Abstract
The world is facing up the most considerable vaccination effort in history to end the Coronavirus disease 2019 (COVID-19) pandemic. Several monoclonal antibodies (mAbs) direct against the Receptor binding domain of the S protein of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) received an Emergency Use Authorization for outpatient management of mild to moderate manifestation from COVID-19. MAbs could prevent the transmission SARS-CoV-2 infection and protect individuals from progression to severe disease. Under the pressure of different treatment strategies, SARS-CoV-2 has been demonstrated to select for different sets of mutations named “variants” that could impair the effectiveness of mAbs by modifying target epitopes. We provide an overview of both completed and unpublished, or ongoing clinical trials of mAbs used and review state of art in order to describe clinical options, possible indications, and the place in therapy for these agents in the treatment of COVID-19 with a particular focus on anti-spike agents. Then, we reassume the current evidence on mutations of the SARS-CoV-2 that might confer resistance to neutralization by multiple mAbs.
Keywords: COVID-19, Monoclonal antibodies, Pneumonia, SARS-CoV-2
1. Introduction
The coronavirus disease 2019 (COVID-19) pandemic has resulted in more than 4.7 million deaths globally and nearly 227 million cases, representing a global challenge that demands pressing prevention and treatment research. Two processes are involved in COVID-19 pathogenesis: in the first phase of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, the damage is induced by the virus entering the host cell through the binding of the S spike protein to ACE-2 receptors and starting to replicate [1]. In the second phase of the infection, the disease is driven by an exaggerated inflammatory response [2]. Several treatments are indicated according to the different stages of the infection.
Remdesivir (RDV) has shown clear clinical benefit if administered early during the viral stage of the disease, resulting in reduced mortality rate in patients who received RDV administration at most 9 days after the onset of symptoms [3].
The timing of therapy is also pivotal for immunomodulatory drugs, such as steroids and tocilizumab since they are employed to manage the hyper inflammation stage associated with acute respiratory distress syndrome (ARDS) [4], [5]
Data from the RECOVERY trial have shown that dexamethasone improves the outcome of 4 weeks mortality in hospitalized convalescents with oxygen or mechanical ventilation. In contrast, it had no apparent effect on patients who were not receiving respiratory support [6]. The NIH panel recommends against the use of corticosteroids in individuals who do not necessitate supplemental oxygen and in outpatients with mild to moderate symptoms because there are insufficient data to support this decision; in addition, corticosteroids may lead to serious adverse effects (e.g., hyperglycemia, neuropsychiatric symptoms, secondary infections) [7]. Similarly, tocilizumab is indicated in combination with dexamethasone in recently hospitalized patients exhibiting rapid respiratory decompensation and significantly increasing inflammation markers [8].
Another treatment still under evaluation is convalescent plasma from donors who have been cured of COVID-19. Early treatment with plasma could increase the patient’s immune response and lower the risk of disease progression.[9] The use of convalescent plasma has lately been associated with improved outcomes in patients with defective immunity after chemo-immunotherapy or with hematologic malignancy in healthcare settings. However, the efficacy of convalescent plasma has not yet been clear, as other trials did not find significant differences in clinical status and mortality among patients treated with plasma from donors who had recovered from SARS-CoV-2 infection and patients treated with placebo [10]
Treatments mentioned earlier are recommended for hospitalized patients with hypoxemia, and no standard therapies were available for outpatients who do not require supplemental oxygen.
Promising drugs that have emerged are monoclonal antibodies (mAbs), mainly aiming at the trimeric spike (S) glycoprotein on the viral surface that mediates entry into host cells. The FDA, in United States [11] , and the EMA, in European countries [12], [13] have issued advice for the use of two combinations of monoclonal antibodies, casirivimab and imdevimab (REGN-COV2) and bamlanivimab and etesevimab in outpatients who are not needing supplemental oxygen and who are at high risk of progressing severe COVID-19.
In this review, we provide an overview of what is currently known about the efficacy and safety of mAbs in COVID-19 patients and provide a new perspective on their potential applications.
2. Material and methods
To rapidly summarize the emerging evidence on the use of new mAb with activity against the S proteins of SARS-CoV-2, all publication types, including peer-reviewed manuscripts, were included in this narrative review. Publications were identified primarily through a search including the following Key search terms: casirivimab and imdevimab OR bamlanivimab and etesevimab OR mAb OR antibody treatment OR monoclonal antibody AND COVID-19 OR SARS-CoV-2 from January 01, 2020, to September 15, 2021. PubMed, Scopus, Google Scholar, and ClinicalTrials.gov were used for this purpose. An overview of the clinical trials addressing this topic was performed by searching on ClinicalTrials.gov using the following condition “SARS-CoV-2, COVID-19”. Studies published in non-English languages were excluded. This search was limited to review articles focused on monoclonal antibodies with specific activity against SARS-CoV-2. Articles focusing on mAb used in the treatment of COVID-19 but without specific activity against SARS-CoV-2 were excluded. All authors reviewed every step of the manuscript.
3. Results
3.1. Monoclonal antibodies anti-SARS-CoV-2
3.1.1. Casirivimab and imdevimab
Casirivimab (REGN10933) and imdevimab (REGN1098) are two noncompeting, neutralizing human IgG1 antibodies that target the receptor-binding domain of the SARS-CoV-2 spike protein, thus blocking the attachment and entry of SARS-CoV-2 into human cells. Based on the result (Table 1 ) of the trial of Weinreich et al., the FDA and EMA have issued Emergency Use Authorization (EUA) for the “cocktail” of casirivimab 1200 mg and imdevimab 1200 mg (REGN-COV2) for outpatients with mild or moderate COVID-19 who do not need oxygen supplementation and who are at high risk of progressing to severe disease [12], [13], [14]. This study suggested that a single intravenous dose of REIGN-COV2 (1200 mg + 1200 mg or 4000 mg + 4000 mg) reduced SARS-CoV-2 viral load (VL), COVID-19 hospitalization days or medical visits when administered within seven days from the onset of symptoms compared to placebo. Patients were treated with a median of 3 days after SARS-CoV-2 infection confirmation. The median time to symptom improvement was five days for the interventional arm then 11 days for the placebo arm. Of note, patients with no initial antibodies for SARS-CoV-2 demonstrated the most significant clinical benefit [14]. The incidence of serious adverse Preliminary data from REGN-COV 2069 [15] trial suggested that a subcutaneous administration casirivimab and imdevimab lowered the risk of symptomatic COVID-19 infection by over 80% SARS-CoV-2 negative individuals household contact with COVID-19 infected individuals. The risk of progression to symptomatic infection was also reduced by 31% in asymptomatic SARS-CoV-2 positive individuals. The [15], [16] trials are assessing the efficacy of REIGN-COV2, respectively, in preventing symptomatic COVID-19 infection in adolescents (greater than12y) who are a household contact of SARS-CoV-2 positive individuals and in reducing SARS-CoV-2 VL in pediatric setting (<18y). The latter trial [16]investigates the efficacy of REIGN-COV2 vs placebo in terms of survival and need of mechanical ventilation in hospitalized individuals who require low-flow oxygen. Dhand et al. reported promising results in 25 solid organ transplant (SOT) patients treated with REIGN-COV2. After a median of 41 days of follow up, none of the patients treated with the cocktail was hospitalized dot to COVID-19[17].
Table 1.
Studies, interventions and results regarding monoclonal antibodies for SARS-CoV-2 infection in use and under investigation.
| Study | Interventions | Results | |
|---|---|---|---|
| Casirivimab and Imdevimab | NCT04452318A Phase III, Randomized, Double-Blind, Placebo-Controlled Study |
|
Active, not recruiting |
|
NCT04425629 A Phase I/Phase II/Phase III, Randomized, Placebo-Controlled Study |
|
Recruiting | |
|
NCT04426695 A Phase I/Phase II/Phase III, Randomized, Placebo-Controlled Study |
Cohort 1 (On Low-Flow Oxygen):
|
Recruiting | |
| Bamlanivimab |
NCT04411628 Phase I, Randomized, Placebo-Controlled, Double-Blind |
|
Completed |
| Bamlanivimab and etesevimab |
NCT04427501 Phase II/III, Randomized, Double-blind, Placebo-Controlled |
|
Recruiting |
|
NCT04497987 Phase III Randomized, Double-Blind, Placebo-Controlled |
Part 1:
|
Active, not recruiting | |
|
NCT04518410 Phase II/III, Randomized |
|
Recruiting | |
|
NCT04501978 Multicenter, Randomized, Blinded Controlled |
|
Recruiting | |
|
NCT04634409 Phase II, Randomized, Double-blind, Placebo-Controlled |
|
Recruiting | |
| CT-P59 |
NCT04525079 Phase I, Randomized, Double-blind, Placebo-controlled |
|
Recruiting |
|
NCT04593641 Pilot Phase I, Randomized, Double-blind, Placebo-controlled |
|
Active, not recruiting | |
|
NCT04602000 Phase II/III, Randomized, Placebo-controlled, Double-Blind |
|
Recruiting | |
| VIR-7831 |
NCT04545060 Phase II/III Randomized, Multi-center, Double-blind, Placebo-controlled |
|
Active, not recruiting |
| TY027 |
NCT04649515 Phase 3 Multi-Site, Randomised, Placebo Controlled, Double Blind |
|
Recruiting |
|
NCT04429529 Phase 1, Randomized, Placebo Controlled, Double Blind |
|
Completed | |
| AZD7442 |
NCT04625972 Phase III Randomized, Double-blind, Placebo-controlled, Multi-center |
|
Active, not recruiting |
|
NCT04723394 Phase III Randomized, Double-blind, Placebo-controlled, Multicenter |
|
Recruiting | |
|
NCT04625725 Phase III Randomized, Double-blind, Placebo-controlled, Multi-center |
|
Active, not recruiting | |
|
NCT04507256 Phase I Double-blind, Placebo-controlled |
|
Active, not recruiting | |
| BRII 198 |
NCT04479644 Phase I Randomized, Single-blind, Placebo-controlled |
|
Completed |
| BRII 196 |
NCT04479631 Phase I, Randomized, Single-blind, Placebo-controlled |
|
Completed |
| SCTA01 |
NCT04483375 Phase I, Randomized, Double-blinded, Placebo-controlled |
|
Completed |
|
NCT04644185 Phase II/III, Multicenter, Adaptive, Randomized, Double-blinded, Placebo-controlled |
|
Recruiting | |
| MW33 |
NCT04533048 Phase I |
|
Completed |
|
NCT04627584 Phase II, Multicenter, Randomized, Double-Blind, Placebo-Controlled |
|
Recruiting | |
| DXP593 |
NCT04532294 Phase I, Randomized, Double-Blind, Placebo Controlled, |
|
Recruiting |
|
NCT04551898 Phase II, Randomized, Double-Blind, Placebo-Controlled |
|
Completed | |
| COVI-AMG |
NCT04734860 Phase Ii, Randomized, Placebo-Controlled, Double-Blind, Multicenter |
|
Recruiting |
| DZIF-10c |
NCT04631705 Phase I/Iia, Open-Label, Randomized |
|
Recruiting |
|
NCT04631666 Phase I/Iia, Open-Label, Randomized |
|
Recruiting | |
| BI767551 |
NCT04822701 Phase II: Randomised, Double-Blind, Placebo Controlled, Doubledummy Phase III: Randomised, Double-Blind, Placebo-Controlled |
Phase II
|
Recruiting |
| COR-101 |
NCT04674566 Phase Ib/II, Randomized, Double-blind, Placebo Controlled |
Cohort 1:
|
Not yet recruiting |
| HLX70 |
NCT04561076 Phase I, Randomized, Double-Blind, Placebo-Controlled, |
|
Not yet recruiting |
| DXP604 |
NCT04669262 Phase I, Randomized, Double-Blind, Placebo Controlled |
Part 1A:
|
Recruiting |
| ADM03820 |
NCT04592549 Phase I, Randomized, Double-Blind, Placebo-Controlled |
Recruiting | |
| HFB30132A |
NCT04590430 Phase I, Randomized, Double-Blind, Placebo-Controlled |
|
Active, not recruiting |
| ABBV-47D11 |
NCT04644120 Phase I, Randomized, Double-Blind, Placebo-Controlled |
|
Recruiting |
| C144-LS and C-135-LS |
NCT04700163 Phase I, Open Label |
|
Active, not recruiting |
| ADG20 |
NCT04805671 Phase I/II/III, Randomized, Double-Blind, Placebo-Controlled |
|
Active, not recruiting |
| VIR-7831 (Gen-2) |
NCT04779879 Phase II, Multicenter, Randomized, Double-Blind |
|
Recruiting |
Abbreviations: SC: subcutaneous; IM: intramuscolar; IV: intravenous
3.1.2. Bamlanivimab and etesevimab
Bamlanivimab (LY-CoV555) and etesevimab (LY-CoV016) are two humanised immunoglobulin G1 (IgG1) kappa neutralising antibodies that act against the receptor-binding domain of SARS-CoV-2 S glycoprotein to prevent the virus from attaching to and entering human cells [18].
Bamlanivimab and etesevimab are co-administered with synergistic intent to target different epitopes of the SARS-CoV-2 spike glycoprotein [18]. The FDA [11] and EMA [13] have issued a EUA for the combined use of these antibodies to treat mild to moderate coronavirus disease 2019 (COVID-19) in adults and children who are not hospitalised. In an animal model, Jones et al. [19] found that prophylactic bamlanivimab reduced viral replication in the upper and lower respiratory tracts. Recently, a phase I randomized, placebo-controlled, double-blind clinical study was commenced to evaluate the safety, tolerability, pharmacokinetics and pharmacodynamics of intravenous bamlanivimab in 24 hospitalised patients with COVID-19 (NCT04411628) (Table 1).
The primary and secondary outcomes included the area under the concentration–time curve and the VL change from the baseline to day 29; however, definitive results are not yet available [20]. In an interim analysis, Chen [21] and then Gottlieb et al. [22] presented data from the BLAZE-1 study. In this study 577 patients were randomized and allocated to three arms to receive one of the following: a single infusion of bamlanivimab (at doses of 700 mg [n = 101], 2800 mg [n = 107] or 7000 mg [n = 101]); a combination treatment of 2800 mg bamlanivimab plus 2800 mg etesevimab (n = 112) or a placebo (n = 156). 533 (92.4%) completed the efficacy evaluation period. The combination therapy of bamlanivimab and etesevimab significantly reduced VL at days 3, 7 and 11. An exploratory analysis showed that fewer patients in the intervention arm had persistently high VL at day 7 compared to the placebo arm (3.0% vs 20.8%, p < 0.0001). Moreover, no emergent putative resistant variants have been found in convalescents treated with combination therapy. The improvement of symptoms at day 11 remained significant in the combination therapy (p = 0.009). The rate of COVID-19-related hospitalizations and emergency room visits decreased in patients treated with the combination therapy (0.9%) vs the placebo (5.8%). The relative risk reduction was 84.5% in the combination arm compared to the placebo arm (p = 0.049) similar to bamlanivimab monotherapy. Treatment-emergent side effects were comparable to the placebo for both bamlanivimab monotherapy and combination therapy, and no drug-related serious adverse events have been reported. The ongoing BLAZE-2[23] phase III trial, which is scheduled to enroll 2,400 patients, and a placebo-controlled phase II/III in outpatients (ACTIV-2, [24]) aims to assess the efficacy of bamlanivimab in preventing SARS-CoV-2 infection in an assisted living facility and outpatients tested positive for COVID-19, respectively. Then, the study will evaluate multiple drugs, including bamlanivimab in the prevention of spreading and progression of SARS-CoV-2 infection [24]. Lastly, Lundgren et al. presented data from the ACTIV-3 study [25], a phase III trial that investigated the effectiveness and safety of bamlanivimab vs remdesivir in hospitalised convalescents. No significant differences in safety outcomes between the groups were found. However, enrolment had been prematurely terminated due to the absence of clinical improvement regardless of the severity of the disease.
3.1.3. Other monoclonal antibodies
A large amount of other monoclonal antibodies is currently under investigation (Table 1). CT-P59 has received emergency use authorization in South Korea (NCT04525079, NCT04593641, NCT04602000), and VIR-7831 has requested EUA to FDA (NCT04545060, NCT04501978). [25], [26], [27], [28], [29].
Phase III trials are ongoing on monoclonal antibodies such as AZD7442 (NCT04625972, NCT04723394, NCT04625725), BRII 198 (NCT04479644, NCT04501978), BRII 196 (NCT04479631, NCT04501978), SCTA01, (NCT04483375, NCT04644185) [30], [31], [32], [33], [34], [35], [36].
Of interest, TY027 is a fully engineered human IgG whose preliminary data from the phase 1 trial (NCT04429529) showed safety and tolerability up to 20 mg/kg. In phase III trials, a total of 1,305 COVID-19 patients are scheduled to be enrolled [37]. The first 15 patients will be randomized 1:1:1 to be given either (i) a single dose of 1,500 mg TY027, (ii) 2,000 mg TY027 or (iii) Placebo for initial safety assessment. Subsequently, patients will be randomized 1:1 to receive either a single fixed dose of 2,000 mg TY027or Placebo (N = 645 per group). All individuals will be admitted to the hospital for up to one-week post-dosing and followed up on Days 14 and 28. Moreover, Phase II trials are ongoing on MW33 (NCT04533048, NCT04627584), DXP593 (NCT04532294, NCT04551898), COVI-AMG (NCT04734860), DZIF-10c (phase II/III NCT04631705, NCT04631666, NCT04822701) [38], [39], [40], [41], [42], [43], [44]. Indeed, Phase I trials are ongoing on COR-101 (phase I/II NCT04674566), HLX70 (NCT04561076), DXP604 (NCT04669262), ADM03820 (NCT04592549), HFB30132A (NCT04590430), ABBV-47D11 (NCT04644120), C144-LS and C-135-LS (NCT04700163), ADG20 (phase I/II/III NCT04805671) [45], [46], [47], [48], [49], [50], [51], [52].
AZD7442 is a combination of two mAbs (AZD8895 and AZD1061) currently investigated in one phase I (NCT04507256) and three safety and efficacy phase III studies vs placebo (NCT04625972; NCT04723394; NCT04625725) [30], [31], [32], [53]. Aminoacidic replacements have been introduced into the ab to prolong their half-lives and their potential benefit and decrease Fc effector function and the potential antibody-dependent disease enhancement. In vitro data showed that both human monoclonal antibodies COV2-2196 and COV2-21301, combined under the name of AZD7442, have neutralising activity against SARS-CoV-2 strain with mutation of concern including E484K, N501Y, and D614G, demonstrating theoretical activity in preventing escape from emerging variant viruses [54].
Recruiting phase III studies to date is a randomized, double-blind, placebo-controlled, multicenter study to assess safety and efficiency in post-exposure prophylaxis of COVID-19 in adults (STORM CHASER; NCT04625972) [32], a randomized, double-blind, placebo-controlled, multicenter study to determine the safety and efficacy of AZD7442 for the treatment of COVID-19 in non-hospitalised adults (TACKLE; NCT04723394 [31]) and a randomized, double-blind, placebo-controlled, multicenter study to determine the safety and efficacy of AZD7442 for pre-exposure prophylaxis of COVID-19 (PROVENT; NCT04625725) [30]. Participating individuals will be randomly divided in a 2:1 ratio to receive a single dose of either 300 mg of AZD7442 or saline placebo on day 1. Primary outcomes will be the SARS CoV-2 RT PCR positive symptomatic illness rate within a period of time of 183 days.
Moreover, ACTIV-2 (NCT04518410) phase II and III trials are evaluating both infused and non-infused agents among AZD7442 (IM or iv), bamlanivimab, BRII-196/BRII-198, inhaled SNG001, camostat or placebo for outpatients, whereas ACTIV-3 (TICO; NCT04501978) is evaluating in a randomized controlled double-blind phase III trial safety and efficacy of multiple investigational agents among soc, LY3819253, VIR-7831, BRII-196/BRII-198, AZD7442 or placebo for inpatients [24], [25].
VIR-7831 is a fully human antiSARS-CoV-2 dual-action mAbs derived from a parent antibody (S309) isolated from memory B cells of a 2003 SARS-CoV-2 infected patient [55]. The engineered “LS” mutation in the Fc region has prolonged serum half-life and potentially enhanced distribution to the respiratory mucosa [55]. In vitro studies showed neutralising activity against wild-type SARS-CoV-2 as well as pseudotyped virus encoding spike protein from the B.1.1.7 English, B.1.351 South African and P.1 Brazilian variants, binding an epitope that does not overlap with mutational sites and it is highly conserved among the current variants, showing a high barrier to resistance in vitro and in vivo [55]. To date, COMET-ICE(phase III) and COMET-PEAK(phase II) trials are ongoing. VIR-7831 has been included in the BLAZE-4 and previously cited ACTIV-3 trials [56]. The COMET-ICE trial (NCT04545060) is a randomized, multi-centre, double-blind, placebo-controlled study to assess the safety and efficacy of 500 mg of VIR-7831 given once intravenously for the early treatment of COVID-19 outpatients [26]. An interim analysis on 583 patients demonstrated a significantly reduced 29-days hospitalization or death hospitalisation in patients receiving VIR-7831 compared to placebo [25]. Based on these results, has been submitted a EUA application to the FDA and the request for authorisation of VIR-7831 usage in other Countries [57].
The COMET-PEAK trial (NCT04779879) is a phase II, a multi-centre, randomized, double-blind, parallel-group, phase II study assessing whether VIR-7831 produced by different processes (gen1 vs gen2) was overlapping in terms of pharmacokinetics and safety in outpatient with mild to moderate COVID-19 [57], [58].
The BLAZE-4 trial (NCT04634409) is a randomized, double-blind, placebo-controlled, phase 2 study that acted to assess the efficiency, safety, and pharmacokinetics of the administration of Bamlanivimab (700 mg) in monotherapy and in combination with Etesevimab or VIR-7831 compared to placebo to assess the proportion of patients with SARS-CoV-2 VL greater than 5.27 ng/ml at Day 7 [59], [60]. Other two phase III trials have not started yet, COMET-TAIL and COMET-STAR, to assess if IM-administered VIR-7831 can lower the rate of hospitalization and fatality induce by COVID-19 and prevent symptomatic infection in an uninfected patient, respectively [57].
CT-P59 is a monoclonal antibody reformatted to a fully human immunoglobulin (IgG1) which binds the receptor-binding domain (RBD) of the spike protein of the virus to inhibit its interaction with the ACE2 receptor and to block the entrance of the virus in cells [18]. Preliminary data suggest the efficacy of CT-P59, against various SARS-CoV-2 isolates in vivo, including the D614G spike protein variant without antibody-dependent enhancement effect [61]. After positive interim results in phase I, randomized, double-blind, placebo-controlled trial (NCT04525079), CT-P59, its efficacy in individuals with mild to moderate symptoms of COVID-19 is currently under investigation [28], [62]. A phase II/III randomized, double-blind, placebo-controlled, parallel-group trial (NCT04602000) [26]. The primary outcome is to assess the treatment efficacy from baseline to Day 14 and 28, measuring the proportion of negative patients, the time needed to negativization and to achieve clinical recovery, and the percentage of hospitalization or death among enrolled patients [26].
Last but not least, a study recently published [63] evaluated intranasal administration in mice of an IgM engineered antibody, IgM-14. So far most neutralizing monoclonal antibodies were IgG1 isotype and were administered intravenously; nevertheless, IgG antibodies have low mucosal penetration. IgM antibodies are usually the first line in the defence of mucous tissues. IgG-14 administered intranasally in a single dose showed persistently high levels in the nasal cavity of mice, while blood and other tissues had minimal antibody exposure [63]. The intranasal delivery of IgM-14 or other sprayed monoclonal antibodies could allow highly effective protection; so it has to be considered as a useful resource in alternative to intravenous administration of other monoclonal antibodies already approved for clinical use. In addition, IgM-14 has shown broad coverage of P1 (Gamma) and B.1.351 (Beta) mouse-adapted variants, with higher efficacy than the intranasally administered corresponding IgG-14 [63].
3.2. Emergence of resistance
Under the pressure of natural immunity and treatment with mAbs, SARS-CoV-2 has the potential to select viral variants with reduced susceptibility to mAbs and vaccines [64].
Of the different viral mutants, including those isolated in Brazil (P.1), the UK (B.1.1.7) and South Africa (B.1.351), the B.1.351 and P.1 variants have shown complete resistance to bamlanivimab and reduced susceptibility to casivirimab [65]. However, VIR-7831 has retained the ability to bind with some SARS-CoV-2 variants, including B.1.351 [56].
In the US, viral variants harbouring E484K and L452R substitutions with reduced susceptibility to bamlanivimab have been described and account for 20% in some regions. For this reason, the FDA revoked the EUA for bamlanivimab monotherapy, but the combination of bamlanivimab and etesevimab remains available [66]. Imdevimab retains activity against some variants, and its association with casivirimab retains sufficient potency to neutralise even novel variants of SARS-CoV-2 [65], [67]. Of concern, the emerging B.1.617.2 (delta) variant was resistant to Bamlanivimab and demonstrated reduced susceptibility to sera from convalescent patients or 3-fold reduced susceptibility to sera from patients vaccinated with BNT162b2 [68]. Another group has demonstrated that using a single antibody to directly spike proteins was associated with the development of variants with reduced susceptibility but not in the presence of ≥ 2 antibodies targeting different viral epitopes [69], [70]. In synthesis, while single mAb demonstrated reduced or very low activity against different viral variants such as B.1.351, B.1.1.28,
B.1.617.1, and B.1.526, combining two mAbs targeting different viral epitopes seems to reduce the probability of selecting escape mutants with retained antiviral activity [23], [71].
On the basis of emerging variants data, on April 16, 2021 the FDA has revoked the approval of the use of bamlanivimab alone; later, FDA gave its authorization for the use of bamlanivimab and etesevimab in combination and according to the most recent studies these MAbs, if administered together maintain activity against Alpha variant (B.1.1.7) and Delta variant (B.1.617.2), while their binding to the Beta (B1.351) and Gamma (P.1) variants has decreased. [72] In the update no. 658, AIFA extended its authorization for the use of casirivimab/indevimab in adult patients hospitalized for COVID-19 requiring low flow oxygen therapy, according to preliminary data that show clinical benefit in this particular category of individuals [73]. At the time we write this review, the cocktail of casirivimab and imdevimab has maintained activity against the most concerning variants of SARS-CoV-2 [74], [75]
4. Discussion
After over a year of the COVID-19 pandemic, morbidity and mortality resulting from severe SARS-CoV-2 infection remain high [76]. Therapeutic approaches are based on antiviral drugs or immune-modulating medications. Of this latter group, there is currently a colossal global attempt to develop and rapidly distribute effective vaccines against SARS-CoV-2. But billions of doses are required for a global vaccination campaign [77].
Meanwhile, attention has been focused on monoclonal antibodies capable of blocking the interaction between viral proteins and human cells. The primary aim of SARS-CoV-2 monoclonal antibodies is surface spike glycoproteins, the key elements that get the virus into the target cells [78] (Table 2 ).
Table 2.
Currently authorized mAbs: way of administration, dosage, exclusion criteria, side effects and role against variants.
| Authorization | Administration | Exclusion criteria | Side effects | Activity Against Variants | |
|---|---|---|---|---|---|
| Bamlanivimab (Eli-Lilly) | AIFA
|
Infusion solutionAdults: 700 mg bamlanivimab given as early as possible after a positive SARS − CoV − 2 test result, and in any case within 10 days of testing. onset of symptoms.Paediatric use:The safety and efficacy of bamlanivimab in children and adolescents under the age of 12 have not yet been established. | patients hospitalized for COVID − 19 patients that receive oxygen therapy for COVID – 19 patients who need, due to COVID − 19, an increase in the flow of chronic oxygen therapy already in place due to pre-existing co-morbidities. | NauseaDiarrhea Dizziness Headache Itching Vomiting |
Impaired Activity:
|
| Bamlanivimab and etesevimab (Eli-Lilly) | AIFA
|
Infusion solution Adults: 700 mg bamlanivimab + 1400 mg etesevimab given as early as possible after a positive SARS − CoV − 2 test result, and in any case within 10 days of testing. onset of symptoms. Paediatric use:The safety and efficacy of bamlanivimab in children and adolescents under the age of 12 have not yet been established. |
patients hospitalized for COVID − 19 patients that receive oxygen therapy for COVID − 19 patients who need, due to COVID − 19, an increase in the flow of chronic oxygen therapy already in place due to pre-existing co-morbidities. | Nausea Diarrhea DizzinessHeadache Itching Vomiting |
Impaired Activity:
|
| Casirivimab and imdevimab (Regeneron/Roche) | AIFA N. 340 GU n.71 23/03/2021 N.912 GU n. 187 06/8/2021 N. 340 GU n.71 23/03/2021 N. 912 GU n. 187 06/08/2021 N. 978n. 209 01/09/2021 |
Infusion solution The recommended dose is 1200 mg casirivimab and 1200 mg imdevimab administered in a single infusion (intravenous infusion).Casirivimab and imdevimab should always be administered ogether. |
patients on high flow oxygen therapy and / or mechanical ventilation. | Fever Chills and chills itchy rash itchy skin abdominal pain reddening of the face |
Retained Activity:
|
| Sotrovimab (GlaxoSmithKline) | AIFA
|
Infusion solution The recommended dose in adults and adolescents (aged 12 years and over and weighing at least 40 kg) is 500 mg. Sotrovimab must be diluted prior to administration. Sotrovimab should be administered as a single intravenous (IV) infusion over 30 min Patients should be monitored during and for at least 1 h after infusion is complete. |
Covid- 19 pneumonie Headache Pneumonia Dyspnoea Nausea Diarrhoea |
Retained Activity
|
|
| Regdanvimab (CT-P59) (Celtrion Healthcare) | A conditional marketing authorization for intravenous formulation of regdanvimab is granted in South Korea and a rolling review for authorization is initiated in European Union and Canada. Clinical development is underway in the US, Spain, Ireland, Hungary, Italy, South Korea and the UK. |
Infusion solution The recommended dosage of regdanvimab is a single intravenous (IV) infusion of 40 mg/kg. Treatment should be initiated as soon as possible after diagnosis, and not later than 7 days after the onset of symptoms. Paediatric use: no data are available |
Neutropenia Hypertriglyceridaemia |
Not Available |
In severely and critically ill SARS-CoV-2 infected patients there is often a dysregulated immune response, usually referred as cytokine release storm (CRS), characterized by high levels of inflammatory markers, like C-reactive proteins, Interleukin-6, ferritin and lactate dehydrogenase. Published data suggest that the administration of mAbs that lower the levels of cytokine, reducing the risk of CRS, could lead to a clinical benefit, reducing mortality from COVID-19 [79], [80]
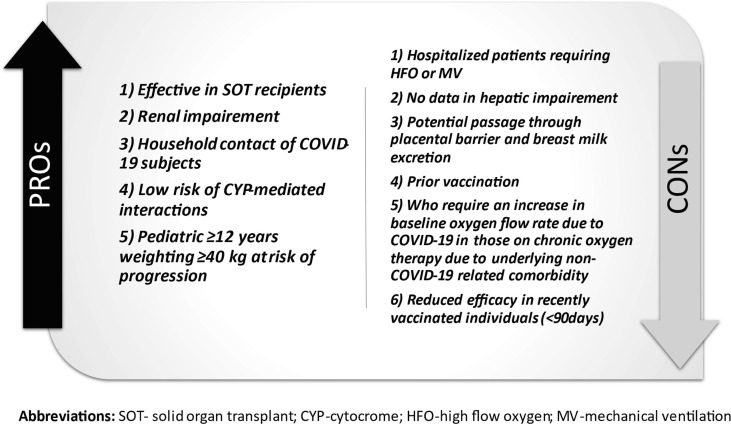
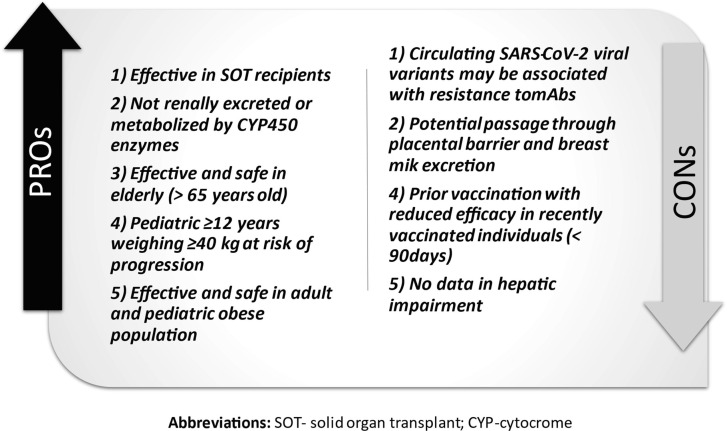
Clinically, the combination of casirivimab and imdevimab or bamlanivimab and etesevimab reduced the hospitalization rate and emergency department admissions in patients with a mild infection, and risk factors for severe manifestation from COVID-19 [23], [81] (Fig. 1, Fig. 2 ). [17], [22]Following these results, the FDA and EMA released a EUA for both the combination of casirivimab and imdevimab and bamlanivimab and etesevimab for mild symptomatic adults with COVID-19 who are not in need of additional oxygen but are at high risk of becoming severely ill. However, monoclonal antibodies are expected to work better in the early infective phase of SARS-CoV2 infection, whereas the late phases are driven by immune dysregulation responsible to severe manifestation in critical patients [82], [83]. Therefore, the “umbrella” provided by mAb is not likely to cover every individual with mild disease, so its use should be prioritised. MAbs should be reserved for individuals at high risk of developing a serious COVID-19 illness, such as people who are obese and elderly or have diabetes, chronic lung diseases and cancer [84]. Early administration might be recommended for household contacts of COVID-19 infected individuals, particularly when the aforementioned comorbidities are present The results of the study NCT04452318 will clarify the issue.
Fig. 1.
Advantages and Weaknesses of Casirivimab and Imdevimab.
Fig. 2.
Advantages and Weaknesses of Bamlanivimab and Etesevimab.
The incidence of COVID-19 infection is higher in SOT patients compared to other groups with high rates of respiratory failure although mortality rates don’t appear constantly higher among studies [85], [86], [87], [88], [89], [90]. One study suggested that casivirimab and indevimab can prevent hospitalization among SOT recipients with mild and moderate COVID-19 [18]. Based on small and relatively heterogenous groups, mAbs could prevent acute respiratory failure in SOT patientsand can be safely applied in this population [91].
Preliminary data from drugmaker suggest that REIGN-COV-2 is effective as a prophylaxis for household contacts of COVID-19 positive individuals by reducing the rate of symptomatic infection by 81% after subcutaneous administration [92], although the effective duration of the protection is not known. This finding could be particularly interesting for people with a poor serological response to vaccination, such as the elderly or patients with haematological malignancy as a complementary or alternative intervention [93], [94]. Moreover, subcutaneous administration is more suitable than IV administration because of greater patient acceptance and easier administration, and it could be widely administered through territory ambulatory services. Recently, Avalon GloboCare (US) and a company led by the University of Helsinki and Eastern Finland, and more recently a group of scientisc in Australia have been working on a nasal-spray vaccine for COVID-19 [95], [96]. In a mice model, the administration of intranasal mAbs (DZIF-10c) has been demonstrated to reduce the VL of SARS-CoV-2 and inflammation in the lungs, including variants of concern [97]. Ideally, even an early administration of aerosolized mAb in asymptomatic COVID-19 people could provide a high concentration of medicine on the respiratory mucosa, which could prevent the subsequent development of respiratory symptoms and pneumonia. The goal of the early administration of mAbs includes reductions in hospitalization, ICU admission and death rates, reducing the current tremendous pressure on hospitals and could be a suitable option in case of variants of concern with reduced susceptibility to vaccines.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Azer S.A. COVID-19: pathophysiology, diagnosis, complications and investigational therapeutics. New Microbes New Infect. 2020;37 doi: 10.1016/j.nmni.2020.100738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cascella M, Rajnik M, Aleem A, Dulebohn SC, Di Napoli R. Features, Evaluation, and Treatment of Coronavirus (COVID-19). 2021 Jul 30. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan–. PMID: 32150360. [PubMed]
- 3.Mehta R.M., Bansal S., Bysani S., Kalpakam H. A shorter symptom onset to remdesivir treatment (SORT) interval is associated with a lower mortality in moderate-to-severe COVID-19: A real-world analysis. Int J Infect Dis. 2021;106:71–77. doi: 10.1016/j.ijid.2021.02.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mongardon N, Piagnerelli M, Grimaldi D, Perrot B, Lascarrou JB; COVADIS study group investigators. Impact of late administration of corticosteroids in COVID-19 ARDS. Intensive Care Med. 2021 Jan;47(1):110-112. doi: 10.1007/s00134-020-06311-z. Epub 2020 Nov 6. PMID: 33156381; PMCID: PMC7645397. [DOI] [PMC free article] [PubMed]
- 5.Liu J., Zhang S., Dong X., Li Z., Xu Q., Feng H., Cai J., Huang S., Guo J., Zhang L., Chen Y., Zhu W., Du H., Liu Y., Wang T., Chen L., Wen Z., Annane D., Qu J., Chen D. Corticosteroid treatment in severe COVID-19 patients with acute respiratory distress syndrome. J Clin Invest. 2020 Dec 1;130(12):6417–6428. doi: 10.1172/JCI140617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med 2021;384:693–704. 10.1056/nejmoa2021436. [DOI] [PMC free article] [PubMed]
- 7.(NIH) NI of H. Therapeutic Management | COVID-19 Treatment Guidelines n.d. https://www.covid19treatmentguidelines.nih.gov/therapeutic-management/ (accessed April 29, 2021).
- 8.(NIH) NI of H. Interleukin-6 Inhibitors | COVID-19 Treatment Guidelines n.d. https://www.covid19treatmentguidelines.nih.gov/immunomodulators/interleukin-6-inhibitors/ (accessed April 29, 2021).
- 9.Casadevall A., Pirofski L.A. The convalescent sera option for containing COVID-19. J Clin Invest. 2020;130:1545–1548. doi: 10.1172/JCI138003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simonovich VA, Burgos Pratx LD, Scibona P, Beruto MV, Vallone MG, Vázquez C, Savoy N, Giunta DH, Pérez LG, Sánchez MDL, Gamarnik AV, Ojeda DS, Santoro DM, Camino PJ, Antelo S, Rainero K, Vidiella GP, Miyazaki EA, Cornistein W, Trabadelo OA, Ross FM, Spotti M, Funtowicz G, Scordo WE, Losso MH, Ferniot I, Pardo PE, Rodriguez E, Rucci P, Pasquali J, Fuentes NA, Esperatti M, Speroni GA, Nannini EC, Matteaccio A, Michelangelo HG, Follmann D, Lane HC, Belloso WH; PlasmAr Study Group. A Randomized Trial of Convalescent Plasma in Covid-19 Severe Pneumonia. N Engl J Med. 2021 Feb 18;384(7):619-629. doi: 10.1056/NEJMoa2031304. Epub 2020 Nov 24. PMID: 33232588; PMCID: PMC7722692. [DOI] [PMC free article] [PubMed]
- 11.Coronavirus (COVID-19) Update: FDA Authorizes Monoclonal Antibodies for Treatment of COVID-19 | FDA n.d. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibodies-treatment-covid-19-0 (accessed April 29, 2021).
- 12.EMA issues advice on use of REGN-COV2 antibody combination (casirivimab / imdevimab) | European Medicines Agency n.d. https://www.ema.europa.eu/en/news/ema-issues-advice-use-regn-cov2-antibody-combination-casirivimab-imdevimab (accessed April 29, 2021).
- 13.EMA issues advice on use of antibody combination (bamlanivimab / etesevimab) | European Medicines Agency n.d. https://www.ema.europa.eu/en/news/ema-issues-advice-use-antibody-combination-bamlanivimab-etesevimab (accessed April 29, 2021).
- 14.Weinreich D.M., Sivapalasingam S., Norton T., Ali S., Gao H., Bhore R., et al. REGN-COV2, a Neutralizing Antibody Cocktail, in Outpatients with Covid-19. N Engl J Med. 2021;384:238–251. doi: 10.1056/NEJMoa2035002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.COVID-19 Study Assessing the Efficacy and Safety of Anti-Spike SARS CoV-2 Monoclonal Antibodies for Prevention of SARS CoV-2 Infection Asymptomatic in Healthy Adults and Adolescents Who Are Household Contacts to an Individual With a Positive SARS-CoV-2 RT-PCR Assay - Full Text View - ClinicalTrials.gov n.d.
- 16.Safety, Tolerability, and Efficacy of Anti-Spike (S) SARS-CoV-2 Monoclonal Antibodies for the Treatment of Ambulatory Adult and Pediatric Patients With COVID-19 - Full Text View - ClinicalTrials.gov n.d.
- 17.Dhand A., Lobo S.A., Wolfe K., Feola N., Lee L., Nog R., et al. Casirivimab-Imdevimab for Treatment of COVID-19 in Solid Organ Transplant Recipients: an Early Experience. Transplantation. 2021 doi: 10.1097/TP.0000000000003737. [DOI] [PubMed] [Google Scholar]
- 18.Tuccori M., Ferraro S., Convertino I., Cappello E., Valdiserra G., Blandizzi C., et al. Anti-SARS-CoV-2 neutralizing monoclonal antibodies: clinical pipeline. MAbs. 2020;12 doi: 10.1080/19420862.2020.1854149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones B.E., Brown-Augsburger P.L., Corbett K.S., Westendorf K., Davies J., Cujec T.P., et al. LY-CoV555, a rapidly isolated potent neutralizing antibody, provides protection in a non-human primate model of SARS-CoV-2 infection. BioRxiv Prepr Serv Biol. 2020 doi: 10.1101/2020.09.30.318972. [DOI] [Google Scholar]
- 20.A Study of LY3819253 (LY-CoV555) in Participants Hospitalized for COVID-19 - Full Text View - ClinicalTrials.gov n.d.
- 21.Chen P., Nirula A., Heller B., Gottlieb R.L., Boscia J., Morris J., et al. SARS-CoV-2 Neutralizing Antibody LY-CoV555 in Outpatients with Covid-19. N Engl J Med. 2021;384:229–237. doi: 10.1056/nejmoa2029849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gottlieb R.L., Nirula A., Chen P., Boscia J., Heller B., Morris J., et al. Effect of Bamlanivimab as Monotherapy or in Combination With Etesevimab on Viral Load in Patients With Mild to Moderate COVID-19. JAMA. 2021;325:632. doi: 10.1001/jama.2021.0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen M.S., Nirula A., Mulligan M.J., Novak R.M., Marovich M., Yen C., et al. Effect of Bamlanivimab vs Placebo on Incidence of COVID-19 among Residents and Staff of Skilled Nursing and Assisted Living Facilities: A Randomized Clinical Trial. JAMA - J Am Med Assoc. 2021 doi: 10.1001/jama.2021.8828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.ACTIV-2: A Study for Outpatients With COVID-19 - Full Text View - ClinicalTrials.gov n.d.
- 25.ACTIV-3: Therapeutics for Inpatients With COVID-19 - Full Text View - ClinicalTrials.gov n.d.
- 26.To Evaluate the Safety and Efficacy of CT-P59 in Patients With Mild to Moderate Syptoms of Severe Acute Respiratory Syndrome COVID-19 - Full Text View - ClinicalTrials.gov n.d.
- 27.This is a Phase 1 Study to Evaluate the Safety,Tolerability and Virology of CT P59 in Patients With Mild Symptoms of Symptoms of Coronavirus Disease (COVID-19) - Full Text View - ClinicalTrials.gov n.d.
- 28.To Evaluate the Safety, Tolerability and Pharmacokinetics of CT-P59 in Healthy Subjects - Full Text View - ClinicalTrials.gov n.d.
- 29.VIR-7831 for the Early Treatment of COVID-19 in Outpatients - Full Text View - ClinicalTrials.gov n.d.
- 30.Phase III Double-blind, Placebo-controlled Study of AZD7442 for Pre-exposure Prophylaxis of COVID-19 in Adult. - Full Text View - ClinicalTrials.gov n.d.
- 31.Phase III Study of AZD7442 for Treatment of COVID-19 in Outpatient Adults - Full Text View - ClinicalTrials.gov n.d.
- 32.Phase III Double-blind, Placebo-controlled Study of AZD7442 for Post- Exposure Prophylaxis of COVID-19 in Adults - Full Text View - ClinicalTrials.gov n.d.
- 33.Safety, Tolerability, and Pharmacokinetics Study of Human Monoclonal Antibody BRII-198 - Full Text View - ClinicalTrials.gov n.d.
- 34.Safety, Tolerability, and Pharmacokinetics Study of Human Monoclonal Antibody BRII-196 - Full Text View - ClinicalTrials.gov n.d.
- 35.The Efficacy and Safety of SCTA01 in Hospitalized Patients With Severe COVID-19 - Full Text View - ClinicalTrials.gov n.d.
- 36.Safety, Tolerability and Pharmacokinetics of SCTA01, an Anti-SARS-CoV-2 Monoclonal Antibody, in Healthy Chinese Subjects - Full Text View - ClinicalTrials.gov n.d.
- 37.Safety of TY027, a Treatment for COVID-19, in Humans - Full Text View - ClinicalTrials.gov n.d.
- 38.A Phase 2 Clinical Trial of MW33 Injection in Patients With COVID-19 - Full Text View - ClinicalTrials.gov n.d.
- 39.A Clinical Study to Evaluate MW33 Injection - Full Text View - ClinicalTrials.gov n.d.
- 40.Safety, Tolerability, Pharmacokinetics, and Immunogenicity of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2/COVID-19) Neutralizing Antibody in Healthy Participants - Full Text View - ClinicalTrials.gov n.d.
- 41.Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Neutralizing Antibody BGB-DXP593 in Participants With Mild-to-Moderate Coronavirus Disease 2019 (COVID-19) - Full Text View - ClinicalTrials.gov n.d.
- 42.Study to Evaluate a Single Dose of STI-2020 (COVI-AMGTM) in Adults With Mild COVID-19 Symptoms - Full Text View - ClinicalTrials.gov n.d.
- 43.SARS-CoV-2-Neutralizing Monoclonal COVID-19 Antibody DZIF-10c by Infusion - Full Text View - ClinicalTrials.gov n.d.
- 44.A Study to Test BI 767551 in People With Mild to Moderate Symptoms of COVID-19 - Full Text View - ClinicalTrials.gov n.d.
- 45.Evaluation of Safety and Tolerability of COR-101 in Hospitalized Patients With Moderate to Severe COVID-19 - Full Text View - ClinicalTrials.gov n.d.
- 46.Evaluate Safety and Pharmacokinetics of HLX70 in Healthy Adult Volunteers - Full Text View - ClinicalTrials.gov n.d.
- 47.BGB-DXP604 Alone and in Combination With BGB DXP593 in Healthy Participants - Full Text View - ClinicalTrials.gov n.d.
- 48.Study of Monoclonal Antibody Cocktail Being Tested for the Prevention of COVID-19 - Full Text View - ClinicalTrials.gov n.d.
- 49.Study to Assess the Safety, Tolerability, and Pharmacokinetics of HFB30132A Against COVID-19 in Healthy Adults - Full Text View - ClinicalTrials.gov n.d.
- 50.Study to Assess Adverse Events and How Intravenous (IV) ABBV-47D11 and IV ABBV-2B04 Given Alone and in Combination Moves Through the Body of Adult Participants With Coronavirus Disease 2019 (COVID-19) - Full Text View - ClinicalTrials.gov n.d.
- 51.RU Anti-SARS-CoV-2 (COVID-19) mAbs in Healthy Volunteers - Full Text View - ClinicalTrials.gov n.d.
- 52.Evaluation of ADG20 for the Treatment of Mild or Moderate COVID-19 - Full Text View - ClinicalTrials.gov n.d.
- 53.AZD7442 - a Potential Combination Therapy for the Prevention and Treatment of COVID-19 - Full Text View - ClinicalTrials.gov n.d.
- 54.Dong J, Zost SJ, Greaney AJ, Starr TN, Chen EC, Chen RE, et al. Genetic and structural basis for recognition of SARS-CoV-2 spike protein by 2 a two-antibody cocktail 3 4. BioRxiv 2021:2021.01.27.428529. 10.1101/2021.01.27.428529.
- 55.Cathcart AL, Havenar-Daughton C, Lempp FA, Ma D, Schmid M, Agostini ML, et al. The dual function monoclonal antibodies VIR-7831 and VIR-7832 demonstrate potent in vitro and 2 in vivo activity against SARS-CoV-2 3 4 5. BioRxiv 2021:2021.03.09.434607. 10.1101/2021.03.09.434607.
- 56.Search of: VIR-7831 - List Results - ClinicalTrials.gov n.d.
- 57.Vir Biotechnology and GSK announce VIR-7831 reduces hospitalisation and risk of death in early treatment of adults with COVID-19 | GSK n.d.
- 58.Safety, Tolerability and Pharmacokinetics of Second Generation VIR-7831 Material in Non-hospitalized Participants With Mild to Moderate COVID-19 - Full Text View - ClinicalTrials.gov n.d.
- 59.A Study of Immune System Proteins in Participants With Mild to Moderate COVID-19 Illness - Full Text View - ClinicalTrials.gov n.d.
- 60.Lilly, Vir Biotechnology and GSK announce positive topline data from the phase 2 BLAZE-4 trial evaluating bamlanivimab with VIR-7831 in low-risk adults with COVID-19 | GSK n.d.
- 61.Kim C., Ryu D.K., Lee J., Il K.Y., Seo J.M., Kim Y.G., et al. A therapeutic neutralizing antibody targeting receptor binding domain of SARS-CoV-2 spike protein. Nat Commun. 2021;12:1–10. doi: 10.1038/s41467-020-20602-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Celltrion Announces Positive Interim Results From Phase I Trial of CT-P59, an Anti-COVID-19 Monoclonal Antibody Treatment Candidate | Business Wire n.d.
- 63.Zhiqiang K.u., Xie X., Hinton P.R., et al. Nasal delivery of an IgM offers broad protection from SARS-CoV-2 variants. Nature. 2021 Jul;595(7869):718–723. doi: 10.1038/s41586-021-03673-2. Epub 2021 Jun 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Harvey W.T., Carabelli A.M., Jackson B., Gupta R.K., Thomson E.C., Harrison E.M., et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat Rev Microbiol. 2021;19:409–424. doi: 10.1038/s41579-021-00573-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hoffmann M, Hofmann-Winkler H, Krüger N, Kempf A, Nehlmeier I, Graichen L, et al. SARS-CoV-2 variant B.1.617 is resistant to Bamlanivimab and evades 1 antibodies induced by infection and vaccination 2 3. BioRxiv 2021:2021.05.04.442663. 10.1101/2021.05.04.442663. [DOI] [PMC free article] [PubMed]
- 66.Coronavirus (COVID-19) Update: FDA Revokes Emergency Use Authorization for Monoclonal Antibody Bamlanivimab | FDA n.d.
- 67.Pharmaceuticals Inc R. FACT SHEET FOR HEALTH CARE PROVIDERS EMERGENCY USE AUTHORIZATION (EUA) OF CASIRIVIMAB AND IMDEVIMAB AUTHORIZED USE. n.d.
- 68.Planas D, Veyer D, Baidaliuk A, Staropoli I, Guivel-Benhassine F, Rajah MM, et al. Reduced sensitivity of infectious SARS-CoV-2 variant B.1.617.2 to monoclonal antibodies and sera from convalescent and vaccinated individuals. BioRxiv 2021:2021.05.26.445838. 10.1101/2021.05.26.445838.
- 69.Bursky JM, Chen RE, Zhang X. Resistance of SARS-CoV-2 variants to neutralization by monoclonal and serum-derived polyclonal antibodies. Nat Med n.d. 10.1038/s41591-021-01294-w. [DOI] [PMC free article] [PubMed]
- 70.Copin R., Baum A., Wloga E., Pascal K.E., Giordano S., Fulton B.O., et al. The monoclonal antibody combination REGEN-COV protects against SARS-CoV-2 mutational escape in preclinical and human studies. Cell. 2021 doi: 10.1016/j.cell.2021.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen RE, Winkler ES, Case JB, Aziati ID, Bricker TL, Joshi A, et al. In vivo monoclonal antibody efficacy against SARS-CoV-2 variant strains n.d. 10.1038/s41586-021. [DOI] [PMC free article] [PubMed]
- 72.Nathan R., Shawa I., De La Torre I., Pustizzi J.M., Haustrup N., Patel D.R., Huhn G. A Narrative Review of the Clinical Practicalities of Bamlanivimab and Etesevimab Antibody Therapies for SARS-CoV-2. Infect Dis Ther. 2021 Aug;10:1–15. doi: 10.1007/s40121-021-00515-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.https://investor.regeneron.com/news-releases/news-release-details/regeneron-announces-encouraging-initial-data-covid-19-antibody.
- 74.Markus Hoffmann, Prerna Arora, Rüdiger Groß et al. SARS-CoV-2 variants B.1.351 and P.1 escape from neutralizing antibodies. [DOI] [PMC free article] [PubMed]
- 75.Falcone M, Tiseo G, Valoriani B. Efficacy of Bamlanivimab/Etesevimab and Casirivimab/Imdevimab in Preventing Progression to Severe COVID-19 and Role of Variants of Concern. [DOI] [PMC free article] [PubMed]
- 76.Koh H.K., Geller A.C., Vanderweele T.J. Deaths from COVID-19. JAMA - J Am Med Assoc. 2021;325:133–134. doi: 10.1001/jama.2020.25381. [DOI] [PubMed] [Google Scholar]
- 77.Irwin A. What it will take to vaccinate the world against COVID-19. Nature. 2021;592:176–178. doi: 10.1038/d41586-021-00727-3. [DOI] [PubMed] [Google Scholar]
- 78.Liu L., Wang P., Nair M.S., Yu J., Rapp M., Wang Q., et al. Potent neutralizing antibodies against multiple epitopes on SARS-CoV-2 spike. Nature. 2020;584:450–456. doi: 10.1038/s41586-020-2571-7. [DOI] [PubMed] [Google Scholar]
- 79.Ali A., Kamjani M.H., Kesselman M.M. The Role of Tocilizumab in Cytokine Storm and Improving Outcomes in COVID-19. Recent Pat Antiinfect Drug Discov. 2020;15(2):104–112. doi: 10.2174/1574891X15666200922155712. [DOI] [PubMed] [Google Scholar]
- 80.Dravid A., Kashiva R., Khan Z., Memon D., Kodre A., Potdar P., Mane M., Borse R., Pawar V., Patil D., Banerjee D., Bhoite K., Pharande R., Kalyani S., Raut P., Bapte M., Mehta A., Reddy M.S., Bhayani K., Laxmi S.S., Vishnu P.D., Srivastava S., Khandelwal S., More S., Shinde R., Pawar M., Harshe A., Kadam S., Mahajan U., Joshi G., Mane D. Combination therapy of Tocilizumab and steroid for management of COVID-19 associated cytokine release syndrome: A single center experience from Pune, Western India. Medicine (Baltimore). 2021 Jul 23;100(29) doi: 10.1097/MD.000000000002670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Phase 3 Data Presented at ATS 2021 Show REGEN-COVTM (casirivimab with imdevimab) Reduced Risk of Hospitalization or Death by 70% in Non-hospitalized COVID-19 Patients | Regeneron Pharmaceuticals Inc. n.d.
- 82.Tay M.Z., Poh C.M., Rénia L., MacAry P.A., Ng L.F.P. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20:363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Giamarellos-Bourboulis E.J., Netea M.G., Rovina N., Akinosoglou K., Antoniadou A., Antonakos N., et al. Complex Immune Dysregulation in COVID-19 Patients with Severe Respiratory Failure. Cell Host Microbe. 2020;27:992–1000.e3. doi: 10.1016/j.chom.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk Factors Associated with Acute Respiratory Distress Syndrome and Death in Patients with Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med 2020:1–10. 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed]
- 85.Trapani S., Masiero L., Puoti F., Rota M.C., Del Manso M., Lombardini L., et al. Incidence and outcome of SARS-CoV-2 infection on solid organ transplantation recipients: A nationwide population-based study. Am J Transplant. 2020;21:2509–2521. doi: 10.1111/ajt.16428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pereira M.R., Mohan S., Cohen D.J., Husain S.A., Dube G.K., Ratner L.E., et al. COVID-19 in solid organ transplant recipients: Initial report from the US epicenter. Am J Transplant. 2020;20:1800–1808. doi: 10.1111/ajt.15941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fernández-Ruiz M., Andrés A., Loinaz C., Delgado J.F., López-Medrano F., San Juan R., et al. COVID-19 in solid organ transplant recipients: A single-center case series from Spain. Am J Transplant. 2020;20:1849–1858. doi: 10.1111/ajt.15929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Linares L, Cofan F, Diekmann F, Herrera S, Marcos MA, Castel MA, et al. A propensity score-matched analysis of mortality in solid organ transplant patients with COVID-19 compared to non-solid organ transplant patients. PLoS One 2021;16. 10.1371/journal.pone.0247251. [DOI] [PMC free article] [PubMed]
- 89.Chaudhry Z.S., Williams J.D., Vahia A., Fadel R., Parraga Acosta T., Prashar R., et al. Clinical characteristics and outcomes of COVID-19 in solid organ transplant recipients: A cohort study. Am J Transplant. 2020;20:3051–3060. doi: 10.1111/ajt.16188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yi S.G., Rogers A.W., Saharia A., Aoun M., Faour R., Abdelrahim M., et al. Early experience with COVID-19 and solid organ transplantation at a US high-volume transplant center. Transplantation. 2020:2208–2214. doi: 10.1097/TP.0000000000003339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Del Bello A, Marion O, Vellas C, Faguer S, Izopet J, Kamar N. Anti-SARS-Cov-2 Monoclonal Antibodies in Solid-Organ-Transplant Patients. Transplantation. 2021 Jun 30. doi: 10.1097/TP.0000000000003883. Epub ahead of print. PMID: 34224543. [DOI] [PMC free article] [PubMed]
- 92.Phase 3 Prevention Trial Showed 81% Reduced Risk of Symptomatic SARS-CoV-2 Infections with Subcutaneous Administration of REGEN-COVTM (casirivimab with imdevimab) | Regeneron Pharmaceuticals Inc. n.d.
- 93.Gavriatopoulou M., Ntanasis-Stathopoulos I., Korompoki E., Terpos E., Dimopoulos M.A. SARS-CoV-2 vaccines in patients with multiple myeloma. HemaSphere. 2021;5 doi: 10.1097/HS9.0000000000000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Agha M, Blake M, Chilleo C, Wells A, Haidar G. Suboptimal response to COVID-19 mRNA vaccines in hematologic malignancies patients. MedRxiv Prepr Serv Heal Sci 2021:2021.04.06.21254949. 10.1101/2021.04.06.21254949.
- 95.Avalon GloboCare to develop intranasal and oral Covid-19 vaccine n.d.
- 96.University of Helsinki and University of Eastern Finland shareholders in a Finnish COVID vaccine company | University of Eastern Finland n.d.
- 97.Halwe S, Kupke A, Vanshylla K, Liberta F, Gruell H, Zehner M, et al. Intranasal administration of a monoclonal neutralizing antibody protects mice against SARS-CoV-2 1 infection 2 n.d. 10.1101/2021.06.09.447662. [DOI] [PMC free article] [PubMed]