Abstract
Background:
Drinking water chlorination by-products have been associated with adverse reproductive outcomes, although the findings for congenital malformations are still inconclusive.
Objective:
We conducted a nationwide register-based prospective study to assess whether first trimester maternal exposure to the four most common trihalomethanes [total trihalomethanes (TTHM)] via municipal drinking water was associated with risk of congenital malformation among newborns.
Methods:
We included all births during 2005–2015 (live and stillbirths) of mothers residing in Swedish localities having inhabitants, two or fewer operating water works, and sufficient municipal TTHM monitoring data. Individual maternal first trimester exposure was obtained by linking TTHM measurements to residential information, categorized into no chlorination and , 5–15, and TTHM/L. We also made chlorination treatment-specific analyses (exclusive use of chloramine or hypochlorite). Outcomes and covariates were obtained via linkage to health care and administrative registers. Odds ratios (ORs) and 95% confidence intervals (CIs) were estimated by logistic regression.
Results:
Based on 623,468 births and a prevalence of congenital malformation of cases/100 births, we observed associations between TTHM exposure in areas using chloramine and malformations of the nervous system (; 95% CI: 1.07, 3.12), urinary system (; 95% CI: 1.53, 2.78), genitals (; 95% CI: 1.38, 2.26), and limbs (; 95% CI: 1.10, 1.64), comparing the highest exposed category with the unexposed. No associations were observed in areas using exclusively hypochlorite as the primary water treatment method. By contrast, for malformations of the heart, a significant inverse association was observed only in areas using hypochlorite.
Discussion:
TTHM exposure was associated with the increased risk of malformations of the nervous system, urinary system, genitals, and limbs in areas exclusively using chloramine. An association between chloramine-related chlorination by-products and congenital malformations has not previously been highlighted and needs further attention. https://doi.org/10.1289/EHP9122
Introduction
Drinking water chlorination is an efficient health intervention, used for decades to inactivate pathogens in the drinking water and to reduce microbial growth in the water distribution system. Owing to its strong oxidative properties, chlorine reacts with substances in the water (e.g., natural organic matter), resulting in the formation of hundreds of chlorination by-products (CBPs) (Richardson et al. 2007). Epidemiological evidence is increasing that CBPs are associated with adverse reproductive outcomes, such as intrauterine growth retardation (Cao et al. 2016; Grazuleviciene et al. 2011; Hinckley et al. 2005; Kramer et al. 1992; Levallois et al. 2012; Lewis et al. 2006; Smith et al. 2016; Säve-Söderbergh et al. 2020; Wright et al. 2003, 2004). Likewise, some epidemiological studies suggest associations may exist between CBP exposures and malformations of the neural tube (Dodds and King 2001; Klotz and Pyrch 1999), urogenital systems (Grazuleviciene et al. 2013; Magnus et al. 1999), and circulatory system (Cedergren et al. 2002; Chisholm et al. 2008; Grazuleviciene et al. 2013; Hwang et al. 2002). However, as reported in a meta-analysis, epidemiological evidence on congenital anomalies resulting from gestational exposure to CBPs remains inconclusive (Nieuwenhuijsen et al. 2009). Because embryonic development is complex and congenital malformations are rare, there is a delicate balance between limited power and pooling of conceivably etiologically heterogenic outcomes. In any case, accurate classification of the exposure is perhaps an even greater challenge that requires careful consideration of any potential bias introduced by misclassifications (Waller et al. 2001; Wright and Bateson 2005).
We conducted a large prospective nationwide register-based cohort study in Sweden to assess the association of the exposure during the first trimester of gestation to the sum of the four most common CBPs, total trihalomethanes (TTHM: chloroform, bromoform, bromodichloromethane, and dibromochloromethane), via drinking tap water, with congenital malformations. All areas included were provided with municipal tap drinking water (chlorinated or nonchlorinated). We also performed chlorination treatment-specific analyses by the two major chlorination treatment methods because they may lead to the formation of different CBPs.
Methods
Study Area and Population
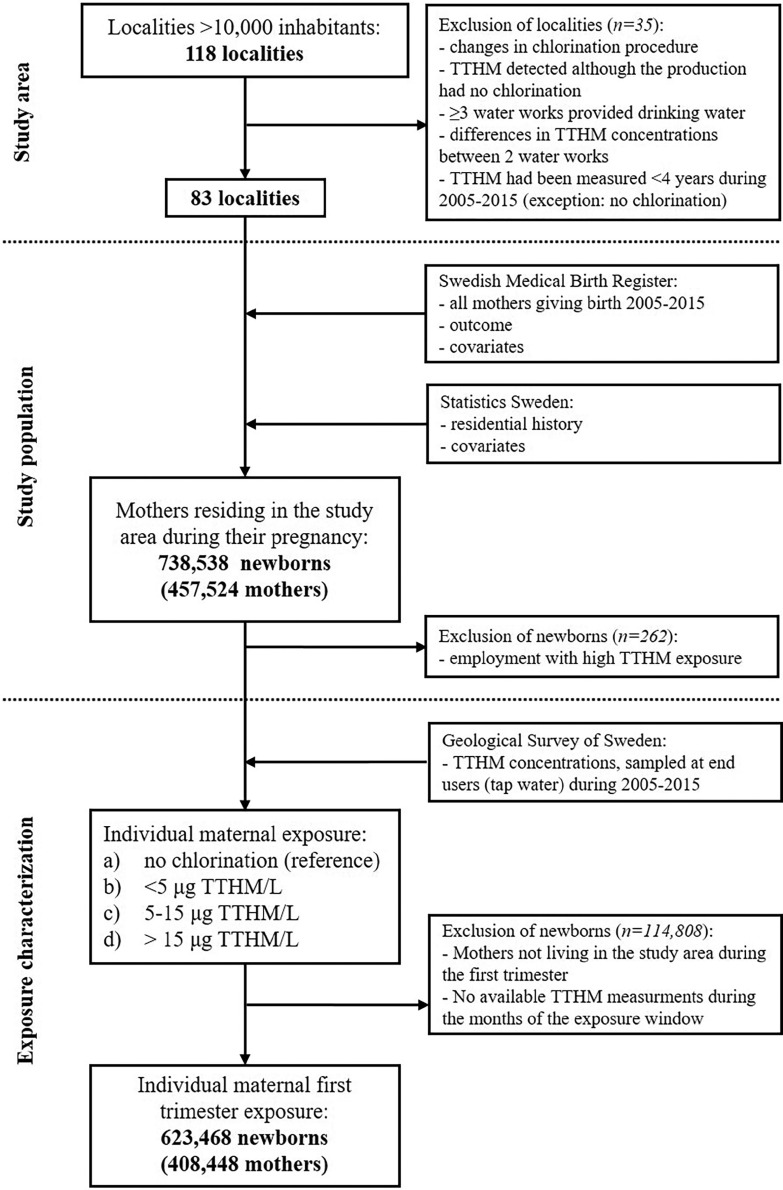
We initially mapped all localities (coherent and densely populated urban areas) in Sweden having a population of inhabitants (, representing of the country’s population) with respect to their drinking water production and raw water source during the period 2005–2015. We then excluded localities if there had been changes in chlorination treatment during 2005–2015 or if the locality or municipality were supplied by three or more water utilities. For localities supplied by nonchlorinated drinking water, we excluded those where CBPs still happened to be detected in the tap water when monitored. Among the localities supplied by chlorinated tap water, we excluded localities in which differences in the mean CBP concentrations between two utilities serving the locality exceeded and those in which CBPs were measured for a period of during the study period (2005–2015). In total, 83 localities remained as our study area (Figure 1). We used a similar study design as in a study assessing TTHM exposure in relation to small for gestational age and preterm delivery (Säve-Söderbergh et al. 2020).
Figure 1.
Study area, study population, and exposure categorization. TTHM, average of the four most common trihalomethanes (total trihalomethanes).
The study population was identified as mothers having their official residential registration in one of the 83 localities at any time during a pregnancy that resulted in a childbirth (live or stillbirth) in Sweden between 1 January 2005 and 31 December 2015. By use of the maternal personal identification number (a unique identification number assigned to all Swedes), health care data linkage was performed by the Swedish Medical Birth Register at the National Board of Health and Welfare. Administrative data linkage included the Longitudinal Integration Database for Health Insurance and Labor Market Studies and a national register for regional divisions based on real estate (Geografidatabasen), both databases at Statistics Sweden. In total, 738,538 newborns (including 2,578 stillbirths) of 457,524 mothers were identified as living in the study area during the pregnancy (Figure 1). Among these mothers, 15% changed locality at some point during their pregnancy, and 7% did so during the first trimester.
Although mothers of full-term newborns were included if they had their residential address in the area at the same locality during a consecutive period of between 6 and 10 months prior to delivery, mothers of preterm newborns were included if they had their residential address in the study area during the entire pregnancy (at birth and 10 months prior to birth). We excluded mothers with a potential high occupational TTHM exposure, that is, if they were registered at the antenatal care as professional swimmers, coaches, or swimming pool personnel (). The study was approved by the regional ethics review board in Stockholm. Because the study was only register-based, no informed consent was required for the data linkage.
Exposure and Covariates
TTHM is a commonly used indicator of the total CBP exposure because trihalomethanes (THMs) are often generated in the highest concentrations (Richardson et al. 2007). We obtained TTHM exposure by summing the concentrations of chloroform, bromoform, bromodichloromethane, and dibromochloromethane in tap water sampled at the end user in the municipal monitoring program from a nationwide database managed by the Geological Survey of Sweden { using confirmed TTHM analyses [in exposed areas: mean (standard deviation) TTHM/L, , TTHM/L]}. The sampling made in the municipal monitoring program was done according to the national drinking water regulations (SLVFS 2001:30). Thus, a minimum of four yearly end-user tap water samples were available, with three additional samplings per every drinking water produced per day (TTHM should not exceed in drinking water). We estimated a trimester-specific 3-month average TTHM exposure for each individual pregnancy by using a locality-specific and multiannual monthly average of TTHM [as outlined by Säve-Söderbergh et al. (2020)]. Briefly, by using a multiannual average for each month and locality for assigning the exposure, we a) accounted for seasonal variations (Andersson et al. 2019), b) reduced the weight of single extreme values and the influence of locality-specific variations in monitoring programs, as well as c) minimized the number of pregnancies excluded due to missing exposure information for a specific month or year.
We used the first trimester because this is the critical window for the development of most human organs and, thus, the effect-window for teratogens (Moore et al. 2016). We are confident that regular consumption of tap water is likely applicable to almost all mothers (Säve-Söderbergh et al. 2018). Based on each individual’s locality-specific and multiannual 3-month average TTHM, the maternal exposure was then categorized into one of the following categories: a) no chlorination (only mothers in localities with no chlorination), b) TTHM/L, c) TTHM/L, and d) TTHM/L.
From the Swedish Medical Birth Register, we obtained maternal information on age, body mass index (BMI), and smoking at registration to antenatal care, parity, self-reported pregestational diabetes, and self-reported use of teratogenic drugs (Class 3) (Nörby et al. 2013). From Statistics Sweden, we obtained data on country of birth, household income, and highest attained education. We also collected locality-related information, such as raw water source, size of the locality, and chlorination treatment used from drinking water producers and the Swedish Water & Wastewater Association.
Outcomes
Information on the diagnosis of congenital malformations [categorized according to the International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD-10 codes Q00–Q99; WHO 2016)] was obtained from the Swedish Medical Birth Register, which covers diagnoses made during the first 28 days postpartum. Major congenital malformations were classified by organ system according to the European Surveillance of Congenital Anomalies (EUROCAT 2014) into nervous system defects, congenital heart defects, orofacial clefts, digestive system defects, urinary system defects, genital defects, and defects in the limbs, as well as chromosomal defects.
Statistical Analyses
We used multivariable logistic regression to estimate odds ratios (ORs) and 95% confidence intervals (CIs) of exposure to TTHM and the risk of major congenital malformations. To reduce dependency on multiple births, the analyses were clustered by anonymized maternal identification number (intragroup correlation), as received by the data provider.
We used inverse probability weighting to account for missing data and for confounding, that is, we used the inverse of the product of selection and exposure probabilities (Hernán and Robins 2020). Confounders included into the model were selected based on prior knowledge of potential risk factors for congenital malformations. Thus, models were adjusted for maternal age (, , , , ), BMI (at registration to antenatal care: , , , ), maternal self-reported pregestational diabetes (yes/no), any use of teratogenic drugs (yes/no), parity (nulliparous, 1, 2, ), smoking at registration to antenatal care (no smoking, 1–9 cigarettes/d, ), highest attained education (elementary school/secondary education/postsecondary education) and household income (yearly quartiles by year of birth). Despite the locality-specific differences, given the risk of overadjusting, we did not cluster by locality because locality was highly linked to the drinking water treatment method.
The median TTHM concentrations for each exposure category was used to assess linear trends. In the main analyses, we used those living in localities with nonchlorinated municipal drinking water as the reference because they represented the most appropriate unexposed population (pregnant women in densely populated areas provided with municipal drinking water, i.e., no private wells). Previous studies generally used the lowest exposed category as the reference [e.g., Nieuwenhuijsen et al. (2008) and Chisholm et al. (2008)]. For comparison and to assess the potential impact of any contextual confounding linked to the use of chlorination, we also performed analyses in which the lowest TTHM category was used as the reference (), excluding localities using nonchlorinated water.
In addition, we performed separate analyses for pregnant women in localities that used either hypochlorite or chloramine as the exclusive drinking water treatment method because these treatments may have varying impact on the formation of CBPs. Statistical significance level was set at 0.05, and all statistical analyses were performed using Stata (Release 14.2; StataCorp).
Results
During 2005–2015, we ascertained 623,468 newborns (live and stillbirths) among mothers assigned to a first trimester TTHM exposure (Figure 1). Of those, 65,154 were newborns of mothers receiving nonchlorinated municipal tap water, whereas 133,071 and 247,497 newborns had mothers receiving tap water chlorinated with only hypochlorite or chloramine, respectively, as drinking water treatment. The total prevalence of major congenital malformations was 20 cases/1,000 births (Table S1). The prevalence per group of major malformation ranged from births for malformation on the nervous system up to births of congenital heart defects. Major malformations with a prevalence of (i.e., malformations of the ear, face and neck, eye, respiratory, and abdominal wall), as well as minor malformations (prevalence cases/10,000 births) were presented only by their prevalence in (Table S1).
We observed some differences in baseline maternal characteristics across the exposure groups, especially for maternal age, attained education, and household income (Table 1). In areas with chlorinated water, the mothers assigned to the lowest exposure category ( TTHM/L) were older, had higher household incomes, lower BMIs, and were less often smokers and diabetics, as compared with those in the highest category ( TTHM/L). Similar differences were also observed among mothers in areas receiving drinking water treated with chloramine alone (Table 1). In contrast, in areas receiving water chlorinated with hypochlorite only, the mothers in the lowest exposed category were younger and had both lower attained educations and household incomes, as compared with those in highest exposure category. Although there were no major overall differences in maternal characteristics between the nonchlorinated and chlorinated study areas, the mothers in the nonchlorinated areas tended to be more similar to those in the highest than the lowest TTHM exposure category (Table 1). For the locality-related characteristics, we observed that areas with nonchlorinated drinking water were generally smaller and their raw water source was more often groundwater as compared with those with chlorinated drinking water. These differences were especially marked when the areas with nonchlorinated drinking water were compared with those using chloramine treatment.
Table 1.
Baseline population characteristics and locality-specific characteristics, by individual first trimester maternal average exposure to the four most common trihalomethanes (TTHM) for all and by chlorination treatment expressed as proportions of all included newborns (%).
| Variables | Categories | No chlorination | All chlorinated areas | Hypochlorite | Chloramine | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TTHM/L | TTHM/L | TTHM/L | TTHM/L | TTHM/L | TTHM/L | TTHM/L | TTHM/L | TTHM/L | |||
| Births included | () | 65,154 | 289,915 | 179,182 | 89,217 | 30,883 | 54,144 | 48,044 | 188,428 | 18,934 | 40,135 |
| Average TTHM | ( TTHM/L) | — | 0.67 | 9.3 | 24 | 1.2 | 10 | 22 | 0.60 | 9.0 | 25 |
| Maternal characteristics | |||||||||||
| Age (y) | 16 | 10 | 12 | 14 | 17 | 16 | 14 | 9 | 10 | 14 | |
| 33 | 26 | 29 | 30 | 33 | 32 | 32 | 24 | 25 | 28 | ||
| 33 | 38 | 37 | 36 | 32 | 34 | 35 | 39 | 38 | 37 | ||
| 15 | 21 | 18 | 17 | 15 | 15 | 16 | 23 | 22 | 18 | ||
| 3.0 | 4.9 | 3.9 | 3.5 | 3.3 | 3.2 | 3.3 | 5.4 | 5.4 | 3.6 | ||
| BMI () | 2.2 | 2.6 | 2.4 | 2.4 | 2.4 | 2.3 | 2.2 | 2.7 | 2.8 | 2.6 | |
| 54 | 59 | 57 | 58 | 54 | 56 | 57 | 61 | 61 | 59 | ||
| 24 | 21 | 22 | 23 | 24 | 24 | 24 | 20 | 21 | 23 | ||
| 12 | 8.8 | 10 | 11 | 13 | 12 | 12 | 7.8 | 8.4 | 9.8 | ||
| Missing data | 7.1 | 8.5 | 8.2 | 5.7 | 6.7 | 6.0 | 6.1 | 9.0 | 7.3 | 5.3 | |
| Pregestational diabetesa | Yes | 1.9 | 1.3 | 1.7 | 2.7 | 1.5 | 1.9 | 1.9 | 1.2 | 2.0 | 3.6 |
| No | 98 | 99 | 98 | 97 | 98 | 98 | 98 | 99 | 98 | 96 | |
| Parity | Nulliparous | 43 | 45 | 46 | 45 | 42 | 44 | 44 | 46 | 48 | 46 |
| 1 | 37 | 38 | 37 | 37 | 37 | 37 | 37 | 37 | 35 | 37 | |
| 2 | 13 | 13 | 12 | 13 | 15 | 13 | 13 | 12 | 12 | 12 | |
| 5.9 | 4.8 | 5.1 | 6.0 | 6.9 | 5.9 | 6.3 | 4.5 | 4.6 | 5.5 | ||
| Smokinga | No smoking | 90 | 90 | 90 | 91 | 89 | 90 | 91 | 91 | 92 | 91 |
| 1–9 cigarettes/d | 4.7 | 3.6 | 4.2 | 4.5 | 5.8 | 4.8 | 4.2 | 3.0 | 3.3 | 4.8 | |
| 1.2 | 1.0 | 1.1 | 1.4 | 1.8 | 1.2 | 1.2 | 0.81 | 0.83 | 1.7 | ||
| Missing data | 4.1 | 5.2 | 4.4 | 3.4 | 3.7 | 3.5 | 4.1 | 5.6 | 3.7 | 2.5 | |
| Use of teratogenic drugsa | Yes | 0.055 | 0.049 | 0.056 | 0.053 | 0.052 | 0.054 | 0.058 | 0.050 | 0.037 | 0.045 |
| No | 99 | 99 | 99 | 99 | 99 | 99 | 99 | 99 | 99 | 99 | |
| Highest attained educational level | Elementary school | 11 | 10 | 11 | 12 | 16 | 12 | 11 | 9.4 | 10 | 12 |
| Secondary education | 39 | 32 | 32 | 31 | 40 | 35 | 33 | 29 | 28 | 28 | |
| Postsecondary education | 50 | 58 | 57 | 58 | 45 | 53 | 56 | 61 | 62 | 60 | |
| Household income (quartiles by year of birth) | 1 | 16 | 15 | 17 | 20 | 19 | 17 | 15 | 15 | 20 | 26 |
| 2 | 29 | 22 | 26 | 27 | 29 | 28 | 28 | 21 | 22 | 26 | |
| 3 | 40 | 30 | 35 | 34 | 36 | 38 | 38 | 28 | 27 | 29 | |
| 4 | 15 | 33 | 22 | 19 | 16 | 18 | 19 | 36 | 32 | 18 | |
| Locality-specific characteristics | |||||||||||
| Water source | Groundwater | 95 | 15 | 21 | 43 | 84 | 63 | 58 | 3.2 | 4.4 | 26 |
| Surface water | 5.2 | 85 | 79 | 57 | 16 | 37 | 42 | 97 | 96 | 74 | |
| Population in locality () | 24 | 1.5 | 2.8 | 3.4 | 7.5 | 8.0 | 6.0 | 0.47 | 0.0053 | 0 | |
| 20,000–200,000 | 76 | 62 | 34 | 41 | 92 | 63 | 51 | 73 | 72 | 30 | |
| 0 | 37 | 64 | 56 | 0 | 29 | 43 | 27 | 28 | 70 | ||
Note: The study included a total of 623,468 children born during 2005–2015 to mothers living in large urban areas ( inhabitants) in Sweden. There were no missing data, with the exception of BMI (8%) and smoking status (6%). —, Not applicable; BMI, body mass index; TTHM, average of the four most common trihalomethanes (total trihalomethanes).
As reported at registration to antenatal care.
When not differentiating by chlorination treatments, a statistically significant increased risk of urinary and genital malformations was observed, multivariable-adjusted (95% CI: 1.10, 1.89; ) and (95% CI: 1.18, 1.81; ), respectively, when comparing the highest TTHM exposure category with unexposed (Table 2). When considering the areas using chloramine treatment exclusively, associations with significantly increased risk of malformations of both the nervous and the urinary systems, as well as of the genitals and limbs, were found: multivariable-adjusted (95% CI: 1.07, 3.12; ), (95% CI: 1.53, 2.78; ), (95% CI: 1.38, 2.26, ), and (95% CI: 1.10, 1.64; ), respectively, when comparing the highest TTHM exposure with unexposed (Table 2). Except for urinary malformations, these associations remained when changing the reference category from unexposed to the lowest exposed category ( TTHM/L) (Table S2). When considering the areas using hypochlorite treatment, no consistent associations were found.
Table 2.
Associations between total trihalomethanes (TTHM) in municipal drinking water during the first trimester of pregnancy and major congenital malformations, expressed as odds ratios (ORs) and 95% confidence intervals (CIs) and using nonchlorinated densely populated areas as reference.
| Groups of congenital malformations | Categories | Total births () | Nonchlorinated (OR) | TTHM/L [OR (95% CI)] | TTHM/L [OR (95% CI)] | TTHM/L [OR (95% CI)] | ||
|---|---|---|---|---|---|---|---|---|
| Nervous system | All | Cases () | 623,468 | 37 | 129 | 67 | 58 | 0.5 |
| Noncases () | 65,117 | 289,786 | 179,115 | 89,159 | ||||
| Crude | 1.00 (Ref) | 0.78 (0.54, 1.13) | 0.66 (0.44, 0.98) | 1.14 (0.76, 1.73) | ||||
| Multivariable-adjusted | 1.00 (Ref) | 0.87 (0.56, 1.34) | 0.71 (0.44, 1.14) | 1.27 (0.78, 2.05) | ||||
| Hypochlorite | Cases () | 198,225 | 37 | 22 | 30 | 20 | 0.3 | |
| Noncases () | 65,117 | 30,861 | 54,114 | 48,024 | ||||
| Crude | 1.00 (Ref) | 1.26 (0.74, 2.13) | 0.98 (0.60, 1.58) | 0.73 (0.43, 1.26) | ||||
| Multivariable-adjusted | 1.00 (Ref) | 1.35 (0.75, 2.45) | 1.07 (0.62, 1.84) | 0.73 (0.39, 1.37) | ||||
| Chloramine | Cases () | 312,651 | 37 | 78 | 9 | 36 | 0.006 | |
| Noncases () | 65,117 | 188,350 | 18,925 | 40,099 | ||||
| Crude | 1.00 (Ref) | 0.73 (0.49, 1.08) | 0.84 (0.40, 1.73) | 1.58 (0.99, 2.50) | ||||
| Multivariable-adjusted | 1.00 (Ref) | 0.85 (0.53, 1.36) | 0.89 (0.35, 2.21) | 1.82 (1.07, 3.12) | ||||
| Heart defects | All | Cases () | 623,468 | 586 | 2,218 | 1,185 | 723 | 0.002 |
| Noncases () | 64,568 | 287,697 | 177,997 | 88,494 | ||||
| Crude | 1.00 (Ref) | 0.85 (0.77, 0.93) | 0.73 (0.66, 0.81) | 0.90 (0.81, 1.00) | ||||
| Multivariable-adjusted | 1.00 (Ref) | 0.82 (0.74, 0.91) | 0.70 (0.63, 0.79) | 0.87 (0.77, 0.99) | ||||
| Hypochlorite | Cases () | 198,225 | 586 | 262 | 453 | 372 | 0.03 | |
| Noncases () | 64,568 | 30,621 | 53,691 | 47,672 | ||||
| Crude | 1.00 (Ref) | 0.94 (0.81, 1.09) | 0.93 (0.82, 1.05) | 0.86 (0.75, 0.98) | ||||
| Multivariable-adjusted | 1.00 (Ref) | 0.91 (0.78, 1.07) | 0.92 (0.81, 1.05) | 0.85 (0.74, 0.98) | ||||
| Chloramine | Cases () | 312,651 | 586 | 1,355 | 139 | 345 | 0.8 | |
| Noncases () | 64,568 | 187,073 | 18,795 | 39,790 | ||||
| Crude | 1.00 (Ref) | 0.80 (0.72, 0.88) | 0.81 (0.68, 0.98) | 0.96 (0.84, 1.09) | ||||
| Multivariable-adjusted | 1.00 (Ref) | 0.75 (0.67, 0.85) | 0.74 (0.60, 0.91) | 0.92 (0.79, 1.06) | ||||
| Orofacial clefts | All | Cases () | 623,468 | 107 | 385 | 246 | 117 | 0.3 |
| Noncases () | 65,047 | 289,530 | 178,936 | 89,100 | ||||
| Crude | 1.00 (Ref) | 0.81 (0.65, 1.00) | 0.84 (0.66, 1.05) | 0.80 (0.61, 1.04) | ||||
| Multivariable-adjusted | 1.00 (Ref) | 0.84 (0.66, 1.06) | 0.86 (0.67, 1.11) | 0.85 (0.64, 1.13) | ||||
| Hypochlorite | Cases () | 198,225 | 107 | 44 | 87 | 64 | 0.4 | |
| Noncases () | 65,047 | 30,839 | 54,057 | 47,980 | ||||
| Crude | 1.00 (Ref) | 0.87 (0.61, 1.23) | 0.98 (0.74, 1.30) | 0.81 (0.59, 1.11) | ||||
| Multivariable-adjusted | 1.00 (Ref) | 0.88 (0.61, 1.28) | 0.94 (0.69, 1.27) | 0.87 (0.63, 1.20) | ||||
| Chloramine | Cases () | 312,651 | 107 | 247 | 27 | 51 | 0.4 | |
| Noncases () | 65,047 | 188,181 | 18,907 | 40,084 | ||||
| Crude | 1.00 (Ref) | 0.80 (0.63, 1.00) | 0.87 (0.57, 1.33) | 0.77 (0.55, 1.08) | ||||
| Multivariable-adjusted | 1.00 (Ref) | 0.83 (0.64, 1.08) | 0.95 (0.61, 1.49) | 0.80 (0.55, 1.15) | ||||
| Digestive system | All | Cases () | 623,468 | 87 | 312 | 198 | 132 | 0.3 |
| Noncases () | 65,067 | 289,603 | 178,984 | 89,085 | ||||
| Crude | 1.00 (Ref) | 0.81 (0.63, 1.03) | 0.83 (0.64, 1.07) | 1.11 (0.84, 1.46) | ||||
| Multivariable-adjusted | 1.00 (Ref) | 0.90 (0.68, 1.18) | 0.89 (0.66, 1.19) | 1.20 (0.88, 1.64) | ||||
| Hypochlorite | Cases () | 198,225 | 87 | 37 | 70 | 77 | 0.2 | |
| Noncases () | 65,067 | 30,846 | 54,074 | 47,967 | ||||
| Crude | 1.00 (Ref) | 0.90 (0.61, 1.32) | 0.97 (0.70, 1.33) | 1.20 (0.88, 1.64) | ||||
| Multivariable-adjusted | 1.00 (Ref) | 0.94 (0.62, 1.44) | 0.96 (0.67, 1.37) | 1.25 (0.88, 1.77) | ||||
| Chloramine | Cases () | 312,651 | 87 | 202 | 14 | 54 | 0.5 | |
| Noncases () | 65,067 | 188,226 | 18,920 | 40,081 | ||||
| Crude | 1.00 (Ref) | 0.80 (0.62, 1.04) | 0.55 (0.31, 0.98) | 1.01 (0.72, 1.42) | ||||
| Multivariable-adjusted | 1.00 (Ref) | 0.92 (0.68, 1.23) | 0.62 (0.33, 1.17) | 1.14 (0.78, 1.68) | ||||
| Urinary | All | Cases () | 623,468 | 101 | 932 | 259 | 195 | 0.2 |
| Noncases () | 65,053 | 288,983 | 178,923 | 89,022 | ||||
| Crude | 1.00 (Ref) | 2.08 (1.69, 2.55) | 0.93 (0.74, 1.17) | 1.41 (1.11, 1.80) | ||||
| Multivariable-adjusted | 1.00 (Ref) | 2.15 (1.70, 2.73) | 0.95 (0.73, 1.23) | 1.44 (1.10, 1.89) | ||||
| Hypochlorite | Cases () | 198,225 | 101 | 63 | 72 | 62 | 0.2 | |
| Noncases () | 65,053 | 30,820 | 54,072 | 47,982 | ||||
| Crude | 1.00 (Ref) | 1.31 (0.96, 1.80) | 0.86 (0.63, 1.16) | 0.83 (0.61, 1.14) | ||||
| Multivariable-adjusted | 1.00 (Ref) | 1.39 (0.99, 1.94) | 0.88 (0.64, 1.22) | 0.90 (0.65, 1.26) | ||||
| Chloramine | Cases () | 312,651 | 101 | 668 | 66 | 128 | 0.001 | |
| Noncases () | 65,053 | 187,760 | 18,868 | 40,007 | ||||
| Crude | 1.00 (Ref) | 2.29 (1.86, 2.83) | 2.25 (1.65, 3.07) | 2.06 (1.58, 2.68) | ||||
| Multivariable-adjusted | 1.00 (Ref) | 2.37 (1.85, 3.04) | 2.33 (1.64, 3.29) | 2.06 (1.53, 2.78) | ||||
| Genital | All | Cases () | 623,468 | 161 | 843 | 491 | 325 | 0.01 |
| Noncases () | 46,993 | 289,072 | 178,691 | 88,892 | ||||
| Crude | 1.00 (Ref) | 1.18 (0.99, 1.40) | 1.11 (0.93, 1.33) | 1.48 (1.22, 1.79) | ||||
| Multivariable-adjusted | 1.00 (Ref) | 1.18 (0.97, 1.42) | 1.14 (0.93, 1.39) | 1.47 (1.18, 1.81) | ||||
| Hypochlorite | Cases () | 198,225 | 161 | 97 | 167 | 145 | 0.09 | |
| Noncases () | 46,993 | 30,786 | 53,977 | 47,899 | ||||
| Crude | 1.00 (Ref) | 1.27 (0.99, 1.64) | 1.25 (1.00, 1.55) | 1.22 (0.97, 1.53) | ||||
| Multivariable-adjusted | 1.00 (Ref) | 1.36 (1.04, 1.77) | 1.30 (1.03, 1.64) | 1.24 (0.97, 1.58) | ||||
| Chloramine | Cases () | 312,651 | 161 | 544 | 63 | 178 | ||
| Noncases () | 64,993 | 187,884 | 18,871 | 39,957 | ||||
| Crude | 1.00 (Ref) | 1.17 (0.98, 1.40) | 1.35 (1.01, 1.81) | 1.80 (1.45, 2.23) | ||||
| Multivariable-adjusted | 1.00 (Ref) | 1.15 (0.93, 1.41) | 1.45 (1.05, 1.98) | 1.77 (1.38, 2.26) | ||||
| Limbs | All | Cases () | 623,468 | 274 | 1,299 | 742 | 430 | 0.3 |
| Noncases () | 64,880 | 288,616 | 178,440 | 88,787 | ||||
| Crude | 1.00 (Ref) | 1.07 (0.93, 1.22) | 0.98 (0.86, 1.13) | 1.15 (0.98, 1.34) | ||||
| Multivariable-adjusted | 1.00 (Ref) | 1.04 (0.89, 1.20) | 0.95 (0.82, 1.11) | 1.14 (0.96, 1.34) | ||||
| Hypochlorite | Cases () | 198,225 | 274 | 146 | 230 | 194 | 0.5 | |
| Noncases () | 64,880 | 30,737 | 53,914 | 47,850 | ||||
| Crude | 1.00 (Ref) | 1.12 (0.92, 1.38) | 1.01 (0.85, 1.21) | 0.96 (0.80, 1.16) | ||||
| Multivariable-adjusted | 1.00 (Ref) | 1.12 (0.90, 1.38) | 0.98 (0.82, 1.19) | 0.96 (0.79, 1.17) | ||||
| Chloramine | Cases () | 312,651 | 274 | 864 | 91 | 232 | 0.02 | |
| Noncases () | 64,880 | 187,564 | 18,843 | 39,903 | ||||
| Crude | 1.00 (Ref) | 1.09 (0.95, 1.25) | 1.14 (0.90, 1.45) | 1.38 (1.15, 1.64) | ||||
| Multivariable-adjusted | 1.00 (Ref) | 1.06 (0.91, 1.25) | 1.02 (0.78, 1.33) | 1.34 (1.10, 1.64) | ||||
| Chromosomal | All | Cases () | 623,468 | 88 | 381 | 229 | 119 | 0.6 |
| Noncases () | 65,066 | 289,534 | 178,953 | 89,098 | ||||
| Crude | 1.00 (Ref) | 0.97 (0.77, 1.23) | 0.95 (0.74, 1.21) | 0.99 (0.75, 1.30) | ||||
| Multivariable-adjusted | 1.00 (Ref) | 0.87 (0.67, 1.14) | 0.87 (0.66, 1.15) | 0.92 (0.68, 1.25) | ||||
| Hypochlorite | Cases () | 198,225 | 88 | 39 | 61 | 68 | 0.8 | |
| Noncases () | 65,066 | 30,844 | 54,083 | 47,976 | ||||
| Crude | 1.00 (Ref) | 0.93 (0.64, 1.37) | 0.83 (0.60, 1.16) | 1.05 (0.76, 1.44) | ||||
| Multivariable-adjusted | 1.00 (Ref) | 0.87 (0.57, 1.31) | 0.81 (0.56, 1.15) | 0.99 (0.70, 1.40) | ||||
| Chloramine | Cases () | 312,651 | 88 | 245 | 24 | 49 | 0.6 | |
| Noncases () | 65,066 | 188,183 | 18,910 | 40,086 | ||||
| Crude | 1.00 (Ref) | 0.96 (0.75, 1.23) | 0.94 (0.60, 1.48) | 0.90 (0.64, 1.28) | ||||
| Multivariable-adjusted | 1.00 (Ref) | 0.83 (0.62, 1.10) | 0.66 (0.38, 1.13) | 0.89 (0.61, 1.30) |
Note: The study included a total of 623,468 children born during 2005–2015 to mothers living in large urban areas ( inhabitants) in Sweden. Models multivariable-adjusted for the following factors: maternal age, BMI, diabetes, any use of teratogenic drugs, parity, smoking at registration to the antenatal care, highest attained education, and household income using inverse probability weighting. BMI, body mass index; Ref, reference; TTHM, average of the four most common trihalomethanes (total trihalomethanes).
On the other hand, comparing the highest exposed mothers ( TTHM/L) to those unexposed (nonchlorinated areas), a significant inverse association was observed for heart defects among the newborns, multivariable-adjusted (95% CI: 0.77, 0.99; ; Table 2). In the chlorination treatment-specific analyses, the inverse association remained only for areas using hypochlorite. However, when the lowest TTHM-exposed area ( TTHM/L) was used as a reference, this association was attenuated and a significant positive association was observed among areas using chloramine, multivariable-adjusted (95% CI: 1.09, 1.41; ; Table S2). No clear indications of any associations were observed for the development of orofacial clefts, malformations of the digestive system, or chromosomal abnormalities.
Discussion
In this large-scale nationwide register-based cohort including over 620,000 newborns of mothers living in areas provided with municipal drinking water, we assessed maternal drinking water-related TTHM exposure during pregnancy and subsequent congenital malformations. Based on the first trimester average TTHM tap water concentrations, we observed an increased risk of malformations of the urinary system and of the genitals among newborns of mothers in the highest exposure category. When analyses were separated by type of chlorination treatment, TTHM was associated with an increased risk of malformations of the nervous system, urinary system, genitals, and limbs in areas using chloramine, but not in areas using exclusively hypochlorite as the primary water treatment method. Except for malformations of the urinary system, these associations were robust and remained after changing the reference from the unexposed women to the lowest exposure category ( TTHM/L). The potential association between TTHM exposure and congenital heart defects was unstable—pointing mainly toward an inverse association—and no clear indications of existing links were observed for the development of orofacial clefts, malformations of the digestive systems, or chromosomal abnormalities.
Based on the most recent meta-analyses of epidemiological studies, evidence of an association between TTHM and congenital malformations is limited (Nieuwenhuijsen et al. 2009). Although some previous studies have reported TTHM exposure (or the use of chlorination as such) to be associated with an increased risk of malformation of the nervous system (Bove et al. 1995; Dodds and King 2001; Klotz and Pyrch 1999), urogenital system (Grazuleviciene et al. 2013; Magnus et al. 1999), and limbs (Kaufman et al. 2020), most studies have reported null findings overall (Chisholm et al. 2008; Dodds et al. 1999; Hwang et al. 2002, 2008; Iszatt et al. 2011; Källén and Robert 2000; Luben et al. 2008; Nieuwenhuijsen et al. 2008; Shaw et al. 2003).
As reviewed by Graves et al. (2001), animal studies lend no support to a suggested association between TTHM exposure and congenital malformations. From animal studies, especially studies on the rat, indications of teratogenic effects affecting heart, limbs, kidney, or urogenital malformations have, however, been observed for CBPs other than TTHM, with the strongest support existing for haloacetic acids and haloacetonitriles (Graves et al. 2001). Nevertheless, even these findings were mainly inconsistent (Graves et al. 2001) and the mechanisms are not yet fully understood (Colman et al. 2011). A proposed pathway is, however, that some CBPs may inhibit methionine synthesis, affecting folic acid metabolism (Dow and Green 2000), which is commonly linked to the development of, for example, neural tube defects. These suggested teratogenic CBPs were not measured in the municipal exposure monitoring programs used in the present study, and thus we cannot exclude that these CBPs were present in the drinking water. Because the method of chlorination treatment affects the types and amount of CBPs formed (Amy et al. 2000), we performed separate analyses for hypochlorite and chloramine, to provide additional support in the interpretations of the findings. Accordingly, we observed treatment-specific differences given that associations were observed for malformations of the nervous system, urinary system, genitals, and limbs in areas using chloramine exclusively, but not hypochlorite. Hypothetically, this could be a result of a proportionally higher formation of potentially teratogenic CBPs, such as haloacetic acids, in areas using chloramine, as compared with hypochlorite, despite similar detected TTHM levels (Sakai et al. 2016). Considering the limited combined evidence from animal and epidemiological studies, we can only speculate on the potential reason behind the chlorination treatment-specific associations observed for chloramine in this study. One potential explanation could be concomitant exposure to CBPs other than those included in the TTHM definition. Alternatively, the chlorinated drinking water may have contained higher levels of single THMs, rather than TTHM, which we were not able to separately account for in this study.
Of all malformations, congenital heart defects have the strongest support for an association with CBP exposure based on experimental and epidemiological studies (Graves et al. 2001; Nieuwenhuijsen et al. 2009). In the present study, however, we observed an inverse association for TTHM exposure and heart defects. One possible explanation for this inverse association observed could be selection bias, commonly introduced when birth records of live births are used (Hernán et al. 2002). Although we were able to include both live and stillbirths in our cohort, there is no information on miscarriage or termination of pregnancies available in the Medical Birth Register. Malformation is common among early pregnancy losses (Philipp et al. 2003), thus any differences in the area-dependent rate of miscarriages or termination of pregnancies due to malformations could also introduce a bias. Although there is no information on the rate of miscarriages, there are indications of regional variations in the rate of termination of pregnancies due to severe malformations in Sweden (NBHW 2018). In some regions with larger cities, there is a higher rate of pregnancies terminated due to some malformations, especially heart defects, compared with less densely populated regions. Potential region-dependent differences in termination of pregnancies may be a reason to the inverse association indicated for heart defects. Unmeasured confounding, such as exposures to teratogens (i.e., chemicals, pathogens, or other stressors) resulting in chlorination- or locality-related differences in, for example, maternal infections could also be an explanation (Ye et al. 2019). Yet, despite the effort to scrutinize the different scenarios, the full explanation for the inverse association observed for heart defects in the present study and whether drinking water chlorination is involved remains ambiguous, but we consider it not to be a result of TTHM exposure per se.
The limitation related to the lack of individual assessments of TTHM exposure in the present study needs special consideration. First, because of the register-based study design, we were forced to use a crude measure of the exposure, potentially resulting in exposure misclassification. However, given that it is estimated that 99.8% of the adult population in Sweden are consumers of unheated tap water (Säve-Söderbergh et al. 2018), locality-specific estimates likely provide a valid individual estimation of TTHM exposure via the consumption of tap water. Still, we cannot fully exclude that there could be regional differences in individual consumption patterns or modification of the consumption due to pregnancy.
Moreover, TTHM exposure unrelated to the consumption of drinking water could also be important, such as exposure via the lung and skin during various water-related activities (e.g., showering) (Backer et al. 2000; Xu et al. 2002). Although we have no information on these activities in our study population, we can still assume that it is likely that women in the unexposed area were exposed to chlorinated water to a much lesser extent, as compared with women living in the exposed areas. An additional asset in reducing exposure misclassification was the availability of data enabling the exclusion of women with high occupational TTHM exposure (professional swimmers or coaches and swimming pool personnel). Second, we used a locality-specific, multiannual, and trimester-specific average of TTHM exposure. This may not fully capture the true external exposure in each first trimester but both handles potential missing exposure data due to variations in municipal sampling strategies (i.e., potentially leading to dropout of pregnancies) and reduces the impact of single extreme values. This strategy has evolved owing to the fact that season is by far the most important determinant of TTHM formation in Northern Europe, peaking in spring and fall (when the natural organic matter content is higher due to rainfalls, snow melting, and low water temperature). Thus, a multiannual monthly TTHM average—as compared with using average of consecutive months—will better capture the trimester-specific exposure.
The use of a single locality-specific average was supported by the fact that most water treatment plants were small, with a fairly rapid turnover time in the drinking water distribution systems, resulting in a low spatial TTHM variation. Thus, although certain exposure misclassification is inevitable in the exposed populations, the inclusion of an unexposed reference area with nonchlorinated drinking water likely considerably reduces its overall impact.
We included only malformations diagnosed up to 28 days postpartum. Although most severe cases are diagnosed during this period, assuring a high specificity, some less severe cases are likely diagnosed later in life. Still, the overall prevalence of major congenital malformations was 2 cases/100 births (Table S1), which agrees well with the reported national prevalence (2.1 cases/100 births, excluding terminated pregnancies) during 2007–2015 (EUROCAT 2020).
The present study has several strengths. To begin with, this is one of the largest prospective studies assessing TTHM exposure and the risk of congenital malformations. The Swedish health care and administrative registers that were included have a coverage of close to 100% (Källén and Källén 2003; Ludvigsson et al. 2016), reducing the risk of selection bias due to missing data. This is explained by the publicly funded antenatal, delivery, and pediatric care and the mandatory reporting to the registers. In addition, the exclusive use of a unique personal identification number in both health care and administrative registers enabled data linkage with high reliability, including information on migratory patterns during gestation, which previous studies were not able to take into account. We observed a high migration rate among the pregnant women, in line with previous findings (Miller et al. 2010), highlighting the relevance of obtaining this information. The extensive data collected in the registers enabled us to adjust for most relevant individual confounders. Although we cannot rule out unmeasured confounders, because the confounders had little impact on the risk estimates, it is unlikely that these potential unmeasured confounders would have affected the results. There may be exceptions, however, such as differences in the use of folic acid, which was not included in any of the models in the present study because it is estimated to be highly underreported in the Medical Birth Register (NBHW 2018). The use of folic acid supplements during early gestation is recommended by authorities because it prevents the formation of malformations, such as neural tube defects (Czeizel et al. 2013), but the population uptake likely varies by socioeconomic factors (Murto et al. 2017). We handled this by controlling for several individual socioeconomic-related factors (e.g., highest attained educational level, household income) as surrogate confounders. In addition, the prevalence of neural tube defects was low in our population ( births), indicating that it is unlikely that folic acid would be an important risk factor.
In conclusion, we observed that first trimester maternal TTHM exposure was associated with the risk of malformations of the nervous and urinary systems, as well as of the genitals and limbs, among the newborns in areas using chloramine as drinking water treatment. There were no indications that TTHM in areas using hypochlorite was associated with congenital malformations among the newborns. This may indicate that chlorination treatment-specific levels of single THMs or other CBPs that are not included in the TTHM definition were formed when treating drinking water with chloramine may be the main CBPs responsible for the associations observed with increased risk of congenital malformations. Congenital malformations linked to a CBP originating from chloramine use has not previously been highlighted, and there is a clear need for further attention.
Supplementary Material
Acknowledgments
This study was funded by the Swedish Research Council Formas (grant 942-2015-425).
References
- Amy G, Bull R, Graun GF, Siddiqui M. 2000. Environmental Health Criteria 216: Disinfectants and Disinfectant By-Products. Geneva, Switzerland: World Health Organization. https://www.who.int/ipcs/publications/ehc/216_disinfectants_part_1.pdf [accessed 17 September 2021]. [Google Scholar]
- Andersson A, Harir M, Gonsior M, Hertkorn N, Schmitt-Kopplin P, Kylin H, et al. 2019. Waterworks-specific composition of drinking water disinfection by-products. Environ Sci (Camb) 5(5):861–872, 10.1039/C9EW00034H. [DOI] [Google Scholar]
- Backer LC, Ashley DL, Bonin MA, Cardinali FL, Kieszak SM, Wooten JV. 2000. Household exposures to drinking water disinfection by-products: whole blood trihalomethane levels. J Expo Anal Environ Epidemiol 10(4):321–326, PMID: 10981726, 10.1038/sj.jea.7500098. [DOI] [PubMed] [Google Scholar]
- Bove FJ, Fulcomer MC, Klotz JB, Esmart J, Dufficy EM, Savrin JE. 1995. Public drinking water contamination and birth outcomes. Am J Epidemiol 141(9):850–862, PMID: 7717362, 10.1093/oxfordjournals.aje.a117521. [DOI] [PubMed] [Google Scholar]
- Cao W-C, Zeng Q, Luo Y, Chen H-X, Miao D-Y, Li L, et al. 2016. Blood biomarkers of late pregnancy exposure to trihalomethanes in drinking water and fetal growth measures and gestational age in a Chinese cohort. Environ Health Perspect 124(4):536–541, PMID: 26340795, 10.1289/ehp.1409234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cedergren MI, Selbing AJ, Löfman O, Källen BA. 2002. Chlorination byproducts and nitrate in drinking water and risk for congenital cardiac defects. Environ Res 89(2):124–130, PMID: 12123645, 10.1006/enrs.2001.4362. [DOI] [PubMed] [Google Scholar]
- Chisholm K, Cook A, Bower C, Weinstein P. 2008. Risk of birth defects in Australian communities with high levels of brominated disinfection by-products. Environ Health Perspect 116(9):1267–1273, PMID: 18795174, 10.1289/ehp.10980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman J, Rice GE, Wright JM, Hunter ES III, Teuschler LK, Lipscomb JC, et al. 2011. Identification of developmentally toxic drinking water disinfection byproducts and evaluation of data relevant to mode of action. Toxicol Appl Pharmacol 254(2):100–126, PMID: 21296098, 10.1016/j.taap.2011.02.002. [DOI] [PubMed] [Google Scholar]
- Czeizel AE, Dudás I, Vereczkey A, Bánhidy F. 2013. Folate deficiency and folic acid supplementation: the prevention of neural-tube defects and congenital heart defects. Nutrients 5(11):4760–4775, PMID: 24284617, 10.3390/nu5114760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodds L, King WD. 2001. Relation between trihalomethane compounds and birth defects. Occup Environ Med 58(7):443–446, PMID: 11404448, 10.1136/oem.58.7.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodds L, King W, Woolcott C, Pole J. 1999. Trihalomethanes in public water supplies and adverse birth outcomes. Epidemiology 10(3):233–237, PMID: 10230830, 10.1097/00001648-199905000-00007. [DOI] [PubMed] [Google Scholar]
- Dow JL, Green T. 2000. Trichloroethylene induced vitamin B12 and folate deficiency leads to increased formic acid excretion in the rat. Toxicology 146(2–3):123–136, PMID: 10814845, 10.1016/S0300-483X(00)00156-6. [DOI] [PubMed] [Google Scholar]
- EUROCAT (European Surveillance of Congenital Anomalies). 2014. Guide 1.4, Chapter 3.3: 3.3 EUROCAT Subgroups of Congenital Anomalies (Version 2014; implemented in EDMP December 2014, used for website prevalence tables from December 2014). https://eu-rd-platform.jrc.ec.europa.eu/sites/default/files/EUROCAT-Guide-1.4-Section-3.3.pdf [accessed 17 September 2021].
- EUROCAT. 2020. Prevalence charts and tables. https://eu-rd-platform.jrc.ec.europa.eu/eurocat/eurocat-data/prevalence_en [accessed 1 December 2020].
- Graves CG, Matanoski GM, Tardiff RG. 2001. Weight of evidence for an association between adverse reproductive and developmental effects and exposure to disinfection by-products: a critical review. Regul Toxicol Pharmacol 34(2):103–124, PMID: 11603954, 10.1006/rtph.2001.1494. [DOI] [PubMed] [Google Scholar]
- Grazuleviciene R, Kapustinskiene V, Vencloviene J, Buinauskiene J, Nieuwenhuijsen MJ. 2013. Risk of congenital anomalies in relation to the uptake of trihalomethane from drinking water during pregnancy. Occup Environ Med 70(4):274–282, PMID: 23404756, 10.1136/oemed-2012-101093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grazuleviciene R, Nieuwenhuijsen MJ, Vencloviene J, Kostopoulou-Karadanelli M, Krasner SW, Danileviciute A, et al. 2011. Individual exposures to drinking water trihalomethanes, low birth weight and small for gestational age risk: a prospective Kaunas cohort study. Environ Health 10:32, PMID: 21501533, 10.1186/1476-069X-10-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernán MA, Hernández-Díaz S, Werler MM, Mitchell AA. 2002. Causal knowledge as a prerequisite for confounding evaluation: an application to birth defects epidemiology. Am J Epidemiol 155(2):176–184, PMID: 11790682, 10.1093/aje/155.2.176. [DOI] [PubMed] [Google Scholar]
- Hernán M, Robins J. 2020. Causal Inference: What If. Boca Raton, FL: Chapman & Hall/CRC. [Google Scholar]
- Hinckley AF, Bachand AM, Reif JS. 2005. Late pregnancy exposures to disinfection by-products and growth-related birth outcomes. Environ Health Perspect 113(12):1808–1813, PMID: 16330369, 10.1289/ehp.8282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang B-F, Jaakkola JJ, Guo H-R. 2008. Water disinfection by-products and the risk of specific birth defects: a population-based cross-sectional study in Taiwan. Environ Health 7:23, PMID: 18518952, 10.1186/1476-069X-7-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang B-F, Magnus P, Jaakkola JJ. 2002. Risk of specific birth defects in relation to chlorination and the amount of natural organic matter in the water supply. Am J Epidemiol 156(4):374–382, PMID: 12181108, 10.1093/aje/kwf038. [DOI] [PubMed] [Google Scholar]
- Iszatt N, Nieuwenhuijsen MJ, Nelson P, Elliott P, Toledano MB. 2011. Water consumption and use, trihalomethane exposure, and the risk of hypospadias. Pediatrics 127(2):e389–e397, PMID: 21220402, 10.1542/peds.2009-3356. [DOI] [PubMed] [Google Scholar]
- Källén B, Källén K. 2003. The Swedish Medical Birth Register—A Summary of Content and Quality. 2003-112-3. Stockholm, Sweden: Socialstyrelsen. http://www.socialstyrelsen.se/NR/rdonlyres/E9BE4DDE-95EE-4E3F-A56F-36CA5125CA8C/1132/20031123.pdf [accessed 17 September 2021]. [Google Scholar]
- Källén BA, Robert E. 2000. Drinking water chlorination and delivery outcome—a registry-based study in Sweden. Reprod Toxicol 14(4):303–309, PMID: 10908833, 10.1016/s0890-6238(00)00086-1. [DOI] [PubMed] [Google Scholar]
- Kaufman JA, Wright JM, Evans A, Rivera-Núñez Z, Meyer A, Narotsky MG. 2020. Disinfection by-product exposures and the risk of musculoskeletal birth defects. Environ Epidemiol 4(1):e081, PMID: 32154492, 10.1097/EE9.0000000000000081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz JB, Pyrch LA. 1999. Neural tube defects and drinking water disinfection by-products. Epidemiology 10(4):383–390, PMID: 10401872, 10.1097/00001648-199907000-00008. [DOI] [PubMed] [Google Scholar]
- Kramer MD, Lynch CF, Isacson P, Hanson JW. 1992. The association of waterborne chloroform with intrauterine growth retardation. Epidemiology 3(5):407–413, PMID: 1391132, 10.1097/00001648-199209000-00005. [DOI] [PubMed] [Google Scholar]
- Levallois P, Gingras S, Marcoux S, Legay C, Catto C, Rodriguez M, et al. 2012. Maternal exposure to drinking-water chlorination by-products and small-for-gestational-age neonates. Epidemiology 23(2):267–276, PMID: 22317810, 10.1097/EDE.0b013e3182468569. [DOI] [PubMed] [Google Scholar]
- Lewis C, Suffet IH, Ritz B. 2006. Estimated effects of disinfection by-products on birth weight in a population served by a single water utility. Am J Epidemiol 163(1):38–47, PMID: 16282238, 10.1093/aje/kwj009. [DOI] [PubMed] [Google Scholar]
- Luben TJ, Nuckols JR, Mosley BS, Hobbs C, Reif JS. 2008. Maternal exposure to water disinfection by-products during gestation and risk of hypospadias. Occup Environ Med 65(6):420–429, PMID: 18032532, 10.1136/oem.2007.034256. [DOI] [PubMed] [Google Scholar]
- Ludvigsson JF, Almqvist C, Bonamy A-KE, Ljung R, Michaëlsson K, Neovius M, et al. 2016. Registers of the Swedish total population and their use in medical research. Eur J Epidemiol 31(2):125–136, PMID: 26769609, 10.1007/s10654-016-0117-y. [DOI] [PubMed] [Google Scholar]
- Magnus P, Jaakkola JJ, Skrondal A, Alexander J, Becher G, Krogh T, et al. 1999. Water chlorination and birth defects. Epidemiology 10(5):513–517, PMID: 10468423, 10.1097/00001648-199909000-00008. [DOI] [PubMed] [Google Scholar]
- Miller A, Siffel C, Correa A. 2010. Residential mobility during pregnancy: patterns and correlates. Matern Child Health J 14(4):625–634, PMID: 19568920, 10.1007/s10995-009-0492-z. [DOI] [PubMed] [Google Scholar]
- Moore KL, Persaud TVN, Torchia MG. 2016. The Developing Human: Clinically Oriented Embryology. 10th ed. Philadelphia, PA: Elsevier Saunders. [Google Scholar]
- Murto T, Yngve A, Skoog Svanberg A, Altmäe S, Salumets A, Wånggren K, et al. 2017. Compliance to the recommended use of folic acid supplements for women in Sweden is higher among those under treatment for infertility than among fertile controls and is also related to socioeconomic status and lifestyle. Food Nutr Res 61(1):1334483, PMID: 28659747, 10.1080/16546628.2017.1334483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NBHW (National Board of Health and Welfare). 2018. Biths defects 2016 [Fosterskador och kromosomavvikelser 2016]. https://www.socialstyrelsen.se/globalassets/sharepoint-dokument/artikelkatalog/statistik/2018-3-13.pdf.
- Nieuwenhuijsen MJ, Martinez D, Grellier J, Bennett J, Best N, Iszatt N, et al. 2009. Chlorination disinfection by-products in drinking water and congenital anomalies: review and meta-analyses. Environ Health Perspect 117(10):1486–1493, PMID: 20019896, 10.1289/ehp.0900677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuijsen MJ, Toledano MB, Bennett J, Best N, Hambly P, de Hoogh C, et al. 2008. Chlorination disinfection by-products and risk of congenital anomalies in England and Wales. Environ Health Perspect 116(2):216–222, PMID: 18288321, 10.1289/ehp.10636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nörby U, Källén K, Eiermann B, Korkmaz S, Winbladh B, Gustafsson LL. 2013. Drugs and Birth Defects: a knowledge database providing risk assessments based on national health registers. Eur J Clin Pharmacol 69(4):889–899, PMID: 23011015, 10.1007/s00228-012-1399-y. [DOI] [PubMed] [Google Scholar]
- Philipp T, Philipp K, Reiner A, Beer F, Kalousek DK. 2003. Embryoscopic and cytogenetic analysis of 233 missed abortions: factors involved in the pathogenesis of developmental defects of early failed pregnancies. Hum Reprod 18(8):1724–1732, PMID: 12871891, 10.1093/humrep/deg309. [DOI] [PubMed] [Google Scholar]
- Richardson SD, Plewa MJ, Wagner ED, Schoeny R, Demarini DM. 2007. Occurrence, genotoxicity, and carcinogenicity of regulated and emerging disinfection by-products in drinking water: a review and roadmap for research. Mutat Res 636(1–3):178–242, PMID: 17980649, 10.1016/j.mrrev.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Sakai H, Tokuhara S, Murakami M, Kosaka K, Oguma K, Takizawa S. 2016. Comparison of chlorination and chloramination in carbonaceous and nitrogenous disinfection byproduct formation potentials with prolonged contact time. Water Res 88:661–670, PMID: 26575475, 10.1016/j.watres.2015.11.002. [DOI] [PubMed] [Google Scholar]
- Säve-Söderbergh M, Toljander J, Donat-Vargas C, Berglund M, Åkesson A. 2020. Exposure to drinking water chlorination by-products and fetal growth and prematurity: a nationwide register-based prospective study. Environ Health Perspect 128(5):057006, PMID: 32438832, 10.1289/EHP6012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Säve-Söderbergh M, Toljander J, Mattisson I, Åkesson A, Simonsson M. 2018. Drinking water consumption patterns among adults—SMS as a novel tool for collection of repeated self-reported water consumption. J Expo Sci Environ Epidemiol 28(2):131–139, PMID: 28612838, 10.1038/jes.2017.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw GM, Ranatunga D, Quach T, Neri E, Correa A, Neutra RR. 2003. Trihalomethane exposures from municipal water supplies and selected congenital malformations. Epidemiology 14(2):191–199, PMID: 12606885, 10.1097/01.EDE.0000050697.18634.A6. [DOI] [PubMed] [Google Scholar]
- Smith RB, Edwards SC, Best N, Wright J, Nieuwenhuijsen MJ, Toledano MB. 2016. Birth weight, ethnicity, and exposure to trihalomethanes and haloacetic acids in drinking water during pregnancy in the Born in Bradford cohort. Environ Health Perspect 124(5):681–689, PMID: 26340797, 10.1289/ehp.1409480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller K, Swan SH, Windham GC, Fenster L. 2001. Influence of exposure assessment methods on risk estimates in an epidemiologic study of total trihalomethane exposure and spontaneous abortion. J Expo Anal Environ Epidemiol 11(6):522–531, PMID: 11791168, 10.1038/sj.jea.7500191. [DOI] [PubMed] [Google Scholar]
- WHO (World Health Organization). 2016. International Statistical Classification of Diseases and Related Health Problems, 10th Revision. http://apps.who.int/classifications/icd10/browse/2016/en [accessed 18 September 2021].
- Wright JM, Bateson TF. 2005. A sensitivity analysis of bias in relative risk estimates due to disinfection by-product exposure misclassification. J Expo Anal Environ Epidemiol 15(3):212–216, PMID: 15226753, 10.1038/sj.jea.7500389. [DOI] [PubMed] [Google Scholar]
- Wright JM, Schwartz J, Dockery DW. 2003. Effect of trihalomethane exposure on fetal development. Occup Environ Med 60(3):173–180, PMID: 12598663, 10.1136/oem.60.3.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright JM, Schwartz J, Dockery DW. 2004. The effect of disinfection by-products and mutagenic activity on birth weight and gestational duration. Environ Health Perspect 112(8):920–925, PMID: 15175183, 10.1289/ehp.6779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Mariano TM, Laskin JD, Weisel CP. 2002. Percutaneous absorption of trihalomethanes, haloacetic acids, and haloketones. Toxicol Appl Pharmacol 184(1):19–26, PMID: 12392965, 10.1006/taap.2002.9494. [DOI] [PubMed] [Google Scholar]
- Ye Z, Wang L, Yang T, Chen L, Wang T, Zhao L, et al. 2019. Maternal viral infection and risk of fetal congenital heart diseases: a meta-analysis of observational studies. J Am Heart Assoc 8(9):e011264, PMID: 30995883, 10.1161/JAHA.118.011264. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.