Abstract
Aims
Ventricular–vascular coupling, the ratio between the right ventricle’s contractile state (Ees) and its afterload (Ea), may be a useful metric in the management of paediatric pulmonary arterial hypertension (PAH). In this study we assess the prognostic capacity of the ventricular–vascular coupling ratio (Ees/Ea) derived using right ventricular (RV) pressure alone in children with PAH.
Methods
One hundred and thirty paediatric patients who were diagnosed with PAH via right heart catheterisation were retrospectively reviewed over a 10-year period. Maximum RV isovolumic pressure and end-systolic pressure were estimated using two single-beat methods from Takeuchi et al (Ees/Ea_(Takeuchi)) and from Kind et al (Ees/Ea_(Kind)) and used with an estimate of end-systolic pressure to compute ventricular–vascular coupling from pressure alone. Patients were identified as either idiopathic/hereditary PAH or associated PAH (IPAH/HPAH and APAH, respectively). Haemodynamic data, clinical functional class and clinical worsening outcomes—separated into soft (mild) and hard (severe) event categories—were assessed. Adverse soft events included functional class worsening, syncopal event, hospitalisation due to a proportional hazard-related event and haemoptysis. Hard events included death, transplantation, initiation of prostanoid therapy and hospitalisation for atrial septostomy and Pott’s shunt. Cox proportional hazard modelling was used to assess whether Ees/Ea was predictive of time-to-event.
Results
In patients with IPAH/HPAH, Ees/Ea_(Kind) and Ees/Ea_(Takeuchi) were both independently associated with time to hard event (p=0.003 and p=0.001, respectively) and when adjusted for indexed pulmonary vascular resistance (p=0.032 and p=0.013, respectively). Neither Ees/Ea_(Kind) nor Ees/Ea_(Takeuchi) were associated with time to soft event. In patients with APAH, neither Ees/Ea_(Kind) nor Ees/Ea_(Takeuchi) were associated with time to hard event or soft event.
Conclusions
Ees/Ea derived from pressure alone is a strong independent predictor of adverse outcome and could be a potential powerful prognostic tool for paediatric PAH.
Keywords: pulmonary arterial hypertension, hypertension, pulmonary, outcome assessment, healthcare
Key questions.
What is already known about this subject?
Ventricular–vascular coupling ratio (Ees/Ea) has shown to be useful metric in management of pulmonary arterial hypertension in adults.
What does this study add?
Pressure-derived Ees/Ea estimated by two different methods (Takeuchi et al and Kind et al) is a strong independent predictor of severe adverse outcome in idiopathic and hereditary paediatric pulmonary arterial hypertension (PAH). Furthermore, pressure-derived Ees/Ea is a stronger multivariable predictor of severe adverse outcome in idiopathic and hereditary paediatric PAH compared with pulmonary vascular resistance alone.
How might this impact on clinical practice?
Pressure-derived Ees/Ea could potentially serve as a powerful prognostic tool in management of PAH in children.
Introduction
Paediatric pulmonary arterial hypertension (PAH) is an incurable disease that, when left untreated, leads to right ventricular (RV) failure and eventual death. RV performance remains the most important determinant of prognosis in PAH in both children and adults,1 2 and the ability to match its contractility with pulmonary arterial (PA) load in order to maintain sufficient cardiac output (CO) is emerging as a critically important index in PAH.3–5 This matching of RV contractility and PA load may be understood as the efficiency of energy transfer between two entities and is referred to as RV–PA coupling. Progression towards uncoupling is fundamental in PAH, where pressure-overloading of the pulmonary vasculature, commonly referred to as afterload, eventually exceeds RV contractility, resulting in RV failure.6 Accordingly, current gold standard metrics for assessment of PAH include both afterload (a composite metric of pulmonary vascular resistance (PVR), arterial compliance and arterial impedance), and RV contractility.6
RV–PA coupling can be derived from ventricular pressure-volume (PV) loops that are generated by altering preload via increasing end-diastolic volume. Conventionally, the slope of the linear relationship between end-systolic points in multiple PV-loops, known as the end-systolic PV relationship (ESPVR), is used to derive RV end-systolic elastance (Ees) as a measurement of RV contractility.7 8 Similarly, effective arterial elastance (Ea) is defined as the slope of a line connecting end-systolic point to the end-diastolic volume intercept.8 The ratio of Ea to Ees is known as the ventricular–vascular coupling ratio (Ees/Ea).
However, the use of multiple PV-loops is impractical within a clinical setting, and novel methods using a single beat with pressure waveforms alone, originally developed by Sunagawa et al for estimation of left ventricular (LV) maximum pressure,8 9 were developed for estimation of RV ESPVR (Ees).10 11 PV analysis is particularly challenging in children due to size limitations and the number of catheters needed to perform this analysis. In the single beat method, first described by Takeuchi et al,10 a maximum theoretical pressure, Pmax and end-systolic pressure (Pes) are estimated through fitting of a sinusoidal function on the corresponding isovolumetric regions of systolic contraction and diastolic relaxation of the RV pressure waveform.10–12 Estimating with RV pressure only was first described by Vanderpool et al and required the estimation of end-systolic pressure. With these two pressures, the ratio of Ea to Ees can then be approximated as the ratio to Pes to Pmax−Pes.12 13
As approximations, single beat methods are only surrogates for true PV-loop based acquisition of ventricular elastance. However, the simplicity of pressure-only (or volume only) estimation cannot be denied. In 2012, Kind14 undertook a systematic examination of the errors of approximation in several single-beat techniques and developed another that used both the RV pressure waveform and its time derivative that overcame previous limitations. It has not been validated in human subjects and could potentially improve the prognostic capacity for RV–PA coupling in PAH. Furthermore, the RV–PA coupling ratio has shown to be an independent predictor of survival in adult proportional hazard (PH) and PAH,13 15 16 however, its prognostic capacity has not been evaluated in children. Here, we compare the original single-beat method of RV single-beat-pressure-derived Ees/Ea (denoted Ees/Ea_(Takeuchi)) versus the method developed by Kind et al (denoted Ees/Ea_(Kind)) and their ability to predict mild to severe adverse clinical outcome in children with PAH. We hypothesise that Ees/Ea derived by pressure alone has a strong prognostic capacity for both mild and severe adverse events, and that RV/PA decoupling is associated with higher likelihood of adverse outcome.
Methods
Patient and public involvement
No patients were involved in the design, conduct, reporting, or dissemination of this retrospective study.
Study population
Patient data were acquired retrospectively from medical records at the Pulmonary Hypertension Clinic at the Children’s Hospital Colorado (CHCO) from 2006 to 2014, to allow for 5 years of follow-up post-right heart catheterisation (RHC). Patients who were referred for PAH underwent RHC. The inclusion criteria for this study were any person age 1–18 years, with mean pulmonary arterial hypertension (mPAP) >25 mm Hg established by RHC before age 18 years at the time of RHC and with available RV-pressure curves allowing for derivation of both Ees/Ea_(Takeuchi) and Ees/Ea_(Kind). Furthermore, patients were subcategorised as idiopathic and hereditary PAH (IPAH/HPAH),17 and associated pulmonary arterial hypertension (APAH), which includes PAH associated with other conditions such as congenital heart disease (CHD), connective tissue diseases and rare blood diseases or infections. Exclusion criteria for this study consisted of diagnostic RHC in which PAH was not confirmed, patients with high altitude pulmonary oedema and patients post-heart transplantation. WHO functional classifications (WHO-FC) were identified at the time of RHC for patients with PAH.
Right heart catheterisation
All patients were under general anaesthesia during RHC. A balloon wedge catheter was inserted through the femoral vein or internal jugular vein and advanced through the right heart to the pulmonary arteries by standard methods. Systolic and end-diastolic RV pressures, mean, systolic and diastolic pulmonary artery pressures, and pulmonary capillary wedge pressure (PCWP) were recorded for haemodynamic data. Fick’s equation using assumed oxygen consumption were used to calculate systemic and pulmonary flows.18 RV cardiac index (RVCI), indexed pulmonary vascular resistance (PVRi) was calculated using standard formulas. Baseline condition was considered room air (21% FiO2).19 RV pressure and dP/dt were recorded from the WITT Series IV catheter system (Philips Medical Systems, Boston, Massachusetts, USA) as discrete data over time, with temporal resolution of approximately 4 ms and a pressure resolution of 0.02 mm Hg/unit.
Ees/Ea estimation
Ees/Ea is the ratio of RV end-systolic elastance (Ees) to PA elastance (Ea) and represents RV contractile response to changes in arterial afterload in order to sustain adequate cardiac output. Ees is the standard measurement for RV contractility10 11 and Ea is an acceptable surrogate of arterial elastance.20 Ees/Ea was estimated using the modified single-beat method, first described by Takeuchi et al (Ees/Ea_(Takeuchi))10 11 19 and the method developed by Kind et al (Ees/Ea_(Kind)).14 Each methodology will be briefly described here.
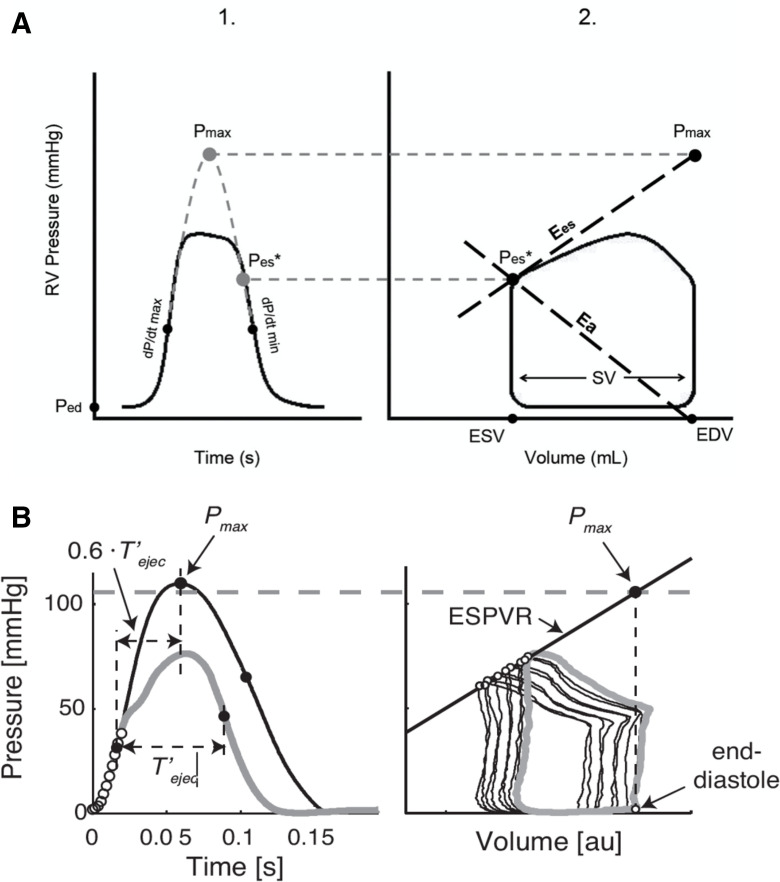
For the method of Takeuchi, maximum pressure (Pmax) was derived by fitting the rising and falling legs of a positive-offset sinusoidal function to the RV pressure waveform isovolumetric phases. Isovolumetric regions were defined as the pressure between end-diastole (assumed to occur when dP/dt exceeds 20% of its peak value) and maximum dP/dt (dP/dtmax), and between minimum dP/dt (dP/dtmin) and the corresponding end-systolic pressure during diastole (figure 1A).21 This method optimises estimation of end-diastole by accounting for variations between individual pressure peaks within a single waveform and differs from the original Takeuchi method, which assumes end diastole when dP/dt exceeds 200 mm Hg/s.
Figure 1.
The Takeuchi and Kind Ees/Ea estimation. (A) The Takeuchi method described by Breeman et al.21 At (1), theoretical maximum pressure of the right ventricle (Pmax) is estimated by fitting a sinusoid from early systolic (end-diastolic pressure (Ped) to maximum dP/dt) and early diastolic (minimum dP/dt to pressure equal to Ped) portion of the RV pressure tracing. This Pmax would occur in isovolumic contraction and is therefore located at end-diastolic volume (EDV) in the pressure-volume loop. End-systolic pressure (Pes) is estimated as the pressure 30 ms before minimum dP/dt (P30ms). At (2), end-systolic elastance (Ees) is estimated by the ratio of Pmax−pes, to stroke volume (SV). Arterial elastance (Ea) is estimated as the ratio of Pes to SV. (B) Pmax estimation developed by Kind et al.14 Illustration of the single-beat method used to estimate the isovolumic pressure curve and its maximum Pmax from a single ejecting beat in the RV. At (1), the normalised isovolumic pressure wave is fitted through the isovolumic contraction period (open circles) with the additional condition of approximately equal slopes in the isovolumic relaxation. In this rat, Pmax is reached at 60% of total contraction time (between maximal and minimal dP/dt). At (2), the reference value of Pmax is estimated by using vena cava occlusion data and extrapolating the ESPVR to EDV of the beat used to estimate isovolumic pressure. The dashed line shows the correspondence of estimated Pmax from the extrapolated ESPVR. The grey line is the last beat before vena cava occlusion. The figure suggests that the moment of dP/dtmax is the same for the isovolumic and the ejecting beat, but that the moment of dP/dtmin differs. Reproduced with permission from original author. EDV, end-diastolic volume; ESPVR, end-systolic pressure-volume relationship; ESV, end-systolic volume; RV, right ventricular.
For the method of Kind et al, maximum isovolumic pressure (Pmax) was derived by fitting the rising leg of a six-harmonic periodic waveform to the RV pressure waveform during isovolumic contraction similar to the Takeuchi method above, and fitting the time-derivative of that six-harmonic periodic waveform to dP/dt during isovolumic relaxation. The isovolumic contraction region started at end-diastole (defined as above) and ended when pressure exceeded 10% of the pressure at maximum dP/dt (see figure 1B).14 The isovolumic relaxation region was defined as starting/ending at ±20% of pressure at minimum dP/dt. Figure 1B shows the representative reference value of Pmax using traditional vena cava occlusion PV loops.
For both methods, each cycle was time-normalised to begin at t=0 prior to fitting using a non-linear least-squares optimisation procedure (MATLAB lsqnonlin()). Multiple cycles were evaluated for each patient. End-systolic pressure (Pes) was defined by the moment d2P/dt2 (the second time derivative of pressure) decreases from its average value during ejection.22 23 Ees/Ea was then computed for both methods as follows:
| (1) |
Where SV is stroke volume. Equation (1) was used to define both Ees/Ea_(Takeuchi) and Ees/Ea_(Kind) in the present study, but other research has reported the inverse (Ea/Ees). A custom code was developed in Matlab (Mathworks, Natick, Massachusetts, USA) for streamlined derivation of Pmax and Pes, and the analysis was performed by two independent researchers (MJD and AL). Rejection criteria for Ees/Ea_(Takeuchi) and Ees/Ea_(Kind) included (1) if the least squares algorithm resulted in multiple sinusoidal peaks fitted to a singular cycle (≥2), (2) if there were fewer than four data points throughout the isovolumic contraction phase, (3) if end diastole preceded dP/dtmin and (4) if there was insufficient data captured during RHC for beginning and end peaks of a waveform. These criteria ensured high fidelity in derivation of Pmax and Pes from beat to beat within an individual patients waveform.
Clinical outcomes
Clinical outcomes were assessed in all patients as described by current clinical standards.20 24 Medical records were reviewed after the initial RHC date for each patient, and adverse outcome was categorised as soft (less severe) or hard (severe), with date of event occurrence. Soft events were defined as WHO-FC worsening (defined by an increase in 1 unit of functional class), a syncopal episode, occurrence of haemoptysis, or hospitalisation due to a PAH-related event. Hard events included endpoints of death, heart or lung transplantation, initiation of intravenous prostanoid therapy and hospitalisation due to atrial septostomy or Pott’s shunt. Each patient was followed up from initial RHC date until occurrence of hard event, or until conclusion of the study period if no hard event occurred.
Statistical analysis
Statistical analyses were done using R V.3.6.025 with a significance level set to 0.05. Demographics were summarised with frequencies and percentages or means and SD. Comparisons of demographics by event status were performed with χ2 tests or two sample independent t-tests. Time to hard event was calculated as the time from RHC date to the first hard event date, if a hard event occurred. Otherwise, the censoring time was the time from RHC date to last follow-up. The same logic was used for time to first soft event.
The Takeuchi and Kind methods of Ees/Ea were plotted using a scatterplot and a Pearson’s correlation coefficient and Bland Altman measure of agreement were reported. Cox PH models were used to model time to hard event separately for patients with IPAH/HPAH and APAH, due to differences in clinical/functional characteristics and prognosis.24 26 27 Univariate models were fit with the following covariates of interest: Kind Ees/Ea, Takeuchi Ees/Ea and PVRi. Multivariable models were used for time to hard event, one with Kind Ees/Ea as the primary predictor and one with Takeuchi Ees/Ea. The models were adjusted for PVRi and also fit separately for patients with IPAH/HPAH and APAH. Akaike information criterion (AIC) was used to compare model fits between the final model with Kind Ees/Ea and the final model with Takeuchi Ees/Ea. This modelling process was repeated for time to soft events.
Results
Clinical outcome analysis
There were 142 paediatric patients with PAH considered for this study. Three patients had invalid Takeuchi Ees/Ea measurements (due to rejection criteria), 7 patients did not have adequate follow-up and 2 patients did not have recorded PVR and so were removed from all analysis, leaving a total of 130 patients for analysis. Over a follow-up of over 10 years, 70 soft events and 31 hard events occurred. Twenty-seven patients experienced both a soft and hard event, 43 patients experienced a soft event only and 4 patients experienced a hard event only. Fifty-nine (45%) patients were diagnosed as IPAH/HPAH and 71 (55%) were diagnosed as APAH. Of the patients with APAH, 64 (90%) were associated with CHD (n=18, repaired, n=46 unrepaired/partial repair), and the remaining 7 (10%) were PAH associated with connective tissue diseases. Medication types included calcium channel blockers, endothelin receptor antagonists, PDE5-inhibitors and prostacyclin analogues. Composite patient demographics and haemodynamics are shown in table 1 and are stratified by aetiology. Of note, IPAH/HPAH children were mildly older than APAH children (p=0.033) and mean right atrial pressure, PCWP, RVCI and heart rate were significantly increased in patients with APAH (p=0.022, p=0.0004, p=0.045 and p=0.0016, respectively). There were also more vasoreactive patients with iPAH/HPAH compared with children with APAH (p=0.018). Patient demographics and clinical characteristics are further stratified by hard event status (table 2) and soft event status (online supplemental table 1).
Table 1.
Patient demographics for patients with IPAH/HPAH and APAH
| Parameter | IPAH/HPAH (n=59) | APAH (n=71) | P value |
| Female, n (%) | 26 (44%) | 43 (60%) | 0.062 |
| Age, years | 10.20±6.34 | 7.77±6.33 | 0.033 |
| Weight, kg | 33.75±24.27 | 29.47±21.14 | 0.286 |
| Height, cm | 123.85±37.85 | 116.88±37.49 | 0.298 |
| Body surface area, m2 | 1.04±0.52 | 0.946±0.49 | 0.307 |
| Treatment at RHC, n (%) | |||
| No treatment | 16 (27%) | 27 (38%) | 0.191 |
| Monotherapy by oral route | 9 (15%) | 15 (13%) | 0.394 |
| Double therapy by oral route | 19 (36%) | 20 (28%) | 0.621 |
| Combination therapy using prostacyclin analogue | 15 (25%) | 9 (13%) | 0.062 |
| Vasoreactivity, n (%) | 15 (25%) | 7 (9%) | 0.018 |
| mRAP, mm Hg | 5.48±2.69 | 6.71±3.29 | 0.022 |
| mPAP, mm Hg | 68.43±41.27 | 82.54±44.22 | 0.087 |
| Systolic RVP, mm Hg | 62.56±11.48 | 58.94±12.20 | 0.082 |
| PVRi, Pa s/m³ | 7.21±7.93 | 8.13±7.01 | 0.489 |
| PCWP, mm Hg | 7.01±3.17 | 9.08±3.28 | 0.0004 |
| RVCI, L/min/m2 | 3.56±0.97 | 4.12±1.65 | 0.045 |
| Heart rate, bpm | 85±19 | 98±26 | 0.0016 |
| Ees/Ea_(Kind) | 1.51±0.49 | 1.50±0.52 | 0.993 |
| Ees/Ea_(Takeuchi) | 0.92±0.68 | 095±0.63 | 0.754 |
Data are expressed as mean values (SD), n (%), unless otherwise noted.
Bold p-values indicate significance.
APAH, associated pulmonary arterial hypertension; Ees/Ea_(Kind), vascular–ventricular coupling ratio Kind method; Ees/Ea_(Takeuchi), vascular–ventricular coupling Takeuchi method; IPAH/HPAH, idiopathic and hereditary pulmonary arterial hypertension; mPAP, mean pulmonary arterial pressure; mRAP, mean right atrial pressure; PCWP, pulmonary capillary wedge pressure; PVRi, indexed pulmonary vascular resistance; RHC, right heart catheterisation; RVCI, right ventricular cardiac index; RVP, right ventricular pressure.
Table 2.
Hard event demographics
| Variable | No hard event (n=99) | Hard event (n=31) | P value |
| Sex | 0.11 | ||
| Female | 48 (49%) | 21 (68%) | |
| Male | 50 (51%) | 10 (32%) | |
| Age at Cath | 8.51±6.36 | 10.29±6.14 | 0.17 |
| Aetiology | 0.02 | ||
| IPAH/HPAH | 39 (39%) | 20 (65%) | |
| APAH | 60 (61%) | 11 (35%) | |
| Time to follow-up or event | 5.36±2.67 | 2.35±2.22 | <0.0001 |
| Ees/Ea_(Kind) | 1.64±0.66 | 1.41±0.45 | 0.0345 |
| Ees/Ea_(Takeuchi) | 1.0±0.61 | 0.73±0.51 | 0.0182 |
| PVRi, Pa s/m³ | 6.15±4.5 | 15.68±10.64 | <0.0001 |
Data are expressed as mean values (SD), n (%), unless otherwise noted.
Bold p-values indicate significance.
APAH, associated pulmonary arterial hypertension; Ees/Ea_(Kind), vascular–ventricular coupling ratio Kind method; Ees/Ea_(Takeuchi), vascular–ventricular coupling Takeuchi method; IPAH/HPAH, idiopathic and hereditary pulmonary arterial hypertension; PVRi, indexed pulmonary vascular resistance.
openhrt-2021-001611supp002.pdf (45KB, pdf)
Patient demographics
Of the 31 hard events that occurred, 7 patients underwent atrial septostomy, 16 patients initiated intravenous prostanoid therapy, 3 underwent POTTs shunt surgery, 1 patient received a heart/lung transplant and 4 patients died. Both Ees/Ea_(Takeuchi) and Ees/Ea_(Kind) were significantly lower in patients who experienced a hard event (p=0.018 and p=0.034, respectively). Sixty-five per cent of patients experiencing a hard event had IPAH/HPAH aetiology, while only 35% of those hard events were of APAH aetiology (p=0.02). Those who experienced hard events were mostly female (68%). The average time to event was 2.35±2.67 years after the RHC date, and the average time to follow-up for those without events was approximately 5.36±2.67 years after the RHC date. These results are summarised in table 2.
Patient demographics
Of the 70 soft events, 7 patients reported haemoptysis, 8 reported a syncopal episode, 42 experienced WHO-FC worsening and 13 were hospitalised due to a PAH-related cause. There were no significant differences between patients who did and not experience a soft event in terms of age, sex or aetiology; however, there was a significant difference in PVRi (p=0.0033). The average time-to-soft event was 4.75±2.84 years, with an average time to follow-up for those without soft events of 4.52±2.91 years. These results are summarised in online supplemental table 1.
Comparison of Ees/Ea_(Kind) and Ees/Ea_(Takeuchi)
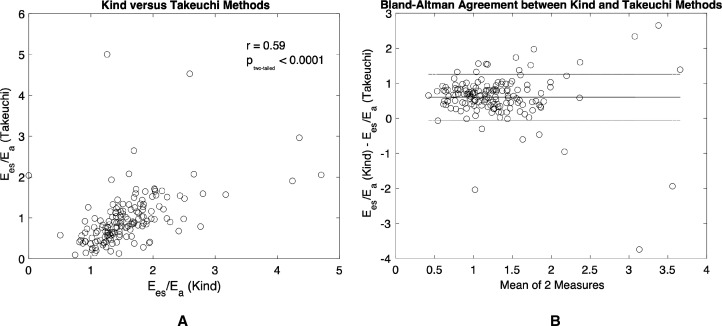
The scatterplot of Ees/Ea_(Kind) vs Ees/Ea_(Takeuchi) is shown in figure 2. Ees/Ea_(Takeuchi) was significantly correlated with Ees/Ea_(Kind) (figure 2A; r=0.59, p<0.0001). Ees/Ea_(Kind) estimated higher values compared with Ees/Ea_(Takeuchi) through Bland-Altman analysis (figure 2B; mean difference, 0.5867; 95% CI −0.1068 to 1.2802). Furthermore, neither Ees/Ea_(Kind) or Ees/Ea_(Takeuchi) demonstrated a significant correlation with PVRi (online supplemental figure 1).
Figure 2.
Pearson’s correlation and Bland-Altman comparison of Ees/Ea_(Takeuchi) and Ees/Ea_(Kind). (A) Ees/Ea_(Takeuchi) and Ees/Ea_(Kind) correlate strongly (r=0.59, p<0.0001) with each other and (B) Bland-Altman of measurement agreement (mean difference, 0.5867; 95% CI −0.1068 to 1.2802).
openhrt-2021-001611supp001.pdf (76.5KB, pdf)
Hard outcomes
In patients with IPAH/HPAH only, univariate Cox PH models showed Ees/Ea_(Takeuchi) was associated with time to hard event (p=0.001): for every one-unit decrease in Ees/Ea_(Takeuchi), the hazard of hard event increased 4.34-fold (95% CI 3.6 to 10) (table 3). Similarly, univariate analysis of Ees/Ea_(Kind) also revealed an association with time to hard event (p=0.003) in patients with IPAH/HPAH: for every one-unit decrease in Ees/Ea_(Kind), the hazard of event increased 2.17-fold (95% CI 1.3 to 3.6) (table 3). Furthermore, PVRi, mPAP and Pes similarly revealed an association time to hard event (p<0.001 for all 3, respectively). Multivariable Cox PH models revealed lower values of Ees/Ea_(Takeuchi) were associated with a higher hazard of hard events after adjusting for PVRi (p=0.013) (table 4). Similarly, for every one-unit decrease in Ees/Ea_(Kind), the hazard of hard event increased by 2.83-fold (95% CI 1.09 to 7.35; p=0.03), after adjusting for PVRi. The use of Ees/Ea_(Takeuchi) demonstrated a lower out of sample prediction error compared with Ees/Ea_(Kind) in the univariate models and the IPAH/HPAH only models. The use of Ees/Ea and PVRi in the same model also had a lower AIC than just Ees/Ea alone or just PVRi alone (AIC in tables 3 and 4). Interestingly, in patients with APAH, neither Ees/Ea_(Takeuchi) nor Ees/Ea_(Kind) showed an association with time to hard event in univariate or multivariable models (adjusted for PVRi).
Table 3.
Time to hard event univariate models, idiopathic and hereditary pulmonary arterial hypertension only
| Covariate | HR | Lower CI | Upper CI | P value | AIC |
| Ees/Ea_(Takeuchi) | 4.34 | 3.6 | 10.0 | 0.001 | 128 |
| Ees/Ea_(Kind) | 2.17 | 1.3 | 3.6 | 0.003 | 135 |
| PVRi, Pa s/m³ | 1.88 | 1.4 | 2.5 | <0.001 | 129 |
| mPAP, mm Hg | 2.78 | 1.8 | 4.3 | <0.001 | 135 |
| RVCI, L/min/m2 | 0.77 | 0.43 | 1.4 | 0.385 | 142 |
| Pes (mm Hg) | 2.57 | 1.6 | 4.1 | <0.001 | 128 |
| RVSV | 0.63 | 0.35 | 1.4 | 0.12 | 139 |
| Pmax_(Takeuchi) | 1.13 | 0.77 | 1.7 | 0.529 | 143 |
| Pmax_(Kind) | 1.56 | 1.002 | 2.4 | 0.049 | 139 |
Data are expressed as Cox proportional hazard regression estimate and HR with CIs, unless otherwise noted. Models are compared using Akaike information criterion (AIC).
Ees/Ea_(Kind), vascular–ventricular coupling ratio Kind method; Ees/Ea_(Takeuchi), vascular–ventricular coupling Takeuchi method.
Bold p-values indicate significance.
Ees/Ea _(Kind), vascular–ventricular coupling ratio Kind method; Ees/Ea _(Takeuchi), vascular–ventricular coupling Takeuchi method; mPAP, mean pulmonary arterial hypertension; Pes, end-systolic pressure; Pmax, maximum theoretical pressure; PVRi, indexed pulmonary vascular resistance; RVCI, right ventricular cardiac index; RVSV, right ventricular stroke volume.
Table 4.
Time to hard event multivariate models, idiopathic and hereditary pulmonary arterial hypertension only
| Covariate | HR | Lower CI | Upper CI | P value | AIC |
| Takeuchi model | 124 | ||||
| PVRi, Pa s/m³ | 2.97 | 1.26 | 6.99 | 0.013 | |
| Ees/Ea_(Takeuchi) | 0.64 | 0.46 | 0.88 | 0.007 | |
| Kind model | 127 | ||||
| PVRi, Pa s/m³ | 1.94 | 1.06 | 3.57 | 0.032 | |
| Ees/Ea_(Kind) | 0.61 | 1.06 | 0.81 | 0.001 |
Data are expressed as Cox proportional hazard regression estimate and HR with CIs, unless otherwise noted. Models are compared using Akaike information criterion (AIC).
A difference in 2–4 points of AIC is considered significant.
Ees/Ea_(Kind), vascular–ventricular coupling ratio Kind method; Ees/Ea_(Takeuchi), vascular–ventricular coupling Takeuchi method; PVRi, indexed pulmonary vascular resistance.
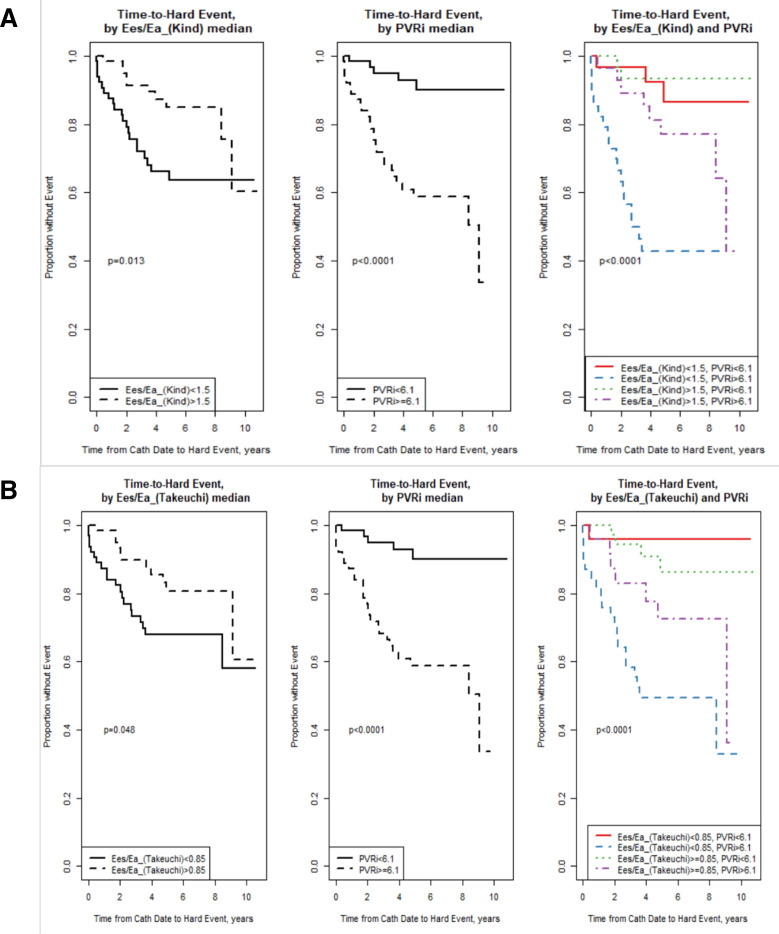
Figure 3 shows Kaplan-Meier survival curves for (1) Ees/Ea_(Kind) and (2) Ees/Ea_(Takeuchi) by splitting the groups according to median values. The median Ees/Ea_(Kind) was 1.5, Ees/Ea_(Takeuchi) was 0.85 and PVRi was 6.1. Both Ees/Ea_(Kind) and PVRi (above/below median) were significantly associated with time-to-hard event (p=0.013 and p<0.0001, respectively). When looking together by all four groups (third panel), there was an overall significant difference by group (p<0.0001). The low Ees/Ea/high PVR (blue line) showed a higher risk of event compared with all other groups. This group of patients, who had both a PVR >6.1 and an Ees/Ea_(Kind) <1.5, has a higher risk of event compared with those with a high PVR >6.1 and an Ees/Ea_(Kind) >1.5 (purple line). There was also a significant association between Ees/Ea_(Takeuchi) (above/below median) and time to event (figure 3B), with an overall significant difference by group (p<0.0001). The low Ees/Ea and high PVRi (blue line) showed a higher risk of event compared with all other groups, except for the higher Ees/Ea and high PVR group (p=0.057). This group of patients, who had both a PVR >6.1 and an Ees/Ea_(Takeuchi) <0.85, has a slightly, though non-significantly, higher risk of event compared with those with a high PVR >6.1 and an Ees/Ea_(Takeuchi) >0.85 (purple line).
Figure 3.
Kaplan-Meier survival curves of (A) Ees/Ea_(Kind) and (B) Ees/Ea_(Takeuchi), stratified by median values with Ees/Ea (left panel), indexed pulmonary vascular resistance (PVRi) (middle panel) and stratified based on both Ees/Ea and PVRi (right panel).
Soft outcomes
Neither Ees/Ea_(Takeuchi) nor Ees/Ea_(Kind) were associated with time to soft event in univariate models in patients with IPAH/HPAH or APAH. Similarly, neither Ees/Ea_(Takeuchi) nor Ees/Ea_(Kind) were associated with time to soft event after adjusting for PVRi.
Discussion
In this study, we present a comparison of the RV pressure-derived estimation of the load-matching capacity of RV contractility with pulmonary afterload using two different methods (Takeuchi et al and Kind et al) for prediction of adverse clinical outcome in children with PAH. Furthermore, clinical outcomes were assessed and classified into two categories of soft adverse events, and hard adverse events. Data from this study of 130 patients have shown that both metrics of ventricular–vascular coupling ratio assessed were (1) an independent predictor of hard events in patients with IPAH/HPAH and (2) a multivariable predictor of hard events in patients with IPAH/HPAH. To the best of our knowledge, we are the first to compare these two methods of ventricular–pulmonary coupling and are the first to demonstrate the prognostic strength of single-beat derived Ees/Ea in children with PAH.
Recent research on survival and mortality in paediatric PAH has warranted reevaluation of prognostic definitions separate from that of adults.2 24 27 Registries from major PH-referral centres have reported WHO-FC worsening, syncope and haemoptysis to be predictive in children27–29; hence, these relatively minor events were classified as endpoints for prognostic assessment of RV contractility/PA afterload matching in the present study. However, neither estimations of Ees/Ea were predictive for these soft outcomes. This could be due to RV–PA decoupling occurring in later stages of PAH, and thus in these patients with the presentation of compensated RV function matched to increased afterload (ie, they remain coupled), Ees/Ea does not predict these minor adverse outcomes. Ees/Ea reflects the efficiency of interaction between increasing RV contractility to match the increasing impedance of the vasculature in order to maintain adequate cardiac output. Eventually, the RV will reach its maximum contractility capacity and will begin to decline, and decompensation, or decoupling between the RV and PA will occur. Thus, it can be argued that in a cohort of mostly compensated RV function, Ees/Ea will not necessarily be associated with soft adverse outcome but is rather predictive of late-stage PAH. Indeed, there was no significant difference for either Ees/Ea_(Takeuchi) or Ees/Ea_(Kind) between those who experienced a soft event and those who did not. Furthermore, these events are relatively more subjective than the defined hard outcomes due to patient-reported observations rather than those discretely observed within the clinic, though they have been indicated to be prognostic in other studies.24 27 Interestingly, although there were no significant differences between aetiology, time to follow-up, Ees/Ea_(Takeuchi) or Ees/Ea_(Kind) in patients who experienced a soft event and those who did not, there was a significant increase in PVRi in those who experienced an event. PVRi has remained a strong predictor of survival in children with PAH,23 27 and thus is identified as one of the ‘gold standards’ of diagnosis and prognosis. The finding of new prognostic metrics in paediatric PAH, such as WHO-FC worsening, reported by van Loon et al24 has prompted a reevaluation of adverse clinical event definitions; however, larger studies are needed to fully discern which soft events defined within the current study demonstrate high prognostic strength.
In agreement with our hypothesis, both the original single-beat method, with a Pmax function developed from LV pressure waveforms in dogs (Ees/Ea_(Takeuchi)10) and one with a Pmax that better models the isovolumic RV pressure waveforms of pulmonary hypertensive rats (Ees/Ea_(Kind)14) were associated with hard outcomes independently in patients with IPAH/HPAH, with higher coupling associated with lower event hazard. These data suggest there is merit to the approach postulated by Kind et al that normalising RV pressure to the standard isovolumetric wave shape thereby reduces the error of approximation of maximum theoretical pressure (Pmax).14 However, Ees/Ea_(Takeuchi) performed better than Ees/Ea_(Kind) with a lower out-of-sample prediction error comparatively (AIC=128 vs AIC=135, respectively). Furthermore, Ees/Ea_(Kind) on average estimated higher values of coupling compared with Ees/Ea_(Takeuchi), and as the mean of the measures increased, the differences between each measure increased (figure 2B), suggesting that at higher values of Ees/Ea (>2) measurements deviate greatly. Other methods using the second derivative of the pressure waveform to define the isovolumetric contraction, reported by Bellofiore et al,23 show reduced interobserver variability in estimation of Pmax, and a novel method by Heerdt et al proposes a Weibull distribution function rather than a sinusoidal fit of the RV pressure waveform.30 Further research in the measurement agreement between Ees/Ea_(Kind) and Ees/Ea_(Takeuchi) and comparison to these other methods mentioned is needed to fully elucidate the differences in their variability in assessment of RV–PA coupling.
Previous studies have shown that separately, pulmonary vascular afterload is only moderately a predictor of survival in PAH, while RV function remains one of the strongest survival determinants.28 29 Thus, a single metric combining RV function with afterload would provide a stronger prognostic metric rather than either afterload or function alone. It is with this advantage that Ees/Ea can provide further insight by representing both components of vascular and ventricular function. Indeed, the Kaplan Meier analysis revealed that both Ees/Ea_(Kind) and Ees/Ea_(Takeuchi) add an extra layer of information to PVRi regarding risk of event (figure 3, third panel). Furthermore, it was demonstrated that though PVRi was a strong independent predictor of hard outcome in patients with IPAH/HPAH (AIC=129), the multivariate Cox PH models demonstrated higher prognosis with combined covariates of PVRi and Ees/Ea_(Takeuchi) and Ees/Ea_(Kind) (AIC=124, AIC=127, respectively), suggesting that Ees/Ea improves prediction in these patients compared with PVRi alone. Finally, PVRi did not show a significant relationship with either Ees/Ea_(Takeuchi) or Ees/Ea_(Kind) (online supplemental figure 1), demonstrating that PVRi and Ees/Ea are indeed non-linear, thus providing further evidence that Ees/Ea offers information beyond PVRi alone.
Ees/Ea_(Takeuchi) also demonstrated similarly strong independent prognosis for hard events (AIC=128) compared with PVRi while Ees/Ea_(Kind) was only mildly independently predictive (AIC=135). Larger studies are needed to determine the differences between Ees/Ea_(Takeuchi), Ees/Ea_(Kind), their prognostic strength in comparison to PVRi, and whether the methods of error reduction postulated by Kind et al are indeed stronger at predicting adverse outcome through the increased accuracy of RV functional assessment. With only 20 patients with IPAH/HPAH and 11 patients with APAH experiencing a hard event, we recognise this limitation and that a larger subject inclusion is necessary to fully elucidate these differences. Interestingly, neither Ees/Ea_(Takeuchi) nor Ees/Ea_(Kind) were predictive of hard outcomes in patients with APAH. This is perhaps due to the heterogenous presentation of cardiac function in patients with APAH that is often confounded by their comorbidity. The presence of intra-cardiac shunts would alter PVRi and RV function that would directly affect an association with patient outcome. With this consideration combined with the low frequency of events, a longer follow-up time with a larger subject population in future studies may fully reveal whether Ees/Ea is prognostic in patients with APAH. In contrast, higher values of both Ees/Ea_(Kind) and Ees/Ea_(Takeuchi) were associated with a decrease in hazard in idiopathic patients. This supports the mechanism of PA/RV decoupling in progression of PAH considering our notation of Ees/Ea as the ratio of Ees to Ea: higher values of Ees/Ea would indicate a higher ratio of RV contractility to afterload. Patients with lower Ees/Ea would have inadequate RV contractility to overcome their PA afterload and would have a higher likelihood of an event occurrence. Because IPAH/HPAH subjects demonstrated a higher likelihood occurrence of a hard event, these patients may be in the progressive decoupled phase would be associated with a higher event hazard.
Limitations
Our study had several limitations. First, though our population is relatively large for a paediatric PAH study, our subpopulations stratified by aetiology are small, and thus the patients experiencing an adverse event are few. Furthermore, the APAH population was highly heterogeneous and was therefore not sufficient to power analysis within subpopulations. Thus, a larger sample size is needed to yield more a more statistically powerful assessment of the prognosis of Ees/Ea in children, with longer retrospective study and follow-up periods. Second, the effect of anaesthetics are known to alter RV–PA coupling in a dose-dependent manner, however, given the conditions of RHC and ubiquity across patients, we argue that these effects are minimal and unavoidable. Third, Ees/Ea derived through PV loops is invasive and requires cardiac catheterisation surgery in order to record ventricular pressures. Current research has focused on derivation of Ees/Ea and RV contractility through volume estimates based on non-invasive imaging alone21; in particular, Heerdt et al have recently postulated an approximation of Ees/Ea as RVEF/(1−RVEF).30 Our group has also previously demonstrated a strong comparison of RHC-derived Ees/Ea to volume-derived Ees/Ea in a study of 27 paediatric patients with PAH.21 The present study focused on interrogation of catheter-derived metrics of coupling, however, future research with our paediatric patients will focus on estimating Ees/Ea and other metrics of coupling, such as RVEF, in a non-invasive capacity. Fourth, due to CHC being a tertiary referral institution for paediatric PAH, possible selection bias may exist, and thus subjects of this study may present with a more severe disease presentation; however, given our inclusion criteria and relatively heterogenous population, this potential bias should not confound our results or conclusions. Finally, though the correlation between Ees/Ea_(Kind) and Ees/Ea_(Takeuchi) was quite strong (p<0.0001), overall Ees/Ea_(Kind) estimated higher values. We acknowledge that a mean difference of 0.58 could indicate a physiological difference between adequate RV–PA coupling and decoupling states. Given the confidence intervals and the suggested disparity of coupling effects between the two estimates, further research of the more accurate method for prediction of event is yet to be determined.
Conclusion
This study assessed the prognostic ability of pressure-derived ventricular–vascular coupling and demonstrated that both the original modified single beat method proposed by Takeuchi et al (Ees/Ea_(Takeuchi)), and Ees/Ea developed by Kind et al (Ees/Ea_(Kind)), is predictive of severe adverse outcomes in children with idiopathic or hereditary aetiology. The data presented show that Ees/Ea is a powerful tool that provides insight to both ventricular and vascular function that can be used in management and treatment of children with PAH.
Acknowledgments
The authors thank all the investigators and all supporting staff, with special thanks to Kathleen Miller-Reed and Danielle Hutchins.
Footnotes
Contributors: MJD performed clinical data acquisition, Ees/Ea analysis, interpretation of data, assisted in project design and implementation, and performed manuscript preparation. DI performed paediatric RHC data collection, assisted with data interpretation and manuscript preparation. KC performed all statistical analyses, interpretation of data, and assisted in project design, implementation, and manuscript preparation. AL performed clinical data acquisition and assisted in Ees/Ea analysis. AR performed original design and implementation of pressure-waveform Ees/Ea derivations. KTNB assisted in Ees/Ea analysis and data acquisition. JMD assisted in clinical data acquisition, defining of outcome endpoints, and manuscript preparation. RMFB assisted in manuscript preparation. VOK assisted in data interpretation and manuscript preparation. KH conceived the project design, designed and implemented the pressure-waveform Ees/Ea derivations analysis, assisted in data interpretation and manuscript preparation.
Funding: This work was supported by NIH/NCATS Colorado CTSA Grant Number UL1 TR002535, NIH K25 HL133481, and the Jayden de Luca foundation. JMD was supported by the Royal Netherlands Academy of Arts and Sciences, Ter Meulen Fund; KTNB and JMD were supported by the Sebald Fund. The University of Colorado has received research support from Actelion Pharmaceuticals Ltd, Bayer, Eli Lilly & Co, Janssen and United Therapeutics for DDI to perform clinical trials; the University of Colorado contracts with Actelion Pharmaceuticals Ltd, Bayer HealthCare, Eli Lilly & Co and United Therapeutics for DD Ivy to be a consultant. The University Medical Centrum Groningen contracts with Actelion Pharmaceuticals, Lilly and Pfizer for advisory board and steering committee activities of RMFB.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available upon reasonable request. The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study was carried out with the approval of the Colorado Institutional Research Board at Childrens Hospital Colorado, in accordance with the Declaration of Helsinki, with waiver of informed consent.
References
- 1.Vonk-Noordegraaf A, Haddad F, Chin KM, et al. Right heart adaptation to pulmonary arterial hypertension: physiology and pathobiology. J Am Coll Cardiol 2013;62:D22–33. 10.1016/j.jacc.2013.10.027 [DOI] [PubMed] [Google Scholar]
- 2.Ivy DD, Abman SH, Barst RJ, et al. Pediatric pulmonary hypertension. J Am Coll Cardiol 2013;62:D117–26. 10.1016/j.jacc.2013.10.028 [DOI] [PubMed] [Google Scholar]
- 3.Wang Z, Chesler NC. Pulmonary vascular wall stiffness: an important contributor to the increased right ventricular afterload with pulmonary hypertension. Pulm Circ 2011;1:212–23. 10.4103/2045-8932.83453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spruijt OA, de Man FS, Groepenhoff H, et al. The effects of exercise on right ventricular contractility and right ventricular-arterial coupling in pulmonary hypertension. Am J Respir Crit Care Med 2015;191:1050–7. 10.1164/rccm.201412-2271OC [DOI] [PubMed] [Google Scholar]
- 5.Vonk Noordegraaf A, Westerhof BE, Westerhof N. The relationship between the right ventricle and its load in pulmonary hypertension. J Am Coll Cardiol 2017;69:236–43. 10.1016/j.jacc.2016.10.047 [DOI] [PubMed] [Google Scholar]
- 6.Tello K, Gall H, Richter M, et al. Right ventricular function in pulmonary (arterial) hypertension. Herz 2019;44:509–16. 10.1007/s00059-019-4815-6 [DOI] [PubMed] [Google Scholar]
- 7.Ryan JJ, Archer SL. The right ventricle in pulmonary arterial hypertension: disorders of metabolism, angiogenesis and adrenergic signaling in right ventricular failure. Circ Res 2014;115:176–88. 10.1161/CIRCRESAHA.113.301129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sunagawa K, Maughan WL, Burkhoff D, et al. Left ventricular interaction with arterial load studied in isolated canine ventricle. Am J Physiol 1983;245:H773–80. 10.1152/ajpheart.1983.245.5.H773 [DOI] [PubMed] [Google Scholar]
- 9.Sunagawa K, Yamada A, Senda Y, et al. Estimation of the hydromotive source pressure from ejecting beats of the left ventricle. IEEE Trans Biomed Eng 1980;27:299–305. 10.1109/TBME.1980.326737 [DOI] [PubMed] [Google Scholar]
- 10.Takeuchi M, Igarashi Y, Tomimoto S, et al. Single-beat estimation of the slope of the end-systolic pressure-volume relation in the human left ventricle. Circulation 1991;83:202–12. 10.1161/01.CIR.83.1.202 [DOI] [PubMed] [Google Scholar]
- 11.Brimioulle S, Wauthy P, Ewalenko P, et al. Single-beat estimation of right ventricular end-systolic pressure-volume relationship. Am J Physiol Heart Circ Physiol 2003;284:H1625–30. 10.1152/ajpheart.01023.2002 [DOI] [PubMed] [Google Scholar]
- 12.Vanderpool RR, Pinsky MR, Naeije R, et al. RV-pulmonary arterial coupling predicts outcome in patients referred for pulmonary hypertension. Heart 2015;101:37–43. 10.1136/heartjnl-2014-306142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richter MJ, Peters D, Ghofrani HA, et al. Evaluation and prognostic relevance of right ventricular-arterial coupling in pulmonary hypertension. Am J Respir Crit Care Med 2020;201:116–9. 10.1164/rccm.201906-1195LE [DOI] [PubMed] [Google Scholar]
- 14.Kind T. “Towards a better description of cardiovascular function in pulmonary hypertension: modeling and clinical practice”, Chapter 6, “Estimation of right ventricular isovolumic pressure from a single ejecting beat in experimental pulmonary hypertension” PhD Thesis Vrije Universiteit Amsterdam; 2012. [Google Scholar]
- 15.Sanz J, Sánchez-Quintana D, Bossone E, et al. Anatomy, Function, and dysfunction of the right ventricle: JACC state-of-the-art review. J Am Coll Cardiol 2019;73:1463–82. 10.1016/j.jacc.2018.12.076 [DOI] [PubMed] [Google Scholar]
- 16.Brewis MJ, Bellofiore A, Vanderpool RR, et al. Imaging right ventricular function to predict outcome in pulmonary arterial hypertension. Int J Cardiol 2016;218:206–11. 10.1016/j.ijcard.2016.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenzweig EB, Abman SH, Adatia I, et al. Paediatric pulmonary arterial hypertension: updates on definition, classification, diagnostics and management. Eur Respir J 2019;53:1801916. 10.1183/13993003.01916-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.LaFarge CG, Miettinen OS. The estimation of oxygen consumption. Cardiovasc Res 1970;4:23–30. 10.1093/cvr/4.1.23 [DOI] [PubMed] [Google Scholar]
- 19.Apitz C, Hansmann G, Schranz D. Hemodynamic assessment and acute pulmonary vasoreactivity testing in the evaluation of children with pulmonary vascular disease. expert consensus statement on the diagnosis and treatment of paediatric pulmonary hypertension. the European paediatric pulmonary vascular disease network, endorsed by ISHLT and DGPK. Heart 2016;102 Suppl 2:ii23–9. 10.1136/heartjnl-2014-307340 [DOI] [PubMed] [Google Scholar]
- 20.Zijlstra WMH, Douwes JM, Rosenzweig EB, et al. Survival differences in pediatric pulmonary arterial hypertension: clues to a better understanding of outcome and optimal treatment strategies. J Am Coll Cardiol 2014;63:2159–69. 10.1016/j.jacc.2014.02.575 [DOI] [PubMed] [Google Scholar]
- 21.Breeman KTN, Dufva M, Ploegstra MJ, et al. Right ventricular-vascular coupling ratio in pediatric pulmonary arterial hypertension: a comparison between cardiac magnetic resonance and right heart catheterization measurements. Int J Cardiol 2019;293:211–7. 10.1016/j.ijcard.2019.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alyono D, Larson VE, Anderson RW. Defining end systole for end-systolic pressure-volume ratio. J Surg Res 1985;39:344–50. 10.1016/0022-4804(85)90113-1 [DOI] [PubMed] [Google Scholar]
- 23.Bellofiore A, Vanderpool R, Brewis MJ, et al. A novel single-beat approach to assess right ventricular systolic function. J Appl Physiol 2018;124:283–90. 10.1152/japplphysiol.00258.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Loon RLE, Roofthooft MTR, Delhaas T, et al. Outcome of pediatric patients with pulmonary arterial hypertension in the era of new medical therapies. Am J Cardiol 2010;106:117–24. 10.1016/j.amjcard.2010.02.023 [DOI] [PubMed] [Google Scholar]
- 25.R Core Team . R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2017. https://www.R-project.org/ [Google Scholar]
- 26.Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019;53. 10.1183/13993003.01913-2018. [Epub ahead of print: 24 01 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McLaughlin VV, Badesch DB, Delcroix M, et al. End points and clinical trial design in pulmonary arterial hypertension. J Am Coll Cardiol 2009;54:S97–107. 10.1016/j.jacc.2009.04.007 [DOI] [PubMed] [Google Scholar]
- 28.Barst RJ, McGoon MD, Elliott CG, et al. Survival in childhood pulmonary arterial hypertension: insights from the registry to evaluate early and long-term pulmonary arterial hypertension disease management. Circulation 2012;125:113–22. 10.1161/CIRCULATIONAHA.111.026591 [DOI] [PubMed] [Google Scholar]
- 29.Badesch DB, Champion HC, Gomez Sanchez MA, Sanchez MA, et al. Diagnosis and assessment of pulmonary arterial hypertension. J Am Coll Cardiol 2009;54:S55–66. 10.1016/j.jacc.2009.04.011 [DOI] [PubMed] [Google Scholar]
- 30.Heerdt PM, Kheyfets V, Charania S, et al. A pressure-based single beat method for estimation of right ventricular ejection fraction: proof of concept. Eur Respir J 2020;55:1901635. 10.1183/13993003.01635-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
openhrt-2021-001611supp002.pdf (45KB, pdf)
openhrt-2021-001611supp001.pdf (76.5KB, pdf)
Data Availability Statement
Data are available upon reasonable request. The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.