Abstract
Background
Hypocalcemia has been identified as a major distinctive feature of COVID-19, predicting poor clinical outcomes. Among the mechanisms underlying this biochemical finding, high prevalence of vitamin D (VD) deficiency in COVID-19 patients reported so far in several studies was advocated. However, robust data in favor of this hypothesis are still lacking. Therefore, aim of our study was to investigate the role of hypovitaminosis D and parathyroid hormone (PTH) levels in the development of hypocalcemia in COVID-19 patients.
Methods
Patients admitted to IRCCS Ospedale San Raffaele for COVID-19 were enrolled in this study, excluding those with comorbidities and therapies influencing calcium and VD metabolism. Serum levels of total calcium (tCa), ionized calcium (Ca2+), 25-OH-VD, and PTH were evaluated at admission. We defined VD deficiency as VD below 20 ng/mL, hypocalcemia as tCa below 2.2 mmol/L or as Ca2+ below 1.18 mmol/L, and hyperparathyroidism as PTH above 65 pg/mL.
Results
A total of 78 patients were included in the study. Median tCa and Ca2+ levels were 2.15 and 1.15 mmol/L, respectively. Total and ionized hypocalcemia were observed in 53 (67.9%) and 55 (70.5%) patients, respectively. VD deficiency was found in 67.9% of patients, but secondary hyperparathyroidism was detected in 20.5% of them, only. tCa levels were significantly lower in patients with VD deficiency and regression analyses showed a positive correlation between VD and tCa.
Conclusions
In conclusion, we confirmed a high prevalence of hypocalcemia in COVID-19 patients and we showed for the first time that it occurred largely in the context of marked hypovitaminosis D not adequately compensated by secondary hyperparathyroidism.
Keywords: Vitamin D, Hypocalcemia, PTH, COVID-19, Calcium, Hypovitaminosis D
Introduction
Hypocalcemia has been identified as a major biochemical distinctive feature of COVID-19, predicting disease severity and poor clinical outcomes [1–3]. Among clinical and biochemical features of COVID-19 initially reported in Chinese studies, no data on calcium levels and mineral metabolism were available [4–6]. In April 2020, we reported the first case of severe acute hypocalcemia in a previously thyroidectomized patient with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and COVID-19 was suggested as possible precipitating cause of a subclinical postsurgical hypoparathyroidism [7].
Subsequently, several studies worldwide reported widespread low calcium levels in patients with COVID-19 closely related to higher biochemical inflammation and thrombotic markers, and negative prognosis including mortality [1, 3, 8–11], suggesting that calcium level may be a useful laboratory marker of disease severity [3].
Despite the consistency of this biochemical finding across different studies, the mechanisms underlying hypocalcemia in COVID-19 remain unclear. In fact, several factors have been hypothesized to play a pathophysiological role including calcium dependent viral mechanisms of action, chronic and acute malnutrition during critical illness, and high levels of unbound and unsaturated fatty acids (UFAs) in inflammatory responses [3, 12–16]. Furthermore, we previously hypothesized that hypovitaminosis D could play a key role in its determinism [17, 18].
Our hypothesis was based on the accepted notion that vitamin D (VD) is a crucial hormone for calcium homeostasis increasing intestinal calcium and phosphate absorption [19] and on the high prevalence of VD deficiency in COVID-19 patients reported by several studies [20–27].
However, in hypovitaminosis D subjects, the compensatory increase in parathyroid hormone (PTH) (secondary hyperparathyroidism) generally maintains eucalcemia [19].
The aim of this study was to investigate the role of VD and PTH in the pathophysiology of hypocalcemia in COVID-19.
Methods
Study design
This was a retrospective substudy on a cohort part of the COVID-BioB study of IRCCS San Raffaele Hospital in Milano, Italy, which, as previously reported, complies with the Declaration of Helsinki and was approved by the Hospital Ethics Committee (protocol no. 34/int/2020) and registered on ClinicalTrials.gov (NCT04318366) [28]. Briefly, adult patients seen at San Raffaele Hospital for COVID-19 during the first wave of the pandemic (March 18 to May 5, 2020) were enrolled in the COVID-BioB study after signing informed consent. COVID-19 was confirmed by positive real-time reverse-transcriptase polymerase chain reaction nasal and/or throat swab in the presence of clinical and/or radiological findings suggestive of COVID-19 pneumonia. Patients admitted for other reasons and subsequently diagnosed with superimposed SARS-CoV-2 infection were excluded [28]. Patients with exhaustive information regarding concomitant comorbidities, chronic therapies, and mineral metabolism biochemical evaluations were included in this substudy cohort.
Data collection
For this substudy, the following variables were collected on hospital admission: age, sex, body mass index (BMI), ionized serum calcium (Ca2+) (measured on arterial blood gas test and adjusted to a standardized pH of 7.4, RapidPoint 500 Analyzer, Siemens Healthcare, VA, USA, mmol/L), serum not-corrected total calcium levels (tCa) (Roche Cobas C 8000 WKC/MET/078 using o-cresolphthalein complexone method assay kit; mmol/L; coefficient of variation (CV) 3%), 25-OH-VD (Roche Cobas 8000 WKC/MET/036 using electrochemiluminescence immunoassays (ECLIA); ng/mL; CV 5%), PTH (PTH 1-84 Roche Cobas C 8000 WKC/MET/034 ECLIA; pg/mL; CV 6%), and estimated glomerular filtration rate (eGFR, as estimated by the CKD-EPI equation and expressed as mL/min/1.73 m2) on admission to the emergency department (ED). As previously reported [29], information on comorbidities at admission (including previous diagnosis of hypertension, osteoporosis, diabetes mellitus, coronary artery disease [CAD], and chronic kidney disease [CKD]) and clinical outcomes (discharge from ED or hospital ward, needs for noninvasive mechanical ventilation, admission to intensive care unit, and mortality rate) for all patients enrolled in our main cohort as well as for those part of this substudy were collected. Exclusion criteria were the same for the main cohort [29] (patients with comorbidities and concomitant active therapies influencing calcium metabolism including chronic glucocorticoids and antiepileptic drugs, VD/calcium, loop/thiazide diuretics) but due to the potential interference of altered renal function and advanced age on the PTH/VD/calcium axis [30, 31], we did not include in this substudy data from patients of our main cohort [29] with previous diagnosis of CKD or eGFR ≤ 60 mL/min/1.73 m2 using creatinine levels at initial evaluation, and patients older than 75 years.
VD deficiency was defined as previously reported for the main cohort, in which the VD levels reported for patients in this substudy were included [29] as 25-OH-VD level below 20 ng/mL, according to the cutoff values reported by Sempos et al. [32]. Hypocalcemia was defined as a Ca2+ level below 1.18 mmol/L or as a tCa level below 2.2 mmol/L [33]. Hyperparathyroidism was defined as a PTH level above 65 pg/mL [34].
Statistical analysis
Descriptive statistics were obtained for all study variables. Categorical variables were summarized as counts and percentages. Kolmogorov–Smirnov normality test was performed (p < 0.05) and continuous variables were expressed as medians and interquartile range (IQR) [25th–75th percentile]. Fisher exact test or χ2 test and the Wilcoxon signed-rank test or the Kruskal–Wallis test were employed to determine the statistical significance of differences in proportions and medians, respectively. Linear regression analyses were used to correlate continuous variables. All statistical tests were two-sided. A p value of <0.05 was considered statistically significant. Statistical analysis was conducted using IBM SPSS Statistics (IBM SPSS Statistics for Windows, Version 23.0. Armonk, NY: IBM Corp.).
Results
Demographics
Seventy-eight COVID-19 patients (part of a previously reported main cohort [29]) were included in the study. Demographic characteristics and disease outcomes of main cohort were previously summarized [29]. Median age of the subgroup of patients included in this study was 55.9 [48.5–64.5] years and 66.7% of patients were male. Twenty-three patients (29.5%) were affected by arterial hypertension, 15 (19.2%) by diabetes mellitus, and 7 (9%) by CAD. Using creatinine levels detected at hospital admission, median eGFR level was 100 [85–108] mL/min/1.73 m2.
Mineral metabolism
Median tCa and Ca2+ levels were 2.15 [2.05–2.23] and 1.15 [1.1–1.18] mmol/L, respectively (Table 1). Total and ionized hypocalcemia were observed in 53 (67.9%) and 55 (70.5%) patients, respectively (Table 1).
Table 1.
Mineral metabolism evaluation in COVID-19 patients
| Variables | No. (%)—median (IQR) |
|---|---|
| Total calcium levels, mmol/L | 2.15 (2.05–2.23) |
| Hypocalcemic | 53 (67.9%) |
| Ionized calcium levels, mmol/L | 1.15 (1.1–1.18) |
| Hypocalcemic | 55 (70.5%) |
| 25-OH-vitamin D, ng/mL | 16.4 (12.6–24) |
| Deficiency | 53 (67.9%) |
| Parathyroid hormone, pg/mL | 45.9 (36.5–63.3) |
| Hyperparathyroidism | 16 (20.5%) |
No. number, IQR interquartile range
Male patients presented median tCa and Ca2+ levels of 2.11 [2.05–2.19] and 1.13 [1.1–1.18] mmol/L, respectively, compared to female patients presenting median tCa and Ca2+ levels of 2.2 [2.15–2.26] and 1.16 [1.12–1.17] mmol/L, respectively, (p = 0.012 and p = 0.29).
Median VD level was 16.4 ng/mL [12.6–24]. VD deficiency was found in 53 (67.9%) patients.
Male patients presented a median VD level of 16.1 ng/mL [10.5–19.5] compared to female patients presenting a median VD level of 19.7 ng/mL [13.2–24.5] (p = 0.048). Age was similar in patients with VD deficiency vs sufficiency (55.5 [49–63] vs 58.3 [47–69] years, p = 0.6).
tCa levels were significantly lower in patients with VD deficiency vs sufficiency (2.12 mmol/L [2.05–2.21] vs 2.18 mmol/L [2.13–2.28]; p = 0.018) (Table 2). On the other hand, a trend toward lower VD levels in tCa-hypocalcemic patients vs tCa-normocalcemic patients (16.2 ng/mL [10.9–21.4] vs 18.5 ng/mL [13–28.8]; p = 0.1) (Table 2) was observed. No statistical differences on Ca2+ levels between VD deficiency and sufficiency groups were found (Table 2).
Table 2.
Differences among mineral metabolism biochemical features in COVID-19 patients
| Variables | p value | ||
|---|---|---|---|
| VD deficiency | VD sufficiency | ||
| tCa levels, mmol/L | 2.12 (2.05–2.21) | 2.18 (2.13–2.28) | p = 0.018 |
| Ca2+ levels, mmol/L | 1.14 (1.1–1.18) | 1.16 (1.12–1.18) | p = 0.24 |
| PTH levels, pg/mL | 49.1 (39.1–64.8) | 41.8 (30.2–63.1) | p = 0.1 |
| tCa-hypocalcemic | tCa-normocalcemic | ||
| VD levels, ng/mL | 16.2 (10.9–21.4) | 18.5 (13–28.8) | p = 0.1 |
| PTH levels, pg/mL | 46 (38.6–65.6) | 44 (29.2–53.6) | p = 0.048 |
| Ca2+-hypocalcemic | Ca2+-normocalcemic | ||
| VD levels, ng/mL | 16.3 (12.8–23) | 16.8 (10.8–28.1) | p = 0.41 |
| PTH levels, pg/mL | 46 (36.7–64.8) | 44.1 (26.1–53.2) | p = 0.19 |
| Hyperparathyroid | Normal PTH | ||
| tCa levels, mmol/L | 2.05 (1.93–2.17) | 2.16 (2.07–2.24) | p = 0.017 |
| Ca2+ levels, mmol/L | 1.1 (1.09–1.14) | 1.16 (1.12–1.18) | p = 0.029 |
| VD levels, ng/mL | 16.1 (10.3–21.8) | 16.5 (13.2–24.1) | p = 0.3 |
P values reported in bold are statistically significant.VD vitamin D, tCa total calcium, Ca2+ ionized calcium, PTH parathyroid hormone
Median PTH level was 45.9 pg/mL [36.5–63.3]. In only 16 patients (20.5%) was found secondary hyperparathyroidism (Table 1).
Male patients presented a median PTH level of 49.1 pg/mL [39.7–64.6] compared to female patients who had median PTH level of 40.7 pg/mL [28–53.4] (p = 0.027). No statistical differences were observed in secondary hyperparathyroidism rate occurrence between male and female patients (23% vs 15.4%, p = 0.55).
We found no significant differences in serum PTH levels between patients with VD deficiency vs sufficiency and between Ca2+-hypocalcemic vs Ca2+-normocalcemic patients (p = 0.1, p = 0.19; respectively), although we found significantly higher PTH levels in tCa-hypocalcemic patients compared to tCa-normocalcemic patients (46 pg/mL [38.6–65.6] vs 44 pg/mL [29.2–53.6], respectively; p = 0.048) (Table 2).
Significantly lower tCa and Ca2+ levels were found in patients with increased vs normal PTH levels (p = 0.017, p = 0.029; respectively), while no differences were found regarding VD (p = 0.3) (Table 2).
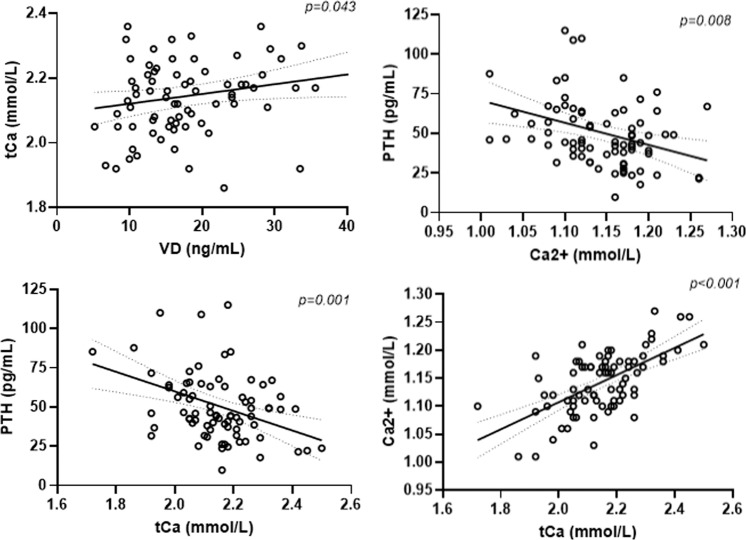
Linear regression analyses showed statistically significant positive correlation between VD and tCa (p = 0.043; r = 0.23) and negative correlations of PTH with tCa (p = 0.001; r = −0.4) and Ca2+ (p = 0.008; r = −0.35) (Fig. 1). Non-significant negative correlation between VD and PTH (p = 0.09; r = −0.18) was also detected. A strong positive correlation was found between tCa and Ca2+ (p < 0.001; r = 0.62) Fig. 1). No statistically significant correlations were observed between PTH and either age or BMI.
Fig. 1.
Statistically significant associations between the different parameters of bone metabolism in COVID-19 patients. VD vitamin D, tCa total calcium, Ca2+ ionized calcium, PTH parathyroid hormone
Discussion
An emerging osteo-metabolic phenotype [35] characterized by low calcium and VD levels as well as by increased risk of vertebral fractures was recently reported among the most prominent endocrine and metabolic conditions in COVID-19 [36–38].
Our data confirmed hypocalcemia as one of the most frequent biochemical finding in COVID-19 patients, as previously reported in the literature [1–3, 39].
Several authors hypothesized different possible pathophysiological mechanisms underlying COVID-19-related hypocalcemia, including calcium depletion due to viral replication mechanisms, malnutrition during critical illness, and UFAs role in inflammatory response [3].
We and others also hypothesized that chronic preexisting hypovitaminosis D may predispose to hypocalcemia in COVID-19. In fact, Sun et al. previously reported in 26 COVID-19 patients a median [IQR] VD level of 10.20 ng/mL [8.20–12.65] and all patients were affected by hypovitaminosis D [8]. The median [IQR] serum calcium level in these 26 patients was 2.13 mmol/L [2.03–2.16], and correlation analyses showed a positive association between calcium and VD levels.
In accordance with these findings, in our study we observed lower calcium levels in patients with VD deficiency compared to those with sufficient levels, and, conversely, in hypocalcemic patients were found lower levels of VD compared to those normocalcemic. Furthermore, linear regression analyses showed a positive correlation between VD and calcium levels. These findings suggest that hypocalcemia in COVID-19 patients may be an epiphenomenon of the widespread hypovitaminosis D which can in turn represent the link between hypocalcemia and negative outcomes in this clinical context [1, 18, 35].
As a matter of facts, chronic hypovitaminosis D is demonstrated to alter calcium metabolism reducing the intestinal absorption of calcium and phosphorus, and COVID-19 may trigger and/or exacerbate hypocalcemia occurrence in patients affected, particularly in those with hypovitaminosis D and severe infection [40, 41].
Interestingly, we showed that hypocalcemia occurred prevalently in a context of a marked hypovitaminosis D not adequately compensated by secondary hyperparathyroidism. Despite most of patients in our cohort presented VD deficiency and hypocalcemia, only one-fifth of them had secondary hyperparathyroidism.
Our findings are in agreement with previously published studies showing a high prevalence of hypovitaminosis D and hypocalcemia in COVID-19 patients concomitantly with a low rate of secondary hyperparathyroidism. Hernández et al. reported a PTH median level of 44.2 pg/mL in 162 COVID-19 patients with VD levels below 20 ng/mL [25]; Mazziotti et al. reported secondary hyperparathyroidism occurrence in only 43.3% of 97 COVID-19 patients characterized by a median VD level of 21 ng/mL [26]; finally, a very recent study reported PTH levels slightly above the normal range (mean level 63.5 pg/mL) in COVID-19 patients with VD levels below 20 ng/mL [27].
These findings seem to confirm the central role of PTH impaired secretion as a strong predictor of acute hypocalcemia occurrence, especially in patients affected by chronic hypovitaminosis D and consequently at higher risk to develop hypocalcemia. Interestingly, preexisting hypovitaminosis D is able to predict poor PTH compensatory response also in other clinical settings such as in post-thyroidectomy hypocalcemia [42].
The mechanism underlying the blunted PTH response to hypovitaminosis D and hypocalcemia in COVID-19 is yet to be understood. In fact, parathyroid gland function may be impaired during systemic critical illness and inflammatory response with increased circulating cytokines [43].
Moreover, previous studies conducted during severe acute respiratory syndrome epidemic in 2003, evaluating tissue samples of patients died of SARS, identified viral RNA and antigenic materials in parathyroid gland acidophilic cells and expression of angiotensin converting enzyme 2 receptors in parathyroid glands cells has been, although not consistently, reported [44–46]. Moreover, in COVID-19, acute worsening of previous well-tolerated primary and/or postsurgical hypoparathyroidism leading to severe hypocalcemia has been reported [7, 47], supporting the hypothesis that widespread hypocalcemia in COVID-19 may derive from a preexisting hypovitaminosis D no more adequately compensated by PTH increase during the SARS-CoV-2 infection.
Moreover, as previously reported, we observed lower levels of VD [48] and calcium [1] in male patients compared to female subjects. Interestingly, in male subjects, despite the above findings, only a modest increase in PTH, as compared to females, was observed with no significant sex differences in prevalence of secondary hyperparathyroidism, supporting the hypothesis of a suboptimal compensatory PTH response in COVID-19 patients.
Limitations of this study are: firstly, its retrospective and cross-sectional nature, which did not allow us to evaluate the longitudinal modifications of these biochemical parameters during disease progression and recovery; secondly, the relatively limited number of patients enrolled due to the strict and rigorous inclusion and exclusion criteria used; thirdly, the evaluation of not-corrected tCa levels since albumin levels were not available (although we included ionized calcium levels analysis); and finally, the lack of data on magnesium levels since the latter were sporadically reported to be decreased in COVID-19 patients [49]. However, it should be noted that hypomagnesemia generally increases PTH secretion and it could hardly explain the impaired compensatory response of PTH observed in COVID-19 patients. Keeping these limitations aside, this is the first study to our knowledge providing mechanistical explanation for hypocalcemia in COVID-19 patients. Therefore, low calcium levels may be an easy to obtain and reliable in emergency conditions marker of VD deficiency which can in turn have, due to the known immune functions of VD [41], negative impact on patient prognosis. Finally, VD prophylaxis in high-risk populations (elderly patients with comorbidities) besides its positive immunomodulatory actions [31, 50, 51] may also be thought to be able to prevent severe acute hypocalcemia, if endogenous PTH/VD axis is disrupted by the infection, which may have an additionally negative prognostic impact [36].
In conclusion, we report for the first time that the highly prevalent low VD is a relevant predisposing factor to hypocalcemia in COVID-19 and that a blunted compensatory PTH response possibly directly related to SARS-CoV-2 infection may facilitate the occurrence of hypocalcemia in these patients.
Data availability
All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Compliance with ethical standards
Conflict of interest
The authors declare no competing interests.
Ethical approval
The study protocol complies with the Declaration of Helsinki, was approved by the Hospital Ethics Committee (protocol no. 34/int/2020), and was registered on ClinicalTrials.gov (NCT04318366).
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Di Filippo L, Formenti AM, Rovere-Querini P, et al. Hypocalcemia is highly prevalent and predicts hospitalization in patients with COVID-19. Endocrine. 2020;68(3):475–478. doi: 10.1007/s12020-020-02383-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.di Filippo L, Formenti AM, Doga M, et al. Hypocalcemia is a distinctive biochemical feature of hospitalized COVID-19 patients. Endocrine. 2021;71(1):9–13. doi: 10.1007/s12020-020-02541-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.L. di Filippo, M. Doga, S. Frara, A. Giustina, Hypocalcemia in COVID-19: prevalence, clinical significance and therapeutic implications. Rev. Endocr. Metab. Disord. 1–10 (2021). 10.1007/s11154-021-09655-z [DOI] [PMC free article] [PubMed]
- 4.Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N. Engl. J. Med. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta A, Madhavan MV, Sehgal K, et al. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020;26(7):1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bossoni S, Chiesa L, Giustina A. Severe hypocalcemia in a thyroidectomized woman with Covid-19 infection. Endocrine. 2020;68(2):253–254. doi: 10.1007/s12020-020-02326-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun JK, Zhang WH, Zou L, et al. Serum calcium as a biomarker of clinical severity and prognosis in patients with coronavirus disease 2019. Aging. 2020;12(12):11287–11295. doi: 10.18632/aging.103526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Torres B, Alcubilla P, González-Cordón A, et al. Impact of low serum calcium at hospital admission on SARS-CoV-2 infection outcome. Int. J. Infect. Dis. 2020;104:164–168. doi: 10.1016/j.ijid.2020.11.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou X, Chen D, Wang L, et al. Low serum calcium: a new, important indicator of COVID-19 patients from mild/moderate to severe/critical. Biosci. Rep. 2020;40(12):BSR20202690. doi: 10.1042/BSR20202690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu J, Han P, Wu J, Gong J, Tian D. Prevalence and predictive value of hypocalcemia in severe COVID-19 patients. J. Infect. Public Health. 2020;13(9):1224–1228. doi: 10.1016/j.jiph.2020.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.di Filippo L, Formenti AM, Giustina A. Hypocalcemia: the quest for the cause of a major biochemical feature of COVID-19. Endocrine. 2020;70(3):463–464. doi: 10.1007/s12020-020-02525-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Millet JK, Whittaker GR. Physiological and molecular triggers for SARS-CoV membrane fusion and entry into host cells. Virology. 2018;517:3–8. doi: 10.1016/j.virol.2017.12.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lai AL, Millet JK, Daniel S, Freed JH, Whittaker GR. The SARS-CoV fusion peptide forms an extended bipartite fusion platform that perturbs membrane order in a calcium-dependent manner. J. Mol. Biol. 2017;429(24):3875–3892. doi: 10.1016/j.jmb.2017.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.L. Di Filippo, R. De Lorenzo, M. D’Amico, et al. COVID-19 is associated with clinically significant weight loss and risk of malnutrition, independent of hospitalisation: a post-hoc analysis of a prospective cohort study. Clin. Nutr. S0261–5614 (2020). 10.1016/j.clnu.2020.10.043 [DOI] [PMC free article] [PubMed]
- 16.Singh VP, Khatua B, El-Kurdi B, Rood C. Mechanistic basis and therapeutic relevance of hypocalcemia during severe COVID-19 infection. Endocrine. 2020;70(3):461–462. doi: 10.1007/s12020-020-02530-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giustina A, Formenti AM. Preventing a covid-19 pandemic: can high prevalence of severe hypovitaminosis D play a role in the high impact of Covid infection in Italy? BMJ. 2020;368:m810. [Google Scholar]
- 18.Ulivieri FM, Banfi G, Camozzi V, et al. Vitamin D in the Covid-19 era: a review with recommendations from a G.I.O.S.E.G. expert panel. Endocrine. 2021;10:1007/s12020–021-02749-3. doi: 10.1007/s12020-021-02749-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blau JE, Collins MT. The PTH-vitamin D-FGF23 axis. Rev. Endocr. Metab. Disord. 2015;16(2):165–174. doi: 10.1007/s11154-015-9318-z. [DOI] [PubMed] [Google Scholar]
- 20.D’Avolio A, Avataneo V, Manca A, et al. 25-Hydroxyvitamin D concentrations are lower in patients with positive PCR for SARS-CoV-2. Nutrients. 2020;12(5):1359. doi: 10.3390/nu12051359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jain A, Chaurasia R, Sengar NS, Singh M, Mahor S, Narain S. Analysis of vitamin D level among asymptomatic and critically ill COVID-19 patients and its correlation with inflammatory markers. Sci. Rep. 2020;10(1):20191. doi: 10.1038/s41598-020-77093-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pizzini A, Aichner M, Sahanic S, et al. Impact of vitamin D deficiency on COVID-19—a prospective analysis from the CovILD Registry. Nutrients. 2020;12(9):2775. doi: 10.3390/nu12092775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.G.E. Carpagnano, V. Di Lecce, V.N. Quaranta, et al. Vitamin D deficiency as a predictor of poor prognosis in patients with acute respiratory failure due to COVID-19. J. Endocrinol. Investig. 1–7 (2020). 10.1007/s40618-020-01370-x [DOI] [PMC free article] [PubMed]
- 24.Hutchings N, Babalyan V, Baghdasaryan S, et al. Patients hospitalized with COVID-19 have low levels of 25-hydroxyvitamin D. Endocrine. 2021;71(2):267–269. doi: 10.1007/s12020-020-02597-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.J.L. Hernández, D. Nan, M. Fernandez-Ayala, et al. Vitamin D status in hospitalized patients with SARS-CoV-2 infection. J. Clin. Endocrinol. Metab. dgaa733 (2020). 10.1210/clinem/dgaa733 [DOI] [PMC free article] [PubMed]
- 26.G. Mazziotti, E. Lavezzi, A. Brunetti, et al. Vitamin D deficiency, secondary hyperparathyroidism and respiratory insufficiency in hospitalized patients with COVID-19. J. Endocrinol. Investig. 1–9 (2021). 10.1007/s40618-021-01535-2 [DOI] [PMC free article] [PubMed]
- 27.Jevalikar G, Mithal A, Singh A, et al. Lack of association of baseline 25-hydroxyvitamin D levels with disease severity and mortality in Indian patients hospitalized for COVID-19. Sci. Rep. 2021;11(1):6258. doi: 10.1038/s41598-021-85809-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.P. Rovere-Querini, C. Tresoldi, C. Conte, A. Ruggeri, S. Ghezzi, R. De Lorenzo, et al. Biobanking for COVID-19 research. Panminerva Med. (2020). 10.23736/S0031-0808.20.04168-3 [DOI] [PubMed]
- 29.L. di Filippo, A. Allora, M. Doga, et al. Vitamin D levels associate with blood glucose and BMI in COVID-19 patients predicting disease severity. J. Clin. Endocrinol. Metab. dgab599 (2021). 10.1210/clinem/dgab599 [DOI] [PMC free article] [PubMed]
- 30.Giustina A, Adler RA, Binkley N, et al. Consensus statement from 2nd International Conference on Controversies in Vitamin D. Rev. Endocr. Metab. Disord. 2020;21(1):89–116. doi: 10.1007/s11154-019-09532-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giustina A, Bouillon R, Binkley N, et al. Controversies in vitamin D: a statement from the Third International Conference. JBMR. 2020;4(12):e10417. doi: 10.1002/jbm4.10417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sempos CT, Heijboer AC, Bikle DD, et al. Vitamin D assays and the definition of hypovitaminosis D: results from the First International Conference on Controversies in Vitamin D. Br. J. Clin. Pharm. 2018;84(10):2194–2207. doi: 10.1111/bcp.13652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.In: H.K. Walker, W.D. Hall, J.W. Hurst (eds), Clinical Methods: the History, Physical, and Laboratory Examinations, 3rd edn. (Butterworths, Boston, 1990) [PubMed]
- 34.Aloia JF, Feuerman M, Yeh JK. Reference range for serum parathyroid hormone. Endocr. Pr. 2006;12(2):137–144. doi: 10.4158/EP.12.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.L. di Filippo, S. Frara, A. Giustina, The emerging osteo-metabolic phenotype of COVID-19: clinical and pathophysiological aspects. Nat. Rev. Endocrinol. 1–2 (2021). 10.1038/s41574-021-00516-y [DOI] [PMC free article] [PubMed]
- 36.Puig-Domingo M, Marazuela M, Giustina A. COVID-19 and endocrine diseases. A statement from the European Society of Endocrinology. Endocrine. 2020;68(1):2–5. doi: 10.1007/s12020-020-02294-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marazuela M, Giustina A, Puig-Domingo M. Endocrine and metabolic aspects of the COVID-19 pandemic. Rev. Endocr. Metab. Disord. 2020;21(4):495–507. doi: 10.1007/s11154-020-09569-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Puig-Domingo M, Marazuela M, Yildiz BO, Giustina A. COVID-19 and endocrine and metabolic diseases. An updated statement from the European Society of Endocrinology. Endocrine. 2021;72(2):301–316. doi: 10.1007/s12020-021-02734-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.di Filippo L, Formenti AM, Doga M, Pedone E, Rovere-Querini P, Giustina A. Radiological thoracic vertebral fractures are highly prevalent in COVID-19 and predict disease outcomes. J. Clin. Endocrinol. Metab. 2021;106(2):e602–e614. doi: 10.1210/clinem/dgaa738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Giustina A. Hypovitaminosis D and the endocrine phenotype of COVID-19. Endocrine. 2021;72(1):1–11. doi: 10.1007/s12020-021-02671-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bilezikian JP, Bikle D, Hewison M, et al. Mechanisms in endocrinology: vitamin D and COVID-19. Eur. J. Endocrinol. 2020;183(5):R133–R147. doi: 10.1530/EJE-20-0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Soares CSP, Tagliarini JV, Mazeto GMFS. Preoperative vitamin D level as a post-total thyroidectomy hypocalcemia predictor: a prospective study. Braz. J. Otorhinolaryngol. 2021;87(1):85–89. doi: 10.1016/j.bjorl.2019.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kelly A, Levine MA. Hypocalcemia in the critically ill patient. J. Intensive Care Med. 2013;28(3):166–177. doi: 10.1177/0885066611411543. [DOI] [PubMed] [Google Scholar]
- 44.Ding Y, He L, Zhang Q, et al. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J. Pathol. 2004;203(2):622–630. doi: 10.1002/path.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.He L, Ding Y, Zhang Q, et al. Expression of elevated levels of pro-inflammatory cytokines in SARS-CoV-infected ACE2+ cells in SARS patients: relation to the acute lung injury and pathogenesis of SARS. J. Pathol. 2006;210(3):288–297. doi: 10.1002/path.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ren P, Gong C, Ma S. Evaluation of COVID-19 based on ACE2 expression in normal and cancer patients. Open Med. 2020;15(1):613–622. doi: 10.1515/med-2020-0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bonnet JB, Berchoux E, Sultan A. Decompensated primary hypoparathyroidism in a patient with COVID-19. Ann. Endocrinol. 2021;82(2):123–124. doi: 10.1016/j.ando.2021.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.De Smet D, De Smet K, Herroelen P, Gryspeerdt S, Martens GA. Serum 25(OH)D level on hospital admission associated with COVID-19 stage and mortality. Am. J. Clin. Pathol. 2021;155(3):381–388. doi: 10.1093/ajcp/aqaa252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Quilliot D, Bonsack O, Jaussaud R, Mazur A. Dysmagnesemia in Covid-19 cohort patients: prevalence and associated factors. Magnes. Res. 2020;33(4):114–122. doi: 10.1684/mrh.2021.0476. [DOI] [PubMed] [Google Scholar]
- 50.Giustina A, Adler RA, Binkley N, et al. Controversies in vitamin D: summary statement from an international conference. J. Clin. Endocrinol. Metab. 2019;104(2):234–240. doi: 10.1210/jc.2018-01414. [DOI] [PubMed] [Google Scholar]
- 51.F. Tecilazich, A.M. Formenti, A. Giustina, Role of vitamin D in diabetic retinopathy: pathophysiological and clinical aspects. Rev. Endocr. Metab. Disord. 1–13 (2020). 10.1007/s11154-020-09575-4 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.