Abstract
Objectives
The risk of contracting SARS-CoV-2 is high among the health care workers (HCW). The comparison between the antibody response to an inactivated Covid19 vaccine and the antibodies that developed during Covid-19 infection has not been elucidated. In this study, vaccine-induced antibody levels were compared with the antibodies developed in naturally infected HCWs.
Methods
Eighty vaccinated individuals and 80 Covid-19 patients enrolled to the study. Both groups were matched on age, gender and antibody testing time. Anti-SARS-CoV-2 total Ig (Roche) and Anti-SARS-CoV-2 ELISA (IgG) (Euroimmun, Germany) were used to detect antibodies.
Results
The anti-S positivity were determined to be 96.2% and 92.5% in vaccinated and patient groups (p=0.303) while the anti-N positivity was 51.2% and 98.8%, respectively (p=<0,0001). The median values for anti-S and anti-N antibodies were statistically significant between both groups. When the vaccinated group was compared with the severe and non-severe patient groups, statistically significant differences were found for both regarding anti-S1 and anti-N antibody titers (p=0,012, p=<0,0001, respectively). For the patient group, there was a positive correlation between the age and anti-S1 antibody titers (r=0.333; p=0.003) and there was also a statistically significant increase in anti-N antibody titers in time (r=0.505; p=0.0001).
Conclusion
The anti-S seroconversion ratio in vaccinated individuals were higher than what was reported by the vaccine manufacturer. The antibody titers in the vaccinated group were lower than the patients group. The decrease in anti-S1 antibody titers in time were considered to be a disadvantage and an undesired phenomenon.
Keywords: Health Care Workers, SARS-CoV-2, Covid19, Vaccination, Anti-S1, Anti-N
Introduction
The devastating effect of SARS-CoV-2 is still ongoing around the globe (WHO, 2019). Virus-infected patients are either asymptomatic or have the disease, with clinical course ranging from mild to severe (Yang et al. 2020). HCWs have a higher risk of encountering SARS-CoV-2. The higher risk comes from likely contact with patients carrying high viral loads and virus-infected stuff in the hospitals. Infection rates rise up to 14% in symptomatic and 7.1% in asymptomatic HCWs. These rates are higher than the general population reported so far and suggest an occupational risk (Shields et al. 2020).
In addition to the effective personal protection measures, the vaccination can prevent the disease and limit the spread of the infection (Dong et al. 2020). The preferred target in nucleic acid vaccines is the S protein and its receptor-binding domain (RBD). S protein, a structural protein of SARS-CoV-2, plays the most important roles in viral attachment, fusion and entry, and it serves as a target for the development of antibodies and vaccines. The S protein mediates viral entry into host cells by binding to a host RBD in the S1 subunit and then allowing the fusion of the viral and host membranes (Tai et al. 2020). The inactivated vaccine also contains other structural proteins of SARS- CoV-2. One of these, the nucleocapsid (N) protein, forms complexes with genomic RNA, plays a critical role in enhancing the efficiency of the virus transcription and assembly. In addition to the S protein, N proteins are also highly immunogenic and are expressed abundantly during infection. Most Covid-19 patients develop neutralizing antibodies specific to S protein and its RBD (Jiang et al. 2020). High titers of IgG antibodies against N protein have also been detected in the sera of Covid-19 patients (Dutta et al. 2020).
CoronaVac, an inactivated vaccine for Covid19, is used for vaccination of the HCWs in Turkey. This vaccine, which is one of the vaccines prepared by conventional methods, contains a large number of viral particles and proteins for immune recognition. Inactivated vaccines have stable expression of conformation-dependent antigenic epitopes (Dong et al. 2020).
Although anti-S and anti-N antibody response titers are known in Covid-19 patients, the level of antibodies developed in those vaccinated with CoronaVac in comparison to those who had a natural infection is not known.
In this study, we compared of an inactivated Covid19 vaccine-induced antibody response with concurrent natural antibodies developed in post-Covid-19 infections in HCWs.
Materials and Methods
Eighty CoronaVac vaccinated HCWs and 80 Covid-19 patients were included to this study. The subjects in both groups were matched by age, gender, and antibody testing time. Individuals with no history of COVID-19 and negative both for RT PCR and for antibody test results were included into the vaccinated group.
Antibody tests were performed for within 44,93 (±14,930) days after the patients were admitted to the hospital due to clinical complaints. PCR tests were positive for all individuals in the patient group. Contacts who were positive during screening and had no clinical complaints were not included in the study.Individuals in the vaccinated group were tested for antibodies for a mean of 44,33 (±15,929) days after the second vaccine.
Covid-19 cases were clinically classified as severe and non-severe patients (Wu and McGoogan 2020). The CoronaVac vaccine used in this study was manufactured by Sinovac Life Sciences (Beijing, China) and contained 3 μg/0.5 mL (equivalent to 600 SU per dose) of inactivated SARS-CoV-2. Two 0.5 mL doses IM (deltoid) with a two-week interval were administered (Palacios et al. 2020).
Anti-SARS-CoV-2 total Ig (Roche) and Anti-SARS-CoV-2 ELISA (IgG) (Euroimmun, Germany) were used to detect antibodies. Ethics permission for this study was granted by the Scientific Research Platform of the Ministry of Health committee (GOKAEK-2020 /14.01).
Categorical variables were presented as numbers (%). Continuous variables were expressed as median (interquartile range, IQR) or mean (± standard deviation, SD). For intergroups comparisons Student’s t-test, Mann-Whitney U-test or Kruskal-Wallis test were used. For continuous variables x2 or Fisher’s exact test were used. Correlations were evaluated using the Spearman’s bivariate correlation test. Statistical significance was defined as P values of <0.05. Statistical analysis was made using IBM SPSS 20.00 (SPSS Inc., Chicago, IL, USA).
Results
A total of 160 people were included into this study. 57 (71.3%) of 80 individual vaccinated were women, 56 (70%) of 80 patients who were diagnosed with Covid19 were women. The median (IQR) age of the individuals in both vaccinated and Covid19 groups were 43,5 (33-50) and 39,5 (31,25-46), respectively. There was no difference between the vaccine and patient groups in terms of gender and median age.
Antibody tests were performed for within 44,93 (±14,930) days after the patients were admitted to the hospital due to clinical complaints. PCR tests were positive for all individuals in the patient group. Individuals in the vaccinated group were tested for antibodies for within 44,33 (±15,929) days after the second vaccine. No statistically significant difference was detected between both groups (Table 1 ).
Table 1.
Demographic characteristics and antibody response in vaccinated group and Covid-19 cases
| Vaccinated group (n=80) | Covid-19 cases (n=80) | P value | |
|---|---|---|---|
| Gender — no. (%) | |||
| Female | 57 (71,3) | 56 (70) | 0,862 |
| Male | 23 (28,8) | 24 (30) | |
| Age —years | 0,081 | ||
| Median (IQR) | 43,5 (33-50) | 39,5 (31,25-46) | |
| Range | 21-58 | 21-64 | |
| Antibody testing time | 0,806 | ||
| Mean (±SD) | 44,33 (±15,929) | 44,93 (±14,930) | |
| Range | 15-75 | 15-74 | |
| Antibody testing time — Mean (±SD) | |||
| Anti-S1 IgG seropositive | 43,91 (±15,635) | 45,55 (±14,433) | 0,502 |
| Anti-S1 IgG seronegative | 55 (±23,580) | 37,17 (±20,064) | 0,358 |
| Anti-S1 IgG seropositive — n (%) | 77 (96,2) | 74 (92,5) | 0,303 |
| Gender in Anti-S1 IgG seropositive — n (%) | |||
| Female | 55 (96,5) | 52 (92,9) | 0,516 |
| Male | 22 (95,7) | 22 (91,7) | |
| Titers of Anti-S1 IgG | |||
| Median (IQR) | 3,169 (2,318-4,275) | 4,367 (2,552-6,407) | 0,012 |
| Range | 0,482-9,145 | 0,169-10,117 | |
| Anti-N total seropositive — n (%) | 41 (51,2) | 79 (98,8) | <0,0001 |
| Gender in Anti-N total seropositive — n (%) | |||
| Female | 32 (56,1) | 55 (98,2) | <0,0001 |
| Male | 9 (39,1) | 24 (100) | |
| Titers of Anti-N total | |||
| Median (IQR) | 0,992 (0,369-2,895) | 34,95 (16,115-63,388) | <0,0001 |
| Range | 0,08-8,85 | 0,737-149,9) |
Anti-S1 and anti-N antibody positivity were 77/80 (96.2%) and 41/80 (51.2%) in the vaccinated group, while the anti-S1 and anti-N antibody positivity were 74/80 (92.5%) and 79/80 (98.8%) in the patient group, respectively (Table 1).
The anti-S1 median value was 3.169 (IQR: 2.318-4.275; range: 0.482-9.145), 4.367 (IQR: 2.552-6.407; range: 0.169- 10.117) in vaccinated and patient groups, respectively. The difference between the anti-S1 titers was statistically significant in two groups (p = 0.012).
The anti-N median value was 0.992 (IQR: 0.369-2.895; range: 0.08-8.85), 34.95 (IQR: 16.115-63.388; range: 0.737-149.9) in the vaccinated and patient groups, respectively. The difference for the anti-N titers between the groups was statistically significant (p = <0.0001) (Table 1).
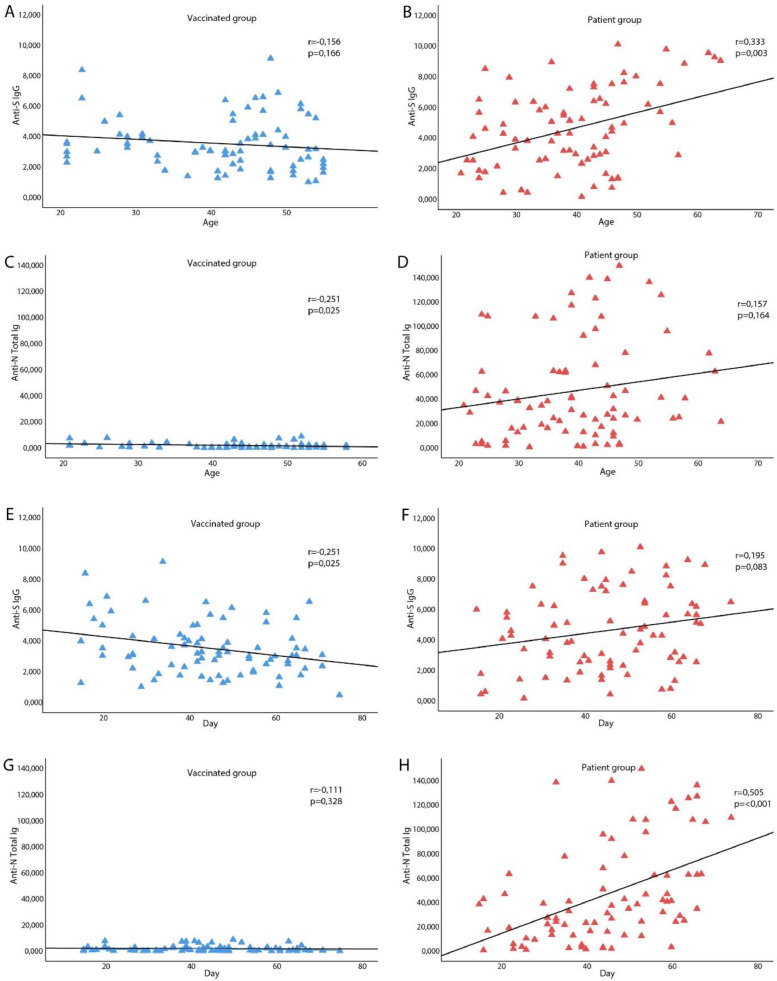
Anti S1 antibody levels decreased significantly in the vaccinated group at around 45 days, while there was no change in the anti S1 antibody levels in the patient group (r= -,251, p=0,025 and r=,195, p=0,83, respectively). In contrast to the vaccinated group, the anti N antibody levels increased at around the same time in the patient group (r= -,111, p=0,328 and r=,505, p=<0,0001, respectively) (Figure 3).
Figure 3.
Antibody titers in vaccinated group and Covid19 patients and correlation with age and time.
A. There was no significant correlation between age and Anti S1 antibody titers in the vaccinated group (r=-,156; p=0,166); B. There was positive and a significant correlation between age and Anti S1 antibody titers in the patient group (r=,333**; p=0,003); C. There was negative significant correlation between the age and Anti N antibody titers in the vaccinated group (r=-,251*; p=0,025); D. There was no significant correlation between age and Anti N antibody titers in the patient group (r=,157; p=0,164); E. Anti S1 antibody titers decreased significantly in the vaccinated group in time (r=-,251*, p=0,025); F. There was no change in the Anti S1 antibody titers in the patient group in time (r=,195, p=0,83); G. There was no change in the Anti N antibody titers in the vaccinated group in time (r=-,111, p=0,328); H. There was positive and significant correlation the anti N antibody titers and in time in the patient group (r=,505**, p=<0,001).
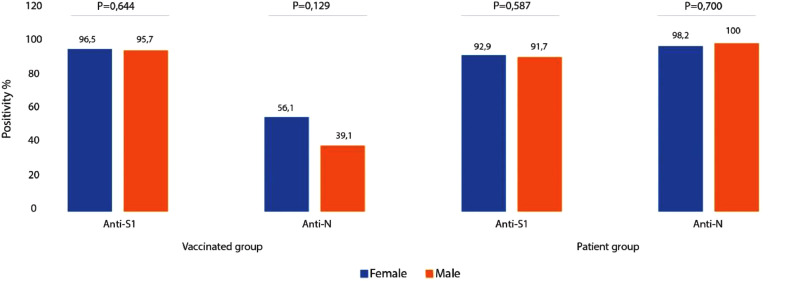
Anti S1 antibody positivity was detected in 55 (96.5%) of 57 women in the vaccinated group and 22 (95.7%) of 23 men. Similarly, 52 (92.9%) of 56 women and 22 (91.7%) of 24 men in the patient group displayed S1 antibody positivity. Anti N antibody positivity was detected in 32 (56.1%) of 57 women and 9 (39.1%) of 23 men in the vaccinated group while those values were determined to be 55 (98.2%) of 56 for women and 24 (100%) of 24 men in the patient group (Figure 1 and Table 1). The antibody responses did not differ for the gender in both groups (p> 0.05). Thirteen (23.2%) of male and 43 (76.8%) of female patients did not display severe clinical course while 11 (45.8%) of male and 13 (54.2%) of female patients displayed severe clinical course (Figure 2 ).
Figure 1.
Evaluation of the antibody titers with gender
Anti-S1antibody positivity was detected in 55 (96.5%) of 57 women in the vaccinated group and 22 (95.7%) of 23 men. Similarly, 52 (92.9%) of 56 women and 22 (91.7%) of 24 men in the patient group displayed S1 antibody positivity.
Anti-N antibody positivity was detected in 32 (56.1%) of 57 women and 9 (39.1%) of 23 men in the vaccinated group while those values were determined to be 55 (98.2%) of 56 for women and 24 (100%) of 24 men in the patient group. The antibody responses did not differ for gender in the vaccinated and the patient groups (p> 0.05).
Figure 2.
Evaluation of the antibody titers with respect to the clinical severity
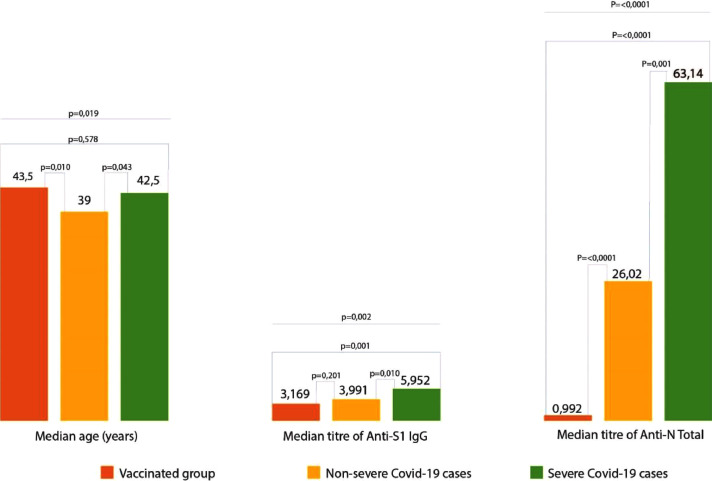
When the vaccinated group was compared with the patient groups in terms of severity of the cases, a significant difference was found in both anti-S1 and anti-N antibody titers between the groups (p=<0,0001, p=0,002). When group to group comparisons were made in terms of anti-S1 antibody titers, no significant difference was found in anti-S1 antibody titers between the vaccinated group and the group that did not display severe disease symptoms (p = 0.201). On the other hand, a significant difference was found between the vaccinated group and the group that displayed severe disease symptoms. (p = 0.001). In terms of anti-N antibody titers; the difference between the vaccinated and both severe and non-severe disease groups was found to be significant (p=0,001).
While there was no significant correlation between age and anti S1antibody levels in the vaccinated group, a negative correlation was found between the age and Anti N antibody levels (r=-,156; p=0,166 and r=-,251*; p=0,025, respectively). In addition, unlike anti N antibody levels, a positive and a significant correlation was found between age and Anti S1 antibody levels in the patient group (r=,157; p=0,164 and r=,333**; p=0,003, respectively). A positive and significant correlation was also found between Anti N antibody and Anti S1 antibody levels between the vaccinated and patient groups (r=,0326**; p=0,003 and r=,552**; p=<0,0001, respectively) (Figure 1). However, the vaccinated group displayed a weak correlation while the patient group displayed a moderate correlation (Figure 3 ).
When the vaccinated group was compared with the patient groups in terms of clinical severity of the cases, a significant difference was found in both anti S1 and anti N antibody levels between groups (p=<0,0001, p=0,002). When two groups comparisons were made in terms of anti S1 antibody levels, no significant difference was found in anti S1 antibody levels between the vaccinated group and the group that did not display severe disease symptoms (p = 0.201). On the other hand, a significant difference was found between the vaccinated group and the group that displayed severe disease symptoms (p = 0.001). In terms of anti N antibody levels; the difference between the vaccinated and both severe and non-severe disease groups was found to be significant (p=0,001)(Table 2 ).
Table 2.
Demographic characteristics and antibody response in the groups of vaccinated, non-severe COVID-19 and severe Covid-19
| Vaccinated group |
Non-severe Covid-19 cases |
Severe Covid-19 cases |
P value |
|
|---|---|---|---|---|
| N:80 | N:56 | N:24 | ||
| Gender — no. (%) | ||||
| Female | 57 (71,3) | 43 (76,8) | 13 (54,2) | 0,124 |
| Male | 23 (28,7) | 13 (23,2) | 11 (45,8) | |
| Age —years | ||||
| Median (IQR) | 43,5 (33-50) | 39 (30-45) | 42,5 (36-54) | 0,019 |
| Range | 21-58 | 21-57 | 22-64 | |
| Titers of Anti-S1 IgG | 0,002 | |||
| Median (IQR) | 3,169 (2,318-4,275) | 3,991 (2,335-5,779) | 5,952 (2,754-8,71) | |
| Range | 0,482-9,145 | 0,169-10,117 | (0,434-9,790) | |
| Titers of Anti-N total | ||||
| Median (IQR) | 0,992 (0,369-2,895) | 26,020(10,998-45,698) | 63,14 (35,515-107,675) | <0,0001 |
| Range | 0,08-8,85 | 0,737-149,9 | 2,010-140,1 |
Discussion
Vaccination has a pivotal role in termination of pandemics. The key question that is being asked regarding all Covid-19 vaccines is how effective they are at controlling the infection. Achieving high titers of neutralizing antibodies to the spike protein of SARS-CoV-2 is likely to be crucial step for effectiveness of a vaccine. Spike glycoprotein contains the S1 subunit which mediates viral binding to functional ACE2 receptors on the susceptible cells. Therefore, antibodies against the SARS CoV-2 S1 protein are important for neutralization of the virus (Poland et al. 2020). Nucleic acid vaccines induce moderate to strong antibody responses. The induction of antibodies seems to correlate well with virus neutralization and protection from the virus (Grigoryan and Pulendran 2020).
Natural SARS-CoV-2 infection results in both humoral and cellular immune responses. Serum antibody testing is becoming one of the most critical methods to assess the response to vaccination (Sui et al. 2021). In this study, the antibody titers developed against S1, and N proteins were investigated in vaccinated HCWs. CoronaVac has the capacity to create a response against not only to S1 but also N protein. Vaccine-induced anti-S1 seroconversion at a rate similar to those observed in naturally infected patients. No SARS-CoV-2 infection was detected in HCWs who were vaccinated with CoronaVac during the study.
There are comparative studies investigated the titers of antibodies in patients with varying clinical course. In our study, Individuals with no history of COVID-19 and negative both for RT PCR and for antibody test results were included into the vaccinated group. The titers of vaccine-induced antibodies in HCWs were compared with antibody levels in age- and gender-matched Covid-19 patients. We also took into consideration of the clinical course. Anti S1 antibody median titers were similar in the vaccinated group and in the non-severe cases of the patient group. In severe cases of the patient group, however, it was found to be significantly high. In comparison to the vaccinated group, anti-N antibody titers were higher both in severe and non-severe cases in the patient group. The difference was more prominent in severe cases. One of the reasons for the increased anti-N antibody titers might be due to the high viral load which causes strong antibody responses in severe cases (Zhang et al. 2020). The quantity of SARS-CoV-2 virus starts to peak 5-6 days after the onset of symptoms in patients and reaches up to 104-107 copies / ml in throat / sputum samples (Pan et al. 2020). Due to the differences in the number of viral particles, differences in the immune response against the virus is observed. The amount of virus in CoronaVac vaccine is 600 SU / 0.5ml (Palacios et al. 2020). This may explain the lower median titers of both anti-S1 and anti-N antibodies in vaccine response compared to severe cases. The increase in natural post-infection antibody levels may be due to the fact that the viral load for each patient various and the virus remains positive for a longer time in some patients (Gaebler et al. 2021). Moreover, CoronaVac may have low immunogenicity since it is an inactivated viral vaccine, that possesses antigen multivalency and alteration of S due to chemicals during inactivation processes (Ophinni et al. 2020). On the other hand, SARS-CoV-2 anti-S antibody titers in BNT162b2 mRNA Covid-19 vaccinated individuals are similar to titers in individuals who have had a mild SARS-CoV-2 infection (Manisty et al. 2021). In our study, although the antibody development rate against the inactivated vaccine was similar to the patient group, the titers of the antibodies formed were found to be lower than the patient group. Anti-N antibody data is not presented in studies using RNA or DNA vaccines, since only the S protein is targeted in the vaccines. In this study, for the first time, data regarding antibodies developed against N protein after vaccination were presented.
When evaluating the effectiveness of vaccines, the emergence and persistence of SARS-CoV-2 variants is of significant concern. Their resistance to existing neutralization antibodies acquired through vaccines may cause a failure in vaccination. Although vaccine-induced antibody titers against N protein were found to be lower than the natural post-infection antibodies in this study, multiple antibodies such as anti-N in addition to anti-S1 are needed to be targeted in new vaccination approaches. Future mutations might necessitate changes for the strains used in vaccine development. The vaccines should be periodically reformulated so that they display better matches for the circulating variants (Xie et al. 2021).
The level of the antibodies developed against S1 protein decreased significantly in the vaccinated group at around 45 days, while it did not change in the patient group during the study. The antibody titers developed against the N protein also increased at around the same time in the patient group.The role of anti-N in permanent immunity in virus-infected individuals is not yet known. It appears that anti S1 antibodies decrease over time in the vaccinated group. If the immune memory is not sufficient, repeated vaccination may be required at regular intervals, especially for risky professions. In this study, the antibody response in patients was tested as late as 75th day after vaccination. Studies in larger populations and long-term follow-up are required to investigate antibody duration and response to the virus. Age is known to influence the immunity (Dong et al. 2020). S1-specific IgG responses were significantly higher in older women (>40 years) than in younger women (<40 years) (Poland et al. 2020). Correlation of post-vaccination anti-spike titer with age in infection-naive participants were detected (Prendecki et al. 2021). In this study, no significant correlation was found between the age and anti S1 titers in the vaccinated group, while the antibody titers developed against S1 protein increased in correlation to the age in the patient group. Some studies also demonstrated that antibody titers correlate well with the disease severity and with gender (Takahashi et al. 2020). Surprisingly, in this study, the anti-S1 and anti N antibody positivity rates in the vaccinated and patient groups did not differ by gender (p> 0.05).
The limitation of the presented study was that we could not compare neutralizing anti-S IgG titers. That was because BSL3 laboratory conditions are required for cultivation of SARS-CoV-2 and BSL3 lab does not exist in our facility (Kellam and Barclay 2020). Yet, in many studies, anti S antibody titers have been found to be correlated with neutralizing antibodies (Grigoryan and Pulendran 2020). Therefore, we believe that antibody responses developed by vaccination or by natural infection will be beneficial for some time in protection from the SARS-CoV-2.
Conclusions
Presenting data is to show the comparable levels of an inactivated vaccine-induced and post-infection antibodies. The anti-S seroconversion ratio in vaccinated individuals were higher than what was reported by the vaccine manufacturer. But the antibody titers in the vaccinated group were lower than the patients group. In addition, the decrease in anti-S1 antibody titers in time were considered to be a disadvantage and an undesired phenomenon. We do not know whether permanent immunity will be effective in case of a decrease in antibody titers. Immune responses that will occur in people infected with the virus after vaccination and antibody development and the course of the disease are still unclear. Clarification of this issue has utmost importance for the fate of pandemic in the future.
Acknowledgments
Declaration of Competing Interest
The authors declare that they have no conflicts of interest.
Acknowledgements
We respectfully acknowledge the healthcare workers who bravely sacrificed their lives during the fight against Covid-19 pandemic.
We thank to Prof. Murat Kasap for language editing and proofreading. We also thank to Nevin Çalık for her assistance in collecting serum samples.
Conflict of interest
None declared.
Funding statement
This study was supported by Kocaeli University Scientific Research Projects Coordination Unit. Project Number: TOA-2020-2222.
Footnotes
(This author contributed equally to this article and share first authorship
References
- Dong Y, Dai T, Wei Y, Zhang L, Zheng M, Zhou F. A systematic review of SARS-CoV-2 vaccine candidates. Signal Transduct Target Ther. 2020;5(1) doi: 10.1038/s41392-020-00352-y. http://dx.doi.org/10.1038/s41392-020-00352-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta NK, Mazumdar K, Gordy JT. The Nucleocapsid Protein of SARS–CoV-2: a Target for Vaccine Development. J Virol. 2020;94(13):1–2. doi: 10.1128/JVI.00647-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaebler C, Wang Z, Lorenzi JCC, Muecksch F, Finkin S, Tokuyama M, et al. Evolution of antibody immunity to SARS-CoV-2. Nature. 2021 doi: 10.1038/s41586-021-03207-w. (November 2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoryan L, Pulendran B. The immunology of SARS-CoV-2 infections and vaccines. Semin Immunol. 2020;50(November) doi: 10.1016/j.smim.2020.101422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellam P, Barclay W. The dynamics of humoral immune responses following SARS-CoV-2 infection and the potential for reinfection. J Gen Virol. 2020;101(8):791–797. doi: 10.1099/jgv.0.001439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manisty C, Otter AD, Treibel TA, McKnight ine, Altmann DM, Brooks T, et al. Correspondence Antibody response to first BNT162b2 dose in previously SARS-CoV-2-infected individuals. Lancet. 2021;6736(21):2–3. doi: 10.1016/S0140-6736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ophinni Y, Hasibuan AS, Widhani A, Maria S, Koesnoe S, Yunihastuti E, et al. COVID-19 vaccines: Current status and implication for use in Indonesia. Acta Med Indones. 2020;52(4):388–412. [PubMed] [Google Scholar]
- Palacios R, Patiño EG, de Oliveira Piorelli R, Conde MTRP, Batista AP, Zeng G, et al. Double-blind, randomized, Placebo-controlled phase III clinical trial to evaluate the efficacy and safety of treating healthcare professionals with the adsorbed COVID-19 (Inactivated) vaccine manufactured by Sinovac – PROFISCOV: A structured summary of a. Trials. 2020;21(1):21–23. doi: 10.1186/s13063-020-04775-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Zhang D, Yang P, Poon LLM, Wang Q. Viral load of SARS-CoV-2 in clinical samples. Lancet Infect Dis. 2020;20(4):411–412. doi: 10.1016/S1473-3099(20)30113-4. http://dx.doi.org/10.1016/S1473-3099(20)30113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poland GA, Ovsyannikova IG, Kennedy RB. SARS-CoV-2 immunity: review and applications to phase 3 vaccine candidates. Lancet. 2020;396(10262):1595–1606. doi: 10.1016/S0140-6736(20)32137-1. http://dx.doi.org/10.1016/S0140-6736(20)32137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendecki M, Clarke C, Brown J, Cox A, Gleeson S, Guckian M, et al. Effect of previous SARS-CoV-2 infection on humoral and T-cell responses to single-dose BNT162b2 vaccine. Lancet. 2021;6736(21):10–12. doi: 10.1016/S0140-6736(21)00502-X. http://www.ncbi.nlm.nih.gov/pubmed/33640037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields A, Faustini SE, Perez-Toledo M, Jossi S, Aldera E, Allen JD, et al. SARS-CoV-2 seroprevalence and asymptomatic viral carriage in healthcare workers: A cross-sectional study. Thorax. 2020;75(12):1089–1094. doi: 10.1136/thoraxjnl-2020-215414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui Y, Bekele Y, Berzofsky JA. Potential SARS-CoV-2 immune correlates of protection in infection and vaccine immunization. Pathogens. 2021;10(2):1–11. doi: 10.3390/pathogens10020138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Ellingson MK, Wong P, Israelow B, Lucas C, Klein J, et al. Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature. 2020;588(7837):315–320. doi: 10.1038/s41586-020-2700-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Coronavirus disease (COVID-19) Weekly Epidemiological Update and Weekly Operational Update. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/
- Wu Z, McGoogan JM. Characteristics of and important lessons from the Coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72314 cases from the Chinese Center for Disease control and prevention. JAMA - J Am Med Assoc. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- Xie X, Liu Y, Liu J, Zhang X, Zou J, Fontes-Garfias CR, et al. Neutralization of SARS-CoV-2 spike 69/70 deletion, E484K and N501Y variants by BNT162b2 vaccine-elicited sera. Nat Med. 2021 doi: 10.1038/s41591-021-01270-4. http://dx.doi.org/10.1038/s41591-021-01270-4. [DOI] [PubMed] [Google Scholar]
- Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Lu S, Li H, Wang Y, Lu Z, Liu Z, et al. Viral and antibody kinetics of COVID-19 patients with different disease severities in acute and convalescent phases: A 6-month follow-up study. Virol Sin. 2020;35(6):820–829. doi: 10.1007/s12250-020-00329-9. [DOI] [PMC free article] [PubMed] [Google Scholar]