Abstract
Background
Easily accessible biomarkers enabling the identification of those patients with multiple sclerosis (MS) who will accumulate irreversible disability in the long term are essential to guide early therapeutic decisions. We here examine the utility of serum neurofilament light chain (sNfL) for forecasting relapse-free disability progression and conversion to secondary progressive MS (SPMS) in the prospective Neurofilamentandlongtermoutcome inMS (NaloMS) cohort.
Methods
The predictive ability of sNfL at Baseline and sNfL follow-up (FU)/ Baseline (BL) ratio with regard to disability progression was assessed within a development cohort (NaloMS, n=196 patients with relapsing-remitting MS (RRMS) or clinically isolated syndrome) and validated with an external independent cohort (Düsseldorf, Essen, n=204). Both relapse-free EDSS-progression (RFP: inflammatory-independent EDSS-increase 12 months prior to FU) and SPMS-transition (minimum EDSS-score of 3.0) were investigated.
Findings
During the study period, 17% (n=34) of NaloMS patients suffered from RFP and 14% (n=27) converted to SPMS at FU (validation cohort RFP n=42, SPMS-conversion n=24). sNfL at BL was increased in patients with RFP (10.8 pg/ml (interquartile range (IQR) 7.7-15.0) vs. 7.2 pg/ml (4.5-12.5), p<0.017). In a multivariable logistic regression model, increased sNfL levels at BL (Odds Ratio (OR) 1.02, 95% confidence interval (CI) 1.01-1.04, p=0.012) remained an independent risk factor for RFP and predicted individual RFP risk with an accuracy of 82% (NaloMS) and 83% (validation cohort) as revealed by support vector machine. In addition, the sNfL FU/BL ratio was increased in SPMS-converters (1.16 (0.89-1.70) vs. 0.96 (0.75-1.23), p=0.011). This was confirmed by a multivariable logistic regression model, as sNfL FU/BL ratio remained in the model (OR 1.476, 95%CI 1.078-2,019, p=0.015) and individual sNfL FU/BL ratios showed a predictive accuracy of 72% in NaloMS (63% in the validation cohort) as revealed by machine learning.
Interpretation
sNfL levels at baseline predict relapse-free disability progression in a prospective longitudinal cohort study 6 years later. While prediction was confirmed in an independent cohort, sNfL further discriminates patients with SPMS at follow-up and supports early identification of patients at risk for later SPMS conversion.
Funding
This work was supported by the German Research Council (CRC-TR-128), Else Kröner Fresenius Foundation and Hertie-Stiftung.
Keywords: Multiple sclerosis, Disease progression, Neurofilament light chain, SPMS transition
Research in context.
Evidence before this study
Serum neurofilament light chain is an emerging biomarker in multiple sclerosis and other neurological disease. We used the terms “Neurofilament light chain”, “NfL”, “multiple sclerosis”, “MS”, “EDSS”, “progression”, “SPMS”, “relapse-free progression” in PubMed to find publications from any date up to January 19, 2021. Whereas numerous publications support an association of sNfL values with signs of current inflammatory activity such as Gadolinium-enhancing lesions on MRI and recent relapse activity, reports on the predictive value of sNfL for clinical meaningful endpoints such as transition into secondary progressive MS (SPMS) and disability progression are sparser and lack external validation.
Added value of this study
Within a prospective cohort study we examined the utility of sNfL for forecasting disability progression and transition into SPMS in patients with relapsing-remitting multiple sclerosis (RRMS) and clinically isolated syndrome (CIS) with a median follow-up of 6 years. We are able to show a predictive value of sNfL for disability progression, as defined by relapse-free EDSS-progression (RFP), confirmed on the individual level with an accuracy of 82% (NaloMS) and validated in an independent cohort (Düsseldorf, Essen; accuracy of 83%) by support vector machine, suggesting that early inflammatory activity has a strong impact on disability accumulation in the long run. Moreover, patients with sNfL levels above 7.2 pg/ml at study entry exhibited a higher risk of disability progression compared to patients with lower values. In addition, patients with sNfL increase at follow-up as shown by sNfL FU/BL ratio had a higher probability for transition into SPMS, which was confirmed on the individual level with an accuracy of 72% in NaloMS and externally validated in an independent validation cohort (Düsseldorf, Essen; accuracy 63%), as revealed by support vector machine. Routine sNfL measurement at follow-up might thereby facilitate early detection of currently mainly retrospectively assessed SPMS-diagnosis.
Implications of all the available evidence
In a prospective cohort study of 196 patients with CIS and RRMS, assessment of sNfL was able to predict relapse-free EDSS-progression at median 6-year follow-up. Especially, when it was measured longitudinally, increased sNfL values, were able to identify patients with transition to SPMS. We here provide sNfL cut-off values for prediction of disability progression and diagnosis of SPMS and were able to externally validate our findings, in order to support routine sNfL measurement and allowing independent comparable studies and thereby fostering the way of sNfL into clinical practice.
Alt-text: Unlabelled box
1. Introduction
Multiple sclerosis (MS) is an autoimmune disease of the CNS associated with neurologic disability and progression over time [1]. Natural history cohorts demonstrate that disability progression and prognosis varies among patients [2], therefore prognostic markers are needed to guide treatment decisions. Neurofilaments, especially neurofilament light chain (NfL), are an emerging marker for neuronal injury in MS [3] and other neurodegenerative [4] and ischemic diseases of the CNS [5]. However, studies focusing on clinically meaningful endpoints for individual patients’ outcome, such as predicting Expanded Disability Status Scale (EDSS)-progression over time [6,7] or the transition into secondary progressive multiple sclerosis (SPMS) [8], [9], [10] lack validation of their findings in external validation cohorts. In light of increasing treatment options for patients with SPMS [11], the current retrospective approach of SPMS diagnosis delays treatment initiation; markers for early identification of patients undergoing SPMS conversion are thus urgently needed to enable timely diagnosis and treatment of SPMS.
Currently, disability progression and the transition to SPMS are determined retrospectively, mainly based on disability accumulation as measured by an increase in EDSS-scores during a period of months or years [12]. This retrospective diagnostic approach has several limitations as shown by a diagnostic delay of up to 3 years in more than 50% of patients[13]: 1) EDSS-assessment is prone to high interrater variability [14,15]. 2) EDSS increase must be confirmed after 3-6 months in order to prevent assessment errors and differentiate between true progression and relapse-related disability [16,17]. 3) EDSS mainly focuses on disability in locomotor function and in case of EDSS scores above 4, further detection of disability progression is limited [18]. 4) MS disease activity such as clinical relapse and magnetic resonance imaging (MRI) activity might also occur in progressive MS [19] and thereby impede the diagnosis of SPMS by the treating physician. With these points in mind, NfL as a marker for neuroaxonal damage might be able to identify patients at risk of SPMS conversion and thereby widen the therapeutic window of newly available SPMS therapies [11].
There is evidence that early initiation of immunomodulatory therapies can delay SPMS conversion [20]. Therefore, prediction of disease progression is essential to guide treatment decisions and support effective immunomodulatory therapy to best preserve the functional independence of MS patients. With this regard, NfL has been associated with inflammatory activity and neurodegeneration in patients with early MS [3,21]. In contrast, data on long-term disability progression and conversion into SPMS is conflicting; data on predictive capacity is lacking. NfL was reported to be associated with EDSS progression [22], however another study was unable to confirm these observations [23]. In line with this, a retrospective study demonstrated an association between T2 lesion load and brain atrophy measured by MRI, but no correlation between baseline NfL values and EDSS after 10 years in a smaller cohort [24]. Similarly, a further study showed an increased risk of reaching EDSS-milestones below 6.0, but no consistent association between NfL levels and sustained disability of EDSS score of 6.0 and conversion to SPMS [7]. We aim to shed light on these conflicting results by examining the value of serum NfL (sNfL) in predicting EDSS progression at six-year follow-up within the Neurofilament and longterm outcome in MS (NaloMS) cohort.
2. Methods
2.1. NaloMS cohort
Patients with relapsing-remitting multiple sclerosis (RRMS) or clinically isolated syndrome (CIS), according to 2010 McDonald criteria who visited the Department of Neurology MS outpatient clinic at the University Medicine Mainz (Germany) between September 2010 and July 2016 were prospectively included in the NaloMS cohort. For classification into RRMS and CIS, we retrospectively applied the 2017 McDonald criteria. Patients with progressive disease forms such as primary progressive MS or SPMS were ineligible. At study entry, data on clinical examination, EDSS score, immunomodulatory therapy and medical history were assessed. This was repeated at follow-up (FU) 1 and FU 2. The time interval between study entry and FU 1 was scheduled as deemed necessary by the treating physician. FU 2 was then fixed for 12 months after FU 1. MR-imaging of the brain was evaluated with regard to number of T2-hyperintense lesions, T1-hypointense lesions and gadolinium-enhancing lesions at Baseline and FU 2 and was assessed by an experienced, blinded reader. Moreover, venous blood was collected at study entry and at FU 2. In order to reliably assess clinical relapses between FU 1 and FU 2, patients underwent clinical examination and EDSS-assessment by a video-trained, blinded investigator. In addition, EDSS-worsening at FU 2 was confirmed after 12 weeks. We followed TRIPOD-Guidelines for prediction model development and reporting (for details see supplemental Table 1).
2.2. Validation cohort
An independent external validation cohort was provided by two additional academic centers (Düsseldorf, Essen). Patients from these centers with RRMS and CIS were prospectively included from January 2010 until September 2016.
2.3. Standard Protocol Approval, Patient consent
The study protocol was approved by the Ethics Committee of the Ärztekammer Rheinland Pfalz (AZ 2018-13133_1); all patients gave written informed consent. The study is in line with the Declaration of Helsinki.
2.4. sNfL measurements
Our sNfL measurement protocol was previously described in detail [5,21]. Briefly, blood was collected in 10 ml Serum-Vacutainer®-tubes (Becton Dickinson, USA); samples were spun at 1300 g at room temperature for 15 minutes within 2 h after sampling. Directly after centrifugation, the serum was evenly transferred to polypropylene tubes and locally stored at -80°C. sNfL levels were then determined in duplicates by single molecule array with a SiMoA HD-1 (Quanterix, USA) using the NF-Light Advantage Kits (Quanterix) according to manufacturer's instructions. Resorufin-β-D-galactopyranoside (RGP) was incubated at 33°C for 60 minutes prior to running the assay. The coefficient of variation (CV, as a percentage) of the two replicates was obtained by dividing the standard deviation of both replicates by the mean of both replicates multiplied by 100. A few samples with intra-assay CV above 20% (or missing replicate result) were measured twice, taking the second measurement into account for further analysis. Finally, the mean intra-assay CV of duplicate determinations for concentration of 5.9% was obtained by averaging all individual sample CVs. Two low and high controls, consisting of recombinant human NfL antigen, were included in each sample run to monitor plate-to-plate variation (low: mean 8.8 pg/ml, inter-assay CV 13.7%; high: mean 190.9 pg/ml, inter-assay CV 13.9%). sNfL measurements were performed in a blinded fashion without information about clinical data and Baseline and FU samples were analyzed in the same run to control for plate-to-plate variation. Nevertheless, to rule out a potential influence of the plate-to-plate variation on our main results, we performed two independent analyses (for details see supplemental Methods).
2.5. Clinical endpoints
In order to assess the predictive value of sNfL and its temporal evolution in patients experiencing EDSS progression and conversion to SPMS, we assessed two clinical outcomes. These endpoints were assessed by two investigators independently from each other. One-year relapse-free EDSS progression (RFP) was defined as EDSS-worsening within 12 months prior to FU with an EDSS-increase of at least 1.5 for patients starting at EDSS 0, at least 1.0 EDSS-points for patients with an initial EDSS between 1 and 4.5 and at least 0.5 points for patients starting with an EDSS equal or greater than 5 [17,25]. In addition, patients experiencing RFP needed to be free of clinical relapse activity and inflammatory MRI-activity (gadolinium-enhancing lesions or new/enlarging T2-lesions, where available) 12 months prior to FU. EDSS-worsening was confirmed 12 weeks after FU assessment. Brain MRI was performed in all patients at study entry and available in 177/196 patients at FU 2. Brain MRI-scans between study entry and FU 2 were performed as deemed necessary by the treating physician.
The transition to SPMS was defined as an increase in EDSS levels at FU compared to study-entry levels (as described above) indicating progression, which had to be confirmed 12 weeks after FU within the same functional system score [17] and in addition, a minimum total EDSS score of 3.0 had to be reached.
In order to assess the ability of sNfL to dissect inflammatory activity and progression at FU, patients were divided into four groups: i) No inflammation or progression (stable), ii) inflammation only, iii) progression only and iv) inflammation and progression. Inflammation was defined as clinical relapse activity or new/enlarging T2-lesions/ gadolinium-enhancing lesions on MR-imaging within the last 12 months prior to FU (where available); progression was defined as EDSS-worsening within the last 12 months under consideration of the EDSS-steps mentioned before or detection of new T1-lesions on follow-up MRI (where available). The distinction of inflammation and progression under consideration of the aforementioned variables was proposed by Lublin et al [25]. for patients with progressive MS; here, we apply the approach to RRMS.
2.6. Individual-level support vector machine analysis
sNfL values at Baseline and sNfL FU/BL ratio for each individual patient were used as predictors of RFP (sNfL Baseline) and SPMS-conversion (sNfL FU/BL ratio) using a machine learning algorithm, namely support vector machine (SVM). The SVM algorithm is able to classify two data sets based on an optimal separating threshold between the data sets by maximizing the margin between classes’ closest points. Points located at the boundaries are called support vectors, whereas points in the middle of the margin are optimal separating thresholds. At the validation step, sNfL values were assessed for the ability to automatically distinguish between patients with progression or SPMS-transition. This procedure was performed independently for the development (NaloMS) and validation cohort (Düsseldorf, Essen)
2.7. Statistics
Statistical analysis was performed using R-Studio version 1.2.5033 (RStudio Inc., USA) with R version 3.6.3 (R Foundation for Statistical Computing, Austria), SPSS version 24 (IBM Corporation, Armonk, NY, USA), and MedCalc version 19.2.1 (MedCalc Software Ltd, Ostend, Belgium). Normal distribution was evaluated by Kolmogorov-Smirnov and Shapiro-Wilk tests. Variables that did not pass normality tests underwent Mann-Whitney or Kruskal-Wallis-tests with Bonferroni-correction for multiple testing, where appropriate. Normally distributed data underwent t-test or one-way/two-way ANOVA. Chi-square test of homogeneity was used to compare differences in proportions. Diagnostic accuracy of sNfL for SPMS diagnosis was assessed by sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV). Multivariable logistic regression analyses were conducted including variables with p<0.05 between different study groups (using Chi-square test, Mann-Whitney-U-test or t-test, as appropriate) and additional clinically relevant variables.
Receiver operating characteristic (ROC) curves were drawn and calculated for estimation of prognostic information on experiencing RFP and diagnostic accuracy in discriminating patients with SPMS at FU. Cumulative incidence curves for the competing risks of relapse-free EDSS-worsening (RFP) and relapse-dependent EDSS-worsening (RDP) were computed by using the R timereg package as proposed by Scheike et al [26]. Area under the curves (AUC) derived from ROC-analysis and Kaplan-Meier survival analysis were compared using the MedCalc for Windows software. All statistical analysis was performed using the original data without modifications. P-values < 0.05 were considered statistically significant.
2.8. Role of the funding source
The funding sources played no role in the study, in the writing of the manuscript or in the decision to submit the paper for publication.
3. Results
3.1. Patient characteristics
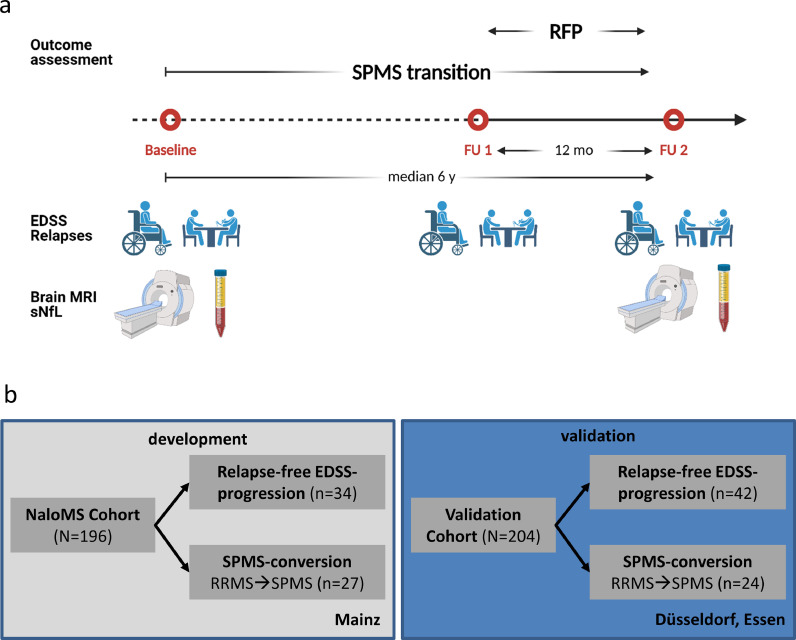
196 patients with RRMS or CIS were prospectively included from September 2010 until July 2016 in NaloMS. Included patients had a median age at diagnosis of 31 years (interquartile range (IQR) 24.9-40.7) and a median age at study entry of 35 years (IQR 27.1-43.1). They were more frequently female (137/196, 69.9%), had a median EDSS of 1 (IQR 0-2), and had a median number of 13 T2-lesions on MR-imaging (IQR 6-21). The median time between baseline and FU was 6 years (IQR 4.3-7.5). Patients in the independent validation cohort (Düsseldorf, Essen) were included from January 2010 until September 2016, had a median age of 31 years at study entry (IQR 26-40), a median age of 31 years at diagnosis (IQR 26-40), were more frequently female (136/204, 66.7%), had a median EDSS of 1.5 (IQR 1.0-2.0) and a median number of 10 T2-lesions on MR-imaging (IQR 6.0-12.0). The median time interval between BL and FU was 6.0 years (IQR 5.9-6.1). The primary outcomes were RFP and transition to SPMS. The study profile is depicted in Fig. 1a and an overview of the outcomes in each independent cohort is displayed in Fig. 1b.
Fig. 1.
Scheduled visits and study profile
a) Scheduled visits within NaloMS. b) Models were developed using the NaloMS cohort (Mainz) and validated with an independent cohort (Düsseldorf, Essen). Abbreviations: RFP, Relapse-free EDSS-progression; SPMS, secondary progressive multiple sclerosis; mo, months; y, years; EDSS, Expanded disability status scale; sNfL, serum neurofilament light chain; MRI, magnetic resonance imaging. Created using Biorender.com
3.2. Serum NfL predicts relapse-free EDSS worsening at six-year follow-up
At FU, 34/196 patients met the criteria for RFP in NaloMS. These patients had a higher age at diagnosis (36.6 years (29.1-45.7) vs. 30.6 years (24.3-39.7), p=0.009), as well as a higher age at study entry (45.7 years (34.2-48.9) vs. 32.9 years (26.3-42.2), p<0.001). In patients with RFP, sNfL levels were elevated at both baseline (10.8 pg/ml (7.7-15.0) vs. 7.2 pg/ml (4.5-12.5), p=0.017, Fig. 2a), and FU (10.0 pg/ml (6.4-13.2) vs. 6.9 pg/ml (5.1-9.1), p=0.008). Moreover, patients with RFP had a higher median EDSS at follow-up (4.3 (2.5-5.6) vs. 1.0 (0-2.0), p<0.001) and a longer disease duration (9.7 years (8.2-16.8) vs. 7.5 years (6.0-9.2), p<0.001). The disease course at baseline was equally distributed between the groups, whereas patients transitioning to SPMS were more frequent in the group with RFP (21/34 (61.8%) vs. 6/162 (3.7%), p<0.001). Patients who were classified as SPMS and did not reach the RFP-endpoint are patients who were classified as SPMS prior to FU1 who did not reach the necessary EDSS-steps mandatory to fulfil RFP-definition. Other baseline characteristics were comparable between patients with and without RFP (Table 1).
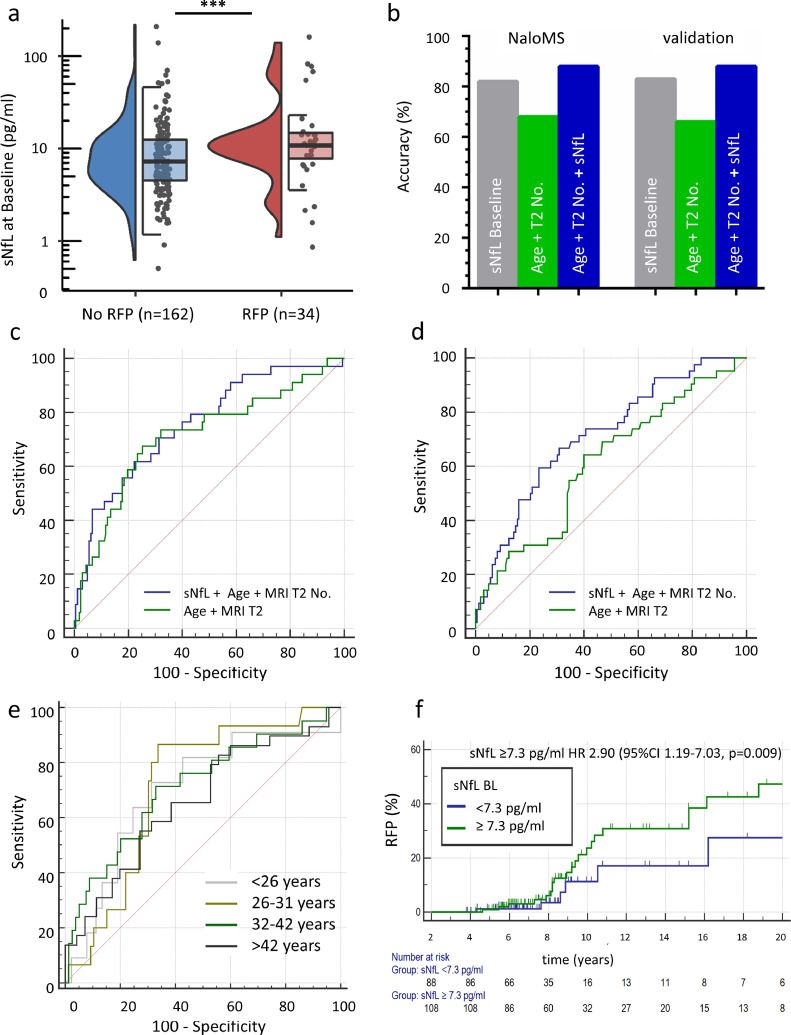
Fig. 2.
sNfL levels at baseline predict relapse-free disability progression in a prospective longitudinal study (NaloMS cohort)
a) Baseline NfL was increased in patients with relapse free EDSS-progression (RFP) (median (IQR) 10.8 (7.7-15.0) vs. 7.2 (4.5-12.5), p=0.017); data is displayed as a violin plot, left side: density of all data points, right side: median and IQR. b) Machine learning by support vector machine assessed the predictive accuracy of sNfL at Baseline for RFP under consideration of covariates (EDSS Baseline, Number of Gadolinium-enhancing lesions at Baseline, T2-hyperintense lesions at Baseline, Age at Baseline, Disease Duration and Relapses within the last 12 months prior to FU; grey bar). In addition, the predictive accuracy for RFP of a combination of Age + T2-hyperintense lesion number (green bar, without covariate adjustment) and a combination of Age + T2-hyperintense lesion number + sNfL at Baseline (blue bar) was similarly analyzed. c,d) Area under the receiver operating characteristic curve (ROC-AUC) for a combination of Age+T2-hyperintense lesions and additional inclusion of sNfL at Baseline in NaloMS (development cohort; c) and validation cohort (Düsseldorf, Essen; d). c) AUC increased from 0.714 (95%CI 0.645-0.776, green line) to 0.755 (95%CI 0.688-0.813, blue line) after additional consideration of sNfL within the NaloMS cohort (development). d) This finding could be confirmed within the validation cohort (Düsseldorf, Essen): AUC increased from 0.613 (95%CI 0.543-0.680) to 0.715 (95%CI 0.648-0.776) after inclusion of sNfL. e) Receiver operating characteristic curve in the combined NaloMS and validation cohort (Düsseldorf, Essen) with regard to prediction of RFP by sNfL at Baseline according to quartiles based on patient age at sampling (<26 years (grey line), 26-31 years (light green line), 32-42 years (dark green line), >42 years (black line)). f) Kaplan-Meier survival analysis for occurrence of RFP in patients with sNfL at baseline ≥7.3 pg/ml (green line) or <7.3 pg/ml (blue line), logrank test p=0.0135. Patients with sNfL ≥7.3 pg/ml at baseline suffer a 190% increased risk of experiencing RFP at follow-up (Hazard Ratio 2.90, 95%CI 1.19-7.03, p=0.009).
Table 1.
Patient cohorts experiencing or not experiencing RFP.
| No RFP | RFP | P-value | |
|---|---|---|---|
| N | 162 | 34 | |
| Age at diagnosis | 30.6 (24.3-39.7) | 36.6 (29.1-45.7) | 0.009 |
| NfL (Baseline) pg/ml | 7.2 (4.5-12.5) | 10.8 (7.7-15.0) | 0.017 |
| Age at Baseline | 32.9 (26.3-42.2) | 45.7 (34.2-48.9) | <0.001 |
| NfL (follow-up) pg/ml | 6.9 (5.1-9.1) | 10.0 (6.4-13.2) | 0.008 |
| sNfL FU/Baseline ratio | 0.99 (0.76-1.23) | 1.00 (0.70-1.32) | 0.995 |
| Age at follow-up | 38.9 (32.4-47.9) | 51.4 (42.9-55.6) | <0.001 |
| Female | 110 (67.9) | 27 (79.4) | 0.183 |
| Disease course at Baseline | 0.683 | ||
| CIS | 3 (1.9) | 1 (1.9) | |
| RRMS | 159 (98.1) | 33 (97.1) | |
| Smoking | 40 (34.8) | 12 (42.9) | 0.426 |
| Pack Years | 14.9 (±10.2) | 20.2 (±18.7) | 0.386 |
| OCB | 141 (87) | 27 (79.4) | 0.248 |
| Relapses | |||
|
73 (45.1) | 8 (23.5) | 0.020 |
|
103 (63.6) | 15 (44.1) | 0.035 |
|
8 (4.9) | 0 (0) | 0.186 |
| EDSS | |||
| Baseline | 1 (0-2) | 1.5 (0-3.1) | 0.148 |
| follow-up | 1 (0-2) | 4.3 (2.5-5.6) | <0.001 |
| Disease duration (years) | 7.5 (6.0-9.2) | 9.7 (8.2-16.8) | <0.001 |
| MRI Baseline | |||
| Gd-enhancement (%) | 47 (29.0) | 9 (26.5) | 0.460 |
| Gd lesion number (No.) | 0.82 (±2.30) | 0.70 (±1.65) | 0.719 |
| T2 lesion number (No) | 15.4 (±12.0) | 18.8 (±15.4) | 0.237 |
| MRI FU | |||
| Gd-enhancement (%) | 7 (4.3) | 0 (0) | 0.217 |
| Gd lesion number (No.) | 0.14 (±0.83) | 0 (0) | 0.392 |
| DMT | 0.125 | ||
| No DMT | 36 (22.2) | 9 (27.3) | |
| Basic | 25 (15.4) | 9 (26.5) | |
| Moderate | 61 (37.7) | 6 (18.2) | |
| High | 40 (24.7) | 10 (30.3) | |
| DMT at FU | 126 (77.8) | 24 (72.7) | 0.530 |
| No. of DMTs initiated | 2 (1-3) | 2 (2-4) | 0.317 |
| Disease course FU | <0.001 | ||
| CIS | 2 (1.2) | 0 (0) | |
| RRMS | 154 (95.1) | 13 (38.2) | |
| SPMS | 6 (3.7) | 21 (61.8) | |
| Relapse activity – FU | |||
| Mean number of Relapses last 5 years | 0.93 (±1.71) | 0.72 (±0.96) | 0.497 |
| Mean number of Relapses last year | 0.18 (±0.5) | 0 (0) | 0.004 |
Data is presented as number (percentages), median (interquartile range 25th-75th percentile) or mean (± standard deviation), as appropriate. sNfL: serum neurofilament light chain; CIS, clinically isolated syndrome; RRMS: relapsing-remitting multiple sclerosis; OCB: Oligoclonal Bands; EDSS: Expanded disability status scale; Gd: Gadolinium; DMT, disease-modifying therapies; basic DMT: interferons and glatirameracetate; moderate DMT: teriflunomide and dimethylfumarate; high DMT: natalizumab, rituximab, fingolimod, ocrelizumab, daclizumab, alemtuzumab and mitoxantrone; SPMS: Secondary progressive MS; FU: follow-up; No: Number; RFP: relapse-free EDSS-progression.
In a next step, we investigated different factors indicative for patients experiencing RFP. In a multivariable logistic regression model, we identified variables unbalanced at the univariate level (p<0.05; sNfL Baseline, sNfL FU, Age at diagnosis, Age at sampling, Disease Duration, Relapses 3 months prior to Baseline, Relapses 12 months prior to Baseline, Relapses last 5 years) and additional factors known to influence disease progression (MRI T2-hyperintense lesion number at Baseline, Number of disease-modifying therapy (DMT)-initiations). Due to association between Age at diagnosis, Age at sampling and Disease duration, we only incorporated Age at sampling in the regression model. We proceeded similarly with sNfL at Baseline and FU and only included sNfL at Baseline. With regard to relapses 3 months and 12 months prior to Baseline, only relapses 3 months prior to Baseline were incorporated in the model. As relapses within the last 5 years prior to follow-up are not available at Baseline, this variable was also not included in the logistic regression model; instead, MRI T2-hyperintense lesion number at Baseline was included as an additional factor known to influence disease progression. Importantly, increased sNfL levels at baseline (Odds ratio (OR) 1.021, 95% confidence interval (CI) 1.005-1.038, p=0.012) and increased age at sampling (OR 1.087, 95%CI 1.034-1.143, p=0.001) as well as patients with relapses within the last 3 months prior to Baseline (OR 0.241, 95%CI 0.068-0.850, p=0.027) remained in the prediction model for RFP at FU. Details of variables included in the logistic regression model are displayed in Table 2. In order to model the time-dependent incidence of RFP and correct for potential confounders, we additionally performed Cox regression analysis and thereby demonstrate that age at diagnosis (Hazard Ratio (HR) 1.092, 95%CI 1.050-1.136, p<0.001), sNfL ≥ 7.3 pg/ml (HR 2.791, 95%CI 1.105-7.047, p=0.030), EDSS at Baseline (HR 0.810, 95%CI 0.657-0.999, p=0.049) and Relapses within 12 months prior to Baseline (HR 3.269, 95%CI 1.315-8.123, p=0.011) are independent predictors of the RFP-outcome within NaloMS (for details see supplemental Table 2).
Table 2.
Logistic Regression Model – Factors associated with relapse-free EDSS-progression.
| Variable | Odds Ratio | 95% CI | P |
|---|---|---|---|
| sNfL BL (pg/ml) | 1.021 | 1.005-1.038 | 0.012 |
| Age at sampling (years) | 1.087 | 1.034-1.143 | 0.001 |
| Relapses 3 months prior BL (yes) | 0.241 | 0.068-0.850 | 0.027 |
| MRI T2 number (No.) | 0.994 | 0.956-1.035 | 0.785 |
| DMT initiations (No.) | 1.178 | 0.839-1.654 | 0.345 |
sNfL: serum neurofilament light chain; EDSS: Expanded disability status scale; BL: Baseline; DMT: disease-modifying therapy; No: Number.
Our results were further validated by an independent machine learning supported analysis using support vector machine (SVM) in order to determine the predictive accuracy of patients’ individual sNfL levels at Baseline for prediction of RFP. In NaloMS, SVM revealed an overall predictive accuracy of sNfL at Baseline for prediction of RFP 12 months prior to FU of 82% under consideration of the following covariates: EDSS Baseline, Number of Gadolinium-enhancing lesions at Baseline, Number of T2-hyperintense lesions at Baseline, Age at Baseline, Disease Duration and Relapses within the last 12 months prior to FU. This observation was confirmed within an independent validation cohort (Düsseldorf Essen) with an accuracy of 83%. In addition, in NaloMS the combination of age and T2-hyperintense lesion number as known risk factors for EDSS-progression showed a predictive accuracy for RFP of 68%, which could be increased to 88% by adding sNfL to the aforementioned variable combination. This was confirmed within the independent validation cohort (Age + T2-hyperintense lesion number 66%, plus sNfL Baseline 88%, Fig. 2b, for further details see also supplemental Table 3).
Moreover, we performed receiver operating characteristic curve (ROC) analysis in order to provide clinically meaningful sNfL cut-offs for stratification of individual patient's risk of RFP. Baseline sNfL exhibited an AUC of 0.631 (95%CI 0.559-0.698, p=0.015) with a sensitivity of 82.4% (95%CI 65.5-93.2) and a negative predictive value (NPV) of 93.2% (95%CI 86.7-96.6) for a sNfL cut-off value of equal or greater than 7.3 pg/ml (derived from the Youden-index of the ROC-AUC), suggesting sNfL as a marker able to identify patients with low risk of long-term disability progression in case of initial values below 7.3 pg/ml. By adding sNfL to a risk score based on clinical and MRI parameters, the ROC-AUC increased from 0.780 for the risk score alone (95%CI 0.714-0.838, p<0.001) to 0.811 with the additional consideration of baseline sNfL (95%CI 0.748-0.865, p<0.001). Details of the diagnostic characteristics of sNfL alone, the risk score alone and a combination of both are given in Table 3. In addition, we provide evidence that the addition of sNfL to a combination of Age and number of T2-hyperintense lesions at Baseline increases the ROC-AUC from 0.714 (95%CI 0.645-0.776) to 0.755 (95%CI 0.688-0.813) in NaloMS (Fig. 2c). This observation was confirmed within the external independent validation cohort (Düsseldorf, Essen: AUC-increase from 0.613, 95%CI 0.543-0.680 to 0.715, 95%CI 0.648-0.776 after inclusion of sNfL, Fig. 2d).
Table 3.
Test effectiveness of sNfL at Baseline and age-dependent sNfL cut-offs for prediction of RFP.
| Item | Sensitivity (95% CI) | Specificity (95%CI) | NPV (95%CI) | PPV (95%CI) |
|---|---|---|---|---|
| NfL Baseline | 82.4 % (65.5-93.2) | 50.6 % (42.7-58.6) | 93.2% (86.7-96.6) | 25.9% (21.9-23.4) |
| Risk Score (Age, Age at diagnosis, Disease duration, EDSS BL, Gd. No., T2 lesion No., Relapses (last 5 years) | 63.3 % (43.9-80.1) | 86.6 % (80.3-91.5) | 90.4% (86.5-93.2) | 47.5% (35.5-59.8) |
| Risk Score + NfL | 70.0 % (50.6-85.3) | 86.6 % (80.3-91.5) | 90.4% (86.4-93.3) | 48.8% (37.2-60.6) |
| Age group | AUC 95%CI | p-value | Criterion | Sens/Spec. |
|---|---|---|---|---|
| <26 years | 0.698 (0.598-0.786) | 0.029 | >11.7 pg/ml | 72.7%/68.5% |
| 26-31 years | 0.713 (0.614-0.798) | 0.001 | >10.8 pg/ml | 86.7%/66.3% |
| 32-42 years | 0.709 (0.610-0.795) | 0.002 | >9.1 pg/ml | 71.4%/67.1% |
| >42 years | 0.657 (0.555-0.750) | 0.012 | >10.4 pg/ml | 55.2%/72.9% |
Test effectiveness of sNfL at Baseline, a risk score and a combination of the risk score and sNfL at Baseline for prediction of relapse-free EDSS-progression in NaloMS. Age-dependent sNfL ROC-AUC with respective sNfL cut-off values and Sensitivity/Specificity derived from a combined cohort of development (NaloMS) and validation cohort (Düsseldorf, Essen).
Abbreviations: NfL: Neurofilament light chain, EDSS: Expanded Disability Status Scale, Gd: Gadolinium-enhancing lesions, No.: Number; NPV: negative predictive value; PPV: positive predictive value and; Sens: Sensitivity; Spec: Specificity.
Due to the known influence of Age on initial sNfL values, we combined the study cohort (NaloMS) and the validation cohort (Düsseldorf, Essen), grouped the patients based on age at sampling and performed ROC-curve analysis separately within these age quartiles (< 26 years, 26-31 years, 32-42 years, >42 years). Notably, we were unable to observe a significant difference in the AUC between age-quartiles (Fig. 2e), but this analysis does enable us to specify age-dependent sNfL cut-offs for prediction of RFP. Details on AUC and specific sNfL cut-offs for RFP-prediction are given in Table 3. Moreover, age-corrected ROC-AUC for prediction of RFP by sNfL at Baseline are given in supplemental Fig. 1.
In order to elucidate the prognostic value of absolute sNfL concentrations, we used the Youden-index of the ROC-AUC of sNFL for further Kaplan-Meier analysis: patients with sNfL ≥7.3 pg/ml showed an increased risk of RFP at FU in time to event analysis (logrank-test, p=0.0135). Similarly, Cox regression analysis revealed a 190% increased risk of experiencing RFP in these patients (Hazard ratio (HR) 2.90, 95%CI 1.19-7.03, p=0.009, Fig. 2f). This finding was confirmed by competing risk analysis of relapse-dependent vs. relapse-free EDSS-progression (For details see supplemental Fig. 2).
3.3. Serum NfL identifies patients with SPMS conversion
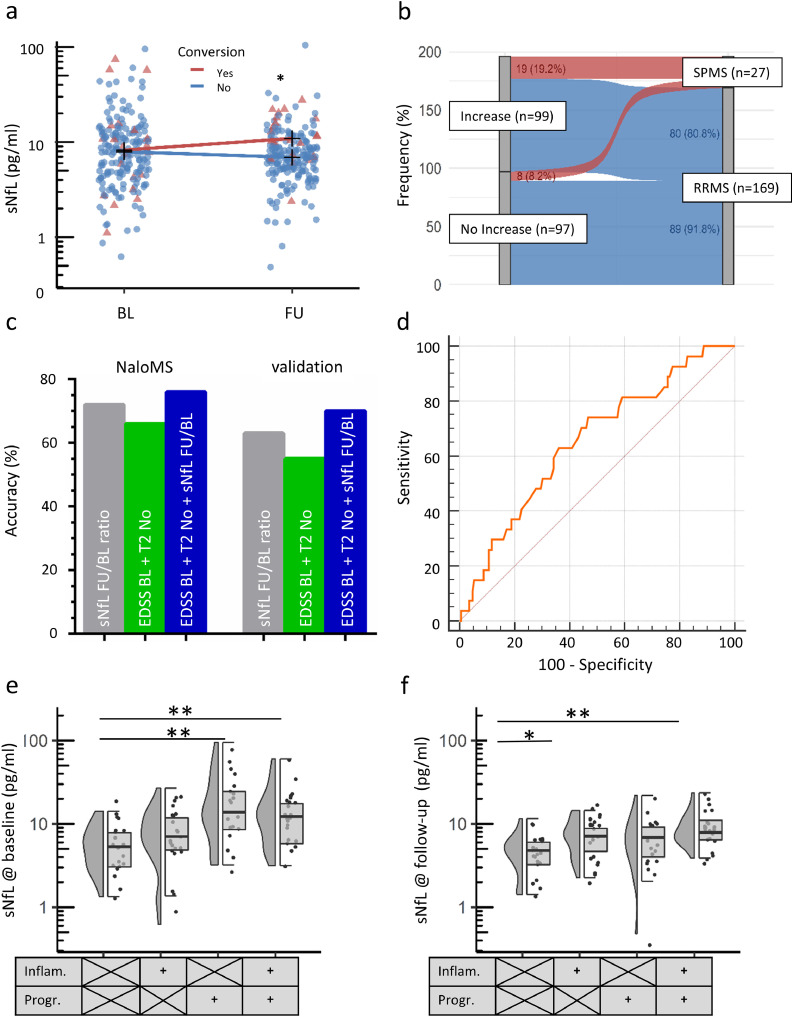
Patients experiencing transition to SPMS had higher sNfL values at FU, compared to patients without transition (conversion 10.4 pg/ml (IQR 6.9-17.6) vs. no conversion 6.9 pg/ml (IQR 5.0-9.2), p<0.001, Fig. 3a), whereas baseline sNfL levels did not differ between later SPMS converters and non-converters. Moreover, individual longitudinal sNfL-increases (FU level higher than Baseline level) were more frequent in patients transitioning to SPMS (19/27, 70.4%) than in non-converters (80/169, 47.3%; Chi2, p=0.026, Fig. 3b), as also shown by patients’ individual sNfL FU/BL ratio (SPMS-converters 1.16 (IQR 0.89-1.70) vs. non-converters 0.96 (IQR 0.75-1.23), p=0.011). Further differences between patients with and without SPMS conversion, as derived from Chi-square test, non-parametric and parametric tests, as appropriate, are provided in Table 4. Multivariate analysis, considering covariates unbalanced at the univariate level as shown in Table 4, revealed sNfL ratio (FU/BL) as independent predictor for SPMS-conversion (OR 1.476, 95%CI 1.078-2.019, p=0.015) in addition to disease duration (OR 1.097, 95%CI 1.030-1.168, p=0.004) and number of DMT-initiations (OR 1.592, 95%CI 1.099-2.305, p=0.014, for details see Table 5). In order to model the time-dependent occurrence of SPMS-conversion, we additionally performed Cox regression analysis, revealing sNfL FU/BL ratio (HR 1.541, 95%CI 1.181-2.010, p=0.001) and EDSS at Baseline (HR 1.364, 1.065-1.746, p=0.014) as independent predictors for transition into SPMS (for details see supplemental Table 4). These findings were refined by SVM, demonstrating that individual sNfL FU/BL ratios identify patients with transition to SPMS with an accuracy of 72% in NaloMS, which was validated within an independent cohort (Düsseldorf, Essen; accuracy 63%). The following covariates were considered for this analysis: EDSS at BL, Number of Gadolinium-enhancing lesions at BL, Number of T2-hyperintense lesions at Baseline, Age at sampling, Disease duration and relapses within the last 12 months prior to FU. Remarkably, accuracy for identification of SPMS increased from 66% for the variable combination of EDSS BL and number of T2-hyperintense lesions at BL to 76% after additional consideration of sNfL FU/BL ratio in NaloMS. These findings could be validated within the independent cohort (increase from 55% to 70% after additional inclusion of sNfL FU/BL ratio, Fig. 3c). For details see also supplemental Table 5.
Fig. 3.
Baseline sNfL level discriminates patients with SPMS conversion at follow-up
a) Patients converting to secondary progressive multiple sclerosis (SPMS) showed similar NfL levels at baseline (conversion 9.2 pg/ml (interquartile range (IQR) 4.5-13.7) vs no conversion 7.9 pg/ml (IQR 4.7-12.9), p=0.657) and an increase in sNfL levels at follow up (conversion 10.4 pg/ml (IQR 6.9-17.6) vs. no conversion 6.9 pg/ml (IQR 5.0-9.2), p<0.001). b) NfL increase at follow-up compared to baseline NfL levels occurred in 70.4% of patients suffering from SPMS-conversion (19 of 27 patients) and in 47.3% of patients without SPMS-conversion (80 out of 169 patients, Chi2-test, p=0.026) supporting the use of sNfL level at FU in case clinical SPMS conversion is suspected. c) Machine learning by support vector machine assessed the accuracy of sNfL FU/BL ratio for identification of patients with SPMS transition. The following covariates were considered: EDSS at BL, Number of gadolinium-enhancing lesions at BL, Number of T2-hyperintense lesions at BL, Age at BL, Disease Duration and Relapses within the last 12 months prior to FU; grey bar). In addition, the accuracy for identification of SPMS-transition of a combination of EDSS at BL + T2-hyperintense lesion number at BL (green bar, without covariate adjustment) and a combination of EDSS at BL + T2-hyperintense lesion number at BL + sNfL FU/BL ratio (blue bar) are also shown. d) A ratio of sNfL at FU divided by sNfL at BL (sNfL FU/BL ratio) was used to draw the Area under the receiver operating curve (ROC-AUC) with regard to discrimination between patients with and without conversion into SPMS at follow-up (AUC 0.651, 95%CI 0.580-0.717, p=0.007). A cut-off above 19% NfL increase at follow-up compared to Baseline levels was determined by Youden's index. e,f) sNfL values at BL and FU in patients with inflammation (Inflam.; new T2-hyperintense lesions, gadolinium-enhancing lesions, clinical relapse) and progression (Progr.; EDSS-progression, MRI signs of atrophy) in an age- and gadolinium-matched subgroup of NaloMS. e) sNfL at BL: Bonferroni-correction: No inflammation no progression, 5.3 (3.0-8.3) pg/ml; inflammation only, 7.1 (4.4-12.2); progression only, 13.8 (5.1-29.0); inflammation and progression, 12.3 (5.2-18.5). p stable vs. inflammation and progression =0.004, stable vs. progression only p=0.004. f) sNfL at FU: Bonferroni-correction: No inflammation no progression, 4.8 (3.0-6.1); inflammation only, 7.1 (4.5-9.6); progression only, 6.0 (3.9-10.1); inflammation and progression, 7.9 (4.5-11.3). p for inflammation and progression vs. stable =0.001, inflammation only vs. stable p=0.039.
Table 4.
Characteristics of patients with and without SPMS-conversion.
| No conversion | SPMS conversion | P-value | |
|---|---|---|---|
| N | 169 | 27 | |
| Age at diagnosis | 30.8 (24.7-40.6) | 36.1 (26.4-45.1) | 0.194 |
| NfL (Baseline) pg/ml | 7.9 (4.7-12.9) | 9.2 (4.5-13.7) | 0.657 |
| Age at Baseline | 33.1 (26.5-42.3) | 42.6 (34.3-48.9) | 0.001 |
| NfL (FU) pg/ml | 6.9 (5.0-9.2) | 10.4 (6.9-17.6) | <0.001 |
| sNfL FU/BL ratio | 0.96 (0.75-1.23) | 1.16 (0.89-1.70) | 0.011 |
| Age at FU | 39.1 (32.6-48.5) | 51.1 (41.0-55.6) | <0.001 |
| Female | 116 (68.6) | 21 (77.8) | 0.336 |
| Disease course at Baseline | 0.450 | ||
|
3 (1.8) | 1 (3.7) | |
|
166 (98.2) | 26 (96.3) | |
| Smoking | 43 (35.8) | 9 (39.1) | 0.763 |
|
15.60 (±12.2) | 18.25 (±14.08) | 0.582 |
| OCB positive | 148 (87.6) | 20 (74.1) | 0.063 |
| Relapses | |||
|
74 (43.8) | 7 (8.6) | 0.080 |
|
108 (64.9) | 10 (37.0) | 0.008 |
|
8 (4.7) | 0 (0) | 0.248 |
| EDSS | |||
|
1 (0-1.5) | 2.5 (1.0-3.5) | <0.001 |
|
1 (0-2.0) | 5.0 (3.5-6.0) | <0.001 |
| Disease duration (years) | 7.4 (6.0-9.2) | 10.4 (8.2-20.2) | <0.001 |
| MRI Baseline | |||
| Gd-enhancement | 48 (28.4) | 8 (29.6) | 0.319 |
| Gd lesion number | 0.67 (±1.80) | 1.62 (±3.86) | 0.231 |
| T2 lesion number | 15.40 (±12.15) | 19.52 (±15.48) | 0.129 |
| MRI FU | |||
|
6 (3.6) | 0 (0) | 1.000 |
|
0.12 (±0.78) | 0 (0) | 0.877 |
|
33 (19.5) | 6 (22.2) | 0.796 |
|
0.54 (±1.42) | 0.62 (±1.16) | 0.813 |
| DMT at FU | 132 (78.1) | 18 (69.2) | 0.317 |
| No. of DMTs initiated | 2 (1-3) | 3 (2-4) | 0.005 |
| Disease course FU | |||
|
2 (1.2) | 0 (0) | |
|
167 (98.8) | 0 (0) | |
|
0 (0) | 27 (100.0) | |
| Relapse-free progression | <0.001 | ||
|
156 (96.3) | 6 (3.7) | |
|
13 (38.2) | 21 (61.8) | |
| Relapse activity - FU | |||
|
0.90 (±1.67) | 0.88 (±1.07) | 0.962 |
|
0.17 (±0.491) | 0 (±0) | 0.017 |
Data is presented as number (percentages), median (interquartile range 25th-75th percentile) or mean (± standard deviation), as appropriate. CIS: clinically isolated syndrome, DMT: disease-modifying treatment, EDSS: Expanded Disability Status Scale, Gd: Gadolinium, FU: follow-up, NfL: Neurofilament light chain, No: Number, OCB: Oligoclonal Bands, RRMS: relapsing-remitting multiple sclerosis, SPMS: secondary progressive MS.
Table 5.
Risk factors for SPMS conversion.
| Variable | Odds Ratio | 95% CI | P |
|---|---|---|---|
| sNfL ratio (FU/BL) | 1.476 | 1.078-2.019 | 0.015 |
| Disease Duration (years) | 1.097 | 1.030-1.168 | 0.004 |
| DMT initiations (No.) | 1.592 | 1.099-2.305 | 0.014 |
| Relases last years (No.) | 0.199 | 0.023-1.742 | 0.145 |
sNfL: serum neurofilament light chain; EDSS: Expanded disability status scale; Gd: Gadolinium; FU: follow-up; BL: baseline; No.: Number; CI: confidence interval.
We also used the sNfL FU/BL ratio to determine the cut-off value of longitudinal sNfL increase in order to discriminate patients with SPMS conversion at FU. ROC analysis of sNfL FU/BL ratio revealed an AUC of 0.651 (95%CI 0.580-0.717, p=0.007, Fig. 3d) with a NPV of 88.2% (95%CI 86.7%-89.5%) and sensitivity of 63.0% (95%CI 42.4%-80.6%) by taking advantage of a sNfL increase above 19% at FU compared to baseline level, as derived from the Youden-index of the ROC-curve. In addition, the 19% increase cut-off showed 65.9% (95%CI 58.2% - 73.0%) specificity and a PPV of 30.3% (17.4%-47.4%). Moreover, age-corrected ROC-AUC for identification of SPMS-transition by sNfL FU/BL ratio is provided in supplemental Fig. 3.
3.4. sNfL measurements reflect inflammation-dependent neuronal damage associated with future disease progression
In order to determine the ability of sNfL to dissect and predict inflammatory activity (New T2-lesions, gadolinium-enhancing lesions, clinical relapse) and disease progression (EDSS-progression, New T1-lesions), we divided patients into four categories based on the presence of inflammation and/or progression (Fig. 3e,f). To reduce the influence of gadolinium-enhancing lesions and age as potential confounding factors on peripheral sNfL levels, we selected a subgroup of the NaloMS cohort and matched for both parameters (Age, Number of gadolinium-enhancing lesions at BL). Of note, we observed no difference in baseline demographics such as age, EDSS or MRI parameters (gadolinium-enhancing lesions, number of T2 hyperintense lesions; for details see supplemental Table 6). In detail, patients with both i) progression only and ii) inflammation and progression at FU showed increased sNfL levels at baseline compared to patients with stable disease (progression only 13.8 (5.1-29.0) pg/ml vs. stable patients, p=0.004; inflammation and progression 12.3 (5.2-18.5) pg/ml vs. stable patients, p=0.004; stable patients 5.3 (3.0-8.3) pg/ml; Bonferroni correction for multiple comparisons Fig. 3e). In addition, patients with inflammation and progression at FU exhibited the highest sNfL values at FU (sNfL inflammation and progression 7.9 pg/ml (4.5-11.3)), representing a significant increase compared to patients without signs of inflammation or progression (sNfL no inflammation/progression 4.8 pg/ml (3.0-6.1), p=0.001). Similarly, patients experiencing inflammation only at FU showed higher sNfL values at FU compared to stable patients (sNfL inflammation only 7.1 pg/ml (4.5-9.6) vs. 4.8 pg/ml (3.0-6.1), p=0.039, Fig. 3f).
4. Discussion
Neuroaxonal loss, due to inflammation and neurodegeneration, is present from the earliest stage of MS-pathology and contributes to neurologic disability [18]. We and others have recently explored a role of sNfL in measuring ongoing neurodegenerative processes starting in very early phases of the disease or even before first clinical manifestation [21,27]. In this study, we prospectively evaluated the temporal development of sNfL in a six-year longitudinal cohort study and examined the ability of sNfL to predict later disability progression and SPMS conversion. Moreover, we aimed to unravel the ability of sNfL assessment to dissect inflammatory activity from disease progression.
Here, we found an increased risk of disability progression as measured by RFP 12 months prior to FU with increased sNfL levels (equal or greater 7.3 pg/ml) at baseline. Moreover, we provide age-dependent cut-offs in order to account for the influence of age on individual NfL values. While interpreting this cut-off value, one has to keep in mind that sNfL quantification methods used by other groups lead to principally higher NfL-values [28]. The high negative predictive value of 93.2% for discriminating patients with/without RFP indicates that patients with sNfL levels below 7.3 pg/ml have a rather low probability of experiencing disability progression. This might help to stratify immunomodulatory treatment strategies on an individual level and thereby enable patients to balance the potential long-term risk of side effects versus necessary treatment strength. Although increased sNfL levels in patients experiencing RFP at FU might also be explained by increased age and longer disease duration, with this cohort sNfL remained in the model after multivariable correction for age at sampling.
Our observations expand previous findings, demonstrating an increased risk of EDSS worsening within the 12 months after sampling in patients with sNfL above the 80th percentile of healthy controls [29]. The probability of EDSS-worsening was 6.7% for patients with sNfL values below the 80th percentile of healthy controls and increased to 15% in patients with sNfL values above the 97.5th percentile [29]. However, in a recent study in a larger cohort with a longer observation period up to 12 years, initial sNfL values, or sNfL-values at other time points collected within the observation period, were not able to differentiate between patients with EDSS-worsening compared to non-progressing patients at FU [23]. These conflicting observations might be explained by differences in the study cohorts, or by neglecting the fact that an EDSS increase might happen due to both relapse activity or relapse-independent disability progression. The unique features of our study are i) the provision of a sNfL cut-off with a high negative predictive value for prediction of relapse free EDSS-progression within 6-year follow-up, ii) the transfer of the findings on patients’ individual sNfL levels by machine learning and iii) the further validation within an independent cohort (Düsseldorf, Essen), thereby fostering the application of sNfL as a biomarker for patient-individualized treatment decisions in clinical practice. In a next step, this patient-individualized treatment approach and the proposed age-dependent sNfL cut-off needs to be validated in prospective studies.
Importantly, patients experiencing SPMS transition were more likely to display increased sNfL levels at follow-up compared to baseline levels (increased sNfL FU/BL ratio). This indicates that longitudinal assessment of sNfL levels might be helpful in shortening the time necessary to diagnose SPMS as early as possible. Assessment of sNfL might therefore reduce the frequent delay in SPMS diagnosis that occurs due to lack of sufficiently sensitive clinical or imaging measures [12]. These observations are contrary to a recent metaanalysis by Martin and colleagues, demonstrating that sNfL level in CSF are associated with inflammatory activity rather than progression, which might be explained by the lack of serial NfL quantifications and CSF instead of serum measurement [30].
Moreover, we assessed the ability of sNfL to dissect inflammatory activity from relapse-independent disability progression. Interestingly, patients who suffered from inflammatory activity (with and without progression) exhibited increased sNfL values at follow-up compared to stable patients (no inflammation, no progression). Whereas previous reports demonstrate a robust association of sNfL with clinical relapses[21,29,31] and subclinical signs of inflammatory activity (T2-lesions[21,32,33], gadolinium-enhancing lesions[3,21,34]), we are able to confine these statements as we observed highest sNfL values in patients with disability relevant inflammatory activity (inflammation and progression), suggesting a role of sNfL for monitoring both disease progression and inflammatory activity. In addition, sNfL at Baseline was increased in patients with progression in presence and absence of inflammatory activity at FU. Thus, we discovered that while elevated sNfL levels reflect current ongoing inflammatory-driven (focal) damage, they more importantly identify those early inflammatory processes resulting in later axonal loss associated with EDSS-progression in the long run.
Besides the strength of the current study, we are aware of potential limitations of the NaloMS cohort. As MRI at FU was lacking in some (less than 10%) patients of the NaloMS cohort, there is the possibility that inflammatory activity might have been missed. In addition, the possible relevance of monitoring the individual temporal evolution of sNfL to examine treatment response and disability progression needs to be validated in future studies in order to enable the next steps for inclusion of sNfL assessment into routine clinical practice.
Taken together, we here report findings from the prospective NaloMS cohort that sNfL measurements are capable of predicting disability progression at 12 months prior to six-year follow-up, due to their ability to reflect disability relevant neuronal damage and are able to discriminate SPMS patients thereby facilitating an earlier diagnosis of patients at risk.
5. Contributors
TU, FS, FZ, SB – conception and design of the study, acquisition and analysis of data, verified the underlying data, drafting a significant proportion of the manuscript and Fig.s.
MM, SG, NR, VF, TR, SM, RP, CK, EE, JL, SE – acquisition and analysis of data.
All authors have read and approved the final version of the manuscript.
Data sharing statement
Data supporting the observation of this study is available upon reasonable request from the corresponding author.
Declaration of Competing Interest
Timo Uphaus has received honoraria from Merck Serono. Tobias Ruck has received travel grants and financial research support from Genzyme and Novartis and received honoraria for lecturing from Roche, Merck, Genzyme, Biogen, and Teva. Sven G. Meuth has received honoraria for lecturing and travel expenses for attending meetings from Almirall, Amicus Therapeutics Germany, Bayer Health Care, Biogen, Celgene, Diamed, Genzyme, MedDay Pharmaceuticals, Merck Serono, Novartis, Novo Nordisk, ONO Pharma, Roche, Sanofi-Aventis, Chugai Pharma, QuintilesIMS, and Teva. His research is funded by the German Ministry for Education and Research (BMBF), Deutsche Forschungsgemeinschaft (DFG), Else Kröner Fresenius Foundation, German Academic Exchange Service, Hertie Foundation, Interdisciplinary Center for Clinical Studies (IZKF) Muenster, German Foundation Neurology and by Almirall, Amicus Therapeutics Germany, Biogen, Diamed, Fresenius Medical Care, Genzyme, Merck Serono, Novartis, ONO Pharma, Roche, and Teva. Frauke Zipp has recently received research grants and/or consultation funds from DFG, BMBF, PMSA, Genzyme, Janssen, Merck Serono, Roche, Novartis, Celgene, and Sanofi-Aventis. Stefan Bittner has received honoraria and compensation for travel from Biogen Idec, Merck Serono, Novartis, Sanofi-Genzyme and Roche. The other authors declare no competing interests.
Acknowledgments
This work was supported by the German Research Council (DFG, CRC-TR-128 to T.U., F.Z. and S.B.), Else Kröner Fresenius Foundation (Else-Kröner Memorial Stipendium to T.U.) and Hertie-Stiftung (to F.S. and S.B.). The authors thank Dr. Cheryl Ernest for proofreading and editing the manuscript.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2021.103590.
Appendix. Supplementary materials
References
- 1.Larochelle C, Uphaus T, Prat A. Secondary Progression in Multiple Sclerosis: Neuronal Exhaustion or Distinct Pathology? Trends Neurosci. 2016;39(5):325–339. doi: 10.1016/j.tins.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Chung KK, Altmann D, Barkhof F. A 30-Year Clinical and Magnetic Resonance Imaging Observational Study of Multiple Sclerosis and Clinically Isolated Syndromes. Ann Neurol. 2020;87(1):63–74. doi: 10.1002/ana.25637. [published Online First: 2019/11/07] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siller N, Kuhle J, Muthuraman M. Serum neurofilament light chain is a biomarker of acute and chronic neuronal damage in early multiple sclerosis. Mult Scler. 2019;25(5):678–686. doi: 10.1177/1352458518765666. [DOI] [PubMed] [Google Scholar]
- 4.Khalil M, Teunissen CE, Otto M. Neurofilaments as biomarkers in neurological disorders. Nat Rev Neurol. 2018;14(10):577–589. doi: 10.1038/s41582-018-0058-z. [DOI] [PubMed] [Google Scholar]
- 5.Uphaus T, Bittner S, Groschel S. NfL (Neurofilament Light Chain) Levels as a Predictive Marker for Long-Term Outcome After Ischemic Stroke. Stroke. 2019;50(11):3077–3084. doi: 10.1161/STROKEAHA.119.026410. [DOI] [PubMed] [Google Scholar]
- 6.Kuhle J, Kropshofer H, Haering DA. Blood neurofilament light chain as a biomarker of MS disease activity and treatment response. Neurology. 2019;92(10):e1007–e1e15. doi: 10.1212/WNL.0000000000007032. [published Online First: 2019/02/10] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manouchehrinia A, Stridh P, Khademi M. Plasma neurofilament light levels are associated with risk of disability in multiple sclerosis. Neurology. 2020;94(23):e2457–e2e67. doi: 10.1212/WNL.0000000000009571. [published Online First: 2020/05/22] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhan A, Jacobsen C, Myhr KM. Neurofilaments and 10-year follow-up in multiple sclerosis. Mult Scler. 2018;24(10):1301–1307. doi: 10.1177/1352458518782005. [published Online First: 2018/08/02] [DOI] [PubMed] [Google Scholar]
- 9.Salzer J, Svenningsson A, Sundstrom P. Neurofilament light as a prognostic marker in multiple sclerosis. Mult Scler. 2010;16(3):287–292. doi: 10.1177/1352458509359725. [published Online First: 2010/01/21] [DOI] [PubMed] [Google Scholar]
- 10.Sellebjerg F, Royen L, Soelberg Sorensen P. Prognostic value of cerebrospinal fluid neurofilament light chain and chitinase-3-like-1 in newly diagnosed patients with multiple sclerosis. Mult Scler. 2019;25(11):1444–1451. doi: 10.1177/1352458518794308. [published Online First: 2018/08/17] [DOI] [PubMed] [Google Scholar]
- 11.Kappos L, Bar-Or A, Cree BAC. Siponimod versus placebo in secondary progressive multiple sclerosis (EXPAND): a double-blind, randomised, phase 3 study. Lancet. 2018;391(10127):1263–1273. doi: 10.1016/S0140-6736(18)30475-6. [published Online First: 2018/03/27] [DOI] [PubMed] [Google Scholar]
- 12.Ferrazzano G, Crisafulli SG, Baione V. Early diagnosis of secondary progressive multiple sclerosis: focus on fluid and neurophysiological biomarkers. J Neurol. 2020 doi: 10.1007/s00415-020-09964-4. [published Online First: 2020/06/07] [DOI] [PubMed] [Google Scholar]
- 13.Katz Sand I, Krieger S, Farrell C. Diagnostic uncertainty during the transition to secondary progressive multiple sclerosis. Mult Scler. 2014;20(12):1654–1657. doi: 10.1177/1352458514521517. [published Online First: 2014/02/05] [DOI] [PubMed] [Google Scholar]
- 14.Ontaneda D, Cohen JA, Amato MP. Clinical outcome measures for progressive MS trials. Mult Scler. 2017;23(12):1627–1635. doi: 10.1177/1352458517729465. [published Online First: 2017/10/19] [DOI] [PubMed] [Google Scholar]
- 15.University of California SFMSET, Cree BA, Gourraud PA. Long-term evolution of multiple sclerosis disability in the treatment era. Ann Neurol. 2016;80(4):499–510. doi: 10.1002/ana.24747. [published Online First: 2016/07/28] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalincik T, Cutter G, Spelman T. Defining reliable disability outcomes in multiple sclerosis. Brain. 2015;138(Pt 11):3287–3298. doi: 10.1093/brain/awv258. [published Online First: 2015/09/12] [DOI] [PubMed] [Google Scholar]
- 17.Lorscheider J, Buzzard K, Jokubaitis V. Defining secondary progressive multiple sclerosis. Brain. 2016;139(Pt 9):2395–2405. doi: 10.1093/brain/aww173. [published Online First: 2016/07/13] [DOI] [PubMed] [Google Scholar]
- 18.Filippi M, Preziosa P, Langdon D. Identifying Progression In Multiple Sclerosis: New Perspectives. Ann Neurol. 2020 doi: 10.1002/ana.25808. [published Online First: 2020/06/09] [DOI] [PubMed] [Google Scholar]
- 19.Waubant E, Goodkin D, Bostrom A. IFNbeta lowers MMP-9/TIMP-1 ratio, which predicts new enhancing lesions in patients with SPMS. Neurology. 2003;60(1):52–57. doi: 10.1212/wnl.60.1.52. [published Online First: 2003/01/15] [DOI] [PubMed] [Google Scholar]
- 20.Brown JWL, Coles A, Horakova D. Association of Initial Disease-Modifying Therapy With Later Conversion to Secondary Progressive Multiple Sclerosis. JAMA. 2019;321(2):175–187. doi: 10.1001/jama.2018.20588. [published Online First: 2019/01/16] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bittner S, Steffen F, Uphaus T. Clinical implications of serum neurofilament in newly diagnosed MS patients: A longitudinal multicentre cohort study. EBioMed. 2020;56 doi: 10.1016/j.ebiom.2020.102807. [published Online First: 2020/05/28] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jakimovski D, Zivadinov R, Ramanthan M. Serum neurofilament light chain level associations with clinical and cognitive performance in multiple sclerosis: A longitudinal retrospective 5-year study. Mult Scler. 2019 doi: 10.1177/1352458519881428. [published Online First: 2019/10/16] [DOI] [PubMed] [Google Scholar]
- 23.Canto E, Barro C, Zhao C. Association Between Serum Neurofilament Light Chain Levels and Long-term Disease Course Among Patients With Multiple Sclerosis Followed up for 12 Years. JAMA Neurol. 2019 doi: 10.1001/jamaneurol.2019.2137. [published Online First: 2019/08/14] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chitnis T, Gonzalez C, Healy BC. Neurofilament light chain serum levels correlate with 10-year MRI outcomes in multiple sclerosis. Ann Clin Translat Neurol. 2018;5(12):1478–1491. doi: 10.1002/acn3.638. [published Online First: 2018/12/20] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lublin FD, Reingold SC, Cohen JA. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology. 2014;83(3):278–286. doi: 10.1212/WNL.0000000000000560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scheike TH, Maiers MJ, Rocha V. Competing risks with missing covariates: effect of haplotypematch on hematopoietic cell transplant patients. Lifetime Data Anal. 2013;19(1):19–32. doi: 10.1007/s10985-012-9229-1. [published Online First: 2012/09/13] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bjornevik K, Munger KL, Cortese M. Serum Neurofilament Light Chain Levels in Patients With Presymptomatic Multiple Sclerosis. JAMA Neurol. 2019 doi: 10.1001/jamaneurol.2019.3238. [published Online First: 2019/09/14] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rahmanzadeh R, Lu PJ, Barakovic M. Myelin and axon pathology in multiple sclerosis assessed by myelin water and multi-shell diffusion imaging. Brain. 2021 doi: 10.1093/brain/awab088. [published Online First: 2021/03/12] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Disanto G, Barro C, Benkert P. Serum Neurofilament light: A biomarker of neuronal damage in multiple sclerosis. Ann Neurol. 2017;81(6):857–870. doi: 10.1002/ana.24954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martin SJ, McGlasson S, Hunt D. Cerebrospinal fluid neurofilament light chain in multiple sclerosis and its subtypes: a meta-analysis of case-control studies. J Neurol Neurosurg Psychiatry. 2019;90(9):1059–1067. doi: 10.1136/jnnp-2018-319190. [published Online First: 2019/05/28] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barro C, Benkert P, Disanto G. Serum neurofilament as a predictor of disease worsening and brain and spinal cord atrophy in multiple sclerosis. Brain. 2018;141(8):2382–2391. doi: 10.1093/brain/awy154. [DOI] [PubMed] [Google Scholar]
- 32.Varhaug KN, Barro C, Bjornevik K. Neurofilament light chain predicts disease activity in relapsing-remitting MS. Neurol Neuroimmunol Neuroinflamm. 2018;5(1):e422. doi: 10.1212/NXI.0000000000000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dalla Costa G, Martinelli V, Sangalli F. Prognostic value of serum neurofilaments in patients with clinically isolated syndromes. Neurology. 2019;92(7) doi: 10.1212/WNL.0000000000006902. e733-e41[published Online First: 2019/01/13] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Novakova L, Zetterberg H, Sundstrom P. Monitoring disease activity in multiple sclerosis using serum neurofilament light protein. Neurology. 2017;89(22):2230–2237. doi: 10.1212/WNL.0000000000004683. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.