Abstract
Background/aims
We developed a novel technology consisting of violet light (VL)-emitting glasses and defined the combination of VL irradiation and riboflavin treatment as KeraVio. Our goal was to evaluate the clinical results of KeraVio in patients with progressive corneal ectasia.
Methods
Eyes were exposed to VL (375 nm, irradiance 310 μW/cm2)-emitting glasses for 3 hours daily for 6 months, and a riboflavin solution was administered onto the corneal epithelium six times during each 3-hour VL irradiation. The primary end point was a change in the maximum keratometry (Kmax) value over 6 months compared with that over the 1 year before baseline.
Results
The efficacy of KeraVio was evaluated in 20 eyes with severe progression, and its safety was evaluated in all 40 eyes. The mean changes in Kmax over the 1 year before baseline and during the 6-month observation period were 6.03±3.41 dioptres (D) and −0.81±3.34 D, respectively (p=0.002). At 6 months, the Kmax value decreased by more than 2 D in 4 eyes (20%), remained within 2 D in 13 eyes (65%), and increased by 2 D or more in 3 eyes (15%). The corneal stromal demarcation line was identified in 16 eyes (80%), and its depth was 206.3±54.9 μm at 1 month. No significant decrease in endothelial cell density, lenticular opacity or transient corneal haze was noted.
Conclusion
Based on our 6-month results, daily treatment of progressive corneal ectasia with KeraVio can halt disease progression without any safety concerns.
Clinical trial registration number
jRCTs032180217.
Keywords: Cornea, Clinical Trial
INTRODUCTION
Keratoconus is a progressive, frequently asymmetric, inflammatory corneal thinning disorder characterised by changes in the structure and organisation of corneal collagen.1 This progressive bilateral disease weakens the cornea, resulting in myopia, irregular astigmatism and central corneal scarring. Corneal cross-linking (CXL) was first introduced by Seiler et al as a promising technique to slow or stop the progression of keratoconus.2 In CXL, riboflavin (vitamin B2) is administered in conjunction with ultraviolet A (UVA, 365 nm). The interaction between riboflavin and UVA leads to the formation of reactive oxygen species, which leads to the formation of additional covalent bonds between collagen molecules, resulting in biomechanical stiffening of the cornea. Since the first clinical study of CXL was published by Wollensak et al, 2 there have been additional published studies reporting the safety and efficacy of the treatment in slowing down or halting the progression of keratoconus and other corneal ecstatic disorders. Standard Dresden CXL received U.S. Food and Drug Administration approval for use in the treatment of progressive corneal ectasia in the USA. However, the current method of CXL requires epithelial removal, which is responsible for most of its major complications, including postoperative pain, vision impairment and an increased risk of infection.3–5 In an attempt to avoid these side effects, transepithelial approaches for loading riboflavin into the corneal stroma have been proposed.6
Currently, CXL is not covered by most health insurance providers in many countries, and information regarding its cost-effectiveness is scarce. The high financial cost of treatment, along with the high cost of the CXL system, has limited ophthalmologists’ and patients’ access to this effective new treatment. A novel approach to corneal CXL without epithelial abrasion uses dietary riboflavin followed by sunlight exposure. A small prospective study using oral administration of riboflavin and 15 min of natural sunlight exposure daily demonstrated no adverse effects. A larger clinical study is ongoing.7 On the basis of prior work, we hypothesised that violet light (VL), which is included in sunlight, may play an important role in corneal ectasia control. According to the international lighting vocabulary of the Commission Internationale de l’Eclairage,8 the lower limits of visible light wavelengths are defined to be between 360 nm and 400 nm, which overlaps with the upper end of the UVA spectrum.9 This range, in fact, is visible as VL, but it is recognised as UVA as well. To perform natural CXL using VL exposure, we developed novel, minimally invasive VL-emitting glasses, the application of which is called KeraVio. The purpose of this proof-of-concept study of KeraVio is twofold: to evaluate the biomechanical effect of combined VL-riboflavin treatment on rabbit corneas and to report the clinical outcomes of KeraVio using VL-emitting glasses and riboflavin drops in patients with progressive corneal ectasia.
METHODS
Rabbit study
Five female Japanese white rabbits weighing 1.5–2.0 kg were used for the experiment. All animals were healthy and free of ocular disease. The right eyes of the rabbits underwent collagen cross-linking with riboflavin and VL (KeraVio group), and the left eyes were treated as the control group. All animals were treated according to the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research.
KeraVio procedure
A topical anaesthetic consisting of 0.4% oxybuprocaine hydrochloride eye drops was applied to the eyes. The process of KeraVio began with the application of 0.05% flavin adenine dinucleotide (FAD) (Santen Pharmaceutical Co., Osaka, Japan), the coenzyme of riboflavin.10 11 The FAD solution was formulated with EDTA and hydroxypropyl methylcellulose, but the concentrations of these components were not disclosed. FAD drops were applied to the corneal epithelium every 30 min for 3 hours during VL irradiation to permeate the cornea. VL irradiation (375 nm) was applied using a single VL diode (Nitride Semiconductors Co., Tokushima, Japan) with an irradiance of 0.31 mW/cm2 for 180 min at a distance of 60 cm from the cornea (total energy dose 3.3 J/cm2). To avoid the cytotoxic threshold of the corneal endothelium, which is 0.36 mW/cm2, we applied a VL intensity of 0.31 mW/cm2 for 3 hours daily.12–14 The KeraVio treatment protocol using FAD drops, and VL irradiation was continued for 7 days (total energy dose 23.4 J/cm2).
Ocular biometry measurements
The eyes of the rabbits were monitored by slit-lamp examination to assess ocular safety; an examination was carried out before KeraVio treatment and repeated every day during the 7-day study. Central cornea thickness and axial length were recorded at baseline (the day before the treatment) and 7 days after the treatment using an ultrasound pachymeter (AL-Scan, Nidek Co., Gamagori, Japan) under topical anaesthesia. Three measurements were taken at each time point, and the mean value of each parameter was recorded, along with the change in its value.
Biomechanical measurements
The rabbits were euthanised with an intravenous overdose of sodium pentobarbital on day 7. The corneas were harvested en bloc along the sclera. A 2–3 mm scleral rim was preserved, and the cornea was attached along a custom-made scale. Then, a corneal strip 5 mm in width was resected vertically along the cornea. After the prepared corneal strip was placed on a computer-controlled electronic universal testing machine (TA.XTplusC Texture Analyser, Stable Micro Systems, London, UK), a fixture was applied to hold the corneoscleral limbus of the corneal strip for a uniaxial tensile test. For the actual measurement, the sample was stretched at a velocity of 1.8 mm/min up to a maximum force of 5 N. The stiffness (Young’s modulus) was calculated as the derivative of the stress–strain curve. For the subsequent statistical analysis, Young’s modulus was consistently evaluated at 10% strain.
Pathological evaluation
The remaining cornea samples were harvested. After being washed with normal saline, the specimens were fixed in 2.5% glutaraldehyde buffer for 2 hours or more, and the corneal cells and collagen arrangement were observed by transmission electron microscopy.
Clinical pilot study
This prospective, three-centre, non-randomised trial assessed the efficacy and safety outcomes of KeraVio. Institutional review board approval was obtained. The study adhered to the tenets of the Declaration of Helsinki. The study subjects completed a written informed consent process. This trial was approved by the Review Board at Keio University and registered in the Japan Registry of Clinical Trials (jRCT): jRCTs032180217.
Inclusion and exclusion criteria
The inclusion and exclusion criteria were similar to those used in the US clinical trials that established the efficacy of CXL.15 16 The inclusion criteria were male or female gender, any race or ethnicity, age 15 years or older, and a diagnosis of keratoconus or corneal ectasia after previous refractive surgery as documented by topography or tomography. Subjects were also required to have exhibited progression within 12 months before the baseline for KeraVio, as defined by one or more of the following: (1) an increase of ≥1.00 dioptre (D) in the maximum keratometry value (Kmax); (2) an increase of ≥1.00 D in cylinder power on subjective manifest refraction; (3) an increase of ≥0.50 D in myopia on subjective manifest refraction; and (4) a documented decrease in visual acuity associated with worsening irregular astigmatism and topographic features of ectasia. Contact lens wearers were required to remove contact lenses before refraction screening for the following lengths of time: 3 days for soft lenses, 1 week for soft extended-wear lenses, 2 weeks for soft toric lenses and 3 weeks for rigid gas-permeable lenses. In this study, we limited the use of contact lenses to VL-transmitting lenses during KeraVio treatment; almost all commercial contact lenses block VL.17
Exclusion criteria included a history of corneal surgery, including intracorneal ring segments, and corneal pachymetry at the thinnest part less than 300 μm. Patients who were pregnant or lactating during the course of the study were excluded.
KeraVio treatment
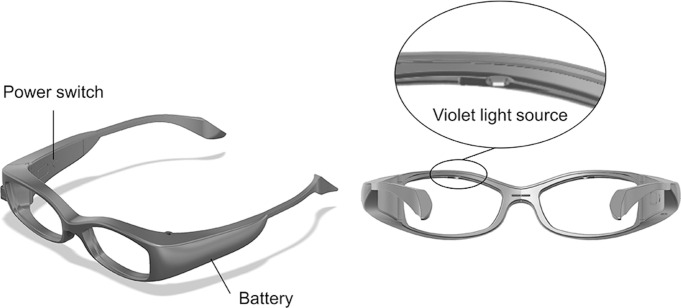
The subjects wore VL-emitting glasses, and their corneas were aligned and exposed to VL (375 nm) for 3 hours daily for 6 months, and 0.05% FAD drops were administered onto the corneal epithelium every 30 min during each 3-hour VL irradiation session to permeate the cornea. Figure 1 shows a photograph of prototype VL-emitting glasses, which have a source of VL on the upper rim of the frame (specifications of device in online supplemental 1). Before each treatment, the desired irradiance of 0.31 mW/cm2 was verified with a UVA metre (LaserMate-Q; LASER 2000, Wessling, Germany) at a 1.2-cm distance from the cornea and, if necessary, regulated with the potentiometer. The aforementioned KeraVio protocol in this clinical study using FAD drops and VL emitting glass was continued daily for 6 months (total energy dose 602.6 J/cm2).
Figure 1.
Images of the TLG-003 prototype illustrated by a coauthor (SK). Declaration of interest: None.
bjophthalmol-2020-316974supp001.pdf (67.6KB, pdf)
Patients could have both eyes treated if the investigator thought the KeraVio treatment could be beneficial in both eyes. Only the more severely affected eye of each patient was considered for the efficacy analysis, whereas all treated eyes were included in the safety analysis.
Outcome measures
Tomography
Tomographic data were obtained using anterior segment optical coherence tomography (AS-OCT) (CASIA, Tomey Corporation, Nagoya, Japan) at baseline and 1, 3 and 6 months after KeraVio. For quantification of keratometric parameters, the minimum corneal thickness and stromal demarcation line (DL) identified by the AS-OCT system were analysed. Kmax was chosen as the primary efficacy outcome because it measures a salient feature of corneal ectasia, that is, the steepness of ectatic tomographic distortion. Moreover, Kmax afforded an objective, quantitative end point and allowed the use of consistent hardware and software among the study sites. Keratometry values along the flat (K1) and steep (K2) meridians were also evaluated. In terms of the repeatability of keratometry measurements, a lower difference was observed between repeated measurements with AS-OCT than with Scheimpflug imaging in keratoconic eyes.18 In this study, we compared the changes in Kmax, K1 and K2 between the 1 year before baseline and the 6-month observation period. To evaluate the success rate, significant corneal flattening 6 months after KeraVio treatment was defined by a decrease in the Kmax of more than 1.00 D compared with the baseline value. Measurements of the DL depth with the AS-OCT scans were taken by two independent observers 1 month after treatment, as analytically reported in our previous studies.19 20 The DL was also assessed visually.
Visual acuity and refraction
The uncorrected distance visual acuity (UDVA), corrected distance visual acuity (CDVA) and manifest refraction spherical equivalent (MRSE) were measured at baseline and 1, 3 and 6 months after KeraVio. Visual acuity measurements were obtained as logarithm of the minimal angle of resolution (logMAR) units using a Landolt C chart.
Safety outcomes
The safety analysis included all treated eyes and the corresponding untreated eyes. The safety outcomes of endothelial cell density, intraocular pressure and axial length were measured at each time point using a specular microscope (NonconRobo, Konan, Nishinomiya, Japan), a tonometer (TONOREF, Nidek Co.) and partial coherence interferometry (IOLMaster 700, Carl Zeiss Meditec AG, Jena, Germany), respectively. Slit-lamp examination was also performed to evaluate adverse events, such as secondary cataracts, conjunctivitis and eyelid sunburn.
Statistical analysis
Analyses were performed with Statistical Analysis Software (version 9.4; SAS Institute, Cary, NC). The outcome measures are reported as the mean±SD. A two-tailed paired t-test was used in the statistical analyses to compare the elastic modulus between the two groups. The Wilcoxon signed-rank test was used for statistical analysis to compare the pretreatment and post-treatment clinical parameters. One-way analysis of variance (ANOVA) was performed to assess the time course of changes, and a post hoc Dunnett’s test was also applied for multiple comparisons. A p value of <0.05 was considered statistically significant.
RESULTS
Rabbit study
KeraVio treatments were performed and continued in all eyes for 7 days without infection or any other incident. There were no adverse events in either group: slit-lamp examinations showed no conjunctival injection, corneal infiltration, corneal stromal inflammation or secondary cataracts throughout the follow-up period.
At baseline and 1 week after treatment, there was no significant difference in central corneal thickness between the two groups (table 1). Similar outcomes were obtained for axial length. The changes in central corneal thickness from baseline to 1 week were not significant in either group. At 1 week after treatment, there was a significant increase in axial length in the KeraVio and control groups, but no significant difference in axial length was found between the two groups.
Table 1.
Comparison of central cornea thickness and axial length in the KeraVio and control groups in rabbit eyes
| KeraVio group | Control group | P value* | ||
|---|---|---|---|---|
| Central corneal thickness (μm) | Baseline | 323.0±23.4 | 329.4±11.6 | 0.675 |
| One week after treatment | 329.2±27.7 | 344.0±26.1 | 0.587 | |
| Change from baseline to 1 week | 6.2±38.1 | 14.6±24.0 | 0.530 | |
| P value† | 0.893 | 0.281 | ||
| Axial length (mm) | Baseline | 14.74±0.16 | 14.64±0.42 | 0.251 |
| One week after treatment | 15.33±0.33 | 15.20±0.27 | 0.530 | |
| Change from baseline to 1 week | 0.59±0.37 | 0.56±0.25 | 0.754 | |
| P value† | 0.043 | 0.043 |
*Compared between the two groups.
†Comparison between baseline and 1 week after treatment.
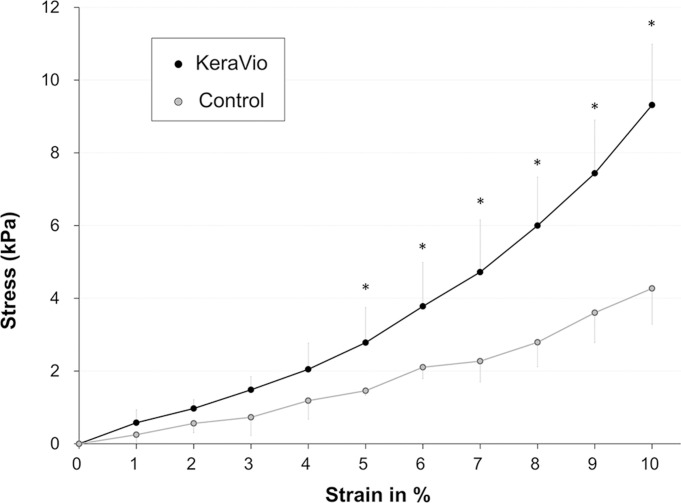
The stress–strain curves showed the exponential increase typical of an elastic solid (figure 2). We found significant differences in stress values between the two groups at 5–10% strain (p<0.05). The average Young’s modulus at 10% strain was 84.3±18.9 kPa for the KeraVio group and 39.1±9.5 kPa for the control group (p=0.013).
Figure 2.
Stress–strain measurements of rabbit corneas treated with KeraVio (n=5). *Pp<0.05 compared with the control.
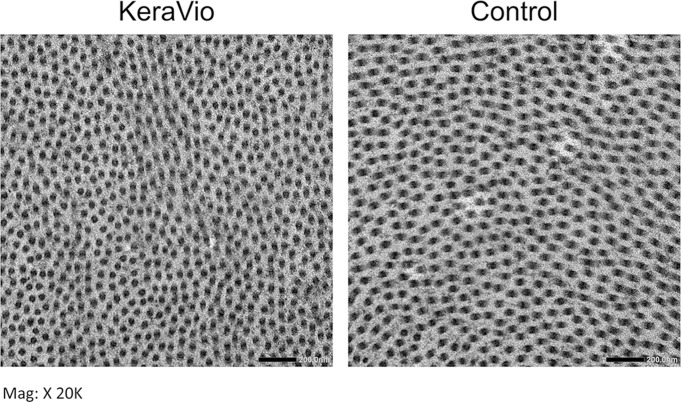
The collagen fibres of the KeraVio group appeared more tightly arranged than those of the control group (figure 3).
Figure 3.
Transmission electron microscopy images of the (left) KeraVio and (right) control corneas. Micrograph demonstrates that feline cornea treated with KeraVio exhibited more cross links between collagen fibrils than untreated control feline corneas.
Clinical study
Forty eyes belonging to 20 patients were treated with KeraVio treatment. Among those eyes, 18 were in the keratoconus subgroup and 2 were in the pellucid marginal degeneration subgroup. The efficacy of KeraVio was evaluated in 20 eyes with severe progression, and its safety was evaluated in all 40 eyes. Participant demographics are presented in table 2. All patients remained in the study through the 6-month follow-up.
Table 2.
Patient demographics in the KeraVio study
| KeraVio | |
|---|---|
| Eyes/patients (n) | 20/20 |
| Age (years), mean±SD | 32.0±10.4 |
| Gender (female/male), n (%) | 5/15 (25.0/75.0) |
| Kmax (diopters), mean±SD | 59.83±8.23 |
Tomography
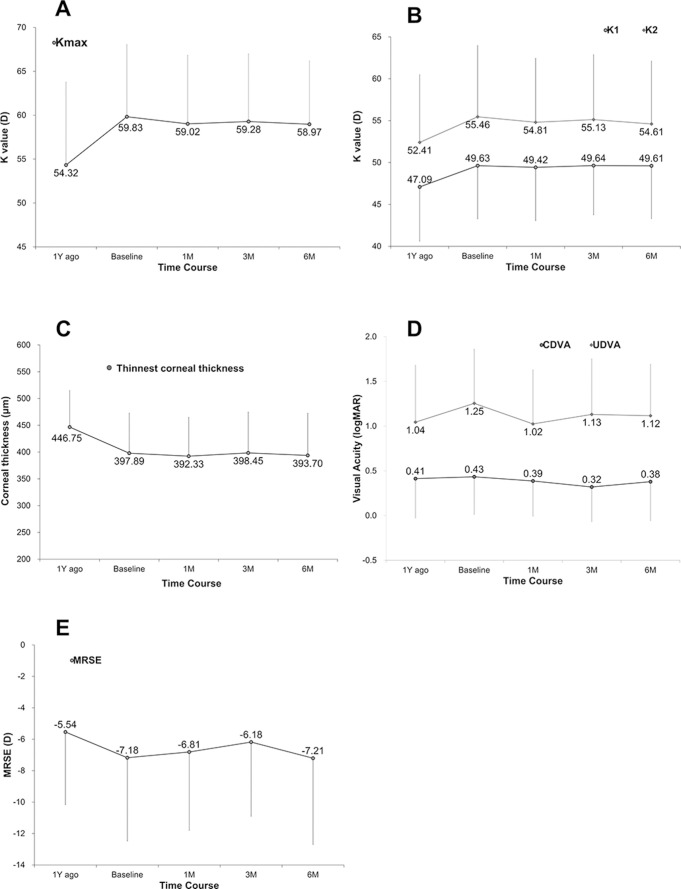
Figure 4A shows the Kmax values from the 1 year before baseline and the 6-month observation period after KeraVio treatment. The mean changes in Kmax over the 1 year before baseline and during the 6-month observation period were 6.03±3.41 D and −0.81±3.34 D, respectively (p=0.002). ANOVA showed no significant difference between baseline and 6 months (p=0.985). The success rate (flattening of the Kmax >1.00 D) was 30% (6 eyes). At 6 months, the Kmax decreased by more than 2 D in 4 eyes (20%), remained within 2 D in 13 eyes (65%) and increased by 2 D or more in 3 eyes (15%). Figure 4B also shows K1 and K2 before and after KeraVio treatment. The mean change in K1 over the preceding 1 year before baseline and during the 6-month observation period was 2.06±5.44 D and −0.02±1.93 D, respectively (p=0.03). Similarly, the mean change in K2 was 3.00±2.75 D and −0.86±3.31 D, respectively (p=0.009). ANOVA showed no significant difference in K1 and K2 between baseline and 6 months (p=0.999 and 0.987, respectively).
Figure 4.
Changes in clinical parameters over time. (A) Kmax. (B) Flat (K1) and steep (K2) keratometry readings. (C) Thinnest corneal thickness. (D) Visual acuities. (E) Manifest refraction spherical equivalent. CDVA, corrected distance visual acuity; logMAR, logarithm of the minimal angle of resolution; MRSE, manifest refraction spherical equivalent; UDVA, uncorrected distance visual acuity.
Figure 4C shows the minimum corneal thickness during the 1 year before baseline and during the 6-month observation period after KeraVio. The mean changes in minimum corneal thickness over the 1 year before baseline and during the 6-month observation period were −62.24±55.96 μm and −9.68±16.00 μm, respectively (p=0.0192). Measurements from eyes treated with KeraVio revealed no significant difference between baseline and 6 months (ANOVA, p=0.993).
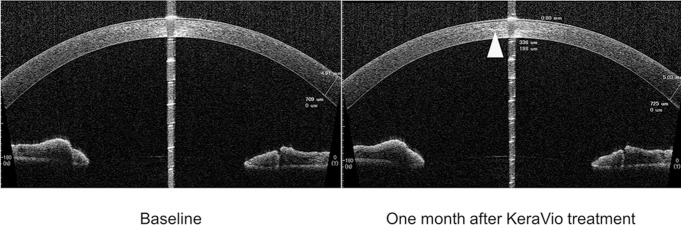
Figure 5 shows representative high-resolution AS-OCT scans of the corneal stromal DL of a KeraVio-treated eye at 1 month. The corneal stromal DL was identified in 16 eyes (80%) by both examiners. The mean depth of the DL was 206.3±54.9 μm at 1 month after treatment.
Figure 5.
High-resolution AS-OCT scan visualising the corneal stromal demarcation line 1 month after KeraVio treatment. The central corneal demarcation line depth is 336 μm.
Visual acuity and refraction
Figure 4D shows the CDVA and UDVA over time. The mean changes in CDVA over the 1 year before baseline and during the 6-month observation period were 0.02±0.02 logMAR and −0.07±0.18 logMAR, respectively (p=0.019). Similarly, the mean changes in UDVA over the same periods were 0.16±0.47 logMAR and −0.19±0.50 logMAR, respectively (p=0.063). ANOVA showed no significant difference in CDVA and UDVA between baseline and 6 months (p=0.866 and 0.697, respectively). Of the 20 eyes evaluated at 6 months, 12 eyes (60%) showed no change in CDVA, 5 eyes (25%) gained two lines or more, 2 eyes (10%) lost one line and 1 eye (5%) lost two lines.
Figure 4E shows the MRSE over time. The mean changes in MRSE over the 1 year before baseline and during the 6-month observation period were −1.42±2.73 D and −0.03±2.58 D, respectively (p=0.142). ANOVA showed no significant difference between baseline and 6 months (p=0.923).
Safety outcomes
Table 3 presents the safety profile of KeraVio treatment. No significant difference was found in any parameter between baseline and 6 months. There were no reported cases of persistent corneal oedema in the study. Regarding adverse events, no vision-threatening complications were found in this study, indicating the safety of this treatment. No pterygium, skin melanoma, lenticular opacity or transient corneal haze was noted in either eye at the 6-month follow-up.
Table 3.
Safety profile of eyes with KeraVio
| Baseline | One month after KeraVio | Three months after KeraVio | Six months after KeraVio | P value* | |
|---|---|---|---|---|---|
| Endothelial cell density (cells/mm2) | 2679.7±295.6 | 2679.4±312.9 | 2752.5±464.2 | 2760.6±604.7 | 0.067 |
| Intraocular pressure (mmHg) | 10.4±3.1 | 11.0±3.7 | 10.5±3.9 | 9.7±3.3 | 0.011 |
| Axial length (mm) | 25.26±1.50 | 25.28±1.52 | 25.24±1.53 | 25.27±1.48 | 0.094 |
*Comparison of changes at 6 months from baseline.
DISCUSSION
Proof-of-concept study
The current study confirmed that cross-linking by FAD and VL (375 nm) had an impressive stiffening effect on rabbit corneas in vivo. KeraVio is composed of a relatively low concentration of FAD drops without epithelial removal and a relatively low intensity of VL irradiation. Young’s modulus at 10% strain in KeraVio-treated corneas was 2.2-fold higher than that of control corneas, which is similar to previously published data.21 22 Our findings support the hypothesis that the KeraVio protocol halts disease progression in eyes with corneal ectasia. According to the photochemical law of reciprocity (Bunsen-Roscoe law),23 the same photochemical effect can be achieved with increased illumination time and correspondingly reduced irradiation intensity. Hammer et al,24 however, concluded that the stiffening effect of CXL decreases with increasing irradiance and decreased treatment times. Those results indicate that the Bunsen-Roscoe law is inapplicable for the evaluation of corneal stiffness. Thus, we selected a VL intensity of 0.31 mW/cm2 for 3 hours daily (3.3 J/cm2/day) in consideration of the cytotoxic threshold for the corneal endothelium.12–14
The corneal epithelium is itself the critical obstacle to the permeation of riboflavin into the corneal stroma, and it affects corneal stiffness because a complete and intact epithelial layer is a tough lipophilic barrier to water-soluble riboflavin.25 FAD drops (0.05%) were administered six times over the course of each 3-hour treatment session, and they contained EDTA, which increases the epithelial permeability and topical medication bioavailability to the corneal stroma.26 However, the concentration of its enhancer has not been disclosed. In our preliminary investigation, we confirmed using eye bank tissue that an adequate riboflavin concentration was achieved in human corneal stroma when one drop of 0.05% FAD was applied every 30 min for 3 hours (Kobashi et al. 2020, unpublished data).
Efficacy of KeraVio
The mean Kmax value of the KeraVio-treated eyes decreased by 0.81 D at 6 months, although the value had increased by 6.03 D in the year before baseline. Thus, our study demonstrates that KeraVio has a beneficial effect on corneal topography in patients with corneal ectasia over the course of 6 months. Kmax, believed to be an important indicator of CXL success, decreased significantly after the procedure. Previous studies reported a reduction of 1–2 D in the mean Kmax of treated keratoconic eyes at 1 year.15 27 Ferdi et al 28 reported a systematic review and meta-analysis of keratoconus natural history data, including 11 529 eyes from 41 publications. Their meta-analysis of Kmax demonstrated a significant increase of 0.7 D at 12 months. Although no untreated control group was included in this pilot study, we believe that the efficacy of KeraVio was demonstrated by the stabilisation of progressive corneal disorder. In our study, the Kmax decreased by 2.0 D or more in four eyes (20%) and increased by 2.0 D or more in three eyes (15%) 6 months after KeraVio. These latter three eyes may be considered treatment failures because keratoconus progressed rather than being stabilised. According to the protocol in this pilot study, we limited the observation period to at least 6 months. A 1-year follow-up is being performed by recruiting voluntary patients after KeraVio treatment to evaluate its efficacy.
A reduction in the minimal corneal thickness was observed before the baseline of KeraVio, but the decrease gradually stopped by 6 months after KeraVio treatment. It is not clear whether this change is clinically meaningful because the SD was high.
Although not synonymous with treatment efficacy, DL has been used as a potential indication of the depth or extent of CXL treatment. Seiler and Hafezi29 described a visible corneal stromal DL theoretically indicating the transition zone between the cross-linked anterior corneal stroma and the untreated posterior corneal stroma. After KeraVio, a corneal stromal DL was detected using AS-OCT in 80% of the patients in this study, and the mean depth of the DL was 206.3±54.9 μm at 1 month after treatment. Previous studies of the CXL technique reported a mean corneal stromal DL depth of more than 250 μm.30 31 Generally, a deeper DL implies better efficacy in CXL,3 but it is unknown whether the mechanism corresponds to that of KeraVio because of its piecemeal approach to stopping disease progression.
In addition to the primary efficacy measurement of Kmax, changes in CDVA may point to additional benefits after KeraVio. The actual clinical significance is best demonstrated by our CDVA results among patients undergoing KeraVio: five eyes (25%) gained two lines or more of CDVA, whereas one eye (5%) lost two lines or more. Therefore, one-quarter of patients enjoyed a clinically meaningful increase in CDVA as a result of KeraVio, whereas only one patient’s eye continued to progress. Our findings are in accordance with those of CXL during the 6-month observation period.32
Safety of KeraVio
Endothelial cell density has been shown to remain unaffected over a 6-month follow-up period after KeraVio treatment. Endothelial cell damage in CXL is a concern and could result from endothelial exposure to free radicals generated from the CXL process. In this study, VL-emitting glasses provided 602.6 J/cm2 over the 6-month treatment period, which was approximately 112-fold higher than the dose delivered by the standard Dresden protocol for CXL. We confirmed that the KeraVio protocol using VL exposure (0.31 mW/cm2 for 3 hours daily for 6 months) did not affect the corneal endothelium.
There are some epidemiological studies that suggest an association between UVA exposure and ocular damage, such as cataracts, pterygium, skin melanoma and/or retinal damage.33–35 No adverse events associated with chronic VL exposure were found in our KeraVio study. The formal definition of VL terminologically differs from that of UVA, whereas their wavelengths overlap with each other.9 Since VL includes only visible light wavelengths, we assume that chronic VL exposure at a low irradiance level does not induce any ocular concerns.
Additional indication for the KeraVio procedure
Our study demonstrated that minor complications after CXL, such as infection, sterile infiltrate and delayed epithelial healing, were not reported because there was no epithelial removal. Although microbial keratitis after CXL is infrequent, it has been reported after epithelium-removing CXL in the paediatric population.36–38 It is suggested that paediatric keratoconus patients might be appropriate candidates for KeraVio as a minimally invasive approach. As patients under 17 years old have a significantly greater risk of keratoconus progression than older patients,28 KeraVio may be a minimally invasive treatment option for paediatric patients with corneal ectasia.
Study limitations
This pilot study has at least three limitations that should be considered. First, the sample size in this study was relatively small, and no placebo-controlled group existed. We are conducting a randomised controlled trial to compare treatment efficacy between a KeraVio group and a placebo group. Second, the observation period in this study was relatively short. More prolonged and careful observation is still required to assess the long-term efficacy and safety of this KeraVio procedure. Third, it is unknown whether the total doses of riboflavin drops and VL irradiation in this study were optimal for preventing the progression of corneal ectasia in the KeraVio procedure. We confirmed the efficacy of KeraVio at the 1-month follow-up when we reviewed the clinical results in this study. Further study is needed to clarify the efficacy of different protocols with different doses of riboflavin drops.
CONCLUSIONS
In conclusion, based on our 6-month results, daily treatment of progressive corneal ectasia with KeraVio can halt disease progression without raising any safety concerns. KeraVio may be a minimally invasive treatment option for patients with corneal ectasia. Further long-term follow-up studies are needed to confirm these findings and to determine the optimal protocol for the KeraVio procedure.
Footnotes
Contributors: HK and KT conceived and designed the study. HK, IT and MI provided patients. HK and HT collected data and performed the statistical analysis. HK, HT and KT performed the literature search and data interpretation. HK and HT wrote the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: HK: consultant and equity owner, Tsubota Laboratory (Tokyo, Japan); Patent, Tsubota Laboratory. HT: Equity owner, Tsubota Laboratory; Patent, Tsubota Laboratory. SK: employee and equity owner, Tsubota Laboratory. KT: employee and equity owner, Tsubota Laboratory; Patent, Tsubota Laboratory.
Patient consent for publication: Not required.
Ethics approval: Human participants: The study adhered to the tenets of the Declaration of Helsinki. The study subjects provided written informed consent. This trial was approved by the Review Board at Keio University and registered in the Japan Registry of Clinical Trials (jRCT): jRCTs032180217. All animals were treated according to the Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
REFERENCES
- 1.Rabinowitz YS. Keratoconus. Surv Ophthalmol 1998;42:297–319. 10.1016/S0039-6257(97)00119-7 [DOI] [PubMed] [Google Scholar]
- 2.Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-a-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol 2003;135:620–7. 10.1016/S0002-9394(02)02220-1 [DOI] [PubMed] [Google Scholar]
- 3.Sorkin N, Varssano D. Corneal collagen crosslinking: a systematic review. Ophthalmologica 2014;232:10–27. 10.1159/000357979 [DOI] [PubMed] [Google Scholar]
- 4.Dhawan S, Rao K, Natrajan S. Complications of corneal collagen cross-linking. J Ophthalmol 2011;2011:869015. 10.1155/2011/869015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kymionis GD, Portaliou DM, Bouzoukis DI, et al. Herpetic keratitis with iritis after corneal crosslinking with riboflavin and ultraviolet A for keratoconus. J Cataract Refract Surg 2007:1982–4 10.1016/j.jcrs.2007.06.036. [DOI] [PubMed] [Google Scholar]
- 6.Filippello M, Stagni E, O’Brart D. Transepithelial corneal collagen crosslinking: bilateral study. J Cataract Refract Surg 2012;38:283–91. 10.1016/j.jcrs.2011.08.030 [DOI] [PubMed] [Google Scholar]
- 7.Dietary riboflavin (Vitamin B-2) and cornea cross-linking. ClinicalTrials.gov. Availablehttps://clinicaltrials.gov/ct2/show/study/NCT03095235(accessed 30 Oct 2019)
- 8.CIE . International standard CEI/IEC 62471 (CIE S 009: 2002) book international standard CEI/IEC 62471 (CIE S 009: 2002) first edition 2006–07. Switzerland: International Electrotechnical Commission, 2006: 89. [Google Scholar]
- 9.Krutmann J, Béhar-Cohen F, Baillet G, et al. Towards standardization of UV eye protection: what can be learned from photodermatology? Photodermatol Photoimmunol Photomed 2014;30:128–36. 10.1111/phpp.12089 [DOI] [PubMed] [Google Scholar]
- 10.Sakata R, Sakisaka T, Matsuo H, et al. Effect of travoprost and nonsteroidal anti-inflammatory drug on diurnal intraocular pressure in normal subjects with low-teen baseline intraocular pressure. J Ocul Pharmacol Ther 2016;32:365–70. 10.1089/jop.2015.0159 [DOI] [PubMed] [Google Scholar]
- 11.Sakata R, Sakisaka T, Matsuo H, et al. Time course of prostaglandin analog-related conjunctival hyperemia and the effect of a nonsteroidal anti-inflammatory ophthalmic solution. J Glaucoma 2016;25:e204–208. 10.1097/IJG.0000000000000227 [DOI] [PubMed] [Google Scholar]
- 12.Wollensak G, Spoerl E, Reber F, et al. Keratocyte cytotoxicity of riboflavin/UVA-treatment in vitro. Eye 2004;18:718–22. 10.1038/sj.eye.6700751 [DOI] [PubMed] [Google Scholar]
- 13.Wollensak G, Spoerl E, Wilsch M, et al. Keratocyte apoptosis after corneal collagen cross-linking using ribofl avin/UVA treatment. Cornea 2004;23:43–9. 10.1097/00003226-200401000-00008 [DOI] [PubMed] [Google Scholar]
- 14.Wollensak G, Spoerl E, Wilsch M, et al. Endothelial cell damage after riboflavin: ultraviolet-A treatment in the rabbit. J Cataract Refract Surg 2003:1786–90 10.1016/s0886-3350(03)00343-2. [DOI] [PubMed] [Google Scholar]
- 15.Hersh PS, Stulting RD, Muller D, et al. United States crosslinking study group. united states multicenter clinical trial of corneal collagen crosslinking for keratoconus treatment. Ophthalmology 2017;124:1259–70. 10.1016/j.ophtha.2017.03.052 [DOI] [PubMed] [Google Scholar]
- 16.Hersh PS, Stulting RD, Muller D, et al. U.S. crosslinking study group. U.S. multicenter clinical trial of corneal collagen crosslinking for treatment of corneal ectasia after refractive surgery. Ophthalmology 2017;124:1475–84. 10.1016/j.ophtha.2017.05.036 [DOI] [PubMed] [Google Scholar]
- 17.Torii H, Kurihara T, Seko Y, et al. Violet light exposure can be a preventive strategy against myopia progression. EBioMedicine 2017;15:210–9. 10.1016/j.ebiom.2016.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Szalai E, Berta A, Hassan Z, et al. Reliability and repeatability of swept-source Fourier-domain optical coherence tomography and Scheimpflug imaging in keratoconus. J Cataract Refract Surg 2012;38:485–94. 10.1016/j.jcrs.2011.10.027 [DOI] [PubMed] [Google Scholar]
- 19.Kymionis GD, Grentzelos MA, Plaka AD, et al. Correlation of the corneal collagen cross-linking demarcation line using confocal microscopy and anterior segment optical coherence tomography in keratoconic patients. Am J Ophthalmol 2014;157:110–115.e1. 10.1016/j.ajo.2013.09.010 [DOI] [PubMed] [Google Scholar]
- 20.Kymionis GD, Tsoulnaras KI, Grentzelos MA, et al. Corneal stroma demarcation line after standard and high-intensity collagen crosslinking determined with anterior segment optical coherence tomography. J Cataract Refract Surg 2014;40:736–40. 10.1016/j.jcrs.2013.10.029 [DOI] [PubMed] [Google Scholar]
- 21.Wollensak G, Spoerl E, Seiler T. Stress-strain measurements of human and porcine corneas after riboflavin-ultraviolet-a-induced cross-linking. J Cataract Refract Surg 2003;29:1780–5. 10.1016/S0886-3350(03)00407-3 [DOI] [PubMed] [Google Scholar]
- 22.Wernli J, Schumacher S, Spoerl E, et al. The efficacy of corneal cross-linking shows a sudden decrease with very high intensity UV light and short treatment time. Invest Ophthalmol Vis Sci 2013;54:1176–80. 10.1167/iovs.12-11409 [DOI] [PubMed] [Google Scholar]
- 23.Bunsen RW, Roscoe HE. Photochemical researches: part V. On the measurement of the chemical action of direct and diffuse sunlight. Proc R Soc Lond 1862;12:306–12 10.1098/rspl.1862.0069. [DOI] [Google Scholar]
- 24.Hammer A, Richoz O, Arba Mosquera S, et al. Corneal biomechanical properties at different corneal cross-linking (CXL) irradiances. Invest Ophthalmol Vis Sci 2014;55:2881–4. 10.1167/iovs.13-13748 [DOI] [PubMed] [Google Scholar]
- 25.Spoerl E, Mrochen M, Sliney D, et al. Safety of UVA-riboflavin cross-linking of the cornea. Cornea 2007;26:385–9. 10.1097/ICO.0b013e3180334f78 [DOI] [PubMed] [Google Scholar]
- 26.Mahaling B, Katti DS. Understanding the influence of surface properties of nanoparticles and penetration enhancers for improving bioavailability in eye tissues in vivo. Int J Pharm 2016;501:1–9. 10.1016/j.ijpharm.2016.01.053 [DOI] [PubMed] [Google Scholar]
- 27.Vinciguerra P, Camesasca FI, Romano MR. Corneal crosslinking and lens opacity. Ophthalmology 2011;118:2519. 10.1016/j.ophtha.2011.07.055 [DOI] [PubMed] [Google Scholar]
- 28.Ferdi AC, Nguyen V, Gore DM, et al. Keratoconus natural progression: a systematic review and meta-analysis of 11 529 eyes. Ophthalmology 2019;126:935–45. 10.1016/j.ophtha.2019.02.029 [DOI] [PubMed] [Google Scholar]
- 29.Seiler T, Hafezi F. Corneal cross-linking-induced stromal demarcation line. Cornea 2006;25:1057–9. 10.1097/01.ico.0000225720.38748.58 [DOI] [PubMed] [Google Scholar]
- 30.Hagem AM, Thorsrud A, Sandvik GF, et al. Collagen crosslinking with conventional and accelerated ultraviolet-A irradiation using riboflavin with hydroxypropyl methylcellulose [published correction appears in J Cataract Refract Surg. 2019 Mar;45(3):390]. J Cataract Refract Surg 2017;43:511–7 10.1016/j.jcrs.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 31.Shetty R, Pahuja NK, Nuijts RM, et al. Current protocols of corneal collagen cross-linking: visual, refractive, and tomographic outcomes. Am J Ophthalmol 2015;160:243–9. 10.1016/j.ajo.2015.05.019 [DOI] [PubMed] [Google Scholar]
- 32.Hashemi H, Fotouhi A, Miraftab M, et al. Short-term comparison of accelerated and standard methods of corneal collagen crosslinking. J Cataract Refract Surg 2015;41:533–40. 10.1016/j.jcrs.2014.07.030 [DOI] [PubMed] [Google Scholar]
- 33.Balasubramanian D. Ultraviolet radiation and cataract. J Ocul Pharmacol Ther 2000;16:285–97. 10.1089/jop.2000.16.285 [DOI] [PubMed] [Google Scholar]
- 34.Delic NC, Lyons JG, Di Girolamo N, et al. Damaging effects of ultraviolet radiation on the cornea. Photochem Photobiol 2017;93:920–9. 10.1111/php.12686 [DOI] [PubMed] [Google Scholar]
- 35.Sample A, He YY. Mechanisms and prevention of UV-induced melanoma. Photodermatol Photoimmunol Photomed 2018;34:13–24. 10.1111/phpp.12329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rana M, Lau A, Aralikatti A, et al. Severe microbial keratitis and associated perforation after corneal crosslinking for keratoconus. Cont Lens Anterior Eye 2015;38:134–7. 10.1016/j.clae.2014.10.004 [DOI] [PubMed] [Google Scholar]
- 37.Sharma N, Maharana P, Singh G, et al. Pseudomonas keratitis after collagen crosslinking for keratoconus: case report and review of literature. J Cataract Refract Surg 2010;36:517–20. 10.1016/j.jcrs.2009.08.041 [DOI] [PubMed] [Google Scholar]
- 38.Shetty R, Kaweri L, Nuijts RM, et al. Profile of microbial keratitis after corneal collagen cross-linking. Biomed Res Int 2014;2014:340509. 10.1155/2014/340509 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bjophthalmol-2020-316974supp001.pdf (67.6KB, pdf)