Abstract
Polycystic Ovary Syndrome (PCOS) is an endocrine/metabolic disorder with an ever-increasing prevalence. It has various clinical characteristics; the cardinals are androgen excess, oligo-anovulatory infertility, polycystic ovaries, insulin resistance (IR), and cardiometabolic alterations. These disturbances are a consequence of PCOS's complex etiology. PCOS is mainly related to women with obesity; however, there are many PCOS lean patients too. Even though they share some aspects in their metabolic profiles, each group has individual differences in body composition and other parameters. Thus, in order to achieve successful therapeutic strategies, they should be tailored to these details. The authors reviewed PubMed's updated and related publications about body composition and nutritional strategies for PCOS lean and obese patients. As previous reports have determined, dietary patterns are essential in PCOS treatment. Several diets have been studied to control and improve IR, infertility, and cardiometabolic dysfunctions in PCOS. This review will explain the specific features in metabolic characterization and body composition among these patients. Finally, the diverse nutritional strategies used in women with PCOS will be analyzed depending on their lean or obese phenotype.
Keywords: Polycystic ovary syndrome, Body composition, Obesity, BMI, Diet modification
1. Introduction
PCOS is a common endocrine dysfunction in women during their fertile years. The estimated prevalence is between 10 and 15% worldwide [1]. The variety of signs and symptoms is characteristic of this syndrome. According to the Rotterdam 2003 diagnostic criteria, PCOS is diagnosed if at least 2 of the following 3 criteria are present: Oligo- and/or anovulation; clinical and/or biochemical signs of hyperandrogenism; polycystic ovaries, taking into consideration that other specific diagnoses such as congenital adrenal hyperplasias, androgen-secreting tumors, Cushing's syndrome, have been studied and excluded [2,3]. Multiple factors are implied to the onset of this syndrome. Many publications focus on the impact of developmental [4], environmental, genetic [5], and epigenetic processes on PCOS pathophysiology [6]. Some of them are present during pregnancy, such as anti-Müllerian hormone levels, intrauterine growth restriction, androgen excess, and endocrine disruptors (e.g. bisphenol A) [7]. These last two also occurred after birth [7]. All of them contribute to the progress of IR and obesity.
Although the mechanism that associates them is not clear, it is known that obesity, IR, compensatory hyperinsulinemia coupled with a chronic low-grade inflammatory state often coexists with this syndrome [8]. These same endocrines and metabolic disorders that develop from PCOS give these patients an increased risk of developing metabolic syndrome, type 2 diabetes mellitus (T2D), infertility [8], cardiovascular diseases (CVD) [9], negative maternal/neonatal outcomes [10], adrenal incidentalomas [11], among others.
It is important to note that PCOS is not exclusive to obese women; it also occurs in lean women. A study by Satyaraddi et al. shows that obese and lean women with PCOS, compared to the control group, are metabolically worse and have more visceral adiposity, concluding that non-obese PCOS presents a metabolic risk similar to that of obese patients because they present a similar amount of visceral adipose tissue [12].
It seems clear that environmental factors such as eating habits play an essential role in preventing and treating women with PCOS. International recommendations indicate that weight control is one of the main treatment strategies for PCOS since obesity worsens the clinical presentation of this syndrome [13]. There is still no consensus on what is the best nutritional treatment for PCOS. However, according to Faghfoori et al., it is recommended to carry out a dietary treatment that has an impact on the control of IR, metabolic functions from a hypocaloric diet, with a low contribution of simple sugars and refined carbohydrates, promoting the intake of foods with a low glycemic index (GI) [14]. Likewise, the reduction of the contribution of saturated and trans fatty acids will be sought, and attention should be paid to possible deficiencies such as vitamin D, chromium, and omega-3 [14]. In turn, it has been observed that, since PCOS can be the consequence of a lipid-induced proinflammatory state, a healthy diet with adequate distribution of macronutrients seems to be a good option [15]. Other diets that have been studied are the low saturated fat weight-loss diet, low GI diet [16], the ketogenic Mediterranean with phytoextracts (KEMEPHY) diet [17], and the very low carbohydrate diet.
In addition, it has been postulated that the Mediterranean diet (MD) can be an adequate dietary treatment for PCOS due to its anti-inflammatory effect and its relationship with the decrease in body weight [8]. The MD is based on the regular consumption of unsaturated fats, low-glycemic carbohydrates, fiber, vitamins, antioxidants, and a moderate animal protein intake [18]. Also, a more elevated adhesion to the MD related to larger Phase Angle has been published as evidence of cell stability, as another positive effect of MD on PCOS [19].
Given the high prevalence of PCOS worldwide and its associated health consequences, the present work aims to know the different nutritional approaches that should be carried out in women with PCOS based on the lean or obese phenotype to take actions to control this syndrome. So, it will be analyzed the specific characteristics in body composition and metabolic profile between these two groups. Later it will be developed the nutritional approach for each of them.
2. Differences in body composition and metabolic profile between lean PCOS and obese PCOS
Adipose tissue is a highly complex organ with profound effects on physiology and pathophysiology. In fact, in recent decades, it has come to be considered a central endocrine organ in energy homeostasis with implications in metabolic disorders [20]. The most common classification scheme distinguishes between subcutaneous and visceral fat, mainly because the latter deposit has a well-known association with metabolic disease, while the former does not [21].
Weight gain and central obesity, although not a diagnostic criterion, are common features of PCOS [22]. Visceral adiposity in PCOS is associated with increased IR, leading to exacerbation of reproductive and metabolic abnormalities [23]. In turn, androgens promote visceral fat accumulation and IR by inhibiting lipolysis and promoting lipogenesis. Additionally, obesity significantly impacts the PCOS phenotype since it is associated with a higher prevalence of menstrual irregularity, hyperandrogenemia, and hirsutism [23]. However, this process has several vicious circles, with two-way relationships between androgen excess, adipose dysfunction, IR, and other actors, such as inflammation [24], and oxidative stress [25,26], cluttering the scene and preventing a breakdown. It is needed a thorough and complete understanding that encompasses the heterogeneity of PCOS [27,28].
Most of the studies that addressed excess body fat in PCOS relied on approximate total or core fat mass indices, such as body mass index (BMI) and waist circumference or waist-to-hip ratio [29]. In practice and research studies, the obese phenotype of PCOS differs from lean based on BMI, that is, if it is above or below, respectively, the limit considered normal for age and ethnicity [30].
It is important to note that BMI is not an accurate descriptor of body composition and is widely surpassed by more specific techniques such as computed tomography (CT), magnetic resonance imaging (MRI), and dual-energy X-ray Absorptiometry (DEXA) [31]. These tools allow the assessment of individual differences in body composition, fat distribution, and adipose tissue function [31]. In this sense, Carmina et al., found an inverse correlation between adiposity assessed by DEXA (total or central fat) and insulin sensitivity (QUICKI) in patients with PCOS [32]. For their part, Barber et al. reported that visceral fat, assessed by MRI, was correlated with IR (HOMA2) in a small group of overweight women with PCOS [33]. Consistent with these findings, Penaforte et al. reported that women with insulin-resistant PCOS, as defined by QUICKI, had higher amounts of visceral and trunk abdominal fat, as measured by CT, than non-insulin-resistant women [34]. In a large cohort of women with PCOS, Tosi et al. demonstrated that total or trunk fat mass is strongly and inversely associated with insulin-induced glucose clearance during clamping, considered the gold standard for evaluating IR [35]. This finding supports the hypothesis that excess body fat may be an essential determinant of IR in PCOS [35]. Satyaraddi et al. in a case-control study with 81 patients with PCOS (42 obese and 39 non-obese), evaluated the disposition of fat using DEXA and concluded that both obese and non-obese women with PCOS, in compared to their age controls with the same BMI, were metabolically worse and had more visceral adiposity [12]. Non-obese PCOS has a similar risk to obese PCOS by having a similar amount of visceral adipose tissue (corrected for body weight) [12].
Beyond obesity, the metabolic abnormalities found in PCOS include dyslipidemias, IR, hyperinsulinemia, and glucose intolerance [36]. When it comes to characterizing the metabolic profiles of the obese and lean phenotypes of women with PCOS, it is essential to do so through the differential patterns of insulin physiology [37]. While non-obese women have lower insulin levels and IR [38] associated with higher levels of sex hormone-binding globulin [39], hyperinsulinemia remains a common finding in this population [40]. A primary alteration in β-cell function has been suggested as a weight-independent pathophysiological component in women with PCOS [41]. Insulin hypersecretion is the probable underlying mechanism in this scenario, present in both lean and obese subjects [42]. A possible link between adipose tissue and insulin sensitivity is provided by mediating adipokines, products of adipose secretory activity. Among them, adiponectin, a protein produced almost exclusively by adipocytes, is considered to exert insulin-sensitizing, anti-atherogenic and anti-inflammatory actions. It has been proposed that adiponectin expression is down-regulated by obesity [43]. In a systematic review and meta-analysis by Toulis et al., regarding adiponectin levels in women with PCOS, they conclude that after controlling for BMI-related effects, adiponectin levels appear to be lower in women with PCOS than in controls without PCOS [44]. Low adiponectin levels in PCOS are likely related to IR, but not testosterone [44]. Lean women with PCOS have shown decreased sensitivity to catecholamine-mediated lipolysis in subcutaneous adipose tissue (SAT), which results in the preservation of this tissue [45]. Because leptin is secreted primarily from SAT, this may partially explain the hyperleptinemia found in normal-weight women with PCOS, and although intrinsic dysregulation of its secretion mechanisms may also be involved, the precise chain of events remains unclear [46]. The role of vitamin D in the pathogenesis of IR associated with PCOS has been postulated, regardless of BMI. The insulin receptor mRNA genomic input is improved by Vitamin D signaling through vitamin D receptor (VDR) [47]. This action initiates insulin synthesis and discharge. It also restrains some pro-inflammatory cytokines directly related to the IR pathogenesis. There is evidence implying that vitamin D deficiency occurs commonly in obese PCOS patients [48,49]. Furthermore, this condition is also related to several other alterations such as cancer progression [50], CVD, autoimmune disorders (e.g., autoimmune thyroid disease) [51], and sleep disruptions. Dyslipidemia is probably the most common metabolic disorder in PCOS detected in 70% of patients according to the National Cholesterol Education Program (NCEP) guidelines [52]. Most studies report a decrease in HDL cholesterol and an increase in triglyceride levels, the same lipid profile that is known to be associated with IR. Lean PCOS women had more diminished HDL and HDL2 levels than their counterparts with normal ovarian function, while obese PCOS individuals also presented increased triglyceride levels [53]. Finally, oxidative stress and low-grade inflammation are two characteristics shared equally by obese and non-obese patients with PCOS [54].
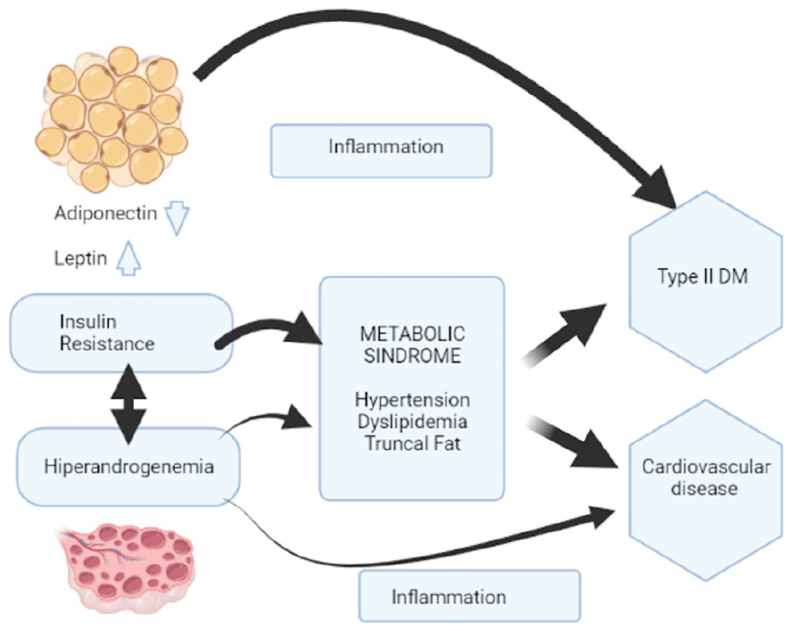
In PCOS women, a particular biological pattern is found worthy of analyzing a complex combination of the consequences of androgen excess, dyslipidemia, and IR (Fig. 1). In general, obese phenotypes have more severe clinical forms of PCOS than their lean equivalent. However, both share many central pathophysiological processes (hyperandrogenemia, IR, oxidative stress, chronic inflammation) [4].
Fig. 1.
Pathophysiology of polycystic ovary syndrome (PCOS)
PCOS: polycystic ovary syndrome; DM: Diabetes mellitus; OH: ovarian hyperandrogenism; IR: insulin resistance; LH: luteinizing hormone; T2D: type 2 diabetes.
Fig. 1. Diagram describing the pathophysiology of polycystic ovary syndrome (PCOS) and the amplifying factor of visceral adiposity in metabolic disorders. The common denominator in PCOS seems to be OH, with IR and consequent counterbalancing hyperinsulinemia, a nonessential but common aggravating factor in this pathophysiology. The propensity for obesity appears to be secondary to the underlying OH and hyperinsulinism. OH can explain the fundamental clinical features of PCOS: hirsutism, oligoanovulation, and polycystic ovaries. Hyperinsulinemia affects the ovary selectively, which persists insulin sensitive, while liver and skeletal muscle evidence IR. In the ovary, hyperinsulinism cooperates with LH to up-regulate androgen generation. These effects cause premature luteinization of granulosa cells complicating hyperandrogenism. Obesity worsens the clinical severity of OH by increasing IR. The mechanisms may include a deficiency of insulin-sensitizing adipokines such as adiponectin in favor of pro-inflammatory cytokines such as TNF-alpha; in turn, hyperinsulinemia also promotes adiposity, closing the vicious circle. Due to its increased lipolytic response to catecholamines, visceral fat gives more IR than subcutaneous abdominal fat. Androgens oppose the effects of insulin on subcutaneous fat stores, while hyperandrogenemia promotes the accumulation of visceral fat. These situations lead to chronic inflammation that mediates long-term cardiometabolic complications and comorbidities seen in women with POCS, including dyslipidemia, metabolic syndrome, T2D, and cardiovascular disease.
3. Nutritional approach to lean PCOS
PCOS is the most common endocrinopathy of females during reproductive age. Hyperandrogenism, IR chronic anovulation, and pathogenesis are still unclear, but there is solid evidence that genetic factors are part of PCOS's etiology since there is a high prevalence of this syndrome in members of the same family. IR, high androgen levels, and dyslipidemia have been found even in non-obese members of patients' families [36].
There is a broad differential disparity of PCOS diagnosis, and it is due to the different criteria used for the syndrome and differences in geographical and environmental factors in relevant studies. PCOS is usually more commonly found in obese women [36]. Obesity is reported in 25–70% of women with PCOS, but a significant proportion of patients have it despite having a normal BMI (≤25 kg/m2) [36]. These findings complicate its diagnostics and therapeutic approach more difficult to determine. These lean PCOS patients may or may not have the cardinal symptoms such as irregular menstrual cycles and acne [30].
Despite androgen and adiposity levels, the majority of PCOS patients have increased serum insulin and IR. Several reports showed the different metabolic disturbances characteristics in PCOS patients, like increased plasma insulin levels 2 h after glucose administration to lean PCOS patients, correlated with the lean, healthy participants. Also, important risen low-density lipoprotein (LDL) and total cholesterol have been associated in lean PCOS women versus controls [1,30,55].
Nutrition is one of the major modifiable environmental risk factors for several non-communicable diseases and plays an essential role in their prevention and initial treatment [56]. Weight loss is usually considered the first-line treatment in women exhibiting the obese phenotype, which in lean women is not needed to lose weight. Instead, for lean PCOS women, the main goal is to maintain their weight. Many lifestyle modifications such as dietary interventions and regular physical activity have demonstrated improved IR and ameliorated hyperandrogenism amongst other beneficial effects on PCOS symptoms [57]. Lean individuals with PCOS must be encouraged to consume a healthier diet in its composition, like increasing vegetables and fruit daily to ensure they have an adequate supply of various minerals, vitamins, and nutrients [30].
Evidence-based guidelines recommend that lifestyle modification regimens incorporate a dietary intake consistent with usual healthy nutritional recommendations that include modified macronutrient composition. These recommendations can be followed for weight maintenance and body composition improvement and the prevention of weight gain and fat mass increase in lean women with PCOS [30].
It is essential to mention and remark that lean women with PCOS need various nutrients, minerals, and vitamins in their diet since they do not need to lose weight or be restricted with their food consumption. PCOS is also associated with low-grade inflammation. This relation could support and demonstrate the therapeutic role of foods and nutrients such as the Mediterranean dietary pattern in the PCOS pathogenesis that likely involving their inflammatory status, IR, and hyperandrogenemia [8]. Nevertheless, the exact dietary macro and micronutrient combination needed for a good nutritional plan to improve PCOS clinical characteristics are undetermined.
The MD is a plant-based, antioxidant-rich diet known for its several health benefits. It is characterized by a high intake of whole grains, fruits, vegetables, tree nuts, legumes, and olive oil daily; a moderate intake of fish and poultry, and low consumption of dairy products, red meat, processed meat and sweets, and finally a moderate consumption of wine with meals. This dietary pattern is considered nutritionally complete and adequate since it is very easy to follow; it is based on the traditional foods that people used to eat in their countries. Higher adherence to this nutritional approach has also been proven to be effective in preserving the skeletal muscle mass in healthy women, likely due to the potential anti-inflammatory and antioxidant properties of micronutrients or through their direct role in muscle metabolism and physiology, such as with magnesium and potassium [56].
As stated before, inflammation and IR are often associated with PCOS. This type of inflammation and hyperandrogenism are linked to saturated fat. As stated before, inflammation and IR are often associated with PCOS. This type of inflammation and hyperandrogenism are linked to saturated fat. A lipid-stimulated proinflammatory status may cause the characteristic IR, hyperandrogenism, and dyslipidemia frequently found in PCOS women. A better macronutrient distribution of the diet despite not being intended for weight loss may improve the constant inflammatory state. So, there is a strategic relevance and importance of the nutritional assessment and body composition evaluation of women with PCOS that needs to be considered a crucial step in managing this syndrome [15].
A better macronutrient distribution of the diet despite not being intended for weight loss may improve the constant inflammatory state. So, there is a strategic relevance and importance of the nutritional assessment and body composition evaluation of women with PCOS that needs to be considered a crucial step in managing this syndrome [15].
One of the goals of patients with PCOS is on supporting their body by promoting healthy hormonal balance, a good uterine lining, regular ovulation, improving estrogen metabolism, and these could be achieved by vitamins and minerals such as calcium and vitamin D, as well as some medicinal herbs that promote hormonal balance and support regular ovulation [58].
There is promising evidence from several studies suggesting that vitamin D may be involved in several features of PCOS, such as infertility [59], hirsutism, IR [60], and CVD risk. For example, vitamin D supplementation may improve reproductive function in women with PCOS by restoring regular menstrual cycles [61,62]. There is a need for more randomized trials in well-delineated and determined populations that will help in defining the role of vitamin D in PCOS.
Some publications state that decreased vitamin D levels and IR are independent body size characteristics in PCOS patients. This assertion is highly relevant since many PCOS women have IR but are not obese [48,49,54]. Lifestyle intervention has been helpful as it improves body composition, hyperandrogenism, and IR in women with PCOS. However, there is a lack of evidence regarding the effect of this lifestyle intervention on improving glucose tolerance or lipid profiles and assessing clinical reproductive outcomes, such as quality of life and treatment satisfaction [63].
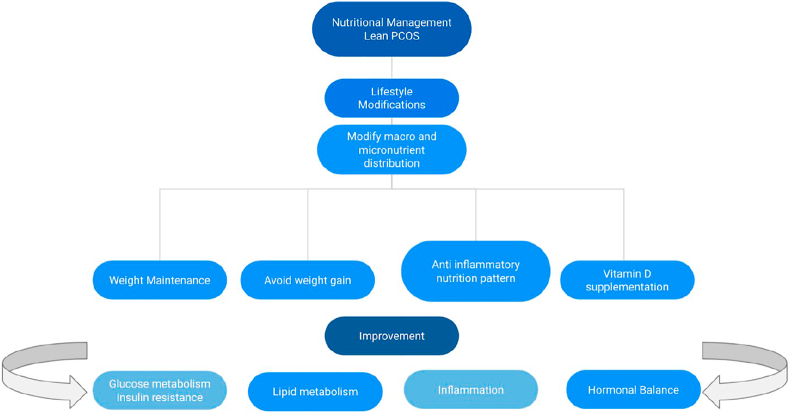
There is still the need for more robust evidence to present a foundation for verifiable nutritional approaches that can help and improve PCOS treatment in this specific population. It is of great interest to find appropriate nutritional interventions that would be highly appealing as a therapeutic lifestyle approach to clinically managing this pathology [58] (Fig. 2).
Fig. 2.
Nutritional management for polycystic ovary syndrome (PCOS) lean patients.
PCOS: polycistic ovary syndrome; IR: insulin resistance.
Fig. 2. Nutritional management for PCOS lean patients. Lifestyle modifications such as dietary interventions and regular physical activity have demonstrated improved IR and ameliorated hyperandrogenism amongst other beneficial effects on PCOS symptoms in PCOS lean patients. These patients must be encouraged to consume a healthier diet in its composition, like increasing vegetables and fruit daily to ensure they have an adequate supply of various minerals, vitamins, and nutrients and prevent weight gain and fat mass increase. Nutritional macro and micronutrient composition have positive effects on glucose and lipid metabolism, inflammation, and hormonal balance. For example, vitamin D supplementation may improve reproductive function in women with PCOS by restoring regular menstrual cycles.
4. Nutritional approach to obese PCOS
The frequency of obese women with PCOS is greater than that of the general female population compared to their lean counterparts [1].
Excessive adiposity in PCOS is associated with increased IR rates, resulting in reproductive and metabolic abnormalities aggravation. Also, excessive weight has a significant impact on the PCOS phenotype since it is associated with a greater prevalence of menstrual and hormonal irregularity, and hirsutism [1].
As for PCOS, no universal treatment is available, and therefore, treatment must always be individualized to the actual needs of the individual patient. Considering that IR is a major issue on PCOS, first-line therapy treatment are lifestyle changes, including modifications of diet and/or physical activity levels should be recommended, given the positive effect that has on improving body composition (abdominal adiposity and obesity), glucose, and/or lipid profile [3,14].
As for dietary treatment, should be taken into consideration a negative energy balance in order to achieve an energy deficit. The specific dietary composition that should be implemented remains quite controversial, which can be considered a challenge for clinicians. In the general population, research evaluating the effects of varying dietary recommendations differs from low-calorie diets with fat modifications, MD, ketogenic diet (KD) and reduction in the dietary GI in addition to overall calorie reduction to induce weight loss may have a positive effect on IR (Table 1) [64,65]. Also, studies that converge weight-loss strategies and the use of inositol have shown that they could bring weight loss in subjects with PCOS [66]. PCOS has been associated with an increased frequency of weight management methods [67]. These findings may be related to women with PCOS being more likely to perceive themselves at risk of excessive weight gain [[67], [68], [69]].
Table 1.
Characteristics of reviewed studies assessing the effect of different dietary compositions on obesity in PCOS.
| Reference | Population | Interventions | Outcomes |
|---|---|---|---|
| Marsh et al., 2010 [16] | PCOS Mean BMI >34 Mean age >30 yo Sydney, Australia Low GI: n > 29 completed Healthy diet: n > 20 completed |
Low saturated fat weight-loss diet Low GI: 1,576 kcal, 50% carbohydrate, 23% protein, 27% fat, 34 g fiber, 40%GI, 74 g GL Standard healthy: 1,569 kcal, 50% carbohydrate, 23% protein, 27% fat, 34 g fiber, 59% GI, 109 g GL |
Low-GI vs healthy diet: Greater improvement in insulin sensitivity, lower fibrinogen, improved menstrual regularity. No significant differences in other outcomes |
| Paoli et al., J 2020 [17] | n: 14 PCOS BMI: 28.84 |
A modified KD protocol was used. The KEMEPHY diet (Mediterranean eucaloric ketogenic) 1600/1700 kcal/day) with phytoextracts, Food supplements are high proteins (19 g/portion) and very low carbohydrate (3.5 g/portion) | Improvement in levels: Testosterone, DHEAs, LH, FSH. Weight loss: 9,43 kg BMI at the end of intervention: 25,49 kg/m2 |
| Le Donne et al., 2019 [64] | n: 43 PCOS 3 groups BMI: 31,8 kg/m2 |
3 groups Group 1(n = 21) diet only Group 2 (n = 10) Diet and MI Group 3 (n = 12) diet inositols (MI + DCI). Duration: 6 months for all groups. The diet (1200 Kcal) administered to all three groups 25% fats, 15–18% proteins and the remaining portion glucids; low GI foods were recommended |
Weight loss: 8.1, 8.5 and 9.8 kg for Group 1,2,3 respectively and significant fat mass lost for Group 1 |
Weight loss, by 5–10%, can improve insulin and testosterone levels and improve menstrual hormones [64,68]. Among the different nutritional strategies, the MD is recognized as a dietary pattern due to its characteristics, like consumption of fatty acids, omega-3 unsaturated fatty acids, and diminished consumption of animal-derived proteins that may decrease many risk factors for metabolic disorders such as endothelial dysfunction, fatty acids alterations and IR [56,67]. The main impact on inflammatory activity is due to the microbiota modulation as a result of the dietary fiber and the high intake of both polyunsaturated fatty acids (PUFA) omega 3 and antioxidants [56].
In the last couple of years, there has been observed that women population presented lower intake of energy-dense nutrient-poor foods and soda drinks, and higher intake of fruits and legumes thus higher adherence to the MD that could support a therapeutic role that has the dietary pattern on PCOS [8,56].
Physical exercise has been shown to improve several factors related to PCOS such as ovulation rates, menstrual regularity, CVD [9], decreasing waist circumference, weight, and total fat mass making exercise a positive non-pharmacological lifestyle change for PCOS [70]. Physical activity has shown improvement in infertility in women. In a retrospective cohort study of obese infertile women undergoing cycles of in-vitro fertilization, the results of patients with regular physical activity were compared with those who were inactive. There were significantly more elevated pregnancy rates in the first group [71,72]. International guidelines for assessing and managing PCOS (2018) indicate that variations on intensity and time of exercise are recommended as a lifestyle change to improve overall health in individuals with PCOS [73]. Daily high-intensity interval training and strength training for at least two nonconsecutive days for a minimum of 150 min per week of moderate-intensity, 75 min per week of vigorous-intensity, or an equivalent of both [70].
As for micronutrient studies, several studies have suggested that 25(OH)D may be involved in complications related to PCOS, such as fertility, hirsutism, glucose metabolism, and CVD risk [48,61]. Vitamin D plays a role in various metabolic pathways, including glucose metabolism. 25(OH)D levels are an independent predictor of total fat mass in PCOS patients. The mechanism behind this effect is still unknown, but the immunomodulatory role is well documented, and the lack of 25(OH)D may cause inflammatory responses, thus worsening IR. Therefore, weight loss should be kept in mind in the management of obese PCOS to restore regular 25(OH)D levels, insulin-sensitivity as a result of total fat mass loss [14,48]. Intervention studies of vitamin D supplementation in women with PCOS have shown improvements in reproductive function, glucose metabolism, and lipid profile by improving reproductive function [48,61,74,75]. Ongoing research is expected to provide answers to whether vitamin D supplementation around 600–800 IU/day depending on age and gender, may be effective [48].
Another micronutrient that is worth discussing is cyclohexane-1,2,3,4,5,6-hexol (Inositol) belongs to the vitamin B complex. The stereoisomers of inositol such as Myo-inositol (MYO-INS) and D-chiro-inositol (DCI) are currently being used for the treatment of PCOS by improving IR and reducing CVD risk factors [51,76].
Bariatric surgery does appear to change the PCOS complications such as ovulation rates, menstrual regularity, CVD, decreasing waist circumference, and total fat mass. A study of women with PCOS who underwent laparoscopic bypass, lowering androgen levels and menstrual cycles, insulin sensitivity, and blood pressure demonstrated that obesity significantly impacts the pathophysiology of obesity-related infertility [72]. The indications for bariatric surgery are not different in women with PCOS (BMI ≥40 kg/m2 or ≥35 kg/m2 and severe morbidity presence. In 2017 a study concluded that weight loss in women with severe obesity and PCOS with gastric surgery resulted in improvement of male hormones like testosterone, hirsutism, and menstrual dysfunction [17].
5. Conclusions
Even though of the high prevalence of PCOS women with obesity, it is not unusual to find a significant proportion of patients with normal BMI. Regardless of androgen concentrations and adiposity levels, these women have elevated serum insulin and IR.
The first line of treatment for PCOS is a decrease in body weight of at least 5–10% in those women who are obese, or if it is a PCOS lean patient, it is recommended to maintain it. A healthy dietary pattern with satisfactory macronutrient distribution is essential in these patients since IR, dyslipidemia, and hyperandrogenism might result from a lipid-induced pro-inflammatory state. Various diets have been studied to treat PCOS, such as the low saturated fat weight-loss diet, low GI diet, the KEMEPHY diet with phytoextracts, and a very low carbohydrate diet. All of them have positively improved some aspects of the PCOS characteristics. Some of them have reached a significant weight loss in those PCOS obese patients. However, the MD has been proposed as a possible eating guide that leads to better results in PCOS individuals because of its anti-inflammatory effect and its relationship with decreased body weight.
In obese women who have undergone bariatric surgery, an improvement has been found by reducing androgen levels and menstrual cycles, insulin sensitivity, and blood pressure. Notably, an increase in the frequency of body weight control methods has been observed, so health personnel must evaluate this aspect in their follow-up visits. Also, vitamin D supplementation has been reported to improve reproductive function in women with PCOS. In itself, cyclohexane-1,2,3,4,5,6-hexol (Inositol) is another of the nutrients being studied for its possible benefits in the treatment of this syndrome. Within the strategies to improve the lifestyle for PCOS treatment, physical activity has a fundamental role. It has been demonstrated that it improves several factors related to PCOS.
Since the dramatically increased prevalence of this syndrome in the last years and considering the consequences for women's health both in the short and long term, it is essential to continue investigating possible non-pharmacological treatments and their impact on PCOS.
CRediT authorship contribution statement
Luigi Barrea: Conceptualization, Methodology, Formal analysis, Writing – original draft, Writing – review & editing. Evelyn Frias-Toral: Investigation, Data curation. Ludovica Verde: Investigation, Data curation. Florencia Ceriani: Investigation, Data curation. Gabriela Cucalón: Investigation, Data curation. Eloisa Garcia-Velasquez: Investigation, Data curation. Dino Moretti: Investigation, Data curation. Silvia Savastano: Conceptualization, Visualization, Supervision. Annamaria Colao: Visualization, Supervision. Giovanna Muscogiuri: Conceptualization, Methodology, Validation, Formal analysis, Writing – original draft, Writing – review & editing, All authors have read and agreed to the published version of the manuscript.
Declaration of competing interest
No conflict of interest has been declared by the authors.
Acknowledgements
The assistance of the staff is gratefully appreciated.
Abbreviations
- BMI
body mass index
- CVD
cardiovascular disease
- CT
Computed tomography
- DCI
D-chiro-inositol
- DEXA
Dual-energy X-ray Absorptiometry
- DHEAs
Dehydroepiandrosterone sulfate
- FSH
Follicle-stimulating hormone
- GI
Glycemic index
- IR
Insulin resistance
- KD
Ketogenic Diet
- KEMEPHY
Ketogenic Mediterranean with phytoextracts
- LH
Luteinizing hormone
- LDL
Low-density lipoprotein
- MD
Mediterranean Diet
- MYO-INS
Myo-inositol MRI magnetic resonance imaging
- NCEP
National Cholesterol Education Program
- PCOS
Polycystic Ovary Syndrome
- PUFA
Polyunsaturated fatty acids
- QUICKI
Quantitative insulin sensitivity check index
- SAT
Subcutaneous adipose tissue
- T2D
Type 2 diabetes mellitus
- VDR
Vitamin D receptor
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Orio F., Muscogiuri G., Nese C., Palomba S., Savastano S., Tafuri D., Colarieti G., La Sala G., Colao A., Yildiz B.O. Obesity, type 2 diabetes mellitus and cardiovascular disease risk: an uptodate in the management of polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2016 Dec;207:214–219. doi: 10.1016/j.ejogrb.2016.08.026. Epub 2016 Aug 12. PMID: 27575870. [DOI] [PubMed] [Google Scholar]
- 2.Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2004 Jan;19(1):41–47. doi: 10.1093/humrep/deh098. PMID: 14688154. [DOI] [PubMed] [Google Scholar]
- 3.Escobar-Morreale H.F. Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nat Rev Endocrinol. 2018 May;14(5):270–284. doi: 10.1038/nrendo.2018.24. Epub 2018 Mar 23. PMID: 29569621. [DOI] [PubMed] [Google Scholar]
- 4.Ganie M.A., Vasudevan V., Wani I.A., Baba M.S., Arif T., Rashid A. Epidemiology, pathogenesis, genetics & management of polycystic ovary syndrome in India. Indian J Med Res. 2019 Oct;150(4):333–344. doi: 10.4103/ijmr.IJMR_1937_17. PMID: 31823915; PMCID: PMC6902362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crespo R.P., Bachega T.A.S.S., Mendonça B.B., Gomes L.G. An update of genetic basis of PCOS pathogenesis. Arch Endocrinol Metab. 2018 Jun;62(3):352–361. doi: 10.20945/2359-3997000000049. PMID: 29972435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bednarska S., Siejka A. The pathogenesis and treatment of polycystic ovary syndrome: what's new? Adv Clin Exp Med. 2017 Mar-Apr;26(2):359–367. doi: 10.17219/acem/59380. PMID: 28791858. [DOI] [PubMed] [Google Scholar]
- 7.Pivonello C., Muscogiuri G., Nardone A., Garifalos F., Provvisiero D.P., Verde N., de Angelis C., Conforti A., Piscopo M., Auriemma R.S., Colao A., Pivonello R. Bisphenol A: an emerging threat to female fertility. Reprod Biol Endocrinol. 2020 Mar 14;18(1):22. doi: 10.1186/s12958-019-0558-8. PMID: 32171313; PMCID: PMC7071611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barrea L., Arnone A., Annunziata G., Muscogiuri G., Laudisio D., Salzano C., Pugliese G., Colao A., Savastano S. Adherence to the Mediterranean diet, dietary patterns and body composition in women with polycystic ovary syndrome (PCOS) Nutrients. 2019 Sep 23;11(10):2278. doi: 10.3390/nu11102278. PMID: 31547562; PMCID: PMC6836220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Orio F., Muscogiuri G., Giallauria F., Savastano S., Bottiglieri P., Tafuri D., Predotti P., Colarieti G., Colao A., Palomba S. Oral contraceptives versus physical exercise on cardiovascular and metabolic risk factors in women with polycystic ovary syndrome: a randomized controlled trial. Clin Endocrinol (Oxf). 2016 Nov;85(5):764–771. doi: 10.1111/cen.13112. Epub 2016 Jul 7. PMID: 27219465. [DOI] [PubMed] [Google Scholar]
- 10.Palomba S., Falbo A., Chiossi G., Muscogiuri G., Fornaciari E., Orio F., Tolino A., Colao A., La Sala G.B., Zullo F. Lipid profile in nonobese pregnant women with polycystic ovary syndrome: a prospective controlled clinical study. Steroids. 2014 Oct;88:36–43. doi: 10.1016/j.steroids.2014.06.005. Epub 2014 Jun 16. PMID: 24945113. [DOI] [PubMed] [Google Scholar]
- 11.Muscogiuri G., Colao A., Orio F. Insulin-mediated diseases: adrenal mass and polycystic ovary syndrome. Trends Endocrinol Metabol. 2015 Oct;26(10):512–514. doi: 10.1016/j.tem.2015.07.010. PMID: 26412152. [DOI] [PubMed] [Google Scholar]
- 12.Satyaraddi A., Cherian K.E., Kapoor N., Kunjummen A.T., Kamath M.S., Thomas N., Paul T.V. Body composition, metabolic characteristics, and insulin resistance in obese and nonobese women with polycystic ovary syndrome. J Hum Reprod Sci. 2019 Apr-Jun;12(2):78–84. doi: 10.4103/jhrs.JHRS_2_19. PMID: 31293320; PMCID: PMC6594114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Teede H.J., Misso M.L., Costello M.F., Dokras A., Laven J., Moran L., Piltonen T., Norman R.J., International PCOS Network Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum Reprod. 2018 Sep 1;33(9):1602–1618. doi: 10.1093/humrep/dey256. Erratum in: Hum Reprod. 2019 Feb 1;34(2):388. PMID: 30052961; PMCID: PMC6112576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faghfoori Z., Fazelian S., Shadnoush M., Goodarzi R. Nutritional management in women with polycystic ovary syndrome: a review study. Diabetes Metab Syndr. 2017 Nov;11(Suppl 1):S429–S432. doi: 10.1016/j.dsx.2017.03.030. Epub 2017 Apr 5. PMID: 28416368. [DOI] [PubMed] [Google Scholar]
- 15.González F., Considine R.V., Abdelhadi O.A., Acton A.J. Inflammation triggered by saturated fat ingestion is linked to insulin resistance and hyperandrogenism in polycystic ovary syndrome. J Clin Endocrinol Metab. 2020 Jun 1;105(6):e2152–e2167. doi: 10.1210/clinem/dgaa108. PMID: 32140727; PMCID: PMC7150616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marsh K.A., Steinbeck K.S., Atkinson F.S., Petocz P., Brand-Miller J.C. Effect of a low glycemic index compared with a conventional healthy diet on polycystic ovary syndrome. Am J Clin Nutr. 2010 Jul;92(1):83–92. doi: 10.3945/ajcn.2010.29261. Epub 2010 May 19. PMID: 20484445. [DOI] [PubMed] [Google Scholar]
- 17.Paoli A., Mancin L., Giacona M.C., Bianco A., Caprio M. Effects of a ketogenic diet in overweight women with polycystic ovary syndrome. J Transl Med. 2020 Feb 27;18(1):104. doi: 10.1186/s12967-020-02277-0. PMID: 32103756; PMCID: PMC7045520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Willett W.C., Sacks F., Trichopoulou A., Drescher G., Ferro-Luzzi A., Helsing E., Trichopoulos D. Mediterranean diet pyramid: a cultural model for healthy eating. Am J Clin Nutr. 1995 Jun;61(6 Suppl):1402S–1406S. doi: 10.1093/ajcn/61.6.1402S. PMID: 7754995. [DOI] [PubMed] [Google Scholar]
- 19.Barrea L., Muscogiuri G., Macchia P.E., Di Somma C., Falco A., Savanelli M.C., Colao A., Savastano S. Mediterranean diet and Phase Angle in a sample of adult population: results of a pilot study. Nutrients. 2017 Feb 17;9(2):151. doi: 10.3390/nu9020151. PMID: 28218645; PMCID: PMC5331582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosen E.D., Spiegelman B.M. What we talk about when we talk about fat. Cell. 2014 Jan 16;156(1–2):20–44. doi: 10.1016/j.cell.2013.12.012. PMID: 24439368; PMCID: PMC3934003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee M.J., Wu Y., Fried S.K. Adipose tissue heterogeneity: implication of depot differences in adipose tissue for obesity complications. Mol Aspect Med. 2013 Feb;34(1):1–11. doi: 10.1016/j.mam.2012.10.001. Epub 2012 Oct 13. PMID: 23068073; PMCID: PMC3549425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Escobar-Morreale H.F., San Millán J.L. Abdominal adiposity and the polycystic ovary syndrome. Trends Endocrinol Metabol. 2007 Sep;18(7):266–272. doi: 10.1016/j.tem.2007.07.003. Epub 2007 Aug 10. PMID: 17693095. [DOI] [PubMed] [Google Scholar]
- 23.Rosenfield R.L., Ehrmann D.A. The pathogenesis of polycystic ovary syndrome (PCOS): the hypothesis of PCOS as functional ovarian hyperandrogenism revisited. Endocr Rev. 2016 Oct;37(5):467–520. doi: 10.1210/er.2015-1104. Epub 2016 Jul 26. PMID: 27459230; PMCID: PMC5045492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rostamtabar M., Esmaeilzadeh S., Tourani M., Rahmani A., Baee M., Shirafkan F., Saleki K., Mirzababayi S.S., Ebrahimpour S., Nouri H.R. Pathophysiological roles of chronic low-grade inflammation mediators in polycystic ovary syndrome. J Cell Physiol. 2020 Jul 2 doi: 10.1002/jcp.29912. Epub ahead of print. PMID: 32617971. [DOI] [PubMed] [Google Scholar]
- 25.Zuo T., Zhu M., Xu W. Roles of oxidative stress in polycystic ovary syndrome and cancers. Oxid Med Cell Longev. 2016:8589318. doi: 10.1155/2016/8589318. Epub 2015 Dec 6. PMID: 26770659; PMCID: PMC4684888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khashchenko E., Vysokikh M., Uvarova E., Krechetova L., Vtorushina V., Ivanets T., Volodina M., Tarasova N., Sukhanova I., Sukhikh G. Activation of systemic inflammation and oxidative stress in adolescent girls with polycystic ovary syndrome in combination with metabolic disorders and excessive body weight. J Clin Med. 2020 May 9;9(5):1399. doi: 10.3390/jcm9051399. PMID: 32397375; PMCID: PMC7291245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moghetti P., Tosi F. Insulin resistance and PCOS: chicken or egg? J Endocrinol Invest. 2021 Feb;44(2):233–244. doi: 10.1007/s40618-020-01351-0. Epub 2020 Jul 9. PMID: 32648001. [DOI] [PubMed] [Google Scholar]
- 28.Dumesic D.A., Abbott D.H., Sanchita S., Chazenbalk G.D. Endocrine-metabolic dysfunction in polycystic ovary syndrome: an evolutionary perspective. Curr Opin Endocr Metab Res. 2020 Jun;12:41–48. doi: 10.1016/j.coemr.2020.02.013. Epub 2020 Mar 9. PMID: 32363240; PMCID: PMC7194185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lim S.S., Davies M.J., Norman R.J., Moran L.J. Overweight, obesity and central obesity in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2012 Nov-Dec;18(6):618–637. doi: 10.1093/humupd/dms030. Epub 2012 Jul 4. PMID: 22767467. [DOI] [PubMed] [Google Scholar]
- 30.Toosy S., Sodi R., Pappachan J.M. Lean polycystic ovary syndrome (PCOS): an evidence-based practical approach. J Diabetes Metab Disord. 2018 Nov 13;17(2):277–285. doi: 10.1007/s40200-018-0371-5. PMID: 30918863; PMCID: PMC6405408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prado C.M., Heymsfield S.B. Lean tissue imaging: a new era for nutritional assessment and intervention. JPEN - J Parenter Enter Nutr. 2014 Nov;38(8):940–953. doi: 10.1177/0148607114550189. Epub 2014 Sep 19. Erratum in: JPEN J Parenter Enteral Nutr. 2016 Jul;40(5):742. PMID: 25239112; PMCID: PMC4361695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carmina E., Bucchieri S., Esposito A., Del Puente A., Mansueto P., Orio F., Di Fede G., Rini G. Abdominal fat quantity and distribution in women with polycystic ovary syndrome and extent of its relation to insulin resistance. J Clin Endocrinol Metab. 2007 Jul;92(7):2500–2505. doi: 10.1210/jc.2006-2725. Epub 2007 Apr 3. PMID: 17405838. [DOI] [PubMed] [Google Scholar]
- 33.Barber T.M., Golding S.J., Alvey C., Wass J.A., Karpe F., Franks S., McCarthy M.I. Global adiposity rather than abnormal regional fat distribution characterizes women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2008 Mar;93(3):999–1004. doi: 10.1210/jc.2007-2117. Epub 2007 Dec 18. PMID: 18089693. [DOI] [PubMed] [Google Scholar]
- 34.Penaforte F.R., Japur C.C., Diez-Garcia R.W., Chiarello P.G. Upper trunk fat assessment and its relationship with metabolic and biochemical variables and body fat in polycystic ovary syndrome. J Hum Nutr Diet. 2011 Feb;24(1):39–46. doi: 10.1111/j.1365-277X.2010.01130.x. PMID: 21210872. [DOI] [PubMed] [Google Scholar]
- 35.Tosi F., Di Sarra D., Kaufman J.M., Bonin C., Moretta R., Bonora E., Zanolin E., Moghetti P. Total body fat and central fat mass independently predict insulin resistance but not hyperandrogenemia in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2015 Feb;100(2):661–669. doi: 10.1210/jc.2014-2786. Epub 2014 Nov 13. PMID: 25393642. [DOI] [PubMed] [Google Scholar]
- 36.Vryonidou A., Paschou S.A., Muscogiuri G., Orio F., Goulis D.G. Mechanisms IN endocrinology: metabolic syndrome through the female life cycle. Eur J Endocrinol. 2015 Nov;173(5):R153–R163. doi: 10.1530/EJE-15-0275. Epub 2015 Jun 1. PMID: 26034072. [DOI] [PubMed] [Google Scholar]
- 37.Shi Y.H., Zhao D.N., Zhao J.L., You L., Liu H., Sun M., Chen Z.J. [Characteristics of glucose metabolism in non-obese and obese women with polycystic ovarian syndrome] Zhonghua Fu Chan Ke Za Zhi. 2010 Aug;45(8):575–577. Chinese. PMID: 21029611. [PubMed] [Google Scholar]
- 38.Unluer A.N., Findik R.B., Sevinc N., Karakaya J. Comparison of HbA1c levels in obese and non-obese polycystic ovarian patients. Clin Exp Obstet Gynecol. 2013;40(1):148–150. PMID: 23724531. [PubMed] [Google Scholar]
- 39.Silfen M.E., Denburg M.R., Manibo A.M., Lobo R.A., Jaffe R., Ferin M., Levine L.S., Oberfield S.E. Early endocrine, metabolic, and sonographic characteristics of polycystic ovary syndrome (PCOS): comparison between nonobese and obese adolescents. J Clin Endocrinol Metab. 2003 Oct;88(10):4682–4688. doi: 10.1210/jc.2003-030617. PMID: 14557441. [DOI] [PubMed] [Google Scholar]
- 40.Dahan M.H., Reaven G. Relationship among obesity, insulin resistance, and hyperinsulinemia in the polycystic ovary syndrome. Endocrine. 2019 Jun;64(3):685–689. doi: 10.1007/s12020-019-01899-9. Epub 2019 Mar 21. PMID: 30900204; PMCID: PMC6557720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Messer C., Boston R., Leroith D., Geer E., Miller J.D., Messer M., Futterweit W. Pancreatic β-cell dysfunction in polycystic ovary syndrome: the role of metformin. Endocr Pract. 2012 Sep-Oct;18(5):685–693. doi: 10.4158/EP11375.OR. PMID: 22548946; PMCID: PMC3722880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vrbíková J., Bendlová B., Hill M., Vanková M., Vondra K., Stárka L. Insulin sensitivity and beta-cell function in women with polycystic ovary syndrome. Diabetes Care. 2002 Jul;25(7):1217–1222. doi: 10.2337/diacare.25.7.1217. PMID: 12087022. [DOI] [PubMed] [Google Scholar]
- 43.Stefan N., Stumvoll M. Adiponectin--its role in metabolism and beyond. Horm Metab Res. 2002 Sep;34(9):469–474. doi: 10.1055/s-2002-34785. PMID: 12384822. [DOI] [PubMed] [Google Scholar]
- 44.Toulis K.A., Goulis D.G., Farmakiotis D., Georgopoulos N.A., Katsikis I., Tarlatzis B.C., Papadimas I., Panidis D. Adiponectin levels in women with polycystic ovary syndrome: a systematic review and a meta-analysis. Hum Reprod Update. 2009 May-Jun;15(3):297–307. doi: 10.1093/humupd/dmp006. Epub 2009 Mar 4. PMID: 19261627. [DOI] [PubMed] [Google Scholar]
- 45.Leung K.L., Sanchita S., Pham C.T., Davis B.A., Okhovat M., Ding X., Dumesic P., Grogan T.R., Williams K.J., Morselli M., Ma F., Carbone L., Li X., Pellegrini M., Dumesic D.A., Chazenbalk G.D. Dynamic changes in chromatin accessibility, altered adipogenic gene expression, and total versus de novo fatty acid synthesis in subcutaneous adipose stem cells of normal-weight polycystic ovary syndrome (PCOS) women during adipogenesis: evidence of cellular programming. Clin Epigenet. 2020 Nov 23;12(1):181. doi: 10.1186/s13148-020-00970-x. PMID: 33228780; PMCID: PMC7686698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yildizhan R., Ilhan G.A., Yildizhan B., Kolusari A., Adali E., Bugdayci G. Serum retinol-binding protein 4, leptin, and plasma asymmetric dimethylarginine levels in obese and nonobese young women with polycystic ovary syndrome. Fertil Steril. 2011 Jul;96(1):246–250. doi: 10.1016/j.fertnstert.2011.04.073. Epub 2011 May 19. PMID: 21600576. [DOI] [PubMed] [Google Scholar]
- 47.Barrea L., Frias-Toral E., Pugliese G., Garcia-Velasquez E., De Los Angeles Carignano M., Savastano S., Colao A., Muscogiuri G. Vitamin D in obesity and obesity-related diseases: an overview. Minerva Endocrinol (Torino) 2021 Jun;46(2):177–192. doi: 10.23736/S0391-1977.20.03299-X. Epub 2020 Nov 19. PMID: 33213116. [DOI] [PubMed] [Google Scholar]
- 48.Muscogiuri G., Policola C., Prioletta A., Sorice G., Mezza T., Lassandro A., Della Casa S., Pontecorvi A., Giaccari A. Low levels of 25(OH)D and insulin-resistance: 2 unrelated features or a cause-effect in PCOS? Clin Nutr. 2012 Aug;31(4):476–480. doi: 10.1016/j.clnu.2011.12.010. Epub 2012 Jan 20. PMID: 22260937. [DOI] [PubMed] [Google Scholar]
- 49.Davis E.M., Peck J.D., Hansen K.R., Neas B.R., Craig L.B. Associations between vitamin D levels and polycystic ovary syndrome phenotypes. Minerva Endocrinol. 2019 Jun;44(2):176–184. doi: 10.23736/S0391-1977.18.02824-9. Epub 2018 Apr 12. PMID: 29652114; PMCID: PMC6467740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Altieri B., Grant W.B., Della Casa S., Orio F., Pontecorvi A., Colao A., Sarno G., Muscogiuri G. Vitamin D and pancreas: the role of sunshine vitamin in the pathogenesis of diabetes mellitus and pancreatic cancer. Crit Rev Food Sci Nutr. 2017 Nov 2;57(16):3472–3488. doi: 10.1080/10408398.2015.131136922. PMID: 27030935. [DOI] [PubMed] [Google Scholar]
- 51.Muscogiuri G., Palomba S., Caggiano M., Tafuri D., Colao A., Orio F. Low 25 (OH) vitamin D levels are associated with autoimmune thyroid disease in polycystic ovary syndrome. Endocrine. 2016 Aug;53(2):538–542. doi: 10.1007/s12020-015-0745-0. Epub 2015 Oct 3. PMID: 26433740. [DOI] [PubMed] [Google Scholar]
- 52.Jacewicz-Święcka M., Kowalska I. Changes in metabolic profile in the women with a history of PCOS-A long-term follow-up study. J Clin Med. 2020 Oct 20;9(10):3367. doi: 10.3390/jcm9103367. PMID: 33092301; PMCID: PMC7589958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Diamanti-Kandarakis E., Papavassiliou A.G., Kandarakis S.A., Chrousos G.P. Pathophysiology and types of dyslipidemia in PCOS. Trends Endocrinol Metabol. 2007 Sep;18(7):280–285. doi: 10.1016/j.tem.2007.07.004. Epub 2007 Aug 10. PMID: 17692530. [DOI] [PubMed] [Google Scholar]
- 54.Frias-Toral E., Garcia-Velasquez E., de Los Angeles Carignano M., Rodriguez-Veintimilla D., Alvarado-Aguilera I., Bautista-Litardo N. Polycystic ovary syndrome and obesity: clinical aspects and nutritional management. Minerva Endocrinol (Torino) 2021 Apr 1 doi: 10.23736/S2724-6507.21.03349-6. Epub ahead of print. PMID: 33792235. [DOI] [PubMed] [Google Scholar]
- 55.Han Y., Kim H.S., Lee H.J., Oh J.Y., Sung Y.A. Metabolic effects of polycystic ovary syndrome in adolescents. Ann Pediatr Endocrinol Metab. 2015 Sep;20(3):136–142. doi: 10.6065/apem.2015.20.3.136. Epub 2015 Sep 30. PMID: 26512349; PMCID: PMC4623341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barrea L., Muscogiuri G., Di Somma C., Tramontano G., De Luca V., Illario M., Colao A., Savastano S. Association between Mediterranean diet and hand grip strength in older adult women. Clin Nutr. 2019 Apr;38(2):721–729. doi: 10.1016/j.clnu.2018.03.012. Epub 2018 Apr 3. PMID: 29643004. [DOI] [PubMed] [Google Scholar]
- 57.Orio F., Muscogiuri G., Ascione A., Marciano F., Volpe A., La Sala G., Savastano S., Colao A., Palomba S. Effects of physical exercise on the female reproductive system. Minerva Endocrinol. 2013 Sep;38(3):305–319. PMID: 24126551. [PubMed] [Google Scholar]
- 58.Rodriguez Paris V., Solon-Biet S.M., Senior A.M., Edwards M.C., Desai R., Tedla N., Cox M.J., Ledger W.L., Gilchrist R.B., Simpson S.J., Handelsman D.J., Walters K.A. Defining the impact of dietary macronutrient balance on PCOS traits. Nat Commun. 2020 Oct 16;11(1):5262. doi: 10.1038/s41467-020-19003-5. PMID: 33067453; PMCID: PMC7568581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Muscogiuri G., Altieri B., de Angelis C., Palomba S., Pivonello R., Colao A., Orio F. Shedding new light on female fertility: the role of vitamin D. Rev Endocr Metab Disord. 2017 Sep;18(3):273–283. doi: 10.1007/s11154-017-9407-2. PMID: 28102491. [DOI] [PubMed] [Google Scholar]
- 60.Sahin S., Eroglu M., Selcuk S., Turkgeldi L., Kozali S., Davutoglu S. Intrinsic factors rather than vitamin D deficiency are related to insulin resistance in lean women with polycystic ovary syndrome. Eur Rev Med Pharmacol Sci. 2014;18:2851–2856. PMID: 25339479. [PubMed] [Google Scholar]
- 61.Muscogiuri G., Mitri J., Mathieu C., Badenhoop K., Tamer G., Orio F., Mezza T., Vieth R., Colao A., Pittas A. Mechanisms in endocrinology: vitamin D as a potential contributor in endocrine health and disease. Eur J Endocrinol. 2014 Sep;171(3):R101–R110. doi: 10.1530/EJE-14-0158. Epub 2014 May 28. PMID: 24872497. [DOI] [PubMed] [Google Scholar]
- 62.Menichini D., Facchinetti F. Effects of vitamin D supplementation in women with polycystic ovary syndrome: a review. Gynecol Endocrinol. 2020;36:1–5. doi: 10.1080/09513590.2019.1625881. PMID: 31187648. [DOI] [PubMed] [Google Scholar]
- 63.Goyal M., Dawood A.S. Debates regarding lean patients with polycystic ovary syndrome: a narrative review. J Hum Reprod Sci. 2017 Jul-Sep;10(3):154–161. doi: 10.4103/jhrs.JHRS_77_17. PMID: 29142442; PMCID: PMC5672719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moran L.J., Ko H., Misso M., Marsh K., Noakes M., Talbot M., Frearson M., Thondan M., Stepto N., Teede H.J. Dietary composition in the treatment of polycystic ovary syndrome: a systematic review to inform evidence-based guidelines. J Acad Nutr Diet. 2013 Apr;113(4):520–545. doi: 10.1016/j.jand.2012.11.018. Epub 2013 Feb 16. PMID: 23420000. [DOI] [PubMed] [Google Scholar]
- 65.Muscogiuri G., Palomba S., Laganà A.S., Orio F. Current insights into inositol isoforms, Mediterranean and ketogenic diets for polycystic ovary syndrome: from bench to bedside. Curr Pharmaceut Des. 2016;22(36):5554–5557. doi: 10.2174/1381612822666160720160634. PMID: 27510483. [DOI] [PubMed] [Google Scholar]
- 66.Le Donne M., Metro D., Alibrandi A., Papa M., Benvenga S. Effects of three treatment modalities (diet, myoinositol or myoinositol associated with D-chiro-inositol) on clinical and body composition outcomes in women with polycystic ovary syndrome. Eur Rev Med Pharmacol Sci. 2019 Mar;23(5):2293–2301. doi: 10.26355/eurrev_201903_17278. PMID: 30915778. [DOI] [PubMed] [Google Scholar]
- 67.Moran L.J., Brown W.J., McNaughton S.A., Joham A.E., Teede H.J. Weight management practices associated with PCOS and their relationships with diet and physical activity. Hum Reprod. 2017 Mar 1;32(3):669–678. doi: 10.1093/humrep/dew348. PMID: 28069732. [DOI] [PubMed] [Google Scholar]
- 68.Muscogiuri G., Barrea L., Caprio M., Ceriani F., Chavez A.O., El Ghoch M., Frias-Toral E., Mehta R.J., Mendez V., Paschou S.A., Pazderska A., Savastano S., Colao A. Nutritional guidelines for the management of insulin resistance. Crit Rev Food Sci Nutr. 2021 Apr 2:1–14. doi: 10.1080/10408398.2021.1908223. Epub ahead of print. PMID: 33797999. [DOI] [PubMed] [Google Scholar]
- 69.Barrea L., Marzullo P., Muscogiuri G., Di Somma C., Scacchi M., Orio F., Aimaretti G., Colao A., Savastano S. Source and amount of carbohydrate in the diet and inflammation in women with polycystic ovary syndrome. Nutr Res Rev. 2018 Dec;31(2):291–301. doi: 10.1017/S0954422418000136. Epub 2018 Jul 23. PMID: 30033891. [DOI] [PubMed] [Google Scholar]
- 70.De Lima Nunes R., Dos Santos I.K., Cobucci R.N., Pichini G.S., Soares G.M., de Oliveira Maranhão T.M., Dantas P.M.S. Lifestyle interventions and quality of life for women with polycystic ovary syndrome: a systematic review and meta-analysis protocol. Medicine (Baltimore) 2019 Dec;98(50) doi: 10.1097/MD.0000000000018323. PMID: 31852122; PMCID: PMC6922537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Palomba S., Falbo A., Valli B., Morini D., Villani M.T., Nicoli A., La Sala G.B. Physical activity before IVF and ICSI cycles in infertile obese women: an observational cohort study. Reprod Biomed Online. 2014 Jul;29(1):72–79. doi: 10.1016/j.rbmo.2014.03.006. Epub 2014 Mar 21. PMID: 24813759. [DOI] [PubMed] [Google Scholar]
- 72.Broughton D.E., Moley K.H. Obesity and female infertility: potential mediators of obesity's impact. Fertil Steril. 2017 Apr;107(4):840–847. doi: 10.1016/j.fertnstert.2017.01.017. Epub 2017 Mar 11. PMID: 28292619. [DOI] [PubMed] [Google Scholar]
- 73.Pani A., Gironi I., Di Vieste G., Mion E., Bertuzzi F., Pintaudi B. From prediabetes to type 2 diabetes mellitus in women with polycystic ovary syndrome: lifestyle and pharmacological management. Internet J Endocrinol. 2020 Jun 8:6276187. doi: 10.1155/2020/6276187. PMID: 32587614; PMCID: PMC7298266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Muscogiuri G., Altieri B., Annweiler C., Balercia G., Pal H.B., Boucher B.J., Cannell J.J., Foresta C., Grübler M.R., Kotsa K., Mascitelli L., März W., Orio F., Pilz S., Tirabassi G., Colao A. Vitamin D and chronic diseases: the current state of the art. Arch Toxicol. 2017 Jan;91(1):97–107. doi: 10.1007/s00204-016-1804-x. Epub 2016 Jul 18. PMID: 27425218. [DOI] [PubMed] [Google Scholar]
- 75.Karamali M., Ashrafi M., Razavi M., Jamilian M., Akbari M., Asemi Z. Effects of calcium, vitamins D and K co-supplementation on markers of insulin metabolism and lipid profiles in vitamin D-deficient women with polycystic ovary syndrome. Exp Clin Endocrinol Diabetes. 2017;125:316–321. doi: 10.1055/s-0043-104530. PMID: 28407660. [DOI] [PubMed] [Google Scholar]
- 76.Muscogiuri G., Palomba S., Laganà A.S., Orio F. Inositols in the treatment of insulin-mediated diseases. Int J Endocrinol. 2016:3058393. doi: 10.1155/2016/3058393. . Epub 2016 Sep 8. Erratum in: Int J Endocrinol. 2016;2016:6189820. PMID: 27688754; PMCID: PMC5027050. [DOI] [PMC free article] [PubMed] [Google Scholar]