We read with high interest the article by Manzo et al. [1] about SARS-CoV-2 triggering polymyalgia rheumatica (PMR). SARS-CoV-2 triggering giant cell arteritis (GCA) have also been described [2]. Interleukin 6 (IL-6) is a key pathway in the pathogenesis of SARS-CoV-2 disease [3], PMR and [4] and IL-6 blockage has been used in all three diseases [3], [4].
COVID-19 vaccine is crucial for patients, especially with chronic rheumatic diseases (CRD). There is only a few data on vaccine tolerance in these population, but recent reports showed reassuring [5], [6], [7] results with at least 85% of patient without flares after the first dose. However, data are scarce regarding COVID-19 vaccine and PMR or GCA. Here we report three cases of PMR and GCA developed soon after COVID-19 vaccine (Fig. 1 ).
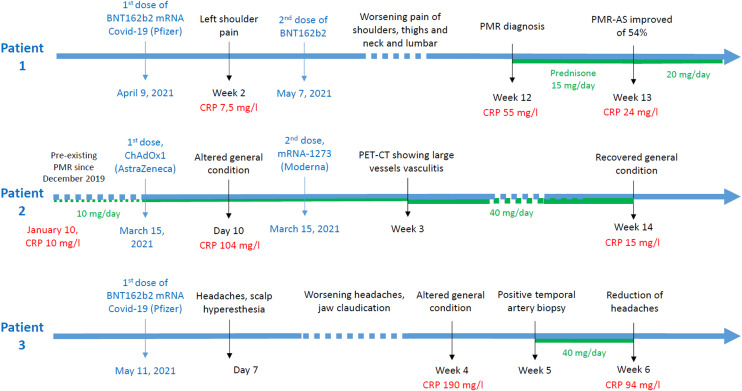
Fig. 1.
Patient's timelines of vaccines and PMR or GCA symptoms.
The first patient was a 71-year-old man with grade 1 obesity, atrial fibrillation, bypass surgery and aortic bioprosthesis. Fourteen days after his first shot, he developed a mild pain in his left shoulder. One month later, he got his second shot. Pain was persistent, but CRP was normal. Slowly, pain worsened with involvement of both shoulders and morning stiffness lasting at least 2 hours. He also developed thigh, neck and lumbar pain. CRP increased at 55 mg/L. Shoulders ultrasound showed subdeltoid bursitis and biceps tenosynovitis. Thoraco-abdominal-pelvic computed tomography (TAP-CT) with injection did not show any carcinologic process or large vessel arteritis. Considering his clinical and biological presentation, the patient met the EULAR 2012 classification criteria of PMR (6 points) [8]. Prednisone was started at 15 mg/day, increased at 20 mg/day after 7 days (54% of improvement of PMR-AS) with good efficiency.
The second patient was a 70-year-old women with a PMR diagnosed in december 2019. One-year later, under 8 mg of prednisone per day, CRP was slightly increased at 10 mg/L without other symptom. She received her first and second shots on march 15 and june 8, respectively. About 10 days after the first shot, she developed an important fatigue and had high CRP levels at 104 mg/l. This systemic inflammation was not associated with typical symptoms of cephalic GCA. A positron emission tomography/computed tomography (PET-CT) showed large vessel vasculitis (Fig. 2 ). Prednisone at 40 mg/day was introduced. One month later, symptoms had decreased and CRP was at 15 mg/L
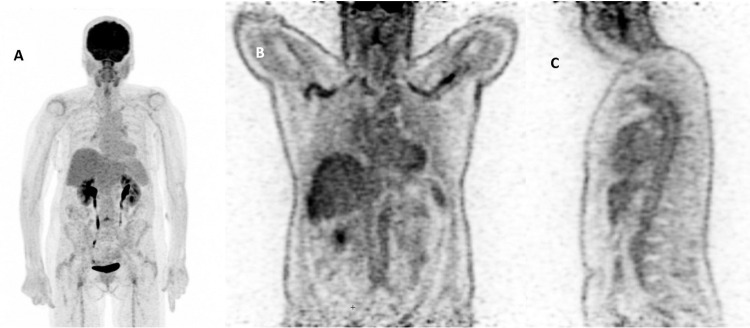
Fig. 2.
PET-CT of patient no 2. A. PET-CT at diagnosis in December 19, showing slight hypermetabolism of glenohumeral and coxofemoral joints without vascular hypermetabolism. B and C. PET-CT 2 years later and 23 days after her first dose of COVID19 vaccine, showing a panaortic and supra-aortic vasculitis.
The last patient was a 74-year-old women with advanced ovarian cancer, stable since 2009. She was vaccinated on 11 may. She slowly developed headache in may 2021, i.e. 7 days after her shot. Gradually, headaches worsened and she developed jaw claudication. Biology showed elevated CRP at 190 mg/L. She was diagnosed with GCA based on the results of her temporal artery biopsy showing granuloma and fragmentation of the internal elastic lamina. Prednisone was initiated at 60 mg per day with improvement of clinical and biological features.
To our knowledge, these cases are the first cases of PMR or GCA diseases shortly developed after COVID-19 vaccination. A specific attention regarding signs of these CRD may be needed in post approval observational studies evaluating vaccine tolerance. The potential role of COVID19 vaccine in relapse of pre-existing PMR and ACG is yet to be determined.
Informed consent
Consent for publication of clinical details was obtained from patients.
Disclosure of interest
The authors declare that they have no competing interest.
References
- 1.Manzo C., Castagna A., Ruotolo G. Can SARS-CoV-2 trigger relapse of polymyalgia rheumatica? Joint Bone Spine. 2021;88:105150. doi: 10.1016/j.jbspin.2021.105150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Riera-Martí N., Romaní J., Calvet J. SARS-CoV-2 infection triggering a giant cell arteritis. Med Clin (Engl Ed) 2021;156:253–254. doi: 10.1016/j.medcle.2020.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tharmarajah E., Buazon A., Patel V., et al. IL-6 inhibition in the treatment of COVID-19: a meta-analysis and meta-regression. J Infect. 2021;82:178–185. doi: 10.1016/j.jinf.2021.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spiera R., Unizony S.H., Bao M., et al. Tocilizumab vs placebo for the treatment of giant cell arteritis with polymyalgia rheumatica symptoms, cranial symptoms or both in a randomized trial. Semin Arthritis Rheum. 2021;51:469–476. doi: 10.1016/j.semarthrit.2021.03.006. [DOI] [PubMed] [Google Scholar]
- 5.Barbhaiya M., Levine J.M., Bykerk V.P., et al. Systemic rheumatic disease flares after SARS-CoV-2 vaccination among rheumatology outpatients in New York City. Ann Rheum Dis. 2021 doi: 10.1136/annrheumdis-2021-220732. [annrheumdis-2021-220732] [DOI] [PubMed] [Google Scholar]
- 6.Braun-Moscovici Y., Kaplan M., Braun M., et al. Disease activity and humoral response in patients with inflammatory rheumatic diseases after two doses of the Pfizer mRNA vaccine against SARS-CoV-2. Ann Rheum Dis. 2021 doi: 10.1136/annrheumdis-2021-220503. [annrheumdis-2021-220503] [DOI] [PubMed] [Google Scholar]
- 7.Cherian S., Paul A., Ahmed S., et al. Safety of the ChAdOx1 nCoV-19 and the BBV152 vaccines in 724 patients with rheumatic diseases: a post-vaccination cross-sectional survey. Rheumatol Int. 2021;41:1441–1445. doi: 10.1007/s00296-021-04917-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dasgupta B., Cimmino M.A., Maradit-Kremers H., et al. 2012 provisional classification criteria for polymyalgia rheumatica: a European League Against Rheumatism/American College of Rheumatology collaborative initiative. Ann Rheum Dis. 2012;71:484–492. doi: 10.1136/annrheumdis-2011-200329. [DOI] [PMC free article] [PubMed] [Google Scholar]