Abstract
We herein report, by using confocal immunofluorescence, the colocalization of the SARS-CoV-2 nucleocapsid within neurons, astrocytes, oligodendrocytes and microglia in three deceased COVID-19 cases, of between 78 and 85 years of age at death. The viral nucleocapsid was detected together with its ACE2 cell entry receptor, as well as the NLRP3 inflammasome in cerebral cortical tissues. It is noteworthy that NLRP3 was colocalized with CD68 + macrophages in the brain and lung of the deceased, suggesting the critical role of this type of inflammasome in SARS-CoV-2 lesions of the nervous system/lungs and supporting its potential role as a therapeutic target.
Keywords: COVID-19, SARS-CoV-2, NLRP3 inflammasome, Macrophages, Human brain
Abbreviations: COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus-2; NC, nucleocapsid; CNS, central nervous system; ACE2, Angiotensin-Converting Enzyme 2
Graphical abstract

1. Introduction
It is widely recognized that patients with coronavirus disease 2019 (COVID-19) present diverse neurological injuries leading to long-term sequels, but the pathogenic mechanisms involved are still largely unknown (Azizi and Azizi, 2020). Using confocal immunofluorescence analysis (Supplementary Materials and Methods), we report the potential role of NLRP3 inflammasome in brain pathogenicity of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) in three deceased patients with COVID-19.
2. Cases report
The patients had a history of distinct comorbidities when they were diagnosed with COVID-19 (Table 1 ). Firstly, cellular tropism of SARS-CoV-2 was investigated in the cerebral cortex of evaluated patients (Fig. 1 ).
Table 1.
Clinical and pathological characteristics of COVID-19 patients included in the present study.
| Cases no. | Gender | Age (years) | Cause of death | Comorbidities | Pathological findings |
|---|---|---|---|---|---|
| 1 | Male | 78 | Sudden cardiac death | paranoid schizophrenia, chronic obstructive pulmonary disease, cardiac insufficiency | Generalized atherosclerotic disease, nephrocalcinosis, pulmonary emphysema, calcified sclerosis tunica media (Monckeberg), acute hepatic steatosis, congestive spleen, moderate cerebral edema, reactive gliosis |
| 2 | Male | 85 | Sepsis, pneumonia | Dementia, left hip fracture | Cerebral atherosclerosis grade III, reactive hepatitis, passive chronic hepatic severe congestion, mild cardiosclerosis, congestive spleen, myocardial stromal fat infiltration, severe cerebral atrophy |
| 3 | Female | 80 | Asthma with respiratory decompensation, multiorgan failure | Asthma, chronic obstructive pulmonary disease, obesity | Generalized atherosclerotic disease, pulmonary edema, severe bronchioloalveolar permeability, moderate cardiosclerosis, hypertensive kidney disease, myocardial stromal fat infiltration, intense hepatic steatosis, acute tubular necrosis, moderate cerebral edema |
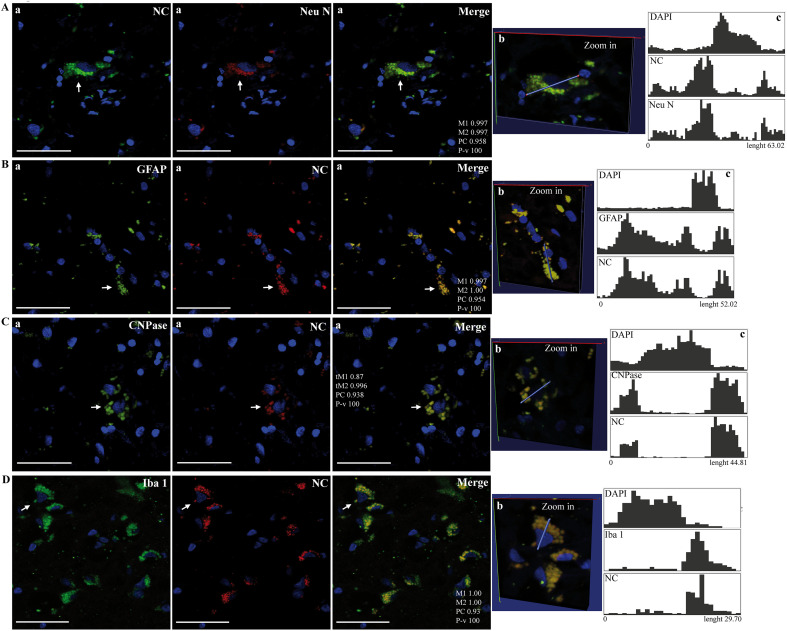
Fig. 1.
Images represent region of interests (Rois) obtained in Supplementary Fig. 2, Supplementary Fig. 3, Supplementary Fig. 4, Supplementary Fig. 5 from confocal microscopy analysis of SARS-CoV-2 nucleocapsid (NC) and cell type markers in the brain cortex of deceased COVID-19 patients. Panels show colocalization of viral NC with Neu N (A), GFAP (B), CNPase (C) and Iba-1 (D). Arrows point to a Roi shown as a Zoom-in view in 3D space (b) and associated quantitative profiling of image voxel intensity of the different dyes along a 3D line segment (c) using the Vaa3D software. Intensity correlation analysis of Rois (a): Manders' Coefficients (M1, M2);Costes' Pearson's Coefficient (CP) and P-value (P-v, %). Bars: 50 μm.
Images represent region of interests (Rois) obtained in Supplementary Figs. 2–5 from confocal microscopy analysis of SARS-CoV-2 nucleocapsid (NC) and cell type markers in the brain cortex of deceased COVID-19 patients. Panels show colocalization of viral NC with Neu N (A), GFAP (B), CNPase (C) and Iba-1 (D). Arrows point to a Roi shown as a Zoom-in view in 3D space (b) and associated quantitative profiling of image voxel intensity of the different dyes along a 3D line segment (c) using the Vaa3D software. Intensity correlation analysis of Rois (a): Manders' Coefficients (M1, M2);Costes' Pearson's Coefficient (CP) and P-value (P-v, %). Bars: 50 μm.
Importantly, the viral nucleocapsid (NC) protein was localized in a variety of typical cells of the central nervous system (CNS), that were identified using antibodies against NeuN (neurons), GFAP (astrocytes), CNPase (oligodendrocytes) and Iba-1 (microglia) (Fig. 1A-D; Supp. Fig. 2–5). In contrast, immunostaining was not detected with only secondary fluorescent probes-conjugated antibodies without primary antibodies in postmortem brain samples from COVID-19 deceased (Supp. Fig. 1).
Next, potential mediators of the pathogenicity of this virus were identified in the human CNS. Interestingly, NC colocalized with the cell entry receptor of SARS-CoV-2, Angiotensin-Converting Enzyme 2 (ACE2), in the brain tissue (Fig. 2A; Supp. Fig. 6). Notably, co-immunostaining of NC with a key player of the neuroinflammatory axis: the inflammasome NLRP3 (Fig. 2B; Supp. Fig. 7) was also observed. Furthermore, NLRP3 was co-detected with CD68, a monocyte/macrophage and microglia marker (Fig. 2C; Supp. Fig. 8).
Fig. 2.
Images represent region of interests (Rois) obtained in Supplementary Fig. 6, Supplementary Fig. 7, Supplementary Fig. 8 from confocal microscopy analysis of SARS-CoV-2 nucleocapsid (NC) and markers of viral host entry and neuroinflammation in the brain cortex of deceased COVID-19 patients. Panels show colocalization of viral NC with ACE2 (A) and NLRP3 (B) or co-detection of CD68 with NLRP3 (C). Arrows point to a Roi shown as a Zoom-in view in 3D space (b) and associated quantitative profiling of image voxel intensity of the different dyes along a 3D line segment (c) using the Vaa3D software. Intensity correlation analysis of Rois (a): Manders' Coefficients (M1, M2);Costes' Pearson's Coefficient (CP) and P-value (P-v, %). Bars: 50 μm.
Images represent region of interests (Rois) obtained in Supplementary Figs. 6–8 from confocal microscopy analysis of SARS-CoV-2 nucleocapsid (NC) and markers of viral host entry and neuroinflammation in the brain cortex of deceased COVID-19 patients. Panels show colocalization of viral NC with ACE2 (A) and NLRP3 (B) or co-detection of CD68 with NLRP3 (C). Arrows point to a Roi shown as a Zoom-in view in 3D space (b) and associated quantitative profiling of image voxel intensity of the different dyes along a 3D line segment (c) using the Vaa3D software. Intensity correlation analysis of Rois (a): Manders' Coefficients (M1, M2);Costes' Pearson's Coefficient (CP) and P-value (P-v, %). Bars: 50 μm.
To determine whether similar viral pathogenic features were observed in lungs and brain, these viral and immune markers were studied in lungs. Remarkably, NC protein colocalized with NLRP3 and CD68 in postmortem lung samples (Fig. 3A and B). In contrast, no immunostaining was detected for NC in a lung sample of a person who died from a cause non-related to COVID-19 (Supp. Fig. 12). Moreover, NLRP3 localized with CD68+ cells (Fig. 3C; Supp 9–11), suggesting that SARS-CoV-2 may regulate the functions of NLRP3 in monocytes/macrophages from lungs and brain. The pathogenesis of virus-related damage to lungs was evidenced by microscopic histochemical examination, showing features of predominant interstitial fibrosis (Fig. 3D).
Fig. 3.
Images represent region of interests (Rois) (A-C) obtained in Supplementary Fig. 9, Supplementary Fig. 10, Supplementary Fig. 11 from confocal microscopy analysis of SARS-CoV-2 nucleocapsid (NC) and markers of innate immune response in lungs of deceased COVID-19 patients. Panels show colocalization of viral NC with NLRP3 (A) and CD68 (B) or co-detection of NLRP3 with CD68 (C). Arrows point to a Roi shown as a Zoom-in view in 3D space (b) and associated quantitative profiling of image voxel intensity of the different dyes along a 3D line segment (c) using the Vaa3D software. Intensity correlation analysis of Rois (a): Manders' Coefficients (M1, M2);Costes' Pearson's Coefficient (CP) and P-value (P-v, %). Bars: 50 μm. D) Representative images of Picro Mallory staining in postmortem lung sections of cases 1 (P1), 2 (P2) and 3 (P3) showing interstitial fibrosis. Collagen is stained in blue. Bars: 200 μm. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Images represent region of interests (Rois) (A-C) obtained in Supplementary Figs. 9–11 from confocal microscopy analysis of SARS-CoV-2 nucleocapsid (NC) and markers of innate immune response in lungs of deceased COVID-19 patients. Panels show colocalization of viral NC with NLRP3 (A) and CD68 (B) or co-detection of NLRP3 with CD68 (C). Arrows point to a Roi shown as a Zoom-in view in 3D space (b) and associated quantitative profiling of image voxel intensity of the different dyes along a 3D line segment (c) using the Vaa3D software. Intensity correlation analysis of Rois (a): Manders' Coefficients (M1, M2);Costes' Pearson's Coefficient (CP) and P-value (P-v, %). Bars: 50 μm. D) Representative images of Picro Mallory staining in postmortem lung sections of cases 1 (P1), 2 (P2) and 3 (P3) showing interstitial fibrosis. Collagen is stained in blue. Bars: 200 μm. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
It is noteworthy that colocalization of NC with markers of CNS's tropism and immune responses, was observed in all deceased COVID-19 patients, evidencing the high cerebral virulence of the SARS-CoV-2.
3. Discussion
SARS-CoV-2 neuroinvasion has been observed in different experimental models, such as human brain organoids, and transgenic mice, overexpressing the human ACE2 (Sanclemente-Alaman et al., 2020). In a study including four brain regions from 43 COVID-19 autopsies, astrogliosis (37/43 cases), microglial activation (43/43 cases) and infiltrated cytotoxic CD8+ T cells (43/43 cases) were observed (Matschke et al., 2020). Out of 40 of these cases, SARS-CoV-2 RNA or proteins (spike or NC) were detected in brain areas of 21 (53%), with 8 cases (20%) containing both viral components, but its presence in the CNS was not associated with the severity of the neuropathological changes (Matschke et al., 2020). Similarly, Song et al. (2021) detected the viral spike protein in brains of 3 deceased COVID-19 patients, specifically in cortical neurons and in the microvasculature. Accordingly, the present study illustrates that NC colocalizes with specific neuronal and glia proteins in the cerebral cortex, suggesting the possibility that the diverse CNS cellular tropism of SARS-CoV-2 could be highly deleterious as previously proposed (Pan et al., 2020). This may promote blood-brain barrier leakage in close association with the viral injury to the CNS vascular endothelium (Lee et al., 2021).
On the other hand, in a series of 41 postmortem cases, microglial activation was found in 80.5% of them (34/41), confirming the important role of this CNS cell type, as well as the infiltration of T cells (38/41 cases) in cerebral SARS-CoV-2 injuries (Thakur et al., 2021). Moreover, brain samples (28/41 cases) had either low viral RNA titers or non-detectable levels of viral structural proteins (Thakur et al., 2021).This fact may reflect the extensive cerebral injuries secondary to viral neuroinvasion. Under these conditions, the capacity of the virus to replicate in brain host cells will be limited, given the CNS heterogeneous expression of genes relevant to viral entry (Matschke et al., 2020), and due to virus clearance by the neuroinflammatory response subsequent to the acute CNS attack (Dogra et al., 2021). In this sense, in a patient who died from COVID-19 with a severe neuropathological condition (large acute cerebral infarction) indisputably related to this disease, the viral RNA was not detected in any of the 16 brain regions studied (Serrano et al., 2021). Therefore, these studies suggest that SARS-CoV-2 CNS infection, replication and clearance mechanisms precede the worsening of COVID-19 neurological complications such as infarctions, hemorrhages and neurodegenerative processes; indicating that appropriate neurotherapeutic interventions should start as early as possible once the virus is detected.
NLRP3 inflammasome activation and the subsequent production of IL-1β in dysregulated reactive microglia, has been associated with CNS pathology (Barclay and Shinohara, 2017). Accordingly, our findings shed light on the plausible pathogenic role, as well as the possible pharmacological targeting, of this intracellular multiprotein complex on SARS-CoV-2 brain damage. We co-detected NLRP3 expression along with CD68 in brain samples, a marker previously related to the ramified state of activated microglia, possibly indicating its phagocytic activity (Hendrickx et al., 2017). Previously, higher NLRP3 levels were also observed in the lungs, in agreement with our observations, indicating that the activation of this inflammasome type may be a crucial SARS-CoV-2 pathogenicity mediator in the lungs and brain (Rodrigues et al., 2020). In the lungs, CD68+ macrophages have been observed within the alveolar space in 10/12 biopsies of COVID-19 patients, taken within 20 days of symptoms onset (Doglioni et al., 2021). These pieces of evidence support the hypothesis of early NLRP3 inflammasome activation in SARS-CoV-2 infected macrophages (lungs) and microglia (brain), promoting the progression to complications in the respiratory and nervous systems, respectively (Brodin, 2021); possibly in a cumulative way, along with its well-known cytokine release syndrome (Moore and June, 2020). Interestingly, an added factor to this puzzle is the bidirectional communication between these organs, as NLRP3 inflammasome components may reach the brain through extracellular vesicles, also possibly carrying SARS-CoV-2 viral particles, coming from infected lungs, and vice versa (Kerr et al., 2018). Considering this, the specific inhibition of NLRP3 inflammasomes could be a rational approach for improving the COVID-19 severity conditions and its CNS-associated injuries. Indeed, several biotechnological and synthetic candidates targeting NLRP3 are already under development (Freeman and Swartz, 2020); while alternative strategies for inhibiting NLRP3 have also been proposed, such as the natural-occurring tetrapyrrolic compound Phycocyanobilin (McCarty et al., 2021). In this sense, cumulative experimental evidence strongly supports the safe application of this compound for COVID-19-induced damage to the nervous system (Pentón-Rol et al., 2021). Moreover, pharmacological synergy may be achieved by combining such NLRP3 inhibition approach with therapies that can restrict the cytokine release syndrome in severe COVID-19 patients, such as the recently described CIGB-258 peptide (JUSVINZA®) (Hernández-Cedeño et al., 2021).
In a broad perspective, our evidence raises the question of whether SARS-CoV-2 may also activate other inflammasome types that are present in neurons or astrocytes, such as NLRP1 and AIM2, or NLRP2, respectively (de Rivero Vaccari et al., 2014). These are relevant topics worth investigating. Further studies with more cases and age/sex-matching controls are also needed for the generalization of our findings. Nonetheless, this paper gives novel clues for dissecting the complex neuroimmunological mechanisms of SARS-CoV-2-induced injury of the human CNS.
4. Conclusion
This study reveals that SARS-CoV-2 infection of the brain occurs in multiple CNS cell types, and induces the activation of the NLRP3 inflammasome in the microglia, which may be involved in diverse neurological complications related to COVID-19. Therefore, our data points to therapeutic strategies focusing on inhibiting microglial NLRP3 inflammasome for preventing neurological sequels, as well as for neuro-recovery promotion of COVID-19 patients.
The following are the supplementary data related to this article.
Supplementary Fig. 1.
Representative images obtained by confocal microscopy negative control of sections incubated only with secondary fluorescent probes-conjugated antibodies without primary antibodies in postmortem brain samples of deceased COVID-19 patients. A) Brain cortex section incubated with fluorescein-conjugated goat anti-mouse and Alexa Fluor 647 (A647)-conjugated goat anti-rabbit IgGs, and DAPI to stain nucleus (blue). B) Brain cortex section incubated with fluorescein-conjugated goat anti-rabbit and Alexa Fluor 647 (A647)-conjugated goat anti-mouse IgGs, and DAPI to stain nucleus (blue). Bars: 50 µm.
Supplementary Fig. 12.
Representative images obtained by confocal microscopy analysis of lung sections from a person who died from a cause non-related to COVID-19 showing no staining for NC, as reference antibodies against NLRP3 (Green) (A) and CD68 (Green) (B) were used. Sections incubated with mouse monoclonal (A) and rabbit polyclonal (B) antibodies against NC and antibodies against NLRP3 (A) and CD68 (B), followed by Alexa Fluor 647-conjugated anti-mouse/rabbit IgGs and fluorescein-conjugated anti-mouse/rabbit IgGs in different combinations, and DAPI to stain nucleus (blue channel). Bars: 50 µm.
Supplementary Materials and Methods.
Funding
This work was supported by the CIGB. J.M.-P., M.C.-Ll., B.P.-M. and G.P.-R. were partially supported by the PN305LH013-036 project of the Ministry of Science, Technology and Environment (Cuba), through the Program for Neuroscience and Neurotechnology.
Declaration of Competing Interest
None.
Acknowledgements
We would like to thank José Suárez-Alba for his technical assistance and Rodolfo Valdés-Véliz and Omar R. Blanco for providing antibodies against the SARS-Cov-2 NC protein.
References
- Azizi S.A., Azizi S.-A. Neurological injuries in COVID-19 patients: direct viral invasion or a bystander injury after infection of epithelial/endothelial cells. J. Neuro-Oncol. 2020;26:631–641. doi: 10.1007/s13365-020-00903-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay W., Shinohara M.L. Inflammasome activation in multiple sclerosis and experimental autoimmune encephalomyelitis (EAE) Brain Pathol. 2017;27:213–219. doi: 10.1111/bpa.12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodin P. Immune determinants of COVID-19 disease presentation and severity. Nat. Med. 2021;27:28–33. doi: 10.1038/s41591-020-01202-8. [DOI] [PubMed] [Google Scholar]
- de Rivero Vaccari J.P., Dietrich W.D., Keane R.W. Activation and regulation of cellular inflammasomes: gaps in our knowledge for central nervous system injury. J. Cereb. Blood Flow Metab. 2014;34:369–375. doi: 10.1038/jcbfm.2013.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doglioni C., Ravaglia C., Chilosi M., Rossi G., Dubini A., Pedica F., Piciucchi S., Vizzuso A., Stella F., Maitan S., Agnoletti V., Puglisi S., Poletti G., Sambri V., Pizzolo G., Bronte V., Wells A.U., Poletti V. COVID-19 interstitial pneumonia: histological and Immunohistochemical features on Cryobiopsies. Respiration. 2021 doi: 10.1159/000514822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogra P., Ruiz-Ramírez J., Sinha K., Butner J.D., Peláez M.J., Rawat M., Yellepeddi V.K., Pasqualini R., Arap W., Sostman H.D., Cristini V., Wang Z. Innate immunity plays a key role in controlling viral load in COVID-19: mechanistic insights from a whole-body infection dynamics model. ACS Pharmacol. Transl. Sci. 2021;4:248–265. doi: 10.1021/acsptsci.0c00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman T.L., Swartz T.H. Targeting the NLRP3 Inflammasome in severe COVID-19. Front. Immunol. 2020;11:1518. doi: 10.3389/fimmu.2020.01518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickx D.A.E., van Eden C.G., Schuurman K.G., Hamann J., Huitinga I. Staining of HLA-DR, Iba1 and CD68 in human microglia reveals partially overlapping expression depending on cellular morphology and pathology. J. Neuroimmunol. 2017;309:12–22. doi: 10.1016/j.jneuroim.2017.04.007. [DOI] [PubMed] [Google Scholar]
- Hernández-Cedeño M., Venegas-Rodriguez R., Peña-Ruiz R., Bequet-Romero M., Santana-Sanchez R., Penton-Arias E., Martinez-Donato G., Guillén-Nieto G., Dominguez-Horta M.C. CIGB-258, a peptide derived from human heat-shock protein 60, decreases hyperinflammation in COVID-19 patients. Cell Stress Chaperones. 2021;26:515–525. doi: 10.1007/s12192-021-01197-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr N.A., de RiveroVaccari J.P., Abbassi S., Kaur H., Zambrano R., Wu S., Dietrich W.D., Keane R.W. Traumatic brain injury-induced acute lung injury: evidence for activation and inhibition of a neural-respiratory-inflammasome axis. J. Neurotrauma. 2018;35:2067–2076. doi: 10.1089/neu.2017.5430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M.H., Perl D.P., Nair G., Li W., Maric D., Murray H., Dodd S.J., Koretsky A.P., Watts J.A., Cheung V., Masliah E., Horkayne-Szakaly I., Jones R., Stram M.N., Moncur J., Hefti M., Folkerth R.D., Nath A. Microvascular injury in the brains of patients with COVID-19. N. Engl. J. Med. 2021;384:481–483. doi: 10.1056/NEJMc2033369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matschke J., Lütgehetmann M., Hagel C., Sperhake J.P., Schröder A.S., Edler C., Mushumba H., Fitzek A., Allweiss L., Dandri M., Dottermusch M., Heinemann A., Pfefferle S., Schwabenland M., Sumner Magruder D., Bonn S., Prinz M., Gerloff C., Püschel K., Krasemann S., Aepfelbacher M., Glatzel M. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol. 2020;19:919–929. doi: 10.1016/S1474-4422(20)30308-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty M.F., Assanga S.B.I., Luján L.L., O’Keefe J.H., DiNicolantonio J.J. Nutraceutical strategies for suppressing NLRP3 Inflammasome activation: pertinence to the management of COVID-19 and beyond. Nutrients. 2021;13:47. doi: 10.3390/nu13010047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J.B., June C.H. Cytokine release syndrome in severe COVID-19. Science. 2020;368:473. doi: 10.1126/science.abb8925. [DOI] [PubMed] [Google Scholar]
- Pan R., Zhang Q., Anthony S.M., Zhou Y., Zou X., Cassell M., Perlman S. Oligodendrocytes that survive acute coronavirus infection induce prolonged inflammatory responses in the CNS. Proc. Natl. Acad. Sci. U. S. A. 2020;117:15902–15910. doi: 10.1073/pnas.2003432117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pentón-Rol G., Marín-Prida J., McCarty M.F. C-Phycocyanin-derived Phycocyanobilin as a potential Nutraceutical approach for major neurodegenerative disorders and COVID-19-induced damage to the nervous system. CurrNeuropharmacol. 2021 doi: 10.2174/1570159X19666210408123807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues T.S., de Sá K.S.G., Ishimoto A.Y., Becerra A., Oliveira S., Almeida L., Gonçalves A.V., Perucello D.B., Andrade W.A., Castro R., Veras F.P., Toller-Kawahisa J.E., Nascimento D.C., de Lima M.H.F., Silva C.M.S., Caetite D.B., Martins R.B., Castro I.A., Pontelli M.C., de Barros F.C., do Amaral N.B., Giannini M.C., Bonjorno L.P., Lopes M.I.F., Santana R.C., Vilar F.C., Auxiliadora-Martins M., Luppino-Assad R., de Almeida S.C.L., de Oliveira F.R., Batah S.S., Siyuan L., Benatti M.N., Cunha T.M., Alves-Filho J.C., Cunha F.Q., Cunha L.D., Frantz F.G., Kohlsdorf T., Fabro A.T., Arruda E., de Oliveira R.D.R., Louzada-Junior P., Zamboni D.S. Inflammasomes are activated in response to SARS-CoV-2 infection and are associated with COVID-19 severity in patients. J. Exp. Med. 2020;218 doi: 10.1084/jem.20201707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanclemente-Alaman I., Moreno-Jiménez L., Benito-Martín M.S., Canales-Aguirre A., Matías-Guiu J.A., Matías-Guiu J., Gómez-Pinedo U. Experimental models for the study of central nervous system infection by SARS-CoV-2. Front. Immunol. 2020;11:2163. doi: 10.3389/fimmu.2020.02163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano G.E., Walker J.E., Arce R., Glass M.J., Vargas D., Sue L.I., Intorcia A.J., Nelson C.M., Oliver J., Papa J., Russell A., Suszczewicz K.E., Borja C.I., Belden C., Goldfarb D., Shprecher D., Atri A., Adler C.H., Shill H.A., Driver-Dunckley E., Mehta S.H., Readhead B., Huentelman M.J., Peters J.L., Alevritis E., Bimi C., Mizgerd J.P., Reiman E.M., Montine T.J., Desforges M., Zehnder J.L., Sahoo M.K., Zhang H., Solis D., Pinsky B.A., Deture M., Dickson D.W., Beach T.G. Mapping of SARS-CoV-2 brain invasion and histopathology in COVID-19 disease. medRxiv. 2021 doi: 10.1101/2021.02.15.21251511. [DOI] [Google Scholar]
- Song E., Zhang C., Israelow B., Lu-Culligan A., Prado A.V., Skriabine S., Lu P., Weizman O.E., Liu F., Dai Y., Szigeti-Buck K., Yasumoto Y., Wang G., Castaldi C., Heltke J., Ng E., Wheeler J., Alfajaro M.M., Levavasseur E., Fontes B., Ravindra N.G., Dijk D.V., Mane S., Gunel M., Ring A., Kazmi S.A.J., Zhang K., Wilen C.B., Horvath T.L., Plu I., Haik S., Thomas J.L., Louvi A., Farhadian S.F., Huttner A., Seilhean D., Renier N., Bilguvar K., Iwasaki A. Neuroinvasion of SARS-CoV-2 in human and mouse brain. J. Exp. Med. 2021;3 doi: 10.1084/jem.20202135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur K.T., Miller E.H., Glendinning M.D., Al-Dalahmah O., Banu M.A., Boehme A.K., Boubour A.L., Bruce S.S., Chong A.M., Claassen J., Faust P.L., Hargus G., Hickman R.A., Jambawalikar S., Khandji A.G., Kim C.Y., Klein R.S., Lignelli-Dipple A., Lin C.C., Liu Y., Miller M.L., Moonis G., Nordvig A.S., Overdevest J.B., Prust M.L., Przedborski S., Roth W.H., Soung A., Tanji K., Teich A.F., Agalliu D., Uhlemann A.C., Goldman J.E., Canoll P. COVID-19 neuropathology at Columbia University Irving medical center/New York Presbyterian hospital. Brain. 2021 doi: 10.1093/brain/awab148. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Materials and Methods.