Abstract
Background and objective
The effect of atrial fibrillation (AF) on outcomes of endovascular treatment (EVT) for acute ischemic stroke (AIS) is controversial. This study aimed to investigate the association of AF with outcomes after EVT in AIS patients.
Methods
Subjects were selected from ANGEL-ACT registry (Endovascular Treatment Key Technique and Emergency Work Flow Improvement of Acute Ischemic Stroke) - a prospective consecutive cohort of AIS patients undergoing EVT at 111 hospitals in China between November 2017 and March 2019, and then grouped according to having a history of AF or not. After 1:1 propensity score matching, the outcome measures including the 90-day modified Rankin Scale (mRS) score, successful recanalization after final attempt, symptomatic intracranial hemorrhage (ICH) within 24 h, and death within 90 days were compared.
Results
A total of 1755 patients, 550 with AF and 1205 without AF, were included. Among 407 pairs of patients identified after matching, no significant differences were found in the mRS score (median: 3 vs. 3 points; P = 0.29), successful recanalization (87.2 vs. 85.3%; P = 0.42), symptomatic ICH (9. 4 vs. 9.1%; P = 0.86) and death (16.3 vs. 18.4%; P = 0.44) between patients with and without AF.
Conclusion
The findings of this matched-control study show comparable outcomes of EVT in Chinese AIS patients with and without AF, which do not support withholding EVT in patients with both AIS and AF.
Trial registration
First registration date: 28/09/2017
First posted date: 13/12/2017
Keywords: Atrial fibrillation, Endovascular treatment, Ischemic stroke, Propensity score matching
Introduction
Atrial fibrillation (AF), as the most common cause of cardioembolic stroke, is associated with a 4-5 times increased risk of acute ischemic stroke (AIS) and accounts for approximately 30–40% of all acute large vessel occlusion (LVO) [1–8]. Patients with AF-related stroke are older, have greater burden of comorbidities and worse neurological deficits, thus have a higher probability of disability or mortality after usual care [9–12]. Furthermore, intravenous thrombolysis (IVT) is less effective on both recanalization and clinical outcome but also increases the risk of intracranial hemorrhage (ICH) in patients with AF. The poor response to IVT could be partly explained by the pathophysiology of AF-related stroke, such as the gaps between patients with and without AF in terms of embolic size and components, collateral status, infarct core volume, and stroke progression [13, 14].
Endovascular treatment (EVT) represented by mechanical thrombectomy with stent-retriever or aspiration catheter has become the standard treatment for selected patients with AIS due to intracranial proximal LVO [15]. However, limited data and conflicting results exist regarding the role of AF on procedural and clinical outcomes after EVT [16–21]. To address this issue and on the hypothesis that the modification of AF was attributed to the effect of case mix; in other words, AF might not independently affect any outcome in EVT-treated patients after adjusting for possible confounders. We therefore performed a matched-control analysis based on a prospective nationwide registry database to assess whether the technical success and functional outcomes differ in LVO patients with and without AF after receiving EVT.
Methods
Study population
Data were extracted from ANGEL-ACT (Endovascular Treatment Key Technique and Emergency Work Flow Improvement of Acute Ischemic Stroke), a prospective nationwide registry of 1793 consecutive patients with AIS caused by LVO undergoing EVT in 111 hospitals in China between November 2017 and March 2019. Full methods of the registry, such as inclusion/exclusion criteria and data collection standards, have been reported earlier [22]. The protocol was approved by the ethics committees of all centers, and all participants (or legal representatives) provided written informed consent. The study procedures were in accordance with the 1964 Helsinki declaration and its later amendments.
In this analysis, patients with missing baseline or procedure data in Table 1 were excluded, and the remainder cases were divided into two groups based on whether they had pre-existing AF, identified by previous medical records.
Table 1.
Baseline and procedure characteristics of patients with AF versus without AF
| Baseline and procedure variables | Pre-matched population (n = 1755) | Post-matched population (n = 814) | ||||||
|---|---|---|---|---|---|---|---|---|
| With AF (n = 550) |
Without AF (n = 1205) |
SD (%) | P-value | With AF (n = 407) |
Without AF (n = 407) |
SD (%) | P-value | |
| Age, median (IQR), years | 71 (64–78) | 63 (54–70) | 72.0 | < 0.01 | 69 (62–76) | 68 (61–75) | 4.3 | 0.32 |
| Male sex | 246 (44.7) | 910 (75.5) | 66.2 | < 0.01 | 213 (52.3) | 221 (54.3) | 3.9 | 0.57 |
| History of hypertension | 333 (60.6) | 673 (55.9) | 9.5 | 0.07 | 232 (57.0) | 245 (60.2) | 6.5 | 0.35 |
| History of diabetes mellitus | 99 (18.0) | 225 (18.7) | 1.8 | 0.74 | 73 (17.9) | 82 (20. 2) | 5.6 | 0.42 |
| Prior ischemic stroke | 130 (23.6) | 207 (17.2) | 16.1 | < 0.01 | 85 (20.9) | 88 (21.6) | 1.8 | 0.80 |
| Pre-stroke mRS score ≥ 1 | 84 (15.3) | 146 (12.1) | 9.2 | 0.07 | 55 (13.5) | 60 (14.7) | 3.5 | 0.61 |
| Cigarette smoking | 56.5 | < 0.01 | 7.3 | 0.27 | ||||
| Never Smoker | 420 (76.4) | 629 (52.2) | 291 | 285 | ||||
| Ex-smoker | 44 (8.0) | 89 (7.4) | 37 | 28 | ||||
| Current smoker | 86 (15.6) | 487 (40. 4) | 79 | 94 | ||||
| Systolic blood pressure, median (IQR), mmHg | 145 (130–160) | 145 (132–162) | 7.5 | 0. 21 | 145 (130–160) | 145 (130–160) | 2.1 | 0.95 |
| NIHSS score, median (IQR) | 18 (14–22) | 15 (11–21) | 29.5 | < 0.01 | 17 (13–21) | 17 (13–22) | 2.6 | 0.87 |
| ASPECTS, median (IQR) a | 10 (7–10) | 9 (7–10) | 13.1 | < 0.01 | 10 (7–10) | 10 (7–10) | 1.5 | 0.91 |
| Occlusion site | 46.4 | < 0.01 | 9.1 | 0.20 | ||||
| Internal carotid artery | 166 (30.2) | 279 (23.2) | 111 (27.3) | 116 (28.5) | ||||
| Middle cerebral artery M1 segment | 266 (48.4) | 493 (40.9) | 197 (48.4) | 187 (45.9) | ||||
| Middle cerebral artery M2 segment | 59 (10.7) | 91 (7.6) | 47 (11.6) | 39 (9.6) | ||||
| Vertebro-basilar artery | 49 (8.9) | 313 (26.0) | 42 (10.3) | 60 (14.7) | ||||
| Other intracranial arteries b | 10 (1.8) | 29 (2.4) | 10 (2.5) | 5 (1. 2) | ||||
| Prior use of antiplatelet agents | 101 (18.4) | 187 (15.5) | 7.8 | 0.14 | 75 (18.4) | 73 (17.9) | 1.3 | 0.86 |
| Prior use of anticoagulants | 51 (9.3) | 20 (1.7) | 34.0 | < 0.01 | 19 (4. 7) | 15 (3.7) | 4.9 | 0.48 |
| Prior intravenous thrombolysis | 145 (26.4) | 368 (30.5) | 9.3 | 0.07 | 115 (28.3) | 102 (25.1) | 7.2 | 0.30 |
| Type of anesthesia | 16.8 | 0.01 | 4.1 | 0.55 | ||||
| Local anesthesia only | 265 (48.2) | 500 (41.5) | 190 (46.7) | 184 (45.2) | ||||
| Local anesthesia plus sedation | 92 (16.7) | 190 (15.8) | 68 (16.7) | 60 (14.7) | ||||
| General anesthesia | 193 (35.1) | 515 (42.7) | 149 (36.6) | 163 (40.1) | ||||
| Stent-retriever thrombectomy | 385 (70.0) | 834 (69.2) | 1.7 | 0.74 | 284 (69.8) | 289 (71.0) | 2.7 | 0.70 |
| Aspiration thrombectomy | 14 (2. 6) | 40 (3.3) | 4.6 | 0.38 | 13 (3.2) | 15 (3.7) | 2.7 | 0.70 |
| Stent-retriever plus aspiration thrombectomy | 124 (22.6) | 180 (14.9) | 19.6 | < 0.01 | 83 (20.4) | 77 (18.9) | 3.7 | 0.60 |
| Pass number of thrombectomy, median (IQR) | 2 (1–3) | 1 (1, 2) | 40.8 | < 0.01 | 2 (1–3) | 2 (1–3) | 1.4 | 0.79 |
| Emergency angioplasty/stenting | 45 (8.2) | 471 (39.1) | 78.1 | < 0.01 | 45 (11.1) | 57 (14.0) | 8.9 | 0.20 |
| Intra-arterial thrombolysis | 33 (6.0) | 111 (9.2) | 12.1 | 0.02 | 31 (7.6) | 31 (7.6) | 0.0 | 1.00 |
| Intra-procedural use of tirofiban | 201 (36.6) | 712 (59.1) | 46.3 | < 0.01 | 167 (41.0) | 186 (45.7) | 9.3 | 0.18 |
| Intra-procedural use of heparin | 251 (45.6) | 606 (50.3) | 9.3 | 0.07 | 187 (46.0) | 178 (43.7) | 4.5 | 0.53 |
| Onset-to-puncture time, median (IQR), min | 260 (195–370) | 325 (225–484) | 30.8 | < 0.01 | 284 (200–390) | 290 (210–410) | 6.0 | 0.22 |
| Puncture-to-recanalization time, median (IQR), min | 80 (50–120) | 89 (54–135) | 11.2 | 0.01 | 79 (50–120) | 87 (53–128) | 5.3 | 0.19 |
Abbreviations: AF atrial fibrillation, ASPECTS Alberta Stroke Program Early CT Score, IQR interquartile range, mRS modified Rankin Scale, NIHSS National Institutes of Health Stroke Scale, pc-ASPECTS posterior circulation Alberta Stroke Program Early CT Score, SD standardized difference
Values are numbers with percentages in parentheses, unless indicated otherwise
aASPECTS for anterior circulation stroke, and pc-ASPECTS for posterior circulation stroke
bincluding anterior cerebral artery A1/A2 segments, posterior cerebral artery P1 segment
Outcome measures
The primary outcome was the 90-day modified Rankin Scale (mRS) score assessed by trained and independent investigators. The secondary outcomes included successful recanalization (modified Thrombolysis in Cerebral Infarction [mTICI] of 2b-3) after first and final attempt, complete recanalization (mTICI of 3) after final attempt, [23] the proportions of mRS 0–1, 0–2 and 0–3 at 90 days. The safety outcomes were intra-procedural complications (e.g., new territorial embolization, arterial perforation, arterial dissection, vasospasm requiring treatment and in-stent thrombosis), any ICH, parenchymal hematoma type 2 (PH2) and symptomatic ICH within 24 hours according to the Heidelberg Bleeding Classification, [24] and death within 90 days.
Statistical analysis
Data were displayed as median (interquartile range [IQR]) or frequency (percentage). Univariable comparisons of baseline characteristics between patients with and without AF were performed using Mann-Whitney or Pearson’s chi-square tests. To improve the comparability between the two groups, a 1:1 propensity score matching (PSM) was performed by using a caliper distance of 0.05 [25]. For comparing the outcomes between both groups, the odds ratios (OR) or common OR with their 95% confidence intervals (CI) were calculated using a binary or ordinal logistic regression model, if applicable. Significance level was set to α = 0.05 (2-sided). Statistical analyses were conducted with SAS software version 9.4 (SAS Institute Inc., Cary, NC).
Results
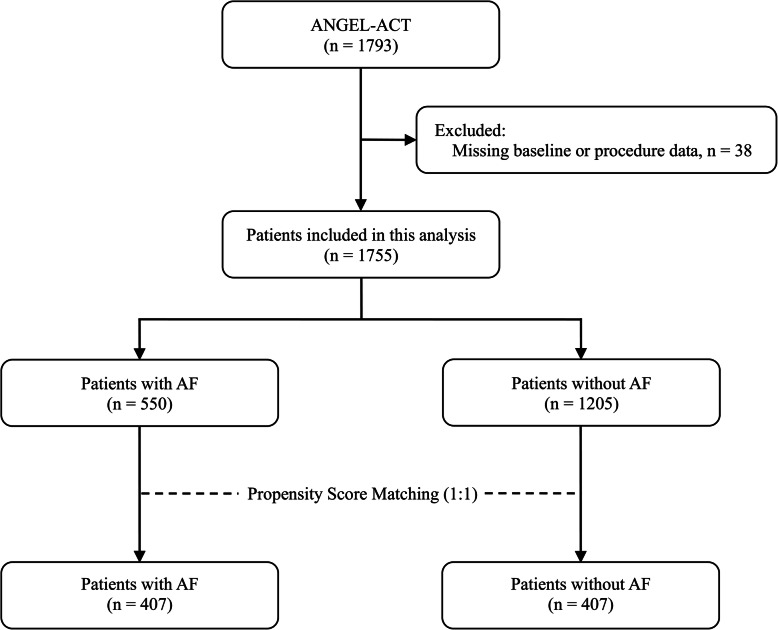
Among 1793 patients enrolled in the ANGEL-ACT registry, 38 patients were excluded due to missing baseline or procedure information, a total of 1755 patients were included in this analysis, including 550 cases with AF and 1205 without AF. After PSM, 814 patients were identified (Fig. 1).
Fig. 1.
Flow chart of patient selection. Abbreviations: AF = atrial fibrillation, ANGEL-ACT = Endovascular Treatment Key Technique and Emergency Work Flow Improvement of Acute Ischemic Stroke
As shown in Table 1, there were significant differences in many baseline and procedure characteristics between pre-matched patients with and without AF. For example, patients with AF were 8 years older, had 3 points higher NIHSS scores, were more frequently given anticoagulants before stroke onset, and received more passes of thrombectomy than those without AF; while patients with AF had lower proportions of male, current smoker, and vertebro-basilar artery occlusion, were less often given tirofiban during the procedure and emergency angioplasty/stenting, and experienced 65 min shorter onset-to-puncture time than those without AF (all P-values < 0.01). After PSM, all baseline and procedure characteristics between groups were well-balanced (Table 1).
Comparisons of outcome measures between patients with and without AF were presented in Table 2. Before matching, there was no significant difference in recanalization rates between the two groups, but patients with AF had a higher 90-day mRS score (P < 0.01) and higher risks of intra-procedural complications (P = 0.02), hemorrhagic transformations within 24 hours (all P < 0.01), and death within 90 days (P = 0.01), whereas they had lower proportions of mRS 0–1, 0–2, and 0–3 points at 90 days (all P < 0.01). After matching, the difference in the primary outcome - 90-day mRS score no longer existed between patients with and without AF (median: 3 vs. 3 points; P = 0.29). In addition, all differences in secondary and safety outcomes that differed between both groups before matching also disappeared.
Table 2.
Outcome measures of patients with AF versus without AF
| Outcome variables | Pre-matched population (n = 1755) | Post-matched population (n = 814) | ||||||
|---|---|---|---|---|---|---|---|---|
| With AF (n = 550) |
Without AF (n = 1205) |
Univariable analysis | With AF (n = 407) |
Without AF (n = 407) |
Univariable analysis | |||
| Effect size (95% CI) | P-value | Effect size (95% CI) | P-value | |||||
| Primary outcome | ||||||||
| mRS at 90 d, median (IQR) | 4 (1–5) | 3 (0–5) | 0.59 (0.47–0.74) a | < 0.01 | 3 (0–5) | 3 (1–5) | 1. 16 (0.82–1.52) a | 0.29 |
| Secondary outcomes | ||||||||
| Successful recanalization after first attempt c | 267/550 (48.6) | 588/1205 (48.8) | 0.99 (0.81–1. 21) b | 0.92 | 209/407 (51.4) | 190/407 (46.7) | 1. 21 (0.92–1.59) b | 0.18 |
| Successful recanalization after final attempt c | 479/550 (87.1) | 1065/1205 (88.4) | 0.89 (0.65–1. 20) b | 0.44 | 355/407 (87.2) | 347/407 (85.3) | 1. 18 (0.79–1.76) b | 0.42 |
| Complete recanalization after final attempt d | 376/550 (68.4) | 789/1205 (65.5) | 1. 14 (0.92–1.41) b | 0. 24 | 279/407 (68.6) | 264/407 (64.9) | 1. 18 (0.88–1.58) b | 0.27 |
| mRS 0–1 at 90 d | 174/518 (33.6) | 521/1162 (44.8) | 0.62 (0.50–0.77) b | < 0.01 | 143/387 (37.0) | 143/386 (37.1) | 1.00 (0.74–1.33) b | 0.98 |
| mRS 0–2 at 90 d | 195/518 (37.6) | 565/1162 (48.6) | 0.64 (0.52–0.79) b | < 0.01 | 160/387 (41.3) | 155/386 (40.2) | 1.05 (0.79–1.40) b | 0.74 |
| mRS 0–3 at 90 d | 252/518 (48.7) | 676/1162 (58.2) | 0.68 (0.55–0.84) b | < 0.01 | 208/387 (53.8) | 192/386 (49.7) | 1. 17 (0.89–1.56) b | 0.27 |
| Safety outcomes | ||||||||
| Intra-procedural complications e | 63/550 (11.5) | 95/1205 (7.9) | 1.51 (1.08–2. 12) b | 0.02 | 43/407 (10.6) | 36/407 (8.8) | 1. 22 (0.76–1.94) b | 0.41 |
| Any ICH within 24 h | 158/516 (30.6) | 222/1163 (19.1) | 1.87 (1.48–2.37) b | < 0.01 | 106/384 (27.6) | 95/388 (24.5) | 1. 18 (0.85–1.62) b | 0.32 |
| PH2 within 24 h f | 35/516 (6.8) | 41/1163 (3.5) | 1.99 (1. 25-3. 17) b | < 0.01 | 25/384 (6.5) | 23/388 (5.9) | 1. 11 (0.62–1.98) b | 0.74 |
| Symptomatic ICH within 24 h f | 54/513 (10.5) | 70/1156 (6.1) | 1.83 (1. 26-2.65) b | < 0.01 | 36/381 (9.4) | 35/386 (9.1) | 1.05 (0.64–1.71) b | 0.86 |
| Death within 90 d | 100/518 (19.3) | 162/1162 (13.9) | 1.48 (1. 12-1.94) b | 0.01 | 63/387 (16.3) | 71/386 (18.4) | 0.86 (0.59–1. 25) b | 0.44 |
Abbreviations: AF atrial fibrillation, CI confidence interval, ICH intracranial hemorrhage, IQR interquartile range, mRS modified Rankin Scale, mTICI modified Thrombolysis in Cerebral Infarction, OR odds ratio, PH2 parenchymal hematoma type 2
Data are shown as the event number/total number (%), unless otherwise indicated
aThe common OR values were calculated using an ordinal logistic regression model and indicated the odds of improvement of 1 point on the mRS at 90 days
bThe OR values were calculated using a binary logistic regression model
cDefined as mTICI of 2b-3
dDefined as mTICI of 3
eIncluding new territorial embolization, arterial perforation, arterial dissection, vasospasm requiring treatment and in-stent thrombosis
fccording to the Heidelberg Bleeding Classification
Discussion
This real-world registry study in China found that patients with AF were older, had more severe symptoms on admission, a lower proportion of posterior circulation occlusions, and a shorter time from onset to puncture. After matching for baseline characteristics using propensity scores, AF was not independently associated with 90-day functional outcomes, recanalization rates, and intra-procedural complications.
A subgroup analysis of the MR CLEAN trial (Multicenter Randomized Clinical Trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands) showed a trend towards a decreased treatment effect of EVT in patients with AF. However, the sample size of AF patients in their study was rather small, thus no definite conclusion could be drawn [16]. A subsequent meta-analysis from the HERMES collaboration (Highly Effective Reperfusion Evaluated in Multiple Endovascular Stroke Trials) demonstrated no interaction between AF and functional outcomes after EVT, but found a trend towards a lower rate of symptomatic ICH in AIS patients with AF (3.4% in AF patients vs. 4.5% in non-AF patients), which might be related to the lower percentage of pre-treatment with IVT (76.3% in AF patients vs. 90.6% in non-AF patients). This is probably mainly due to the fact that patients with AF are more likely to taking oral anticoagulants, which is a contraindication for the administration of tPA [17]. Conversely, a post-hoc analysis of a multi-center head-to-head clinical trial revealed that AF was an independent risk factor for any ICH in AIS patients undergoing stent-retriever thrombectomy, which was partly attributable to the adjusted anticoagulation status and more retrieval attempts by mediation analyses [18]. Furthermore, a national registry study assessing post-thrombectomy outcomes found no difference in either in-hospital or discharge outcomes between matched patients with or without AF, [19] whereas two other studies suggested faster procedural time, fewer passes, higher rates of first pass effect, successful reperfusion and good functional outcome with AF-related stroke [20, 21].
Previous observations found patients with AIS caused by AF tend to have more bleedings and worse outcomes after EVT than those without AF [16, 18]. However, special cautions should be taken when interpreting these results, such a statement could lead to misconclusions to suspecting or even denying EVT to patients with AF. We may expect that AIS caused by a sudden embolus from the cardiovascular circulation can progress faster than AIS caused by progressive carotid or intracranial artery stenosis, where there may be time for development of collaterals [26]. In this study, patients with AF were treated about 1 hour earlier (median time from onset to puncture: 260 min vs. 325 min) compared to those without AF, suggesting a faster infarct growth rate and a stronger time dependence of reperfusion therapy in AF-related stroke.
Strengths of this study were the large sample size of enrolled patients (n = 1755) and the high prevalence of AF (31. 3%), resulting in more reliable estimations. Also, comparison of outcomes after PSM was a strength. Finally, all radiological and clinical outcomes in this analysis were centrally adjudicated by the independent imaging core laboratory or clinical events committee, except those intra-procedural complications were locally scored by site investigators. Nevertheless, our study has some limitations. First, the collateral status has been shown to be an excellent predictor of stroke outcomes, [27] so a major limitation of this study is the lack of assessment of collateral status, which has been postulated as a possible reason for difference in functional outcomes post-EVT of LVO patients with vs. without AF [28, 29]. Second, this study was conducted in Chinese population, where the prevalence of intracranial atherosclerotic disease (ICAD) is very high [30]. In this context, an underlying ICAD stenotic lesion is often cited as a possible reason for immediate re-occlusion after thrombectomy that results in bailout intracranial angioplasty or stenting, thus potentially having an impact on the outcomes [31]. Our findings should be interpreted with caution and could not easily be extrapolated to other populations. Third, patients with AF may have more comorbidities (e.g., decreased ejection fraction, valvular heart disease, other organ failure), larger infarct core, and different texture of thrombus compared to those without AF. However, these variables were not collected in the ANGEL-ACT registry, so their confounding effects could not be ruled out. Finally, no information on antithrombotic therapy from post-procedure to discharge, treatment adherence and rehabilitation training after discharge was recorded, therefore limiting comments on the association between them and functional outcomes.
Conclusion
The present study found no difference in the radiological and clinical outcomes following EVT between Chinese AIS patients with and without AF, implying AF status should not hamper the decision making to proceed to EVT. Furthermore, our results were in contrast to the increased hemorrhage rates and worse functional outcomes observed in AF-related stroke treated with supportive care or IVT. It is known that thrombolysis is less used in patients with AF-related LVO and, if used, has only limited effect. Thus, the fact is EVT might be the best chance for these patients.
Acknowledgements
We thank all participating hospitals, relevant clinicians, statisticians, and imaging and laboratory technicians.
The ANGEL-ACT study group
Zhongrong Miao1; Liqiang Gui8; Cunfeng Song9; Ya Peng10; Jin Wu11; Shijun Zhao12; Junfeng Zhao13; Zhiming Zhou14; Yongli Li15; Ping Jing16; Lei Yang17; Yajie Liu18; Qingshi Zhao19; Yan Liu20; Xiaoxiang Peng21; Qingchun Gao22; Zaiyu Guo23; Wenhuo Chen24; Weirong Li25; Xiaojiang Cheng26; Yun Xu27; Yongqiang Zhang28; Guilian Zhang29; Yijiu Lu30; Xinyu Lu31; Dengxiang Wang32; Yan Wang33; Hao Li34; Yang Hua35; Deqin Geng36; Haicheng Yuan37; Hongwei Wang38; Haihua Yang39; Zengwu Wang40; Liping Wei41; Xuancong Liufu42; Xiangqun Shi43; Juntao Li44; Wenwu Yang45; Wenji Jing46; Xiang Yong47; Leyuan Wang48; Chunlei Li49; Yibin Cao50; Qingfeng Zhu51; Peng Zhang52; Xiang Luo53; Shengli Chen54; WenWu Peng55; Lixin Wang56; Xue Wen57; Shugui Shi58; Wanming Wang59; Wang Bo60; Pu Yuan61; Dong Wang62; Haitao Guan63; Wenbao Liang64; Daliang Ma65; Long Chen66; Yan Xiao67; Xiangdong Xie68; Zhonghua Shi69; Xiangjun Zeng70; Fanfan Su71; MingZe Chang72; Jijun Yin73; Hongxia Sun74; Chong Li75; Yong Bi76; Gang Xie77; Yuwu Zhao78; Chao Wang79; Peng Zhang80; Xianjun Wang81; Dongqun Li82; Hui Liang83; Zhonglun Chen84; Yan Wang85; Yuxin Wang86; Lin Yin87; HongKai Qiu88; Jun Wei89; Yaxuan Sun90; Xiaoya Feng91; Weihua Wu92; Lianbo Gao93; Zhibing Ai94; Lan Tan95; Li Ding96; Qilong Liang97; Zhimin Wang98; Jianwen Yang99; Ping Xu100; Wei Dong101; Quanle Zheng102; Zhenyun Zhu103; Liyue Zhao104; Qingbo Meng105; Yuqing Wei106; Xianglin Chen107; Wei Wang108; Dong Sun109; Yongxing Yan110; Guangxiong Yuan111; Yadong Yang112; Jianfeng Zhou113; Zhi Yang114; Zhenzhong Zhang115; Ning Guan116; Huihong Wang117
1Beijing Tiantan Hospital, Beijing, China
8Langfang Changzheng Hospital, Hebei, China
9Liaocheng Third People’s Hospital, Shandong, China
10The First People’s Hospital of Changzhou, Jiangsu, China
11The Second Affiliated Hospital of Nanjing Medical University, Jiangsu, China
12Fengrun District People’s Hospital of Tangshan City, Hebei, China
13SiPing Central People’s Hospital, Jilin, China
14Yijishan Hospital of Wannan Medical College, Anhui, China
15The 2nd Affiliated Hospital of Harbin Medical University, Heilongjiang, China
16The Central Hospital of Wuhan, Hubei, China
17The First Hospital of Shijiazhuang, Hebei, China
18Shenzhen Hospital of Southern Medical University, Guangdong, China
19The People’s Hospital of Longhua, Guangdong, China
20Jingjiang People’s Hospital, Jiangsu, China
21The Third People’s Hospital of Hubei Province, Hubei, China
22The Second Affiliated Hospital of Guangzhou Medical University, Guangdong, China
23Tianjin TEDA Hospital, Tianjin, China
24Zhangzhou Affiliated Hospital of Fujian Medical University, Fujian, China
25Taiyuan Central Hospital, Shanxi, China
26The First Affiliated Hospital of Xinjiang Medical University, Xinjiang, China
27Affiliated Drum Tower Hospital of Nanjing University Medical School, Jiangsu, China
28The First People’s Hospital of Wenling, Zhejiang, China
29The Second Affiliated Hospital of Xi’an Jiaotong University, Shaanxi, China
30The First People’s Hospital of Yulin, Guangxi, China
31Zhenjiang First People’s Hospital, Jiangsu, China
32Qitaihe Coal General Hospital Heilongjiang, China
33People’s Hospital of Tangshan City, Hebei, China
34Affiliated Hospital of Guilin Medical University, Guangxi, China
35The Affiliated Hospital of Guizhou Medical University, Guizhou Province, China
36The Affiliated Hospital of Xuzhou Medical University, Jiangsu, China
37Qingdao Central Hospital, Shandong, China
38The Fourth People’s Hospital of Langfang City, Hebei, China
39Beijing Daxing hospital, Beijing, China
40Weifang People’s Hospital, Shandong, China
41Luoyang General Hospital Affiliated to Zhengzhou University, Henan, China
42Dongguan Kanghua Hospital, Guangdong, China
43Shunde Hospital of Southern Medical University, Guangdong, China
44Handan Central Hospital, Hebei, China
45The 981 hospital of the Chinese People’s Liberation Army, Hebei, China
46Linfen people’s Hospital, Shanxi, China
47Anshun people’s Hospital of Guizhou, China
48Changle People’s Hospital, Shandong, China
49The Second People’s Hospital of Dongying, Shandong, China
50Tangshan Gongren hospital, Hebei, China
51PLA 985 Hospital of the Joint Logistics Support Force, Shanxi, China
52Gaomi People’s Hospital, Shandong, China
53Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Hubei, China
54Chongqing Sanxia Center Hospital, Chongqing, China
55Hospital of Traditional Chinese Medicine of Qiannan, Guizhou, China
56Guangdong Hospital of Chinese Medicine, Guangdong, China
57People’s hospital of Yangjiang, Guangdong, China
58The Third Affiliated Hospital of CQMU, Chongqing, China
59General Hospital of The Yangtze River Shipping, Hubei, China
60First People’s Hospital of Bijie City, Guizhou, China
61Suqian People’s Hospital of Nanjing Drum-Tower Hospital Group, Jiangsu, China
62Weifang TCM Hospital, Shandong, China
63The Third Affiliated Hospital of Guangzhou Medical University, Guangdong, China
64Karamay Central hospital, Xinjiang, China
65The third people’s Hospital of Xinjiang Uygur Autonomous Region, Xinjiang, China
66Wulanchabu City Central Hospital, Inner Mongolia, China
67Hospital of Xinjiang Production & Construction Corps, Xinjiang, China
68Jiaozuo Second people’s hospital, Henan, China
69The 904 Hospital of Joint Logistic Support Force of PLA, Jiangsu, China
70Ganzhou People’s Hospital, Jiangxi, China
71The 967 Hospital of the Joint Logistics Support Force of PLA, Liaoning, China
72The Affiliated Hospital of Northwest University Xi’an No.3 Hospital, Shaanxi, China
73The Second Hospital of Liao Cheng, Shandong, China
74Jilin Province People’s Hospital, Jilin, China
75People’s Hospital of Huanghua City, Hebei, China
76Shanghai Forth People’s Hospital, Shanghai, China
77Wanbei Coal-electricity Group General Hospital, Anhui, China
78Shanghai Jiao Tong University Affiliated Sixth People’s Hospital, Shanghai, China
79Binzhou Medical University Hospital, Shandong, China
80The 988 hospital of the people’s liberation army, Henan, China
81Linyi People’s Hospital, Shandong, China
82Yingkou City Central Hospital, Liaoning, China
83Yantaishan Hospital, Shandong, China
84Mianyang Central hospital, Sichuan, China
85Chengdu Fifth People’s Hospital, Sichuan, China
86Hengshui Fifth Hospital of Heng shui City, Hebei, China
87Second Hospital of Dalian Medical University, Liaoning, China
88Boai Hospital of Zhongshan, Guangdong, China
89The First People’s Hospital of Yibin, Sichuan, China
90Shanxi provincial people’s hospital, Shanxi, China
91Shandong Provincial Third Hospital, Cheeloo College of Medicine, Shandong University, Shandong, China
92Chuxiong State People’s Hospital, Chuxiong, Yunnan, China
93The Fourth Affiliated Hospital of China Medical University, Liaoning, China
94Taihe Hospital, Shiyan, Hubei, China
95Qingdao Municipal Hospital, Shandong, China
96The First People’s Hospital of Yunnan Province, Yunnan, China
97The NO.2 People’s Hospital of Lanzhou, Gansu, China
98Taizhou First People’s Hospital, Zhejiang, China
99Hunan Provincial People’s Hospital, Hunan, China
100First People’s Hospital of Changde City, Hunan, China
101Zhejiang Yuyao People’s Hospital, Zhejiang, China
102Aidebao Hospital, Hebei, China
103The First Hospital of Fangshan District, Beijing, China
104Tianjin Xiqing Hospital, Tianjin, China
105People’s Hospital of Zunhua, Hebei, China
106Xingtai Third Hospital, Hebei, China
107Qingyuan People’s Hospital, Guangdong, China
108Fengcheng City Central Hospital, Liaoning, China
109People’s Hospital of Hejian City, Hebei, China
110Hangzhou Third People’s Hospital, Zhejiang, China
111Xiangtan Central Hospital, Hunan, China
112People’s Hospital of Nanpi Country, Hebei, China
113Liuzhou Railway Central Hospital, Guangxi, China
114Maoming People’s Hospital, Guangdong, China
115Tongde Hospital of Zhejiang Province, Zhejiang, China
116The First Affiliated Hospital of Jinzhou Medical University, Liaoning, China
117Xishan coal electricity group worker general hospital, Shaanxi, China
Abbreviations
- AF
Atrial fibrillation
- AIS
Acute ischemic stroke
- ANGEL-ACT
Endovascular Treatment Key Technique and Emergency Work Flow Improvement of Acute Ischemic Stroke
- ASPECTS
Alberta Stroke Program Early CT Score
- CI
Confidence interval
- EVT
Endovascular treatment
- HERMES
Highly Effective Reperfusion Evaluated in Multiple Endovascular Stroke Trials
- ICH
Intracranial hemorrhage
- IQR
Interquartile range
- IVT
Intravenous thrombolysis
- LVO
Large vessel occlusion
- MR CLEAN
Multicenter Randomized Clinical Trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands
- mRS
Modified Rankin Scale
- mTICI
Modified Thrombolysis in Cerebral Infarction
- NIHSS
National Institutes of Health Stroke Scale
- OR
Odds ratio
- pc-ASPECTS
Posterior circulation Alberta Stroke Program Early CT Score
- PH2
Parenchymal hematoma type 2
- PSM
Propensity score matching
- SD
Standardized difference
Authors’ contributions
XT, DM and ZM designed the study; XT, SL and WL wrote the main manuscript text and prepared figures; ZR and RL made the critical revision of manuscript; AW performed the statistical analysis; BJ, XZ, XH, GL and GM conducted the study and acquired the data; YW, YW and ZM supervised the study; DM and ZM analyzed and interpreted the data. All authors reviewed the manuscript. The author(s) read and approved the final manuscript
Funding
This study was funded by the National Key Research and Development Program of China (2018YFC1312801, 2016YFC1301500), China Postdoctoral Science Foundation (2019 M650773). The funding body did not play any role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author Dapeng Mo (bjttmodp@163.com) or Zhongrong Miao (zhongrongm@163.com) upon reasonable request.
Declarations
Ethics approval and consent to participate
The protocol was approved by the ethics committees of Beijing Tiantan Hospital and each participating site. Each participant or his/her representative gave written informed consent before being enrolled in the study. The study procedures were in accordance with the 1964 Helsinki declaration and its later amendments.
Consent for publication
Not applicable.
Competing interests
The authors have no financial conflicts of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xu Tong, Shijing Li and Wei Liu contributed equally to this work.
Contributor Information
Zhongrong Miao, Email: zhongrongm@163.com.
Dapeng Mo, Email: bjttmodp@163.com.
on behalf of ANGEL-ACT study group:
Zhongrong Miao, Liqiang Gui, Cunfeng Song, Ya Peng, Jin Wu, Shijun Zhao, Junfeng Zhao, Zhiming Zhou, Yongli Li, Ping Jing, Lei Yang, Yajie Liu, Qingshi Zhao, Yan Liu, Xiaoxiang Peng, Qingchun Gao, Zaiyu Guo, Wenhuo Chen, Weirong Li, Xiaojiang Cheng, Yun Xu, Yongqiang Zhang, Guilian Zhang, Yijiu Lu, Xinyu Lu, Dengxiang Wang, Yan Wang, Hao Li, Yang Hua, Deqin Geng, Haicheng Yuan, Hongwei Wang, Haihua Yang, Zengwu Wang, Liping Wei, Xuancong Liufu, Xiangqun Shi, Juntao Li, Wenwu Yang, Wenji Jing, Xiang Yong, Leyuan Wang, Chunlei Li, Yibin Cao, Qingfeng Zhu, Peng Zhang, Xiang Luo, Shengli Chen, Wen Wu Peng, Lixin Wang, Xue Wen, Shugui Shi, Wanming Wang, Wang Bo, Pu Yuan, Dong Wang, Haitao Guan, Wenbao Liang, Daliang Ma, Long Chen, Yan Xiao, Xiangdong Xie, Zhonghua Shi, Xiangjun Zeng, Fanfan Su, Ming Ze Chang, Jijun Yin, Hongxia Sun, Chong Li, Yong Bi, Gang Xie, Yuwu Zhao, Chao Wang, Peng Zhang, Xianjun Wang, Dongqun Li, Hui Liang, Zhonglun Chen, Yan Wang, Yuxin Wang, Lin Yin, Hong Kai Qiu, Jun Wei, Yaxuan Sun, Xiaoya Feng, Weihua Wu, Lianbo Gao, Zhibing Ai, Lan Tan, Li Ding, Qilong Liang, Zhimin Wang, Jianwen Yang, Ping Xu, Wei Dong, Quanle Zheng, Zhenyun Zhu, Liyue Zhao, Qingbo Meng, Yuqing Wei, Xianglin Chen, Wei Wang, Dong Sun, Yongxing Yan, Guangxiong Yuan, Yadong Yang, Jianfeng Zhou, Zhi Yang, Zhenzhong Zhang, Ning Guan, and Huihong Wang
References
- 1.January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC, Jr, Ellinor PT, Ezekowitz MD, Field ME, Furie KL, Heidenreich PA, Murray KT, Shea JB, Tracy CM, Yancy CW. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the Management of Patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2019;74(1):104–132. doi: 10.1016/j.jacc.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 2.Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, Roy D, Jovin TG, Willinsky RA, Sapkota BL, Dowlatshahi D, Frei DF, Kamal NR, Montanera WJ, Poppe AY, Ryckborst KJ, Silver FL, Shuaib A, Tampieri D, Williams D, Bang OY, Baxter BW, Burns PA, Choe H, Heo JH, Holmstedt CA, Jankowitz B, Kelly M, Linares G, Mandzia JL, Shankar J, Sohn SI, Swartz RH, Barber PA, Coutts SB, Smith EE, Morrish WF, Weill A, Subramaniam S, Mitha AP, Wong JH, Lowerison MW, Sajobi TT, Hill MD. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372:1019–1030. doi: 10.1056/NEJMoa1414905. [DOI] [PubMed] [Google Scholar]
- 3.Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, Yan B, Dowling RJ, Parsons MW, Oxley TJ, Wu TY, Brooks M, Simpson MA, Miteff F, Levi CR, Krause M, Harrington TJ, Faulder KC, Steinfort BS, Priglinger M, Ang T, Scroop R, Barber PA, McGuinness B, Wijeratne T, Phan TG, Chong W, Chandra RV, Bladin CF, Badve M, Rice H, de Villiers L, Ma H, Desmond PM, Donnan GA, Davis SM. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372(11):1009–1018. doi: 10.1056/NEJMoa1414792. [DOI] [PubMed] [Google Scholar]
- 4.Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, Schonewille WJ, Vos JA, Nederkoorn PJ, Wermer MJ, van Walderveen MA, Staals J, Hofmeijer J, van Oostayen JA, Nijeholt GJ L à, Boiten J, Brouwer PA, Emmer BJ, de Bruijn SF, van Dijk LC, Kappelle LJ, Lo RH, van Dijk EJ, de Vries J, de Kort PL, van Rooij WJ, van den Berg JS, van Hasselt BA, Aerden LA, Dallinga RJ, Visser MC, Bot JC, Vroomen PC, Eshghi O, Schreuder TH, Heijboer RJ, Keizer K, Tielbeek AV, den Hertog HM, Gerrits DG, van den Berg-Vos RM, Karas GB, Steyerberg EW, Flach HZ, Marquering HA, Sprengers ME, Jenniskens SF, Beenen LF, van den Berg R, Koudstaal PJ, van Zwam WH, Roos YB, van der Lugt A, van Oostenbrugge RJ, Majoie CB, Dippel DW. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372:11–20. doi: 10.1056/NEJMoa1411587. [DOI] [PubMed] [Google Scholar]
- 5.Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, San Román L, Serena J, Abilleira S, Ribó M, Millán M, Urra X, Cardona P, López-Cancio E, Tomasello A, Castaño C, Blasco J, Aja L, Dorado L, Quesada H, Rubiera M, Hernandez-Pérez M, Goyal M, Demchuk AM, von Kummer R, Gallofré M, Dávalos A. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372(24):2296–2306. doi: 10.1056/NEJMoa1503780. [DOI] [PubMed] [Google Scholar]
- 6.Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, Albers GW, Cognard C, Cohen DJ, Hacke W, Jansen O, Jovin TG, Mattle HP, Nogueira RG, Siddiqui AH, Yavagal DR, Baxter BW, Devlin TG, Lopes DK, Reddy VK, du Mesnil de Rochemont R, Singer OC, Jahan R. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015;372(24):2285–2295. doi: 10.1056/NEJMoa1415061. [DOI] [PubMed] [Google Scholar]
- 7.Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, Yavagal DR, Ribo M, Cognard C, Hanel RA, Sila CA, Hassan AE, Millan M, Levy EI, Mitchell P, Chen M, English JD, Shah QA, Silver FL, Pereira VM, Mehta BP, Baxter BW, Abraham MG, Cardona P, Veznedaroglu E, Hellinger FR, Feng L, Kirmani JF, Lopes DK, Jankowitz BT, Frankel MR, Costalat V, Vora NA, Yoo AJ, Malik AM, Furlan AJ, Rubiera M, Aghaebrahim A, Olivot JM, Tekle WG, Shields R, Graves T, Lewis RJ, Smith WS, Liebeskind DS, Saver JL, Jovin TG. Thrombectomy 6 to 24 Hours after Stroke with a Mismatch between Deficit and Infarct. N Engl J Med. 2018;378:11–21. doi: 10.1056/NEJMoa1706442. [DOI] [PubMed] [Google Scholar]
- 8.Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, McTaggart RA, Torbey MT, Kim-Tenser M, Leslie-Mazwi T, Sarraj A, Kasner SE, Ansari SA, Yeatts SD, Hamilton S, Mlynash M, Heit JJ, Zaharchuk G, Kim S, Carrozzella J, Palesch YY, Demchuk AM, Bammer R, Lavori PW, Broderick JP, Lansberg MG. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378(8):708–718. doi: 10.1056/NEJMoa1713973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin S, Wu B, Hao ZL, Kong FY, Tao WD, Wang DR, He S, Liu M. Characteristics, treatment and outcome of ischemic stroke with atrial fibrillation in a Chinese hospital-based stroke study. Cerebrovasc Dis. 2011;31(5):419–426. doi: 10.1159/000323221. [DOI] [PubMed] [Google Scholar]
- 10.Kimura K, Minematsu K, Yamaguchi T. Atrial fibrillation as a predictive factor for severe stroke and early death in 15,831 patients with acute ischaemic stroke. J Neurol Neurosurg Psychiatry. 2005;76(5):679–683. doi: 10.1136/jnnp.2004.048827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henninger N, Goddeau RP, Jr, Karmarkar A, Helenius J, McManus DD. Atrial fibrillation is associated with a worse 90-day outcome than other Cardioembolic stroke subtypes. Stroke. 2016;47(6):1486–1492. doi: 10.1161/STROKEAHA.116.012865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steger C, Pratter A, Martinek-Bregel M, Avanzini M, Valentin A, Slany J, Stöllberger C. Stroke patients with atrial fibrillation have a worse prognosis than patients without: data from the Austrian stroke registry. Eur Heart J. 2004;25(19):1734–1740. doi: 10.1016/j.ehj.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 13.Yue R, Li D, Yu J, Li S, Ma Y, Huang S, Zeng Z, Zeng R, Sun X. Atrial Fibrillation is Associated With Poor Outcomes in Thrombolyzed Patients With Acute Ischemic Stroke: A Systematic Review and Meta-Analysis. Medicine (Baltimore) 2016;95:e3054. doi: 10.1097/MD.0000000000003054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu Y, Ji C. Efficacy and safety of thrombolysis for acute ischemic stroke with atrial fibrillation: a meta-analysis. BMC Neurol. 2021;21(1):66. doi: 10.1186/s12883-021-02095-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, Jauch EC, Kidwell CS, Leslie-Mazwi TM, Ovbiagele B, Scott PA, Sheth KN, Southerland AM, Summers DV, Tirschwell DL. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 update to the 2018 guidelines for the early Management of Acute Ischemic Stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344–e418. doi: 10.1161/STR.0000000000000211. [DOI] [PubMed] [Google Scholar]
- 16.Heshmatollah A, Fransen P, Berkhemer OA, Beumer D, van der Lugt A, Majoie C, Oostenbrugge RJ, van Zwam WH, Koudstaal PJ, Roos Y, Dippel D. Endovascular thrombectomy in patients with acute ischaemic stroke and atrial fibrillation: a MR CLEAN subgroup analysis. EuroIntervention. 2017;13(8):996–1002. doi: 10.4244/EIJ-D-16-00905. [DOI] [PubMed] [Google Scholar]
- 17.Smaal JA, de Ridder IR, Heshmatollah A, van Zwam WH, Dippel D, Majoie CB, Brown S, Goyal M, Campbell B, Muir KW, Demchuck AM, Davalos A, Jovin TG, Mitchell PJ, White P, Saver JL, Hill MD, Roos YB, van der Lugt A, van Oostenbrugge RJ. Effect of atrial fibrillation on endovascular thrombectomy for acute ischemic stroke. A meta-analysis of individual patient data from six randomised trials: results from the HERMES collaboration. Eur Stroke J. 2020;5(3):245–251. doi: 10.1177/2396987320923447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang K, Zha M, Gao J, Du J, Liu R, Liu X. Increased intracranial hemorrhage of mechanical thrombectomy in acute ischemic stroke patients with atrial fibrillation. J Thromb Thrombolysis. 2021;51(2):536–544. doi: 10.1007/s11239-020-02269-3. [DOI] [PubMed] [Google Scholar]
- 19.Munir MB, Alqahtani F, Beltagy A, Tarabishy A, Alkhouli M. Comparative outcomes of mechanical Thrombectomy for acute ischemic stroke in patients with and without atrial fibrillation. J Vasc Interv Radiol. 2017;28(11):1604–1605. doi: 10.1016/j.jvir.2017.06.024. [DOI] [PubMed] [Google Scholar]
- 20.Akbik F, Alawieh A, Cawley CM, Howard BM, Tong FC, Nahab F, et al. Differential effect of mechanical thrombectomy and intravenous thrombolysis in atrial fibrillation associated stroke. J Neurointerv Surg. 2021;13(10):883–8. 10.1136/neurintsurg-2020-016720. [DOI] [PMC free article] [PubMed]
- 21.Lin CJ, Luo CB, Chien C, Chang FC, Lin CJ, Lee IH, Hsu LC, Chung CP, Liu HY, Chi NF, How CK, Wang SJ, Guo WY, Lin YY. Better endovascular mechanical thrombectomy outcome in atrial fibrillation patients with acute ischemic stroke: a single-center experience. J Chin Med Assoc. 2020;83(8):756–760. doi: 10.1097/JCMA.0000000000000377. [DOI] [PubMed] [Google Scholar]
- 22.Jia B, Ren Z, Mokin M, Burgin WS, Bauer CT, Fiehler J, Mo D, Ma N, Gao F, Huo X, Luo G, Wang A, Pan Y, Song L, Sun X, Zhang X, Gui L, Song C, Peng Y, Wu J, Zhao S, Zhao J, Zhou Z, Li Y, Jing P, Yang L, Liu Y, Zhao Q, Liu Y, Peng X, Gao Q, Guo Z, Chen W, Li W, Cheng X, Xu Y, Zhang Y, Zhang G, Lu Y, Lu X, Wang D, Wang Y, Li H, Ling L, Peng G, Zhang J, Zhang K, Li S, Qi Z, Xu H, Tong X, Ma G, Liu R, Guo X, Deng Y, Leng X, Leung TW, Liebeskind DS, Wang Y, Wang Y, Miao Z. Current Status of Endovascular Treatment for Acute Large Vessel Occlusion in China: A Real-World Nationwide Registry. Stroke. 2021;52:STROKEAHA120031869. doi: 10.1161/STROKEAHA.120.031869. [DOI] [PubMed] [Google Scholar]
- 23.Zaidat OO, Yoo AJ, Khatri P, Tomsick TA, von Kummer R, Saver JL, Marks MP, Prabhakaran S, Kallmes DF, Fitzsimmons BF, Mocco J, Wardlaw JM, Barnwell SL, Jovin TG, Linfante I, Siddiqui AH, Alexander MJ, Hirsch JA, Wintermark M, Albers G, Woo HH, Heck DV, Lev M, Aviv R, Hacke W, Warach S, Broderick J, Derdeyn CP, Furlan A, Nogueira RG, Yavagal DR, Goyal M, Demchuk AM, Bendszus M, Liebeskind DS, Cerebral Angiographic Revascularization Grading (CARG) Collaborators. STIR Revascularization working group. STIR Thrombolysis in Cerebral Infarction (TICI) Task Force Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke. 2013;44(9):2650–2663. doi: 10.1161/STROKEAHA.113.001972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.von Kummer R, Broderick JP, Campbell BC, et al. The Heidelberg bleeding classification: classification of bleeding events after ischemic stroke and reperfusion therapy. Stroke. 2015;46(10):2981–2986. doi: 10.1161/STROKEAHA.115.010049. [DOI] [PubMed] [Google Scholar]
- 25.Tong X, Wang Y, Fiehler J, Bauer CT, Jia B, Zhang X, Huo X, Luo G, Wang A, Pan Y, Ma N, Gao F, Mo D, Song L, Sun X, Liu L, Deng Y, Li X, Wang B, Ma G, Wang Y, Ren Z, Miao Z. Thrombectomy Versus Combined Thrombolysis and Thrombectomy in Patients With Acute Stroke: A Matched-Control Study. Stroke. 2021;52:STROKEAHA120031599. doi: 10.1161/STROKEAHA.120.031599. [DOI] [PubMed] [Google Scholar]
- 26.Widimsky P. Acute ischaemic stroke in atrial fibrillation: worse outcomes unrelated to treatment methods. EuroIntervention. 2017;13(8):905–906. doi: 10.4244/EIJV13I8A134. [DOI] [PubMed] [Google Scholar]
- 27.Tan BY, Kong WY, Ngiam JN, Teoh HL, Sharma VK, Yeo LL. The role of topographic collaterals in predicting functional outcome after thrombolysis in anterior circulation ischemic stroke. J Neuroimaging. 2017;27(2):217–220. doi: 10.1111/jon.12387. [DOI] [PubMed] [Google Scholar]
- 28.Sun B, Shi Z, Pu J, Yang S, Wang H, Yang D, Hao Y, Lin M, Ke W, Liu W, Guo F, Bai Y, Zhang S, Li Z, Li S, Zuo M, Xu G, Zi W, Liu X. Effects of mechanical thrombectomy for acute stroke patients with etiology of large artery atherosclerosis. J Neurol Sci. 2019;396:178–183. doi: 10.1016/j.jns.2018.10.017. [DOI] [PubMed] [Google Scholar]
- 29.Guglielmi V, LeCouffe NE, Zinkstok SM, Compagne K, Eker R, Treurniet KM, Tolhuisen ML, van der Worp HB, Jansen I, van Oostenbrugge RJ, Marquering HA, Dippel D, Emmer BJ, Majoie C, Roos Y, Coutinho JM. Collateral circulation and outcome in atherosclerotic versus Cardioembolic cerebral large vessel occlusion. Stroke. 2019;50(12):3360–3368. doi: 10.1161/STROKEAHA.119.026299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Y, Zhao X, Liu L, Soo YO, Pu Y, Pan Y, Wang Y, Zou X, Leung TW, Cai Y, Bai Q, Wu Y, Wang C, Pan X, Luo B, Wong KS. Prevalence and outcomes of symptomatic intracranial large artery stenoses and occlusions in China: the Chinese intracranial atherosclerosis (CICAS) study. Stroke. 2014;45(3):663–669. doi: 10.1161/STROKEAHA.113.003508. [DOI] [PubMed] [Google Scholar]
- 31.Yeo L, Bhogal P, Gopinathan A, Cunli Y, Tan B, Andersson T. Why does mechanical Thrombectomy in large vessel occlusion sometimes fail? : A review of the literature. Clin Neuroradiol. 2019;29(3):401–414. doi: 10.1007/s00062-019-00777-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author Dapeng Mo (bjttmodp@163.com) or Zhongrong Miao (zhongrongm@163.com) upon reasonable request.