large number of patients have no known risk factor for the disease and are described as idiopathic.2 Although the pathogenesis of ONFH is still unclear, various mechanisms leading to ischaemia have been postulated, including vascular interruption by trauma, intravascular occlusion by thrombi and fat emboli, and intraosseous extravascular compression. ONFH has classically been considered a vascular disease with secondary changes in the subchondral bone. However, increasing evidence suggests that certain changes in bone tissue distant from the necrotic lesion, including the intertrochanteric and metaphyseal region3-5 and the transiliac bone,6 are not easily explained as secondary changes but could be part of the primary disease. In fact, abnormal cancellous bone in the proximal femur has been discussed as a major reason for a higher risk of revision surgery in patients with ONFH than in patients with primary osteoarthritis (OA).7,8
The mechanism of mechanical failure of the femoral head in patients with nontraumatic ONFH is associated with the formation of microfractures at the junction between the reparative zone and the necrotic bone due to extensive remodelling and insufficient bone formation.9-11 The rate of bone formation is largely determined by the number of osteoblasts and their ability to synthesize and mineralize bone matrix. Osteoblasts also contribute to bone repair by regulating angiogenesis and bone resorption through the secretion of paracrine factors, including vascular endothelial growth factor (VEGF), osteoprotegerin (OPG), receptor activator of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) ligand (RANKL), and other cytokines.12 Abnormalities in the replicative capacity of osteoblasts from the proximal femur have been found in patients with ONFH associated with glucocorticoid therapy when compared with patients with OA.13 Moreover, osteoblast apoptosis is frequent in the femoral head of patients with glucocorticoid-induced ONFH.4,14 These findings might be expected, given that glucocorticoids inhibit osteoblastogenesis, promote osteoblast apoptosis, and reduce bone formation and strength.15 Nevertheless, the mechanisms involved in the pathogenesis and progression of ONFH not induced by glucocorticoids remain poorly understood. In this study, we investigated whether ONFH without an obvious aetiological factor, referred to as idiopathic, could be related to abnormal osteoblast activities. To this end, we evaluated the cell viability, mineralization capacity, and secretory activity in osteoblasts obtained from the intertrochanteric region, located distant from the necrotic lesion, of patients with idiopathic ONFH and those with OA. We also explored the biological processes that could be affected by ONFH by comparing the in vitro function of osteoblasts obtained from the femoral head and the intertrochanteric region of patients with ONFH.
Methods
Patients and bone sample collection
Patients younger than 66 years of age undergoing THA between January 2017 and December 2018 in our institution were considered for inclusion, with nine patients with non-traumatic ONFH ultimately included in this study. We defined ONFH through imaging after assessing conventional radiographs and MRI. All patients had Ficat and Arlet stage IV ONFH and required THA.16,17 The period of time between hip early symptoms and severe loss of joint function varied for each patient and ranged from a minimum of six months to three years. Nine patients with primary OA of the hip were considered as controls, with the groups matched by age and sex. OA was confirmed by radiography. Informed consent was obtained from all participants. All procedures were approved by the Clinical Research Ethics Committee of Hospital Universitario La Paz. The ONFH group includes cases of ONFH without an obvious aetiological factor. Patients with risk factors such as smoking, obesity, dyslipidaemia, and hypertension, which had a high prevalence in both groups, were also included. Patients with a history of glucocorticoid therapy, alcoholism, metabolic bone diseases, and major trauma of the hips were excluded from the study. Patients with congenital hip dysplasia, Gaucher disease, sickle cell disease, decompression sickness, haematopoietic or solid organ transplant, or who were receiving chemotherapy, were also excluded to limit confounding from these conditions that lead to a particularly high risk for ONFH. Table I presents the demographic and clinical characteristics. The mean age was 56 years (50 to 63) for the ONFH group and 60 years (55 to 65) for the OA group. The demographic characteristics were similar between the groups in terms of sex, weight, and BMI. Among the nine patients with ONFH, a patient had a history of breast cancer in their medical records and another patient had a history of melanoma. Both were diagnosed more than ten years earlier and did not require chemotherapy. Two patients had hypothyroidism and were on thyroxine replacement with normal thyroid function and no associated metabolic bone diseases. Although the percentage of patients who smoked was greater in the ONFH group than in the OA group, we could not detect statistical differences with the number of patients available. There were no significant differences in the prevalence of diabetes, obesity, dyslipidaemia, or other conditions between the groups.
Table I.
Demographic and clinical characteristics of patients.
| Variable | OA (n = 9) | ONFH (n = 9) | p-value |
|---|---|---|---|
| Mean age, yrs (SD) | 60 (6) | 56 (8) | 0.309* |
| Mean weight, kg (SD) | 75 (10) | 79 (10) | 0.335* |
| Mean BMI, kg/m2 (SD) | 28 (4) | 28 (3) | 0.923* |
| Male sex, n (%) | 3 (33) | 4 (44) | 0.629† |
| Smoking, n (%) | 2 (22) | 6 (67) | 0.058† |
| Hypertension, n (%) | 6 (67) | 2 (22) | 0.058† |
| Dyslipidaemia, n (%) | 4 (44) | 5 (56) | 0.634† |
| Obesity, n (%) | 4 (44) | 4 (44) | 1.000† |
| Diabetes, n (%) | 1 (11) | 1 (11) | 1.000† |
| Cancer, n (%) | 0 (0) | 2 (22) | 0.134† |
| Hypothyroidism, n (%) | 1 (11) | 2 (22) | 0.527† |
Mann–Whitney U test.
Chi-squared test.
OA, osteoarthritis; ONFH, osteonecrosis of the femoral head; SD, standard deviation.
Trabecular bone explants were taken intraoperatively from the intertrochanteric region of the femur of patients in both groups by using a box osteotome during femoral preparation. In the ONFH group, bone explants from the femoral head were also obtained with a curette (Figure 1).
Fig. 1.
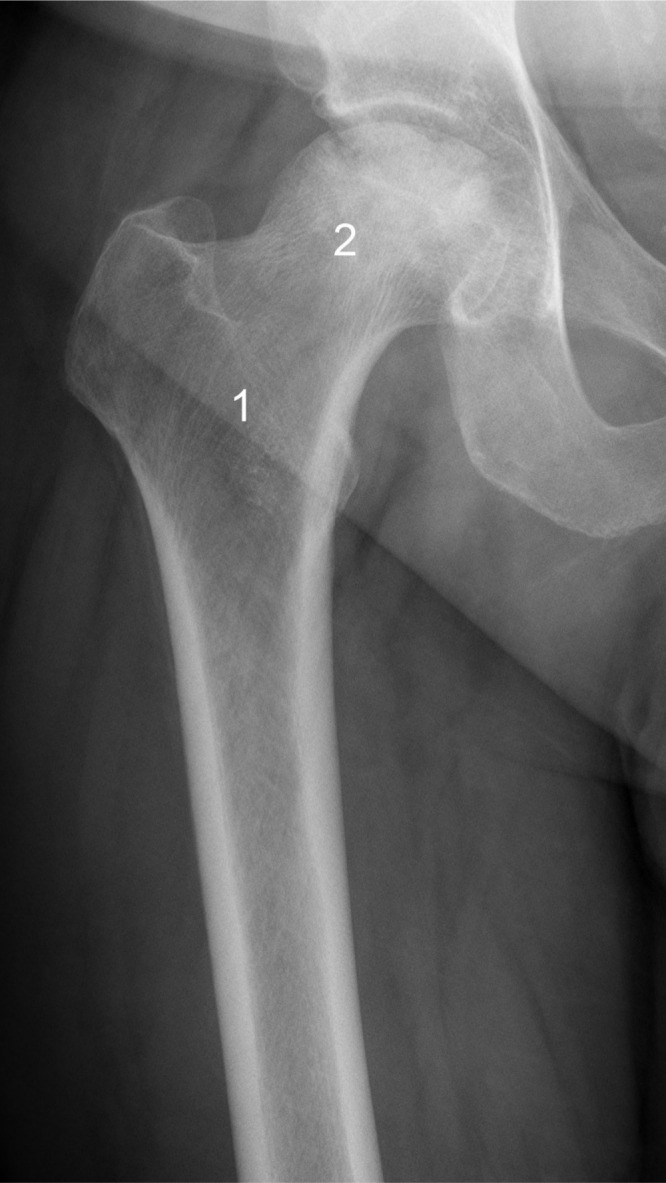
Preoperative anteroposterior radiograph of a right hip in a 50-year-old man with osteonecrosis of the femoral head (ONFH). Trabecular bone explants were taken from the femoral head (1) and from the intertrochanteric region (2) of patients with ONFH.
Cell culture and viability
Osteoblasts were isolated from bone explants and cultured using a standardized technique, as previously described.18 Each bone sample was processed in a separated primary culture and experiments were performed using independent cultures at passage 1. In the ONFH group, osteoblast cultures from the intertrochanteric region were compared with those from the femoral head of the same patient. All the in vitro studies were conducted on osteoblasts derived from each of the 18 patients included in this study. The bone fragments were cultured in growth medium consisting of Dulbecco's Modified Eagle Medium (DMEM; Lonza, Switzerland) supplemented with 15% fetal bovine serum and antibiotics. The cells were maintained at 37°C in a humidified 5% carbon dioxide (CO2) incubator. Cells were seeded at a density of 5 × 104 cells/well in 24-well plates and cultured in 500 μl of growth medium for one, four, and seven days. Cell viability was evaluated using the alamarBlue assay (Biosource, Belgium). Briefly, cells were incubated for three hours in DMEM containing 10% alamarBlue dye and the fluorescence emitted by cell-reduced alamarBlue was quantified using a spectrofluorometer (Synergy 4; BioTek, France).
Immunoenzymatic assays
Cells were seeded at a density of 105 cells/well in 12-well plates and incubated in 1 ml of growth medium for three days. The culture media were collected, clarified by centrifugation at 1,200 g for ten minutes, supplemented with 2 μg/ml of aprotinin, 17.5 μg/ml of phenylmethylsulphonyl fluoride, 1 μg/ml of pepstatin A, and 50 μg/ml of bacitracin (all from MilliporeSigma, Spain), and stored at -80°C. The levels of interleukin (IL)-6, IL-8, VEGF, and monocyte chemoattractant protein-1 (MCP-1) were determined in cell culture media using BD CBA Flex Sets (BD Biosciences, USA). The data were acquired using a FACSCalibur flow cytometer and analyzed with the FCAP Array Software version 3.0 (BD Biosciences). Human-specific enzyme-linked immunosorbent assay (ELISA) kits were used to measure the levels of OPG (Bender MedSystems GmbH, Austria), RANKL (Biomedica Gruppe, Austria), macrophage colony-stimulating factor (M-CSF) (R&D Systems, Germany), and prostaglandin E2 (PGE2) (Cayman Chemical Company, USA), according to the manufacturer’s instructions.
Alkaline phosphatase activity and alizarin red staining
Cells were seeded at a density of 2 × 105 cells/well in six-well plates, and incubated in 2 ml of osteogenic medium consisting of growth medium supplemented with 10-7 M dexamethasone, 3 × 10−4 M ascorbic acid, and 10−2 M β-glycerophosphate (all from MilliporeSigma) for 12 and 18 days. Cells incubated in growth medium were used as controls. In all cases, the culture medium was partially replaced with an equal volume of fresh medium every three days. After 12 days of culture, cell layers were extracted with 5 × 10−1 M sodium chloride (NaCl), 5 × 10−2 M Tris-Hydrochloride (HCl) pH 8.0, and 1% Triton X-100 (all from MilliporeSigma), and supplemented with protease inhibitors as described above. Alkaline phosphatase (ALP) activity was measured in the cell layers by determining the release of p-nitrophenol from p-nitrophenyl phosphate (MilliporeSigma). The data were normalized to the total protein amount in the cell extracts, determined by a Bradford-based protein assay (Bio-Rad Laboratories, USA). The degree of cell layer calcification was assessed in cells cultured for 18 days using alizarin red staining. Cells fixed with ethanol were stained with 40 mM alizarin red S in deionized water at a pH of 4.2. Images of stained cell layers were captured using a phase-contrast microscope. The bound stain was eluted with 10% cetylpyridinium chloride (MilliporeSigma), and the absorbance at 562 nm was measured using a spectrofluorometer.
Gene expression
Cells were seeded at a density of 2 × 105 cells/well in six-well plates and incubated in 2 ml of osteogenic medium for 12 and 18 days. Total RNA was isolated using TRI Reagent (Molecular Research Center, USA). Complementary DNA was prepared from the total RNA using the Transcriptor Reverse Transcriptase and an anchored-oligo (dT)18 primer (Roche, Spain). Real-time quantitative polymerase chain reaction was performed using the LightCycler FastStart DNA Master SYBR Green I and a LightCycler instrument (Roche). Quantitative expression values were normalized to the mean of the expression values of HPRT1 and GUSB. Specific oligonucleotide primers are shown in Supplementary Table i.
Immunofluorescence assays
Cells were seeded at a density of 2 × 104 cells/well in eight-well chamber slides and incubated in 200 μl of growth medium for seven days or in 200 μl of osteogenic medium for 18 days. Cells were fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS), permeabilized with 0.1% Triton X-100 in PBS, blocked in PBS containing 2% bovine serum albumin (BSA) and 0.05% Tween 20 (all from MilliporeSigma), and incubated with mouse anti-human fibronectin monoclonal antibody (Santa Cruz Biotechnology, Germany) or with mouse anti-human osteocalcin (Biogenesis, UK) diluted 1:50 in 1% BSA in PBS. After washing with 0.05% Tween 20 in PBS, cells were incubated with goat anti-mouse Alexa-Fluor 488 (Molecular Probes, The Netherlands) diluted 1:1,000 in 1% BSA in PBS. To label the actin cytoskeleton, cells were additionally incubated with PBS containing 4 × 10−7 M rhodamine-phalloidin (MilliporeSigma). For nuclei labelling, cells were incubated with PBS containing 3 × 10−6 M 4′,6-Diamidino-2-phenylindole (DAPI; MilliporeSigma). Cells were imaged using a confocal microscope (Leica TCS SPE; Leica, Germany).
Statistical analysis
The statistical analyses were performed using SPSS version 25.0 (IBM, USA). Exact chi-squared tests were performed to compare the proportions between the groups. Cell culture experiments were performed in duplicate and data are presented as means (standard deviation (SD)). Pairwise comparisons between the groups were analyzed by using the Mann-Whitney U test and the paired Wilcoxon test. Statistical significance was set at p < 0.05.
Results
Cell viability and fibronectin matrix formation
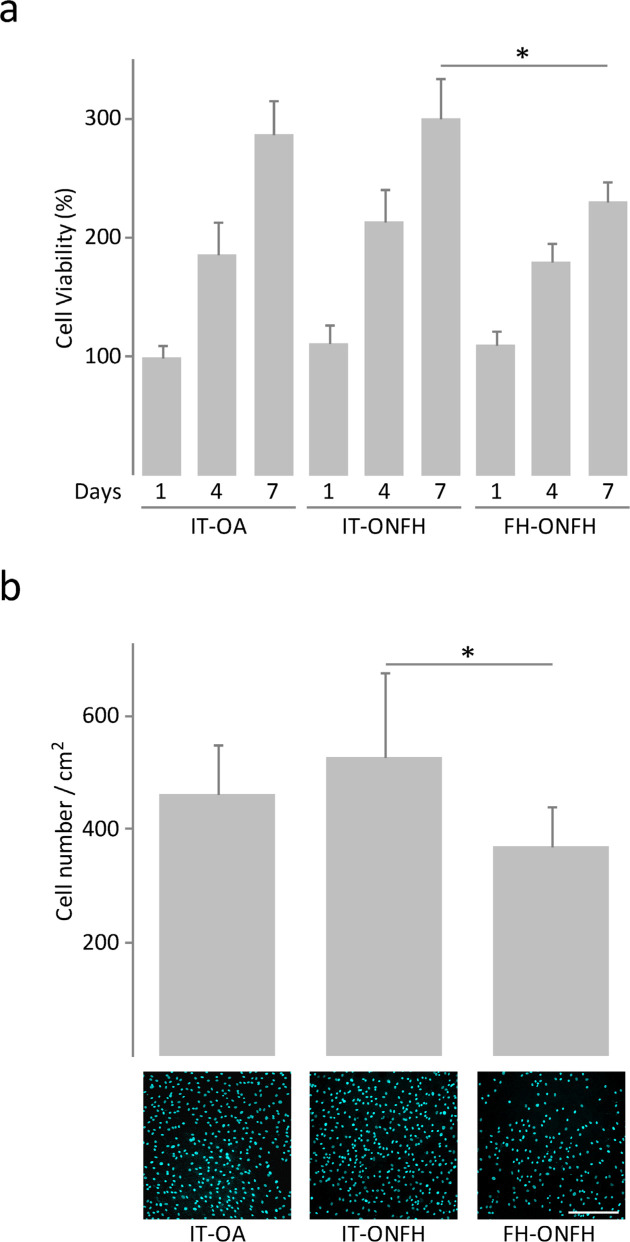
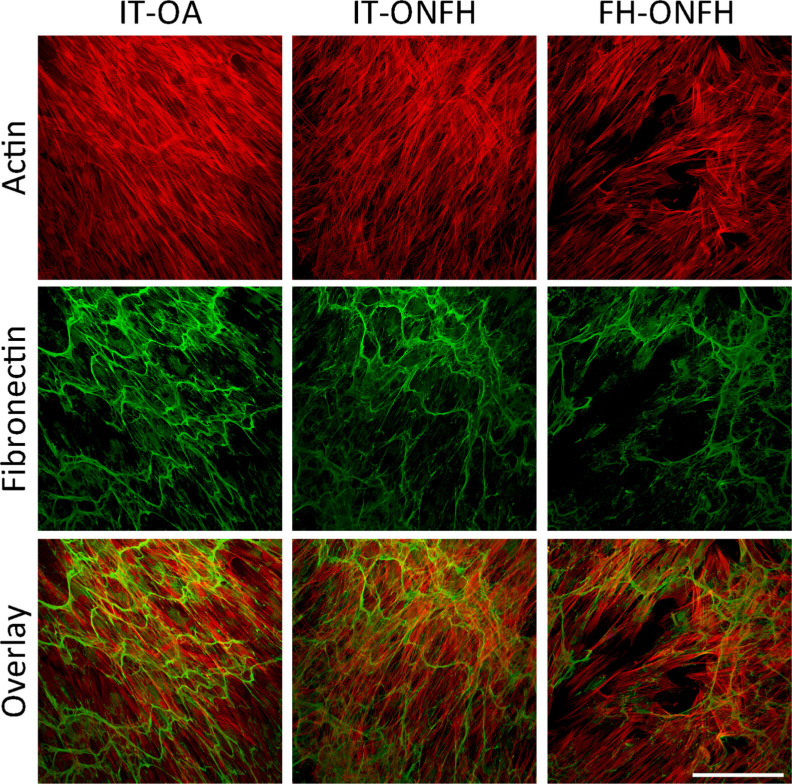
We conducted cell viability assays after culturing osteoblasts from patients with ONFH or OA for one, four, and seven days in growth medium. In all cases, cell viability increased throughout the incubation period (Figure 2a). There were no differences in cell viability between osteoblasts from the intertrochanteric region of patients with ONFH and those from the same skeletal site in patients with OA at any timepoint (Figure 2a). In patients with ONFH, cell viability at seven days was lower in osteoblasts from the femoral head than in those from the intertrochanteric region, which was associated with lower cell number, as revealed in images of DAPI-stained cells (Figure 2b). However, osteoblasts obtained from the femoral head of patients with ONFH displayed a well-spread morphology with a dense interconnected fibronectin network similar to that observed in osteoblasts from the intertrochanteric region of patients with ONFH or OA (Figure 3).
Fig. 2.
Viability of osteoblasts from patients with osteonecrosis of the femoral head (ONFH). a) Viability of osteoblasts obtained from the intertrochanteric region (IT) of patients with osteoarthritis (OA) or ONFH and from the femoral head (FH) of patients with ONFH, and cultured in growth medium for one, four, and seven days. Data were normalized relative to those measured in osteoblasts from IT-OA at day 1, which were given an arbitrary value of 100. b) 4′,6-Diamidino-2-phenylindole (DAPI) staining and number of osteoblasts after seven days of culture. Bar = 400 μm. *p < 0.05 between the indicated conditions, paired Wilcoxon test.
Fig. 3.
Actin cytoskeleton and fibronectin matrix in osteoblasts from patients with osteonecrosis of the femoral head (ONFH). Osteoblasts obtained from the intertrochanteric region (IT) of patients with osteoarthritis (OA) or ONFH and from the femoral head (FH) of patients with ONFH were cultured in growth medium for seven days. Confocal maximum projections showing cells stained for actin (red) and fibronectin (green). Bar = 200 μm.
Matrix maturation and mineralization
After culturing for 12 days in osteogenic medium, osteoblasts from the intertrochanteric region of patients with ONFH showed lower ALP activity and alkaline phosphatase (ALPL) messenger RNA (mRNA) levels than those from the same skeletal site in patients with OA (Figure 4). The osteoblast ability to develop a mineralized matrix was assessed in cells cultured in osteogenic medium for 18 days. Cultures were stained with alizarin red to detect calcium deposits, or immunostained for osteocalcin, an osteoblast-specific protein produced by osteoblasts in late stages of mineralization. Interestingly, cultures of osteoblasts from the intertrochanteric region of patients with ONFH showed a reduction in calcium deposition compared with those from patients with OA (Figure 5a). Close examination of alizarin red staining revealed that nodular aggregates were smaller and stained less intensely in cultures from patients with ONFH than those with OA (Figure 5b). Furthermore, osteocalcin accumulation in cell layers was less evident in cultures from patients with ONFH (Figure 5b). Having observed that ONFH could be associated with reduced mineralized nodule formation, we investigated whether the expression, at the mRNA level, of bone gamma-carboxyglutamate protein (BGLAP, osteocalcin), integrin-binding sialoprotein (IBSP, bone sialoprotein), and secreted phosphoprotein 1 (SPP1, osteopontin) was different in osteoblasts from patients with ONFH and in those from patients with OA. We found that BGLAP and IBSP mRNA levels were lower in osteoblasts from patients with ONFH than from those with OA (Figure 6). In contrast, mRNA levels of SPP1 increased in osteoblasts from patients with ONFH.
Fig. 4.
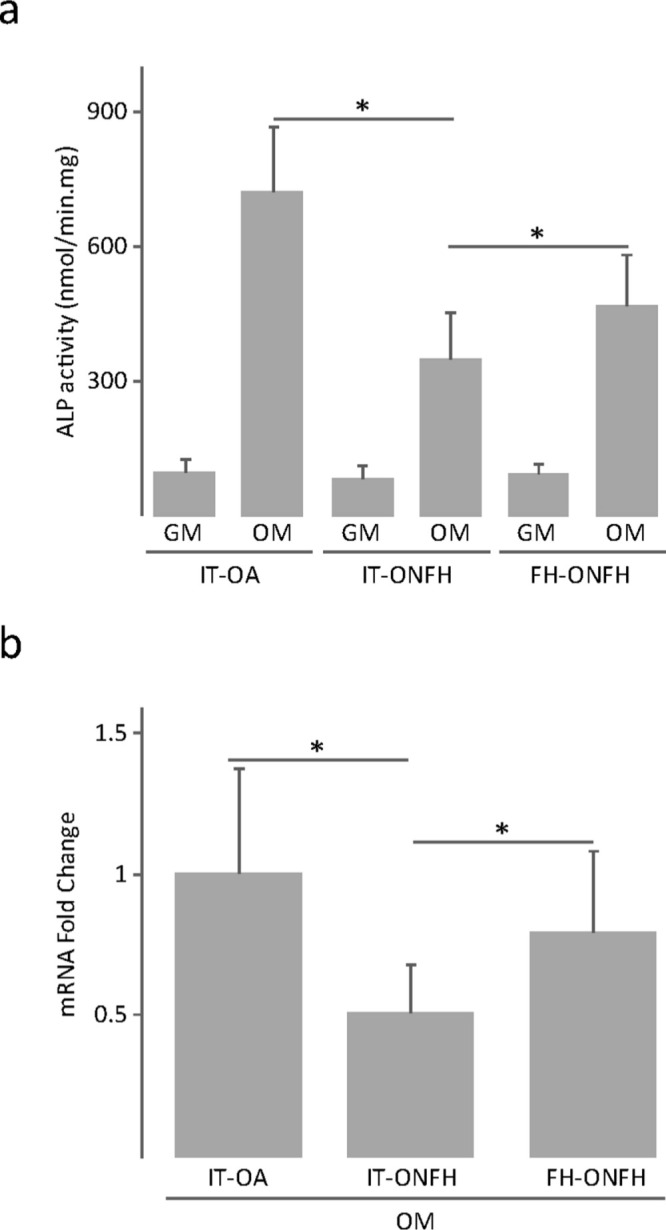
Alkaline phosphatase (ALP) activity in osteoblasts from patients with osteonecrosis of the femoral head (ONFH). a) ALP activity and b) ALPL messenger RNA (mRNA) levels in osteoblasts obtained from the intertrochanteric region (IT) of patients with osteoarthritis (OA) or ONFH and from the femoral head (FH) of patients with ONFH, and cultured in growth medium (GM) or osteogenic medium (OM) for 12 days. The data in (b) were normalized relative to those measured in osteoblasts from IT-OA, which were given an arbitrary value of 1. *p < 0.05 between the indicated conditions, Mann–Whitney U test and paired Wilcoxon test.
Fig. 5.
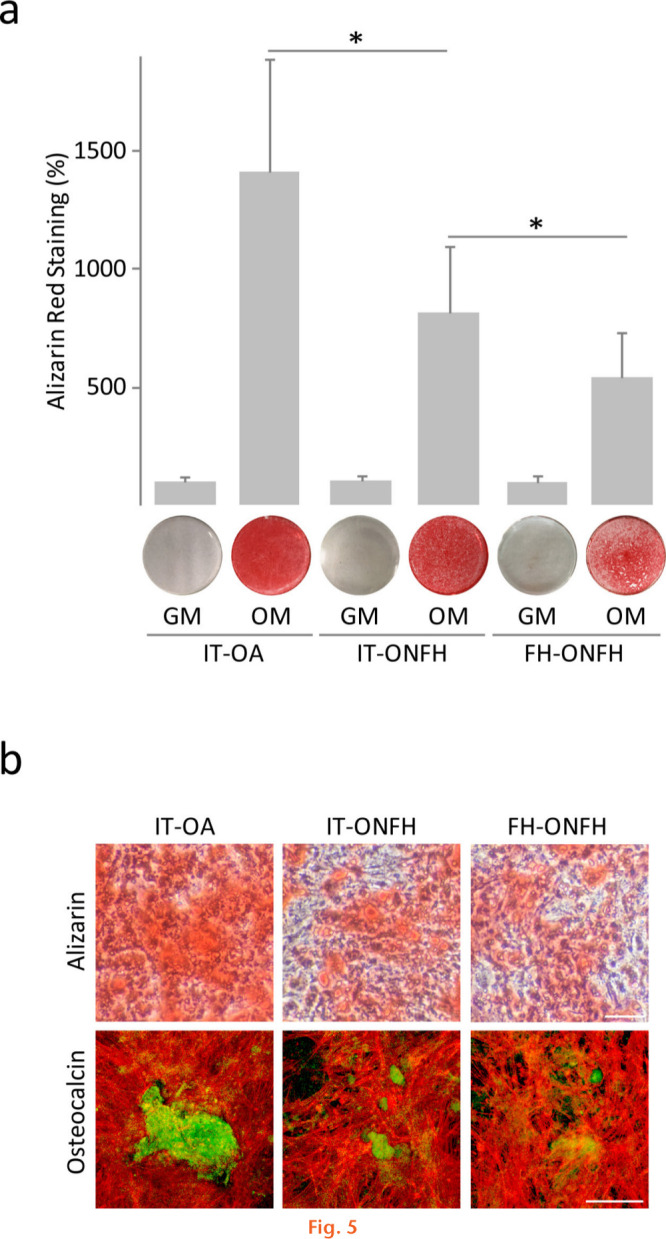
Mineralized nodule formation in osteoblasts from patients with osteonecrosis of the femoral head (ONFH). a) Alizarin red quantification in layers of osteoblasts obtained from the intertrochanteric region (IT) of patients with osteoarthritis (OA) or ONFH and from the femoral head (FH) of patients with ONFH, and cultured in growth medium (GM) or osteogenic medium (OM) for 18 days. The data were normalized relative to those measured in osteoblasts from IT-OA, which were given an arbitrary value of 100. b) Images showing alizarin red staining (upper panel) and osteocalcin (green) and actin (red) staining (lower panel) in cultures incubated in OM. Bar = 100 μm. *p < 0.05 between the indicated conditions, Mann–Whitney U test and paired Wilcoxon test.
Fig. 6.
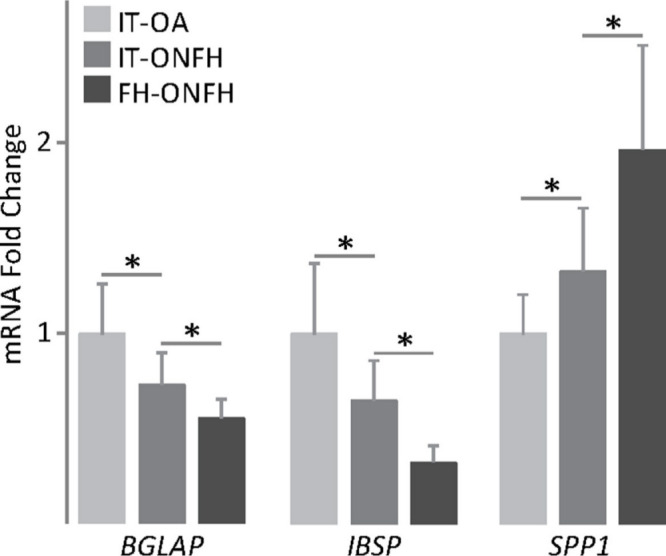
Expression of mineralization-related genes in osteoblasts from patients with osteonecrosis of the femoral head (ONFH). Bone gamma-carboxyglutamate protein (BGLAP), integrin binding sialoprotein (IBSP), and secreted phosphoprotein 1 (SPP1) messenger RNA (mRNA) levels in osteoblasts obtained from the intertrochanteric region (IT) of patients with osteoarthritis (OA) or ONFH and from the femoral head of patients with ONFH, and cultured in osteogenic medium for 18 days. The data were normalized relative to those measured in osteoblasts from IT-OA, which were given an arbitrary value of 1. *p < 0.05 between the indicated conditions, Mann–Whitney U test and paired Wilcoxon test.
A comparison between osteoblasts from the femoral head and from the intertrochanteric region of patients with ONFH revealed distinct mineralization capacity. ALP activity and mRNA levels were higher in osteoblasts from the femoral head than in those from the intertrochanteric region (Figure 4), whereas the degree of cell layer calcification was lower (Figure 5a). In fact, osteoblast cultures from the femoral head showed a more diffuse and discrete staining for alizarin red and osteocalcin than cultures from the intertrochanteric region (Figure 5b). Lower mineralization capacity of osteoblasts from the femoral head correlated with lower expression, at the mRNA level, of BGLAP and IBSP and with higher SPP1 mRNA levels (Figure 6).
Secretion of soluble factors
Lastly, we investigated the secretory profile of factors linked to bone remodelling and regeneration in osteoblasts from patients with idiopathic ONFH (Table II). The inflammatory factors IL-6, PGE2, MCP-1, IL-8, and M-CSF and the growth factor VEGF were secreted in similar amounts by osteoblasts from the intertrochanteric region of patients with ONFH and those from the same location in patients with OA. Interestingly, osteoblasts from patients with ONFH secreted lower OPG levels than cultures from patients with OA, which resulted in a higher RANKL-to-OPG molar ratio. In patients with ONFH, there were noticeable differences in the secretion of inflammatory factors between osteoblasts from the femoral head and those from the intertrochanteric region. IL-6 and PGE2 levels in the culture media were higher in cultures from the femoral head than in those from the intertrochanteric region. Conversely, osteoblasts from the femoral head secreted lower levels of MCP-1 and IL-8. Production levels of M-CSF, VEGF, and OPG were similar in osteoblasts from both skeletal sites. Of note, RANKL levels were markedly higher in cultures from the femoral head than in those from the intertrochanteric region, which correlated with a significant increase in the RANKL-to-OPG ratio.
Table II.
Secretion of soluble factors.
| Factor | IT-OA | IT-ONFH | FH-ONFH |
|---|---|---|---|
| IL-6 | 39.7 (14.7) | 37.3 (14.2) | 53.2 (18.6)* |
| PGE2 | 16.4 (7.2) | 15.2 (6.4) | 27.5 (8.7)* |
| MCP-1 | 6.0 (1.9) | 4.3 (1.4) | 3.3 (1.2)* |
| IL-8 | 7.6 (2.6) | 6.8 (2.0) | 4.1 (1.6)* |
| M-CSF | 3.2 (1.1) | 3.7 (0.9) | 2.7 (0.6) |
| VEGF | 3.2 (1.1) | 3.1 (1.3) | 3.2 (1.1) |
| OPG | 5.1 (1.4) | 3.6 (1.2)† | 3.8 (1.6) |
| RANKL | 66 (14) × 10-3 | 78 (17) × 10-3 | 152 (32) × 10-3 * |
| RANKL/OPG ratio (% of OA) | 100 (19) | 143 (31)† | 251 (83)* |
The data are expressed as pg/ml per μg of total proteins. Each value represents the mean (standard deviation).
Paired Wilcoxon test. p < 0.05 compared to IT-ONFH.
Mann-Whitney U test. p < 0.05 compared to IT-OA.
FH, femoral head; IL, interleukin; IT, intertrochanteric region; MCP-1, monocyte chemoattractant protein-1; M-CSF, macrophage colony-stimulating factor; OA, osteoarthritis; ONFH, osteonecrosis of the femoral head; OPG, osteoprotegerin; PGE2, prostaglandin E2; RANKL, receptor activator of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) ligand; VEGF, vascular endothelial growth factor.
Discussion
Studies on the pathophysiology of non-traumatic ONFH have been conducted mainly using bone explants from patients exposed to glucocorticoids. Other studies often combined different causes of ONFH together in one group, or poorly described patients’ clinical features, making it difficult to determine the disease mechanisms. To our knowledge, the present study is the first to investigate the osteoblast function in patients with idiopathic ONFH. The ONFH and OA groups were adjusted by age and sex, which are known to affect osteoblast activities.19-21 Patients with risk factors such as smoking, obesity, dyslipidaemia, and hypertension, which could be associated with idiopathic ONFH, were included in this study.22 However, there were no statistically significant differences in the proportion of each of these risk factors between patients with ONFH and those with OA, which could be attributed to their high prevalence in both groups, as well as to the small sample size.
Osteoblasts from the intertrochanteric region of patients with ONFH and those of patients with OA proliferated at a similar rate and assembled a dense fibronectin matrix, required for collagen polymerization and matrix integrity.23 However, ALP activity and alizarin red staining were lower in cultures from patients with ONFH than those with OA. In addition, the deposition of osteocalcin, which binds to hydroxyapatite during bone matrix mineralization, was lower in cultures from patients with ONFH. The abnormal mineralization capacity of osteoblasts could be responsible for the insufficient bone repair that occurs after ONFH and its progression to femoral head collapse. Non-collagenous proteins expressed by osteoblasts regulate the nucleation and growth of hydroxyapatite crystals. Abnormalities in non-collagenous protein expression have been found in several bone diseases, including osteoporosis24 and osteogenesis imperfecta.25 Compared to patients with OA, osteoblasts from the intertrochanteric region of patients with ONFH showed lower mRNA levels of genes encoding osteocalcin and bone sialoprotein, while the opposite trend was observed for osteopontin expression. Bone sialoprotein and osteocalcin appear to promote apatite crystal nucleation,26,27 whereas high osteopontin levels can prevent mineral growth.28 Although the levels of these proteins have not been quantified in the present study, different trends of expression of mineralization-related genes in osteoblasts from patients with ONFH could account for their reduced mineralization capacity.
Osteoblasts regulate osteoclastogenesis and bone resorption through the production of RANKL and OPG. The latter inhibits bone resorption by disrupting the interaction of RANKL with its receptor RANK, expressed on the surface of osteoclasts and their progenitors. Polymorphisms in the OPG and RANKL genes have been found in patients with ONFH associated with alcohol consumption.29 Our data suggest that idiopathic ONFH could be related to abnormal OPG expression by osteoblasts in the intertrochanteric region, as its secretion was lower in cultures from patients with ONFH than in those from the same anatomical site in patients with OA.
Accumulation of apoptotic osteocytes in the superior part of the femoral head is a feature of ONFH, independent of its aetiology.14 In addition, osteocyte apoptosis and bone marrow abnormalities in the deeper part of the head have been reported in bone biopsies.30,31 Osteocytes sense mechanical forces and then regulate osteoclast and osteoblast functions via the production of soluble factors that reach the bone marrow.32 It is therefore expected that osteocyte death affects osteoblast activity in the femoral head. Our data indicate that cell viability and mineralization in osteoblasts obtained from trabecular bone in the femoral head of patients with ONFH was lower than in osteoblasts from the intertrochanteric region. The increase in ALP activity levels, together with reduced matrix calcification, suggests that osteoblasts from the femoral head initiate matrix maturation but fail to undergo complete mineralization, which could be influenced by altered expression of non-collagenous proteins. In this regard, a histological study found fewer osteocalcin-stained cells in the femoral head than in the neck of patients with ONFH.33 However, there were no differences in the number of osteocalcin-stained cells between the femoral head and the neck of patients without ONFH, supporting the notion that the necrotic lesion affects osteoblast function.
Changes in the mechanical properties and microstructure of bone in the femoral head of patients with ONFH have been attributed to osteoclastic hyperactivity as a result of osteocyte death.10,11 A previous study with bone biopsies found that RANKL expression increased proximally to the necrotic region of patients with ONFH.11 Our results suggest that osteoblasts might boost osteoclast activity in the femoral head through the secretion of high levels of RANKL, as well as of IL-6 and PGE2, which collaborate in the production of pro-resorptive factors.34 Curiously, the increased secretion of pro-resorptive factors by osteoblasts from the femoral head correlated with a reduction in the levels of MCP-1 and IL-8, chemokines involved in osteoclast formation,35 which may be interpreted as a compensatory mechanism to prevent excessive bone resorption. Although further studies on the role of osteoclasts in ONFH are needed, therapeutic strategies targeting RANKL could be beneficial for patients with ONFH. Currently, the benefit/risk profile of RANKL-targeted therapy for the treatment of bone diseases is being investigated in clinical trials.36
Our study has several limitations. The control group consisted of patients with OA undergoing THA because of the ethical difficulties in obtaining bone biopsies from healthy donors. Moreover, the sample size in the study was relatively small, and therefore the results should be checked in future studies with a larger sample size in both groups. Lastly, osteoblasts from the femoral head and those from the intertrochanteric region might differ in patients with other bone diseases or in healthy individuals.
In conclusion, the present study indicates that osteoblasts from the intertrochanteric region of patients with idiopathic ONFH show reduced mineralization capacity and OPG secretion compared with osteoblasts obtained from the same skeletal site in sex- and age-matched patients with OA. We also found that mineralization decreases in osteoblasts from the femoral head of patients with ONFH compared with those from the intertrochanteric region, whereas the secretion of pro-osteoclastogenic factors increases. These findings provide new insight into the mechanisms involved in the pathogenesis and progression of idiopathic ONFH and might help in developing future treatment strategies. It should be noted that ONFH is frequently observed in patients younger than 50 years of age.37 The mean age of patients with ONFH included in this study was 56 years (SD 8), which is similar to that reported in European databases.7,38 Further studies on a series of younger patients with ONFH are needed to confirm our results.
Contributor Information
Leila Maestro-Paramio, Email: leila_maestro24@hotmail.com.
Eduardo García-Rey, Email: edugrey@yahoo.es.
Fátima Bensiamar, Email: fatima_B83@hotmail.com.
Laura Saldaña, Email: laura.saldana@salud.madrid.org.
Author contributions
L. Maestro-Paramio: Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing.
E. García-Rey: Conceptualization, Resources, Funding acquisition, Writing – review & editing.
F. Bensiamar: Investigation, Methodology, Writing – review & editing.
L. Saldaña: Conceptualization, Formal analysis, Funding acquisition, Supervision, Writing – original draft, Writing – review & editing.
Funding statement
This work (including open access funding) was supported by grant PI18/00643 from Instituto de Salud Carlos III (ISCIII)-Fondo Europeo de Desarrollo Regional (FEDER), Ministerio de Ciencia, Innovación y Universidades (MICINN)-AES. L.M.P was supported by a predoctoral contract (PEJD-2018-PRE/BMD-7965) from the Comunidad de Madrid (CAM). No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
ICMJE COI statement
The authors report institutional grants (paid to Hospital Universitario La Paz-IdiPAZ) from Instituto de Salud Carlos III (ISCIII)-Fondo Europeo de Desarrollo Regional (FEDER)- Ministerio de Economía y Competitividad (MINECO)-AES and Comunidad de Madrid (CAM) (grant numbers: PI18/00643 and PEJD-2018-PRE/BMD-7965, respectively), related to this study.
Acknowledgements
We are indebted to the Cell Culture and Image Facilities of the Hospital Universitario La Paz-IdiPAZ for their technical help. The authors would like to thank Dr N. Vilaboa and Dr G. Vallés for critically reviewing the manuscript.
Ethical review statement
The protocol of this study was reviewed and approved by the Clinical Research Ethics Committee of Hospital Universitario La Paz. All experiments were carried out in accordance with the approved guidelines and regulations.
Supplementary material
Table showing primer sequences used in polymerase chain reaction.
References
- 1.Lieberman JR, Berry DJ, Mont MA, et al. . Osteonecrosis of the hip: management in the 21st century. Instr Course Lect. 2003;52:337–355. [PubMed] [Google Scholar]
- 2.Fukushima W, Fujioka M, Kubo T, et al. . Nationwide epidemiologic survey of idiopathic osteonecrosis of the femoral head. Clin Orthop Relat Res. 2010;468(10):2715–2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laroche M, Costa L, Bernard J, et al. . Dual-energy X-ray absorptiometry in osteonecrosis of the femoral head. Rev Rhum Engl Ed. 1998;65(6):393–396. [PubMed] [Google Scholar]
- 4.Calder JDF, Pearse MF, Revell PA. The extent of osteocyte death in the proximal femur of patients with osteonecrosis of the femoral head. J Bone Joint Surg Br. 2001;83-B(3):419–422. [DOI] [PubMed] [Google Scholar]
- 5.Tingart M, Beckmann J, Opolka A, et al. . Analysis of bone matrix composition and trabecular microarchitecture of the femoral metaphysis in patients with osteonecrosis of the femoral head. J Orthop Res. 2009;27(9):1175–1181. [DOI] [PubMed] [Google Scholar]
- 6.Arlot ME, Bonjean M, Chavassieux PM, Meunier PJ. Bone histology in adults with aseptic necrosis. Histomorphometric evaluation of iliac biopsies in seventy-seven patients. J Bone Joint Surg Am. 1983;65-A(9):1319–1327. [PubMed] [Google Scholar]
- 7.Bergh C, Fenstad AM, Furnes O, et al. . Increased risk of revision in patients with non-traumatic femoral head necrosis. Acta Orthop. 2014;85(1):11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hart A, Janz V, Trousdale RT, et al. . Long-term survivorship of total hip arthroplasty with highly cross-linked polyethylene for osteonecrosis. J Bone Joint Surg Am. 2019;101-A(17):1563–1568. [DOI] [PubMed] [Google Scholar]
- 9.Narayanan A, Khanchandani P, Borkar RM, et al. . Avascular necrosis of femoral head: a metabolomic, biophysical, biochemical, electron microscopic and histopathological characterization. Sci Rep. 2017;7(1):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang C, Meng H, Wang Y, et al. . Analysis of early stage osteonecrosis of the human femoral head and the mechanism of femoral head collapse. Int J Biol Sci. 2018;14(2):156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang C, Wang X, Xu XL, et al. . Bone microstructure and regional distribution of osteoblast and osteoclast activity in the osteonecrotic femoral head. PLoS One. 2014;9(5):e96361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han Y, You X, Xing W, Zhang Z, Zou W. Paracrine and endocrine actions of bone-the functions of secretory proteins from osteoblasts, osteocytes, and osteoclasts. Bone Res. 2018;6:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gangji V, Hauzeur JP, Schoutens A, Hinsenkamp M, Appelboom T, Egrise D. Abnormalities in the replicative capacity of osteoblastic cells in the proximal femur of patients with osteonecrosis of the femoral head. J Rheumatol. 2003;30(2):348–351. [PubMed] [Google Scholar]
- 14.Mutijima E, Maertelaer D, Deprez M, Malaise M, Hauzeur JP. The apoptosis of osteoblasts and osteocytes in femoral head osteonecrosis: its specificity and its distribution. Clin Rheumatol. 2014;33(12):1791–1795. [DOI] [PubMed] [Google Scholar]
- 15.O’Brien CA, Jia D, Plotkin LI, et al. . Glucocorticoids act directly on osteoblasts and osteocytes to induce their apoptosis and reduce bone formation and strength. Endocrinology. 2004;145(4):1835–1841. [DOI] [PubMed] [Google Scholar]
- 16.Ficat RP, Arlet J. Forage-biopsie de la tete femorale dans I’osteonecrose primative. Observations histo-pathologiques portant sur huit forages. Rev Rhum. 1964;31:257–264. [Google Scholar]
- 17.Kuroda Y, Tanaka T, Miyagawa T, et al. . Classification of osteonecrosis of the femoral head: Who should have surgery? Bone Joint Res. 2019;8(10):451–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saldaña L, Bensiamar F, Boré A, Vilaboa N. In search of representative models of human bone-forming cells for cytocompatibility studies. Acta Biomater. 2011;7(12):4210–4221. [DOI] [PubMed] [Google Scholar]
- 19.Becerikli M, Jaurich H, Schira J, et al. . Age-dependent alterations in osteoblast and osteoclast activity in human cancellous bone. J Cell Mol Med. 2017;21(11):2773–2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ingram RT, Park YK, Clarke BL, Fitzpatrick LA. Age- and gender-related changes in the distribution of osteocalcin in the extracellular matrix of normal male and female bone. Possible involvement of osteocalcin in bone remodeling. J Clin Invest. 1994;93(3):989–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Starlinger J, Kaiser G, Thomas A, Sarahrudi K. The impact of nonosteogenic factors on the expression of osteoprotegerin and RANKL during human fracture healing. Bone Joint Res. 2019;8(7):349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao DW, Yu M, Hu K, et al. . Prevalence of Nontraumatic Osteonecrosis of the Femoral Head and its Associated Risk Factors in the Chinese Population: Results from a Nationally Representative Survey. Chin Med J. 2015;128(21):2843–2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sottile J, Hocking DC. Fibronectin polymerization regulates the composition and stability of extracellular matrix fibrils and cell-matrix adhesions. Mol Biol Cell. 2002;13(10):3546–3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruiz-Gaspà S, Blanch-Rubió J, Ciria-Recasens M, et al. . Reduced proliferation and osteocalcin expression in osteoblasts of male idiopathic osteoporosis. Calcif Tissue Int. 2010;86(3):220–226. [DOI] [PubMed] [Google Scholar]
- 25.Vetter U, Fisher LW, Mintz KP, et al. . Osteogenesis imperfecta: changes in noncollagenous proteins in bone. J Bone Miner Res. 1991;6(5):501–505. [DOI] [PubMed] [Google Scholar]
- 26.Hunter GK, Goldberg HA. Nucleation of hydroxyapatite by bone sialoprotein. Proc Natl Acad Sci U S A. 1993;90(18):8562–8565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen L, Jacquet R, Lowder E, Landis WJ. Refinement of collagen-mineral interaction: a possible role for osteocalcin in apatite crystal nucleation, growth and development. Bone. 2015;71:7–16. [DOI] [PubMed] [Google Scholar]
- 28.Gericke A, Qin C, Spevak L, et al. . Importance of phosphorylation for osteopontin regulation of biomineralization. Calcif Tissue Int. 2005;77(1):45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Y, Wang Y, Guo Y, et al. . OPG and RANKL polymorphisms are associated with alcohol-induced osteonecrosis of the femoral head in the north area of China population in men. Medicine (Baltimore). 2016;95(25):e3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hauzeur JP, Pasteels JL. Pathology of bone marrow distant from the sequestrum in nontraumatic, aseptic necrosis of the femoral head. : Arlet J, Mazières B. eds. Bone Circulation and Bone Necrosis. Berlin: Springer-Verlag. 1990:73–76. [Google Scholar]
- 31.Inoue A, Ono K. A histological study of idiopathic avascular necrosis of the head of the femur. J Bone Joint Surg Br. 1979;61-B(2):138–143. [DOI] [PubMed] [Google Scholar]
- 32.Jilka RL, Noble B, Weinstein RS. Osteocyte apoptosis. Bone. 2013;54(2):264–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tingart M, Beckmann J, Opolka A, et al. . Influence of factors regulating bone formation and remodeling on bone quality in osteonecrosis of the femoral head. Calcif Tissue Int. 2008;82(4):300–308. [DOI] [PubMed] [Google Scholar]
- 34.Liu XH, Kirschenbaum A, Yao S, Levine AC. Interactive effect of interleukin-6 and prostaglandin E2 on osteoclastogenesis via the OPG/RANKL/RANK system. Ann N Y Acad Sci. 2006;1068:225–233. [DOI] [PubMed] [Google Scholar]
- 35.Brylka LJ, Schinke T. Chemokines in physiological and pathological bone remodeling. Front Immunol. 2019;10:2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang N, Zhang ZK, Yu Y, Zhuo Z, Zhang G, Zhang BT. Pros and Cons of Denosumab Treatment for Osteoporosis and Implication for RANKL Aptamer Therapy. Front Cell Dev Biol. 2020;8:325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu F, Wang W, Yang L, et al. . An epidemiological study of etiology and clinical characteristics in patients with nontraumatic osteonecrosis of the femoral head. J Res Med Sci. 2017;22:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cooper C, Steinbuch M, Stevenson R, Miday R, Watts NB. The epidemiology of osteonecrosis: findings from the GPRD and THIN databases in the UK. Osteoporos Int. 2010;21(4):569–577. [DOI] [PMC free article] [PubMed] [Google Scholar]