Summary
Recovering waste solvent for reuse presents an excellent alternative to improving the greenness of industrial processes. Implementing solvent recovery practices in the chemical industry is necessary, given the increasing focus on sustainability to promote a circular economy. However, the systematic design of recovery processes is a daunting task due to the complexities associated with waste stream composition, techno-economic analysis, and environmental assessment. Furthermore, the challenges to satisfy the desired product specifications, particularly in pharmaceuticals and specialty chemical industries, may also deter solvent recovery and reuse practices. To this end, this review presents a systems-level approach including various methodologies that can be implemented to design and evaluate efficient solvent recovery pathways.
Subject areas: Chemistry, Chemical engineering, Industrial chemistry, Process engineering, Organic chemistry, Green chemistry, Green engineering
Graphical abstract
Chemistry; Chemical engineering; Industrial chemistry; Process engineering; Organic chemistry; Green chemistry; Green engineering
Introduction
Solvents are an integral part of industrial processes. Almost all industrial processes rely on solvents with varying levels. The pharmaceutical industry is one of the major consumers of solvents for its active pharmaceutical ingredient (API) purification and refinement processes (Constable et al., 2007; Dunn Peter et al., 2010). Furthermore, the importance of solvents can be extended to the food, cosmetics, nutraceuticals, biofuels, paints, and fine chemical industries (Benvenutti, 2019; Breil et al., 2016; Farrán et al., 2015; Fernández-Agulló et al., 2013; Jiménez-González et al., 2011; Sá, 2017; Sharma and Kanwar, 2014; Zhang, 2016). The continuous growth in demand for solvents has inadvertently increased waste generation. For example, approximately 25–100 kg of waste is generated per kg of a product by the pharmaceutical industry (Sheldon, 2017). The forefront of this generation issue is the inefficiencies associated with industrial processes and the poor solvent selection criteria (Cseri et al., 2018). Undeniably, there is excessive use of solvents to achieve desired purities and quantities of products. Therefore, the increasing trends in waste solvent generation have necessitated process intensification methods such as solvent recovery to curb the growing environmental, health, and safety concerns.
Incineration, offsite, and onsite disposal techniques have been the conventional waste handling methods practiced by most industries. However, these methods present challenges regarding the emissions, safety, handling, and fate of the waste solvents within the ecosystem. The annual Disability-Adjusted Life Years (DALY) associated with transportation for offsite disposal has been estimated to be 0.35–35.03 (Das et al., 2012; Goedkoop and Spriensma, 2001; Jolliet et al., 2003). Incineration is typically a more expensive waste management technique because it requires high energy input to maintain continuous operation. The thermal destruction capability by incineration is highly effective at reducing solvent waste volume. However, chemicals resulting from the combustion of solvents can be hazardous to the surrounding ecosystem (Federal Remediation Technologies Roundtable, 2020; Incineration, 2009; Oppelt, 1990; Riber, 2007; World Health Organization, 2001). Offsite and onsite disposals are not energy-intensive, but the waste solvents are prone to leakage into the nearby water supply and land, contaminating the affected resources (Muralikrishna and Manickam, 2017;US EPA and OW, 2015).
Thus, solvent recovery presents a better mitigation option than conventional disposal methods due to lower implementation costs and fewer emissions (Chea et al., 2020; Raymond et al., 2010). The recovery and reuse of organic waste solvents are essential to improving the sustainability and circularity of industrial processes. The use of conventional waste handling methods tends to increase the overall energy and ecological footprint of an industry (Das et al., 2012; Oppelt, 1990; Slater et al., 2010a; US EPA, 2003;Villanueva and Wenzel, 2007). Over the years, governmental policies have been enacted to help industries practice the disposal of hazardous waste solvents to reduce their life cycle impacts. The Resource Conservation and Recovery Act (RCRA) was implemented to promote industrial sustainability (US EPA, 2016). This act encourages environmentally sound methods for managing waste. It establishes a national framework for hazardous waste control by mandating the United States Environmental Protection Agency (US EPA) to develop regulations, guidelines, and policies that ensure safe handling of hazardous waste and programs to ensure its reuse. Based on the RCRA requirements, solvent recovery presents an opportunity that fosters a robust industrial sustainability backbone by ensuring responsible management practices (Chea et al., 2020; Cseri et al., 2018; Abejon et al., 2015; Slater et al., 2010b).
The implementation of solvent recovery processes comes with various challenges. In the modern-day capitalistic economy, the cost has been the driving force for most industrial policies because it determines if solvent recovery can be used in waste management. Technology selection is another challenge that should be considered when designing a solvent recovery process because different technologies can perform similar tasks. Therefore, selecting technologies that can achieve the required specification at minimum cost can be challenging without systematic evaluation. Furthermore, the need to integrate a sustainability metric that quantitatively evaluates the greenness of solvent recovery processes has not been extensively investigated. Lastly, the waste solvent characteristics are a major concern for most industries when considering recycling and reuse options within the same process. However, there is a hidden opportunity for research and development to devise better solutions to every challenge.
Several metrics have been developed to help assess the sustainability of industrial processes. The E factor quantifies the amount of waste generated per kilogram of a product obtained. Solvents have been used in large quantities in the pharmaceutical field for the past 25 years (Sheldon, 2007, 2017). The American Chemical Society Green Chemistry Institute Pharmaceutical Roundtable (ACS-GCIPR) has adopted the Process Mass Intensity (PMI) as the benchmark for evaluating the greenness of pharmaceutical processes (Jimenez-Gonzalez et al., 2011). However, these metrics are mass-dependent and usually do not account for the energy demand along the supply chain of processes. Emergy is one of the fundamental assessments that has gained popularity in its industrial applications over the past two decades (Brown and Herendeen, 1996; Brown and Ulgiati, 2002; Cheng et al., 2020; Guo, 2019; Odum, 1988; Ulgiati et al., 1994). It can quantify the available energy used up in transformations to make a product. Emissions in gaseous states have also been represented in terms of carbon dioxide equivalent (CO2-eq). CO2 is known to persist in the atmosphere and trap heat for an extended period. The release of other chemicals is compared directly to CO2 based on a 100-year global warming potential (GWP), in which CO2 is assigned a reference value of 1. The release of 1 kg of methane is equivalent to 25 kg of CO2 released into the atmosphere (US EPA, 2014). Other substances such as 1 kg of sulfur hexafluoride may equate up to 22,800 kg CO2 released (Climate Change Connection, 2020). The difference in CO2-eq values is dependent on the ability of a substance to absorb energy and its persistence in the atmosphere with respect to CO2 (US EPA, 2020). In solvent-intensive operation, fugitive emissions, spills, and evaporations can create gaseous phase emissions that can negatively impact the environment over time. Therefore, CO2-eq is a valuable addition to evaluating the impacts from process waste (E factor) and energy consumption (Emergy).
In this paper, we discuss the body of works that have been accomplished in solvent use and recovery practices. Furthermore, we present a superstructure optimization approach for designing an all-inclusive solvent recovery roadmap and integrating multiple sustainability indicators in the assessment framework. We further discuss energy efficient ways to recovery and the need to incorporate Quality by Design and Control (QbD&C) in industrial process design. We finally discuss the importance of process intensification, and the application of Machine Learning (ML) and Artificial Intelligence (AI) to improve solvent recovery processes.
Supporting legislation for solvent recovery
Solvent recovery may have begun as early as the Industrial Revolution, as the production of chemicals has shifted to larger scales (Greatorex, 1985). However, the focus on material recovery has been treated as a secondary feature rather than a primary objective. The primary goals of any chemical company have always been to obtain a product with high yield and purity with minimal expenditure. Many small companies did not have a proper solvent recovery system because the production process was too expensive, complex, and required a workforce to maintain and operate (Greatorex, 1985). Until the mid-1970s, contaminated solvents were treated as liquid waste and then discarded in the ground or water sources because the solvents could evaporate over time, leaving behind dissolved contaminants.
The practice of burying hazardous waste has severely harmed the surrounding land and water because the waste containments are susceptible to corrosions, causing leaks over time (Cheremisinoff, 1997; Kjeldsen et al., 2002). Dumping hazardous waste in the local water source also creates an issue involving the accumulation of toxic pollutants. One of the most notable events that sparked the creation of the US Environmental Protection Agency was the incident involving the Cuyahoga River fire in 1969 (Rotman, 2019; US EPA, 2021). A spark from a train track ignited debris on the surface of the Cuyahoga River, propagating the flames along the surface of the polluted river. This event increased public awareness and flaws regarding the viability of the existing waste management and eventually led to the creation of the US EPA.
Additionally, the continuous growth of the economy and population led to an increase in production scale to meet demand, leading to a rise in waste generation. The need to develop mitigation plans becomes paramount as a result. In 1965, the chemical industry in the US produced over four million tons of chemicals. The boost in production resulted in the generation and release of many toxic by-products, which were unregulated. By 1973, the total amount of unregulated solid was 144 million tons (Andersen, 1978). Due to the unrestrictive disposal, severe health and environmental issues were associated with water and air pollution. For example, the 30 inches of rain in early 1978 resulted in the overflow of the Stringfellow Acid Pits, culminating in the release of over a million gallons of contaminated water into the Glen Avon community (Butler and Fukurai, 1988; USEPA, 2021). Table 1 summarizes the various legislations that supported solvent recovery, the agencies that enacted them, and their major features.
Table 1.
Legislations that gave recognition to hazardous waste and promoted solvent recovery
| Legislation/Act/Policy | Agency | Features |
|---|---|---|
| Environmental Action Program (1973) | European Community (EC) | Established the first environmental framework by focusing on finding solutions to waste, toxicity, and non-biodegradability issues |
| Polluter Pays Principle (1973) | European Community (EC) | Charged polluters with clean-up costs and also advocated for greener production processes |
| The Framework Directives on Waste (1975) | European Community (EC) | Defined “waste” and “disposal” and proposed various disposal methods. It also advocated for the recovery and recycling of chemical waste by outlining the basic disposal requirements needed to protect human health and the environment |
| The Resource Conservation and Recovery Act (1976) | United States Congress | Mandated the US EPA to promulgate regulations controlling mainly hazardous solid waste from “cradle to grave.” |
| Environmental Action Program (1977) | European Community (EC) | This legislation was enacted to reduce the quality of non-recoverable waste, promote recovery, recycling, and reuse of waste, and proper management practices for the disposal of non-recoverable waste |
| Hazardous and Solid Waste Amendment (1984) | United States Congress | Banned the disposal of hazardous liquid waste via landfills, provided the guidelines to disposal facilities on how to handle hazardous liquid waste |
| Environmental Protection Law (1989) | National People’s Congress, China | Enacted to provide environmental standards, monitoring, planning, pollutant discharge, and pollution control. It further provided the administrative, criminal and civil liabilities for the infringement of the environmental laws |
| The Waste Framework Directive (2008) | European Union (EU) | Laid down basic waste management principles. Presented a 5-step hierarchical benchmark for waste management, namely, prevention, reuse, recycling, recovery, and disposal |
| Environmental Protection Law (2015) | National People’s Congress, China | Sets forth a stringent legal framework, stresses the need for scientific and technological advancement to solve environmental issues |
Resource Conservation and Recovery Act (RCRA)
In late 1976, US Congress passed environmental legislation called the Resource Conservation and Recovery Act (RCRA) (Andersen, 1978; US EPA and OLEM, 2015). The act was established to ensure economically sound and environmentally safe disposal of hazardous waste, primarily solids. The scope of this act ensured that these hazardous wastes are regulated from cradle to grave. The US EPA established benchmarks for generators, transporters, and disposers of hazardous waste through RCRA. However, the initial administration and implementation of RCRA had its challenges. For example, while US EPA mandated the individual states to establish their hazardous waste program to complement RCRA, there was inadequate knowledge of hazardous waste sites’ numbers, location, and cleanup costs across the nation (Andersen, 1978; Phifer, 2010).
The 1976 RCRA became a source of hope to citizens and institutional bodies such as the American Chemical Society (ACS), advocating for mitigation plans to dispose of hazardous solid wastes safely. However, this hope was short-lived. The vast “secured” landfills that were supposed to contain solid waste began to leach toxic substances into the surrounding groundwater (Phifer, 2010). Furthermore, the ACS indicated that wastes generated by laboratories were of varying characteristics and predominantly liquid. These wastes were not well captured in the RCRA. As a result, there was an amendment in the RCRA act in 1984 known as the Hazardous and Solid Waste Amendment (HSWA) (Epley, 1987; US EPA, 2013). This amendment paved the way for the proper regulation of liquid hazardous waste. For waste generators, the HSWA meant direct disposal to landfills sites was not cost-effective as there was an immediate ban on releases into these sites. Therefore, incineration and other recovery methods for the solvent and its reuse became the most economical way to handle solvent-containing liquid waste (Long and Schweitzer, 1982; Opp, 2012; Patterson, 1989; Spaanstra, 1986). The treatment of chemical waste before disposal resulted in solvent recovery gaining popularity among chemical industries.
European Union (E.U.) hazardous waste legislation
Various legislations have been enacted by the European Community (EC) to help mitigate hazardous waste accumulation (Simonsson, 1994). However, during the 1970s, there was a surge in enacting these legislations (European Environment Agency, 2007) due to public awareness of the negative environmental impacts due to growth in industrialization (Rootes, 2003). Furthermore, the geographic proximity of countries meant each member state was affected directly by the environmental practices of the other. For example, the pollution of the river Rhine in Switzerland directly affected the Netherlands downstream, leading to the harmonization of the independent member-state legislation (Bernauer, 1995; Pieter et al., 2000).
The Environmental Action Program (EAP) in 1973 was the first framework established by the EC to help address environmental issues (Simonsson, 1994). This legislation aimed to find solutions to eliminate waste accumulation problems within the EC. The first EAP was followed by the second in 1977. While the first EAP was mainly used to establish the need for the harmonization of environmental policies by the participating countries, the second EAP was specifically focused on three main objectives, namely: (1) the prevention and reduction of quality non-recoverable waste, (2) the recycling, recovery, and reuse of waste for raw materials and energy, and (3) proper management and disposal of non-recoverable waste. The recovery of waste for reuse objective was one of the pillars that helped industries rethink their waste disposal methods by resorting to more environmentally friendly options (Barnes, 2007; Ryan, 2007; Wilkinson, 1997).
Other legislation, such as the 1973 Polluter Pays Principle (PPP), encouraged proper waste disposal. The 1975 Framework Directives on Waste (FDW) was the first hazardous waste-related legislation passed by the EC (Simonsson, 1994). The 1983 disappearance of a shipment of barrels containing waste dioxins transported from Italy triggered the Transfrontier Movement of Hazardous Waste (TMHW). This event presented a benchmark for the shipment of waste across the EC. However, the need to recover hazardous waste for reuse was highly encouraged due to the dangers associated with transportation. The Waste Framework Directive (WFD) is the current management principle being implemented by the European Union (EU) (European Union, 2011, 2018, 2019; European Environment Agency, 2017). This directive strongly encourages the recovery of hazardous waste solvents for reuse.
Environmental protection policies by China
Economic growth had been the main emphasis for the Chinese government. However, this increased growth directly scales with the burdens of environmental issues. Environmental protection was incorporated in the constitution when it was revised in 1978, leading to the enactment of the Environmental Protection Law (EPL) (China Congress, 1979). This law helped regulate pollution by making a general provision for handling industrial waste (Beyer, 2006; Zhang and Wen, 2008). However, there was no stringent adherence to the enacted policies due to the liberality in its implementation. In addition, specific details of the various ways to handle waste were not clearly indicated and defined within the law; in fact, some referred to it as a “trial and error” framework (Pang, 2020; Zhang et al., 2016). Therefore, the law had a major revision in 1989, which provided environmental standards, monitoring, planning, and pollutant discharge declaration and registration for industries (Zhang and Wen, 2008). Thus, these reforms helped improve the scope of the EPL. However, some of the provisions within the law indicated that industries could still dispose of hazardous chemicals into the ecosystem as long as they paid the associated fees (Zhang and Wen, 2008). Therefore, solvent recovery was still not promoted within the law reformations. The implementation of a revised EPL in 2015 has raised the awareness of the detrimental effects of the irresponsible disposal of hazardous waste into the environment (Beyer, 2006; Zhang et al, 2015, 2016; Zhang and Wen, 2008). The amended EPL is more stringent on the release of waste into the environment and hence has strong support for solvent recovery.
Environmental policies by other countries
The Australian Standard 1940 (AS1940) plays a critical role by guiding the safe handling and storage of flammable and combustible liquids. This standard helps industries enact good industrial practices that minimize the risks associated with solvent wastes handling by promoting solvent recovery (Australia Standards, 2004; WHS Act, 2018). The Canadian Environmental Protection Act (CEPA) enacted in 1999 is the legislation that helps regulate the management of hazardous waste recycling and disposal. The CEPA sets criteria and standards to assess environmentally friendly ways of hazardous waste materials. It promotes the export and import of hazardous waste based on set guidelines (Canada, 2021;CEPA, 1999). Rapid industrialization in Japan during the 1960s and 70s resulted in high economic growth and, consequently, the generation of hazardous wastes such as organic solvents. To curb the growing concerns posed by these wastes, the Japanese government amended the Waste Management and Public Cleansing Act in 1970, which offered comprehensive steps needed to safely dispose of wastes (Ministry of Environment, 2014). In addition, Japan currently has a waste management act that advocates for resource utilization, promoting solvent recovery by industries (Ministry of Environment, 2014).
Evolution of solvent recovery technologies
As seen in the previous section, there is tremendous emphasis on chemical recovery and minimizing hazardous waste disposal in most countries worldwide. However, it is essential to devise efficient recovery methods to ensure that these practices are implemented to their fullest potential. To this end, we present an overview of solvent recovery practices.
Conventional solvent recovery practices
Between the late 1930s and the early 1970s, solvent recovery was practiced on smaller scales to accomplish the objective of removing contaminants from chemical solvents (Greatorex, 1985). The drive to advance solvent recovery, however, was heavily focused on economic profit. Distillation has, historically, been the most commonly used technique because of its ability to separate components from a fluid mixture at a wide range of flow rates, regardless of the initial concentration, and with high purity (Smith and Jobson, 2000). Flash, steam, fractional, extractive, and azeotropic distillation were the most common distillation types to remove contaminants from solvent waste (Cargua-Sagbay et al., 2020; Geankoplis, 2003; Huang et al., 2010; Kiss et al., 2014). Additional processing steps such as carbon adsorption have been reported to improve the final appearance of the solvent. Flash distillation is a single-stage process that partially vaporizes the liquid feed under vacuum or atmospheric pressure in a column, creating two phases in thermodynamic equilibrium (Cargua-Sagbay et al., 2020). Steam distillation does not require vacuum-like flash distillation. However, this method subjects the solvent waste to high temperatures, which may cause the impurities and non-volatile substances to react, decompose or change the quality of the distilled product. Water is mixed with the organic solvent waste and heated to a boil, creating a vapor mixture of water and organic solvent. The gaseous phase substance is condensed into liquid and separated from water in the downstream process (Rostagno and Prado, 2013). Fractional distillation is used to separate multiple volatile components from a waste mixture if azeotropes are not present, i.e., components do not possess similar boiling points. The liquid waste feed is heated to a high temperature and fed into the fractionation column. Volatile components travel toward the top of the column and condense at different locations in the column, based on their boiling points (Geankoplis, 2003; Towler and Sinnott, 2012). Extractive distillation separates close boiling components and azeotropes by introducing a new relatively non-volatile component to serve as an entrainer. The newly added component does not form an azeotrope with any other substance in the mixture, allowing easier separation between the components in the original mixture (Kiss et al., 2014). Azeotropic distillation also introduces an entrainer. However, this component can form new azeotropes with other components from the mixture of interest. The newly formed azeotropes can be separated in another distillation column and recovered for reuse (Huang et al., 2010). The distillation process requires high energy usage despite the separation capabilities, which greatly affects the operation costs. Alternative options to the conventional solvent recovery methods are later discussed in Emerging trends in designing solvent recovery section.
Solvent selection and its influence on recovery
Solvent recovery technology selection is a function of the solvent choices selected for use during the process. The resulting waste stream establishes the properties of the final streams, altering the recovery methods required to recover valuable materials. Poor solvent selection may lead to low production yield, difficulty in separation, and excess material consumption. Many companies such as AstraZeneca, Pfizer, GSK, and Sanofi have taken the necessary steps to publish their guides on solvent selection (Alder et al., 2016; Byrne et al., 2016; Henderson et al., 2011). The solvent selection guides were later enhanced to consider safety, health, environmental impacts (SHE), and process requirements to ensure that green chemistry is incorporated at every design stage. The latest solvent selection guide has been designed with inspiration from other companies, including a database of 272 known, new, and green solvents typically used in processes. Solvents were grouped and differentiated based on chemical functionality, categorized in different solvent classes such as acid, alcohol, alkene, ester, hydrocarbon, amine, and aromatics (Diorazio et al., 2016). In addition, seven SHE categories from AstraZeneca: health, air impact, water impact, life cycle analysis, flammability, static potential, and VOC (volatile organic carbon) potentials, were included. Some solvents may take on the characteristics of one or more classes because of the functional groups present, enhancing the solubility of the desired solutes. Classifying the solvent system during the design phase or recovery phase provides a better understanding of the physical properties and chemical interactions that may cause a change in density, affinity toward a specific substance, or solution stability. Solvents can thus be analyzed and compared based on their physical and chemical properties, safety, health, and environmental impacts to suit the process needs (Diorazio et al., 2016).
When designing a solvent-intensive process, it is crucial to consider the factors listed in the solvent selection guide to ensure that the process objective can be achieved efficiently and not adversely impact the environment. The GlaxoSmithKline (GSK) solvent selection guide, in particular, provides scoring assessments based on available data for a selected list of solvents on incineration, recycling, biotreatment, VOC emissions, aquatic impact, air impact, health hazard, exposure potential, flammability & explosion, reactivity, and life cycle analysis. Each category is assigned a score of 1 (least green) to 10 (most green) with a unique criteria. For instance, the VOC emission score is determined according to vapor pressure and risk of spillage and loss during storage, transport, and waste management of a specific solvent. Solvents with a low boiling point are rated lower due to the increased volatility (Alder et al., 2016). High vapor pressure of individual solvents also negatively impacts other factors such as biotreatment, VOC emissions, air, exposure potential, flammability, and explosion. These factors are categorized into waste management, environment, health, and safety (Henderson et al., 2011). For a given solvent, scores are determined through various evaluation metrics recommended by the GSK solvent selection guide. The individual scores are combined into a geometric mean, resulting in a composite (overall) score for waste, environment, health, and safety categories.
Other groups such as the American Chemical Society Green Chemistry Institute Pharmaceutical Roundtable (ACS GCI PR) contributed a solvent selection tool that considers the properties of individual solvents and identifies a shortlist of solvents appropriate to a process needs (ACS Green Chemistry Institute Pharmaceutical Roundtable, 2019). Slater and Savelski developed a solvent selection table in collaboration with Bristol-Myers Squibb and the US Environmental Protection Agency to compare green solvents and process routes based on 12 environmental parameters (Slater and Savelski, 2007). The Innovative Medicines Initiatives (IMI)-CHEM21 contributed a solvent selection tool that analyzes and ranks classical solvents used within the pharmaceutical industry. Unlike the previous tools, the CHEM21 selection guide is not generalized for all applications by default. Additional criteria and solvent lists are needed for use in other applications (Prat et al., 2016).
A solvent selection guide should be considered in the design stage to improve the recyclability of a chemical solvent. Table 2 provides the recommended criteria to improve the process greenness. However, it is not necessary to satisfy all criteria to ensure recyclability. The solvent waste categories present the largest impact on the ease of recycling in the solvent end-of-life phase. The chosen solvent should ideally have low miscibility with water, low vapor pressure, high boiling point, and no reactivity with other substances in the process. This solvent can easily be separated from a mixture through conventional separation techniques. These considerations ultimately provide the ease of separation and purification. For instance, in the design phase, a process engineer may choose a multi-component system that possesses significantly different boiling points and no azeotropes over the solvents with close boiling points and azeotropes intentionally. The solvent selection guide generally favored low boiling point solvents in the recycling score because it correlates with a lower vapor pressure at the given operating temperature than solvents with a higher boiling point (Alder et al., 2016). Consideration for environmental and human health hazards can also influence the outcome of the process by allowing the process engineer to account for the potential releases and implement the necessary mitigation strategies.
Table 2.
A summary of solvent selection criteria required to maximize solvent recycling potentials and minimize impacts on the environment, health, and safety (Alder et al., 2016; Henderson et al., 2011; Jiménez-González et al., 2002)
| Categories | Criteria |
|---|---|
| Waste | Low water miscibility and can be separated from water easily |
| Easily separable from a mixture of multiple solvents | |
| Low vapor pressure and high boiling point | |
| Environment | Low photochemical ozone creation potential (POCP), odor score (high vapor pressure → low score) |
| Low toxicity (acute and chronic) toward the environment and aquatic species can biodegrade | |
| Health | Low carcinogenic, mutagenic effects, and not considered harmful to reproductive health Solvents exposure level falls within the occupational exposure limits (OEL) |
| Safety | Low flammability and explosivity (based on boiling point, flash point, and auto ignition temperature) |
| Low to no intrinsic reactivity of solvents (self-reaction, thermal decomposition, reaction with acidic or basic reagents, forms peroxide over time) |
Isoni et al. (2016) created an LCA-based methodology called Q-SA√ESS to further assist in decision-making in the manufacturing stage of a chemical process by using a cradle-to-grave analysis. The general hierarchy of green processes begins with using no solvent as the most green, followed by water, renewable solvent, and petroleum-based solvents as the least green option. Q-SA√ESS method used metrics such as carbon footprint, acidification potential, eutrophication, human toxicity, the total energy used per batch, and the product obtained per batch to evaluate the safety, health, and environmental impacts of the organic solvents used (Isoni et al., 2016). These categories were scored according to the ACS GCI Pharmaceutical roundtable solvent selection guide (American Chemical Society, 2011). Trade-off relationships between social, environmental, and economic factors were observed, creating complexity in decision making. Although social and environmental impacts are important to consider, economic profit possesses the largest influence on the final decision of a company (Isoni et al., 2016). Therefore, an acceptable balance between social, environmental, and economic factors should be decided at the process design stage to ensure efficient recovery.
Judicious solvent selection in the initial design stage served as a preventive strategy, while recovery processes exist to minimize process waste that were generated as a result of the existing chemical processes. Depending on the process, a combination of dissolved solids, suspended solids, chemical impurities, and multiple solvent components could be present as multiple phases in the waste stream. Therefore, solvent recovery processes should be designed according to the components and properties of the waste stream unique to the process. Selecting the most appropriate separation and purification technologies to recover valuable solvent at a desirable purity level remains the largest obstacle to date.
Solvent recovery in practice
Earlier solvent recovery works stressed the development of separation and purification technologies for solvent recovery and their implementation. For example, Blaney (1986) proposed various technologies that could be used to treat hazardous waste solvents. Technologies such as sedimentation, filtration, centrifugation, flotation, and evaporation were proposed. Lau and Koenig (2001) also indicated that applying solvent recovery techniques during machine cleaning, dry cleaning, and screen cleaning can help reduce the cost of industrial processes by using a case study associated with Chemical Waste Treatment Center (CWTC) in Hong Kong. However, recovery costs became predominant as industries sought a way to cut down production costs.
Recently, there have been efforts with developing emerging technologies, including sustainability indicators, and applying computational tools and optimization methods due to technological advancements. For example, García et al. (2013) presented an extensive study on the recovery of organic waste solvents using pervaporation technology. They studied an aqueous solvent mixture consisting of n-butanol, dichloromethane, and sodium chloride and observed a 100% rejection rate of sodium chloride when a pervaporation unit with a hydrophobic membrane is used followed by a hydrophilic membrane. Therefore, the permeate from the first stage consisting of n-butanol (50–90 %wt), dichloromethane (5–45 %wt), and water (5–20 %wt) served as the feed to the second pervaporation unit. The resulting permeate consisted of 97.6 %wt of water and 2.4 %wt of n-butanol with 100% dichloromethane retained by the membrane. Pervaporation, therefore, has a higher capability of recovering waste solvents from wastewater streams and thus should be one of the key technologies industries should consider as a recovery option due to the greenness of the process. Raymond et al. (2010) presented a life cycle assessment approach to pharmaceutical waste solvent treatment. In their work, they estimated the life cycle inventories using SimaPro® and EcoSolvent software. They also presented a comparison of off-site disposal (base case) with on-site incineration (with energy recovery) and solvent recovery based on three case studies and observed that life cycle assessment of solvent recovery should be done from a cradle-to-grave perspective rather than gate-to-gate. Chea et al. (2020) developed a framework for waste solvent recovery using a superstructure-based optimization approach (Chea et al., 2020). They evaluated the techno-economic feasibility of the framework by using two case studies of varying complexities and formulated their recovery framework model as a mixed-integer non-linear programming problem (MINLP). Their first case study, which entailed the recovery of isopropanol (IPA) from an IPA/water mixture, resulted in an optimal cost of 0.14 $/kg of IPA processed using a pervaporation-ultrafiltration pathway. Their second case study comprising a waste stream of 21.3% dimethoxyethane (DME), 35.3% water, 41.3% toluene, and 1.3% 1-ethoxy-1-methoxy ethane (EME) resulted in a recovery cost of 4.12 $/kg of processed feed. Solvent recovery proves to be economical at higher flow rates of solvent-containing waste streams. Their work presents a paradigm shift from a hierarchical to superstructure-based optimization approach to solvent recovery.
Ooi et al. (2019) proposed a Computer-Aided Molecular Design (CAMD) framework that simultaneously factors solute extraction and solvent recovery. They aim to design solvents that can be recovered with low economics, environmental impacts, and health hazard. Their framework can systematically predict, estimate, and design solvents in separation processes by analyzing their molecular properties. Their approach follows similar techniques to Chea et al. (2020), which screens for existing separation technologies, determines the best recovery pathway combinations, identifies crucial parameters, and determines costs. However, this framework extended beyond cost and targeted safety and health criteria. An objective function was formulated, which considers weighting factors for the multiple objectives. The authors concluded that by selecting solvent recovery methods with consideration for its intended application, process performance and overall cost savings could be improved (Ooi et al., 2019). Wang and Lakerveld presented a systematic approach to optimize continuous crystallization process conditions, solvent selection, and recycling in pharmaceutical applications. The Perturbed-Chain Statistical Associating Fluid Theory (PC-SAFT) method of continuous mapping was used to simplify the optimization from a mixed-integer non-linear programming problem (MINLP) to a non-linear programming problem (NLP). The PC-SAFT identifies thermodynamic parameters and can estimate the effects of interaction parameters for unique solvents with limited data. The crystallization process considered multiple solvent options before choosing the ideal solvent or antisolvent. Based on the selected solvent, the process conditions were modified to reflect the optimization result. The case study presented provided three different anti-solvent optimized processes and demonstrated the PC-SAFT’s ability to reasonably predict the solubility and vapor-vapor equilibrium of the system (Wang and Lakerveld, 2018). Table 3 summarizes some previous work done in the field of solvent recovery and the limitations associated with each work. The limitations of the various works suggest that a systems-level approach to solvent recovery presents a better and holistic methodology where there is a convolution of all prior techniques implemented.
Table 3.
Previous studies on solvent recovery with features and limitations
| Reference | Features | Limitations |
|---|---|---|
| Lau and Koenig (2001) | Evaluated the economic feasibility of the solvent recycling process. Presented some industrial activities from which solvent usage can be minimized | No comparison of alternate technologies for the recycling process. Analysis based on mass balance, no energy balance |
| Capello et al. (2005) | Presented a statistical analysis of estimating life cycle data inventory associated with the separation of waste solvents via distillation. | No sensitivity analysis using the estimated parameters for the LCI |
| Raymond et al. (2010) | Demonstrated the need to perform a life cycle assessment on pharmaceutical solvents. They further applied solvent recovery to API manufacturing by considering the entire supply chain of the process. | Case-specific studies. No process design of the solvent recovery options and alternatives |
| Slater et al., (2012a) | Coupled distillation with pervaporation and demonstrated that over 92% of emissions associated with the solvent recovery and incineration can be reduced when recovering isopropyl alcohol (IPA) from water | Process and solvent specific. Only binary mixture was considered; no multi-component analysis |
| Slater et al., (2012b) | Coupled a constant volume distillation with pervaporation and demonstrated the recovery of tetrahydrofuran (THF) from water, as compared to azeotropic distillation. | Alternate technologies should have been considered aside from pervaporation and distillation |
| Cavanagh et al. (2014) | Developed a software toolbox to assess binary solvent recoverability from both the economic and environmental perspectives | Only distillation and pervaporation technologies were considered. Only binary solvents were considered; no multi-component solvents |
| Chaniago et al. (2015) | Implemented the box and quadratic programming approach to minimize the energy required for the distillation-based solvent recovery process in the semiconductor industry. About 40% of energy savings can be made based on the developed energy-efficient distillation system as compared to conventional sequences | No comparison with other distillation configurations and not alternate technologies. Distillation is an energy-intensive process. No LCA analysis |
| Wang and Lakerveld (2018) | Proposed a methodology for solvent selection and recycling for crystallization. This was achieved by transforming an MINLP problem into an NLP using PC-SAFT methodology | No economic and sustainability assessment of the process |
| Ooi et al. (2019) | Proposes a CAMD approach for the selection of solvents with higher recoverability properties. Focus on the Safety, Health, and Environmental (SHE) impact of the solvent generated | Only energy balance is incorporated in the CAMD approach. |
| Chea et al. (2020) | Generated a generic superstructure for solvent recovery and implemented an MINLP approach to minimize the cost associated with the process. | Case studies were specific. No LCA or sustainability assessment |
Emerging trends in designing solvent recovery
Solvent waste processing
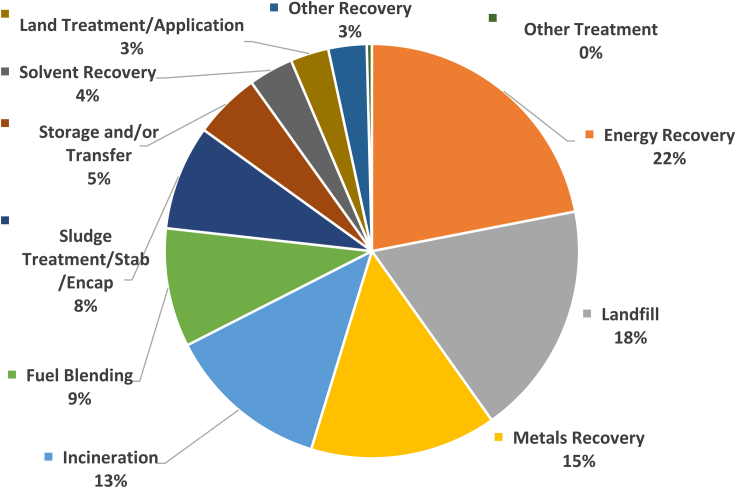
According to the US EPA, energy recovery proves to be the most typical form of hazardous waste management (22.0%) in 2019, followed by landfills, contributing 18.3%. Solvent Recovery ranks eighth with a percentage contribution of 3.5%. Figure 1 suggests that minimum efforts are directed toward the implementation of solvent recovery by the chemical industries. Ever since the enactment of major legislation by the US, EU, China, and other countries, industries, and academia have dedicated considerable research efforts to find alternative treatment methods (Blaney, 1986; Phifer, 2010; US EPA and OLEM, 2015). Recent advances in hazardous waste disposal indicate a gradual transition from conventional treatment techniques such as deep injection wells and incineration to modern recovery and reuse methods.
Figure 1.
Hazardous management techniques
Top 12 hazardous waste management techniques in 2019 (total tons managed: 6,613,468) (EPA, 2019a, 2019b).
Membrane separation technologies began to find usage in scientific research and various industries in the 1970s (Saleh and Gupta, 2016; Xiang et al., 2020). This method has been implemented primarily in wastewater treatment and desalination to remove solutes and produce high-purity water. Membrane separation may use pressure as a driving force to separate the component of interest through either polymeric or ceramic membranes as they permeate. Other components that do not diffuse through the membrane exit as the retentate. Microfiltration, ultrafiltration, nanofiltration, and reverse osmosis are prime examples of pressure-driven membrane separation. Alternatively, membrane separation may also be osmotically driven, which uses osmotic pressure as the driving force. Forward osmosis and pressure-retarded osmosis are typically used to perform osmotically-driven membrane processes (Goh et al., 2020).
Despite the popularities in water-based applications, organic solvent-resistant membranes are an emerging option for separating organic solvents (Van der Bruggen, 2009). Compared to distillation, the relatively low energy consumption allows membranes to serve as an alternative option in solvent recovery. Liquid–liquid extraction is also gaining importance by proving its viability as an alternative to distillation and various usages in bio-based product applications. In some cases, the components in chemical solvent waste may be heat-sensitive and become susceptible to creating unwanted by-products. The presence of azeotropes and a similar boiling-point mixture may also deter distillation and encourage liquid-liquid extraction as a separation option. The chemical solvent of interest may have its impurities be extracted using another immiscible solvent with a strong affinity for the impurities. First, the two immiscible solvents are mixed to provide sufficient contact time between the impurities and the extracting solvent. Then, the two phases are allowed to separate and be collected according to density difference. Multiple extraction cycles may be used with fresh extracting solvent to reduce the impurity levels after the initial extraction (Geankoplis, 2003; Green and Perry, 2019).
Table 4 displays the alternative strategies that can be used to recover chemical solvents and the advantages and disadvantages of selecting each method. In deciding the most optimal solvent recovery technique for a waste mixture, the limitation of the solvent feed system should be identified. For instance, ethanol and water are known to form an azeotrope with each other (Peng et al., 2017). Therefore, distillation is a less favorable approach over other techniques that can break the azeotropes with minimal energy cost.
Table 4.
Conventional solvent recovery technologies, driving forces, important specifications, and key advantages and disadvantages
| Technology | Principle/driving force | Specifications and important conditions | Advantages | Disadvantages | Literature sources |
|---|---|---|---|---|---|
| Physical separation | |||||
| Precipitation | Charge solubility | Antisolvent, supersaturation, temperature, pH change | Low cost, selective removal possible, high yield, can remove dissolved solids | Impurities, coprecipitates | (Green and Perry, 2019; Harvey, 2019; Mersmann and Kind, 1988; Wu et al., 2019) |
| Sedimentation or decantation | Density gradient, Settling velocity | Size, density, tank depth, residence time | Effective at removing dense particles, cheap to implement | Require large space, must be designed based on maximum volume, cannot remove dissolved solids | (Belter et al., 1988; Green and Perry, 2019; Kwok-Keung and LeChevallier, 2013) |
| Centrifugation | Settling velocity Centrifugal force | Size, density, angular speed, the ratio of centrifugal to gravitational force, and settling distance | Effective at removing low-density and colloidal particles in a shorter time frame than sedimentation | Energy-intensive, cannot remove dissolved solids, generates high heat, and poses a safety hazard when processing volatile solvents | (Agena et al., 1998; Ambler, 1961; Green and Perry, 2019; Price, 1970; Taulbee and Mercedes Maroto-Valer, 2000) |
| High-temperature separation | |||||
| Distillation | Relative volatility | Relative volatility >1.05 Heat of vaporization and energy requirements | Designed for a large variety of flow rates, it can separate a homogeneous fluid mixture | Energy-intensive, difficult to separate azeotropes unless a modification is made | (Diwekar, 2011; Górak and Sorensen, 2014; Green and Perry, 2019; Smith and Jobson, 2000; Towler and Sinnott, 2012) |
| Membrane processes | |||||
| Membranes | Particle/molecular size/permeability Sorption/Diffusion Pressure |
Pore size, Mol. wt. cut-off, average flux, Pressure gradient, type of membranes – M.F., U.F., NF, and R.O. | Lower energy requirement than distillation, highly selective with products, break azeotropes | Fouling, cannot operate at high temperature, may not be compatible with all solvents | (Green and Perry, 2019; Ho and Sirkar, 1992; Lewis, 1996; van Reis and Zydney, 2007; Xiang et al., 2020) |
| Pervaporation | Sorption/Diffusion Partial pressure | The heat of vaporization, chemical potential gradient, pressure gradient, average flux, membrane selectivity | Can break azeotropes, separate close-boiling point mixture, lower energy requirement than distillation, | Low-permeate flow rate, reduced membrane stability | (Green and Perry, 2019; Luis, 2018; Shao and Kumar, 2011; Slater et al., 2012b; Zarzo, 2018) |
| Liquid–liquid extraction | |||||
| Liquid–liquid extraction | Selective partitioning of solutes | Partition coefficient, the solubility of solutes, low solubility of the added solvent in water | Extracts dissolved solids from solvents, high selectivity, separates azeotrope mixture, does not require high temperature | Solvent-intensive, requires, limited by solubility | (Belter et al., 1988; Birajdar et al., 2014; Green and Perry, 2019; Kennedy and Cabral, 1993; Seader et al., 2010; Towler and Sinnott, 2012; Wu and Tu, 2016) |
| Aqueous two-phase extraction | Partitioning of solute, bioselectivity | Solubility, the composition of two phases, molecular weight | Highly practical with separating bioproducts | Macromolecule partition differently than smaller molecules | (Asenjo and Andrews, 2012; Benavides et al., 2011; Johansson et al., 1998; Sikdar et al., 1991; Wu et al., 2011) |
[Recreated with permission from Chea et al. (2020).Copyright 2021American Chemical Society]
Process intensification
Solvent recovery technology selection does not necessarily need to adhere to conventional methods. The theory of process intensification aims to optimize the existing processes by condensing multiple methods into fewer units or steps without sacrificing the efficiencies or changing the driving forces. Effects such as a reduced equipment size, energy consumption per product mass, and by-product formation may be achieved (Stankiewicz and Moulijn, 2000). There is no single definition that can fully describe process intensification. However, the general principle holds that the intensified process should maximize the effectiveness of molecular events, provide a similar processing experience for all molecules, optimize the driving forces and the associated surface area of contact, and maximize the synergistic effects between the combined processes. There should be no extra chemicals, solvents, or equipment used in the new process. The overall size of the process would ideally be reduced due to combining multiple functions (Dimian et al., 2014; Sitter et al., 2019; Van Gerven and Stankiewicz, 2009). Process synthesis can therefore be improved using process intensification as long as the requirements and limitations are specified.
Process intensification can be performed with interest to the spatial, thermodynamic, functional, and temporal domains. The spatial domain aims to create a structure that would minimize randomness in a process. Thus, a controlled process can be directed to reach the desired outcome more consistently. The thermodynamic domain aims to optimize the transfer of energy at various stages to minimize energy dissipation and waste. The functional domain seeks to synergize the traits from different processes into one unit. Yadav et al. designed an intensified process to extract algae oil and convert the biomass to biodiesel using CO2 and methanol. This intensification was done by premixing the algae extract stream with methanol solvent before sending the material in for transesterification. Supercritical CO2 was added (Yadav et al., 2021). The temporal domain modifies the timescale of the process to create the possibility of obtaining the product at a smaller timescale. One instance of timescale manipulation occurs when a continuously stirred tank is fed periodic feed, creating a state of oscillating liquid volume and changing the mixing characteristic similar to a plug-flow reactor. The conversion of a batch to a continuous process may also be treated as a case of timescale manipulation (Van Gerven and Stankiewicz, 2009).
The approaches to process intensification can be applied to all design scales. In engineering designs, multi-functional reactors, hybrid separators, alternative energy sources, and specially designed equipment have been introduced. Specifically, with solvent recovery, separators such as dividing-wall column, membrane distillation, pervaporation, membrane adsorption, adsorptive distillation, and liquid membrane can be viable choices (Boi et al., 2020; Chang, 2020; Dejanović et al., 2010; González et al., 2017; León and Fontalvo, 2018; Luis, 2018; Megawati et al., 2017; Shao and Kumar, 2011; Slater et al., 2012b). A dividing wall column is an alternative to conventional distillation that uses a longitudinal partition wall to separate multiple components in one unit. This method provides a considerable advantage over traditional distillation column-in series and in parallel because it requires less energy and space to operate. An existing distillation column can be retrofitted to include a dividing wall to reduce up to 20–50% operation and capital cost (DWC Innovations, 2020) while achieving multi-component separations (Dejanović et al., 2010). Membrane distillation combines the separation functions of reverse osmosis and evaporation into one unit by using a porous membrane to transfer volatile components in the liquid feed to the permeate side as vapor, followed by condensation into the liquid phase. This method has effectively rejected 100% ions, macromolecules, colloids, cells, and other non-volatile substances using temperature as the driving force. The operating temperature and pressure of membrane distillation remain lower than conventional membrane and distillation processes, creating a safer environment for heat-sensitive materials. Pervaporation combines the idea of permselective and evaporation to separate the component of interest based on its permeability through the membrane. The entering liquid feed enters the pervaporation unit and comes in contact with a dense membrane. The vacuum is pulled on the permeate side to serve as a driving force for separation. Materials permeate through the membrane in the vapor phase, which later gets condensed into a liquid. This method is less energy-intensive than conventional distillation and can break azeotropes and separate components with similar boiling points (Chea et al., 2020; Luis, 2018). Membrane adsorption uses a polymeric membrane to allow specific substances to selectively adsorb onto the surface through functional groups present on the membrane. Sorbent may be incorporated as part of the membrane to enhance the adsorption capability. This method has been used to remove contaminants from drinking water (Khulbe and Matsuura, 2018). Adsorptive distillation adds selective adsorbents into the distillation feed to remove impurities, azeotropes, and components with similar relative volatilities (Stankiewicz and Moulijn, 2000). Chang (2020) discusses the use of green solvents in extraction and liquid membrane. Liquid membrane (LM) is an emerging technology promoting solute removal and solvent extraction into one unit. The use of LM can reduce the energy requirement and provide non-equilibrium mass transfer and greater solute diffusion coefficients than solid membranes. The solute transport in LM is governed by solution-diffusion. Although promising at the lab scale, LM has not been used in large-scale applications due to poor membrane stability. The performance of using green organic solvents, conventional organic solvents, and a mixture of the two were compared. While green organic solvent can help achieve similar efficiency as conventional solvents, this cost may present the largest barrier for using a green organic solvent. Food security may also be affected because they are derived from agricultural food commodities extracted from plants (palm oil, soybean oil, sunflower oil, coconut oil, etc.) (Chang, 2020). The temperature-swing molecular imprinting technology designed by Voros et al. (2019) can extract bio-based compounds and recover solvents at over 97% efficiency (Voros et al., 2019). In reaction applications, Kisszekelyi et al. (2019) implemented a synthesis and separation hybrid using a flow reactor with an in-line membrane separation unit to recover catalysts and solvents. Their reaction process achieved up to 95% yield while recovering 100% of the catalysts and 50% of the solvents. Challenges involving products precipitating during membrane separation were reduced by adding heat, followed by a subsequent crystallization in the collection vessel to further purify the products (Kisszekelyi et al., 2019). The examples shown merely represent a small pool of the intensified solvent recovery processes that have been implemented.
Selecting the most viable process intensification option for optimizing solvent recovery processes may be as complex as designing a new solvent recovery system because of the lack of precedent, data, simulations, and safety concerns on the proposed process (Sitter et al., 2019). Lutze et al. have created a framework for minimizing the feasible process intensification search space based on a six-step method (Lutze et al., 2010). This framework requires that the designer (1) defines the objectives, process scenario, and constraints, (2) collects information about the process and identifies limitations, (3) creates mathematical models to describe the process, (4) synthesizes a superstructure that encompasses all of the possible intensified processes and incorporates logical constraints and binary variables, (5) uses shortcut methods or semi-rigorous simulations to eliminate infeasible options, and (6) performs a multi-objective optimization on the feasible intensified process methods. The selected intensified process may be validated through experiments (Lutze et al., 2010). It should be noted that intensifying a process does not need to satisfy every criterion in the existing definition. However, the process viability, sustainability, and net profit can be expected to shift greatly toward the favorable outcome with each successful process intensification.
Solvent recovery is one of the alternative approaches to improving the sustainability and greenness of industrial processes. However, the design of an effective recovery process requires a systems-level approach. In the subsequent sections, we discuss some of the key elements that can help improve the integration of solvent recovery into industrial processes.
Solvent recovery process synthesis
The problem formulation step first defines the objective functions for solvent recovery, aiming to maximize material recovery while minimizing both process costs and environmental impacts (Chea et al., 2020). This step identifies the solvent waste streams and process constraints to establish the feed stream characteristics and limitations. Feed stream information such as solvent identity and composition, possible azeotropes, impurity types, and quantities proves valuable in the superstructure generation stage. The limitations identified may include temperature limits to avoid degradations, equipment types available for usage, and the minimum purity and recovery requirement. Process synthesis typically employs two methods involving (1) sequential methods and (2) superstructure-based optimization.
Sequential method
The sequential method relies on using past engineering designs and decisions to generate a process flow sheet. The primary process is designed one at a time to achieve a specific goal irrespective of the decisions made in the previous stages (Douglas, 1988). Some prominent examples may include reactor networks, gas cleaning systems, and heat recovery networks (Henao and Maravelias, 2011; Towler and Sinnott, 2012). The existing systems and networks serve as a general starting point and can be modified to fit the process needs. Many unique combinations of process units may also be possible because process design is partially dependent on the creativity of the engineers. However, such an approach can be time-consuming because alternative methods are not being compared simultaneously.
Superstructure optimization approach
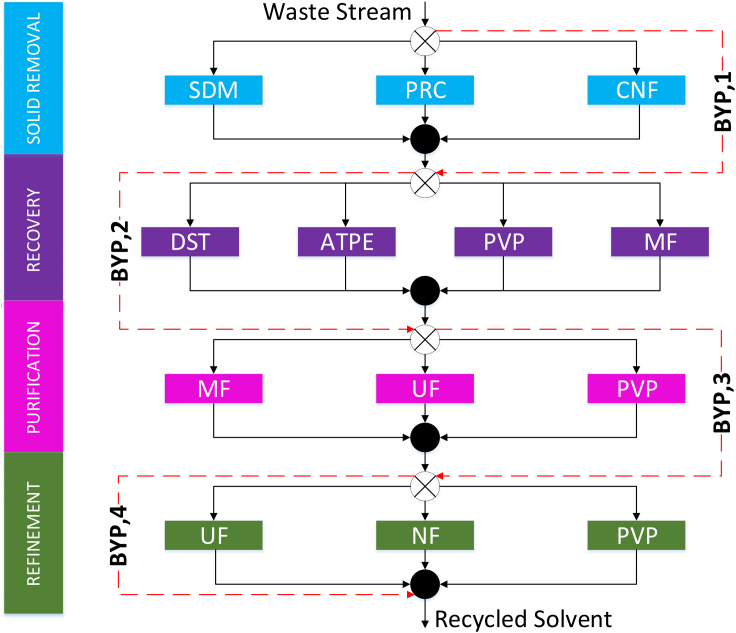
A superstructure-based optimization approach is a powerful alternative method that has been used in process synthesis because it considers all of the possibilities to perform the desired process and connects the techniques between the stages (Chea et al., 2020; Henao and Maravelias, 2011; Mencarelli et al., 2020; Wu et al., 2017; Yenkie et al., 2017; Yeomans and Grossmann, 1999). The superstructure-based optimization is formulated as mathematical equations, including constraints, logical constraints between units and stages, and process unit models (Henao and Maravelias, 2011). The generation of a superstructure begins with the problem formulation, followed by superstructure generation, and finding a solution to the optimization problem (Bertran et al., 2016). For example, Chea et al., (2020) have developed a generic superstructure-based solvent recovery framework that uses a stage-wise approach to separate and purify waste solvent from a chemical process. This solvent recovery framework is illustrated in Figure 2.
Figure 2.
Generic solvent recovery superstructure
A generic solvent recovery superstructure with considerations for sedimentation (SDM), precipitation (PRC), centrifugation (CNF), distillation (DST), aqueous two-phase extraction (ATPE), pervaporation (PVP), microfiltration (MF), ultrafiltration (UF), nanofiltration (NF).
[Reprinted (adapted) with permission from Chea et al. (2020). Copyright 2021 American Chemical Society]
By default, a superstructure approach considers the traditional and emerging technologies without imposing any special restrictions. Technology restrictions may be included as needed, based on the property of the waste solvent. For instance, distillation may be excluded from consideration because the waste solvent contains components with similar boiling points or form azeotropes.
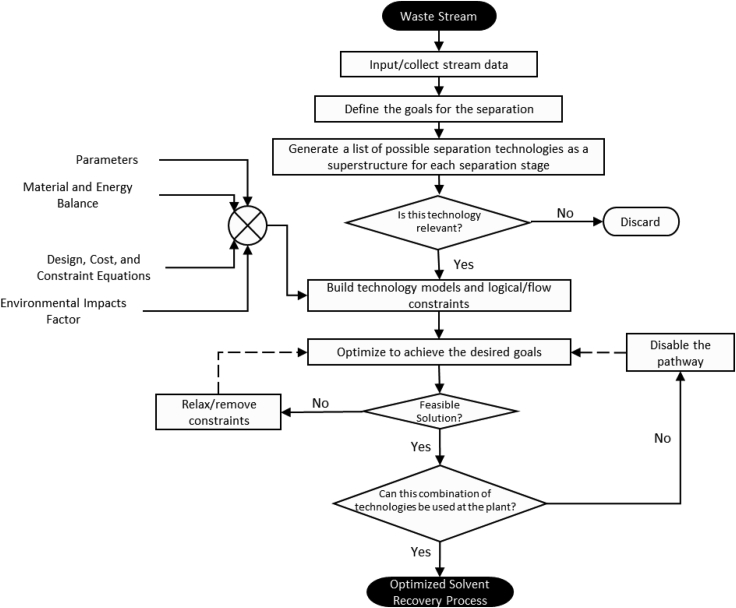
The superstructure generation method begins by identifying all of the possible technologies relevant to performing a specific task. The technologies may be grouped at various stages according to their characteristics. Given that solvent recovery may require multiple stages to be completed, the interconnections between the technologies at each stage should also be included to show the relationships and possible interactions with the previous stages. Each technology presented in the superstructure can be represented in the optimization model as mathematical equations that include designs, user-specified and typical constraints, costs, and environmental impacts. These calculations can be customized and incorporated into the model based on the interest. The superstructure-based optimization problem can be solved as either mixed-integer non-linear (MINLP) or linear programming (MILP) because the decisions to choose between alternative technologies for a given stage are represented by integer variables (Kılınç and Sahinidis, 2018). This method analyzes all non-constrained options simultaneously and selects the best combination of technologies that satisfy the objectives (Bertran et al., 2016; Chea et al., 2020; Mencarelli et al., 2020; Wu et al., 2017, 2017, 2017). The process design engineer may also choose to exclude technologies deemed infeasible or unavailable to reduce the number of calculations in the analysis. Figure 3 summarizes the solvent recovery superstructure methodology and the necessary steps required to acquire the most feasible design. This method provides a degree of flexibility by allowing simultaneous comparison of potential options with reasonable approximation at the screening level stage. Detailed design pathways can be compiled as a shorter list based on the constraints set forth by the designer.
Figure 3.
Flowchart for implementing superstructure optimization approach
The recommended flow path for optimizing solvent recovery processes using the superstructure approach. The diamond-shaped decision box is implemented via binary (1-yes/0-no) selection variables, which then enables the logical flow constraints for active/inactive technologies and their corresponding stream flows.
Economic analysis
Economic assessment is a crucial component of any design process to ensure viability. Such estimation should be performed in the early stages of design because there is greater flexibility in changing the initial concept over a process that is already in operation. However, retrofitting an existing process to implement solvent recovery is also a feasible approach. Both cases ultimately determine the cost of installing, maintaining, and operating a solvent recovery system. If the projected cost and payback period of solvent recovery for a given waste stream is deemed unfavorable, further optimization can be performed by comparing alternative options based on the initial assessment. In some instances, the capital cost required to implement a solvent recovery system may be too high due to low waste volume. Savelski et al. (2017) identified this challenge and completed a relevant case study on the economic and environmental feasibility of solvent recovery at an active pharmaceutical ingredient production facility. They concluded that the recovery of solvent from one instance of waste would net negative cost. However, a flexible recovery design that can combine low-volume waste streams into larger quantities can lead to more favorable results. Their life cycle impact assessment also demonstrated that impacts on human health, ecosystems, and resources are unavoidable but can be drastically reduced by recovering the materials and reducing the need to create manufacture new raw materials (Savelski et al., 2017). Chea et al. (2020)expanded upon the work by creating a generic and systematic approach to determine the economic feasibility of solvent recovery methods at a large scale. They presented two test cases of solvent waste processing from different sectors in the chemical industry (pharmaceutical and specialty chemicals) at the screening phase before design. The screening phase examines many options and narrows down the possibilities to the most favorable candidates for further analyses. The outcome of the economic analysis of the selected solvent recovery processes generally favors recycling instead of using waste incineration and purchasing fresh solvents. The recovered materials can also reduce process waste and unwanted chemical releases, reducing the E factor of the process and environmental impacts (Chea et al., 2020).
The cost estimation of solvent recovery processes can be approached using multiple methods. The quickest way of plant cost assessment within ±25% accuracy can be made by scaling the capital cost of a process with the capacity (Douglas, 1988). The capacity of the unit can be calculated according to chemical engineering principles, design equations, and common constraints. Equation 1 describes the cost scaling method, where Ci represents the estimated capital cost based on the new capacity, Qi, while C0 and Qo represent the standard cost and capacity, respectively. The exponent n is the scaling factor that can vary depending on the type of process. However, in the chemical industry, n = 0.6 is considered an average (Towler and Sinnott, 2012). This exponent is always less than 1.0 because larger equipment generally costs less at a specific capacity than smaller equipment.
| (Equation 1) |
Standard cost and capacity data are not always available, especially if the process is novel. A step count method has been reported, which can allow an order-of-magnitude cost estimate of the overall process by correlating the total capital cost to the number of functional units with considerable costs in a plant N, total plant capacity Q, and the reactor conversion rate s of product per mass fed to the unit. The reactor conversion rate can be treated as the expected recovery fraction in estimating the solvent recovery process sequence. Equations 2 and 3 describe the correlation, with 60,000 metric tons/yr as the threshold (Green and Perry, 2019; Towler and Sinnott, 2012).
| (Equation 2) |
| (Equation 3) |
The cost scale and the step count methods should be used to provide screening level estimation for determining the process viability. The uncertainty of the estimated capital cost can be further reduced by creating a list of required parts and equipment, considering material types, the fabrication process, and the labor required. A direct vendor price is also a valid option because it provides the actual cost tailored toward a specific process condition and purpose of the equipment. The installation cost of solvent recovery unit conservatively can be estimated as 100% of the capital cost (Cavanagh et al., 2014).
In addition to the capital cost of the equipment, variable costs, fixed costs, revenues, and profits are the remaining essential components to the total process cost. Variable costs are defined as costs that scale according to the plant output and operation, including raw materials, utilities, consumables, disposals, packaging, and shipping. The fixed costs, including labor, overhead, maintenance, taxes, insurance, and rent, do not change with the plant output and operation. Labor cost can be considered a variable cost if it is treated to be scalable to the working capacity of a processing unit (Chea et al., 2020). Equation 4 displays a method to approximate the number of laborers required to operate a technology under a different capacity. The standard number of labors (Nlabor,std) corresponds to the standard capacity (Qstd) and vice versa for the scaled number of labors (Nlabor) and new capacity (Qnew). The overhead cost in solvent recovery processes primarily consider research and development and general administrative costs. Research and development involving material recovery may account for up to 15% of the total process revenue, while the general administrative cost may be assumed to be a minimum of 65% of the calculated labor cost (Towler and Sinnott, 2012).
| (Equation 4) |
The process revenues are typically contributed by the main products and by-products (Towler and Sinnott, 2012). However, process waste may contain valuable materials (e.g., solvents) that can be recovered, provided that the substance meets the minimum standards for reuse. This standard may vary between different sectors. For instance, the pharmaceutical industry may require a more stringent requirement for solvent reuse than the paint industry (Chea et al., 2020). Nevertheless, recovered solvents have values and can improve the overall process viability. This value is a function of the solvent purity level obtained through the separation and purification techniques used.
Raw materials are defined as substances used in the process, which may include solvents. Consumables include materials that require regular replacement, including membranes, acids, bases, and adsorbents. Solvents are typically treated as a consumable. However, in solvent recovery processes, solvents can be treated as raw materials that can be recovered in large quantities. The price of raw material and consumables can be calculated by multiplying the price ($/mass unit) by the input rate. The largest uncertainty with raw material pricing originates from fluctuating prices over time, constantly altering the total process cost. The raw material price becomes higher than the final product in some instances, rendering the process infeasible and unnecessary (Sepiacci and Manca, 2015). Although assuming an average price is possible, this approach does not fully represent the market’s volatility. Future trends must be evaluated based on the price history of the material of interest through a deterministic and stochastic approach (Rasello and Manca, 2014). This approach is shown in Equation 5, where is the price of the material at time i, is the material price in the previous time step, is the standard deviation of the price, is the average price, and RAND is a random function that produces a set of values within a normal distribution.
| (Equation 5) |
Utilities include fuel, steam, cooling water, electricity, and other gas required to maintain operation. Likewise, with other variable costs, utility cost is subjected to price volatility, which requires a similar stochastic approach to estimating the cost of the materials (Weron, 2014). Utility pricing can be estimated using statistical forecasting methods such as interval, density, threshold, and point. Statistical forecasting considers the random nature of the price possibilities and suggests a prediction interval with a calculated probability. Interval forecast uses probabilistic intervals to account for the possibility of price fluctuation in the future. The forecasted value of the utility price falls within an interval with a specific probability. Density forecast predicts utility pricing through a probability integral transform (PIT). The densities of utility prices can be generated and evaluated for quality by calculating the average Continuous Ranked Probability Scores (CRPS). A threshold forecast can determine a critical price point that no longer yields a profitable process. The accuracy of the utility price through threshold forecast is less important than determining a specific price threshold that cannot be exceeded. Point forecast predicts the utility price at a given time using the average of the forecasted price (Clements, 2005; Hyndman and Athanasopoulos, 2018; Weron, 2014).
Waste disposal costs are considered when materials produced from a given process cannot be recycled or sold. Chemical processes and solvent recovery, in general, do not have 100% efficiency and thus generates waste. However, organic chemical solvents contain a large amount of stored energy within the chemical bonds, thus reducing energy costs. By knowing the heat of combustion, the waste solvent value ($/mass unit) can be approximated using Equation 6, where is the heat of combustion and PF is the price of fuel ($/energy unit) (Towler and Sinnott, 2012).
| (Equation 6) |
The evaluation of fixed and variable costs is the bare minimum calculation required to estimate the preliminary cost of the solvent recovery process. However, other techniques such as cost-volume-profit, break-even, and cash flow analysis may be incorporated to acquire more information regarding process viability (Arnaboldi et al., 2015). Short-term cost projection can be determined through cost-volume-profit analysis by analyzing the relationship between the output and the changes in revenues, process cost, and profit. In solvent recovery, the revenue generated may include recovered solvents sold to other industries. Other sources of revenue are expected to come from the main process. The process cost accounts for the variable and fixed costs to operate the process. Profit may be determined by the amount of solvent sold to other industries and money saved by reusing the recovered solvents. The economic viability with solvent recovery should occur where the recovered solvent can offset new solvent purchases.
Energy-efficient ways for solvent recovery
Energy usage is one of the paramount factors to consider in the design of solvent recovery systems. Higher energy consumption by the recovery process tends to reduce the attractiveness of its implementation by industries. In addition, energy from non-renewable sources tends to increase the overall carbon footprint of the process. Thus, there is the need to improve the energy demand of the process to make it economically and environmentally viable for implementation. Earlier implementation of the solvent recovery process by Case and Toy (1987) indicated an energy-efficient, activated carbon stripping system to recover 2721 kg/h of toluene-naphtha-lactane solvent mixture. They showed that preheating the water before being used for steam generation by the boiler unit improved the energy demand of the recovery process by reducing the amount of steam required. Recent works predominantly explore the use of distillation columns for the recovery of used industrial solvents. There has been tremendous research conducted in separation processes using distillation to improve the energy efficiency of distillation processes. Chaniago et al. (2015) proposed an enhanced distillation system for the recovery of a waste solvent mixture comprising isopropanolamine, water, monoisopropanolamine, methyl diglycol, n-methylformamide, 1-poperazineethanol, photoresist in the semiconductor industry. They observed that by thermally coupling the distillation columns in sequence and implementing a heat pump, about 40% energy savings are made compared to conventional methods. They presented further advanced combinations of the distillation units in sequence, which achieved the required outlet solvent specifications at a reduced reboiler heat duty.
The use of Switchable Hydrophilicity Solvents (SHSs) for industrial processes has begun to gain attention in the past decade (Han et al., 2020; Jessop et al, 2005, 2012). The idea with SHSs is to “switch” the hydrophobicity and hydrophilicity of solvents in the presence of water and CO2 (Han et al., 2020). These unique physicochemical properties of certain nitrogenous organic solvents present a new frontier for distillation-free solvent utilization and recovery processes. Thus with increased pressure on distillation, which is an energy- and cost-intensive process, the use of SHSs presents an energy-efficient way of designing solvent recovery processes. Expanding the research to find non-nitrogenous organic solvents that exhibit this “switchable” property should be vital to improving solvent-based processes and recoveries.
Membrane processes also present cheaper alternatives to energy-intensive technologies. For example, White and Nitsch (2000) presented a solvent recovery of lube oil filtrates using a polyimide membrane, which was later commercialized. Given that fewer boiling processes are associated with the implementation of membrane processes, they should be preferred to distillation in terms of energy efficiency.
Environmental impact assessments
Evaluating the greenness of the solvent recovery process helps in its comparison with other conventional disposal methods. Quantitative analysis helps with the comparative assessment of the various techniques. The disposal of waste solvents contributes greatly to the release of greenhouse gases and other emissions. Incineration, the conventional waste solvent disposal technique, tends to increase the overall carbon footprint, increasing the pressure on governmental bodies to enact legislation seeking to address sustainability issues. Implementing solvent recovery into industrial processes can offer significant benefits to both the industry and the environment. Solvent recovery benefits include increasing compliance with environmental regulations, reducing fresh solvent purchases, transportations, and human exposure.
Life Cycle Impact Assessments (LCIA) offer a unique perspective into a company’s carbon footprint by analyzing all the emissions along the entire supply chain or process. The International Standard Organization (ISO) has standardized documentation that depicts the development and interpretation of LCIA. In analyzing the LCA of solvent recovery, the human health, climate change, environmental, and resource utilization impacts must be evaluated. One crucial aspect of L.C.A. is estimating the Life Cycle Inventory (LCI) for the chemical or process in question (Cseri et al., 2018; Savelski et al., 2017; Slater et al., 2010b). Software package such as SimaPro® has an extensive database that helps to quantify the LCIs of processes (Goedkoop et al., 2016). Slater et al. (2012a) presented an LCA of solvent recovery alternatives to minimize and recover isopropanol (IPA) for a pharmaceutical process of celecoxib production using SimaPro®. They examined various combinations of pervaporation and distillation units for the recovery process. The use of distillation followed by incineration yielded the highest emissions of 12,287 kg-CO2/batch, while distillation followed by pervaporation with the sale of concentrated mother liquor resulted in emissions of ∼837 kg-CO2/batch. The use of their approach presents essential insights into the techno-sustainability assessment of the recovery process. However, their methodology is more case-specific to IPA process rather than it being generic. Further, the consideration of technologies alternative to distillation was not examined. In addition, the membrane area for the pervaporation (PV) should have been varied to determine the optimal surface that maximizes the recovery process.Slater et al. (2012b) showed an LCA of the recovery of tetrahydrofuran (THF) using a pervaporation unit coupled with a constant-volume distillation (CVD) technology. Their methodology implements the use of SimaPro® to generate LCIs, while EcoSolvent® was used for the waste disposal analysis. The work indicated that over 93.8% of total emissions could be prevented if the CVD-PV combination was preferred to only CVD. The key to achieving this reduction percentage is the optimization of the membrane area needed for the pervaporation technology.
There has been recent works that present solvent recovery from the broader sustainability perspective. For example, Voros et al. (2019) introduced a temperature-swing molecular imprinting technology to extract bio-based compounds with in-line nanofiltration to purify and recycle solvents. They used the E factor and carbon footprint as their sustainability analysis indices and reported a reduction of 61% in the E factor of the process and a 49% reduction in carbon footprints (Voros et al., 2019). Kisszekelyi et al. (2019) implemented a synthesis and separation hybrid using a flow reactor with an in-line membrane separation unit to recover catalysts and solvents in reaction applications. Their reaction process achieved up to 95% yield while recovering 100% of the catalysts and 50% of the solvents. Challenges involving products precipitating during membrane separation were reduced by adding heat, followed by a subsequent crystallization in the collection vessel to further purify the products (Kisszekelyi et al., 2019).
The ecological footprint is another sustainability methodology incorporated in the superstructure approach to solvent recovery (Singh et al., 2009). Ecological footprints map the impact of the entire process on the land area. The use of the land area is very beneficial concerning interpreting the results of the process. The main advantage of having an ecological footprint is quantifying the environmental burden and pressure associated with the process. Most ecological footprints consider the air, water, and soil emissions related to the process. Therefore, most ecological assessments inherently incorporate human health impacts. The Sustainable Process Index (SPI) developed by Narodoslawsky and Krotscheck has been quite extensively to measure the ecological performance of the industrial processes (Krotscheck and Narodoslawsky, 1996; Narodoslawsky and Krotscheck, 1995). It utilizes the idea of the dual nature of the earth as a solar energy recipient and production factor to measure the impact of the processes and maps it to arable land area. SPI presents an integral and systematic approach, where all mass and energy flows are accounted for and converted to land equivalents. The five main areas associated with SPI are the area needed for (1) raw material, (2) energy consumption, (3) staff accommodation, (4) installations, and (5) infrastructure, and to embed air, water, and soil emissions sustainably into the ecosystem. Thus, SPI helps tackle the insufficient interdependencies and relations between society and nature by presenting the effects of anthropogenic activities on the ecosystem(Narodoslawsky, 2015; Narodoslawsky and Krotscheck, 2004). Figure 4 shows the essential components of the SPI.
Figure 4.
Components that entail the Sustainable Process Index (SPI)
Illustration of sustainable process index showing what entails the input and output areas. The raw material area considers the environmental pressure exerted by provision of renewable and non-renewable resources for the process. For processes that are labor intensive, provision is made for staff accommodation in the evaluation process. The infrastructure considers the direct and indirect installations of building and apparatus. The output area is usually the decisive factor in the SPI analysis besides the raw material area.
The use of ecological indicators presents a better way of relating the environment to human activities. However, these indicators are insufficient and ineffective at estimating the energy demand of processes. With energy consumption being one of the main driving forces in implementing solvent recovery processes, there is a need to incorporate such indicators. Measuring the sustainability of energy sources is often complicated, and the decision lies mainly within a qualitative assessment of the various energy sources (Brown and Ulgiati, 2002). Emergy, a quantitative technique developed by Odum in 1988, has gained tremendous popularity over the years (Odum, 1988; Odum and Odum, 2000; Ulgiati et al., 1994). Emergy is the amount of available energy of one kind used on transformation processes to make a product. In terms of entropy, Emergy can be considered as a measure of the produced entropy along the entire supply chain of the entire process. The integration of Emergy helps in estimating the actual energy demand of the process. It further identifies various energy hotspots within the process, which is less visible in an ecological footprint. Therefore, incorporating both ecological and energy indicators is essential as each addresses specific problems within their jurisdiction and presents a better way of improving the greenness of the process.
The Techno-Ecological Synergy (TES) methodology developed by Bakshi et al is a framework that quantitatively assesses the interdependencies of the technological and ecological systems to determine the extent of ecological overshoot from specific human activities. Most eco-efficient methodologies consider the demand for ecosystem services but completely ignore how the carrying capacity of the ecosystems can supply the required services. Furthermore, the demand for the services does not consider the spatial distribution of the requested services. The implementation of the TES methodology helps in addressing these issues. Thus, their work presents a mutualistic benefit from both environmental and economic perspectives and can be integrated into the design of solvent recovery processes (Bakshi et al., 2015).
The Greenhouse gases, Regulated Emissions, and Energy use in Technologies (GREET) model developed by Argonne National Laboratory is also a good source of LCI data (Argonne GREET Model, 2021;Dang et al., 2014; Quinn et al., 2014). The GREET model is mainly geared toward the life cycle analysis of transportation fuels and vehicle technologies in the transportation sector. However, the model can be used to estimate LCI data for renewable energy processes (Huo et al., 2009). The Reducing Embodied-Energy and Decreasing Emissions (REMADE) calculator is another tool that can be used to estimate the emissions due to the recovery process. The REMADE-calculator is used in the plastics industry to estimate the embodied-energy and CO2-equivalent emissions of the recycling process. However, this calculator can easily be transformed and used in the area of solvent recovery (REMADE Institute, 2020). Another prominent sustainability assessment tool is GREENSCOPE (Gauging Reaction Effectiveness for Environmental Sustainability of Chemistries with a multi-Objective Process Evaluator). The US EPA developed this process evaluation tool to determine the environmental impacts, accounting for human toxicity, terrestrial toxicity, acidification, photochemical oxidation, global warming, and ozone depletion. Close to 140 sustainability indicators relating to environmental, economic, efficiency, and energy can be calculated if process data, such as equipment, operating conditions, streams, utilities, and material properties, are available. The result of each indicator is normalized into a sustainability score between 0 (worst) to 100% (best), based on the boundary limits. A generic process may possess indicators in the profile described in Figure 5. Weaknesses within the process can be identified using GREENSCOPE. Modifications to the process can be made to perform tradeoffs that raise the sustainability score of a specific indicator (Ruiz-Mercado et al., 2012a, 2012b, 2013). Areas needed for improvement can thus be identified, and modifications to the process can be made. For instance, the process data may indicate too much heat loss in one part of a distillation column. Extra expenses can be made to provide the necessary insulation and minimize energy waste. GREENSCOPE can thus prove to be a valuable tool to analyze solvent recovery process design because it provides a visual representation of the four major aspects of sustainability in a process (economics, environmental, efficiency, and energy), allowing the user to target specific areas with low sustainability scores.
Figure 5.
Generic GREEENSCOPE analysis of a process
Generic GREENSCOPE analysis of a process, with the blue line representing the sustainability score in various categories (E11–E34).
[Recreated from Gonzalez et al., 2011 and Ruiz-Mercado et al. (2013). Copyright 2013American Chemical Society]
Knowing both the economic and environmental impacts of the designed process can help determine the process viability and identify areas that may require modification or upgrades. Table 5 summarizes the methodologies and tools that can be used for an effective design of solvent recovery processes. It should be noted that process modification and the upgrade do not necessarily limit to only using higher quality material, better insulation, and process control. The recovery process may include elements of process intensification to provide the benefits that individual units cannot fully provide.
Table 5.
Summary of tools and techniques that can be implemented for solvent recovery
| Methodology/Tool | Advantages | Deficiencies | Reference |
|---|---|---|---|
| Tools | |||
| GREET | Able to capture the emissions associated with the transportation system used by industry. Able to compare detailed alternate renewable sources of energy | Only geared toward LCIs for vehicular and fuel combinations | (Argonne GREET Model, 2021) |
| GREENSCOPE | Able to evaluate process sustainability in four main areas namely: material efficiency, energy, economics, and environment. Can be applied at both unit and system level | Over 139 indicators. Need expert interpretation of results for potential applications | (Ruiz-Mercado et al., 2012a, 2012b, 2013) |
| SimaPro | Used to estimate the life cycle inventories and impact assessment of processes. Helps in comparative assessment of alternate processes. Captures detailed health, climate, and resource impacts | Lack of detailed ecological analysis of the process | (Goedkoop et al., 2016) |
| REMADE | Simple and easy to use. Able to evaluate the impact change of the recycling process | Designed for energy and CO2-equivalent evaluation in plastics recycling | (REMADE Institute, 2020) |
| Methodology | |||
| Life Cycle Assessment (LCA) | Estimates the emissions associated with processes | Data gathering is tedious. Focuses only on emissions | (ISO 14040, 1997; ISO 14044, 2006) |
| Sustainable Process Index (SPI) | Estimates the ecological burden of processes. Detailed environmental cost of the process. Social impacts of processes are inherent during the estimation process | Not able to capture detailed energy demand of the process | (Krotscheck and Narodoslawsky, 1996; Narodoslawsky and Krotscheck, 1995) |
| Emergy | Presents an approach to estimating the available energy needed for the process. Helps in the determination of the economic pressure associated with imported resources for a process | Lack of detailed models for transformity estimation. Data collection and handling are laborious | (Odum, 1988; Odum and Odum, 2000; Ulgiati et al., 1994) |
| Techno-Ecological Synergy (TES) | Presents an approach to estimate the ecological overshoot of the process and the interdependencies of human activities and nature. Considers spatial distribution of ecosystem resources | Unable to encapsulate the dynamics of the ecological system | (Bakshi et al., 2015) |
Quality by design and control
Another approach to designing efficient recovery systems is the concept of Quality by Design and Control (QbD&C). The application of QbD&C principles to solvent recovery can be focused on building quality into the method during the development phase, as opposed to testing methods for quality after development. Quality is related to customer satisfaction regarding a product, process, and service (Juran et al., 1974; Juran and Godfrey, 1999; Sangshetti et al., 2017). The “Juran Trilogy” proposed by J.M. Juran has transformed the design of processes from the aspect of quality over the years. He indicated that the underlying concept for quality consists of three essential elements: quality planning, quality control, and quality improvement (Juran, 1986). Thus, incorporating solvent recovery in the design framework improves the quality of the process. Quality by Design (QbD) and Quality by Control (QbC) have gained tremendous acceptance by policy-makers. Developed by a quality expert, Juran, he argues that quality should be designed into a product. This principle has been used to advance the process and product quality of industries. Major environmental issues such as emissions associated with conventional waste solvent treatment such as deep injection wells and incineration can be prevented if solvent recovery is included at the onset of industrial process design. Not only does solvent recovery improves the quality of the environment, but it also improves the quality of the entire process. With QbD&C incorporated, method-specific risk assessment, control strategies, and validations can be strategized to improve the optimal performance, reliability, and robustness of industrial processes and hence improve solvent recovery.
Machine learning/artificial intelligence
Machine Learning (ML) and Artificial Intelligence (AI) has gained tremendous importance and applications in the chemical industries (Boobier et al., 2020; Dimiduk et al., 2018; Ge et al., 2017; Liu et al., 2017; Shang and You, 2019) They are emerging fields that can transform solvent and technology selection. In recent times, ML algorithms have been used to predict the physicochemical properties of organic solvents. An example is the prediction of the solubility of organic solvents in water presented by Boobier et al. (2020). In their work, they coupled ML algorithms such as artificial neural networks (ANN), support vector machine (SVM), and random forest (RF) with computational chemistry models. They observed that models such as multiple linear regression (MLR) and partial least square (PLS) poorly predicted the solubility of organic solvents such as benzene, acetone, and ethanol in water. This observation supports the notion that predicting the solubility of solvents is a complex process as numerous variables such as temperature, molar volume, enthalpies of fusion and vaporizations, must be considered for accurate predictions (Chen et al., 2002). Furthermore, it has been observed that based on the Hansen Solubility Parameter (HSP) theory, solvents can be designed by engineers to improve their recoverability (Seay, 2020). Thus, HSP theory is an area that ML can be applied extensively to develop novel benign solvents that are environmentally friendly and recyclable. Another area where ML and AI can be applied in selecting efficient technologies for the recovery process. The selection of membranes for processes such as ultra-, micro-, nano-filtrations, and reverse osmosis can be improved using ML and AI methodologies. This selection process can be observed in the work presented by Hu et al. (2021), where they implemented ANN, SVM, and RF algorithms to predict membrane permeance and rejection rates for nanofiltration technology. Similar works in the area of membranes have shown that the application of ML algorithms can predict optimal membrane properties and, hence, help in selecting efficient membrane technologies for solvent recovery (Fetanat et al., 2021; Goebel and Skiborowski, 2020; Liu et al., 2020). The implementation of ML algorithms can also be used to select energy-efficient technologies during the design phase of the recovery process (Narciso and Martins, 2020). Furthermore, ML can assist in the comparative assessment and selection of renewable energy sources to reduce the recovery process's emissions (Shahab and Singh, 2019).
Concluding remarks
Implementing solvent recovery presents an opportunity to reduce manufacturing costs, improve the greenness of the process, and reduce the emissions and environmental impacts associated with industrial processes. With increasing stringent governmental regulations for hazardous waste disposal, solvent recovery offers industries a greener alternative to meet these legislations. A superstructure-based approach for designing solvent recovery systems presents a decisive advantage over the sequential methods because it can simultaneously analyze multiple options. The interconnections between the separation and purification technologies are preserved, while sequential design targets the specific needs using conventional schemes and one-at-a-time analysis. The sequential method may not always reach the global optimum, leaving room for improvement. Sustainability metrics can serve as an indicator for assessing the chosen process viability. In cases when improvements are needed, there are opportunities to optimize recovery processes further. For instance, direct process modification can solve the specific limitations in the process by targeting the areas of interest and crucial bottlenecks. Alternatively, process intensification aims to combine the functionality of two or more separation technologies by reducing the equipment sizes without drastically changing the process characteristics, which can help reduce the fixed costs and land footprint. The overall benefits of solvent recovery highlight the need for a paradigm shift from traditional waste handling methods to environmentally friendly and green alternatives. Energy resource utilization are always immensely at the forefront of industrial decisions. The emergence of switchable hydrophilicity solvents could improve greatly the architecture of energy demands for solvent recovery processes. The rise in data generation at a geometric rate, indicates that AI/ML have become indispensable tools for industrial research. Advances in ML/AI can help with critical steps in addressing solvent recovery processes in industries.
Acknowledgments
This review is based upon work supported by the United States Environmental Protection Agency’s (USEPA) Pollution Prevention (P2) Program (NP96259218). The authors would like to thank the Rowan Chemical Engineering Department for the procurement of resources and maintenance of computational licenses required for this work. The authors would also like to acknowledge the undergraduate students Jake Stengel, Austin Lehr, Kayla Heider, Michael Mackley, and James Geier from the Sustainable Design and Systems Medicine Lab for their contributions to this work.
Declaration of interests
The authors declare no competing interests.
References
- ACS Green Chemistry Institute Pharmaceutical Roundtable . ACS Green Chemistry Institute Pharmaceutical Roundtable; 2019. Tools for Innovation in Chemistry.https://www.acsgcipr.org/tools-for-innovation-in-chemistry/ [Google Scholar]
- Argonne GREET Model The greenhouse gases, regulated emissions, and energy use in technologies model. 2021. https://greet.es.anl.gov/
- Abejon R., Garea A., Irabien A. Organic solvent recovery and reuse in pharmaceutical purification processes by nanofiltration membrane cascades. Chem. Eng. Trans. 2015;43:1057–1062. doi: 10.3303/CET1543177. [DOI] [Google Scholar]
- Agena S.M., Bogle I.D.L., Cornish A.R.H. Process synthesis for particle separations using centrifuges. Comput. Chem. Eng. 1998;22:351–356. doi: 10.1016/S0098-1354(97)00249-4. [DOI] [Google Scholar]
- Alder C.M., Hayler J.D., Henderson R.K., Redman A.M., Shukla L., Shuster L.E., Sneddon H.F. Updating and further expanding GSK’s solvent sustainability guide. Green. Chem. 2016;18:3879–3890. doi: 10.1039/C6GC00611F. [DOI] [Google Scholar]
- Ambler C.M. The fundamentals of separation, including sharples“Sigma value” for predicting equipment performance. Ind. Eng. Chem. Res. 1961;53:430–433. [Google Scholar]
- American Chemical Society . ACS Green Chemistry Institute; 2011. ACS GCI Pharmaceutical Roundtable Solvent Selection Guide. [Google Scholar]
- Andersen R.W. TheResourceConservationandRecoveryAct of 1976: closingthegap. Wisconsin Law Rev. 1978;83:633. [Google Scholar]
- Arnaboldi M., Azzone G., Giorgino M. Performance Measurement and Management for Engineers. Elsevier; 2015. Long- and short-term decision making; pp. 107–115. [DOI] [Google Scholar]
- Asenjo J.A., Andrews B.A. Aqueous two-phase systems for protein separation: phase separation and applications. J. Chromatogr. A. 2012;1238:1–10. doi: 10.1016/j.chroma.2012.03.049. [DOI] [PubMed] [Google Scholar]
- Australia Standards . Standards Australia International; 2004. The Storage and Handling of Flammable and Combustible Liquids. [Google Scholar]
- Bakshi B.R., Ziv G., Lepech M.D. Techno-ecological SYNERGY: aframework for sustainable engineering. Environ. Sci. Technol. 2015;49:1752–1760. doi: 10.1021/es5041442. [DOI] [PubMed] [Google Scholar]
- Barnes P. Towards sustainability: implementing the European Community’s strategy for sustainable development. Eur. Environ. 2007;2:5–9. doi: 10.1002/eet.3320020604. [DOI] [Google Scholar]
- Belter P.A., Cussler E.L., Hu W.-S. John Wiley & Sons, Ltd; 1988. Bioseparations: Downstream Processing for Biotechnology, 1st ed. [Google Scholar]
- Benavides J., Rito-Palomares M., Asenjo J.A. Comprehensive Biotechnology. Elsevier; 2011. Aqueous two-phase systems; pp. 697–713. [DOI] [Google Scholar]
- Benvenutti, L., 2019. Which is the Best Food Emerging Solvent_ IL, DES or NADES? 14.
- Bernauer T. The international financing of environmental protection: lessons from efforts to protect the river Rhine against chloride pollution. Environ. Polit. 1995;4:369–390. doi: 10.1080/09644019508414212. [DOI] [Google Scholar]
- Bertran M.-O., Frauzem R., Zhang L., Gani R. Computer Aided Chemical Engineering. Elsevier; 2016. A generic methodology for superstructure optimization of different processing networks; pp. 685–690. [DOI] [Google Scholar]
- Beyer S. Environmental law and policy in the People’s Republic of China. Chin. J. Int. Law. 2006;5:185–211. doi: 10.1093/chinesejil/jmk002. [DOI] [Google Scholar]
- Birajdar S.D., Padmanabhan S., Rajagopalan S. Rapid solvent screening using thermodynamic models for recovery of 2,3-butanediol from fermentation by liquid–liquid extraction. J. Chem. Eng. Data. 2014;59:2456–2463. doi: 10.1021/je500196e. [DOI] [Google Scholar]
- Blaney B.L. Treatment technologies for hazardous wastes: part II alternative techniques for managing solvent wastes. J. Air Pollut.Control Assoc. 1986;36:275–285. doi: 10.1080/00022470.1986.10466070. [DOI] [Google Scholar]
- Boi C., Malavasi A., Carbonell R.G., Gilleskie G. A direct comparison between membrane adsorber and packed column chromatography performance. J. Chromatogr. A. 2020;1612:460629. doi: 10.1016/j.chroma.2019.460629. [DOI] [PubMed] [Google Scholar]
- Boobier S., Hose D.R.J., Blacker A.J., Nguyen B.N. Machine learning with physicochemical relationships: solubility prediction in organic solvents and water. Nat. Commun. 2020;11:5753. doi: 10.1038/s41467-020-19594-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breil C., Meullemiestre A., Vian M., Chemat F. Bio-based solvents for green extraction of lipids from oleaginous yeast biomass for sustainable aviation biofuel. Molecules. 2016;21:196. doi: 10.3390/molecules21020196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M.T., Herendeen R.A. Embodied energy analysis and EMERGY analysis: a comparative view. Ecol. Econ. 1996;19:219–235. doi: 10.1016/S0921-8009(96)00046-8. [DOI] [Google Scholar]
- Brown M.T., Ulgiati S. Emergy evaluations and environmental loading of electricity production systems. J. Clean. Prod. 2002;10:321–334. doi: 10.1016/S0959-6526(01)00043-9. [DOI] [Google Scholar]
- Van der Bruggen B. In: Membrane Operations. Drioli E., Giorno L., editors. Wiley-VCH Verlag GmbH & Co. KGaA; 2009. Fundamentals of membrane solvent separation and pervaporation; pp. 45–61. [DOI] [Google Scholar]
- Butler E.W., Fukurai H. Acid pits and birth defects: a case study of the Stringfellow acid pits dump site and congenital anomalies. Int. J. Environ. Stud. 1988;32:151–167. doi: 10.1080/00207238808710456. [DOI] [Google Scholar]
- Byrne F.P., Jin S., Paggiola G., Petchey T.H.M., Clark J.H., Farmer T.J., Hunt A.J., Robert McElroy C., Sherwood J. Tools and techniques for solvent selection: green solvent selection guides. Sustain. Chem. Process. 2016;4:1–24. doi: 10.1186/s40508-016-0051-z. [DOI] [Google Scholar]
- Canada E. Understanding the Canadian Environmental Protection Act. 2021. https://www.canada.ca/en/services/environment/pollution-waste-management/understanding-environmental-protection-act.html
- Capello C., Hellweg S., Badertscher B., Hungerbühler K. Life-cycle inventory of waste solvent distillation: statistical analysis of empirical data. Environ. Sci. Technol. 2005;39:5885–5892. doi: 10.1021/es048114o. [DOI] [PubMed] [Google Scholar]
- Cargua-Sagbay D., Palomo-Lema E., Camacho O., Alvarez H. Flash distillation control using a feasible operating region: asliding mode control approach. Ind. Eng. Chem. Res. 2020;59:2013–2024. doi: 10.1021/acs.iecr.9b05688. [DOI] [Google Scholar]
- Case M.D., Toy D.A. Chem. Process.; 1987. Energy Efficient Solvent Recovery System Nets Two Year Return on Investment. [Google Scholar]
- Cavanagh E.J., Savelski M.J., Slater C.S. Optimization of environmental impact reduction and economic feasibility of solvent waste recovery using a new software tool. Chem. Eng. Res. Des. 2014;92:1942–1954. doi: 10.1016/j.cherd.2014.02.022. [DOI] [Google Scholar]
- CEPA, 1999. The Canadian Environmental Protection Act, 1999.
- Chang S.H. Utilization of green organic solvents in solvent extraction and liquid membrane for sustainable wastewater treatment and resource recovery—a review. Environ. Sci. Pollut. Res. 2020;27:32371–32388. doi: 10.1007/s11356-020-09639-7. [DOI] [PubMed] [Google Scholar]
- Chaniago Y.D., Minh L.Q., Khan M.S., Koo K.-K., Bahadori A., Lee M. Optimal design of advanced distillation configuration for enhanced energy efficiency of waste solvent recovery process in semiconductor industry. Energy Convers.Manag. 2015;102:92–103. doi: 10.1016/j.enconman.2015.03.086. [DOI] [Google Scholar]
- Chea J.D., Lehr A.L., Stengel J.P., Savelski M.J., Slater C.S., Yenkie K.M. Evaluation of solvent recovery options for economic feasibility through a superstructure-based optimization framework. Ind. Eng. Chem. Res. 2020;59:5931–5944. doi: 10.1021/acs.iecr.9b06725. [DOI] [Google Scholar]
- Chen X., Cho S.J., Li Y., Venkatesh S. Prediction of aqueous solubility of organic compounds using a quantitative structure–property relationship. J. Pharm. Sci. 2002;91:1838–1852. doi: 10.1002/jps.10178. [DOI] [PubMed] [Google Scholar]
- Cheng J., Zhang C., Sun J., Qiu L. Assessing the sustainable abilities of a pilot hybrid solar-pyrolysis energy system using emergy synthesis. Int. J.Energy Res. 2020;44:2909–2924. doi: 10.1002/er.5110. [DOI] [Google Scholar]
- Cheremisinoff N.P. Groundwater Remediation and Treatment Technologies. Elsevier; 1997. Treating contaminated groundwater and leachate; pp. 259–308. [DOI] [Google Scholar]
- China Congress, S.C., 1979. Environmental Protection Law of the People’s Republic of China.
- Clements M. Evaluating Econometric Forecasts of Economic and Financial Variables. Palgrave Macmillan; 2005. Density forecasts. [Google Scholar]
- Climate Change Connection . Climate Change Connection; 2020. CO2 Equivalents.https://climatechangeconnection.org/emissions/co2-equivalents/ [Google Scholar]
- Constable D.J.C., Jimenez-Gonzalez C., Henderson R.K. Perspective on solvent use in the pharmaceutical industry. Org. Process. Res. Dev. 2007;11:133–137. doi: 10.1021/op060170h. [DOI] [Google Scholar]
- Cseri L., Razali M., Pogany P., Szekely G. Green Chemistry. Elsevier; 2018. Organic solvents in sustainable synthesis and engineering; pp. 513–553. [DOI] [Google Scholar]
- Dang Q., Yu C., Luo Z. Environmental life cycle assessment of bio-fuel production via fast pyrolysis of corn stover and hydroprocessing. Fuel. 2014;131:36–42. doi: 10.1016/j.fuel.2014.04.029. [DOI] [Google Scholar]
- Das A., Gupta A.K., Mazumder T.N. A comprehensive risk assessment framework for offsite transportation of inflammable hazardous waste. J. Hazard. Mater. 2012;227(228):88–96. doi: 10.1016/j.jhazmat.2012.05.014. [DOI] [PubMed] [Google Scholar]
- Dejanović I., Matijašević L.j., Olujić Ž. Dividing wall column—a breakthrough towards sustainable distilling. Chem. Eng. Process. Process Intensifi. 2010;49:559–580. doi: 10.1016/j.cep.2010.04.001. [DOI] [Google Scholar]
- Dimian A.C., Bildea C.S., Kiss A.A. Computer Aided Chemical Engineering. Elsevier; 2014. Process intensification; pp. 397–448. [DOI] [Google Scholar]
- Dimiduk D.M., Holm E.A., Niezgoda S.R. Perspectives on the impact of machine learning, deep learning, and artificial intelligence on materials, processes, and structures engineering. Integr. Mater. Manuf Innov. 2018;7:157–172. doi: 10.1007/s40192-018-0117-8. [DOI] [Google Scholar]
- Diorazio L.J., Hose D.R.J., Adlington N.K. Toward a more holistic framework for solvent selection. Org. Process Res.Dev. 2016;20:760–773. doi: 10.1021/acs.oprd.6b00015. [DOI] [Google Scholar]
- Diwekar U. CRC Press: Taylor & Francis Group; 2011. Batch Distillation: Simulation, Optimal Design, and Control, 2nd ed. [Google Scholar]
- Douglas J. McGraw-Hill; 1988. Conceptual Design of Chemical Processes. [Google Scholar]
- Dunn Peter J., Wells Andrew S., Williams Michael T. Wiley Online Books; 2010. Future trends for green chemistry in the pharmaceutical industry. Green Chemistry in the Pharmaceutical Industry. [DOI] [Google Scholar]
- DWC Innovations . DWC Innovations; 2020. Dividing Wall Column.https://www.dwcinnovations.com/dividing-wall-column/ [Google Scholar]
- EPA U. Management methods, limited to wastes received from Off-Site for National, Wastewater Only | US Environmental Protection Agency. 2019. https://rcrapublic.epa.gov/rcrainfoweb/action/modules/br/management
- EPA U. Management Methods, Limited to Wastes Generated On-Site for National, Non-wastewater Only | US Environmental Protection Agency. 2019. https://rcrapublic.epa.gov/rcrainfoweb/action/modules/br/management
- Epley J.B. Review of hazardous waste regulation–the new era: an analysis and guide to RCRA and the 1984 amendments. Ecol. Law Q. 1987;14:181–187. [Google Scholar]
- European Environment Agency, 2007. 1970s European Environment Policies.
- European Environment Agency . Publications Office; 2017. Prevention of Hazardous Waste in Europe: The Status in 2015. [Google Scholar]
- European Union Commission Decision of 18 November 2011 establishing rules and calculation methods for verifying compliance with the targets set in Article 11(2) of Directive 2008/98/EC of the European Parliament and of the Council (notified under document C(2011) 8165) Off. J. Eur. Union. 2011;L 310:11–16. [Google Scholar]
- European Union Consolidated Text: Directive 2008/98/EC of the European Parliament and of the Council of 19 November 2008 on waste and repealing certain Directives. Off. J. Eur. Union. 2018;3:1–59. [Google Scholar]
- European Union Commission Implementing Decision (EU) 2019/1004 - of 7 June 2019 - laying down rules for the calculation, verification and reporting of data on waste in accordance with Directive 2008/98/EC of the European Parliament and of the Council and repealing Commission Implementing Decision C(2012) 2384 - (notified under document C(2019) 4114) Off. J. Eur. Union. 2019;L 163:66–100. [Google Scholar]
- Farrán A., Cai C., Sandoval M., Xu Y., Liu J., Hernáiz M.J., Linhardt R.J. Green solvents in carbohydrate chemistry: from raw materials to fine chemicals. Chem. Rev. 2015;115:6811–6853. doi: 10.1021/cr500719h. [DOI] [PubMed] [Google Scholar]
- Federal Remediation Technologies Roundtable . Federal Remediation Technologies Roundtable (FRTR); 2020. Chapter 24 - Incineration.https://frtr.gov/matrix2/health_safety/chapter_24.html [Google Scholar]
- Fernández-Agulló A., Pereira E., Freire M.S., Valentão P., Andrade P.B., González-Álvarez J., Pereira J.A. Influence of solvent on the antioxidant and antimicrobial properties of walnut (Juglans regia L.) green husk extracts. Ind. Crops Prod. 2013;42:126–132. doi: 10.1016/j.indcrop.2012.05.021. [DOI] [Google Scholar]
- Fetanat M., Keshtiara M., Keyikoglu R., Khataee A., Daiyan R., Razmjou A. Machine learning for design of thin-film nanocomposite membranes. Sep. Purif.Technol. 2021;270:118383. doi: 10.1016/j.seppur.2021.118383. [DOI] [Google Scholar]
- García V., Pongrácz E., Phillips P.S., Keiski R.L. From waste treatment to resource efficiency in the chemical industry: recovery of organic solvents from waters containing electrolytes by pervaporation. J. Clean. Prod. 2013;39:146–153. doi: 10.1016/j.jclepro.2012.08.020. [DOI] [Google Scholar]
- Ge Z., Song Z., Ding S.X., Huang B. Data mining and analytics in the process industry: the role of machine learning. IEEE Access. 2017;5:20590–20616. doi: 10.1109/ACCESS.2017.2756872. [DOI] [Google Scholar]
- Geankoplis C.J. P T R Prentice-Hall, Inc.; 2003. Transport Processes and Unit Operations, 4th ed. [Google Scholar]
- Van Gerven T., Stankiewicz A. Structure, energy, synergy, time—the fundamentals of process intensification. Ind. Eng. Chem. Res. 2009;48:2465–2474. doi: 10.1021/ie801501y. [DOI] [Google Scholar]
- Goebel R., Skiborowski M. Machine-based learning of predictive models in organic solvent nanofiltration: pure and mixed solvent flux. Sep. Purif.Technol. 2020;237:116363. doi: 10.1016/j.seppur.2019.116363. [DOI] [Google Scholar]
- Goedkoop, M., Spriensma, R., 2001. The Eco-indicator 99 A Damage Oriented Method for Life Cycle Impact Assessment. Annex report Eco-indicator 99, 87.
- Goedkoop, M., Oele, M., Leijting, J., Ponsioen, T., Meijer, E., 2016. Introduction to LCA with SimaPro.
- Goh P.S., Wong T.W., Lim J.W., Ismail A.F., Hilal N. Innovation Strategies in Environmental Science. Elsevier; 2020. Innovative and sustainable membrane technology for wastewater treatment and desalination application; pp. 291–319. [DOI] [Google Scholar]
- González D., Amigo J., Suárez F. Membrane distillation: perspectives for sustainable and improved desalination. Renew.Sustain. Energy Rev. 2017;80:238–259. doi: 10.1016/j.rser.2017.05.078. [DOI] [Google Scholar]
- Gonzalez M., Smith R., Ruiz-Mercado G. GREENSCOPE: Sustainable Process Modeling. National Service Center for Environmental Publications; Washington, D.C., USA: 2011. [Google Scholar]
- Górak A., Sorensen E. Academic Press; 2014. Distillation: Fundamentals and Principles. [Google Scholar]
- Greatorex N.H. Solvents (external recycling): history and development. Pigment Resin Technol. 1985;14:4–9. doi: 10.1108/eb042111. [DOI] [Google Scholar]
- Green D.W., Perry R.H. McGraw-Hill; 2019. Perry’s Chemical Engineers Handbook, 9th ed. [Google Scholar]
- Guo X.J. Emergy-based urban ecosystem health evaluation for a typical resource-based city: a case study of Taiyuan, China. Appl. Ecol. Environ. Res. 2019;17:15131–15149. doi: 10.15666/aeer/1706_1513115149. [DOI] [Google Scholar]
- Han S., Raghuvanshi K., Abolhasani M. Accelerated material-efficient investigation of switchable hydrophilicity solvents for energy-efficient solvent recovery. ACS Sustain. Chem. Eng. 2020;8:3347–3356. doi: 10.1021/acssuschemeng.9b07304. [DOI] [Google Scholar]
- Harvey, D., 2019. 8.2: Precipitation Gravimetry. LibreTexts Libraries [online] URL https://chem.libretexts.org/Bookshelves/Analytical_Chemistry/Analytical_Chemistry_2.1_(Harvey)/08%3A_Gravimetric_Methods/8.02%3A_Precipitation_Gravimetry (accessed 09.12.19)
- Henao C.A., Maravelias C.T. Surrogate-based superstructure optimization framework. AIChE J. 2011;57:1216–1232. doi: 10.1002/aic.12341. [DOI] [Google Scholar]
- Henderson R.K., Jiménez-González C., Constable D.J.C., Alston S.R., Inglis G.G.A., Fisher G., Sherwood J., Binks S.P., Curzons A.D. Expanding GSK’s solvent selection guide – embedding sustainability into solvent selection starting at medicinal chemistry. Green. Chem. 2011;13:854. doi: 10.1039/c0gc00918k. [DOI] [Google Scholar]
- Ho W., Sirkar K. Springer; 1992. Membrane Handbook. [Google Scholar]
- Hu J., Kim C., Halasz P., Kim J.F., Kim J., Szekely G. Artificial intelligence for performance prediction of organic solvent nanofiltration membranes. J. Membr. Sci. 2021;619:118513. doi: 10.1016/j.memsci.2020.118513. [DOI] [Google Scholar]
- Huang H.-J., Ramaswamy S., Tschirner U.W., Ramarao B.V. Bioalcohol Production. Elsevier; 2010. Separation and purification processes for lignocellulose-to-bioalcohol production; pp. 246–277. [DOI] [Google Scholar]
- Huo H., Wang M., Bloyd C., Putsche V. Life-cycle assessment of energy use and greenhouse gas emissions of soybean-derived biodiesel and renewable fuels. Environ. Sci. Technol. 2009;43:750–756. doi: 10.1021/es8011436. [DOI] [PubMed] [Google Scholar]
- Hyndman R.J., Athanasopoulos G. OTexts; Melbourne Australia: 2018. Forecasting: Principles and. Practice 504. [Google Scholar]
- Incineration Waste management resources. 2009. http://www.wrfound.org.uk/articles/incineration.html
- ISO 14044 . International Organization for Standardization; Geneva, Switzerland: 1997. Environmental Management - Life Cycle Assessment - Principles and Framework. [Google Scholar]
- ISO 14044 . International Organization for Standardization; Geneva, Switzerland: 2006. Environmental Management - Life Cycle Assessment - Requirements and Guidelines. [Google Scholar]
- Isoni V., Wong L.L., Khoo H.H., Halim I., Sharratt P. Q-SA√ESS: a methodology to help solvent selection for pharmaceutical manufacture at the early process development stage. Green. Chem. 2016;18:6564–6572. doi: 10.1039/C6GC02440H. [DOI] [Google Scholar]
- Jessop P.G., Heldebrant D.J., Li X., Eckert C.A., Liotta C.L. Reversible nonpolar-to-polar solvent. Nature. 2005;436:1102. doi: 10.1038/4361102a. [DOI] [PubMed] [Google Scholar]
- Jessop P.G., Mercer S.M., Heldebrant D.J. CO2-triggered switchable solvents, surfactants, and other materials. Energy Environ. Sci. 2012;5:7240. doi: 10.1039/c2ee02912j. [DOI] [Google Scholar]
- Jiménez-González C., Constable D.J., Curzons A.D., Cunningham V.L. Developing GSK’s green technology guidance: methodology for case-scenario comparison of technologies. Clean. Technol. Environ.Policy. 2002;4:44–53. doi: 10.1007/s10098-001-0134-7. [DOI] [Google Scholar]
- Jiménez-González C., Poechlauer P., Broxterman Q.B., Yang B.-S., am Ende D., Baird J., Bertsch C., Hannah R.E., Dell’Orco P., Noorman H. Key green engineering research areas for sustainable manufacturing: aperspective from pharmaceutical and fine chemicals manufacturers. Org. Process. Res. Dev. 2011;15:900–911. doi: 10.1021/op100327d. [DOI] [Google Scholar]
- Jimenez-Gonzalez C., Ponder C.S., Broxterman Q.B., Manley J.B. Using the right green yardstick: why process mass intensity is used in the pharmaceutical industry to drive more sustainable processes. Org. Process. Res. Dev. 2011;15:912–917. doi: 10.1021/op200097d. [DOI] [Google Scholar]
- Johansson H.-O., Karlström G., Tjerneld F., Haynes C.A. Driving forces for phase separation and partitioning in aqueous two-phase systems. J. Chromatogr. B: Biomed. Sci. Appl. 1998;711:3–17. doi: 10.1016/S0378-4347(97)00585-9. [DOI] [PubMed] [Google Scholar]
- Jolliet O., Margni M., Charles R., Humbert S., Payet J., Rebitzer G., Rosenbaum R. Impact 2002+: a new life cycle impact assessment methodology. Int. J. LCA. 2003;8:324–330. doi: 10.1007/BF02978505. [DOI] [Google Scholar]
- Juran, J.M., 1986. The Quality Trilogy.
- Juran J.M., Godfrey A.B., editors. Juran’s Quality Handbook, 5th ed. McGraw Hill; 1999. [Google Scholar]
- Juran J., Gryna F., Bingham R. Quality Control Handbook. McGraw-Hill, Inc; New York, USA: 1974. [Google Scholar]
- Kennedy J.,F., Cabral J.M. Wiley; 1993. Recovery Processes for Biological Materials, 1st ed. [Google Scholar]
- Khulbe K.C., Matsuura T. Removal of heavy metals and pollutants by membrane adsorption techniques. Appl. Water Sci. 2018;8:1–30. doi: 10.1007/s13201-018-0661-6. [DOI] [Google Scholar]
- Kiss A.A., Ignat R.M., Bildea C.S. Computer Aided Chemical Engineering. Elsevier; 2014. Optimal extractive distillation process for bioethanol dehydration; pp. 1333–1338. [DOI] [Google Scholar]
- Kisszekelyi P., Alammar A., Kupai J., Huszthy P., Barabas J., Holtzl T., Szente L., Bawn C., Adams R., Szekely G. Asymmetric synthesis with cinchona-decorated cyclodextrin in a continuous-flow membrane reactor. J. Catal. 2019;371:255–261. doi: 10.1016/j.jcat.2019.01.041. [DOI] [Google Scholar]
- Kjeldsen P., Barlaz M.A., Rooker A.P., Baun A., Ledin A., Christensen T.H. Present and long-term composition of MSWlandfill leachate: areview. Crit. Rev. Environ. Sci.Technol. 2002;32:297–336. doi: 10.1080/10643380290813462. [DOI] [Google Scholar]
- Krotscheck C., Narodoslawsky M. The Sustainable Process Index a new dimension in ecological evaluation. Ecol. Eng. 1996;6:241–258. doi: 10.1016/0925-8574(95)00060-7. [DOI] [Google Scholar]
- Kwok-Keung A., LeChevallier M.W. WHO; 2013. Water Treatment and Pathogen Control Process Efficiency in Achieving Safe Drinking-Water. [Google Scholar]
- Kılınç M.R., Sahinidis N.V. Exploiting integrality in the global optimization of mixed-integer nonlinear programming problems with BARON. Optim. Methods Softw. 2018;33:540–562. doi: 10.1080/10556788.2017.1350178. [DOI] [Google Scholar]
- Lau P.K.W., Koenig A. Management, disposal and recycling of waste industrial organic solvents in Hong Kong. Chemosphere. 2001;44:9–15. doi: 10.1016/S0045-6535(00)00378-7. [DOI] [PubMed] [Google Scholar]
- León J.A., Fontalvo J. Tools for the design of hybrid distillation–pervaporation columns in a single unit: hybrid rectifying–pervaporation section. Ind. Eng. Chem. Res. 2018;57:11970–11980. doi: 10.1021/acs.iecr.8b02078. [DOI] [Google Scholar]
- Lewis M.J. Woodhead Publishing Limited; 1996. Separation Processes in the Food and Biotechnology Industries. [Google Scholar]
- Liu Y., Zhao T., Ju W., Shi S. Materials discovery and design using machine learning. J. Materiomics. 2017;3:159–177. doi: 10.1016/j.jmat.2017.08.002. [DOI] [Google Scholar]
- Liu T., Liu L., Cui F., Ding F., Zhang Q., Li Y. Predicting the performance of polyvinylidene fluoride, polyethersulfone and polysulfone filtration membranes using machine learning. J. Mater. Chem. A. 2020;8:21862–21871. doi: 10.1039/D0TA07607D. [DOI] [Google Scholar]
- Long F.A., Schweitzer G.E., editors. Risk Assessment at Hazardous Waste Sites, ACS Symposium Series. American Chemical Society; 1982. [DOI] [Google Scholar]
- Luis P. Fundamental Modelling of Membrane Systems. Elsevier; 2018. Pervaporation; pp. 71–102. [DOI] [Google Scholar]
- Lutze P., Gani R., Woodley J.M. Process intensification: a perspective on process synthesis. Chem. Eng. Process. Process Intensif. 2010;49:547–558. doi: 10.1016/j.cep.2010.05.002. [DOI] [Google Scholar]
- Megawati, Wicaksono D., Abdullah M.S. Experimental study on the adsorptive-distillation for dehydration of ethanol-water mixture using natural and synthetic zeolites. Presented at the Engineering International Conference (EIC) 2016: Proceedings of the 5th International Conference on Education, Concept, and Application of Green Technology, Semarang, Indonesia, 020032. 2017. [DOI]
- Mencarelli L., Chen Q., Pagot A., Grossmann I.E. A review on superstructure optimization approaches in process system engineering. Comput. Chem. Eng. 2020;136:106808. doi: 10.1016/j.compchemeng.2020.106808. [DOI] [Google Scholar]
- Mersmann A., Kind M. Chemical engineering aspects of precipitation from solution. Chem. Eng.Technol. 1988;11:264–276. doi: 10.1002/ceat.270110136. [DOI] [Google Scholar]
- Ministry of Environment . History and Current State of Waste Management in Japan. Japan Environmental Sanitation Center; Tokyo, Japan: 2014. [Google Scholar]
- Muralikrishna I.V., Manickam V. Environmental Management. Elsevier; 2017. Hazardous waste management; pp. 463–494. [DOI] [Google Scholar]
- Narciso D.A.C., Martins F.G. Application of machine learning tools for energy efficiency in industry: a review. Energy Rep. 2020;6:1181–1199. doi: 10.1016/j.egyr.2020.04.035. [DOI] [Google Scholar]
- Narodoslawsky M. In: Assessing and Measuring Environmental Impact and Sustainability. Klemeš J.J., editor. Butterworth-Heinemann; 2015. Chapter 3 - sustainable process index; pp. 73–86. [DOI] [Google Scholar]
- Narodoslawsky M., Krotscheck C. The sustainable process index (SPI): evaluating processes according to environmental compatibility. J. Hazard. Mater. Sel. Pap.Present. Conf. Hazard. Waste Remediat. 1995;41:383–397. doi: 10.1016/0304-3894(94)00114-V. [DOI] [Google Scholar]
- Narodoslawsky M., Krotscheck C.h. What can we learn from ecological valuation of processes with the sustainable process index (SPI) — the case study of energy production systems. J. Clean. Prod. Adv.Clean.Prod.Technol. 2004;12:111–115. doi: 10.1016/S0959-6526(02)00184-1. [DOI] [Google Scholar]
- Odum H.T. Self-organization, transformity, and information. Science. 1988;242:1132–1139. doi: 10.1126/science.242.4882.1132. [DOI] [PubMed] [Google Scholar]
- Odum H.T., Odum E.C. Modeling for All Scales, 479. Academic Press; San Diego, California, USA: 2000. [Google Scholar]
- Ooi J., Ng D.K.S., Chemmangattuvalappil N.G. A systematic molecular design framework with the consideration of competing solvent recovery processes. Ind. Eng. Chem. Res. 2019;58:13210–13226. doi: 10.1021/acs.iecr.9b01894. [DOI] [Google Scholar]
- Opp S.M. Environmental justice and the resource conservation recovery act inspection and enforcement process. Int. Rev. Public Adm. 2012;17:179–192. doi: 10.1080/12264431.2012.10805222. [DOI] [Google Scholar]
- Oppelt E.T. Air emissions from the incineration of hazardous waste. Toxicol. Ind. Health. 1990;6:23–51. [PubMed] [Google Scholar]
- Pang P.C. China’s evolving environmental protection laws - environment - China. 2020. https://www.mondaq.com/china/clean-air-pollution/955486/china39s-evolving-environmental-protection-laws
- Patterson J. Industrial wastes reduction. Environ. Sci. Technol. 1989;23:1032–1038. doi: 10.1021/es00067a609. [DOI] [Google Scholar]
- Peng Y., Lu X., Liu B., Zhu J. Separation of azeotropic mixtures (ethanol and water) enhanced by deep eutectic solvents. Fluid Phase Equilib. 2017;448:128–134. doi: 10.1016/j.fluid.2017.03.010. [DOI] [Google Scholar]
- Phifer R. Rcra–the first 30 years of hazardous waste regulation. J. Chem. Health Saf. 2010;17:4–7. doi: 10.1016/j.jchas.2010.01.002. [DOI] [Google Scholar]
- Pieter H., Joost de J., Koos W. Transboundary cooperation in shared reiver basins: experiences from the Rhine, Meuse and North Sea. Water Policy. 2000;2:83–97. [Google Scholar]
- Prat D., Wells A., Hayler J., Sneddon H., McElroy C.R., Abou-Shehada S., Dunn P.J. CHEM21 selection guide of classical- and less classical-solvents. Green. Chem. 2016;18:288–296. doi: 10.1039/C5GC01008J. [DOI] [Google Scholar]
- Price E.B. Solvents in centrifuge operations. Ind. Eng. Chem. Res. 1970:48–52. doi: 10.1021/ie50721a008. [DOI] [Google Scholar]
- Quinn J.C., Smith T.G., Downes C.M., Quinn C. Microalgae to biofuels lifecycle assessment —multiple pathway evaluation. Algal Res. 2014;4:116–122. doi: 10.1016/j.algal.2013.11.002. [DOI] [Google Scholar]
- Rasello R., Manca D. Computer Aided Chemical Engineering. Elsevier; 2014. Stochastic price/cost models for supply chain management of refineries; pp. 433–438. [DOI] [Google Scholar]
- Raymond M.J., Slater C.S., Savelski M.J. LCA approach to the analysis of solvent waste issues in the pharmaceutical industry. Green. Chem. 2010;12:1826. doi: 10.1039/c003666h. [DOI] [Google Scholar]
- van Reis R., Zydney A. Bioprocess membrane technology. J. Membr. Sci. 2007;297:16–50. [Google Scholar]
- REMADE Institute REMADE Institute Technology Roadmap 2020. 2020. https://static1.squarespace.com/static/59678de486e6c0c5f27e2a3c/t/6079e8dbc3b4da7dd48d15b7/1618602220624/REMADE+2020_Roadmap.pdf
- Riber C. Kgs. Lyngby; 2007. Evaluation of Waste Specific Environmental Impacts from Incineration. [Google Scholar]
- Rootes C. Oxford University Press; 2003. Environmental Protest in Western Europe. [DOI] [Google Scholar]
- Rostagno M.A., Prado J.M., editors. Green Chemistry Series. Royal Society of Chemistry; 2013. Natural product extraction principles and applications; pp. P001–P004. [DOI] [Google Scholar]
- Rotman M. Cleveland Historical; 2019. Cuyahoga River Fire.https://clevelandhistorical.org/items/show/63 [Google Scholar]
- Ruiz-Mercado G.J., Smith R.L., Gonzalez M.A. Sustainability indicators for chemical processes: I. Taxonomy. Ind. Eng. Chem. Res. 2012;51:2309–2328. doi: 10.1021/ie102116e. [DOI] [Google Scholar]
- Ruiz-Mercado G.J., Smith R.L., Gonzalez M.A. Sustainability indicators for chemical processes: II. Data needs. Ind. Eng. Chem. Res. 2012;51:2329–2353. doi: 10.1021/ie200755k. [DOI] [Google Scholar]
- Ruiz-Mercado G.J., Gonzalez M.A., Smith R.L. Sustainability indicators for chemical processes: III. Biodiesel case study. Ind. Eng. Chem. Res. 2013;52:6747–6760. doi: 10.1021/ie302804x. [DOI] [Google Scholar]
- Ryan P. The European community’s environment policy: meeting the challenges of the 90’s. Eur. Environ. 2007;1:1–6. doi: 10.1002/eet.3320010602. [DOI] [Google Scholar]
- Sá A.G.A. A review on enzymatic synthesis of aromatic esters used as flavor ingredients for food, cosmetics and pharmaceuticals industries. Trends Food Sci. Technol. 2017;69:95–105. [Google Scholar]
- Saleh T.A., Gupta V.K. An overview of membrane science and technology. Nanomater. Polym. Membr. 2016;23 doi: 10.1016/B978-0-12-804703-3.00001-2. [DOI] [Google Scholar]
- Sangshetti J.N., Deshpande M., Zaheer Z., Shinde D.B., Arote R. Quality by design approach: regulatory need. Arabian J. Chem. 2017;10:S3412–S3425. doi: 10.1016/j.arabjc.2014.01.025. [DOI] [Google Scholar]
- Savelski M.J., Slater C.S., Tozzi P.V., Wisniewski C.M. On the simulation, economic analysis, and life cycle assessment of batch-mode organic solvent recovery alternatives for the pharmaceutical industry. Clean. Technol. Environ.Policy. 2017;19:2467–2477. doi: 10.1007/s10098-017-1444-8. [DOI] [Google Scholar]
- Seader J.D., Henley E.J., Roper D.K. Wiley; 2010. Separation Process Principles with Applications Using Process Simulators, 4th ed. [Google Scholar]
- Seay J.R. Waste plastic: challenges and opportunities for the chemical industry. Chem. Eng. Prog. 2020;116:22–29. [Google Scholar]
- Sepiacci P., Manca D. Economic assessment of chemical plants supported by environmental and social sustainability. Chem. Eng. Trans. 2015;43:2209–2214. doi: 10.3303/CET1543369. [DOI] [Google Scholar]
- Shahab A., Singh M.P. 2019 International Conference on Communication and Signal Processing (ICCSP). Presented at the 2019 International Conference on Communication and Signal Processing (ICCSP), IEEE, Chennai, India. 2019. Comparative analysis of different machine learning algorithms in classification of suitability of renewable energy resource; pp. 0360–0364. [DOI] [Google Scholar]
- Shang C., You F. Data analytics and machine learning for smart process manufacturing: recent advances and perspectives in the big data era. Engineering. 2019;5:1010–1016. doi: 10.1016/j.eng.2019.01.019. [DOI] [Google Scholar]
- Shao P., Kumar A. Process energy efficiency in pervaporative and vacuum membrane distillation separation of 2,3-butanediol. Can. J. Chem. Eng. 2011;89:1255–1265. doi: 10.1002/cjce.20468. [DOI] [Google Scholar]
- Sharma S., Kanwar S.S. Organic solvent tolerant lipases and applications. Sci. World J. 2014;2014:1–15. doi: 10.1155/2014/625258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon R.A. The E factor: fifteen years on. Green. Chem. 2007;9:1273–1283. doi: 10.1039/B713736M. [DOI] [Google Scholar]
- Sheldon R.A. The E factor 25 years on: the rise of green chemistry and sustainability. Green. Chem. 2017;19:18–43. doi: 10.1039/C6GC02157C. [DOI] [Google Scholar]
- Sikdar S.K., Cole K.D., Stewart R.M., Szlag D.C., Todd P., Cabezas H. Aqueous two-phase extraction in bioseparations: an assessment. Nat. Biotechnol. 1991;9:252–256. doi: 10.1038/nbt0391-252. [DOI] [PubMed] [Google Scholar]
- Simonsson E. Hazardous Waste Management Policy in the European Union: Case of Germany and Unification Illustrates Need to Consider Principle of Subsidiarity, 13. Biennial European Community Studies Association Conference; Charleston, South Carolina, USA: 1994. [Google Scholar]
- Singh R.K., Murty H.R., Gupta S.K., Dikshit A.K. An overview of sustainability assessment methodologies. Ecol. Indic. 2009;9:189–212. doi: 10.1016/j.ecolind.2008.05.011. [DOI] [Google Scholar]
- Sitter S., Chen Q., Grossmann I.E. An overview of process intensification methods. Curr. Opin. Chem. Eng. 2019;25:87–94. doi: 10.1016/j.coche.2018.12.006. [DOI] [Google Scholar]
- Slater C.S., Savelski M. A method to characterize the greenness of solvents used in pharmaceutical manufacture. J. Environ. Sci. Health A. 2007;42:1595–1605. doi: 10.1080/10934520701517747. [DOI] [PubMed] [Google Scholar]
- Slater C.S., Savelski M.J., Carole W.A., Constable D.J.C. Green Chemistry in the Pharmaceutical Industry. Wiley-Blackwell; 2010. Solvent use and waste issues; pp. 49–82. [DOI] [Google Scholar]
- Slater, C.S., Savelski, M.J., Hounsell, G., Urbanski, F., Geiger, J., Knoechel, D., 2010b. Green engineering analysis of a multi-use solvent recovery system for small volume waste steams in the pharmaceutical industry. Presented at the 2010 Meeting of the American Institute of Chemical Engineers, Salt Lake City, UT.
- Slater C.S., Savelski M., Hounsell G., Pilipauskas D., Urbanski F. Green design alternatives for isopropanol recovery in the celecoxib process. Clean. Technol. Environ.Policy. 2012;14:687–698. [Google Scholar]
- Slater C.S., Savelski M.J., Moroz T.M., Raymond M.J. Pervaporation as a green drying process for tetrahydrofuran recovery in pharmaceutical synthesis. Green. Chem. Lett. Rev. 2012;5:55–64. doi: 10.1080/17518253.2011.578590. [DOI] [Google Scholar]
- Smith R., Jobson M. Encyclopedia of Separation Science. Academic Press; 2000. Distillation; pp. 84–103. [Google Scholar]
- Spaanstra J.R. The 1984 HSWA Amendments: The Land Disposal Restrictions, 30. Getting a Handle on Hazardous Waste Control, Summer Conference. University of Colorado Law School; Denver , Colorado, USA: 1986. [Google Scholar]
- Stankiewicz A.I., Moulijn J.A. Process intensification: transforming chemical engineering. Chem. Eng. Prog. 2000;13:22–34. [Google Scholar]
- Taulbee D.N., Mercedes Maroto-Valer M. Encyclopedia Separation Science. 2000. Centrifugation; pp. 17–40. [Google Scholar]
- Towler G., Sinnott R.K. Butterworth-Heinemann; 2012. Chemical Engineering Design, Second Edition: Principles, Practice and Economics of Plant and Process Design, 2 edn. [Google Scholar]
- Ulgiati S., Odum H.T., Bastianoni S. Emergy use, environmental loading and sustainability an emergy analysis of Italy. Ecol. Model. 1994;73:215–268. doi: 10.1016/0304-3800(94)90064-7. [DOI] [Google Scholar]
- US EPA . Air Pollution Control Technology Fact Sheet - Thermal Incinerator. National Service Center for Environmental Publications; Washington, D.C., USA: 2003. [Google Scholar]
- US EPA . US EPA; 2013. Summary of the Resource Conservation and Recovery Act.https://www.epa.gov/laws-regulations/summary-resource-conservation-and-recovery-act [Google Scholar]
- US EPA, 2014. Emission Factors for Greenhouse Gas Inventories.
- US EPA . Environmental Protection Agency; 2016. Solvents in the Workplace - How to Determine if They Are Hazardous Waste. [Google Scholar]
- US EPA . United States Environmental Protection Agency; 2020. Understanding Global Warming Potentials.https://www.epa.gov/ghgemissions/understanding-global-warming-potentials [Google Scholar]
- US EPA . United States Environmental Protection Agency; 2021. The Origins of EPA.https://www.epa.gov/history/origins-epa [Google Scholar]
- US EPA. OLEM . US EPA; 2015. History of the Resource Conservation and Recovery Act (RCRA)https://www.epa.gov/rcra/history-resource-conservation-and-recovery-act-rcra [Google Scholar]
- US EPA. OW . US EPA; 2015. General Information About Injection Wells.https://www.epa.gov/uic/general-information-about-injection-wells [Google Scholar]
- Villanueva A., Wenzel H. Paper waste –recycling, incineration or landfilling? A review of existing life cycle assessments. Waste Manag. 2007;27:S29–S46. doi: 10.1016/j.wasman.2007.02.019. [DOI] [PubMed] [Google Scholar]
- Voros V., Drioli E., Fonte C., Szekely G. Process intensification via continuous and simultaneous isolation of antioxidants: an upcycling approach for olive leaf waste. ACS Sustain. Chem. Eng. 2019;7:18444–18452. doi: 10.1021/acssuschemeng.9b04245. [DOI] [Google Scholar]
- Wang J., Lakerveld R. Integrated solvent and process design for continuous crystallization and solvent recycling using PC-SAFT. AIChE J. 2018;64:1205–1216. doi: 10.1002/aic.15998. [DOI] [Google Scholar]
- Weron R. Electricity price forecasting: a review of the state-of-the-art with a look into the future. Int. J. Forecast. 2014;30:1030–1081. doi: 10.1016/j.ijforecast.2014.08.008. [DOI] [Google Scholar]
- White L.S., Nitsch A.R. Solvent recovery from lube oil filtrates with a polyimide membrane. J. Membr. Sci. 2000;179:267–274. doi: 10.1016/S0376-7388(00)00517-2. [DOI] [Google Scholar]
- WHS Act . Office of Industrial Relations Workplace Health and Safety; Queensland, Australia: 2018. A Guide for Flammable and Combustible Liquids under the Work Health and Safety Act 2011, 18. [Google Scholar]
- Wilkinson D. Towards sustainability in the European Union? Steps within the European commission towards integrating the environment into other European Union policy sectors. Environ. Polit. 1997;6:153–173. doi: 10.1080/09644019708414315. [DOI] [Google Scholar]
- World Health Organization . WHO; 2001. Exposure and Health Risks from Incineration. [Google Scholar]
- Wu C., Tu X. Handbook of Biofuels Production. Elsevier; 2016. Biological and fermentative conversion of syngas; pp. 335–357. [DOI] [Google Scholar]
- Wu X., Liang L., Zou Y., Zhao T., Zhao J., Li F., Yang L. Aqueous two-phase extraction, identification and antioxidant activity of anthocyanins from mulberry (Morus atropurpurea Roxb.) Food Chem. 2011;129:443–453. doi: 10.1016/j.foodchem.2011.04.097. [DOI] [PubMed] [Google Scholar]
- Wu W., Yenkie K., Maravelias C.T. A superstructure-based framework for bio-separation network synthesis. Comput. Chem. Eng. 2017;96:1–17. doi: 10.1016/j.compchemeng.2016.10.007. [DOI] [Google Scholar]
- Wu M., Feng Z., Deng Y., Zhong C., Liu Y., Liu J., Zhao X., Fu Y. Liquid antisolvent precipitation: an effective method for ocular targeting of lutein esters. Int. J. Nanomed. 2019;14:2667–2681. doi: 10.2147/IJN.S194068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang J., Liu T., Hua X., Cheng P., Zhang L. Elsevier; 2020. Chapter 17 - the future prospect of China’s independent R8D technology (ITK) in water resources utilization and wastewater treatment. Water Conservation and Wastewater Treatment in BRICS Nations 329–351. [DOI] [Google Scholar]
- Yadav G., Fabiano L.A., Soh L., Zimmerman J., Sen R., Seider W.D. CO2 process intensification of algae oil extraction to biodiesel. AIChE J. 2021;67:e16992. doi: 10.1002/aic.16992. [DOI] [Google Scholar]
- Yenkie K.M., Wu W., Maravelias C.T. Synthesis and analysis of separation networks for the recovery of intracellular chemicals generated from microbial-based conversions. Biotechnol. Biofuels. 2017;10:1–22. doi: 10.1186/s13068-017-0804-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeomans H., Grossmann I.E. A systematic modeling framework of superstructure optimization in process synthesis. Comput. Chem. Eng. 1999;23:709–731. doi: 10.1016/S0098-1354(99)00003-4. [DOI] [Google Scholar]
- Zarzo D. Emerging Technologies for Sustainable Desalination Handbook. Elsevier; 2018. Beneficial uses and valorization of reverse osmosis brines; pp. 365–397. [DOI] [Google Scholar]
- Zhang K. Organic solvent pretreatment of lignocellulosic biomass for biofuels and biochemicals: a review. Bioresour.Technol. 2016;199:21–33. doi: 10.1016/j.biortech.2015.08.102. [DOI] [PubMed] [Google Scholar]
- Zhang K., Wen Z. Review and challenges of policies of environmental protection and sustainable development in China. J. Environ. Manag. 2008;88:1249–1261. doi: 10.1016/j.jenvman.2007.06.019. [DOI] [PubMed] [Google Scholar]
- Zhang L., He G., Mol A.P.J. China’s new environmental protection law: a game changer? Environ.Dev. 2015;13:1–3. doi: 10.1016/j.envdev.2014.10.001. [DOI] [Google Scholar]
- Zhang B., Cao C., Gu J., Liu T. A new environmental protection law, many old problems? Challenges to environmental governance in China. J. Environ. Law. 2016;28:325–335. doi: 10.1093/jel/eqw014. [DOI] [Google Scholar]