Key Points
Question
What are the clinical characteristics and outcomes of patients with cerebral venous sinus thrombosis with thrombocytopenia syndrome after SARS-CoV-2 vaccination?
Findings
In this cohort study of 116 patients with cerebral venous sinus thrombosis after SARS-CoV-2 vaccination, 78 (67.2%) had thrombosis with thrombocytopenia syndrome. Patients with thrombosis with thrombocytopenia syndrome were frequently comatose at presentation (24%) and often had intracerebral hemorrhage (68%) and concomitant thromboembolism (36%), and 47% died during hospitalization.
Meaning
Patients with cerebral venous sinus thrombosis after SARS-CoV-2 vaccination who met criteria for thrombosis with thrombocytopenia syndrome had a distinct clinical profile and high mortality rate.
This cohort study describes the clinical characteristics and outcome of patients with cerebral venous sinus thrombosis after SARS-CoV-2 vaccination with and without thrombosis with thrombocytopenia syndrome.
Abstract
Importance
Thrombosis with thrombocytopenia syndrome (TTS) has been reported after vaccination with the SARS-CoV-2 vaccines ChAdOx1 nCov-19 (Oxford–AstraZeneca) and Ad26.COV2.S (Janssen/Johnson & Johnson).
Objective
To describe the clinical characteristics and outcome of patients with cerebral venous sinus thrombosis (CVST) after SARS-CoV-2 vaccination with and without TTS.
Design, Setting, and Participants
This cohort study used data from an international registry of consecutive patients with CVST within 28 days of SARS-CoV-2 vaccination included between March 29 and June 18, 2021, from 81 hospitals in 19 countries. For reference, data from patients with CVST between 2015 and 2018 were derived from an existing international registry. Clinical characteristics and mortality rate were described for adults with (1) CVST in the setting of SARS-CoV-2 vaccine–induced immune thrombotic thrombocytopenia, (2) CVST after SARS-CoV-2 vaccination not fulling criteria for TTS, and (3) CVST unrelated to SARS-CoV-2 vaccination.
Exposures
Patients were classified as having TTS if they had new-onset thrombocytopenia without recent exposure to heparin, in accordance with the Brighton Collaboration interim criteria.
Main Outcomes and Measures
Clinical characteristics and mortality rate.
Results
Of 116 patients with postvaccination CVST, 78 (67.2%) had TTS, of whom 76 had been vaccinated with ChAdOx1 nCov-19; 38 (32.8%) had no indication of TTS. The control group included 207 patients with CVST before the COVID-19 pandemic. A total of 63 of 78 (81%), 30 of 38 (79%), and 145 of 207 (70.0%) patients, respectively, were female, and the mean (SD) age was 45 (14), 55 (20), and 42 (16) years, respectively. Concomitant thromboembolism occurred in 25 of 70 patients (36%) in the TTS group, 2 of 35 (6%) in the no TTS group, and 10 of 206 (4.9%) in the control group, and in-hospital mortality rates were 47% (36 of 76; 95% CI, 37-58), 5% (2 of 37; 95% CI, 1-18), and 3.9% (8 of 207; 95% CI, 2.0-7.4), respectively. The mortality rate was 61% (14 of 23) among patients in the TTS group diagnosed before the condition garnered attention in the scientific community and 42% (22 of 53) among patients diagnosed later.
Conclusions and Relevance
In this cohort study of patients with CVST, a distinct clinical profile and high mortality rate was observed in patients meeting criteria for TTS after SARS-CoV-2 vaccination.
Introduction
Reports of cerebral venous sinus thrombosis (CVST) and other thromboses at unusual sites in combination with thrombocytopenia occurring within 4 to 28 days of vaccination with the SARS-CoV-2 vaccines ChAdOx1 nCov-19 (Oxford–AstraZeneca) and Ad26.COV2.S (Janssen/Johnson & Johnson) have prompted several countries to restrict the use of these vaccines, especially in younger patients.1,2,3,4,5 The similarities between the clinical syndrome reported in these patients and spontaneous heparin-induced thrombocytopenia6 led investigators to the identification of circulating platelet-activating platelet factor 4 (PF4) antibodies in many of these patients. The condition has been named vaccine-induced immune thrombotic thrombocytopenia.1 To identify cases and promote research aimed at confirming whether this condition is indeed linked to vaccination, the Brighton Collaboration has proposed a definition of thrombosis with thrombocytopenia syndrome (TTS), which relies on evidence of thrombosis and new-onset thrombocytopenia without known recent exposure to heparin.7
To gain better insight into the clinical characteristics and outcomes of patients with CVST with TTS (CVST-TTS), we initiated an international registry of patients with CVST after SARS-CoV-2 vaccination. We hypothesized that patients with CVST-TTS would have distinct clinical features and a worse prognosis, while patients with postvaccination CVST without TTS would have a clinical profile resembling that of patients with CVST prior to the COVID-19 pandemic.
Methods
Patient Selection
We report results of a registry-based study on adult patients diagnosed with CVST after vaccination with any SARS-CoV-2 vaccine. Patients with CVST with symptom onset within 28 days of SARS-CoV-2 vaccination were included. Through the existing International Cerebral Venous Thrombosis Consortium,8,9 we asked physicians to disseminate this research initiative within national and international stroke care networks. The study was endorsed by the European Academy of Neurology and European Stroke Organisation. Participating investigators were requested to report consecutive patients with postvaccination CVST from their hospital. The ethical review committee of the Amsterdam UMC gave a waiver of formal approval for this observational study. Each center was responsible for obtaining adequate permission from local authorities for study participation and for acquiring informed consent for the use of pseudonymized care data if required by national law and hospital regulation. We collected data from a total of 81 hospitals in 19 countries. Data were collected from March 29 to June 18, 2021, and included both prospectively and retrospectively enrolled patients. All included patients were diagnosed with CVST between January 30 and June 14, 2021.
In accordance with the Brighton Collaboration level 1 interim case definition of TTS (version 10.16.2; May 18, 2021),7 patients with CVST after SARS-CoV-2 vaccination were classified as having TTS if they met all of the following criteria: (1) confirmed thrombosis, (2) new-onset thrombocytopenia, and (3) no known recent exposure to heparin. We refer to the group of patients classified as having TTS as the TTS group and patients with CVST after SARS-CoV-2 vaccination who were classified as not having TTS as the no TTS group.
The control group consisted of adult patients diagnosed with CVST prior to the COVID-19 pandemic who were included in the International Cerebral Venous Thrombosis Consortium registry between January 2, 2015, and February 24, 2018, from 6 hospitals in Finland, the Netherlands, Switzerland, Mexico, Iran, and Costa Rica. All these centers are tertiary hospitals with experience in the management of patients with CVST. Each hospital had permission from their ethical review board for the collection of observational data and obtained written informed consent for the use of pseudonymized care data if required under applicable national laws. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Data Collection and Definitions
A standardized case report form was used to collect detailed information on demographic characteristics, CVST risk factors, clinical manifestations, laboratory and imaging characteristics, treatment, and outcome in both the post–SARS-CoV-2 vaccination and control groups. Vaccination details were collected for the post–SARS-CoV-2 vaccination CVST group. In both groups, CVST had to be confirmed with computed tomography venography, magnetic resonance imaging, magnetic resonance venography, catheter angiography, or autopsy, in accordance with international guidelines.10,11 Patients’ sex and, for the post–SARS-CoV-2 vaccination registry, race were reported in the case report form by the local investigator. The race of the participants was observed by investigators. CVST risk factors included oral contraceptive use, hormone therapy use, pregnancy, recent delivery (up to 12 weeks prior to CVST diagnosis), cancer, concomitant infection, previous thromboembolism, and known thrombophilia.
Thrombocytopenia was defined as a platelet count of less than 150 × 103/μL (to convert to ×109 per liter, multiply by 1) and was further categorized into mild (100 to 150 × 103/μL), moderate (50 to 100 × 103/μL), or severe (less than 50 × 103/μL).12 Intracerebral hemorrhage was defined as hemorrhagic infarction or intracerebral hematoma. Functional outcome at hospital discharge among patients after SARS-CoV-2 vaccination was assessed using the modified Rankin Scale.13
Statistical Analysis
We did not perform an a priori power calculation but used data of all patients included in the study up to June 18, 2021. For the primary analysis, we used descriptive statistics to describe characteristics of the 3 groups (TTS, no TTS, and control). The number of missing values for each variable is reported. In exploratory secondary analyses, we calculated 95% CIs using the Wilson method for the frequencies of concomitant venous thromboembolism, new major bleeding during admission, and mortality at hospital discharge in each of the 3 groups. To compare mortality at hospital discharge between patients in the TTS group and the control group, we calculated unadjusted and age- and sex-adjusted odds ratios (ORs) with 95% CIs using logistic regression. For this specific analysis, we excluded patients in the TTS group who were still admitted to the hospital at the time of analysis. Data on age, sex, and mortality at discharge were otherwise complete in both groups, and age had a linear association with log(odds[mortality]). In a descriptive secondary analysis, we report treatment details and outcome of patients with CVST-TTS diagnosed before and after March 19, 2021, which is the date when the scientific community first became aware of this new syndrome and suggestions for treatment were circulated.
Results
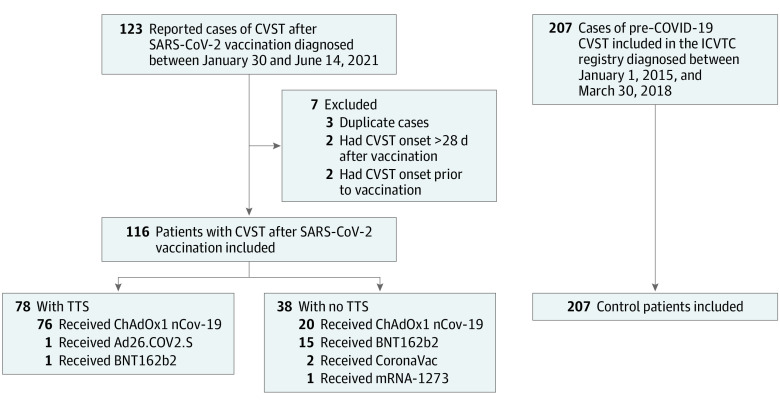
Of 123 reported patients with CVST after SARS-CoV-2 vaccination, 7 were excluded: 3 patients were reported in duplicate, 2 had CVST symptom onset more than 28 days after vaccination, and 2 had CVST symptom onset prior to vaccination (Figure). Thus, we included 116 patients with postvaccination CVST, diagnosed between January 30 and June 14, 2021. In total, 96 patients (82.8%) were vaccinated with ChAdOx1 nCov-19, 16 (13.8%) with BNT162b2 (Pfizer/BioNTech), 2 (1.7%) with CoronaVac (Sinovac), 1 (0.9%) with Ad26.COV2.S, and 1 (0.9%) with mRNA-1273 (Moderna). The pre–COVID-19 control group consisted of 207 patients. The distribution of patients per country is provided in eTables 1 and 2 in the Supplement.
Figure. Flowchart of Patient Selection.
CVST indicates cerebral venous sinus thrombosis; ICVTC, International Cerebral Venous Thrombosis Consortium; TTS, thrombosis with thrombocytopenia syndrome.
Among the 116 patients with CVST after SARS-CoV-2 vaccination, 78 (67.2%) met the Brighton criteria for TTS. In the control group, 2 patients (1.0%) had new-onset thrombocytopenia without recent exposure to heparin and would therefore have met criteria for TTS. In both of these patients, thrombocytopenia was due to infection.
Of the 78 patients with TTS after SARS-CoV-2 vaccination, 76 (97%) had received ChAdOx1 nCov-19, 1 (1%) had received Ad26.COV2.S, and 1 (1%) had received BNT162b2. One patient developed CVST-TTS after the second ChAdOx1 nCov-19 vaccination. The patient who had received BNT162b2 presented with mild thrombocytopenia (platelet count of 148 × 103/μL) and had an alternative explanation for the thrombocytopenia (cyclosporine use). Two patients who received ChAdOx1 nCov-19 also had an alternative explanation for the thrombocytopenia (suspected Behçet disease [baseline platelet count of 90 × 103/μL] and suspected antiphospholipid antibody syndrome [baseline platelet count of 47 × 103/μL]).
The median (IQR) time from vaccination to CVST symptom onset was 9 (7-10) days in the TTS group and 7 (3-16) days in the no TTS group (Table 1). The median (IQR) platelet count at hospital admission among patients with postvaccination CVST-TTS was 45 (25-71) ×103/μL. A total of 3 of 78 patients (4%) presented with a normal platelet count and developed thrombocytopenia during admission, 2 (3%) presented with mild thrombocytopenia, 30 (38%) with moderate thrombocytopenia, and 43 (55%) with severe thrombocytopenia. Details on the 5 patients with CVST-TTS with normal or mildly decreased platelet count at presentation are provided in eTable 3 in the Supplement. The median (IQR) platelet count nadir in the TTS group was 30 (14-53) ×103/μL. PF4 antibodies were measured in 69 patients with TTS, of whom 63 (91%) tested positive (62 of 67 [93%] after ChAdOx1 nCov-19; 1 of 1 after Ad26.COV2.S; and 0 of 1 after BNT162b2). Of the 6 patients with negative PF4 antibody test findings, 3 were tested with an enzyme-linked immunosorbent assay (ELISA) and 3 with a rapid test. Findings of platelet activation assays were positive in all 36 tested patients (35 after ChAdOx1 nCov-19 and 1 after Ad26.COV2.S).
Table 1. Details of SARS-CoV-2 Vaccination, Previous COVID-19 Infection, and Thrombocytopenia.
| Variable | Group, No./total No. (%) | |
|---|---|---|
| TTS (n = 78) | No TTS (n = 38) | |
| SARS-CoV-2 vaccinea | ||
| ChAdOx1 nCov-19 | 76/78 (97)b | 20/38 (53) |
| Ad26.COV2.S | 1/78 (1) | 0 |
| BNT162b2 | 1/78 (1) | 15/38 (40)c |
| mRNA-1273 | 0 | 1/38 (3) |
| CoronaVac | 0 | 2/38 (5) |
| Time from vaccination to CVST symptom onset, median (IQR), dd | 9 (7-10) | 7 (3-16) |
| Previous COVID-19 infection | 1/72 (1) | 4/36 (11) |
| Confirmede | 0 | 4/36 (11) |
| Suspected | 1/72 (1) | 0 |
| Thrombocytopenia details | ||
| Platelet count at admission, median (IQR), ×103/μL | 45 (25-71) | 272 (224-319) |
| Platelet count nadir, median (IQR), ×103/μL | 30 (14-53)f | NA |
| Positive for PF4 antibodies | 63/69 (91)g | NA |
| Positive findings on platelet activation assays | 36/36 (100) | NA |
| Positive for antiphospholipid antibodies | 8/56 (14)h | 0i |
Abbreviations: CVST, cerebral venous sinus thrombosis; NA, not applicable; TTS, thrombosis with thrombocytopenia syndrome.
SI conversion factor: To convert platelet count to ×109 per liter, multiply by 1.
In the ChAdOx1 nCov-19 group, 29 different vaccine batch numbers could be identified among 53 patients; in the BNT162b2 group, 9 different batch numbers among 10 patients; and in the CoronaVac group, 1 batch number in 1 patient.
One patient developed CVST with TTS after the second ChAdOx1 nCov-19 vaccination; PF4 antibodies were not tested in this patient.
Nine patients developed CVST without TTS after the second BNT162b2 vaccination.
Data for 1 patient was missing in each group.
Positive findings on nucleic acid amplification test.
Data missing for 11 patients.
Of the 6 patients who tested negative for PF4 antibodies, 3 were tested with an enzyme-linked immunosorbent assay and 3 with a rapid test.
Not performed in 22 patients.
Not performed in 9 patients.
Baseline characteristics for those in the TTS, no TTS, and control groups are provided in Table 2. In the TTS, no TTS, and control groups, a conventional CVST risk factor was identified in 19 (24%), 16 (42%), and 110 (64.0%) patients, respectively. One patient in the TTS group and 1 in the no TTS group developed a COVID-19 infection after vaccination. In the TTS group, 7 patients (9%) presented with petechiae, 2 (3%) with purpura, and 4 (5%) with mucosal bleeding. Intracerebral hemorrhage at baseline was present in 53 patients (68%) in the TTS group.
Table 2. Baseline Characteristics of Patients With Cerebral Venous Sinus Thrombosis (CVST) After SARS-CoV-2 Vaccination With and Without Thrombosis With Thrombocytopenia Syndrome (TTS).
| Characteristic | Group, No./total No. (%) | ||
|---|---|---|---|
| TTS (n = 78) | No TTS (n = 38) | Control (n = 207) | |
| Demographic characteristics | |||
| Age, mean (SD), y | 45 (14) | 55 (20) | 42 (16) |
| Sex | |||
| Female | 63/78 (81) | 30/38 (79) | 145/207 (70.0) |
| Male | 15/78 (19) | 8/38 (21) | 62/207 (30.0) |
| Race | |||
| Asian | 4/77 (5) | 2/38 (5) | ND |
| Black | 0 | 1/38 (3) | ND |
| White | 73/77 (95) | 35/38 (92) | ND |
| CVST risk factors | |||
| Any conventional CVST risk factor | 19/78 (24) | 16/38 (42) | 110/172 (64.0) |
| Oral contraceptivesa | 11/63 (17) | 9/30 (30) | 61/144 (42.4) |
| Hormone therapy among womena | 2/63 (3) | 1/30 (3) | 0 |
| Pregnancya | 0 | 0 | 14/145 (9.7) |
| Recent deliverya | 0 | 1/30 (3) | 4/145 (2.8) |
| Any infection | 5/78 (6) | 0 | 19/206 (9.2) |
| Previous thromboembolism | 1/78 (1)b | 1/38 (3)c | 17/202 (8.4) |
| Known thrombophilia | 1/78 (1)d | 1/38 (3)e | 17/181 (9.4) |
| Cancer | 3/78 (4) | 3/38 (8) | 20/207 (9.7) |
| Clinical presentation of CVST | |||
| Time from symptom onset to CVST diagnosis, median (IQR), d | 3 (2-4) | 3 (0-8) | 5 (2-11) |
| Headache | 75/78 (96) | 30/38 (79) | 185/207 (89.4) |
| Focal neurologic deficits | 41/78 (53) | 14/38 (37) | 128/206 (62.1) |
| Seizure | 8/78 (10) | 13/38 (34) | 62/205 (30.2) |
| Coma | 18/75 (24) | 1/37 (3) | 10/207 (4.8) |
| Baseline imaging characteristics of CVST | |||
| Thrombosis locationf | |||
| Sinus | |||
| Superior sagittal | 43/77 (56) | 15/38 (39) | 102/207 (49.3) |
| Lateralg | 67/77 (87) | 27/38 (71) | 129/207 (62.3) |
| Straight | 17/77 (22) | 3/38 (8) | 24/207 (11.6) |
| Deep venous systemh | 10/77 (13) | 1/38 (3) | 23/207 (11.1) |
| Cortical vein | 17/77 (22) | 12/38 (32) | 38/207 (18.4) |
| Intracerebral hemorrhage | 53/78 (68) | 10/38 (26) | 72/207 (34.8) |
| Focal edema only | 4/75 (5) | 4/38 (11) | 30/207 (14.5) |
Abbreviation: ND, not determined.
Includes female patients only.
Deep vein thrombosis.
Cerebral venous thrombosis.
Prothrombin G20210A variant.
MTHFR heterozygous variant.
Multiple locations are possible for each individual.
Transverse or sigmoid sinus.
Vein of Galen, basal vein of Rosenthal, internal cerebral vein, inferior longitudinal sinus, thalamostriate, or caudate veins.
Anticoagulant treatment was initiated in 67 patients with TTS (86%) (Table 3). Heparin treatment was initiated in 30 patients (38%) in the TTS group and 32 (84%) in the no TTS group, while nonheparin anticoagulants were started in 37 (47%) in the TTS group and in 6 (16%) in the no TTS group (eTable 4 in the Supplement). In the TTS group, 52 patients (67%) received immunomodulation therapy, most often intravenous immunoglobulins (47 [60%]). Of the patients with TTS, 60 (81%) were admitted to the intensive care unit, 16 (21%) underwent endovascular treatment, and 23 (30%) decompressive hemicraniectomy, compared with 8 (21%), 1 (3%), and 1 (3%), respectively, in the no TTS group, and 32 (17.9%), 1 (0.5%), and 10 (4.8%) in the control group.
Table 3. Clinical Course, Treatment, and Outcomes of Patients With Cerebral Venous Sinus Thrombosis (CVST) After SARS-CoV-2 Vaccination With and Without Thrombosis With Thrombocytopenia Syndrome (TTS).
| Treatment and outcome | Group, No./total No. (%) | ||
|---|---|---|---|
| TTS (n = 78) | No TTS (n = 38) | Control (n = 207) | |
| CVST treatment | |||
| Any anticoagulant treatment | 67/78 (86)a | 38/38 (100) | 200/206 (97.1) |
| Intensive care unit admission | 60/74 (81) | 8/38 (21) | 32/179 (17.9) |
| Endovascular treatment | 16/77 (21) | 1/38 (3) | 1/207 (0.5) |
| Hemicraniectomy | 23/77 (30) | 1/38 (3) | 10/207 (4.8) |
| Immunomodulation treatment | |||
| Any immunomodulation treatment | 52/78 (67) | NA | NA |
| Intravenous immunoglobulins | 47/78 (60) | NA | NA |
| Plasma exchange | 6/78 (8) | NA | NA |
| Corticosteroids | 25/78 (32) | NA | NA |
| Eculizumab | 2/78 (3) | NA | NA |
| Rituximab | 1/78 (1) | NA | NA |
| Platelet transfusion | 20/78 (26) | NA | NA |
| Because of neurosurgical intervention | 13/78 (17) | NA | NA |
| Outcomes | |||
| Any concomitant thromboembolism | 25/70 (36) | 2/35 (6) | 10/206 (4.9) |
| Splanchnic vein thrombosis | 10/70 (14) | 1/35 (3) | 0 |
| Deep vein thrombosis | 6/70 (9) | 0 | 3/206 (1.5) |
| Pulmonary embolism | 16/70 (23) | 0 | 3/206 (1.5) |
| Pelvic vein thrombosis | 6/70 (9) | 1/35 (3) | 0 |
| Other thrombosis | 6/70 (9)b | 1/35 (3)c | 1/206 (0.5) |
| Any major bleeding complicationd | 9/76 (12) | 3/37 (8) | 9/206 (4.4) |
| New intracranial hemorrhage | 8/76 (11) | 3/37 (8) | ND |
| Other major bleeding | 4/76 (5)e | 0 | ND |
| Mortality at discharge | 36/76 (47) | 2/37 (5) | 8/207 (3.9) |
Abbreviations: NA, not applicable; ND, not determined.
Reasons for withholding anticoagulant treatment included early death (n = 7), intracranial hemorrhage (n = 3), and CVST diagnosed at autopsy (n = 1).
Kidney vein (n = 1), inferior vena cava (n = 3), aortic arch thrombus (n = 1), ventricular thrombus (n = 1), myocardial infarction (n = 2), and basilar artery (n = 1).
Inferior vena cava thrombosis and brainstem ischemic stroke (n = 1).
According to the criteria of the International Society on Thrombosis and Haemostasis.
Adrenal (n = 2), intraocular (n = 1), and thigh (n = 1).
A total of 25 patients (36%; 95% CI, 26-47) in the TTS group, 2 (6%; 95% CI, 2-19) in the no TTS group, and 10 (4.9%; 95% CI, 2.7-8.7) in the control group had concomitant thromboembolism in addition to CVST (Table 3). A new major bleeding complication during hospital admission occurred in 9 patients (12%; 95% CI, 6-21) in the TTS group, 3 (8%; 95% CI, 2-21) in the no TTS group, and 9 (4.4%; 95% CI, 2.3-8.1) in the control group. At the time of analysis, 76 patients (97%) in the TTS group and 37 (97%) in the no TTS group had been discharged from the hospital or had died. In-hospital mortality was 47% (36 of 76; 95% CI, 37-58) in the TTS group and 3.9% (8 of 207; 95% CI, 2.0-7.4) in the control group (unadjusted OR, 22; 95% CI, 10-52; age- and sex-adjusted OR, 46; 95% CI, 15-147). In-hospital mortality rate in the no TTS group was 5% (2 of 37; 95% CI, 1-18). All deaths in the TTS group were due to brain herniation. Within the TTS group, the mortality rate among patients receiving heparin as the first anticoagulant treatment was 41% (12 of 29). The mortality rate in patients with CVST-TTS treated with intravenous immunoglobulins was 28% (13 of 46). The median (IQR) time from hospital admission to death was 2 (1-4) days. The full range of the modified Rankin Scale scores at discharge among patients in the TTS and no TTS groups are depicted in the eFigure in the Supplement.
Prior to March 19, 2021, when the scientific community first became aware of this new syndrome, 8 of 23 patients with CVST-TTS (35%) were treated with intravenous immunoglobulins and 17 of 23 (74%) with heparin (Table 4). After March 19, 2021, 39 of 55 patients (71%) and 13 of 55 (24%) were treated with intravenous immunoglobulins and heparin, respectively. In-hospital mortality rate of patients with CVST-TTS before and after March 19, 2021, were 14 of 23 (61%) and 22 of 53 (42%), respectively.
Table 4. Treatment and Outcome of 78 Patients With Cerebral Venous Sinus Thrombosis (CVST) With Thrombosis With Thrombocytopenia Syndrome Before and After March 19, 2021a.
| Outcome | No./total No. (%) | |
|---|---|---|
| Before March 19, 2021 (n = 23) | After March 19, 2021 (n = 55) | |
| Baseline characteristics | ||
| Age, mean (SD), y | 44 (11) | 46 (15) |
| Sex | ||
| Female | 20/23 (87) | 43/55 (78) |
| Male | 3/23 (13) | 12/55 (22) |
| Time from symptom onset to CVST diagnosis, median (IQR), d | 3 (1-4) | 4 (2-5) |
| Coma | 6/23 (26) | 12/52 (23) |
| Intracerebral hemorrhage | 20/23 (87) | 33/55 (60) |
| Treatment | ||
| Intravenous immunoglobulins | 8/23 (35) | 39/55 (71) |
| Plasma exchange | 3/23 (13) | 3/55 (6) |
| Corticosteroids | 9/23 (39) | 16/55 (29) |
| Eculizumab | 1/23 (4) | 1/55 (2) |
| Rituximab | 0 | 1/55 (2) |
| Platelet transfusion | 10/23 (44) | 10/55 (18) |
| First started anticoagulant treatment | ||
| Heparinb | 17/23 (74) | 13/55 (24) |
| Nonheparin anticoagulantc | 2/23 (9) | 35/55 (64) |
| No anticoagulant treatment started | 4/23 (17)d | 7/55 (13)e |
| Decompressive hemicraniectomy | 9/23 (39) | 14/54 (26) |
| Intensive care unit admission | 20/23 (87) | 40/51 (78) |
| Outcome | ||
| New VTE during admission | 6/21 (29) | 6/49 (12) |
| New major bleeding during admissionf | 4/22 (18) | 5/54 (9) |
| In-hospital mortality | 14/23 (61) | 22/53 (42) |
Abbreviation: VTE, venous thromboembolism.
The proportion of included patients who were classified as having thrombosis with thrombocytopenia syndrome was 66% (23 of 35) prior to March 19 and 68% (55 of 81) thereafter.
Low-molecular-weight heparin or unfractionated heparin.
Fondaparinux, argatroban, danaparoid, or direct oral anticoagulant.
Reasons for withholding anticoagulant treatment included early death (n = 2), intracranial hemorrhage (n = 1), and CVST diagnosed at autopsy (n = 1).
Reasons for withholding anticoagulant treatment included early death (n = 5) and intracranial hemorrhage (n = 2).
According to the criteria of the International Society on Thrombosis and Haemostasis.
Discussion
In this study, patients with CVST after recent SARS-CoV-2 vaccination who fulfilled the criteria for TTS had a severe clinical profile, with high rates of coma and intracerebral hemorrhage at presentation, high rates of concomitant thromboembolism, and death due to brain herniation occurring in approximately half of all patients. This clinical profile was distinct from both patients with postvaccination CVST who did not fulfill the criteria for TTS as well as a control group of patients with CVST from prior to the COVID-19 pandemic. The age- and sex-adjusted risk of death was higher for patients with CVST-TTS than for control patients with CVST. In contrast, patients with postvaccination CVST who did not fulfill the TTS criteria were largely similar to those in the control group with regard to both clinical manifestations and outcome.
The current findings support the usefulness of the interim case definition for TTS from the Brighton Collaboration for case finding purposes.7 Nevertheless, it is important to note that 3 patients with TTS in our study had a normal platelet count at baseline and developed thrombocytopenia only during hospital admission. Two of these patients also were positive for PF4 antibodies, while this was not tested with ELISA in the third patient. On the other hand, while the Brighton case definition uses a platelet count cutoff of 150 × 103/μL, 73 of 78 patients (94%) in this study had a baseline platelet count of less than 100 × 103/μL. Positivity for PF4 antibodies was also high in the TTS group (63 of 69 tested patients [91%]). As also noted in previous reports,1 a small minority of patients (3 in our study) fulfilling criteria for TTS tested negative for PF4 antibodies despite the use of an ELISA test. However, the sensitivity of different PF4 ELISA tests has been found to vary.14
In accordance with prior reports,1,2 most patients fulfilling the criteria for TTS presented after vaccination with ChAdOx1 nCov-19. One patient who fulfilled the criteria for TTS presented after vaccination with Ad26.COV2.S and 1 after vaccination with BNT162b2. The patient with TTS after BNT162b2 vaccination, however, had mild thrombocytopenia for which an alternative explanation was found and negative findings on PF4 antibody test. As a result, while this patient fulfilled the diagnostic criteria for TTS, a causal relation with the BNT162b2 vaccine is unlikely. As the criteria of the Brighton Collaboration for TTS are designed for case finding and surveillance purposes, it is expected that some TTS cases do not correspond to vaccine-induced immune thrombotic thrombocytopenia, as is exemplified by the fact that 1% of patients from the control group also fulfilled the criteria for TTS.
Half of patients with CVST-TTS died in this study. While the proportion of patients treated with decompressive hemicraniectomy and endovascular intervention was high, several patients with TTS did not receive medical treatment according to current recommendations for this condition, namely the use of nonheparin anticoagulants and intravenous immunoglobulins.15,16,17 Of note, this was especially common among patients diagnosed before March 19, 2021, when the syndrome was first brought to wide attention. The mortality rate appeared to be lower in CVST-TTS cases after March 19, 2021; however, given the small sample size and inability to properly adjust for confounding variables, we could not assess whether the outcome has truly improved with the evolution of treatment specifically aimed at TTS. Of note, even if the mortality rate improved after implementation of treatment guidelines, it persisted to be very high in more recent cases, highlighting the need for further studies to improve recognition and determine optimal treatment of CVST-TTS.
Although CVST-TTS is clearly a severe condition, it is important to note that our study was not aimed at determining whether the risks of any SARS-CoV-2 vaccine outweigh the benefits. The denominator of persons receiving each of the SARS-CoV-2 vaccines is unknown, and therefore, we were not able to determine the absolute risk of CVST-TTS after vaccination. COVID-19 is associated with a substantial risk of hospitalization and death as well as an increased risk of thrombotic events, including ischemic stroke and CVST.18,19,20 Of note, one study found that patients hospitalized for COVID-19 have a higher risk of developing CVST than people vaccinated against SARS-CoV-2.21
Strengths and Limitations
This study has a number of strengths. To our knowledge, this is the largest registry of post–SARS-CoV-2 vaccination CVST described to date and includes detailed information on risk factors, clinical manifestations, and imaging of CVST. The network of stroke centers that was recruited for this project has a wide geographical coverage, and we collected consecutive cases, irrespective of vaccine type or platelet count. This is crucial to guarantee a broad overview of the full spectrum of cases possibly related to SARS-CoV-2 vaccination. This is important to avoid early assumptions on the expected clinical profile of CVST in the setting of SARS-CoV-2 vaccine–induced immune thrombotic thrombocytopenia, which could lead to circular reasoning. Also, we have included a large control group of historical nonexposed cases. Data collection was standardized, and information on SARS-CoV-2 testing and PF4 antibodies was available in a high proportion of patients.
The study has several limitations. First, the lack of central adjudication of CVST diagnosis and study outcome measures is a possible source of imprecision and bias, although we have focused our analysis mainly on hard end points, such as mortality. Second, information was available up to hospital discharge only, precluding evaluation of the long-term consequences of CVST-TTS, which should be the focus of future studies. Third, differences in patients with postvaccination CVST and the historical control group should be taken into account. For example, many countries used different SARS-CoV-2 vaccines for different age groups, and pregnant women were partly excluded from SARS-CoV-2 vaccination at the time of study recruitment. Furthermore, the postvaccination CVST cohort included patients from countries not included in the historical control group, each with their own unique health care context. Unfortunately, the limited sample size precluded us from performing a secondary analysis comparing only cases from similar countries. Fourth, although we collected detailed information on treatment approach, the sample size and observational design of the study limits conclusions on the contribution of each specific approach to prognosis.
Conclusions
In this cohort study of patients with CVST, those with CVST-TTS after SARS-CoV-2 vaccination had a clinical profile distinct from patients with CVST before the COVID-19 pandemic, with high rates of coma and intracerebral hemorrhage and a high mortality rate. By contrast, patients with post–SARS-CoV-2 vaccination CVST who did not fulfill the criteria for TTS appeared to have a similar clinical presentation and prognosis as patients with CVST before the COVID-19 pandemic.
eTable 1. Number of included CVST cases after SARS-CoV-2 vaccination per country.
eTable 2. Number of CVST cases in the pre–COVID-19 control group per country.
eTable 3. Case description of patients with CVST-TTS with normal platelet count or mild thrombocytopenia at presentation.
eTable 4. First-started anticoagulant treatment in patients with CVST with and without TTS.
eFigure. Modified Rankin Scale score at hospital discharge among patients with CVST after SARS-CoV-2 vaccination with and without TTS.
References
- 1.Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021;384(22):2092-2101. doi: 10.1056/NEJMoa2104840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384(22):2124-2130. doi: 10.1056/NEJMoa2104882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.See I, Su JR, Lale A, et al. US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, March 2 to April 21, 2021. JAMA. 2021;325(24):2448-2456. doi: 10.1001/jama.2021.7517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.European Medicines Agency . Use of Vaxzevira to prevent COVID-19—article 5(3) procedure: assessment report. Accessed July 13, 2021. https://www.ema.europa.eu/en/documents/referral/use-vaxzevria-prevent-covid-19-article-53-procedure-assessment-report_en.pdf
- 5.MacNeil JR, Su JR, Broder KR, et al. Updated recommendations from the Advisory Committee on Immunization Practices for use of the Janssen (Johnson & Johnson) COVID-19 vaccine after reports of thrombosis with thrombocytopenia syndrome among vaccine recipients—United States, April 2021. MMWR Morb Mortal Wkly Rep. 2021;70(17):651-656. doi: 10.15585/mmwr.mm7017e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greinacher A, Selleng K, Warkentin TE. Autoimmune heparin-induced thrombocytopenia. J Thromb Haemost. 2017;15(11):2099-2114. doi: 10.1111/jth.13813 [DOI] [PubMed] [Google Scholar]
- 7.Brighton Collaboration . Interim case definition of thrombosis with thrombocytopenia syndrome (TTS). Accessed July 13, 2021. https://brightoncollaboration.us/thrombosis-with-thrombocytopenia-syndrome-interim-case-definition
- 8.Zuurbier SM, Arnold M, Middeldorp S, et al. Risk of cerebral venous thrombosis in obese women. JAMA Neurol. 2016;73(5):579-584. doi: 10.1001/jamaneurol.2016.0001 [DOI] [PubMed] [Google Scholar]
- 9.Lindgren E, Silvis SM, Hiltunen S, et al. Acute symptomatic seizures in cerebral venous thrombosis. Neurology. 2020;95(12):e1706-e1715. doi: 10.1212/WNL.0000000000010577 [DOI] [PubMed] [Google Scholar]
- 10.Ferro JM, Bousser MG, Canhão P, et al. ; European Stroke Organization . European Stroke Organization guideline for the diagnosis and treatment of cerebral venous thrombosis—endorsed by the European Academy of Neurology. Eur J Neurol. 2017;24(10):1203-1213. doi: 10.1111/ene.13381 [DOI] [PubMed] [Google Scholar]
- 11.Saposnik G, Barinagarrementeria F, Brown RD Jr, et al. ; American Heart Association Stroke Council and the Council on Epidemiology and Prevention . Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(4):1158-1192. doi: 10.1161/STR.0b013e31820a8364 [DOI] [PubMed] [Google Scholar]
- 12.Williamson DR, Albert M, Heels-Ansdell D, et al. ; PROTECT Collaborators, the Canadian Critical Care Trials Group, and the Australian and New Zealand Intensive Care Society Clinical Trials Group . Thrombocytopenia in critically ill patients receiving thromboprophylaxis: frequency, risk factors, and outcomes. Chest. 2013;144(4):1207-1215. doi: 10.1378/chest.13-0121 [DOI] [PubMed] [Google Scholar]
- 13.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19(5):604-607. doi: 10.1161/01.STR.19.5.604 [DOI] [PubMed] [Google Scholar]
- 14.Vayne C, Rollin J, Gruel Y, et al. PF4 immunoassays in vaccine-induced thrombotic thrombocytopenia. N Engl J Med. 2021;385(4):376-378. doi: 10.1056/NEJMc2106383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nazy I, Sachs UJ, Arnold DM, et al. Recommendations for the clinical and laboratory diagnosis of VITT against COVID-19: communication from the ISTH SSC Subcommittee on Platelet Immunology. J Thromb Haemost. 2021;19(6):1585-1588. doi: 10.1111/jth.15341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Furie KL, Cushman M, Elkind MSV, Lyden PD, Saposnik G; American Heart Association/American Stroke Association Stroke Council Leadership . Diagnosis and management of cerebral venous sinus thrombosis with vaccine-induced immune thrombotic thrombocytopenia. Stroke. 2021;52(7):2478-2482. doi: 10.1161/STROKEAHA.121.035564 [DOI] [PubMed] [Google Scholar]
- 17.Ferro JM, de Sousa DA, Coutinho JM, Martinelli I. European Stroke Organization interim expert opinion on cerebral venous thrombosis occurring after SARS-CoV-2 vaccination. Eur Stroke J. Published online July 20, 2021. doi: 10.1177/23969873211030842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baldini T, Asioli GM, Romoli M, et al. Cerebral venous thrombosis and severe acute respiratory syndrome coronavirus-2 infection: a systematic review and meta-analysis. Eur J Neurol. Published online January 11, 2021. doi: 10.1111/ene.14727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mowla A, Shakibajahromi B, Shahjouei S, et al. Cerebral venous sinus thrombosis associated with SARS-CoV-2: a multinational case series. J Neurol Sci. 2020;419:117183. doi: 10.1016/j.jns.2020.117183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al-Mufti F, Amuluru K, Sahni R, et al. Cerebral venous thrombosis in COVID-19: a New York Metropolitan cohort study. AJNR Am J Neuroradiol. 2021;42(7):1196-1200. doi: 10.3174/ajnr.A7134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bikdeli B, Chatterjee S, Arora S, et al. Cerebral venous sinus thrombosis in the U.S. population, after adenovirus-based SARS-CoV-2 vaccination, and after COVID-19. J Am Coll Cardiol. 2021;78(4):408-411. doi: 10.1016/j.jacc.2021.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Number of included CVST cases after SARS-CoV-2 vaccination per country.
eTable 2. Number of CVST cases in the pre–COVID-19 control group per country.
eTable 3. Case description of patients with CVST-TTS with normal platelet count or mild thrombocytopenia at presentation.
eTable 4. First-started anticoagulant treatment in patients with CVST with and without TTS.
eFigure. Modified Rankin Scale score at hospital discharge among patients with CVST after SARS-CoV-2 vaccination with and without TTS.