Abstract
Bone morphogenetic protein-2 (BMP-2) is a clinically used osteoinductive growth factor. With a short half-life and side effects, alternative delivery approaches are needed. This work examines thiolation of BMP-2 for chemical attachment to a poly(ethylene glycol) hydrogel using thiol-norbornene click chemistry. BMP-2 retained bioactivity post-thiolation and was successfully tethered into the hydrogel. To assess tethered BMP-2 on osteogenesis, MC3T3-E1 pre-osteoblasts were encapsulated in matrix metalloproteinase (MMP)-sensitive hydrogels containing RGD and either no BMP-2, soluble BMP-2 (5 nM) or tethered BMP-2 (40–200 nM) and cultured in a chemically defined medium containing dexamethasone for seven days. The hydrogel culture supported MC3T3-E1 osteogenesis regardless of BMP-2 presentation, but tethered BMP-2 augmented the osteogenic response, leading to significant increases in osteomarkers, Bglap and Ibsp. The ratio, Ibsp-to-Dmp1, highlighted differences in the extent of differentiation, revealing that without BMP-2, MC3T3-E1 cells showed a higher expression of Dmp1 (low ratio), but an equivalent expression with tethered BMP-2 and more abundant bone sialoprotein. In addition, this work identified that dexamethasone contributed to Ibsp expression, but not Bglap or Dmp1 and confirmed that tethered BMP-2 induced the BMP canonical signaling pathway. This work presents an effective method for the modification and incorporation of BMP-2 into hydrogels to enhance osteogenesis.
Graphical Abstract
Introduction
Growth factors of the extracellular environment are important signaling molecules for tissue development.1 Extracellular matrix (ECM)-bound growth factors have been described as serving as a “memory function” for future cells.2,3 Essentially, the stored growth factors signal important information about the tissue environment, which encodes a set of instructions for cell behavior. Leveraging growth factors found within native ECM is a powerful approach for tissue engineering.4 Bone morphogenetic proteins (BMPs) are one class of growth factors that have been implicated in a number of cellular functions from embryonic to adult cells.5–7 BMP-2, specifically, has essential roles in maintaining homeostasis of the postnatal skeleton and providing osteogenic signals necessary for intrinsic initiation of bone fracture repair.8–10
Although BMP-2 is highly effective in its ability to affect cell behavior and stimulate bone formation, there are numerous challenges in delivering BMP-2 in vivo. Direct injection of BMP-2 at the injury site is largely ineffective because it is rapidly cleared in vivo due to its relatively short half-life (e.g., 7–16 minutes in the blood stream).4,11–13 Therefore, supraphysiological levels of the growth factor are often required to enhance the efficacy of exogenous BMP-2.11 These levels can lead to serious complications; including, ectopic bone formation, increased risk of cancer, bone resorption, and development of antibodies against BMP-2, which was observed in a small subset of patients.14–18 Given the importance of growth factors, there is a need to deliver them in a manner that does not require excessive concentrations and that maintains their bioactivity in a localized domain.14
Several methods have been developed to deliver growth factors in a therapeutically efficient mode.19 Examples include direct loading of the growth factor into a polymer matrix for controlled release or binding of the protein directly to a polymer matrix via intermolecular interactions or covalent bonds.20 Direct loading requires no modification of the exogenous growth factor and relies on the material to retain and then release the protein in a controlled manner.21–23 One shortcoming of this method is a burst release that results in a large initial loss of the growth factor, most of which is proteolytically degraded. This shortcoming necessitates much higher loading to sustain the signal and in turn, increases the risk of negative side effects.11,20 Alternative methods that bind the protein to the delivery system have been developed. These methods more closely mimic the presentation of growth factors by the ECM.19 This matrix-bound delivery system provides a means to stabilize the active form of the protein, which protects it from immediate clearance and proteolytic inactivation, and reduces off-target effects. In addition, such a system may provide a mechanism for controlled dosing and localization to enhance the efficacy and safety of the use of growth factors.
Non-covalent methods of protein adsorption have been widely studied and utilized as a method for binding the growth factor to the matrix.24 These relatively weak interactions can result in similar burst release profiles of the protein, limiting the effectiveness of this approach.25 Research has shown that irrespective of the system used, delivery platforms that maintain a greater concentration of localized growth factor over time are more effective at promoting bone regeneration. These limitations have led researchers to explore covalent binding as an alternative to improve the stability and retention of the growth factor at the site of interest.24 It has been suggested that covalent immobilization may permit prolonged signal transduction of the protein relative to a soluble counterpart, because once immobilized, the protein cannot be internalized or easily deactivated by cells and proteolytic degradation would be reduced.25,26 There are several chemistries that have been leveraged to covalently tether BMP-2; including, attachment to a chitosan film via carbodiimide chemistry, covalent linking of BMP-2 to gold nanoparticles via amidation reaction with a heterobifunctional linker, and the coupling of BMP-2 to TiO2 nanorods by N,N-carbonyldiimiadazole reaction.25,27,28 The work presented herein investigates a thiol-ene reaction to covalently bind BMP-2 to a poly(ethylene glycol) (PEG) hydrogel.
When designing a growth factor delivery platform, several important considerations are the concentration of the growth factor delivered by the system and its bioactivity. In the work described herein, BMP-2 was modified with the addition of free thiols onto primary amines to enable its incorporation into a PEG network (Schematics 1 and 2). Similar methods have been previously described for use with TGF-β1 and TGF-β3.29–31 Photoclick reactions occur under mild conditions (e.g., room temperature and in PBS) and have been shown to support encapsulation of proteins, making this system promising for use with growth factors.32,33 The overarching goal for this work was to investigate the effectiveness of tethered BMP-2 on the osteogenic response of MC3T3-E1 pre-osteoblast cells, encapsulated in a PEG hydrogel and cultured in a chemically defined medium. A schematic of cell encapsulation and hydrogel formation is shown in Scheme 1. There were four primary aims for this study (Figure 1). The first was to assess the bioactivity of the BMP-2 after thiolation and after tethering into the hydrogel, utilizing the thiol-norbornene click reaction. The second aim was to evaluate the effects of the BMP-2 presentation and concentration of immobilized BMP-2 on MC3T3-E1 cells by the expression of osteogenic markers. The third and fourth aims were to identify the contribution of dexamethasone, which is known to induce osteogenesis, on the cellular osteogenic response of the encapsulated MC3T3-E1 cells, thus providing insights into the specific role of the hydrogel alone (study 3a) and the tethered BMP-2 (study 4a).34–36 Additionally, these aims sought to determine if the BMP canonical signaling pathway via activation of SMAD 1/5/8 was responsible for the increased osteogenic response in both the hydrogel alone, by activation from endogenously produced biomolecules (study 3b), and in the tethered condition by the immobilized BMP2-SH (study 4b). MC3T3-E1 cells were chosen because they have been used extensively to study the biological effects of BMP-2 in 2D culture and in several 3D culture systems; including, nanorods, titanium substrates, and hydrogels.37–43 Ultimately, this work demonstrates an effective method for the modification and incorporation of BMP-2 into a PEG hydrogel.
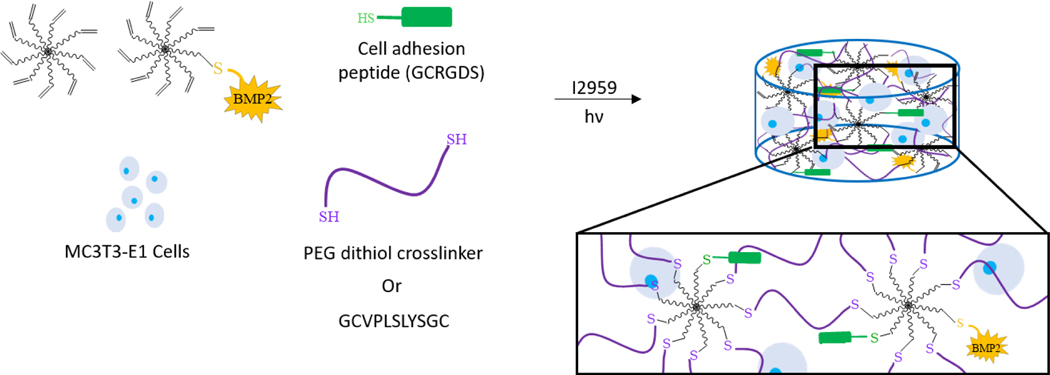
Scheme 1.
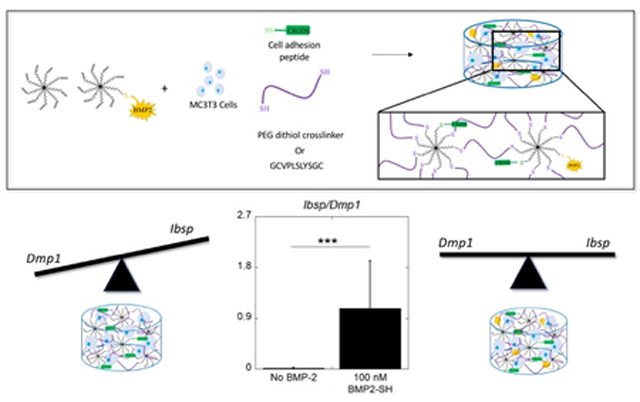
The formation of the BMP-2 tethered hydrogel with MC3T3-E1 cells. Hydrogels were formed from a precursor solution containing 8-arm PEG-norbornene, 8-arm PEG-norbornene-BMP-2, crosslinker (PEG-dithiol or matrix- metalloproteinase (MMP)-sensitive crosslinker), cell adhesion peptide, and MC3T3-E1 cells. The BMP-2 characterization studies utilized hydrogels with the PEG-dithiol crosslinker without cells. Close-up box highlights the crosslinked network. Not drawn to scale.
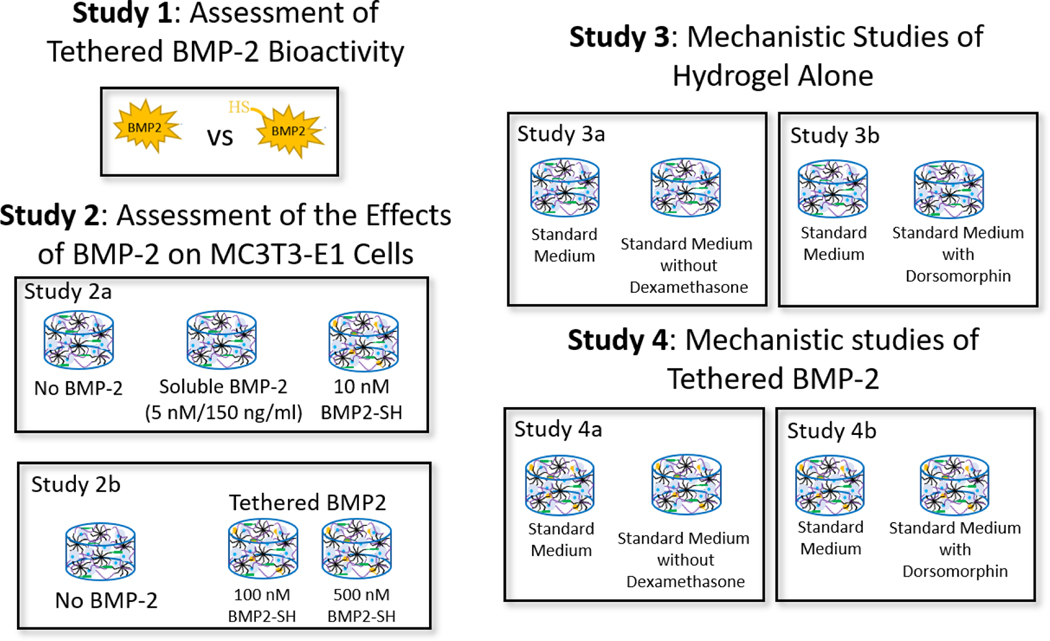
Figure 1.
An overview of the experimental design to assess the effectiveness of immobilizing BMP-2, via a thiol-norbornene click reaction, into PEG hydrogels. Study 1 assessed the bioactivity of thiolated BMP-2 in solution (study 1a) and the efficacy of tethering the functionalized growth factor to the PEG network (study 1b). A cell-reporter assay for SMAD 1/5/8 signaling and a modified-ELISA were used. Study 2 assessed the effects of BMP-2 (soluble and tethered) (study 2a) and concentration effects of tethered BMP-2 (study 2b) on MC3T3-E1 cells encapsulated in a PEG hydrogel and cultured in defined osteogenic medium (study 2a). Study 3 assessed the contribution of dexamethasone, which is present in the chemically defined medium (i.e., standard medium), on MC3T3-E1 cells encapsulated in the PEG hydrogel. The hydrogels were cultured in standard medium or standard medium without dexamethasone. Study 4 investigated if tethered BMP-2 signals via its receptors, that activates the SMAD 1/5/8 pathway, to induce the osteogenic response of the encapsulated cells. MC3T3-E1 cells were encapsulated in the PEG network and cultured in standard medium or standard medium with the inhibitor, dorsomorphin. In Studies 2–4, cell-laden hydrogels were cultured for up to seven days.
Materials and Methods
Macromer Synthesis
Both 10 kDa and 20 kDa 8-arm poly(ethylene glycol) (PEG) amine (JenKem) were modified with a norbornene group at the end of each arm.44 Briefly, the PEG amine, dissolved in equal volumes of dimethylformamide (DMF, Sigma-Aldrich) and dichloromethane (DCM, Sigma-Aldrich), was reacted overnight, under argon, with eight times molar excess of 5-norbornene-2-carboxylic acid in the presence of three molar excess 1-[Bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxide hexafluorophosphate (HATU, Chem-Impex International) and four molar excess N,N-diisopropylethylamine (DIPEA, Sigma-Aldrich). The product was recovered by precipitation in ice cold ethyl ether (Sigma-Aldrich), dialyzed for 3 days in deionized water, filtered (0.2 μm), and lyophilized. Comparison of the area under the peak for the allylic hydrogen closest to the norbornene hydrocarbon (𝛿=3.1–3.2 ppm) and the peak for the PEG backbone methyl group (𝛿=3.4–3.85 ppm) in an 1H-NMR spectrum were used and a ~100% conjugation of norbornene to the 8-arm PEG was determined.
Thiolation and assessment of BMP-2 bioactivity
BMP-2 was thiolated following an adapted protocol.30 Briefly, 2-iminothiolane (Traut’s reagent, Thermo Fisher Scientific) was reacted with recombinant, CHO-derived human/murine/rat BMP-2 (Peprotech, Catalog Number 120–02C) at a 4M excess for 1 h in buffer at room temperature to produce the product BMP2-SH. Assessment of bioactivity was performed using the SMAD 1/5/8 luciferase reporter HEK293 cell line (Signosis, SL-0051) per manufacturer provided instructions. Briefly, HEK293 cells were expanded in complete growth medium containing Dulbecco’s modified Eagle media (DMEM, Invitrogen), 10% fetal bovine serum (FBS, Atlanta Biologicals), and 1% penicillin/streptavidin (Corning) to ~90% confluency. Cells were trypsinized (Life Technologies), seeded into a 96-well plate at a concentration of 1×104 cells per well, and incubated at 37oC with 5% CO2 overnight. The next day, cells were incubated for 16 hours in media containing DMEM, 0.1% FBS and increasing concentrations of BMP-2 or BMP2-SH. Cells were washed with PBS and lysed with lysis buffer. Lysates were treated with luciferase substrate (Promega) and luminescence was recorded on a plate reader (BMG LabTech). Luminescence was subtracted from a blank containing lysis buffer and luciferase substrate.
Evaluation of the degree of thiolation of BMP-2
A fluorescent assay was used to assess the average degree of thiolation of BMP-2 post-reaction with 2-iminothiolane. A model protein of bovine serum albumin (BSA, Sigma-Aldrich) was used to develop the assay. BSA was reacted at a range of 0:1 to 20:1 mole ratio of 2-iminothiolane to BSA in PBS at a pH of 7.4. The product, BSA-SH, was reacted at 100:1 molar ratio of maleimide-fluorophore to BSA-SH in PBS at a pH of 7.4 for two hours at room temperature in the dark. At pH values between 6.5 and 7.5, the maleimide preferentially reacts with free thiols, thus adding a fluorophore to each free thiol. Samples were then run through a 7kDa MWCO desalting column (Thermo Fisher Scientific), as per manufacturer instructions, to remove unreacted maleimide-fluorophore. Fluorescence was measured at an excitation of 515 nm and emission of 580 nm on a plate reader (Molecular Devices) with a standard curve of fluorescence versus picomoles of fluorophore-tagged maleimide. Fluorescence of all BSA-SH samples were compared to the standard curve to determine the moles of free thiol that were added by the 2-iminothiolane reaction, after accounting for the background fluorescence of the naive protein. To determine the number of free thiols per BMP-2 molecule, BMP-2 was reacted at 4 moles 2-iminothiolane to 1 mole BMP-2, as previously described. A control consisted of BMP-2 exposed to the same conditions, but without 2-imionthiolane. The same procedures were followed as described for the BSA. Average degree of thiolation was determined by subtracting the picomoles of the control BMP-2 condition (i.e., no 2-iminotiolane, but reacted with maleimide-fluorophore) from the experimental BMP2-SH condition. This difference was divided by the total moles of BMP-2 reacted.
Evaluation of incorporation of thiolated BMP-2 into PEG network
Hydrogels were prepared by reaction of 10% (g/g) 20 kDa PEG-norbornene with 1 kDa PEG-dithiol crosslinker (Sigma-Aldrich) and either no BMP-2, 400 nM naive BMP-2, or 400 nM 2-iminothiolane-treated BMP-2 under UV light at 352 nm (5 mW/cm2) with 0.05 wt% (g/g) photoinitiator, 1-(4-(2- Hydroxyethoxy)-phenyl)-2-hydroxy-2-methyl- 1-propane-1-one (I2959; BASF), for 8 minutes (n = 5). In order to define experimental groups, conditions incorporating 2-iminotiolane-treated BMP-2 will subsequently be referred to as BMP2-SH. Hydrogels were swollen in media containing DMEM and 0.1% FBS at 37oC for 24 hours to release untethered BMP-2 and BMP2-SH. Luciferase reporter cells were treated with media from the hydrogels to determine the relative quantities of BMP-2 and BMP2-SH released. Cells were washed with PBS and lysed with lysis buffer. Lysates were transferred to a white 96-well plate, treated with luciferase substrate (Promega), and luminescence was recorded on a plate reader. Luminescence was subtracted from a blank containing lysis buffer and luciferase substrate.
BMP-2 was thiolated and then pre-reacted with 10 kDa, 8-arm PEG-NB under UV light at 352 nm (5 mW/cm2) with 0.05 wt% (g/g) I2959 for 1 minute to achieve a final precursor concentration of 0, 100, 250, and 500 nM BMP2-SH (n = 3). A precursor solution of 9 wt% (g/g) BMP2-tethered PEG-norbornene, 3.4 kDa PEG-dithiol (1:1 thiol:ene ratio), and 0.05 wt% (g/g) I2959 was polymerized in a cylindrical mold under UV light at 352 nm (5 mW/cm2) for 8 minutes. Following polymerization, hydrogels were washed 3x in PBS over 24 hours. An adaptation of a previously described modified-ELISA protocol using components of the DuoSet ELISA kit for BMP-2 (R&D Biosystems) was used to validate the thiolation and subsequent tethering of the growth factor to the PEG network.45 Briefly, hydrogels were transferred to a low-bind 96 well-plate and incubated in a solution of anti-BMP-2 capture antibody (5μg mL−1) for 2 hours at room temperature. Hydrogels were rinsed and incubated in a solution of biotin-labeled anti-BMP-2 detection antibody (250 ng mL−1) for 2 hours at room temperature. Gels were rinsed and incubated for 30 minutes at room temperature in a solution containing streptavidin- horseradish-peroxidase (1:40 dilution). Samples were washed and then transferred to a clean well-plate and incubated with tetramethylbenzidine/H2O2 solution and incubated for 20 minutes at room temperature and then quenched. The absorbance of the solution was measured using a spectrophotometer (Molecular Devices) at 450 nm with a 550 nm baseline.
Cell Culture and Encapsulation
MC3T3-E1 murine pre-osteoblast cells (ATCC, CRL-2593) were expanded in growth media (α- minimum essential medium, (Gibco)) supplemented with 10% of fetal bovine serum (Atlanta Biologicals) and 1% antibiotics (5000 Units mL–1 Penicillin, 5000 μg mL−1 Streptomycin, Corning) in an incubator at 37 °C with 5% CO2. The medium was exchanged three times weekly and the cells were passaged at ∼90% confluency with 0.25% Trypsin-EDTA (Gibco). Cell-laden hydrogels were formed by encapsulation of MC3T3-E1 cells at a cell concentration of 50×106 cells mL−1 of filter-sterilized (0.22 mm filter) precursor solution. Precursor solution contained 6% (g/g) 10 kDa-8-arm PEG-norbornene pre-reacted with either 0, 10, 100, or 500 nM BMP2-SH, 2mM CRGDS (GenScript), MMP-sensitive crosslinker (GCVPLS-LYSGC, GenScript) at a 0.8:1 thiol-ene ratio, and was reacted with 0.05 wt% I2959 under UV light at 352 nm (5 mW/cm2) for 8 minutes.
Cell-laden hydrogels were cultured in chemically defined osteogenic differentiation medium, referred to as standard medium. Standard medium consisted of MEM α (Gibco), 1×ITS + premix (Corning), 100 nM dexamethasone (Sigma-Aldrich), 50 μg mL−1 ascorbate-2-phosphate (Sigma-Aldrich), 1× MEM non-essential amino acids (Gibco), 10mM β-glycerophosphate (Sigma-Aldrich), penicillin/streptomycin (Invitrogen), and fungizone (Invitrogen). Study 2 utilized the standard medium for the no BMP-2 and tethered BMP2-SH hydrogels. A subset of gels containing no tethered BMP-2 were cultured in the standard medium supplemented with 5 nM (130 ng mL−1) BMP-2 (Peprotech, Catalog Number 120–02C). In studies 3a and 4a, the standard medium without dexamethasone was used. In studies 3b and 4b, the standard medium was supplemented with 10μM dorsomorphin (Cayman Chemical Company). Dorsomorphin in a small molecule inhibitor of the bone morphogenetic protein type I receptors; ALK 2, 3 and 6. Fresh dorsomorphin was added to the standard medium at every medium exchange. All cell-laden hydrogels were cultured under standard cell conditions of 37oC with 5% CO2 for one or seven days. All procedures were performed inside a biosafety cabinet using sterilized instruments.
ALP Assay
Hydrogel samples were removed from the culture after one or seven days and rinsed in PBS for 1 h, lysed in deionized water (diH2O), frozen in liquid nitrogen, and stored at −80 °C. Samples were disrupted and lysed using a tissue lyser (Qiagen) and subsequent freeze−thaw-sonication cycles. DNA content was measured using a Quant-iT PicoGreen dsDNA assay kit (Thermo Fisher Scientific) by fluorescence with excitation at 485 nm and emission at 520 nm according to the manufacturer specifications. Alkaline phosphatase activity was determined by measuring the number of moles of p-nitrophenol phosphate catalyzed to p-nitrophenol, which was measured by absorbance at 450 nm using a spectrophotometer (Molecular Devices).
Gene expression
Samples were collected at days one and seven and placed in TRK lysis buffer (Omega) and stored at −80 °C. Samples were disrupted using a tissue lyser (Qiagen), and RNA was isolated using E.Z.N.A. microelute kit (Omega) as per the manufacturer instructions. The amount of pure RNA was quantified using a Nanodrop instrument (ND-1000, Thermo Fisher Scientific) with A260/280 greater than 1.90. Purified RNA was reverse-transcribed into cDNA using a high-capacity cDNA reverse transcription kit (Applied Biosystems) as per the manufacturer instructions. Quantitative real-time polymerase chain reaction (qPCR) was performed with Fast SYBR Green Master Mix (Applied Biosystems) on a 7500 Fast Real-Time PCR Machine (Applied Biosystems). Primers were designed and evaluated for efficiencies within the MIQE guidelines of acceptable range (80–120%, IDT).46 Genes, primer sequences, accession numbers, and efficiencies are reported in Table 1. All genes of interest (GOI) are relative to the housekeeping gene, L32. Data are presented as normalized expression (NE) given by:
where the gene expression is normalized to a control of the no BMP-2 condition in standard medium at day one.47
Table 1.
Primer sequences, accession numbers, and efficiencies for genes used in this study.
| Gene | Forward Primer | Reverse Primer | Accession # | Efficiency (E) |
|---|---|---|---|---|
| L32 | CCATCTGTTTTACGGCATCATG | TGAACTTCTTGGTCCTCTTTTTGA | NM_172086 | 83% |
| Id1 | AACGGCGAGATCAGTGCCTT | CCTCAGCGACACAAGATGCGAT | NM_001355113 | 106% |
| Bglap | CAGACACCATGAGGAGGACCATCTT | GATAGCTCGTCACAAGCAGG | NM_007541 | 118% |
| Ibsp | TTCGTTTGAAGTCTCCTCTTCC | CTCCTCTGAAACGGTTTCCA | NM_008318 | 94% |
| Dmp1 | GCTTCTCTGAGATCCCTCTTCG | GCGATTCCTCTACCCTCTCT | NM_016779.2 | 97% |
Immunohistochemistry (IHC)
At day seven, MC3T3-E1-laden hydrogels (n=3) were removed from culture and prepared for IHC. Samples were fixed overnight at 4 oC in 4% paraformaldehyde and transferred to sterile PBS for storage at 4 oC. Samples were dehydrated following standard methods, embedded in paraffin, and sectioned at 5 μm. Samples were pretreated with Retrievagen A (BD Pharmingen) for antigen retrieval. Following permeabilization and blocking, sections were treated overnight at 4 oC with the primary antibody: 1:15 anti-bone sialoprotein II (DSHB) and 1:100 anti-DMP-1 (Millipore Sigma) in blocking solution. Samples were treated for two hours at room temperature with goat-anti-mouse IgG labelled Alexa Fluor 488 (1:200, Abcam) or goat-anti-mouse IgG labelled AlexaFluor 546 (1:200, Fisher Thermo Scientific) and the nuclei counterstained with DAPI (Life Technologies).
Statistical Analysis
Data are represented as mean with standard deviation shown parenthetically in the text or as error bars in the figures. Statistical analysis was performed using the Real Statistics add-in for Excel. Normal distribution and homogeneous variances were confirmed prior to performing analyses. Two-way ANOVAs were performed with α = 0.05 where factors included time and BMP-2 presentation as described in the results. If a significant interaction between the two factors was observed, one-way ANOVAs were performed holding each factor constant. For significant main effects, Tukey’s post-hoc was performed. In comparisons that were limited to two groups, a Student’s t test was performed assuming independent samples and equal variances. Data were analyzed using a one-way ANOVA (α = 0.05) for comparisons with more than two groups but only one factor. Significant results were followed up with a Bonferroni comparison test. Additionally, p-values from the analyses are provided to indicate the level of significance, with p < 0.05 being considered statistically significant. The sample size was n = 3 unless otherwise noted.
Results and Discussion
BMP-2 maintains biological activity post-thiolation
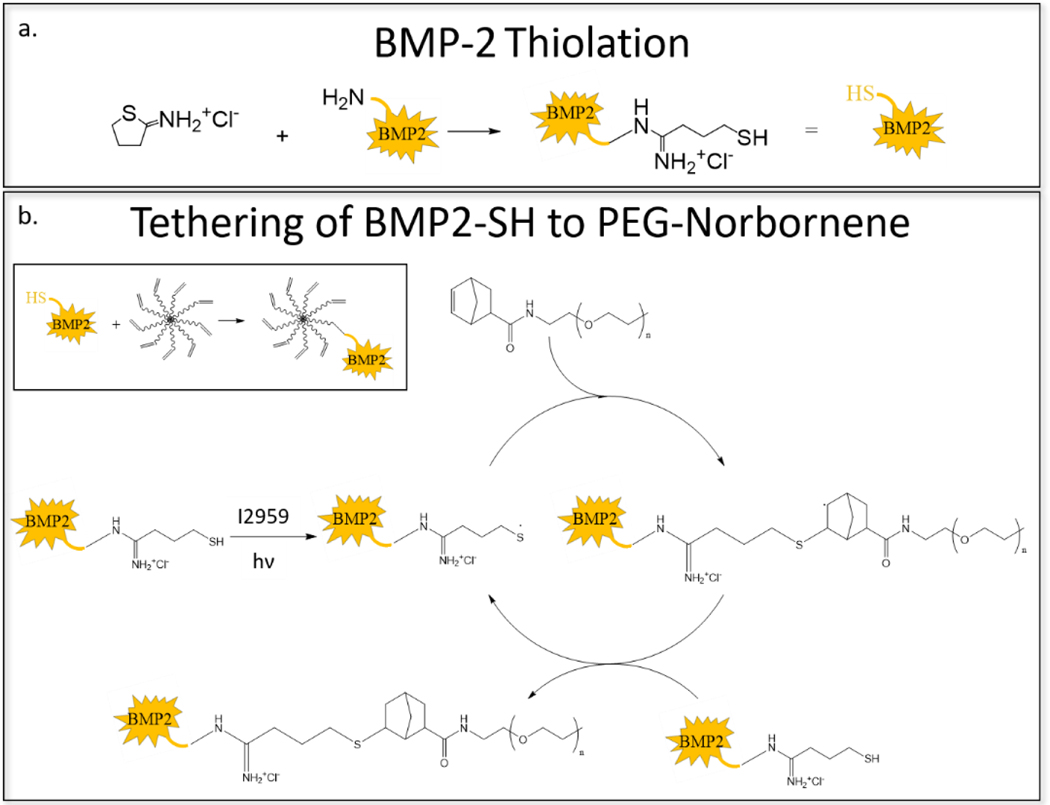
To immobilize BMP-2 to the PEG hydrogel, naive BMP-2 was first reacted with 2-iminothiolane, which adds a free thiol to a primary amine, which are located on lysine residues and the N-terminus position of the protein (Scheme 2a). The BMP2-SH is then reacted with the 8-arm PEG-norbornene, through the thiol-norbornene click reaction in the presence of a photoinitiator and light, to produce a stable sulfenamide bond (Scheme 2b). Once the BMP-2 is tethered and subsequently reacted into the hydrogel, the BMP-2 will remain tethered to the PEG and remain in the hydrogel until the hydrogel degrades.
Scheme 2.
(a) The addition of a free thiol to BMP-2 by reaction with 2-iminothiolane. (b) The radical-mediated, photoclick, thiol-norbornene reaction between norbornene-functionalized PEG and thiol-functionalized BMP-2 (i.e., BMP2-SH). BMP-2 is tethered via a stable bond.
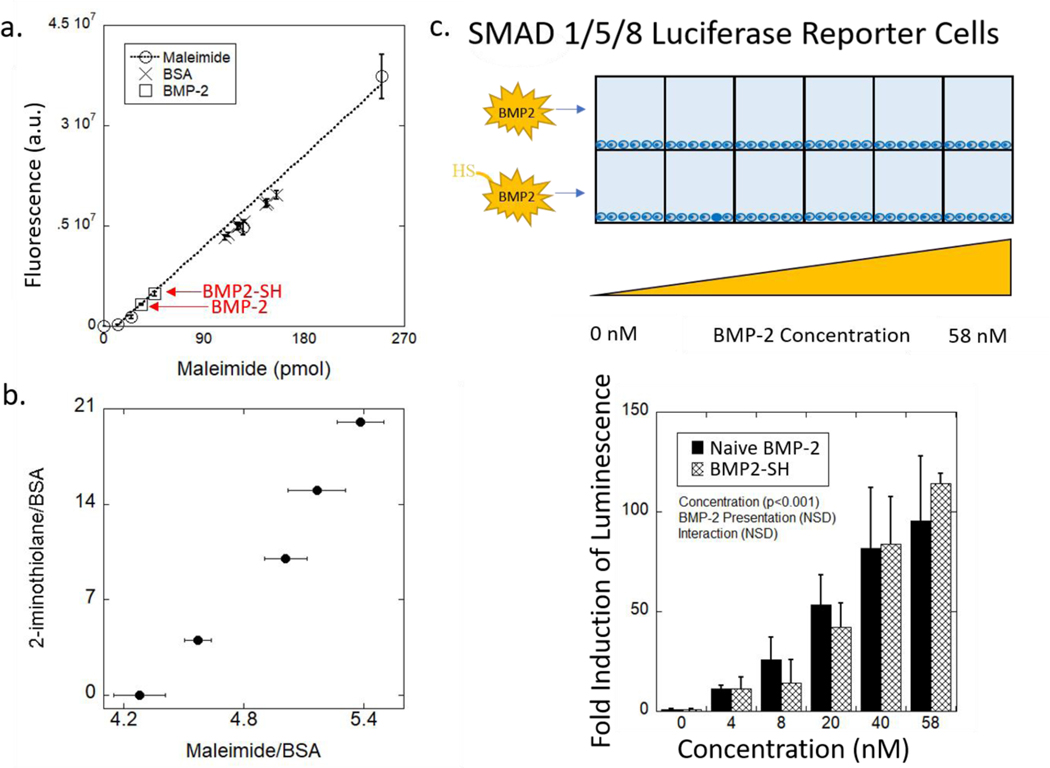
The thiolation efficiency of the reaction was assessed by utilizing a fluorophore-tagged maleimide, which reacts efficiently with free thiols, and measuring the resultant fluorescence. To ensure the rigor of this method, bovine serum albumin (BSA) was used as a model protein that was reacted with 2-iminothiolane at different molar ratios to provide a range of thiols added per BSA molecule (BSA-SH). The fluorescence of BSA-SH from each reaction was overlaid on the standard curve of fluorescence versus maleimide (Figure 2a). An increase in the ratio of 2-iminothiolane to the BSA resulted in a relatively linear increase in the number of thiols added (Figure 2b). BMP2-SH, reacted with a fluorophore-tagged maleimide, was also overlaid on this same plot. This result indicates, that approximately 52% of the BMP-2 molecules were modified, on average, with a single thiol post-reaction with 2-iminothiolane.
Figure 2.
(a) Fluorescence measured as a function of picomoles of fluorescently tagged-maleimide is used to quantify the average number of free thiols added to BMP-2 and BSA. Red notation on graph identifies data point related to the BMP2-SH used in this study. Data represent average with standard deviation error bars (n = 2). (b) Molar ratio of 2-iminothiolane to BSA as a function of the average number of molecules of maleimide added per macromolecule of BSA. Data represent average with standard deviation error bars (n = 2–4). (c) Schematic of the experimental system to test for biological activity of BMP2-SH as compared to naive BMP-2 growth factor using SMAD 1/5/8 reporter cells. Fold-induction of luminescence of the reporter cells as a function of concentration for naive BMP-2 and BMP2-SH relative to the 0 nM condition. p > 0.05 between each condition at all concentrations. Data represent average with standard deviation error bars; n = 3. Data were analyzed using a two-way ANOVA and Tukey’s post-hoc test.
The relatively low efficiency of thiolation of both proteins is attributed to the pH of the reaction (i.e., pH 7.4), which was chosen to maintain the bioactivity of the growth factor. However, 2-iminothiolane is more reactive at a higher pH due to a greater proportion of unprotonated primary amines. The pKa values for the N-terminus amine and the primary amine on lysine residues are 7.6–8.0 and 10.0–10.2, respectively, indicating that a large number of the primary amines will be protonated and less reactive. The Henderson-Hasselbalch equation estimates ~68% of the N-terminus primary amines would be deprotonated and readily reactive with the 2-iminothiolane, which is consistent with our findings that 52% of the BMP-2 was successfully thiolated. Our findings are supported by other studies in the literature. For example, studies that have PEGylated BMP-2 via its primary amines reported ~30–42% was unmodified and that increasing the PEGylating agent (i.e., PEG-aldehyde) to a 10-molar excess improved the reaction efficiency.48 The results from BSA-SH suggest that an increased molar ratio between 2-iminothiolane and the protein of interest will result in increased thiolation. Future studies will require a higher molar excess to achieve a higher degree of BMP-2 thiolation.
It is important to note that the maleimide fluorophore reacted with a small number of primary amines on the proteins. This was evident by the fluorescence of naive BSA and BMP-2 after reaction with maleimide fluorophore. Each BSA molecule has one free thiol, but ~4 maleimide molecules were added per naive BSA, indicating that roughly three of the 30–35 free lysine residues found on BSA were reacted with maleimide.49–51 Similarly for BMP-2, which does not have any free thiols, ~1.5 maleimide molecules (out of 20 available primary amines) were added per naive protein. Due to the large (100:1) molar excess of maleimide and the reaction pH (i.e., 7.4) that favors free thiols over primary amines, it is reasonable to assume that the increased fluorescence over the naive protein is attributed to the free thiols that were added by the 2-iminothiolane.
As with many biomolecules, structure dictates function and it has been demonstrated that growth factors, including BMP-2, have structures that are sensitive to environmental changes.1,13 Studies on the structure of BMP-2 have shown that, in addition to the primary amine on the N-terminus, each monomeric chain contains four lysine residues within a flexible N-terminal segment and an additional five are found in a highly conserved cysteine knot domain, which is crucial for receptor binding.48 It has been previously demonstrated that the formation of amidine bonds by reaction between primary amines in proteins with imidoesters (e.g., 2-iminothiolane) produces minimal changes in the conformational domains and biological activities of proteins, as long as the modified lysine residue is not a vital component of the reactive site.52,53 These findings suggest that the structure of BMP-2, and thus its bioactivity, should be maintained as long as there are no modifications to lysine residues within the cysteine knot domain.
The bioactivity of BMP2-SH was assessed and compared to naive BMP-2. HEK293 reporter cells that stably express the firefly luciferase gene, when SMAD 1/5/8 is activated, were used. SMAD 1/5/8 is the canonical signaling pathway of BMP-2, therefore luminescence is indicative of BMP-2 activity.7 Plated HEK293 cells were incubated with either the naive (i.e., unmodified) BMP-2 or BMP2-SH at increasing concentration from 0 to 58 nM (Figure 2c). Thiolation with 2-iminothiolane did not change (p > 0.05) the bioactivity of BMP2-SH relative to naive BMP-2 across the entire concentration range (Figure 2c). These results indicate that thiolation of the primary amine on BMP-2 did not affect the cysteine knot domain, which is required for BMP-2 activity.
BMP2-SH was successfully tethered into the network at a range of concentrations
Once functionalized with a thiol group, BMP2-SH was tethered into the network via thiol-norbornene click chemistry (Scheme 1 and 2b).54,55 Only BMP-2 that is thiolated is expected to be covalently tethered to the hydrogel. To assess thiolation and tethering, the BMP-2 incorporated into the hydrogel was indirectly assessed by measuring BMP-2 that was released. Hydrogels were formed with no BMP-2, naive BMP-2, or BMP2-SH and then swollen in cell-culture media for 24 hours to allow untethered BMP-2 to diffuse out of the hydrogel (Figure 3a). The released BMP-2 was assessed using the SMAD 1/5/8 reporter cell line and luminescence was measured (Figure 3b). Minimal luminescence was measured in the negative control (i.e., with no BMP-2). A 40% decrease in luminescence was observed between the BMP-2 and BMP2-SH conditions. This result suggests that an average of 40% of the 2-iminothiolane-reacted BMP-2 was tethered into the network. This finding is consistent with our results from Figure 2a that an average of 52% of the BMP-2 molecules have a single free thiol. Since it is possible that some BMP-2 molecules may have more than one free thiol, a lower percent of BMP-2 that is tethered into the hydrogel, is expected. We further surmise that the polymerization did not cause a loss of bioactivity.
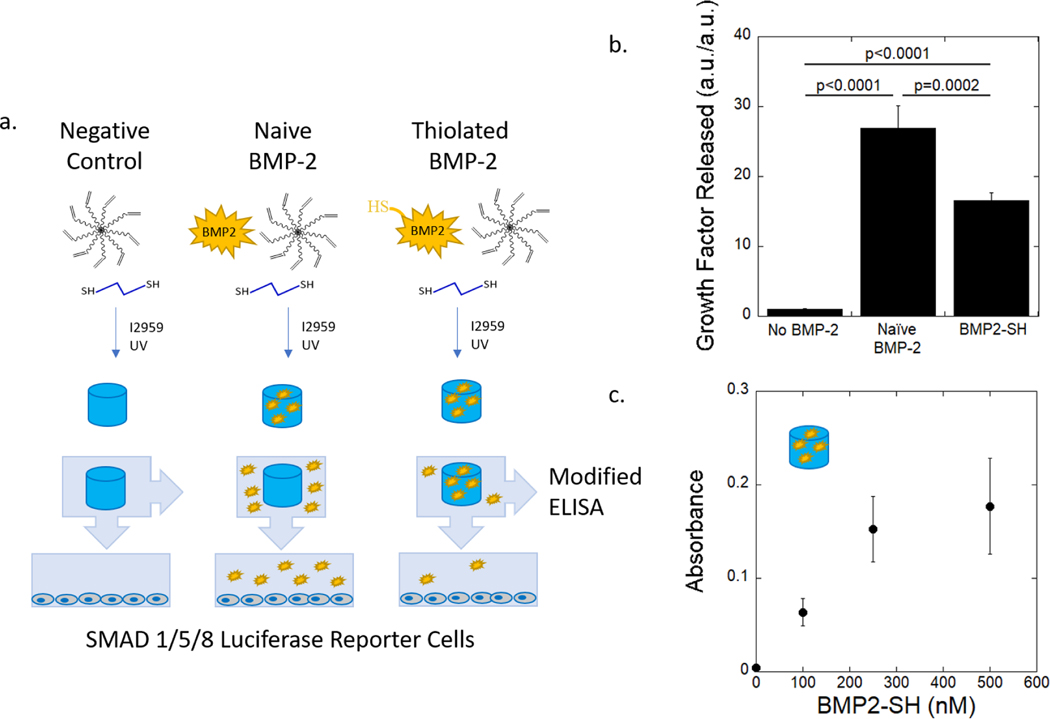
Figure 3.
(a) Schematic of experimental design to test for efficacy of immobilizing BMP2-SH in hydrogel using SMAD 1/5/8 reporter cells and a modified-ELISA. Hydrogels were formed from a precursor solution containing no BMP-2, naive BMP-2, or BMP2-SH and then photopolymerized. The precursor solution contained 8-arm PEG-norbornene, PEG-dithiol, and photoiniator (I2959). Once formed, the hydrogels were swelled in cell-culture media for 24 hours to release any untethered BMP-2 or BMP2-SH. Luciferase reporter cells were treated with released BMP. (b) Luminescence of the reporter cells as a function of BMP-2 and BMP2-SH released from hydrogels. Data are normalized to the no BMP-2 condition. (n = 4). Data were analyzed using a one-way ANOVA and a Bonferroni’s post-hoc comparison test.(c) Absorbance measured from a modified ELISA quantifies the presence of tethered BMP2-SH on the surface of the PEG hydrogel (n = 3). Data represent average with standard deviation error bars.
The ability to vary the concentration of BMP2-SH incorporated into the hydrogel was also investigated. A modified-ELISA protocol was used to evaluate the amount of tethered BMP2-SH relative to the BMP2-SH concentration in the precursor solution. Hydrogels were formed by first pre-reacting 0–500 nM BMP2-SH (referring to the concentration of BMP-2 used in the thiolation reaction) with the 8-arm PEG-norbornene, where there are 4.4 × 106 norbornene molecules per BMP2-SH molecule. The hydrogels were swollen in PBS for 24 hours to allow any untethered BMP2-SH to diffuse out of the network. A dose-dependent increase in absorbance was observed up to 250 nM BMP2-SH (Figure 3c). These results confirm that BMP2-SH is incorporated into the hydrogel and that the amount of BMP2-SH incorporated can be controlled. A saturation in the absorbance was observed at the 500 nM BMP2-SH concentration, which is attributed to the technical limitation of the modified ELISA. The ELISA uses ~1.6 × 10−13 detection antibody molecules per sample; in the 500 nM BMP2-SH condition, assuming 40% of the protein successfully tethered, there would be ~2.3 × 10−13 molecules of BMP2-SH per gel. It is unlikely that all of the detection antibody molecules in solution would be bound to a tethered BMP-2 molecule. It is also unlikely that BMP-2 or the antibodies would be transport limited due to the low crosslink density of the hydrogel (and hence a large mesh size),56 which is also supported by the results in Figure 3b. Nonetheless, these data demonstrate that the concentration of the BMP2-SH incorporated into the hydrogel can be readily modified by modulating the amount of BMP2-SH added to precursor solution prior to forming the hydrogel.
MC3T3-E1 cells undergo osteogenesis in 3D hydrogels regardless of BMP-2 presentation
To assess the bioactivity of the tethered BMP2-SH, this study measured alkaline phosphatase (ALP) activity and the gene expression of known osteogenic markers: Id1, Bglap, Ibsp, and Dmp1. MC3T3-E1 pre-osteoblasts were encapsulated in MMP-sensitive, PEG hydrogels and cultured for up to one week. Three experimental groups were compared and included: a hydrogel with 10 nM BMP2-SH (equivalent to 4 nM or 100 ng mL−1 of hydrogel volume, assuming 40% tethering efficiency, Table 2), a hydrogel treated with 5 nM soluble BMP-2 (equivalent to 130 ng mL−1 of media), and a no BMP-2 condition (Figure 1). The soluble BMP-2 concentration was selected based on previous osteogenic studies.35,57–61 A defined medium was selected to avoid complicating the interpretation due to fetal bovine serum and its plethora of undefined growth factors.
Table 2.
Nomenclature and concentrations of BMP-2 used in this work.
| Nomenclature | BMP-2 (nM)1 | Tethered BMP-2 (nM)2 | Tethered BMP-2 (ng mL−1)2 |
|---|---|---|---|
| 10 nM BMP2-SH | 10 | 4 | 100 |
| 100 nM BMP2-SH | 100 | 40 | 1000 |
| 500 nM BMP2-SH | 500 | 200 | 5000 |
Concentration of BMP-2 used in the thiolation reaction.
Estimated concentration of tethered BMP-2 concentration in the gel after thiolation and immobilization based on modified ELISA, Figure 3c.
Alkaline phosphatase activity, which is an indicator of osteoblast differentiation, was measurable across all groups. Time was a significant factor (p = 0.02) for ALP activity across all conditions but there were no significant pairwise comparisons (Figure 4a). BMP-2 presentation was not a factor for ALP activity. This result is contrary to 2D studies, which have reported an upregulation in ALP activity in MC3T3-E1 cells under BMP-2 treatment.62 However consistent with our findings, a different study reported minimal changes in ALP levels in MC3T3-E1 cells when encapsulated in alginate hydrogels with the addition of BMP-2 in the medium or tethered to the hydrogel.38 Taken together, these studies suggest that a 3D-culture environment may be sufficient to regulate ALP activity independent of exogenous BMP-2 signaling in MC3T3-E1 cells.
Figure 4.
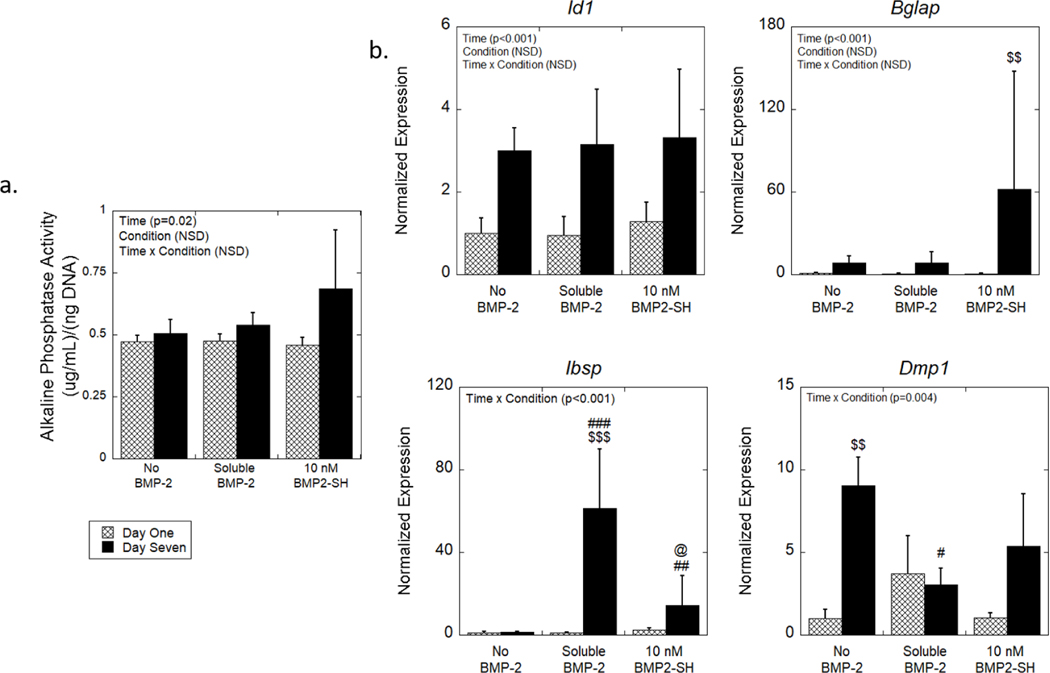
The effects of BMP-2 presentation on osteogenesis of MC3T3-E1 cells encapsulated in MMP-sensitive hydrogels. (a) ALP activity normalized to DNA content as a function of time. (b) Relative expression of osteogenic genes normalized to no BMP-2 condition at day one. Data are represented as the mean with standard deviation error bars (n = 3). Data were analyzed by two-way ANOVA. Statistical significance determined by student’s t-test within a condition between days indicated by $. Statistical significance on the same day as determined by one-way ANOVA indicated by # for those relative to the no BMP-2 condition and by @ for those relative to the soluble condition. One symbol indicates p < 0.05, two symbols indicate p < 0.01, and three symbols indicate p < 0.0001. NSD = Not statistically different.
Osteogenesis was also measured by gene expression of four bone markers (Figure 4b). Each gene was normalized to the expression in MC3T3-E1 cells encapsulated in the no BMP-2 condition at day one, to determine the contribution of BMP-2 and its presentation as well as the change in expression over time (i.e., from day one to seven). Expression of Id1, a direct target of BMP-2, significantly increased with time across all conditions (p < 0.001; no significant pairwise comparisons were found) but was not dependent on BMP-2 presentation. Expression of Bglap, a gene expressed primarily by osteoblasts, was also significantly increased with time (p < 0.001); pairwise comparisons revealed an 88-fold increase (p = 0.005) from day one to seven for the tethered condition. For the late stage osteoblast marker, Ibsp, there was a significant interaction between time and condition. Follow-up analyses indicate that both the soluble and tethered conditions were significantly upregulated (p < 0.001 and p = 0.02, respectively) in expression at day seven relative to no BMP-2. Additionally, there was a four-fold increase (p = 0.03) at day seven for the soluble condition relative to the tethered condition. Pairwise comparisons revealed a 60-fold increase (p = 0.009) from day one to seven for the soluble BMP-2 condition, but no other pairwise comparisons were significant. Similarly, expression for Dmp1, a marker of osteocytes, resulted in a significant interaction between time and BMP-2 presentation.63 Follow-up analyses found a nine-fold increase (p = 0.001) in Dmp1 expression from day one to seven for the no BMP-2 condition. Dmp1 expression for the soluble condition was lower (p = 0.03) relative to the no BMP-2 condition at day seven, but there were no differences in its expression between the soluble and tethered condition.
These data indicate that the 3D hydrogel culture supported osteogenic differentiation of encapsulated MC3T3-E1 cells, further supporting the ALP results. Several factors could have led to an osteoinductive environment in the hydrogel. Studies have indicated that RGD, when incorporated into a 3D hydrogel environment, has osteogenic capabilities.64,65 For example, the addition of RGD into a PEG hydrogel increased ALP activity and Cbfa1/Runx2 expression in encapsulated goat bone marrow stromal cells when cultured in osteogenic medium.64 Previous work by our group reported that MC3T3-E1 cells encapsulated in a similar MMP-sensitive hydrogel with RGD had elevated ALP activity on day 14 when cultured in growth medium containing serum, but without additional osteogenic factors.65 The latter study suggests that timing of the experiment may have impacted the exact ALP activity levels; ALP activity often peaks during the early stages of osteogenesis and then declines.15 In this study and in the hydrogel alone, ALP activity was measured and osteogenic genes increased over time indicating differentiation. The incorporation of soluble or tethered BMP-2, however, resulted in a significant increase in Ibsp by day seven, which is consistent with other findings.38,66,67 Taken together, these findings demonstrate that the hydrogel supported osteogenesis, but that BMP-2, regardless of its presentation, further enhanced osteogenesis of MC3T3-E1 cells within the hydrogel.
Increasing tethered BMP-2 concentration enhances osteoblast differentiation of MC3T3-E1 cells
Given that the culture environment (hydrogel + chemically defined medium), irrespective of the presence of BMP-2, supported osteogenesis of encapsulated cells, we next investigated whether higher concentrations of tethered BMP2-SH could further induce the osteogenesis of the encapsulated MC3T3-E1 cells. Following the same hydrogel and culture conditions as described above, 100 and 500 nM BMP2-SH was introduced into the hydrogel and compared to the no BMP-2 control. Bioactivity was assessed by ALP activity and gene level expression (Figure 5). For this study we chose not to include a soluble condition, as the amounts needed to provide a comparable concentration in the media would have been unrealistically high. Additionally, as previously discussed, it has been demonstrated that the use of such high concentrations in the soluble form can lead to complications when translating to in vivo applications.
Figure 5.
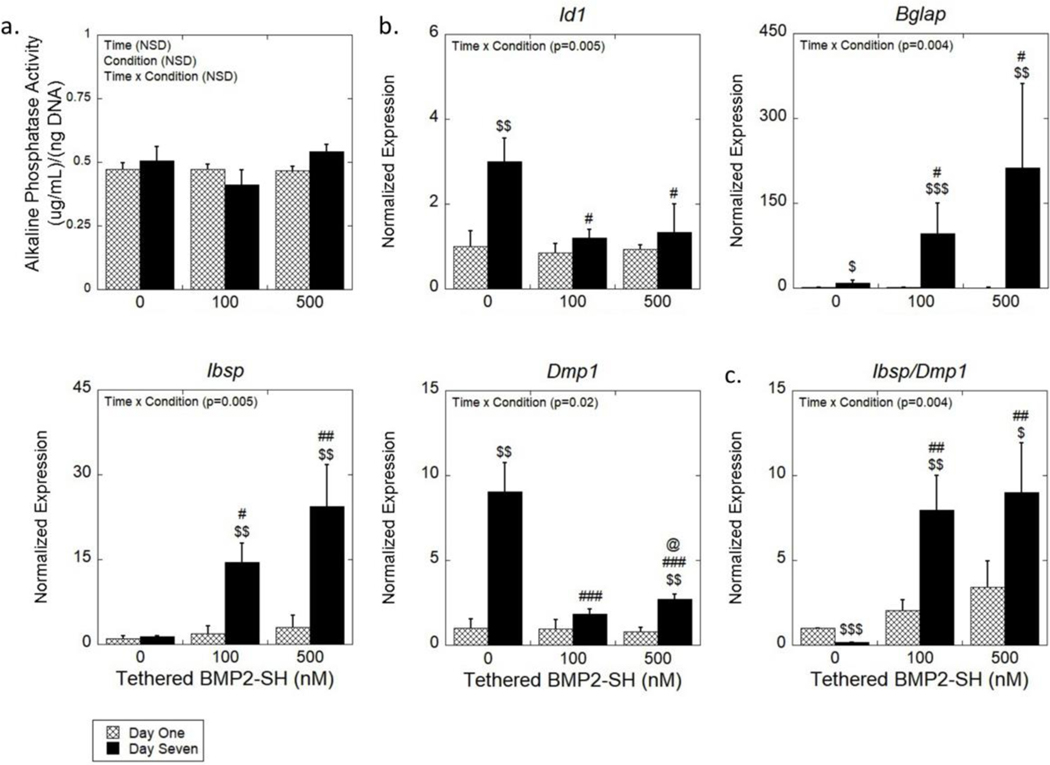
(a) The ALP activity normalized to DNA content as a function of time. Data are represented as the mean with standard deviation error bars (n = 3). (b) Expression of osteogenic genes (Id1, Bglap, Ibsp, Dmp1) of encapsulated MC3T3-E1 cells normalized to gene expression of no BMP-2 condition at day one. (c) A ratio of the gene expression of Ibsp and Dmp1 of encapsulated MC3T3-E1. Data represented as the mean with standard deviation error bars (n = 3). Data were analyzed by two-way ANOVA. Statistical significance determined by student’s t-test within a condition between days indicated by $. Statistical significance on the same day as determined by one-way ANOVA indicated by # for those relative to the no BMP-2 condition and by @ for those relative to the 100 nM condition. One symbol indicates p < 0.05, two symbols indicate p < 0.01, and three symbols indicate p < 0.0001. NSD = Not statistically different.
Consistent with previous results, ALP activity was measurable across all groups but was not affected by time or BMP-2 presentation (Figure 5a). These findings further support the hypothesis that BMP-2 does not enhance ALP activity beyond that of dexamethasone. The 100 nM BMP2-SH condition at day seven resulted in significant differential gene expression relative to the no BMP-2 formulation for all genes analyzed. However, the 500 nM BMP-SH condition did not lead to further increases in the genes analyzed with the exception of Dmp1. Expression of Id1 was significantly lower (p = 0.01 and p = 0.02) by 2.25 and 2.5-fold for the tethered conditions for the 100 and 500 nM, respectively, compared to the no BMP-2 condition. There was also a significant increase (p = 0.006) in expression for no BMP-2 between day one and seven, but neither tethered condition was affected by time. The expression of Bglap was 11-fold higher (p = 0.03) for the 100 nM BMP2-SH condition and 24-fold higher (p = 0.01) for the 500 nM BMP2-SH condition relative to no BMP-2. In addition, no BMP-2 and both tethered conditions, showed a significant increase (p = 0.03, p < 0.001 and p = 0.002, respectively) in Bglap expression from day one to seven. Expression of Ibsp significantly increased (p = 0.03 and p = 0.002) by 11-fold and 18-fold for the 100 nM and 500 nM BMP2-SH conditions, respectively, as compared to no BMP-2. In both tethered conditions, there was also a significant upregulation (p = 0.004 and p = 0.009, respectively) in from day one to day seven. Finally, the tethered conditions showed 3 to 5-fold lower Dmp1 levels relative to no BMP-2 (p < 0.001). No BMP-2 and 500 nM BMP2-SH both showed significant upregulation (p = 0.003 and p = 0.002, respectively) of Dmp1 from day one to day seven. At day seven there was a significant increase (p = 0.04) in expression in the 500 nM BMP-2 condition relative to the 100 nM condition.
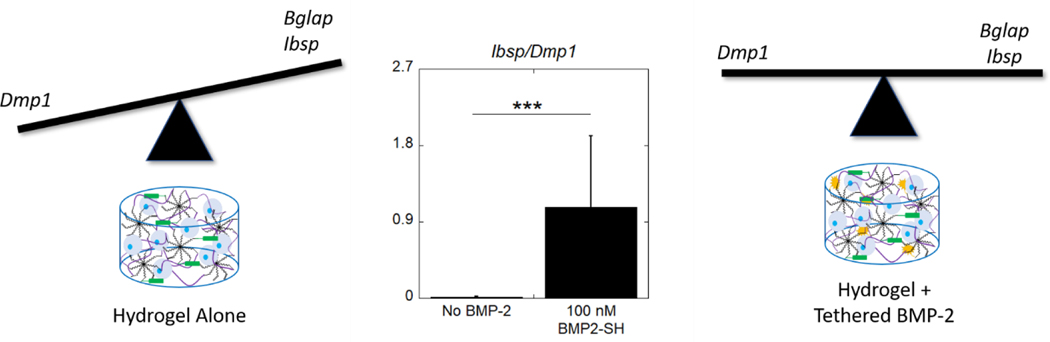
To understand the extent of differentiation in the hydrogels with tethered BMP-2, the relative degree of an osteoblastic to an osteocytic phenotype was assessed by the ratio of Ibsp-to-Dmp1 (Figure 5c). For both tethered conditions, the ratio significantly increased (p = 0.008 and p = 0.004, respectively), relative to no BMP-2, at day seven. For the 100 nM and 500 nM conditions, the ratio was higher (p = 0.007 and p = 0.04, respectively) at day seven compared to day one, but was lower (p < 0.001) for the no BMP-2 condition at day seven. These results suggest a difference in the extent of differentiation of the MC3T3-E1 cells depending on the presence of tethered BMP-2.
These results indicate that the tethered BMP-2 conditions enhanced the osteoblastic phenotype of the encapsulated MC3T3-E1 cells, as increases in osteoblast specific gene expression were observed. Dmp1, provides information about the extent of osteogenic differentiation and is associated with an osteocytic phenotype.68 This gene is involved in osteoblast differentiation and the protein DMP-1 is critical for proper mineralization of bone ECM.69 Interestingly, our findings suggest that the hydrogel alone (i.e., without BMP-2) exhibits a greater osteocytic phenotype. Contrarily, the presence of BMP-2 in this hydrogel system, appears to retain the MC3T3-E1 differentiation closer to an osteoblastic phenotype. ECM.69 Interestingly, our findings suggest that the hydrogel alone without BMP-2 exhibits a greater osteocytic phenotype. Contrarily, the presence of BMP-2 in this hydrogel system, appears to retain the MC3T3-E1 differentiation closer to an osteoblastic phenotype.
Osteogenic differentiation of MC3T3-E1 cells in the hydrogel alone is mediated by the canonical BMP signaling pathway
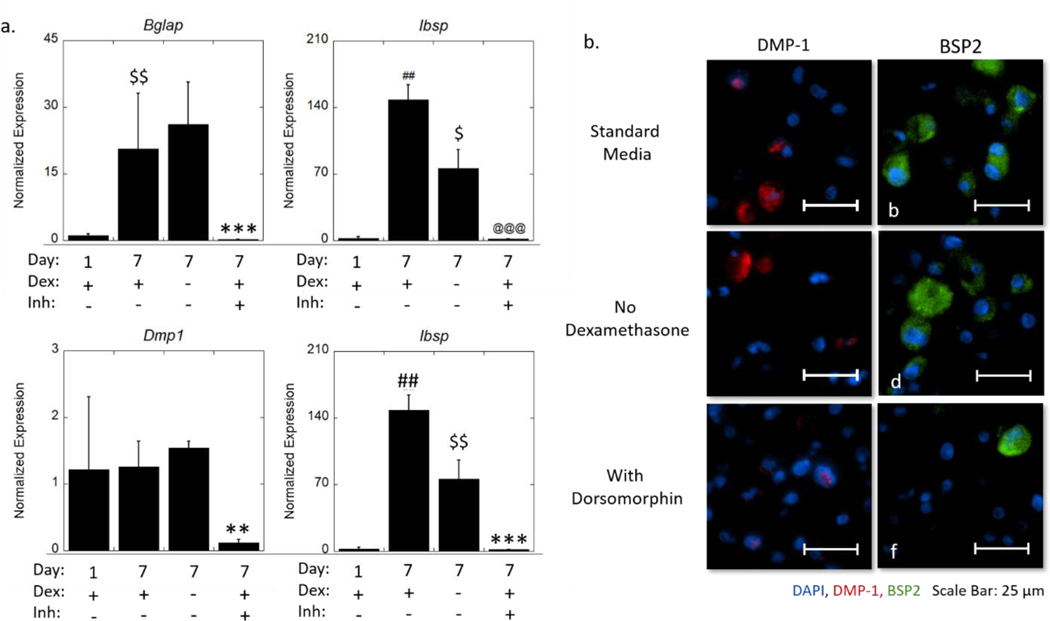
We next sought to determine the mechanisms by which the hydrogel, without BMP-2, induces osteogenesis (Figure 6 and Supplementary Information Figure S1). The hydrogels were cultured in chemically defined medium supplemented with dexamethasone, which is known to be osteoinductive. MC3T3-E1 cells have been reported to respond to dexamethasone by increased ALP activity.34,36,70 Other studies have also reported that dexamethasone promoted Dmp1 expression in rat mesenchymal progenitor (ROB-C26) cells.71 Therefore, dexamethasone may be responsible for inducing osteogenesis. In a follow-up study, we investigated expression of Bglap, Ibsp, and Dmp1 and protein expression of DMP-1 and bone sialoprotein, in MC3T3-E1 cells that were encapsulated in the hydrogels and cultured in chemically defined medium, but without dexamethasone. Interestingly, Bglap and Dmp1 were not affected by dexamethasone. This result is consistent with the DMP-1 protein expression, which showed that cytosolic DMP-1 remained present in most of the cells without dexamethasone. However, the elevated expression of Ibsp from day one to day seven required dexamethasone. There was a seven-fold decrease (p = 0.002) in Ibsp expression at day seven when dexamethasone was removed. Contrarily, bone sialoprotein was still detected, indicating that a sufficient level of Ibsp expression remained without dexamethasone. These results suggest that dexamethasone is not required for osteogenesis of the MC3T3-E1 cells in the hydrogels.
Figure 6.
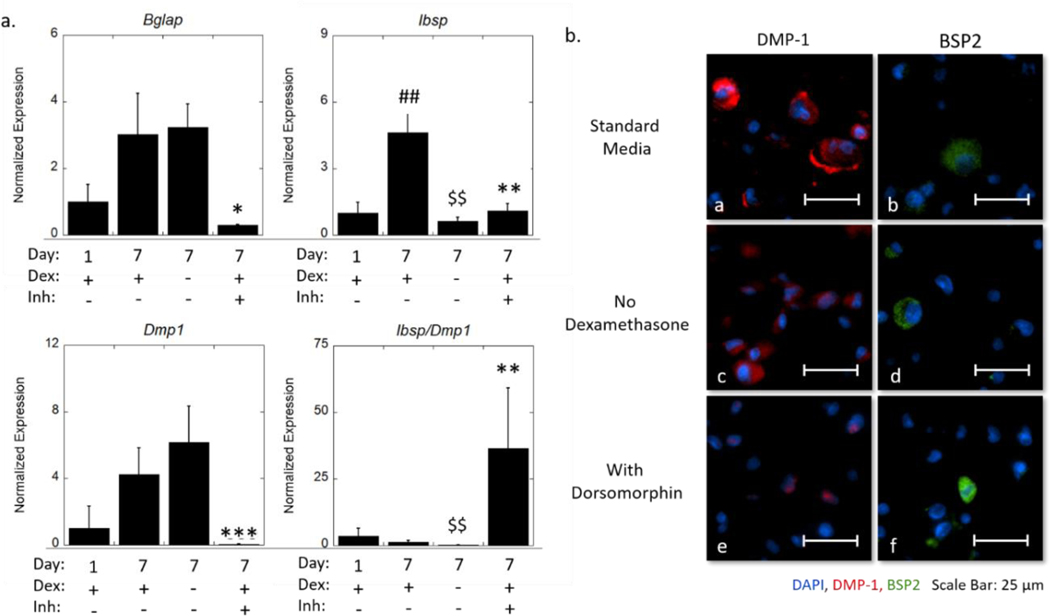
(a) Expression of osteogenic genes (Id1, Bglap, Dmp1) normalized to gene expression of no BMP-2 in standard medium at day one and a ratio of expression of Ibsp and Dmp1 of encapsulated MC3T3-E1 cells. Data are represented as the mean with standard deviation error bars (n=3). (b) Representative widefield fluorescent microscopy images for DMP-1 (red) and BSP2 (green) at day seven cultured in standard medium (a-b), medium without dexamethasone (c-d), and medium with dorsomorphin (e-f). Nuclei are counterstained blue. Scale bar = 25 μm. Data were analyzed by student’s t-test. Statistical significance between day seven standard media and day one indicated by #. Statistical difference between day seven no dexamethasone and day seven standard media indicated by $. Statistical significance between day seven with dorsomorphin and day seven standard media indicated by *. One symbol indicates p < 0.05, two symbols indicate p < 0.01, and three symbols indicate p < 0.001.
As MC3T3-E1 cells are known to constitutively express BMP-2 during osteogenesis, we treated the cells with dorsomorphin, an inhibitor of the BMP canonical pathway, which occurs via Smad signaling.72 Dorsomorphin treatment significantly inhibited Bglap, Ibsp, and Dmp1. At day seven Bglap expression decreased (p = 0.02) by 10-fold, Ibsp expression decreased (p = 0.004) by four-fold and Dmp1 expression decreased (p < 0.001) by 95-fold with the inhibitor. Qualitatively, the spatial presentation of the DMP-1 protein was distinctly different, where it was restricted to the nucleus with dorsomorphin. This finding is in agreement with the current understanding of the dual biological role of DMP-1. It has been reported in MC3T3-E1 cells, that DMP-1 behaves as a transcriptional regulator to initiate osteoblastic differentiation when localized to the nucleus; and with differentiation, DMP-1 is transported out of the cell, where it initiates biomineralization.73 In the standard medium, DMP-1 is localized in the cytoplasm, indicating that the cells have matured beyond the initial state of osteoblastic differentiation. This process appears to have been arrested with dorsomorphin. This suggests that the cells were still in the pre-osteoblastic stage at day seven when BMP-2 signaling is inhibited. There also appeared fewer cells staining positive for bone sialoprotein with the inhibitor. Taken together, these results confirm that the hydrogel alone is capable of supporting osteogenesis of encapsulated MC3T3-E1 through autocrine and paracrine BMP signaling.
Tethered BMP2 signals through the BMP canonical pathway and augments osteogenic response of MC3T3-E1 cells
In our final study, we sought to determine the mechanisms by which tethered BMP-2 enhances MC3T3-E1 osteogenesis. We first teased apart the contribution of dexamethasone. It has been reported that the combination of BMP-2 and dexamethasone can significantly impact the osteogenic expression of MC3T3-E1 cells, particularly as related to their constitutive expression of BMP-2.74 Herein, a follow-up study was performed with the 100 nM BMP2-SH condition. We investigated the expression of Bglap, Ibsp, Dmp1 and protein expression of DMP-1 and bone sialoprotein (Figure 7 and Supplementary Information Figure S2). Similar to our findings in the previous section, Bglap and Dmp1 were not affected by dexamethasone. Visually, DMP-1 protein expression was markedly lower in the tethered BMP-2 condition (comparing to Figure 6), showing minimal cytosolic staining and some positive nuclear staining, but no obvious difference without dexamethasone. The expression of Ibsp was once again significantly reduced in the absence of dexamethasone, resulting in a two-fold decrease (p = 0.02) in expression at day seven. Qualitatively, there were no observable differences in the extent of bone sialoprotein staining, indicating that differences at the gene level, were not significant enough to lead to large shifts in the protein distribution. Taken together, it appears that dexamethasone has a limited contribution to osteogenesis of MC3T3-E1 cells encapsulated in this 3D hydrogel environment, primarily influencing Ibsp expression.
Figure 7.
(a) Expression of osteogenic genes (Id1, Bglap, Dmp1) normalized to gene expression of no BMP-2 in standard medium at day one and a ratio of expression of Ibsp and Dmp1 of encapsulated MC3T3-E1 cells. Data are represented as the mean with standard deviation error bars (n=3). (b) Representative widefield fluorescent microscopy images for DMP-1 (red) and BSP2 (green) at day seven cultured in standard medium (a-b), medium without dexamethasone (c-d), and medium with dorsomorphin (e-f). Nuclei are counterstained blue. Scale bar = 25 μm. Data were analyzed by student’s t-test. Statistical significance between day seven standard media and day one indicated by #. Statistical difference between day seven no dexamethasone and day seven standard media indicated by $. Statistical significance between day seven with dorsomorphin and day seven standard media indicated by *. One symbol indicates p < 0.05, two symbols indicate p < 0.01, and three symbols indicate p < 0.001.
To determine that the tethered BMP-2 is capable of signaling via its canonical pathway, the hydrogels with tethered BMP-2 were treated with dorsomorphin. A significant downregulation of Bglap, Ibsp, and Dmp1 was observed. At day seven, Bglap expression decreased (p < 0.001) by 68-fold, Ibsp expression decreased (p < 0.001) by 98-fold, and Dmp1 expression decreased (p = 0.007) by 11-fold with the inhibitor. There was similar DMP1 protein expression with the tethered BMP-2 (comparing to Figure 6) and the cells that did stain positive for DMP1, were similarly restricted to the nucleus. Bone sialoprotein appeared more abundant with the tethered BMP-2 (comparing to Figure 6). A decrease in the degree of positive staining for bone sialoprotein was evident with the inhibitor, indicating that tethered BMP-2 enhanced maturation of the cells. These data confirm that tethered BMP-2 signals to the encapsulated MC3T3-E1 cells through the BMP-2 canonical signaling pathway and enhances osteogenesis.
It is important to note several limitations of this study. This study was limited to a seven-day culture, which may not have captured the peak in ALP-activity. The MC3T3-E1 cells are considered a pre-osteoblast and thus already have a propensity to differentiate towards an osteoblast in differentiation medium. Thus, the effects of tethered BMP-2 on the initial stages of osteogenesis was not examined. This may have contributed to the few observable differences between the soluble and tethered BMP-2 at the 4–5 nM concentrations, which are commonly used to induce osteogenesis of stem cells. Due to the differences when study 2 and study 3 and 4 were performed, different lots of FBS were used to expand the cells. The differences in gene expression level for Ibsp is attributed to batch-to-batch variations of the FBS.75 Nonetheless the same trends were observed. Despite these limitations, this study demonstrates that tethered BMP-2 is biologically active and enhances osteogenesis. Future studies will investigate the response of encapsulated human mesenchymal stem cells to tethered BMP-2 to assess its role in initiating osteogenesis.
Conclusions
This work demonstrates that BMP-2 can be successfully thiolated by reaction with 2-iminothiolane without loss of function and can be tethered into a PEG hydrogel via the photoclick thiol-norbornene reaction in a concentration-controlled manner. Moreover, tethered BMP-2 enhanced osteogenesis of encapsulated MC3T3-E1 cells, further supporting the conclusion that the growth factor maintained bioactivity and osteoinductive properties post-tethering. When there is no BMP-2 present in the hydrogel, encapsulated MC3T3-E1 cells demonstrate both a greater expression of Dmp1 relative to Ibsp and an increased expression of DMP-1 at the protein level. In contrast, the presence of a relatively high concentration (40–200 nM) of immobilized BMP-2, results in an equivalent expression of Dmp1 and Ibsp, but with more cells expressing bone sialoprotein over DMP-1. Overall, the study suggests that the hydrogel environment promotes osteogenesis and maturation of MC3T3-E1 cells towards an osteocytic phenotype, while the inclusion of tethered BMP-2 restricts the cells in an osteoblast differentiation state (Figure 8). Additionally, this work showed that dexamethasone has limited contribution to the osteogenic response, enhancing expression of Ibsp, but not affecting Bglap or Dmp1. Lastly, it was confirmed that tethered BMP-2 enhances the osteogenic response of the MC3T3-E1 cells by signaling through its canonical SMAD 1/5/8 pathway. Overall, this relatively simple method may provide an alternative approach to BMP-2 delivery for inducing osteogenesis locally while minimizing side effects associated with soluble delivery, warranting future in vivo studies.
Figure 8.
The different effects of the hydrogel environment without and with tethered BMP-2 on MC3T3-E1 osteogenic differentiation. The balance in expression of osteogenic markers Ibsp and Bglap compared to the osteocytic marker Dmp1 shows distinctly different osteogenic fates depending on the inclusion of tethered BMP-2. A ratio of expression of Ibsp and Dmp1 of encapsulated MC3T3-E1 cells. Data are represented as the mean with standard deviation error bars (n=6). Data were analyzed by student’s t-test. Statistical significance *** indicates p < 0.001.
Supplementary Material
ACKNOWLEDGMENT
The authors would like to thank Dr. Kurt Hankenson for his expert advice in discussing these data and for his and Yadav Wagley’s assistance in primer development. We would also like to acknowledge Archish Muralidharan for his assistance to SAS in the initial cell studies. The research was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases under the award R01AR069060 and by the National Institute of Child Health and Human Development under the award R21HD092109. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors also acknowledge support from a NIH Training Grant in Molecular Biophysics to SAS under award number T32 GM065103.
ABBREVIATIONS
- BMP-2
bone morphogenetic protein-2
- BMP2-SH
2-iminothiolane-treated bone morphogenetic protein-2
- PEG
poly (ethylene glycol)
- MMP
matrix metalloproteinase
- RGD
Arginylglycylaspartic acid
- ECM
extracellular matrix
- SMAD
small mothers against decapentaplegic
- ELISA
enzyme-linked immunosorbent assay
- ALP
alkaline phosphatase
- Id1
inhibitor of DNA binding 1
- Bglap
bone gamma-carboxyglutamate protein
- Ibsp
integrin binding sialoprotein
- Dmp1
dentin matrix acidic phosphoprotein 1
Footnotes
Supplemental Information
Fluorescent images from immunohistochemistry stain DMP1 and BSP2 from studies 3 and 4. These images provide additional context for the higher magnification images included in the body of the manuscript. Figure S1 includes images related to study 3 with the no BMP-2 hydrogels and Figure S2 includes images related to study 4 with the 100 nM BMP2-SH hydrogel (PDF).
References
- (1).Caballero Aguilar LM; Silva SM; Moulton SE Growth Factor Delivery: Defining the next Generation Platforms for Tissue Engineering. J. Control. Release 2019, 306 (May), 40–58. 10.1016/j.jconrel.2019.05.028. [DOI] [PubMed] [Google Scholar]
- (2).Taipale J; Keski-Oja J. Growth Factors in the Extracellular Matrix. FASEB J. 1997, 11 (1), 51–59. [DOI] [PubMed] [Google Scholar]
- (3).Chen FM; Zhang M; Wu ZF Toward Delivery of Multiple Growth Factors in Tissue Engineering. Biomaterials 2010, 31 (24), 6279–6308. 10.1016/j.biomaterials.2010.04.053. [DOI] [PubMed] [Google Scholar]
- (4).Tabata Y. The Importance of Drug Delivery. Pharm. Sci. Technolo. Today 2000, 3 (3), 80–89. [DOI] [PubMed] [Google Scholar]
- (5).Lutolf MP; Weber FE; Schmoekel HG; Schense JC; Kohler T; Müller R; Hubbell JA Repair of Bone Defects Using Synthetic Mimetics of Collagenous Extracellular Matrices. Nat. Biotechnol 2003, 21 (5), 513–518. 10.1038/nbt818. [DOI] [PubMed] [Google Scholar]
- (6).Sakou T. Bone Morphogenetic Protein (BMP): From Basic Studies to Clinical Approaches. Bone 1998, 22 (6), 591–603. [DOI] [PubMed] [Google Scholar]
- (7).Wang RN; Green J; Wang Z; Deng Y; Qiao M; Peabody M; Zhang Q; Ye J; Yan Z; Denduluri S; Idowu O; Li M; Shen C; Hu A; Haydon RC; Kang R; Mok J; Lee MJ; Luu HL; Shi LL Bone Morphogenetic Protein (BMP) Signaling in Development and Human Diseases. Genes Dis. 2014, 1 (1), 87–105. 10.1016/j.gendis.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Boontheekul T; Mooney DJ Protein-Based Signaling Systems in Tissue Engineering. Curr. Opin. Biotechnol 2003, 14 (5), 559–565. 10.1016/j.copbio.2003.08.004. [DOI] [PubMed] [Google Scholar]
- (9).Rosen V. BMP2 Signaling in Bone Development and Repair. Cytokine Growth Factor Rev. 2009, 20 (5–6), 475–480. 10.1016/j.cytogfr.2009.10.018. [DOI] [PubMed] [Google Scholar]
- (10).Tsuji K; Bandyopadhyay A; Harfe BD; Cox K; Kakar S; Gerstenfeld L; Einhorn T; Tabin CJ; Rosen V. BMP2 Activity, Although Dispensable for Bone Formation, Is Required for the Initiation of Fracture Healing. Nat. Genet 2006, 38 (12), 1424–1429. 10.1038/ng1916. [DOI] [PubMed] [Google Scholar]
- (11).Tabisz B; Schmitz W; Schmitz M; Luehmann T; Heusler E; Rybak JC; Meinel L; Fiebig JE; Mueller TD; Nickel J. Site-Directed Immobilization of BMP-2: Two Approaches for the Production of Innovative Osteoinductive Scaffolds. Biomacromolecules 2017, 18 (3), 695–708. 10.1021/acs.biomac.6b01407. [DOI] [PubMed] [Google Scholar]
- (12).Martinek V; Fu FH; Huard J. Gene Therapy and Tissue Engineering in Sports Medicine. Phys. Sportsmed 2000, 28 (2), 34–51. 10.3810/psm.2000.02.691. [DOI] [PubMed] [Google Scholar]
- (13).Huang B; Yuan Y; Liu C. Biomaterial-Guided Immobilization and Osteoactivity of Bone Morphogenetic Protein-2. Appl. Mater Today 2020, 19, 100599. 10.1016/j.apmt.2020.100599. [DOI] [Google Scholar]
- (14).Calori GM; Donati D; Di Bella C; Tagliabue L. Bone Morphogenetic Proteins and Tissue Engineering: Future Directions. Injury 2009, 40 Suppl 3 (April 2016), S67–S76. 10.1016/s0020-1383(09)70015-4. [DOI] [PubMed] [Google Scholar]
- (15).Madl CM; Mehta M; Duda GN; Heilshorn SC; Mooney DJ Presentation of BMP-2 Mimicking Peptides in 3D Hydrogels Directs Cell Fate Commitment in Osteoblasts and Mesenchymal Stem Cells. Biomacromolecules 2014, 15 (2), 445–455. 10.1021/bm401726u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Carlisle E; Fischgrund JS Bone Morphogenetic Proteins for Spinal Fusion. Spine J. 2005, 5 (6 SUPPL.), S240–S249. 10.1016/j.spinee.2005.02.014. [DOI] [PubMed] [Google Scholar]
- (17).Gautschi OP; Frey SP; Zellweger R. Bone Morphogenetic Proteins in Clinical Applications. ANZ J. Surg 2007, 77 (8), 626–631. 10.1111/j.1445-2197.2007.04175.x. [DOI] [PubMed] [Google Scholar]
- (18).Tannoury CA; An HS Complications with the Use of Bone Morphogenetic Protein 2 (BMP-2) in Spine Surgery. Spine J. 2014, 14 (3), 552–559. 10.1016/j.spinee.2013.08.060. [DOI] [PubMed] [Google Scholar]
- (19).Zisch AH; Lutolf MP; Hubbell JA Biopolymeric Delivery Matrices for Angiogenic Growth Factors. Cardiovasc. Pathol 2003, 12 (6), 295–310. 10.1016/S1054-8807(03)00089-9. [DOI] [PubMed] [Google Scholar]
- (20).Silva AKA; Richard C; Bessodes M; Scherman D; Merten OW Growth Factor Delivery Approaches in Hydrogels. Biomacromolecules 2009, 10 (1), 9–18. 10.1021/bm801103c. [DOI] [PubMed] [Google Scholar]
- (21).Hiemstra C; Zhong Z; van Steenbergen MJ; Hennink WE; Feijen J. Release of Model Proteins and Basic Fibroblast Growth Factor from in Situ Forming Degradable Dextran Hydrogels. J. Control. Release 2007, 122 (1), 71–78. 10.1016/j.jconrel.2007.06.011. [DOI] [PubMed] [Google Scholar]
- (22).Ennett A; Kaigler D; Mooney D. Temporally Regulated Delivery of VEGF in Vitro and in Vivo. J. Biomed. Mater. Res. Part A 2006, 79 (1), 176–184. https://doi.org/ DOI: 10.1002/jbm.a.30771 Abstract:a. [DOI] [PubMed] [Google Scholar]
- (23).Kanematsu A; Yamamoto S; Ozeki M; Noguchi T; Kanatani I; Ogawa O; Tabata Y. Collagenous Matrices as Release Carriers of Exogenous Growth Factors. Biomaterials 2004, 25 (18), 4513–4520. 10.1016/j.biomaterials.2003.11.035. [DOI] [PubMed] [Google Scholar]
- (24).King WJ; Krebsbach PH Growth Factor Delivery: How Surface Interactions Modulate Release in Vitro and in Vivo. Adv. Drug Deliv. Rev 2012, 64 (12), 1239–1256. 10.1038/jid.2014.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Budiraharjo R; Neoh KG; Kang ET Enhancing Bioactivity of Chitosan Film for Osteogenesis and Wound Healing by Covalent Immobilization of BMP-2 or FGF-2. J. Biomater. Sci. Polym. Ed 2013, 24 (6), 645–662. 10.1080/09205063.2012.703949. [DOI] [PubMed] [Google Scholar]
- (26).Ito Y. Covalently Immobilized Biosignal Molecule Materials for Tissue Engineering. Soft Matter 2007, 4 (1), 46–56. 10.1039/b708359a. [DOI] [PubMed] [Google Scholar]
- (27).Schwab EH; Pohl TLM; Haraszti T; Schwaerzer GK; Hiepen C; Spatz JP; Knaus P; Cavalcanti-Adam EA Nanoscale Control of Surface Immobilized BMP-2: Toward a Quantitative Assessment of BMP-Mediated Signaling Events. Nano Lett. 2015, 15 (3), 1526–1534. 10.1021/acs.nanolett.5b00315. [DOI] [PubMed] [Google Scholar]
- (28).Bauer S; Park J; Pittrof A; Song Y-Y; Von Der Mark K; Schmuki P. Covalent Functionalization of TiO2 Nanotube Arrays with EGF and BMP-2 for Modified Behavior towards Mesenchymal Stem Cells. Intergrative Biol. 2011, 3 (9), 927–936. 10.1039/c0ib00155d. [DOI] [PubMed] [Google Scholar]
- (29).Mccall JD; Luoma JE; Anseth KS Covalently Tethered Transforming Growth Factor Beta in PEG Hydrogels Promotes Chondrogenic Differentiation of Encapsulated Human Mesenchymal Stem Cells. Drug Deliv. Transl. Res 2012, 2 (5), 305–312. 10.1007/s13346-012-0090-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Sridhar BV; Doyle NR; Randolph MA; Anseth KS Covalently Tethered TGF-Β1 with Encapsulated Chondrocytes in a PEG Hydrogel System Enhances Extracellular Matrix Production. J. Biomed. Mater. Res., Part A 2014, pp 4464–4472. 10.1002/jbm.a.35115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Schneider MC; Chu S; Randolph MA; Bryant SJ An in Vitro and in Vivo Comparison of Cartilage Growth in Chondrocyte-Laden Matrix Metalloproteinase-Sensitive Poly(Ethylene Glycol) Hydrogels with Localized Transforming Growth Factor Β3. Acta Biomater. 2019, 93, 97–110. 10.1016/j.actbio.2019.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Lin CC; Metters AT Enhanced Protein Delivery from Photopolymerized Hydrogels Using a Pseudospecific Metal Chelating Ligand. Pharm. Res 2006, 23 (3), 614–622. 10.1007/s11095-005-9395-x. [DOI] [PubMed] [Google Scholar]
- (33).McCall J; Anseth KS Photopolymerizations Provide a Facile Method To Encapsulate Proteins and Maintain Their Bioactivity Copy.Pdf. Biomacromolecules. 2012, pp 2410–2417. 10.1021/bm300671s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Beloti MM; Rosa AL Osteoblast Differentiation of Human Bone Marrow Cells under Continuous and Discontinuous Treatment with Dexamethasone. Braz. Dent. J 2005, 16 (2), 156–161. 10.1590/s0103-64402005000200013. [DOI] [PubMed] [Google Scholar]
- (35).Jørgensen NR; Henriksen Z; Sørensen OH; Civitelli R. Dexamethasone, BMP-2, and 1,25-Dihydroxyvitamin D Enhance a More Differentiated Osteoblast Phenotype: Validation of an in Vitro Model for Human Bone Marrow-Derived Primary Osteoblasts. Steroids 2004, 69 (4), 219–226. 10.1016/j.steroids.2003.12.005. [DOI] [PubMed] [Google Scholar]
- (36).Park JB The Effects of Dexamethasone, Ascorbic Acid, and β-Glycerophosphate on Osteoblastic Differentiation by Regulating Estrogen Receptor and Osteopontin Expression. J. Surg. Res 2012, pp 99–104. 10.1016/j.jss.2010.09.010. [DOI] [PubMed] [Google Scholar]
- (37).Takuwa Y; Ohse C; Wang EA; Wozney JM; Yamashita K. Bone Morphogenetic Protein-2 Stimulates Alkaline Phosphatase Activity and Collagen Synthesis in Cultured Osteoblastic Cells, MC3T3-E1. Biochem. Biophys. Res. Commun 1991, 174 (1), 96–101. 10.1016/0006-291X(91)90490-X. [DOI] [PubMed] [Google Scholar]
- (38).Igwe JC; Mikael PE; Nukavarapu SP Design, Fabrication and in Vitro Evaluation of a Novel Polymer-Hydrogel Hybrid Scaffold for Bone Tissue Engineering. J. Tissue Eng. Regen. Med 2014, 8, 131–142. 10.1002/term.1506. [DOI] [PubMed] [Google Scholar]
- (39).Hiraki Y; Inoue H; Shigeno C; Sanma Y; Bentz H; Rosen DM; Asada A; Suzuki F. Bone Morphogenetic Proteins (BMP-2 and BMP-3) Promote Growth and Expression of the Differentiated Phenotype of Rabbit Chondrocytes and Osteoblastic MC3T3-E1 Cells in Vitro. J. Bone Miner. Res 1991, pp 1373–1385. 10.1002/jbmr.5650061215. [DOI] [PubMed] [Google Scholar]
- (40).Jadlowiec J; Koch H; Zhang X; Campbell PG; Seyedain M; Sfeir C. Phosphophoryn Regulates the Gene Expression and Differentiation of NIH3T3, MC3T3-E1, and Human Mesenchymal Stem Cells via the Integrin/MAPK Signaling Pathway. J. Biol. Chem 2004, 279 (51), 53323–53330. 10.1074/jbc.M404934200. [DOI] [PubMed] [Google Scholar]
- (41).Lemonnier J; Ghayor C; Guicheux J; Caverzasio J. Protein Kinase C-Independent Activation of Protein Kinase D Is Involved in BMP-2-Induced Activation of Stress Mitogen-Activated Protein Kinases JNK and P38 and Osteoblastic Cell Differentiation. J. Biol. Chem 2004, 279 (1), 259–264. 10.1074/jbc.M308665200. [DOI] [PubMed] [Google Scholar]
- (42).Dai G; Wan W; Chen J; Wu J; Shuai X; Wang Y. Enhanced Osteogenic Differentiation of MC3T3-E1 on RhBMP-2 Immobilized Titanium Surface through Polymer-Mediated Electrostatic Interaction. Appl. Surf. Sci 2019, 471 (August 2018), 986–998. 10.1016/j.apsusc.2018.11.243. [DOI] [Google Scholar]
- (43).Liu M; Shu M; Xu W; Liu X; Hou Z; Xing B; Lin J. BMP-2-Loaded HAp: Ln3+ (Ln = Yb, Er, Gd) Nanorods with Dual-Mode Imaging for Efficient MC3t3-E1 Cell Differentiation Regulation. Langmuir 2019, 35 (47), 15287–15294. 10.1021/acs.langmuir.9b02824. [DOI] [PubMed] [Google Scholar]
- (44).Aisenbrey EA; Bryant SJ Mechanical Loading Inhibits Hypertrophy in Chondrogenically Differentiating HMSCs within a Biomimetic Hydrogel. J. Mater. Chem. B 2016, 4, 3562–3574. 10.1039/c6tb00006a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Hume PS; He J; Haskins K; Anseth KS Strategies to Reduce Dendritic Cell Activation through Functional Biomaterial Design. Biomaterials 2012, 33 (14), 3615–3625. 10.1016/j.biomaterials.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Bustin SA; Benes V; Garson JA; Hellemans J; Huggett J; Kubista M; Mueller R; Nolan T; Pfaffl MW; Shipley GL; Vandesompele J; Wittwer CT The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. Clin. Chem 2009, 55 (4), 611–622. 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- (47).Pfaffl MW; Horgan GW; Dempfle L. Relative Expression Software Tool (REST©) for Group-Wise Comparison and Statistical Analysis of Relative Expression Results in Real-Time PCR. Nucleic Acids Res. 2002, 30 (9), 2002–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Hu J; Sebald W. N-Terminal Specificity of PEGylation of Human Bone Morphogenetic Protein-2 at Acidic PH. Int. J. Pharm 2011, 413 (1–2), 140–146. 10.1016/j.ijpharm.2011.04.043. [DOI] [PubMed] [Google Scholar]
- (49).Hermanson GT The Reactions of Bioconjugation. In Bioconjugate Techniques; 2013; pp 229–258. 10.1016/b978-0-12-382239-0.00003-0. [DOI] [Google Scholar]
- (50).Brewer CF; Riehm JP Evidence for Possible Nonspecific Reactions between N-Ethylmaleimide and Proteins. Anal. Biochem 1967, 18 (2), 248–255. 10.1016/0003-2697(67)90007-3. [DOI] [Google Scholar]
- (51).Hermanson GT Vaccines and Immunogen Conjugates. In Bioconjugate Techniques; 2013; pp 839–865. 10.1016/b978-0-12-382239-0.00019-4. [DOI] [Google Scholar]
- (52).Koniev O; Wagner A. Developments and Recent Advancements in the Field of Endogenous Amino Acid Selective Bond Forming Reactions for Bioconjugation. Chem. Soc. Rev 2015, 44 (15), 5495–5551. 10.1039/c5cs00048c. [DOI] [PubMed] [Google Scholar]
- (53).Wofsy L; Singer SJ Effects of the Amidination Reaction on Antibody Activity and on the Physical Properties of Some Proteins. Biochemistry 1963, 2 (1), 104–116. 10.1021/bi00901a019. [DOI] [PubMed] [Google Scholar]
- (54).Hoyle CE; Bowman CN Thiol-Ene Click Chemistry. Angew. Chemie - Int. Ed 2010, 49 (9), 1540–1573. 10.1002/anie.200903924. [DOI] [PubMed] [Google Scholar]
- (55).Lin C-C; Ki CS; Shih H. Thiol-Norbornene Photo-Click Hydrogels for Tissue Engineering Applications. J. Appl. Polym. Sci 2015, 132 (8), 41563. 10.1002/app.41563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Jha AK; Yang W; Kirn-Safran CB; Farach-Carson MC; Jia X. Perlecan Domain I-Conjugated, Hyaluronic Acid-Based Hydrogel Particles for Enhanced Chondrogenic Differentiation via BMP-2 Release. Biomaterials 2009, 30 (36), 6964–6975. 10.1016/j.biomaterials.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Kim HKW; Oxendine I; Kamiya N. High-Concentration of BMP2 Reduces Cell Proliferation and Increases Apoptosis via DKK1 and SOST in Human Primary Periosteal Cells. Bone 2013, 54 (1), 141–150. 10.1016/j.bone.2013.01.031. [DOI] [PubMed] [Google Scholar]
- (58).Jiao X; Billings PC; O’Connell MP; Kaplan FS; Shore EM; Glaser DL Heparan Sulfate Proteoglycans (HSPGs) Modulate BMP2 Osteogenic Bioactivity in C2C12 Cells. J. Biol. Chem 2007, 282 (2), 1080–1086. 10.1074/jbc.M513414200. [DOI] [PubMed] [Google Scholar]
- (59).Ghayor C; Ehrbar M; Miguel BS; Grätz KW; Weber FE CAMP Enhances BMP2-Signaling through PKA and MKP1-Dependent Mechanisms. Biochem. Biophys. Res. Commun 2009, 381 (2), 247–252. 10.1016/j.bbrc.2009.02.032. [DOI] [PubMed] [Google Scholar]
- (60).Zheng Y; Wang L; Zhang X; Zhang X; Gu Z; Wu G. BMP2/7 Heterodimer Can Modulate All Cellular Events of the in Vitro RANKL-Mediated Osteoclastogenesis, Respectively, in Different Dose Patterns. Tissue Eng. - Part A 2012, 18 (5–6), 621–627. 10.1089/ten.tea.2011.0366. [DOI] [PubMed] [Google Scholar]
- (61).Ishibashi O; Ikegame M; Takizawa F; Yoshizawa T; Moksed MA; Iizawa F; Mera H; Matsuda A; Kawashima H. Endoglin Is Involved in BMP-2-Induced Osteogenic Differentiation of Periodontal Ligament Cells through a Pathway Independent of Smad-1/5/8 Phosphorylation. J. Cell. Physiol 2010, pp 465–473. 10.1002/jcp.21968. [DOI] [PubMed] [Google Scholar]
- (62).Choi JY; Lee BH; Song KB; Park RW; Kim IS; Sohn KY; Jo JS; Ryoo HM Expression Patterns of Bone-Related Proteins during Osteoblastic Differentiation in MC3T3-E1 Cells. J. Cell. Biochem 1996, 61 (4), 609–618. . [DOI] [PubMed] [Google Scholar]
- (63).Sawa N; Fujimoto H; Sawa Y; Yamashita J. Alternating Differentiation and Dedifferentiation between Mature Osteoblasts and Osteocytes. Sci. Rep 2019, 9 (1), 13842. 10.1038/s41598-019-50236-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Yang F; Williams CG; Wang DA; Lee H; Manson PN; Elisseeff J. The Effect of Incorporating RGD Adhesive Peptide in Polyethylene Glycol Diacrylate Hydrogel on Osteogenesis of Bone Marrow Stromal Cells. Biomaterials 2005, 26 (30), 5991–5998. 10.1016/j.biomaterials.2005.03.018. [DOI] [PubMed] [Google Scholar]
- (65).Carles-Carner M; Saleh LS; Bryant SJ The Effects of Hydroxyapatite Nanoparticles Embedded in a MMP-Sensitive Photoclickable PEG Hydrogel on Encapsulated MC3T3-E1 Pre-Osteoblasts. Biomed. Mater 2018, 13 (4), 045009. 10.1088/1748-605X/aabb31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Diefenderfer DL; Osyczka AM; Garino JP; Leboy PS Regulation of BMP-Induced Transcription in Cultured Human Bone Marrow Stromal Cells. J. Bone Jt. Surgery, Am Vol. 2003, 85, 19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Lecanda F; Cheng SL; Zhang SF; Avioli LV Regulation of Bone Matrix Protein Expression and Signaling Pathways in the Induction of Differentiation of Human Osteoblasts and Human Bone Marrow Stromal Cells by Bone Morphogenetic Protein-2. J. Investig. Med 1996, 44 (3), 386–398. [PubMed] [Google Scholar]
- (68).Komori T. Regulation of Bone Development and Extracellular Matrix Protein Genes by RUNX2. Cell Tissue Res. 2010, 339 (1), 189–195. 10.1007/s00441-009-0832-8. [DOI] [PubMed] [Google Scholar]
- (69).Padovano JD; Ramachandran A; Bahmanyar S; Ravindran S; George A. Bone-Specific Overexpression of DMP1 Influences Osteogenic Gene Expression during Endochondral and Intramembranous Ossification. Connect. Tissue Res 2014, 55 (SUPPL. 1), 121–124. 10.3109/03008207.2014.923878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Rousseau M; Pereira-Mouriès L; Almeida MJ; Milet C; Lopez E. The Water-Soluble Matrix Fraction from the Nacre of Pinctada Maxima Produces Earlier Mineralization of MC3T3-E1 Mouse Pre-Osteoblasts. Comp. Biochem. Physiol. - B Biochem. Mol. Biol 2003, 135 (1), 1–7. 10.1016/S1096-4959(03)00032-0. [DOI] [PubMed] [Google Scholar]
- (71).Mikami Y; Takahashi T; Kato S; Takagi M. Dexamethasone Promotes DMP1 MRNA Expression by Inhibiting Negative Regulation of Runx2 in Multipotential Mesenchymal Progenitor, ROB-C26. Cell Biol. Int 2008, 32 (2), 239–246. 10.1016/j.cellbi.2007.08.033. [DOI] [PubMed] [Google Scholar]
- (72).Xiao G; Gopalakrishnan R; Jiang D; Reith E; Benson MD; Franceschi RT Bone Morphogenetic Proteins, Extracellular Matrix, and Mitogen-Activated Protein Kinase Signaling Pathways Are Required for Osteoblast-Specific Gene Expression and Differentiation in MC3T3-E1 Cells. J. Bone Miner. Res 2002, pp 101–110. 10.1359/jbmr.2002.17.1.101. [DOI] [PubMed] [Google Scholar]
- (73).Narayanan K; Ramachandran A; Hao J; He G; Park KW; Cho M; George A. Dual Functional Roles of Dentin Matrix Protein 1. Implications in Biomineralization and Gene Transcription by Activation of Intracellular Ca2+ Store. J. Biol. Chem 2003, 278 (19), 17500–17508. 10.1074/jbc.M212700200. [DOI] [PubMed] [Google Scholar]
- (74).Luppen CA; Leclerc N; Noh T; Barski A; Khokhar A; Boskey AL; Smith E; Frenkel B. Brief Bone Morphogenetic Protein 2 Treatment of Glucocorticoid-Inhibited MC3T3-E1 Osteoblasts Rescues Commitment-Associated Cell Cycle and Mineralization without Alteration of Runx2. J. Biol. Chem 2003, 278 (45), 44995–45003. 10.1074/jbc.M306730200. [DOI] [PubMed] [Google Scholar]
- (75).Gstraunthaler G; Lindl T; Van Der Valk J. A Plea to Reduce or Replace Fetal Bovine Serum in Cell Culture Media. Cytotechnology 2013, 65 (5), 791–793. 10.1007/s10616-013-9633-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.