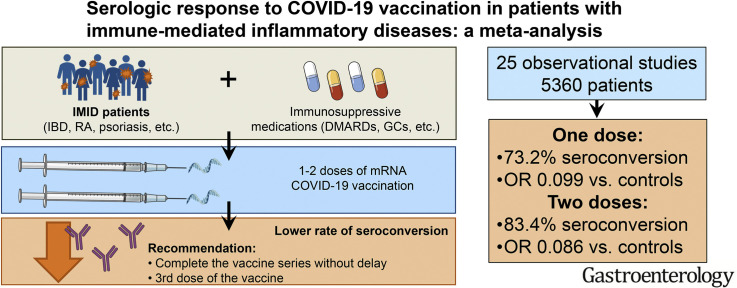
Abstract
Background & Aims
Patients with immune-mediated inflammatory diseases (IMIDs) have an increased risk of coronavirus disease 2019 (COVID-19), primarily attributed to the use of immunosuppressive drugs such as glucocorticoids, which may attenuate the response to vaccines. This meta-analysis assessed the serologic response to COVID-19 vaccination in patients with IMIDs.
Methods
Electronic databases were searched on August 1, 2021, for observational studies. Data extracted included reference population, medications, vaccination, and proportion of patients achieving a serologic response.
Results
The analysis included 25 observational studies (5360 patients). Most of the studies used messenger RNA (mRNA) vaccines (BNT162b2, mRNA-1273), with a small number of studies including other types of vaccines (AZD1222, CoronaVac, BBV152, Ad26.COV2.S). Serologic response after 1 dose (6 studies) and 2 doses (17 studies) of mRNA vaccine were 73.2% (95% confidence interval [CI], 65.7%-79.5%) and 83.4% (95% CI, 76.8%-88.4%), respectively. On meta-regression, anti-CD20 therapy was associated with lower response rates (P < .001) and anti-tumor necrosis factor therapy also showed a trend toward lower response rates (P = .058). Patients with IMIDs were less likely to achieve a serologic response compared with controls after 2 doses of mRNA vaccine (6 studies; odds ratio, 0.086; 95% CI, 0.036–0.206; P < .001). There were not enough studies to assess response to the adenoviral or inactivated vaccines.
Conclusions
Our meta-analysis demonstrated that patients with IMIDs have a reduced response to mRNA COVID-19 vaccines. These results suggest that IMID patients receiving mRNA vaccines should complete the vaccine series without delay and support the strategy of providing a third dose of the vaccine.
Keywords: Vaccine, Outcomes, Immune-Mediated Inflammatory Diseases, Inflammatory Bowel Disease, Rheumatic Disease
Abbreviations used in this paper: b/ts, biological/targeted synthetic; CD, cluster of differentiation; CI, confidence interval; COVID-19, coronavirus disease 2019; csDMARDs, conventional synthetic disease-modifying antirheumatic drugs; GCs, glucocorticoids; IBD, inflammatory bowel disease; IL, interleukin; IMID, immune-mediated inflammatory disease; mRNA, messenger RNA; OR, odds ratio; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TNF, tumor necrosis factor
Graphical abstract
Patients with immune-mediated inflammatory disease had low serologic response after both doses of messenger RNA vaccines, and so should complete the vaccine series without delay and consider receiving a third dose of the vaccine.
See Covering the Cover synopsis on page 1.
What You Need to Know.
Background and Context
Some medications used to treat immune-mediated inflammatory diseases increase the risk of coronavirus disease 2019 and also hamper vaccine response, but little is known about the effectiveness of coronavirus disease 2019 vaccines in patients with immune-mediated inflammatory diseases.
New Findings
Patients with immune-mediated inflammatory diseases had low serologic response after both doses of messenger RNA vaccines, which was lower compared with controls. Anti-cluster of differentiation 20 and anti-tumor necrosis factor therapies were associated with lower serologic responses.
Limitations
Continued studies assessing both humoral and cellular immunity to the various types of vaccine will be required to assess both vaccine effectiveness and durability.
Impact
The results of our study suggest that patients with immune-mediated inflammatory diseases should complete the vaccine series without delay and support the Food and Drug Administration decision that patients with immune-mediated inflammatory diseases on immunosuppressives need a third dose.
A novel RNA coronavirus called severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is responsible for the coronavirus disease 2019 (COVID-19) pandemic and global health emergency.1 Patients with pre-existing conditions, such as immune-mediated inflammatory diseases (IMIDs), may be more susceptible to infection with SARS-CoV-2, and there is concern that certain immunosuppressive therapies may lead to worse outcomes.2 , 3 To understand the incidence and prognosis of COVID-19 in IMIDs, international registries of patients with inflammatory bowel disease (IBD) (Surveillance Epidemiology of Coronavirus Under Research Exclusion [SECURE]-IBD registry) or rheumatic diseases (COVID-19 Global Rheumatology Alliance [C19-GRA]) diagnosed with COVID-19 were developed and analyzed with respect to individual patient outcomes.4 These studies have demonstrated that similar to the general population, age and underlying comorbidities were poor prognostic factors among patients with IMIDs developing COVID-19.5 , 6
We previously reported in a meta-analysis of 62 studies that the prevalence of COVID-19 was elevated in rheumatic diseases.3 Studies in IBD suggest that the risk is similar to the general population.7 With respect to the therapies often used in the management of patients with IMIDs, glucocorticoids (GCs), conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), and biological/targeted synthetic (b/ts) DMARDs-csDMARDs combination therapy significantly increased the risk of severe outcomes, whereas b/tsDMARDs monotherapy, in particular anti-tumor necrosis factor (TNF) therapy, reduced the risk of severe COVID-19.3
With the lack of effective treatments, prevention strategies, including vaccination, are of paramount importance in reducing the risk of COVID-19.8 Rapidly emerging data have shown that messenger RNA (mRNA)-based COVID-19 vaccines are safe and effective in the general population. However, the efficacy of COVID-19 vaccines in patients with IMIDs is unknown because patients with IMIDs or those treated with immunosuppressing therapies were excluded from regulatory vaccine trials.9
Guidelines currently recommend that patients with IMIDs should be vaccinated against SARS-CoV-2 due to the ongoing pandemic and risk of death.10 , 11 Data from other vaccine-preventable illnesses suggest attenuated responses in patients receiving GCs, TNF antagonists, and immunosuppressive drugs such as cyclophosphamide and azathioprine.10 , 12 A recent study by Kennedy et al13 reported that patients with IBD patients receiving infliximab had an attenuated immunogenicity to a single-dose of the BNT162b2 and ChAdOx1 nCoV-19/AZD1222 SARS-CoV-2 vaccines compared with those receiving vedolizumab, a gut-selective biologic.13 Additional studies investigating the effectiveness of COVID-19 vaccines in IMIDs are limited and mostly include small sample sizes. Therefore, there is a need to integrate findings across studies to better understand the effectiveness of COVID-19 vaccines in patients with IMIDs. In this systematic review and meta-analysis, our aim was to determine the serologic response rate to COVID-19 vaccination in patients with IMIDs.
Materials and Methods
Search Strategy and Study Selection
This meta-analysis was conducted according to an a priori-defined protocol that is in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guideline.14 The protocol of this meta-analysis was submitted to the International Prospective Register of Systematic Reviews (PROSPERO).15 We searched PubMed/MEDLINE, Embase, and medRxiv (https://www.medrxiv.org/) from inception to August 1, 2021, to identify studies assessing the response to COVID-19 vaccination in patients with IMIDs.
We considered for inclusion observational studies reporting the outcomes of COVID-19 vaccination in IMIDs patients. There were no restrictions regarding age, sex, or duration of the study. We imposed no geographic or language restrictions. Two authors (A.L., A.S.) independently screened each of the potential studies to determine whether they were eligible for inclusion. Areas of disagreement or uncertainty were resolved by consensus among the authors.
Studies were identified with the following terms: “COVID-19,” “SARS-CoV-2,” “vaccine,” “immunization,” “inflammatory bowel diseases” or “rheumatoid arthritis” or “psoriasis” or “rheumatic diseases” or “systemic lupus erythematosus,” “psoriatic arthritis,” “ankylosing spondylitis,” “Crohn’s disease,” “ulcerative colitis” or “multiple sclerosis,” “immune mediated diseases,” or “autoimmune diseases.”
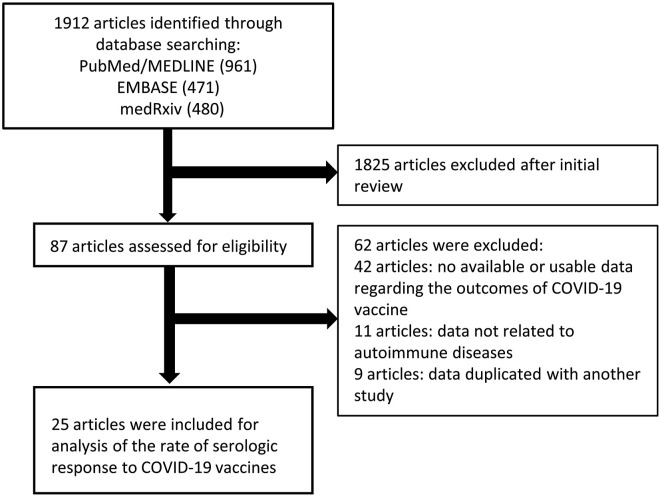
A search was also performed of bibliographies of identified articles for additional references. Abstracts of Digestive Disease Week 2021, European Crohn’s and Colitis Organisation 2021, and the Annual Meeting of the American Academy of Neurology 2021 were also searched because they were held after the vaccines became available. The search was restricted to human studies. Manuscripts published in languages other than English were translated if necessary. Single case reports were excluded. Studies that reported only adverse outcomes to COVID-19 vaccination were excluded. The search strategy is described in Figure 1 , and the PubMed/MEDLINE search strategy is summarized in Supplementary Table 1.
Figure 1.
Flow chart of the assessment of the studies identified in the meta-analysis.
Data Extraction and Quality Assessment
All data were independently abstracted in duplicate by 2 authors (A.L., A.S.) by using a data extraction form. Data on the study characteristics, including author name, year of publication, study design, duration, study location, sample size, diagnosis of IMIDs, concomitant medication use, age and sex of patients, type and frequency of vaccination, and type and outcome of serologic testing were collected. We divided medication use into the following 3 categories: (1) GCs, (2) csDMARDs, and (3) b/tsDMARDs.3 Budesonide, which is used as an ileal release form in IBD, was not included in the GCs when data were available. csDMARDs included hydroxychloroquine, chloroquine, thiopurines, cyclophosphamide, cyclosporine, tacrolimus, leflunomide, methotrexate, mycophenolate mofetil/mycophenolic acid, and sulfasalazine. b/tsDMARDs included abatacept, belimumab, cluster of differentiation (CD) 20, interleukin (IL)1, IL6, IL12/23, IL23, IL17, TNF, α4β7 integrin, and Janus kinase inhibitors. We also divided b/tsDMARDs into monotherapy and b/tsDMARDs-csDMARDs combination therapy if studies separately presented the data. If not, we considered b/tsDMARDs as used as a monotherapy.
The risk of bias of included studies was assessed using the Joanna Briggs Institute Critical Appraisal Checklist.16 We rated the quality of evidence according to the Grades of Recommendation, Assessment, Development and Evaluation (GRADE) approach to assess the certainty of evidence obtained from the present meta-analysis.17
Outcome Assessment
The primary outcome was the rate of serologic response to COVID-19 vaccination in patients with IMIDs. Response was assessed separately after 1 or 2 doses of vaccine when data were available. The secondary outcome of interest was the rate of serologic response in patients with IMIDs compared with control patients without IMIDs. We extracted the number of patients who achieved an above cutoff antibody level among the total number of patients tested in each study. Using a common cutoff value between studies was not possible because each study used a different serologic testing method. When tests were performed multiple times in a study, we chose the date closest to 4 weeks after the vaccination.
To conduct subgroup analyses with each disease, we classified IMIDs based on the target organ, such as digestive, musculoskeletal, urinary (kidney), and integumentary systems. Diseases of the digestive system were categorized into IBD. Rheumatic diseases included rheumatoid arthritis, systemic lupus erythematosus, psoriatic arthritis, spondyloarthritis, ankylosing spondylitis, vasculitis, polymyalgia rheumatica, Sjögren’s syndrome, systemic sclerosis, and other autoimmune-mediated diseases, including Behçet’s disease, sarcoidosis, vasculitis, and inflammatory myopathies. Diseases of the skin were categorized as psoriasis/autoimmune skin diseases. Immune-mediated kidney diseases included antineutrophil cytoplasmic antibody–associated vasculitis, minimal change disease/focal and segmental glomerulosclerosis, and membranous nephropathy. Studies that included various IMIDs were classified as mixed group.
Subgroup analyses or meta-regression according to type of vaccine, age, disease, or medication use were undertaken when data were available.
Statistical Analysis
We undertook a meta-analysis of the rate of serologic response to COVID-19 vaccination among individuals with IMIDs from observational studies by using a random-effects model. Inverse variance of each study’s effect estimator was used to allocate the weight to each study in the synthesis. The presence of heterogeneity across studies was assessed by using the I 2 statistic. An I 2 value of <25% indicates low heterogeneity, 25% to 75% as moderate heterogeneity, and >75% as considerable heterogeneity.18 Heterogeneity was evaluated by using Cochran’s Q-statistics with a significance level of P < .10.19 Begg’s and Egger’s tests were performed to assess publication bias, and funnel plots were constructed to visualize asymmetry when ≥3 studies were available.20 , 21 Univariate and multivariate meta-regression models were used to assess the contributions of each of potential risk factors and medication class to the outcome of vaccine response. Multivariate meta-regression was undertaken with the variables that had a P < .05 on univariate meta-regression. When the number of available studies for each analysis was <10, funnel plot construction and meta-regression analysis were undertaken for reference purposes due to its low reliability.
We included preprints because they form a substantial part of the available COVID-19 evidence, but due to their lack of peer review, we conducted a sensitivity analysis by excluding preprints.22 We also performed one study-removed analyses to assess whether the results are strongly influenced by any single study. When comparing serologic response to controls, zero-event studies were excluded from analysis, but we also performed sensitivity analysis including all studies by applying the standard continuity correction of 0.5 to 0-event studies.23
Statistical analyses were performed using Comprehensive Meta Analysis Software version 3 (Biostat, Englewood, NJ). All statistical tests, except for the Q statistics, used a 2-sided P value of .05 for significance.
Data Sharing and Access
Data will be made available upon request to the corresponding author. All authors had access to the study data and reviewed and approved the final manuscript.
Patient and Public Involvement
We did not directly include patient and public involvement in this study. Patients were not invited to comment on the study design and were not consulted to interpret the results. Patients were not invited to contribute to the writing or editing of this manuscript.
Ethics Statement
This study did not involve human participants, so ethics approval by an Institutional Review Board was not needed.
Results
Study Characteristics
We identified 1912 citations through the literature search and excluded 1825 titles and abstracts after initial screening. We assessed 87 studies for eligibility, and 25 articles including 5360 patients met eligibility criteria (Figure 1). As reported in Table 1 , 12 were full-text articles,13 , 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34 9 were correspondence/letters,35, 36, 37, 38, 39, 40, 41, 42, 43 and 4 were preprints.44, 45, 46, 47 Eleven studies included patients with rheumatologic diseases,27 , 28 , 32, 33, 34 , 36 , 38 , 40 , 42 , 43 , 46 4 studies included patients with IBD,13 , 25 , 26 , 47 2 studies included patients with psoriasis,30 , 35 1 study included patients with immune-mediated kidney diseases,39 and 7 studies included patients with various IMIDs.24 , 29 , 31 , 37 , 41 , 44 , 45 Of the 25 studies, 23 used BNT162b2 (Pfizer-BioNTech; Pfizer, New York, NY) or mRNA-1273 (Moderna, Cambridge, MA). Three studies included 50% to 75.9% of patients who used ChAdOx1 nCoV-19/AZD1222 (Oxford-AstraZeneca, Cambridge, United Kingdom), and data were separately reported.13 , 37 , 46 Three studies included a small proportion (1.8%-17.2%) of patients who used Ad26.COV2.S (Janssen/Johnson & Johnson, New Brunswick, NJ),41 , 45 , 47 but data were not reported separately, so these studies were included in the analyses of mRNA vaccines. Two studies used CoronaVac (Sinovac, Beijing, China) only.31 , 33 One study included patients who used BBV152 (Covaxin; Bharat Biotech, Hyderabad, India), and data were separately reported.46
Table 1.
Characteristics and Outcomes of the Included Studies
| Author | Country | Year | Patient numbers and description | Control numbers and description | Age of patients, median (y) | Sex of patients (% females) | Cases, concomitant biologics/DMARDs | Cases, concomitant steroids | Type of vaccine | No. of patients receiving 1 dose | No. of patients receiving 2 doses |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Damiani | Italy | 2021 Letter | 4 (psoriasis 100%) | None | 46.8 | 25 | 100% (secukinumab 50%, ixekizumab 25%, risankizumab 25%) | NA | BNT162b2 (Pfizer-BioNTech) 100% | 0 | 4 |
| Deepak | United States | 2021 Preprint | 133 (CD 16.5%, UC 13.5%, RA 28.6%, SpA 15%, SLE 11.3%, Sjögren’s syndrome 6.0%, MS 6.8%, etc) | 53 (HCW and patients) | Cases 45.5,Controls 43.4 | Cases 74.4%,Controls 54.7% | 93.20% (methotrexate 21.8%, hydroxychloroquine 22.6%, MMF 6.8%, AZA 3.0%, leflunomide 1.5%, sulfasalazine 5.3%, JAK inhibitors 8.3%, TNFi 28.6%, etc) | 12.8% | BNT162b2 (Pfizer-BioNTech) NA%, mRNA-1273 (Moderna) NA% | 0 | 133 |
| Geisen | Germany | 2021 Full-text article | 26 (RA 31%, psoriasis/PsA 23%, spondyloarthropathy 12%, SLE 8%, CD 8%, etc) | 42 (HCW) | Cases 50.5 (range 24–89), Controls 37.5 (range 22–61) | Cases 64.3%,Controls 69.2% | 92.3% (cDMARDs 30.8%, bDMARDs 76.9%, both 11.5%) | 26.9% | BNT162b2 (Pfizer-BioNTech) 92.6%, mRNA-1273 (Moderna) 7.3% | 0 | 68 |
| Kennedy | United Kingdom | 2021 Full-text article | 1293 (UC 57.2%, CD 42.8%) | None | 43.8 (32.8–57.6) | 49.2% | 100% (infliximab 66.9%, vedolizumab 33.1%, immunomodulators 48.5%, mesalazine, 25.9%) | 4.8% | AZD1222 (Oxford-AstraZeneca) 54.4%, BNT162b2 (Pfizer-BioNTech) 45.6% |
1293 | 27 |
| Wong | United States | 2021 Full-text article | 48 (UC 52%, CD 48%) | 43 (HCW and volunteers) | Cases 48.8, HCW 35.2, Volunteers 31.5 | Cases 50%,HCW 50%, Volunteers 39% | 85% (TNFi 33%, vedolizumab monotherapy 42%, vedolizumab combination therapy with thiopurine 6%, ustekinumab 8%, guselkumab 2%) | 6% | BNT162b2 (Pfizer-BioNTech) 59%, mRNA-1273 (Moderna) 41% | 36 (22 IBD, 0 HCW, 14 volunteers) | 66 (26 IBD, 14 HCW, 26 volunteers) |
| Boyarsky | United States | 2021 Letter | 123 (inflammatory arthritis 28%, SLE 20%, Sjögren’s syndrome 13%, overlap connective tissue diseases 29%) | None | 50 | 95% | 72% (csDMARDs 19%, bDMARDs 14%, combination therapy 37%) | 3% | BNT162b2 (Pfizer-BioNTech) 52%, mRNA-1273 (Moderna) 48% | 123 | 0 |
| Kappelman | United States | 2021 Full-text article | 317 (IBD 100%) | None | Mean 50.9 | 75.1% | 95.2% (TNFi monotherapy 34.1%, TNFi combination therapy 7.6%, 6MP/AZA/MTX alone 6.3%, mesalazine, sulfasalazine, budesonide, or no medication 20.5%, vedolizumab monotherapy 14.5%, ustekinumab monotherapy 12.3%) | 4.1% | BNT162b2 (Pfizer-BioNTech) 54.6%, mRNA-1273 (Moderna) 45.4% | 0 | 317 |
| Furer | Israel | 2021 Full-text article | 686 (RA 38.3%, PsA 24.1%, AxSpA, 9.9%, SLE 14.7%, AAV 3.8%, etc) | 121 (mainly HCWs) | Cases mean: 56.76, Controls mean: 50.76 | Cases 69.2%, Controls 64.5% | 95.2% (MTX 25.7%, TNFi 25.1%, IL6 inhibitors 5.4%, Anti-CD20 12.7%, abatacept 2.3%, JAK inhibitors 7.1%, IL17 inhibitors 7.0%, MMF 4.1%) | 18.95% | BNT162b2 (Pfizer-BioNTech) 100% | 0 | 807 |
| Shenoy | India | 2021 Preprint | 102 (RA 37.2%, palindromic rheumatism 16.7%, inflammatory polyarthritis 15.7%, spondyloarthropathies 12.7%, SLE 8.8%, vasculitis 5.9%, scleroderma 2.9%, myositis 1.0%) |
60 (Volunteers) | Cases mean: autoimmune 52, other RMD 54.12, Controls mean: ChAdOx1 43.60, BBV152 44.20 | Cases 77.2%, Controls 93.3% | 100% (MTX 56.9%, sulfasalazine 19.6%, leflunomide 8.8%, hydroxychloroquine 69.6%, tofacitinib 5.9%, rituximab 5.9%, MMF 4.9%, etc) | 19.9% | AZD1222 (Oxford-AstraZeneca) 75.9%, BBV152 (Covaxin) 24.1% | 0 | 162 |
| Haidar | United States | 2021 Preprint | 160 (IBD 38.1%, rheumatologic tdiseases 45.6%, other 16.3%) | 107 (HCWs) | Cases mean: 54.2, Controls mean: 43.7 | Cases 70%, Controls 72.0% | 48.1% (TNFi 45%, anti-CD20 3.1%) | NA | Overall population: mRNA-1273 (Moderna) 48.5%, BNT162b2 (Pfizer-BioNTech) 49.7%, Ad26.COV2.S (Janssen/Johnson & Johnson) 1.8% | 0 | 267 |
| Haberman | United States | 2021 Full-text article | 26 (psoriasis/PsA 47.1%, RA 43.1%, other (vasculitis, dermatomyositis, adult-onset Still’s disease, sarcoidosis and polymyalgia rheumatica) 9.8%) | 51 (Healthy subjects) | Cases No MTX mean: 49.1, MTX mean: 63.2, Controls mean: 49.2 | Cases 70.6%, Controls 61.5% | 100% (MTX 49.0%, TNFi 39.2%, other anticytokines/JAK inhibitors 19.6%, immunomodulators 25.5%) | 5.9% | BNT162b2 (Pfizer-BioNTech) 100% | 0 | 77 |
| Mahil | United Kingdom | 2021 Full-text article | 84 (Psoriasis 100%) | 17 (Volunteers) | 43.0 (IQR 31.0–52.0) | 44.5% | 100% (MTX 20.2%, TNFi 32.1%, IL17 inhibitors 17.9%, IL23 inhibitors 29.8%) | 0% | BNT162b2 (Pfizer-BioNTech) 100% | 94 | 0 |
| Simon | Germany | 2021 Full-text article | 84 (SpA 32.1%, RA 29.8%, IBD 9.5%, psoriasis 9.5%, systemic 19.1%) | 182 (Clinic patients) | Cases mean 53.1, Controls mean 40.8 | Cases 65.5%, Controls 57.1% | 66.7% (csDMARDs monotherapy 23.9%, bDMARDs/tsDMARDs 42.9%) | 11.9% | BNT162b2 (Pfizer-BioNTech) 100% | NA | 266 |
| Al-Janabi | United Kingdom | 2021 Letter | 120 (psoriasis 89.2%, PsA 20.8%, RA 8.3%, SLE 0.83%, CD 2.5%) | None | 53 (IQR 33–73) | 40.8% | 74.2% (biologics 67.5%, immunomodulators 25.8%, biologic and immunomodulator 6.7%) | 2.5% | BNT162b2 (Pfizer-BioNTech) 50%, AZD1222 (Oxford-AstraZeneca) 50% | 120 | 0 |
| Bugatti | Italy | 2021 Letter | 120 (RA 57.5%, PsA 21.7%, SpA 20.8%) | None | Mean 56.7 | 67.5% | 100% (csDMARDs 55.8%, b/tsDMARDs 100%) | 39.2% | BNT162b2 (Pfizer-BioNTech) 100% | 120 | 0 |
| Braun-Moscovici | Israel | 2021 Full-text article | 264 (inflammatory arthritis 57.8%, connective tissue diseases 33.0%, vasculitis 7.2%, other 2.3%) | None | Mean 57.6 | 76% | 100% (csDMARDs 60.6%, b/tsDMARDs 67.4%, colchicine 2.3%, nintedanib 1.1%, combination therapy 36.0%) | 34.8% | BNT162b2 (Pfizer-BioNTech) 100% | 0 | 156 |
| Demoulin | Belgium | 2021 Letter | 11 (AAV 63.6%,Minimal change disease/focal and segmental glomerulosclerosis 27.3%, membranous nephropathy 9.1%) | None | 38 (IQR 36–61) | 45.5% | 100% (rituximab monotherapy 100%) | 0% | BNT162b2 (Pfizer-BioNTech) 100% | 0 | 11 |
| Seyahi | Turkey | 2021 Full-text article | 104 (RA 18.3%, SLE 8.7%, Sjögren’s syndrome 6.7%, polymyositis 1.0%, ankylosing spondylitis 16.3%, psoriasis/PsA 6.7%, IBD 4.8%, vasculitis 6.7%, MS 4.8%, etc) | 347 (HCWs, elderly patients) | Cases HCWs mean 42.2, Elderly patients mean 71.4, Controls HCWs mean 41.7, Elderly patients mean 70.9 |
Cases 66.3%, Controls 62.5% | 68.3% (biologics 30.8%, csDMARDs 26.0%, colchicine 15.4%, hydroxychloroquine 11.5%) | 16.3% | CoronaVac (Sinovac) 100% | 0 | 451 |
| Ruddy | United States | 2021 Letter | 404 (inflammatory diseases 44.6%, SLE 21.5%, Sjögren’s syndrome 4.7%, myositis 5.9%, SSc 0.5%, vasculitis 2.0%, overlap connective tissue disease 20.8%) |
None | 44 (IQR 36–57) | 95.3% | 100% (hydroxychloroquine 42.1%, MTX 23.3%, TNFi 24.3%, belimumab 13.9%, mycophenolate 10.1%, AZA 8.7%, IL inhibitors 7.7%, etc) | 29.0% | BNT162b2 (Pfizer-BioNTech) 49.0%, mRNA-1273 (Moderna) 51.0% | 0 | 404 |
| Mrak | Austria | 2021 Full-text article | 74 (IgG4-related disease 2.7%, Connective tissue diseases 29.7%, RA 44.6%, Vasculitis 23.0%) | 10 (Healthy blood donors) | Mean 61.7 | 77.0% | 100% (rituximab 100%, MTX 32.4%, MMF 10.8%, Hydroxychloroquine 9.5%, AZA 6.8%, Leflunomide 5.4%, Sulfasalazine 1.4%, Ig therapy 4.1%) | 29.7% | Cases BNT162b2 (Pfizer-BioNTech) 82.4%, mRNA-1273 (Moderna) 17.6%, Controls BNT162b2 (Pfizer-BioNTech) 100% |
0 | 84 |
| Chung | United States | 2021 Correspondence | 15 (AAV 26.7%, RA 26.7%, SSc 20%, SLE 13.3%, IgG4-related disease 6.7%, IgA vasculitis 6.7%) | None | 57 (IQR 46-65) | 66.7% | 100% (rituximab 93.3%, belimumab 6.7%, MTX 20%, MMF 20%, hydroxychloroquine 13.3%) | 40% | mRNA vaccines (BNT162b2 or mRNA-1273) 93.3%, Ad26.COV2.S (Janssen/Johnson & Johnson) 6.7% | 0 | 15 |
| Spiera | United States | 2021 Letter | 89 (RA 25.8%, SLE 10.1%, Sjögren’s syndrome 11.2%, SSc 5.6%, PsA 6.7%, granulomatosis with polyangiitis 13.5%, giant cell arteritis 2.2%, etc) | None | Mean 61.3 | 76.4% | 100% (csDMARDs 62.9%, bDMARDs 52.6%) | 19.1% | BNT162b2 (Pfizer-BioNTech) 57.3%, mRNA-1273 (Moderna) 42.7% | 6 | 83 |
| Ammitzbøll | Denmark | 2021 Report | 134 (RA 54.5%, SLE 45.5%) | None | SLE 60.2 (IQR 46.3–67.1), RA 70.3 (IQR 66.9–73.5) |
67.2% | RA 100%, SLE 91.8% | 27.6% | BNT162b2 (Pfizer-BioNTech) 100% | 0 | 134 |
| Medeiros-Ribeiro | Brazil | 2021 Full-text article | 910 (chronic inflammatory arthritis (RA, AxSpA, PsA) 49.6%, other ARD (SLE, primary vasculitis, SSc, pSS, IIM, PAPS) 50.4%) | 182 (HCWs) | Cases 51 (IQR 40–60), Controls (50, IQR 41–60) | Cases 76.9%, Controls 76.9% | 100% (Biologics 35.3%, Immunosuppressive drugs 63.0%, Hydroxychloroquine 29.6%, Sulfasalazine 8.0%) | 38.2% | CoronaVac (Sinovac) 100% | 0 | 1038 |
| Dailey | United States | 2021 Preprint | 29 (IBD 100%) | None | Entire study Mean 17.0 (range 2–26), Vaccinated IBD patients NA |
Entire study 42.0%, Vaccinated IBD cohort NA |
100% (vedolizumab monotherapy [4, 13.8%], infliximab monotherapy [22, 75.9%], infliximab + MTX [3, 10.3%]) | NA | mRNA vaccines (BNT162b2 or mRNA-1273) 82.8%, Ad26.COV2.S (Janssen/Johnson & Johnson) 17.2% | 0 | 29 |
| Author | Test used to check antibody response | Timing of test | After 1 dose |
After 2 doses |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cases responders | Controls responders | Cases Ab titers | Controls Ab titers | Cases responders | Controls responders | Cases Ab titers | Controls Ab titers | |||
| Damiani | Anti-S1-receptor binding domain IgG against SARS-CoV-2 | After the second vaccination (days not specified) | … | … | … | … | 100% (4/4) | … | … | … |
| Deepak | Anti-S IgG quantification performed using enzyme-linked immunosorbent assay | 1–2 weeks post-vaccination (mean 8.5 days) | … | … | … | … | Overall 88.7% (118/133), 92% in cases not taking steroids (107/116), 65% in cases taking steroids (11/17) | 98% (52/53) | 33% compared to controls | NA |
| Geisen | IgG antibodies against the SARS-CoV-2 S1 antigen (EUROIMMUN QuantiVac) | 7 days after the second vaccination | … | … | … | … | 100% (26/26) | 100% (42/42) | 2053 ± 1218 (binding antibody units) | 2685 ± 1102 (binding antibody units) |
| Kennedy | Antibodies (including IgG) to the SARS-CoV-2 spike (S) protein receptor binding domain (Roche Elecsys Anti-SARS-CoV-2 S) | 3–10 weeks after the first vaccination | Total 38.2% (494/1293) ADZ1222 33.0% (232/704) BNT162b2 44.5% (262/589) | … | BNT162b2: infliximab GMT 191 U/mL (SD 12.5), vedolizumab GMT 1865 U/mL (SD 8.0), ADZ1222: Infliximab GMT 185 U/mL (SD 9.3), vedolizumab GMT 752 U/mL (SD 12.5) | … | BNT162b2 85.2% (23/27) | … | 158 U/mL in infliximab, 562 U/mL in vedolizumab | - |
| Wong | Siemens Healthineers COV2T and sCOVG assays which test for total immunoglobulins (Igs) and IgG to the receptor binding domain of the SARS-CoV-2 S protein, Roche assay for antibodies to nucleocapsid protein, In-house ELISA testing for IgG against full-length S protein | IBD patients: 14 (3–28) days after the first vaccination, 18 (2–36) days after the second vaccination HCW: 30 (7–37) days after second the vaccination Volunteers: 9 (1–40) days after the first vaccination, 8 (6–18) days after the second vaccination |
67% (6/9) | NA | NA | NA | 100% (26/26) | 100% (40/40) | Similar titers to controls | NA |
| Boyarsky | Antibodies (including IgG) to the SARS-CoV-2 spike (S) protein receptor binding domain (Roche Elecsys Anti-SARS-CoV-2 S) | 22 (18–26) days after the first vaccination | 74.0% (91/123) | … | … | … | … | … | … | … |
| Kappelman | LabCorp Cov2Quant IgG assay | Median 64.0 days (IQR 59.0–2.5) after second vaccination | … | … | … | … | 94.6% (300/317) | … | Median: 17.0 μg/mL (IQR 7.8–30.0) Mean: 28.6 μg/mL (SD 47.5) |
… |
| Furer | DiaSorin LIAISON SARS-CoV-2 anti-S1/S2 IgG assay | 2–6 weeks after second vaccination | … | … | … | … | 86.0% (590/686) | 100% (121/121) | Mean 132.9 BAU/mL (SD 91.7) | Mean 218.6 BAU/mL (SD 82.06) |
| Shenoy | Roche commercial chemiluminescent assay | Cases: Mean 27.6 days (SD 11.7) after second vaccination; Controls: Mean 43 days (SD 10.6) after second vaccination` | … | … | … | … | Total 90.2% (92/102), AZD1222 93.5% (87/93), BBV152 55.6% (5/9) |
ADZ1222 100% (30/30), BBV152 76.7% (23/30) |
Median 223.60 (IQR 53.06–656.40) | ADZ1222 Median 278 (IQR 205–603.12), BBV152 Median 73.89 (IQR 0.85–306.25) |
| Haidar | Beckman Coulter SARS-CoV-2 Spike RBD IgG platform | Median 78 days (IQR 58–105) after second vaccination | … | … | … | … | 83.8% (134/160) | 98.1% (105/107) | Mean antibody level: 8.2 (SD 8.3) | Mean antibody level: 10.1 (SD 8.7) |
| Haberman | Direct ELISA | 1 week after second vaccination | … | … | … | … | 42/51 | 25/26 | No MTX Median 113,608 (range 25–737,310), MTX Median 46,901 units (range 25–694,528) |
Median 104,354 (141–601,185) |
| Mahil | SARS-CoV-2 Spike-specific IgG ELISA | 28 days (±2 days) after first vaccination | 77.9% (60/77) | 100% (17/17) | Responder Median EC50: 43 (IQR 25–162) | Responder Median EC50: 101 (IQR 55–200) | … | … | … | … |
| Simon | Euroimmun anti-S1 IgG ELISA | More than 10 days before serum collection | … | … | … | … | 94.0% (79/84) | 100% (182/182) | Mean optical density 6.47 (SD 3.14) | Mean optical density 9.36 (SD 1.85) |
| Al-Janabi | Roche Diagnostics Elecsys Anti-SARS-CoV-2 S immunoassay | Median: 34 days (IQR 23–46) | 85% (102/120), ADZ1222 78.3% (47/60), BNT162b2 91.7% (55/60) |
… | NA | … | … | … | … | … |
| Bugatti | DiaSorin LIAISON SARS-CoV-2 S1/S2 IgG chemiluminescent assay | 21 days after first vaccination | 55% (66/120) | … | NA | … | … | … | … | … |
| Braun-Moscovici | Abbott SARS-Cov-2 IgG II Quant chemiluminescent microparticle immunoassay (CMIA) | 4–6 weeks after second vaccination | … | … | … | … | 86.0% (227/264) | … | Mean 5830.8 AU/mL (SD 8937) | … |
| Demoulin | Roche Diagnostics SARS-CoV-2 anti-RBD electrochemiluminescent immunoassay | 28 days after second vaccination | … | … | … | … | 45.5% (5/11) | … | Median <0.8 U/mL | … |
| Seyahi | Roche Diagnostics Elecsys Anti-SARS-CoV-2 assay | 30.7 ± 9.0 days after second vaccination | … | … | … | … | 89.4% (93/104) | 99.4% (345/347) | NA | NA |
| Ruddy | Roche Elecsys anti-SARS-CoV-2 S enzyme immunoassay | Median 29 days after second vaccination | … | … | … | … | 93.6% (378/404) | … | >250 U/mL | … |
| Mrak | Roche Diagnostics Elecsys Anti-SARS-CoV-2 S immunoassay | Median 21.9 days (Range 7–49) after second vaccination | … | … | … | … | 39.2% (29/74) | 100% (10/10) | Median 64.9 U/mL (IQR 16.2–2161.0) | NA |
| Chung | Euroimmun IgG binding SARS-CoV-2 spike protein S1 assay (14, 93.3%), DiaSorin Liaison SARS-CoV-2 S1/S2 IgG assay (1, 6.7%) | Median 39 days (IQR 17.5–59.5) after second vaccination | … | … | … | … | 0% (0/15) | … | NA | … |
| Spiera | Roche Elecsys anti-SARS-CoV-2 (84, 94.4%), Siemens healthineers SARS-CoV-2 Total Assay Atellica IM or ADVIA Centaur XP/XPT‡ (5, 5.6%) | NA | … | … | … | … | 76.4% (68/89) | … | NA | … |
| Ammitzbøll | Ortho Clinical Diagnostics VITROS Immunodiagnostic Products Anti-SARS-CoV-2 Total test | 1 week after second vaccination | … | … | … | … | 76.9% (103/134) | … | NA | … |
| Medeiros-Ribeiro | DiaSorin LIAISON SARS-CoV-2 S1/S2 IgG chemiluminescent assay | 28 and 69 days after second vaccination | … | … | … | … | Day 28 18.7% (161/859), Day 69 70.4% (605/859) |
Day 28 34.6% (62/179), Day 69 95.5% (171/179) |
Day 28 geometric mean titer: 5.1 AU/mL (4.7–5.5) Day 69 geometric mean titer: 10.3 AU/mL (8.5–12.5) |
Day 28 geometric mean titer: 10.3 AU/mL (8.5–12.5) Day 69 geometric mean titer: 67.0 AU/mL (59.8–74.9) |
| Dailey | Fluorescent bead-based immunoassay, flow cytometry | mRNA vaccines: mean 3.3 weeks (range 1–10) after second vaccination, adenovirus vector vaccine: mean 3.1 weeks (range 1.6–3.6) after second vaccination |
… | … | … | … | 29/29 | … | NA | … |
6MP, 6-mercaptopurine; AAV, antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis; Ab, antibody; ARD, autoimmune rheumatic diseases; AxSpA, axial spondyloarthritis; AZA, azathioprine; CD, Crohn’s disease; DMARD, disease-modifying antirheumatic drug; ELISA, enzyme-linked immunosorbent assay; HCW, health care worker; Ig, immunoglobulin; IIM, idiopathic inflammatory myopathy; IQR, interquartile range; JAK, Janus kinase; MMF, mycophenolate mofetil; MS, multiple sclerosis; MTX, methotrexate; NA, not available; PAPS, primary antiphospholipid syndrome; PsA, psoriatic arthritis; pSS, primary Sjögren’s syndrome; RA, rheumatoid arthritis; SD, standard deviation; SLE, systemic lupus erythematosus; SpA, spondyloarthritis; SSc, systemic sclerosis; TNFi, TNF inhibitors; UC, ulcerative colitis.
For the analyses on the rate of serologic response to COVID-19 vaccination, 6 and 20 studies were available for assessment after 1 dose13 , 25 , 30 , 36, 37, 38 and 2 doses13 , 24, 25, 26, 27, 28, 29 , 31, 32, 33, 34, 35 , 39, 40, 41, 42, 43, 44, 45, 46 doses, respectively. Nine studies compared outcomes after 2 doses against a control population without IMIDs,27, 28, 29 , 31, 32, 33 , 44, 45, 46 but only 1 study that had a control population after 1 dose.30 Most of the studies that assessed serologic response after the first vaccination performed testing 2 to 3 weeks after vaccination. Most studies that assessed serologic response after the second vaccination performed testing between 1 and 3 weeks after vaccination. Characteristics and outcomes of the included studies are summarized in Table 1. The risk of bias of included studies assessed using the Joanna Briggs Institute Critical Appraisal Checklist is shown in Supplementary Table 2. Most of the studies were of medium to high quality.
Rate of Serologic Response After a Single Dose of Coronavirus Disease 2019 Vaccine
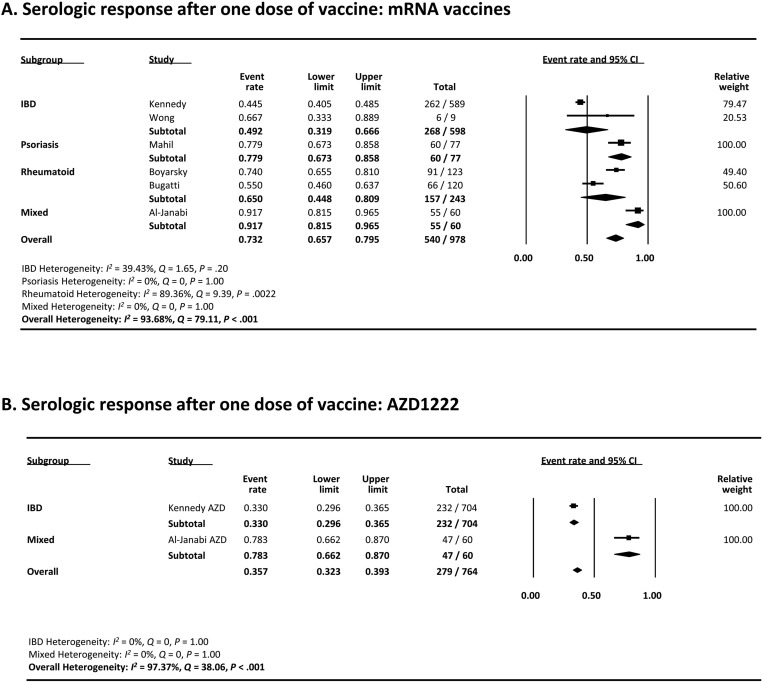
Eight studies (6 mRNA studies13 , 25 , 30 , 36, 37, 38 and 2 AZD1222 studies13 , 37) assessed the serologic response after the first dose of mRNA vaccine in patients with IMIDs. As shown in Figure 2 A, the pooled proportion of patients achieving a serologic response was 73.2% (95% confidence interval [CI], 65.7–79.5) with mRNA vaccines. On multivariate meta-regression, a greater proportion of patients taking anti-TNF agents among studies was associated with lower serologic response rates (coefficient, −2.60; 95% CI, −4.49 to −0.72; P = .0069), which likely contributed to the difference in serologic response rates and overall heterogeneity (I 2 = 93.68%) (Supplementary Table 3). In regards to disease type, studies that included patients with IBD had a lower rate of serologic response compared with rheumatoid studies (49.2% vs 65.00%), which likely reflects the greater proportion of patients using anti-TNF agents in IBD studies. The funnel plot showed no publication bias (Begg’s test P = .26, Egger’s test P = 0.39) (Supplementary Figure 1).
Figure 2.
(A) Meta-analysis of serologic response after 1 dose of mRNA vaccine. (B) Meta-analysis of serologic response after 1 dose of AZD1222 vaccine. The size of the solid squares denotes the mean difference, and the horizontal lines represent the 95% CIs. The diamond denotes the weighted mean difference, and the lateral tips of the diamond indicate the associated CIs.
Supplementary Figure 1.
Funnel plot of studies included in the meta-analysis of serologic response after 1 dose of mRNA vaccine.
Sensitivity analyses were done to assess whether individual studies influenced the results (Supplementary Figure 2). When individual studies were removed one at a time from the analyses, the corresponding pooled rates were not markedly altered by any single study (rates ranging from 63.7% to 74.7%), confirming the stability of the results. Specifically, when the study with the largest sample size was removed, the result was similar. No preprint studies were included for this analysis, so sensitivity analysis excluding preprints was not performed.
Supplementary Figure 2.
Sensitivity analysis excluding 1 study at a time for serologic response after 1 dose of mRNA vaccine.
Two studies assessed the serologic response after the first dose of the AZD1222 vaccine (Figure 2 B). The pooled proportion of patients achieving a serologic response was 35.7% (95% CI, 32.3%-39.3%).
Rate of Serologic Response After 2 Doses of Coronavirus Disease 2019 Vaccine
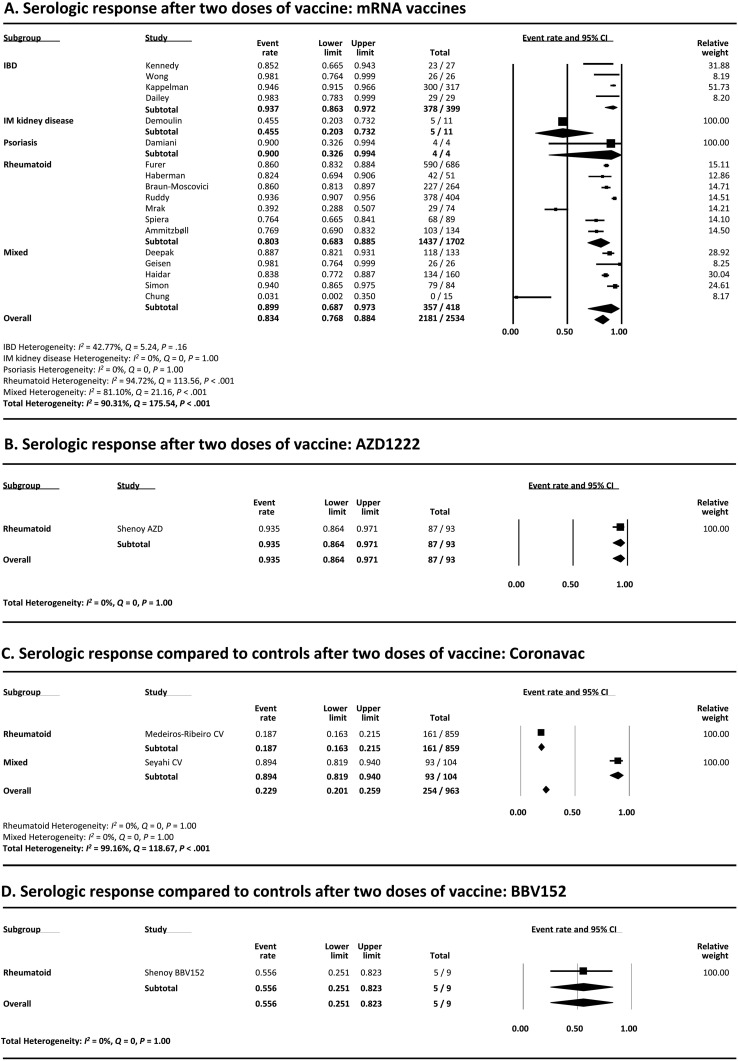
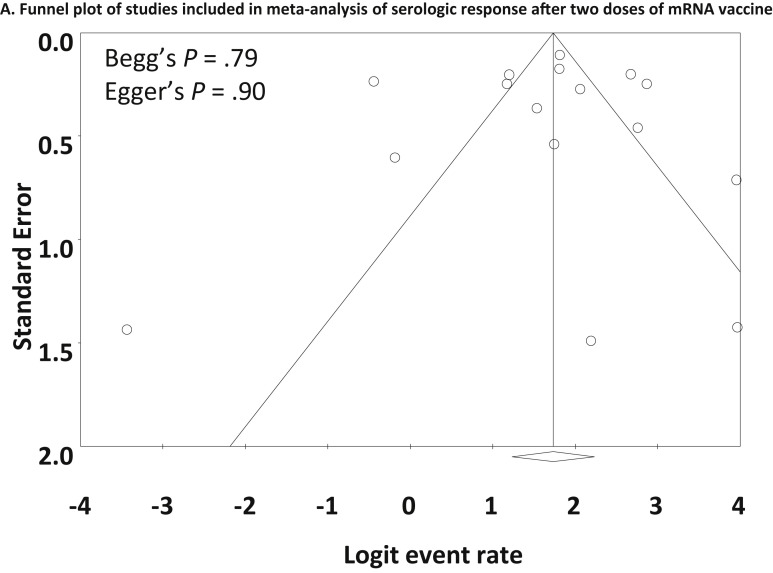
The serologic response after 2 doses of mRNA vaccines was assessed in 18 studies.13 , 24, 25, 26, 27, 28, 29 , 32 , 34 , 35 , 39, 40, 41, 42, 43, 44, 45 , 47 As shown in Figure 3 A, the pooled proportion of patients achieving a serologic response was 83.4% (95% CI, 76.8%-88.4%). Heterogeneity was present (I 2 = 90.31%), and multivariate meta-regression showed that a greater proportion of patients taking anti-CD20 therapies was associated with lower serologic response rates (coefficient, −6.08; 95% CI −9.40 to −2.76; P < .001). Older age was also associated with lower serologic response rates (coefficient, −0.044; 95% CI, −0.083 to −0.0050; P = .027), but the coefficient was very small. Anti-TNF agent use among studies was associated with numerically, but not statistically, lower serologic response rates (coefficient, −3.19; 95% CI, −6.48 to 0.10; P = .058) (Supplementary Table 4). The funnel plot showed no publication bias (Begg’s test P = .79, Egger’s test P = .90) (Supplementary Figure 3).
Figure 3.
(A) Meta-analysis of serologic response after 2 doses of mRNA vaccine. (B) Meta-analysis of serologic response after 2 doses of AZD1222 vaccine. (C) Meta-analysis of serologic response after two doses of CoronaVac vaccine. (D) Meta-analysis of serologic response after 2 doses of BBV152 vaccine. The size of the solid squares denotes the mean difference, and the horizontal lines represent the 95% CIs. The diamond denotes the weighted mean difference, and the lateral tips of the diamond indicate the associated CIs. IM, immune-mediated.
Supplementary Figure 3.
Funnel plot of studies included in the meta-analysis of serologic response after 2 doses of mRNA vaccine.
Sensitivity analysis that excluded 3 preprint studies (Supplementary Figure 4 A)44 , 45 , 47 demonstrated a serologic response rate similar to when they were included (81.2% vs 83.4%). Remove-one study analysis also showed that pooled rates were not markedly altered by any single study (Supplementary Figure 4 B).
Supplementary Figure 4.
(A) Sensitivity analysis excluding preprints for serologic response after 2 doses of mRNA vaccine. (B) Sensitivity analysis excluding 1 study at a time for serologic response after 2 doses of mRNA vaccine. The size of the solid squares denotes the mean difference, and the horizontal lines represent the 95% CIs. The diamond denotes the weighted mean difference, and the lateral tips of the diamond indicate the associated CIs. IM, immune-mediated.
Only a small number of studies reported the serologic response after 2 doses of other vaccine types (Figure 3 B–D). The rates were 93.5% (95% CI, 86.4%-97.1%), 22.9% (95% CI, 20.1%-25.9%), and 55.6% (95% CI, 25.1%-82.3%) with AZD1222 (1 study46), CoronaVac (2 studies31 , 33), and BBV152 (1 study46), respectively. The low response rate seen with CoronaVac is due to 1 study reporting a low rate at 28 days after the vaccination.33 Interestingly, this study reported a higher response rate at 69 days after the vaccination.
Serologic Response Compared With Controls
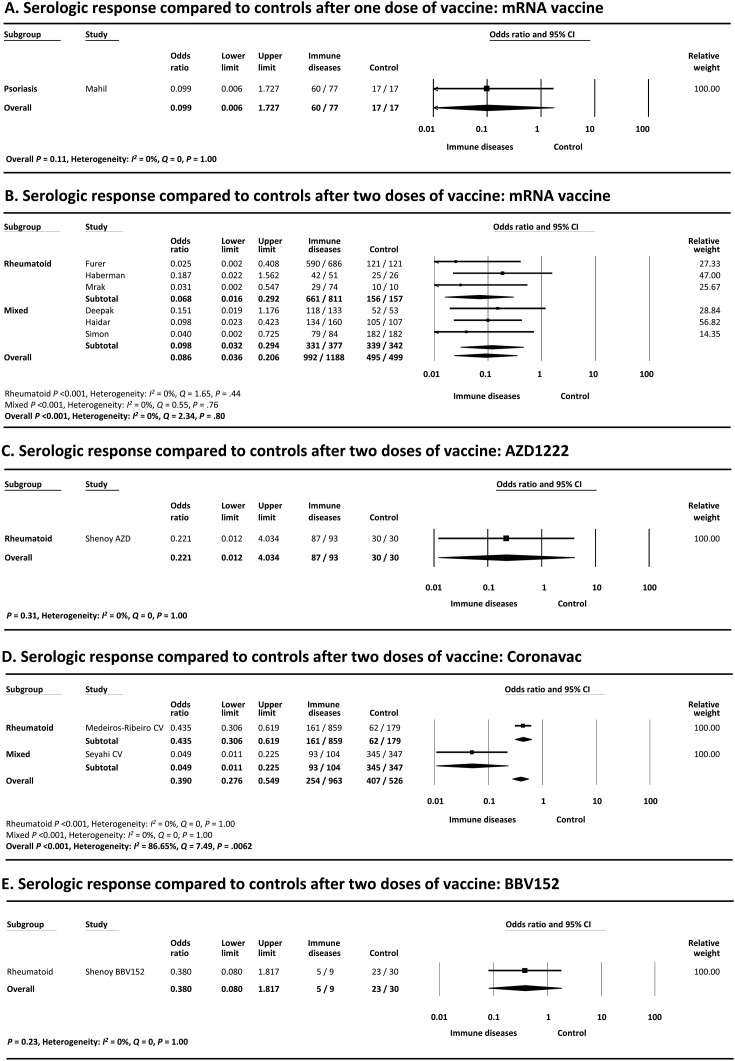
Only 1 study30 compared the serologic response with control patients after 1 dose of mRNA vaccine (odds ratio [OR], 0.099; 95% CI, 0.006–1.73; P = .11) (Figure 4 A).
Figure 4.
Meta-analysis of serologic response compared with controls after (A) 1 dose of after (B) 2 doses of mRNA vaccine. after (C) 2 doses of AZD1222 vaccine, after (D) 2 doses of CoronaVac vaccine and after (E) 2 doses of BBV152 vaccine. The size of the solid squares denotes the mean difference, and the horizontal lines represent the 95% CIs. The diamond denotes the weighted mean difference, and the lateral tips of the diamond indicate the associated CIs.
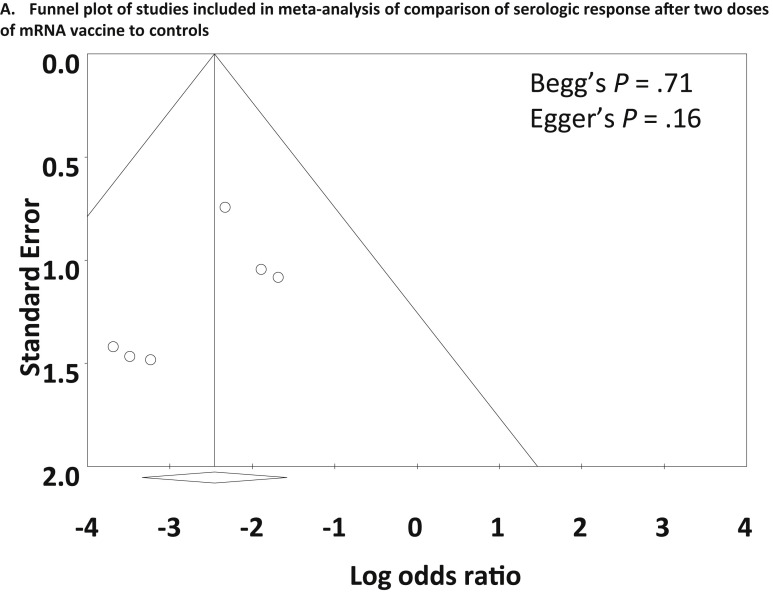
As shown in Figure 4 B, meta-analysis of 6 studies27, 28, 29 , 32 , 44 , 45 that included control patients without IMIDs demonstrated that a significantly smaller proportion of patients with IMIDs achieved a serologic response compared with control patients after 2 doses of vaccine (OR, 0.086; 95% CI, 0.036–0.21; P < .001). Most of the studies also reported lower levels of antibody titers or concentrations in patients with IMIDs compared with controls24 , 25 , 27, 28, 29 , 44 , 45 (Table 1). Heterogeneity was absent (I 2 = 0%), and the funnel plot showed no publication bias (Begg’s test P = .71, Egger’s test P = .16) (Supplementary Figure 5).
Supplementary Figure 5.
Funnel plot of studies included in meta-analysis of comparison of serologic response after 2 doses of mRNA vaccine compared with controls.
Sensitivity analysis was undertaken by excluding 2 preprint studies44 , 45 (Supplementary Figure 6 A). Exclusion of preprints demonstrated an OR similar to when they were included (0.061 vs 0.086). Remove-one study analysis also showed that ORs were not markedly altered by any single study (Supplementary Figure 6 B). When 2 studies with 0 events were included in analysis, the results were similar (OR, 0.10; 95% CI, 0.044–0.23; P < .001) (Supplementary Figure 6 B).
Supplementary Figure 6.
(A) Sensitivity analysis excluding preprints for comparison of serologic response after 2 doses of mRNA vaccine to controls. (B) Sensitivity analysis excluding 1 study at a time for comparison of serologic response after 2 doses of mRNA vaccine to controls. (C) Sensitivity analysis including zero event studies for comparison of serologic response after 2 doses of mRNA vaccine to controls. The size of the solid squares denotes the mean difference, and the horizontal lines represent the 95% CIs. The diamond denotes the weighted mean difference, and the lateral tips of the diamond indicate the associated CIs.
Only a small number of studies compared the serologic response with controls after 2 doses of other types of vaccine (Figure 4 C–E). The OR were 0.22 (95% CI, 0.012–4.03; P = .31), 0.39 (95% CI, 0.27–0.55; P < .001), and 0.38 (95% CI, 0.080–1.82; P = .23) with AZD1222 (1 study46), CoronaVac (2 studies31 , 33), and BBV152 (1 study46), respectively.
Grading the Quality of Evidence
Based on the GRADE approach, an overall quality of evidence for this analysis was low because the data were obtained from observational studies (Supplementary Tables 5 and 6).
Discussion
In the present meta-analysis, we assessed the serologic responses to COVID-19 vaccination in patients with IMIDs. We demonstrated that a significantly lower proportion of patients with IMIDs (82.3%) achieved a serologic response to 2 doses of COVID-19 mRNA vaccines compared with control patients. The response after 1 dose (73.2%) appeared to be lower than the rates reported in healthy controls in the literature, so patients with IMIDs should receive the complete vaccination series without delay. The lower serologic response to a 2-dose vaccine strategy for the mRNA-based vaccines suggests patients should be considered to receive a third dose of the vaccine. Only a limited number of studies included non-mRNA vaccines, so further studies assessing the response to other types of vaccine are warranted.
Patients with IMIDs, especially those with rheumatic diseases, are known to have a higher prevalence of COVID-19.3 Medications used to treat IMIDs such as GCs, csDMARDs, and b/tsDMARDs-csDMARDs combination therapy may increase the risk of severe COVID-19.3 Owing to the lack of effective therapies to treat COVID-19, it is important to know the effectiveness of COVID-19 vaccines in patients with IMIDs. Kennedy et al13 , 48 reported that seroconversion of anti–SARS-CoV-2 antibody after COVID-19 infection as well as immunogenicity to a single dose of vaccine were attenuated in patients with IBD treated with infliximab. Interestingly, they reported that most patients seroconverted after the second dose; however, only a small number of other studies have assessed the effectiveness of COVID-19 vaccines in patients with IMIDs taking immunosuppressive therapies, and there is a large variation in reported outcomes.
A recent study by Shrotri et al49 found that among 8517 adults in the United Kingdom, 96.42% who received BNT162b2 or ChAdOx1 nCoV-19/AZD1222 vaccines developed antibodies 4 to 6 weeks after the first dose. That rate rose to 99.08% within 2 weeks of the second dose. While seroprevalence was found in nearly all patients after 2 doses of the vaccines, they found lower antibody levels in elderly people, those with a chronic condition, such as diabetes and cardiovascular disease, or those with cancer.49 Our study showed that the proportion of patients achieving a serologic response after a single dose of COVID-19 mRNA vaccine was 73.2%, which is lower than the rates reported by Shrotri et al.49 The serologic response after 2 doses of mRNA vaccine was 82.3% in our study.
Among studies that included control patients without IMIDs, patients with IMIDs had a significantly lower likelihood of achieving a serologic response after 2 doses of mRNA vaccines (OR, 0.086). Recently, Jena et al50 reported the results of a meta-analysis of response to SARS-CoV-2 vaccination in IMIDs. The response rates reported in their study were similar to our study, and they reported that the response was attenuated in patients with rheumatoid arthritis or those on anti- CD20 or anti-cytotoxic T lymphocyte–associated antigen therapies, suggesting the need of assessing seroconversion in these patients. The results of our subgroup analysis and multivariate meta-regression confirm their results, but we consider that the response rates in patient with IMID are suboptimal, that they should complete the vaccine series without delay, and should be considered for a third dose of the vaccine. Only a few studies included patients with ChAdOx1 nCoV-19/AZD1222 or other types of vaccine, so future studies will also need to include patients treated with different vaccines.
This study has some limitations. Only 8 months have passed since the United Kingdom first approved BNT162b2, so available studies in the patient population with IMIDs mostly included limited numbers of participants. There are currently 9 different vaccines on the global market, but the included studies mostly used mRNA vaccines requiring 2 doses: either BNT162b2 (Pfizer-BioNTech) or mRNA-1273 (Moderna), so further research is needed to determine whether the results of our study can be generalized to other vaccines. Most of the included studies only assessed humoral responses to vaccination, and the extent to which cell-mediated immunity is involved remains unclear.51
Included studies were heterogeneous in sample size, type of IMIDs, medication use, and type of and timing of antibody testing. We analyzed IMIDs together due to limited number of studies, but further research of the individual disease states is warranted because they consist of different age-groups and are often treated with different types of biologics (anti-CD20 therapy, anti-TNF agents, anti-integrins, etc), which have a different degree of influence on vaccine response.
A small number of studies were preprints, but sensitivity analyses demonstrated that their exclusion did not influence the results. Despite some potential drawbacks, preprints have an increasing role in creating timely evidence during the current pandemic.52
Furthermore, due to novel variants of SARS-CoV-2 and waning antibody response, effectiveness of or serologic response to COVID-19 vaccines may vary depending on the timing of testing. Research and knowledge of COVID-19 vaccine are rapidly evolving and updated living meta-analyses are warranted in the future.
Conclusion
This study is the first meta-analysis to analyze the rate of seroconversion to COVID-19 vaccines in patients with IMIDs. Our meta-analysis demonstrated that 82.3% of patients with IMIDs achieved a serologic response to 2 doses of COVID-19 mRNA vaccines, which was statistically lower compared with controls. The results of our study suggest that patients with IMIDs should receive the series of mRNA vaccines without delay and be considered for the third dose of the vaccine. Further studies assessing the response to different types of vaccines are warranted.
Acknowledgments
CRediT Authorship Contributions
Atsushi Sakuraba, MD, PhD (Conceptualization: Lead; Data curation: Equal; Formal analysis: Lead; Methodology: Lead; Writing – original draft: Equal; Writing – review & editing: Lead). Alexander Luna, BA (Data curation: Equal). Dejan Micic, MD (Writing – review & editing: Equal).
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding No funding was received.
This article has an accompanying continuing medical education activity, also eligible for MOC credit, on page e16. Learning Objective: Upon completion of this CME activity, successful learners will be able to identify the effectiveness of mRNA COVID-19 vaccination in patients with immune mediated inflammatory diseases (IMIDs) and review the necessity of a third vaccination in this patient population.
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at https://doi.org/10.1053/j.gastro.2021.09.055.
Supplementary Material
Supplementary Table 1.
PubMed Search Strategy
| PubMed search strategy | No. of studies | |
|---|---|---|
| #1 | “COVID-19” OR “SARS-CoV-2” [MeSH Terms] | 98,051 |
| #2 | “inflammatory bowel diseases” OR “rheumatoid arthritis” OR “psoriasis” OR “rheumatic diseases” OR “systemic lupus erythematosus”, “psoriatic arthritis”, “ankylosing spondylitis”, “Crohn’s disease”, “ulcerative colitis” OR “multiple sclerosis”, “immune mediated diseases” OR “autoimmune diseases” [MeSH Terms] | 85,734 |
| #3 | “vaccine” OR “immunization” [MeSH Terms] | 162,867 |
| #4 | #1 AND #2 AND #3 | 961 |
MeSH, Medical Subject Headings.
Supplementary Table 2.
Risk of Bias Assessment by Joanna Briggs Institute Critical Appraisal Checklist
| Author | Year | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 |
|---|---|---|---|---|---|---|---|---|---|---|
| Damiani | 2021 | Yes | Yes | Yes | No | No | NA | Yes | NA | NA |
| Deepak | 2021 | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes |
| Geisen | 2021 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Kennedy | 2021 | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes |
| Wong | 2021 | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes |
| Boyarsky | 2021 | Yes | Yes | Yes | No | No | NA | No | Yes | Yes |
| Kappelman | 2021 | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes |
| Furer | 2021 | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes |
| Shenoy | 2021 | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes |
| Haidar | 2021 | Yes | Unclear | Unclear | Yes | No | Yes | Yes | Yes | Yes |
| Haberman | 2021 | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes |
| Mahil | 2021 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Simon | 2021 | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes |
| Al-Janabi | 2021 | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes |
| Bugatti | 2021 | Unclear | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes |
| Braun-Moscovici | 2021 | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes |
| Demoulin | 2021 | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes |
| Seyahi | 2021 | Yes | Yes | Yes | Yes | Na | Yes | Yes | Yes | Unclear |
| Ruddy | 2021 | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes |
| Mrak | 2021 | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes |
| Chung | 2021 | Yes | Yes | Yes | No | No | Yes | No | Yes | Yes |
| Benucci | 2021 | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes |
| Spiera | 2021 | Yes | Yes | Yes | No | No | No | No | Yes | Yes |
| Ammitzbøll | 2021 | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes |
| Medeiros-Ribeiro | 2021 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Dailey | 2021 | Yes | Yes | Yes | Unclear | No | Yes | Yes | Yes | Yes |
NOTE. Q1: Is it clear in the study what is the “cause” and what is the “effect” (ie, there is no confusion about which variable comes first)?; Q2: Were the participants included in any comparisons similar?; Q3: Were the participants included in any comparisons receiving similar treatment/care, other than the exposure or intervention of interest?; Q4: Was there a control group?; Q5: Were there multiple measurements of the outcome both pre and post the intervention/exposure?; Q6: Was follow-up complete and if not, were differences between groups in terms of their follow-up adequately described and analyzed?; Q7: Were the outcomes of participants included in any comparisons measured in the same way?; Q8: Were outcomes measured in a reliable way?; Q9: Was appropriate statistical analysis used?
NA, not applicable.
Supplementary Table 3.
Univariate and Multivariate Meta-regression Models of Variables Associated With Serologic Response After 1 Dose of Messenger RNA Vaccine
| Variable | Univariate meta-regression |
Multivariate meta-regression |
||||
|---|---|---|---|---|---|---|
| Coefficient | 95% CI | P value | Coefficient | 95% CI | P value | |
| Biologic/DMARD | −4.30 | −.91 to 0.31 | .68 | |||
| Methotrexate | NA | |||||
| Steroid | −2.58 | −8.58 to 3.42 | .40 | -0.90 | −3.29 to 1.49 | .46 |
| Anti-CD20 | NA | |||||
| Anti-TNF | −2.69 | −4.05 to −1.32 | <.001 | -2.60 | −4.49 to −0.72 | .0069 |
| Age | 0.027 | −0.14 to 0.20 | .76 | |||
NA, not applicable.
Supplementary Table 4.
Univariate and Multivariate Meta-regression Models of Variables Associated With Serologic Response After 2 Doses of Messenger RNA Vaccine
| Variable | Univariate meta-regression |
Multivariate meta-regression |
||||
|---|---|---|---|---|---|---|
| Coefficient | 95% CI | P value | Coefficient | 95% CI | P value | |
| Biologic/DMARD | −1.70 | −4.94 to 1.53 | .30 | |||
| Methotrexate | −3.00 | −8.80 to 2.81 | .31 | |||
| Steroid | −3.13 | −7.65 to 1.37 | .17 | |||
| Anti-CD20 | −2.74 | −3.56 to −1.94) | <.001 | -6.08 | −9.40 to −2.76) | <.001 |
| Anti-TNF | 1.86 | −0.47 to -4.18 | .018 | -3.19 | −6.48 to 0.10 | .058 |
| Age | −0.048 | −0.092 to −0.0036 | .034 | -0.044 | −0.083 to −0.0050) | .027 |
Supplementary Table 5.
Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) Criteria for Studies Included in the Meta-analysis of Observational Studies Assessing Serologic Response After 2 Doses of Messenger RNA Vaccine
| No. of participants | Starting level of evidence | Quality assessment |
Reasons to increase level of evidence (large magnitude of effect; dose-response gradient; potential confounding) | Overall quality of evidence | ||||
|---|---|---|---|---|---|---|---|---|
| Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | ||||
| 2505 | Low | Not serious | Not serious | Serious | Not serious | Not serious | Not applicable | Low |
Supplementary Table 6.
Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) Criteria for Studies Included in the Meta-Analysis of Case Control Studies Comparing Serologic Response After 2 Doses of Messenger RNA Vaccine to Controls
| No. of participants | Starting level of evidence | Quality assessment |
Reasons to increase level of evidence (large magnitude of effect; dose-response gradient; potential confounding) | Overall quality of evidence | ||||
|---|---|---|---|---|---|---|---|---|
| Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | ||||
| 1188 (cases) and 499 (controls) | Low | Not serious | Serious | Serious | Not serious | Not serious | N/A | Low |
N/A, not applicable.
References
- 1.Chen N., Zhou M., Dong X., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sparks J.A., Wallace Z.S., Seet A.M., et al. Associations of baseline use of biologic or targeted synthetic DMARDs with COVID-19 severity in rheumatoid arthritis: results from the COVID-19 Global Rheumatology Alliance physician registry. Ann Rheum Dis. 2021;80:1137–1146. doi: 10.1136/annrheumdis-2021-220418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akiyama S., Hamdeh S., Micic D., et al. Prevalence and clinical outcomes of COVID-19 in patients with autoimmune diseases: a systematic review and meta-analysis. Ann Rheum Dis. 2021;80:384–391. doi: 10.1136/annrheumdis-2020-218946. [DOI] [PubMed] [Google Scholar]
- 4.Brenner E.J., Ungaro R.C., Gearry R.B., et al. Corticosteroids, but not TNF antagonists, are associated with adverse COVID-19 outcomes in patients with inflammatory bowel diseases: results from an international registry. Gastroenterology. 2020;159:481–491.e3. doi: 10.1053/j.gastro.2020.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gianfrancesco M., Yazdany J., Robinson P.C. Epidemiology and outcomes of novel coronavirus 2019 in patients with immune-mediated inflammatory diseases. Curr Opin Rheumatol. 2020;32:434–440. doi: 10.1097/BOR.0000000000000725. [DOI] [PubMed] [Google Scholar]
- 6.Gianfrancesco M., Hyrich K.L., Al-Adely S., et al. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis. 2020;79:859–866. doi: 10.1136/annrheumdis-2020-217871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allocca M., Chaparro M., Gonzalez H.A., et al. Patients with inflammatory bowel disease are not at increased risk of COVID-19: a large multinational cohort study. J Clin Med. 2020;9:3533. doi: 10.3390/jcm9113533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Polack F.P., Thomas S.J., Kitchin N., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jackson L.A., Anderson E.J., Rouphael N.G., et al. An mRNA vaccine against SARS-CoV-2-preliminary report. N Engl J Med. 2020;383:1920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wellens J., Colombel J.F., Satsangi J.J., et al. SARS-CoV-2 vaccination in IBD: past lessons, current evidence and future challenges. J Crohns Colitis. 2021;15:1376–1386. doi: 10.1093/ecco-jcc/jjab046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Battafarano D.F., Battafarano N.J., Larsen L., et al. Antigen-specific antibody responses in lupus patients following immunization. Arthritis Rheum. 1998;41:1828–1834. doi: 10.1002/1529-0131(199810)41:10<1828::AID-ART15>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 12.Elkayam O., Bashkin A., Mandelboim M., et al. The effect of infliximab and timing of vaccination on the humoral response to influenza vaccination in patients with rheumatoid arthritis and ankylosing spondylitis. Semin Arthritis Rheum. 2010;39:442–447. doi: 10.1016/j.semarthrit.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Kennedy N.A., Lin S., Goodhand J.R., et al. Infliximab is associated with attenuated immunogenicity to BNT162b2 and ChAdOx1 nCoV-19 SARS-CoV-2 vaccines in patients with IBD. Gut. 2021;70:1884–1893. doi: 10.1136/gutjnl-2021-324789. [DOI] [PubMed] [Google Scholar]
- 14.Moher D., Liberati A., Tetzlaff J., et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 15.Booth A. PROSPERO’s progress and activities 2012/13. Syst Rev. 2013;2:111. doi: 10.1186/2046-4053-2-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aromataris E, Munn Z, eds. JBI Manual for Evidence Synthesis. 2020. Available at: https://synthesismanual.jbi.global. Accessed July 1, 2021.
- 17.Guyatt G.H., Oxman A.D., Vist G., et al. GRADE guidelines: 4. Rating the quality of evidence—study limitations (risk of bias) J Clin Epidemiol. 2011;64:407–415. doi: 10.1016/j.jclinepi.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 18.Higgins J.P., Thompson S.G., Deeks J.J., et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins J., Green S. The Cochrane Collaboration; 2011. Cochrane Handbook for Systematic Reviews of Interventions. [Google Scholar]
- 20.Begg C.B., Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 21.Egger M., Davey Smith G., Schneider M., et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clyne B., Walsh K.A., O’Murchu E., et al. Using preprints in evidence synthesis: commentary on experience during the COVID-19 pandemic [published online ahead of print May 19, 2021]. J Clin Epidemiol. https://doi.org/10.1016/j.jclinepi.2021.05.010 [DOI] [PMC free article] [PubMed]
- 23.Friedrich J.O., Adhikari N.K.J., Beyene J. Inclusion of zero total event trials in meta-analyses maintains analytic consistency and incorporates all available data. BMC Med Res Methodol. 2007;7:5. doi: 10.1186/1471-2288-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geisen U.M., Berner D.K., Tran F., et al. Immunogenicity and safety of anti-SARS-CoV-2 mRNA vaccines in patients with chronic inflammatory conditions and immunosuppressive therapy in a monocentric cohort. Ann Rheum Dis. 2021;80:1306–1311. doi: 10.1136/annrheumdis-2021-220272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wong S.Y., Dixon R., Pazos V.M., et al. Serological response to mRNA COVID-19 vaccines in IBD patients receiving biological therapies. Gastroenterology. 2021;161:715–718.e4. doi: 10.1053/j.gastro.2021.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kappelman M.D., Weaver K.N., Boccieri M., et al. Humoral immune response to messenger RNA COVID-19 vaccines among patients with inflammatory bowel disease. Gastroenterology. 2021;161:1340–1343.e2. doi: 10.1053/j.gastro.2021.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Furer V., Eviatar T., Zisman D., et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis. 2021;80:1330–1338. doi: 10.1136/annrheumdis-2021-220647. [DOI] [PubMed] [Google Scholar]
- 28.Haberman R.H., Herati R., Simon D., et al. Methotrexate hampers immunogenicity to BNT162b2 mRNA COVID-19 vaccine in immune-mediated inflammatory disease. Ann Rheum Dis. 2021;80:1339–1344. doi: 10.1136/annrheumdis-2021-220597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simon D., Tascilar K., Fagni F., et al. SARS-CoV-2 vaccination responses in untreated, conventionally treated and anticytokine-treated patients with immune-mediated inflammatory diseases. Ann Rheum Dis. 2021;80:1312–1316. doi: 10.1136/annrheumdis-2021-220461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mahil S.K., Bechman K., Raharja A., et al. The effect of methotrexate and targeted immunosuppression on humoral and cellular immune responses to the COVID-19 vaccine BNT162b2: a cohort study. Lancet Rheumatol. 2021;3:e627–e637. doi: 10.1016/S2665-9913(21)00212-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seyahi E., Bakhdiyarli G., Oztas M., et al. Antibody response to inactivated COVID-19 vaccine (CoronaVac) in immune-mediated diseases: a controlled study among hospital workers and elderly. Rheumatol Int. 2021;41:1429–1440. doi: 10.1007/s00296-021-04910-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mrak D., Tobudic S., Koblischke M., et al. SARS-CoV-2 vaccination in rituximab-treated patients: B cells promote humoral immune responses in the presence of T-cell-mediated immunity. Ann Rheum Dis. 2021;80:1345–1350. doi: 10.1136/annrheumdis-2021-220781. [DOI] [PubMed] [Google Scholar]
- 33.Medeiros-Ribeiro A.C., Aikawa N.E., Saad C.G.S., et al. Immunogenicity and safety of the CoronaVac inactivated vaccine in patients with autoimmune rheumatic diseases: a phase 4 trial [published online ahead of print July 30, 2021]. Nat Med. https://doi.org/10.1038/s41591-021-01469-5 [DOI] [PubMed]
- 34.Braun-Moscovici Y., Kaplan M., Braun M., et al. Disease activity and humoral response in patients with inflammatory rheumatic diseases after two doses of the Pfizer mRNA vaccine against SARS-CoV-2. Ann Rheum Dis. 2021;80:1317–1321. doi: 10.1136/annrheumdis-2021-220503. [DOI] [PubMed] [Google Scholar]
- 35.Damiani G., Allocco F., Malagoli P.O. COVID-19 vaccination and psoriatic patients under biologics: real-life evidence on safety and effectiveness from Italian vaccinated healthcare workers. Clin Exp Dermatol. 2021;46:1106–1108. doi: 10.1111/ced.14631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boyarsky B.J., Ruddy J.A., Connolly C.M., et al. Antibody response to a single dose of SARS-CoV-2 mRNA vaccine in patients with rheumatic and musculoskeletal diseases. Ann Rheum Dis. 2021;80:1098–1099. doi: 10.1136/annrheumdis-2021-220289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Al-Janabi A., Littlewood Z., Griffiths C.E.M., et al. Antibody responses to single-dose SARS-CoV-2 vaccination in patients receiving immunomodulators for immune-mediated inflammatory disease. Br J Dermatol. 2021;185:646–648. doi: 10.1111/bjd.20479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bugatti S., De Stefano L., Balduzzi S., et al. Methotrexate and glucocorticoids, but not anticytokine therapy, impair the immunogenicity of a single dose of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic inflammatory arthritis [published online ahead of print June 25, 2021]. Ann Rheum Dis. https://doi.org/10.1136/annrheumdis-2021-220862 [DOI] [PubMed]
- 39.Demoulin N., Scohy A., Gillion V., et al. Low rates of humoural response to BNT162b2 SARS-CoV-2 vaccination in patients with immune-mediated kidney diseases treated with rituximab. Clin Kidney J. 2021;14:2132–2133. doi: 10.1093/ckj/sfab102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruddy J.A., Connolly C.M., Boyarsky B.J., et al. High antibody response to two-dose SARS-CoV-2 messenger RNA vaccination in patients with rheumatic and musculoskeletal diseases. Ann Rheum Dis. 2021;80:1351–1352. doi: 10.1136/annrheumdis-2021-220656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chung S.H., Wener M., Bays A.M., et al. Correspondence on “SARS-CoV-2 vaccination in rituximab-treated patients: evidence for impaired humoral but inducible cellular immune response” by Bonelli et al. Ann Rheum Dis. 2021;80:e165. doi: 10.1136/annrheumdis-2021-220957. [DOI] [PubMed] [Google Scholar]
- 42.Spiera R., Jinich S., Jannat-Khah D. Rituximab, but not other antirheumatic therapies, is associated with impaired serological response to SARS-CoV-2 vaccination in patients with rheumatic diseases. Ann Rheum Dis. 2021;80:1357–1359. doi: 10.1136/annrheumdis-2021-220604. [DOI] [PubMed] [Google Scholar]
- 43.Ammitzboll C., Bartels L.E., Bogh Andersen J., et al. Impaired antibody response to the BNT162b2 messenger RNA coronavirus disease 2019 vaccine in patients with systemic lupus erythematosus and rheumatoid arthritis. ACR Open Rheumatol. 2021;3:622–628. doi: 10.1002/acr2.11299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deepak P., Kim W., Paley M.A., et al. Glucocorticoids and B cell depleting agents substantially impair immunogenicity of mRNA vaccines to SARS-CoV-2. medRxiv. 2021 2021.04.05.21254656. [Google Scholar]
- 45.Haidar G., Agha M., Lukanski A., et al. Immunogenicity of COVID-19 Vaccination in immunocompromised patients: an observational, prospective cohort study interim analysis. medRxiv. 2021 2021.06.28.21259576. [Google Scholar]
- 46.Shenoy P., Ahmed S., Cherian S., et al. Immunogenicity of the ChAdOx1 nCoV-19 and the BBV152 vaccines in patients with autoimmune rheumatic diseases. medRxiv. 2021 doi: 10.1007/s00296-021-04917-0. 2021.06.06.21258417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dailey J., Kozhaya L., Dogan M., et al. Antibody Responses to SARS-CoV-2 after infection or vaccination in children and young adults with inflammatory bowel disease. medRxiv. 2021 doi: 10.1093/ibd/izab207. 2021.06.12.21258810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kennedy N.A., Goodhand J.R., Bewshea C., et al. Anti-SARS-CoV-2 antibody responses are attenuated in patients with IBD treated with infliximab. Gut. 2021;70:865–875. doi: 10.1136/gutjnl-2021-324388. [DOI] [PubMed] [Google Scholar]
- 49.Shrotri M., Fragaszy E., Geismar C., et al. Spike-antibody responses to ChAdOx1 and BNT162b2 vaccines by demographic and clinical factors (Virus Watch study) medRxiv. 2021 2021.05.12.21257102. [Google Scholar]
- 50.Jena A., Mishra S., Deepak P., et al. Response to SARS-CoV-2 vaccination in immune mediated inflammatory diseases: systematic review and meta-analysis [published online ahead of print August 30, 2021]. Autoimmun Rev. https://doi.org/10.1016/j.autrev.2021.102927 [DOI] [PMC free article] [PubMed]
- 51.Iqbal H. The importance of cell-mediated immunity in COVID-19-An opinion. Medical Hypotheses. 2020;143 doi: 10.1016/j.mehy.2020.110152. 110152-110152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nabavi Nouri S., Cohen Y.A., Madhavan M.V., et al. Preprint manuscripts and servers in the era of coronavirus disease 2019. J Eval Clin Pract. 2021;27:16–21. doi: 10.1111/jep.13498. [DOI] [PubMed] [Google Scholar]