Abstract
Background:
Per- and polyfluoroalkyl substances (PFAS) may impair bone accrual and strength via endocrine disruption and nuclear receptor agonism, but human studies are primarily of adults or cross-sectional.
Objectives:
We assessed associations of individual PFAS and their mixture during pregnancy with child bone mineral content (BMC) and areal bone mineral density (aBMD) at age 12 y.
Methods:
Among 206 mother–child pairs enrolled in a prospective cohort (2003–2006), we quantified perfluorooctanoic acid (PFOA), perfluorononanoic acid (PFNA), perfluorohexane sulfonic acid (PFHxS), and perfluorooctane sulfonic acid (PFOS) in maternal serum collected during gestation or delivery. When children were age 12 y, we performed dual energy X-ray absorptiometry and calculated BMC, aBMD, and bone mineral apparent density (BMAD) -scores for six skeletal sites. We estimated covariate-adjusted -score differences per doubling of individual PFAS using linear regression and assessed the PFAS mixture using quantile g-computation and Bayesian kernel machine regression. We explored whether associations were modified by child’s sex or mediated by whole-body lean mass.
Results:
In covariate-adjusted models, we found that higher maternal serum concentrations of PFOA, PFNA, and the PFAS mixture were associated with lower total hip and forearm (one-third distal radius) BMC -scores in children. Differences in forearm BMC -scores were [95% confidence interval (CI): , 0.01] and (95% CI: , ) per doubling of PFOA and PFNA, respectively, and (95% CI: , ) per quartile increase in the PFAS mixture. Child’s sex modified PFOA associations for some skeletal sites; for example, differences in spine BMAD -score per doubling were (95% CI: , ) among males and 0.07 (95% CI: , 0.30) among females (modification ). Except for PFNA among females, these associations were not mediated by whole-body lean mass.
Discussion:
Maternal PFAS concentrations during pregnancy may be associated with lower bone mineral accrual and strength in early adolescence. https://doi.org/10.1289/EHP9424
Introduction
Bone mass in adolescence is a determinant of fractures in adolescence (Clark et al. 2006; Kalkwarf et al. 2011), peak bone mass in early adulthood (Weaver et al. 2016), and the development of osteoporosis later in life (Wright et al. 2014). Low bone mineral density (BMD) and osteoporosis affect more than U.S. adults over age 50 y (Wright et al. 2014), with more than fractures occurring annually in people with osteoporosis (Haentjens et al. 2010; Singer et al. 2015). Because bone mass accrual increases rapidly during adolescence and peaks within a few years of puberty, achieving optimal bone mass during this developmental period is vital to lifelong bone health (Weaver et al. 2016). Bone acquisition begins before birth (Harrast and Kalkwarf 1998), but few human studies have evaluated the influence of environmental chemical exposures during gestation on bone accrual and strength later in life (Jeddy et al. 2018; van Zwol-Janssens et al. 2020).
Per- and polyfluoroalkyl substances (PFAS) are a class of thousands of fluorinated chemicals used in diverse applications, such as stain- and water-resistant coatings for textiles, nonstick cookware, food container coatings, floor polish, fire-fighting foam, and industrial surfactants (Buck et al. 2011; EFSA 2008). PFAS bioaccumulate in the environment, and primary routes of human exposures include oral ingestion of contaminated food, water, and dust (Fromme et al. 2009). Half-lives in humans range from 2 to 9 y for long-alkyl chain PFAS, including perfluorooctanoic acid (PFOA), perfluorononanoic acid (PFNA), perfluorohexane sulfonic acid (PFHxS), and perfluorooctane sulfonic acid (PFOS) (Li et al. 2018; Olsen et al. 2007). These four PFAS, which are detected in the serum of more than 95% of Americans (CDC 2019; Kato et al. 2011), persist in humans (Buck et al. 2011) and have been detected in bone (Koskela et al. 2017; Pérez et al. 2013). In addition, PFAS have been detected in fetal tissue, and PFOA, PFOS, and PFNA have been detected in placenta (Mamsen et al. 2019).
PFAS may act as environmental osteotoxicants, chemicals that adversely affect skeletal tissue, causing deficits in bone morphogenesis, mineralization, or remodeling (Fernández et al. 2018). Two in vitro studies reported concentration-dependent changes in osteoblast and osteoclast differentiation of bone marrow stromal cells treated with PFOA (Koskela et al. 2016, 2017). In female mice, PFOA exposure during gestation and lactation caused decreased cortical tibia BMD that persisted into adulthood (Koskela et al. 2016).
PFAS exposures have been associated with lower BMD and related bone measures in cross-sectional studies of children (Cluett et al. 2019; Khalil et al. 2018) and adults (Di Nisio et al. 2020a; Hu et al. 2019; Khalil et al. 2016; Lin et al. 2014). In a prospective study of 294 adults age 30–70 y, higher plasma concentrations of PFOS, PFNA, and perfluorodecanoic acid at baseline were associated with faster rate of total hip BMD loss over 2 y (Hu et al. 2019). In a prospective study of PFAS exposures during pregnancy, higher maternal serum PFAS concentrations were associated with lower whole-body bone mineral content (BMC), areal BMD (aBMD), and bone area among 17-y-old females (Jeddy et al. 2018). Estimates were attenuated on adjustment for lean body mass (Jeddy et al. 2018), which may mediate associations, given that maternal PFAS concentrations during pregnancy have been associated with offspring body composition (Braun 2017, 2020b; Liu et al. 2020). The Jeddy et al. study was limited to females and unable to evaluate possible sex differences that may be expected due to endocrine activity of PFAS (Jensen and Leffers 2008) and sexually dimorphic patterns of skeletal development (Weaver et al. 2016).
Our objective was to assess associations of serum concentrations of individual PFAS and their mixture, measured during pregnancy, with BMC and BMD at multiple skeletal sites among 12-y-old children in the Health Outcomes and Measures of the Environment (HOME) Study. We also explored whether associations were modified by child’s sex or mediated by whole-body lean mass.
Methods
Study Design and Population
The HOME Study is an ongoing prospective cohort study that enrolled pregnant women from March 2003 to January 2006 (Braun et al. 2017, 2020a). A detailed flowchart of study eligibility and enrollment criteria is provided in Figure S1. Women were recruited from nine prenatal practices in affiliation with three hospitals in Cincinnati, Ohio, USA. Enrollment eligibility criteria included: of age; 13–19 wk gestation; living in a residence built prior to 1978 that was not a mobile/trailer home; HIV negative; not receiving seizure, thyroid, or chemotherapy/radiation medications; intention to continue prenatal care and deliver at a collaborating clinic; English fluency; no diagnosis of diabetes, bipolar disorder, or schizophrenia; and no cancer requiring radiation or chemotherapy. Of 1,263 eligible pregnant women, 468 were enrolled and 401 remained in the study at delivery. Of 67 women who dropped before delivery, 11 were reenrolled at a later follow-up visit. Of the total 412 enrolled mothers, 368 had singleton live-born infants with maternal gestational PFAS measures available. After restricting to those with available bone outcome measures from dual energy X-ray absorptiometry (DXA) scans, we included 206 children in the current study. Despite study attrition over time, maternal baseline characteristics of children followed at age 12 y were similar to those of the full cohort (Braun et al. 2020a).
The HOME Study protocol was approved by the institutional review boards of the Cincinnati Children’s Hospital Medical Center (CCHMC) and collaborating hospitals. The U.S. Centers for Disease Control and Prevention (CDC) deferred to CCHMC Internal Review Board (IRB) as the IRB of record. Written informed consent was provided by women for themselves and their children. At the 12-y study visit, children also provided written informed assent.
Quantification of Serum PFAS Concentrations
We measured PFAS concentrations in serum separated from clotted maternal venous blood samples collected at wk gestation (), 26 wk gestation (), or within 1 day of delivery (). We stored serum at before shipment on dry ice to the CDC for quantification of PFOA, PFNA, PFHxS, and PFOS concentrations using on-line solid phase extraction coupled with high performance liquid chromatography-isotope dilution tandem mass spectrometry (Kato et al. 2011). Each analytic batch included quality control (QC) materials and reagent blanks. Coefficients of variation for QC materials were . Limits of detection were , depending on the PFAS.
Bone Outcomes
At the 12-y follow-up visit, experienced research staff measured standing height to the nearest , in triplicate, with an Ayrton Stadiometer Model S100. Trained technicians performed DXA scans of the whole-body, lumbar spine, hip, and radius with a Hologic Horizon densitometer. We analyzed scans using Apex software (version 5.5; Hologic Inc) to estimate BMC (g) and areal BMD (, grams per square centimeter) for the whole-body less head, lumbar spine, total hip, femoral neck, one-third radius, and ultradistal radius. We calculated spine bone mineral apparent density (BMAD, grams per cubic centimeter) as the sum of BMC (grams) of the lumbar spine vertebrae divided by the sum of the volumetric bone of the lumbar spine vertebrae (Kindler et al. 2019). We used whole-body less head because the proportion of BMC contained in the skull varies inconsistently relative to body size in children (Taylor et al. 1997). We monitored long-term calibration stability of our DXA scanner by scanning the Hologic anthropomorphic spine phantom daily. Reproducibility of duplicate scans, expressed as the percent coefficient of variation, range from 0.75% for total hip aBMD to 1.85% for spine BMC in children 10–13 years of age (Shepherd et al. 2011).
Because DXA is a 2-dimensional technology, values are affected by bone size. To prevent bias because of short or tall stature, we calculated height-for-age adjusted age-, sex-, and population ancestry-specific whole-body less head, lumbar spine, total hip, femoral neck, and forearm (one-third distal radius) BMC and aBMD -scores and ultradistal forearm aBMD -scores using reference ranges from the Bone Mineral Density in Childhood Study (Kindler et al. 2020; Zemel et al. 2011). To estimate volumetric BMD (grams per cubic centimeter) not affected by height, we calculated age-, sex-, and population ancestry-specific spine bone mineral apparent density (BMAD) -scores (Kindler et al. 2019). We also adjusted for child’s sex and age at follow-up to account for any residual influence of bone size on bone outcome -scores. Compared with BMC, aBMD measures in childhood exhibit stronger tracking during skeletal and sexual maturity (Wren et al. 2014) and tend to be more precise (Crabtree et al. 2014).
Covariates
We selected covariates a priori based on a directed acyclic graph (Figure S2). Using standardized, computer-assisted interviews and medical chart reviews, trained research assistants collected information on maternal and perinatal characteristics, including maternal age at delivery, race/ethnicity, household income, parity, and prenatal vitamin use during the second or third trimester of pregnancy as well as child’s sex. We collected maternal whole blood at up to three time points (16 wk gestation, 26 wk gestation, and delivery), and stored samples at until shipment on dry ice to the CDC to measure lead concentrations using inductively coupled plasma mass spectrometry (Jones et al. 2017). We took the average of the available maternal blood lead concentrations as a measure of lead exposure during pregnancy.
We assessed children’s characteristics at the 12-y follow-up visit, including age in months, body composition, and pubertal status (Braun et al. 2020a). We measured whole-body lean mass by DXA at the same time as the bone measurements. We calculated lean body mass index (LBMI) as lean mass (kilograms) divided by height (square meters) and then calculated age- and sex-specific LBMI -scores based on a reference population of U.S. children and adolescents (Weber et al. 2013). Participants conducted self-assessments of pubertal stage using Tanner stage line drawings (Morris and Udry 1980; Taylor et al. 2001), and girls self-reported attainment of menarche.
Statistical Analyses
We conducted descriptive statistics including the proportion of participants within categories of discrete variables or missing data, mean and range of continuous variables, and visualized continuous variables using histograms. We PFAS concentrations because their distributions were log-normally distributed on visual inspection. We calculated Spearman’s correlation coefficients to assess correlations among PFAS. To understand the relation between LBMI and body mass index (BMI) among children in our sample, we calculated the Pearson’s correlation coefficient between LBMI and BMI -scores at age 12 y.
We used linear regression to estimate unadjusted and covariate-adjusted differences and 95% confidence intervals (CIs) in BMC, aBMD, and BMAD -scores per doubling of maternal serum PFAS concentrations. We used full-information maximum likelihood to account for missing covariate data (Enders 2001). Adjusted models included potential confounders identified with our directed acyclic graph, including maternal age at delivery (continuous), maternal BMI at 16 wk gestation (continuous), maternal race/ethnicity (non-Hispanic White, other race/ethnicity), household income (continuous), parity (nulliparous, 1, ), prenatal vitamin use (ever/never), and average maternal blood lead concentration (continuous). Given the small number of participants in the “other” race/ethnicity category (), we grouped race/ethnicity as non-Hispanic White and other race/ethnicity to retain all individuals in our analysis. As noted above, we also adjusted for child age at follow-up (continuous) and child sex (male/female) to account for residual influence of bone size on 2-dimensional bone outcome -score measures. In overall models, we additionally included a product term for child’s sex by child’s age to allow for the associations of age with bone outcomes to differ by sex, thus accounting for potential sex-dependent confounding (Buckley et al. 2017). To evaluate effect measure modification (EMM) of associations by child’s sex, we estimated sex-specific associations in fully stratified models and calculated EMM -values using a two-sample -test (Buckley et al. 2017).
We considered associations to be statistically significant at for main effects and for effect measure modification. We conducted descriptive and linear regression analyses using Stata (version 15.1; StataCorp).
Secondary Analyses
Mixtures.
We estimated effects of the PFAS mixture on bone outcomes using two approaches: quantile g-computation and Bayesian kernel machine regression (BKMR). Quantile g-computation estimates parameters of a marginal structural model to determine the change in the expected potential outcome per quantile increase in all mixture components (Keil et al. 2020). With this model, we estimated a) the overall mixture effect, , as the difference (95% CI) in outcome -score per quartile increase in all PFAS; b) the difference in outcome -score per quartile increase in all PFAS with associations in either the positive or negative direction (i.e., directional scaled effects); and c) weights that indicate the percent contribution of each PFAS to , the overall mixture effect. We estimated standard errors using 5,000 bootstrapped samples and fit these models using the R package qgcomp.
We also assessed PFAS mixture associations using BKMR, which flexibly models the relation between a set of exposures (i.e., PFAS concentrations) and a given outcome by allowing the exposures to relate nonlinearly and nonadditively via a kernel function (Bobb et al. 2015). Individual PFAS concentrations are selected for inclusion into the model at each iteration, resulting in posterior inclusion probabilities (PIPs) detailing the importance of each PFAS to the mixture. For all BKMR models, we estimated parameters from four parallel chains, each run with 12,500 burn-in and 12,500 additional Markov chain Monte Carlo (MCMC) iterations, and default priors for all parameters. To assess associations of the PFAS mixture with bone outcomes across values of the mixture, we estimated covariate-adjusted outcome -score differences and 95% credible intervals (CrI) setting the mixture to selected percentiles compared to the 50th percentile. We fit these models using the R package bkmr.
Mediation.
We also explored potential mediation of associations by LBMI. We focused on lean rather than fat mass because LBMI is a stronger predictor of bone mass in children (Sayers and Tobias 2010). In addition, a prior study found that adjustment for lean mass attenuated associations of PFAS with bone outcomes more than adjustment for fat mass (Jeddy et al. 2018). We estimated natural direct effects (NDE) and natural indirect effects (NIE) of individual PFAS on each outcome using structural equation models (Muthén and Asparouhov 2015). We used maximum likelihood estimation for fitting model parameters and a bias-corrected bootstrapping procedure for standard errors (10,000 samples) (Mackinnon et al. 2004). We accounted for missing covariate information using full-information maximum likelihood (Enders 2001) and fit the structural equation models using MPlus (version 8.6; Muthén & Muthén).
For assessing LBMI as a mediator of PFAS mixture effects, we used BKMR-causal mediation analysis (BKMR-CMA) (Devick et al. 2018) with the same number of chains and MCMC iterations as those used in the standard BKMR models to estimate counterfactually defined estimates of NDE and NIE (Valeri and Vanderweele 2013). For this analysis, NDEs represent the average difference in the counterfactual outcomes for a change in PFAS concentrations from the 75th percentile to the 25th percentile, fixing the mediator to the level it would have taken if the concentrations were at the 25th percentile. NIEs represent the average difference in counterfactual outcomes when the concentrations are fixed to the 75th percentile, but the mediator varies from the value it would have taken if the concentrations were set to the 75th in comparison with the 25th percentile. We fit these models with R using sample code published by the authors (https://github.com/kdevick/BKMR-CMA) (Devick et al. 2018).
For secondary analyses fitting quantile g-computation, BKMR, and BKMR-CMA models, we conducted analyses using R (version 4.05; R Development Core Team). For these analyses, we accounted for missing data by using a single stochastic imputation by chained equations. We included as predictors in the imputation process all selected covariates as well as maternal serum cotinine concentration during pregnancy (first available measure from either 16- or 26-wk gestation), child race/ethnicity, and pubertal status.
Sensitivity Analysis
As a sensitivity analysis, we additionally adjusted for Tanner stage as a continuous variable to account for the influence of pubertal status on bone outcomes. We did not include pubertal status in primary models because it is a potential causal intermediate (Figure S2).
Results
Population Characteristics
Mothers in our study sample were predominantly 25 years of age and older (73%) and non-Hispanic White (56%) and had a household income per year (58%) (Table 1). The four PFAS were detected in of maternal serum samples (Table S1). Spearman’s rank order correlation coefficients among PFAS ranged from 0.32 for PFNA and PFHxS to 0.61 for PFOS and PFHxS (Table S2). LBMI and BMI -scores were highly correlated in our sample (Pearson’s ). Children were on average years of age (range: ) at follow-up (Table 2).
Table 1.
Maternal characteristics during pregnancy (): The Health Outcomes and Measures of the Environment (HOME) Study.
| Characteristic | (%) |
|---|---|
| Age at delivery (y) | |
| 56 (27) | |
| 25–35 | 122 (59) |
| 28 (14) | |
| Race/ethnicity | |
| Non-Hispanic white | 116 (56) |
| Non-Hispanic black | 80 (39) |
| Other | 10 (5) |
| Maternal BMI () at 16 wk gestation | |
| (underweight) | 3 (2) |
| (normal weight) | 79 (41) |
| (overweight) | 64 (34) |
| (obese) | 45 (24) |
| Missing | 15 |
| Household income ( per y) | |
| 53 (26) | |
| 34 (17) | |
| 70 (34) | |
| 49 (24) | |
| Parity | |
| Nulliparous (0) | 82 (41) |
| 1 | 64 (32) |
| 53 (27) | |
| Missing | 7 |
| Prenatal vitamin use | |
| Never used | 24 (12) |
| Ever used | 174 (88) |
| Missing | 8 |
| Average blood lead concentration ()a | |
| 36 (18) | |
| 141 (68) | |
| 29 (14) | |
Note: BMI, body mass index: .
Average of up to three lead measures in maternal blood collected at 16 wk gestation, 26 wk gestation, and delivery.
Table 2.
Child characteristics at follow-up, overall and by sex () (): The Health Outcomes and Measures of the Environment (HOME) Study.
| Variable | Overall () | Males () | Females () |
|---|---|---|---|
| Age (y) | 12.3 (0.7) | 12.4 (0.7) | 12.3 (0.7) |
| Tanner stage [n (%)] | |||
| 1 | 22 (11) | 16 (17) | 6 (5) |
| 2 | 54 (26) | 30 (32) | 24 (21) |
| 3 | 58 (28) | 23 (25) | 35 (31) |
| 4 | 42 (20) | 15 (16) | 27 (24) |
| 5 | 29 (14) | 9 (10) | 20 (18) |
| Missing | 1 | 0 | 1 |
| Whole-body lean mass (kg) | 32.8 (8.3) | 32.3 (8.6) | 33.2 (8.0) |
| LBMI -scorea | (1.22) | (1.19) | (1.24) |
| BMC (g) | |||
| Whole-body less head | 1,215.5 (321.7) | 1,158.5 (318.4) | 1,262.4 (318.2) |
| Total hip | 25.0 (6.7) | 25.4 (7.5) | 24.7 (5.9) |
| Femoral neck | 3.7 (0.8) | 3.4 (0.8) | 3.7 (0.8) |
| Forearm | 1.4 (0.3) | 1.4 (0.3) | 1.5 (0.3) |
| Spine | 38.5 (11.6) | 35.0 (10.3) | 41.4 (12.0) |
| BMC -score | |||
| Whole-body less headb | (0.82) | (0.78) | 0.06 (0.84) |
| Total hipb | (0.92) | (0.95) | (0.89) |
| Femoral neckb | 0.23 (0.97) | 0.12 (0.95) | 0.32 (0.99) |
| Forearmb | 0.10 (0.97) | (0.93) | 0.22 (0.98) |
| Spineb | 0.10 (0.85) | 0.02 (0.85) | 0.16 (0.85) |
| BMD | |||
| Whole-body less head aBMD () | 0.82 (0.09) | 0.80 (0.09) | 0.84 (0.09) |
| Total hip aBMD () | 0.85 (0.14) | 0.83 (0.13) | 0.86 (0.14) |
| Femoral neck aBMD () | 0.79 (0.14) | 0.78 (0.12) | 0.81 (0.15) |
| Forearm aBMD () | 0.60 (0.06) | 0.59 (0.06) | 0.61 (0.06) |
| Ultradistal forearm aBMD () | 0.35 (0.06) | 0.34 (0.05) | 0.35 (0.06) |
| Spine BMAD () | 0.23 (0.04) | 0.21 (0.03) | 0.24 (0.04) |
| BMD -score | |||
| Whole-body less head aBMDb | (0.90) | (0.87) | (0.93) |
| Total hip aBMDb | (1.07) | (1.09) | 0.03 (1.06) |
| Femoral neck aBMDb | (1.07) | (1.01) | 0.04 (1.04) |
| Forearm aBMDb | 0.24 (0.93) | 0.20 (0.88) | 0.27 (0.98) |
| Ultradistal forearm aBMDb | 0.26 (1.15) | 0.12 (0.98) | 0.38 (1.26) |
| Spine BMADc | 0.31 (1.02) | 0.27 (1.00) | 0.35 (1.04) |
Note: aBMD, areal bone mineral density; BMC, bone mineral content; BMD, bone mineral density; BMAD, bone mineral apparent density; BMI, body mass index; LBMI, lean body mass index; SD, standard deviation.
Age- and sex-specific whole-body LBMI -score.
Height-for-age adjusted age-, sex-, and population ancestry-specific -score.
Age-, sex-, and population ancestry-specific -score.
Single PFAS Models
In covariate-adjusted linear regression models, maternal serum PFOA and PFNA concentrations were associated with lower BMC -scores at the total hip and forearm but not at other skeletal sites (Figure 1A and Table S3). For example, each doubling of maternal serum PFNA concentration was associated with a (95% CI: , ) standard deviation (SD) difference in forearm BMC -score. PFHxS and PFOS were not significantly associated with BMC -scores.
Figure 1.
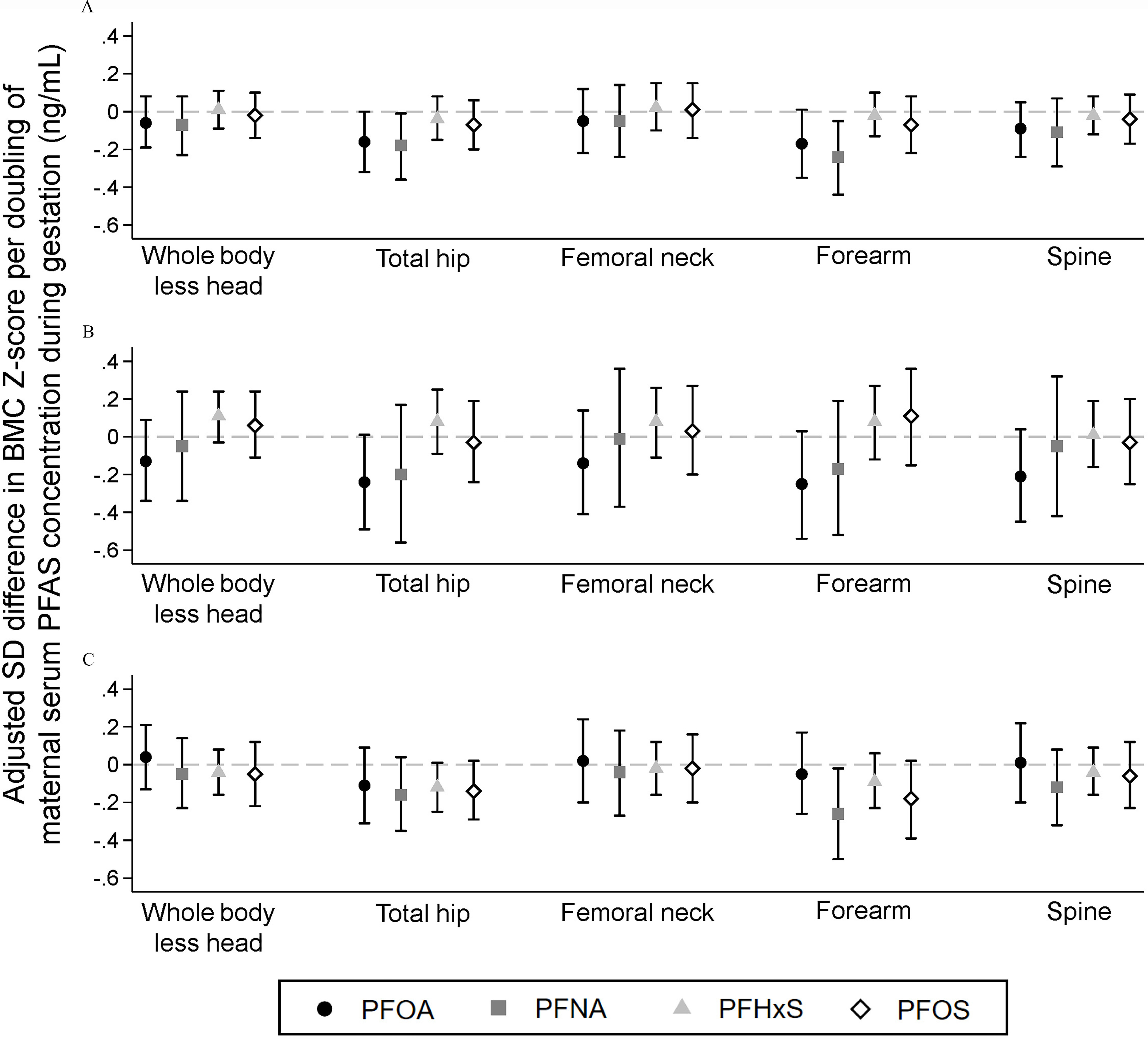
Adjusted associations of maternal serum perfluoroalkyl substances concentrations with BMC z-scores at 12 years of age: The Health Outcomes and Measures of the Environment (HOME) Study. (A) overall (), (B) males (), (C) females (). Difference (95% confidence interval) in BMC z-score per unit increase in perfluoroalkyl substance concentrations estimated in separate linear regression models adjusted for maternal age at delivery, midpregnancy BMI, race/ethnicity, household income, parity, prenatal vitamin use, average blood lead concentration, and child age at follow-up. Models for the overall population are additionally adjusted for child sex and child sex by child age at follow-up. Missing covariate information accounted for using full-information maximum likelihood. Numeric results are reported in Tables S3 and S4. Note: BMC, bone mineral content; BMI, body mass index; PFHxS, perfluorohexane sulfonic acid; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonic acid.
Associations with BMD -scores were similar in magnitude but tended to be slightly weaker than associations with BMC -scores (Table S3). Maternal serum PFOA concentrations were associated with lower forearm aBMD -score (, 95% CI: , 0.00). Maternal serum PFHxS concentrations were weakly associated with lower forearm aBMD (, 95% CI: , 0.02) and spine BMAD (, 95% CI: , 0.02) -scores.
For PFOA, associations of maternal serum concentrations with bone outcome -scores were generally stronger among males than females (Figure 1B,C and Table S4). We observed statistically significant EMM for spine BMC (), femoral neck aBMD (), ultradistal forearm aBMD (), and spine BMAD () (Table S4). Sex differences in PFOA associations were strongest for spine BMAD, where each doubling of maternal serum PFOA concentrations was associated with a (95% CI: , ) SD difference in spine BMAD -score in males, but there was no association in females (, 95% CI: , 0.30).
Child’s sex also significantly modified associations of PFHxS with whole-body BMC (EMM ), total hip BMC (EMM ), forearm BMC (EMM ), and total hip aBMD (EMM ) and of PFOS with forearm BMC (EMM ) (Figure 1B,C and Table S4). In contrast to PFOA, these sex-specific estimates were positive in males and negative in females; however, associations were generally small in magnitude and not statistically significant in either sex.
Overall and sex-specific estimates from unadjusted linear regression models were similar to the adjusted results (Table S5).
PFAS Mixture Models
In quantile g-computation models, higher concentrations of the PFAS mixture were associated with lower total hip and forearm BMC and aBMD -scores (Table 3 and Table S6). In the overall population, SD differences per quartile increase in all maternal serum PFAS concentrations were (95%CI: , 0.02) for total hip and (95% CI: , ) for forearm BMC -score (Table 3). As we found with the single PFAS models, we observed null associations of the PFAS mixture with other skeletal sites (Table 3). Although magnitudes of the overall mixture effects () were similar among males and females, PFOA tended to have the largest negative scaled effect weights among males, whereas PFOS tended to have the largest negative scaled effect weights among females (Table S6).
Table 3.
Adjusted associations of the maternal serum perfluoroalkyl substance mixture with BMC -scores at age 12 y estimated using quantile g-computation, overall and by child sex (): The Health Outcomes and Measures of the Environment (HOME) Study.
| Outcome | Overall () | Males () | Females () |
|---|---|---|---|
| Whole-body less head | (, 0.10) | 0.01 (, 0.22) | (, 0.15) |
| Total hip | (, 0.02) | (, 0.16) | (, 0.05) |
| Femoral neck | (, 0.15) | 0.03 (, 0.29) | (, 0.22) |
| Forearm | (, ) | (, 0.07) | (, 0.02) |
| Spine | (, 0.08) | (, 0.16) | (, 0.21) |
Note: Difference (95% confidence interval) in outcome -score per quartile increase in all perfluoroalkyl substances estimated using quantile g-computation. Adjusted for maternal age at delivery, midpregnancy BMI, race/ethnicity, household income, parity, prenatal vitamin use, average blood lead concentration, and child age at follow-up. Models for the overall population are additionally adjusted for child sex and child sex by child age at follow-up. Missing covariate information accounted for using single stochastic imputation by chained equations. Note: BMC, bone mineral content; BMI, body mass index.
Results from BKMR models in the overall population were similar to results from quantile g-computation (Table S7). In sex-stratified analyses, patterns of association tended to differ by child’s sex and exhibit nonlinear relationships with bone outcomes (Table S7). For example, we observed a nonlinear positive association of the PFAS mixture with femoral neck aBMD in females and a weak inverted U-shaped association in males (Figure 2A). For spine BMAD, we observed a nonlinear negative association of the PFAS mixture in males and no association in females (Figure 2B). Among males, holding all PFAS at the 90th percentile was associated with a (95% CrI: , ) SD difference in spine BMAD -score in comparison with holding all PFAS at the median (Table S7). PIPs from the BKMR models tended to be highest for PFOA; the highest PIP was 0.82 for PFOA in the spine BMAD model (Table S8).
Figure 2.
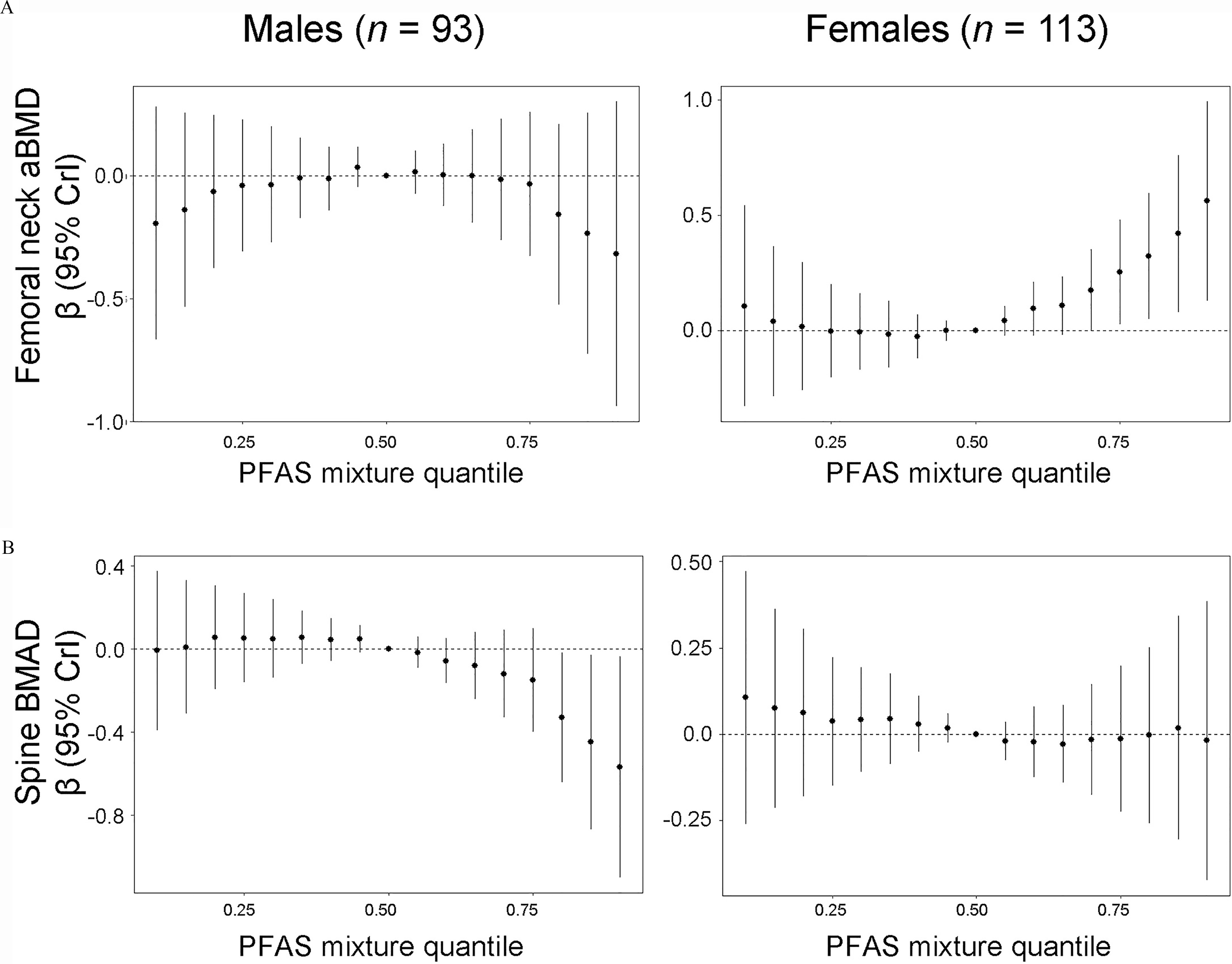
Adjusted associations of the maternal serum perfluoroalkyl substances mixture with bone outcome -scores at age 12 y by child’s sex (): The Health Outcomes and Measures of the Environment (HOME) Study. (A) femoral neck aBMD, (B) spine BMAD. Difference (95% CrI) in outcome -score setting all PFAS to selected quantiles compared to the median, estimated using Bayesian kernel machine regression models adjusted for maternal age at delivery, mid-pregnancy BMI, race/ethnicity, household income, parity, prenatal vitamin use, average blood lead concentration, and child age at follow-up. Missing covariate information accounted for using single stochastic imputation by chained equations. Numeric results are reported in Table S7. Note: aBMD, areal bone mineral density; BMAD, spine bone mineral apparent density; BMI, body mass index; CrI, credible interval; PFAS, per- and polyfluoroalkyl substances.
Mediation Analyses
We observed limited evidence that LBMI mediated associations between individual PFAS and BMC (Table 4) or BMD (Tables S9-S11) -scores. The strongest evidence for LBMI mediation was for PFNA associations among females, where NIEs were negative and statistically significant for most bone outcomes (Table 4; Table S11). For example, the LBMI-mediated NIE and NDE on forearm BMC -score per doubling of PFNA among females were (95% CI: , ) and (95% CI: , ), respectively. There was also weak evidence that LBMI mediated associations of maternal serum PFOA with bone outcome -scores among males (Table 4; Table S10). LBMI did not mediate associations of the maternal serum PFAS mixture with bone outcomes (Table S12).
Table 4.
Adjusted estimates of natural indirect (lean body mass index mediated) and natural direct effects of maternal serum perfluoroalkyl substances concentrations on BMC -scores at age 12 y: The Health Outcomes and Measures of the Environment (HOME) Study.
| PFAS/Outcome | Effect | Overall () | Males () | Females () |
|---|---|---|---|---|
| PFOA | ||||
| Whole-body less head | NIE | (, 0.05) | (, 0.05) | (, 0.11) |
| NDE | (, 0.09) | (, 0.13) | 0.05 (, 0.22) | |
| Total hip | NIE | (, 0.05) | (, 0.04) | (, 0.11) |
| NDE | (, 0.01) | (, 0.06) | (, 0.10) | |
| Femoral neck | NIE | (, 0.05) | (, 0.05) | (, 0.12) |
| NDE | (, 0.13) | (, 0.16) | 0.03 (, 0.24) | |
| Forearm | NIE | (, 0.04) | (, 0.04) | (, 0.11) |
| NDE | (, 0.03) | (, 0.06) | (, 0.21) | |
| Spine | NIE | (, 0.04) | (, 0.04) | (, 0.08) |
| NDE | (, 0.09) | (, 0.11) | 0.02 (, 0.26) | |
| PFNA | ||||
| Whole-body less head | NIE | (, 0.04) | 0.01 (, 0.22) | (, 0.00) |
| NDE | (, 0.14) | (, 0.24) | 0.05 (, 0.24) | |
| Total hip | NIE | (, 0.04) | 0.01 (, 0.23) | (, 0.00) |
| NDE | (, 0.06) | (, 0.16) | (, 0.16) | |
| Femoral neck | NIE | (, 0.04) | 0.01 (, 0.23) | (, 0.01) |
| NDE | 0.01 (, 0.20) | (, 0.37) | 0.06 (, 0.29) | |
| Forearm | NIE | (, 0.03) | 0.01 (, 0.20) | (, ) |
| NDE | (, 0.00) | (, 0.15) | (, 0.09) | |
| Spine | NIE | (, 0.03) | 0.01 (, 0.20) | (, ) |
| NDE | (, 0.14) | (, 0.29) | (, 0.22) | |
| PFHxS | ||||
| Whole-body less head | NIE | 0.01 (, 0.08) | 0.08 (, 0.23) | (, 0.04) |
| NDE | (, 0.08) | 0.03 (, 0.16) | (, 0.10) | |
| Total hip | NIE | 0.02 (, 0.08) | 0.08 (, 0.24) | (, 0.04) |
| NDE | (, 0.05) | 0.00 (, 0.18) | (, 0.04) | |
| Femoral neck | NIE | 0.02 (, 0.08) | 0.08 (, 0.24) | (, 0.04) |
| NDE | 0.01 (, 0.12) | 0.00 (, 0.19) | 0.00 (, 0.14) | |
| Forearm | NIE | 0.01 (, 0.07) | 0.07 (, 0.20) | (, 0.03) |
| NDE | (, 0.07) | 0.01 (, 0.17) | (, 0.08) | |
| Spine | NIE | 0.01 (, 0.07) | 0.07 (, 0.20) | (, 0.03) |
| NDE | (, 0.07) | (, 0.11) | (, 0.11) | |
| PFOS | ||||
| Whole-body less head | NIE | (, 0.04) | 0.00 (, 0.15) | (, 0.02) |
| NDE | 0.01 (, 0.11) | 0.06 (, 0.22) | 0.01 (, 0.16) | |
| Total hip | NIE | (, 0.04) | 0.00 (, 0.16) | (, 0.02) |
| NDE | (, 0.08) | (, 0.19) | (, 0.07) | |
| Femoral neck | NIE | (, 0.04) | 0.00 (, 0.15) | (, 0.03) |
| NDE | 0.04 (, 0.17) | 0.03 (, 0.24) | 0.04 (, 0.22) | |
| Forearm | NIE | (, 0.03) | 0.00 (, 0.13) | (, 0.01) |
| NDE | (, 0.10) | 0.10 (, 0.32) | (, 0.08) | |
| Spine | NIE | (, 0.03) | 0.00 (, 0.13) | (, 0.01) |
| NDE | (, 0.12) | (, 0.21) | 0.00 (, 0.17) | |
Note: Difference (95% confidence interval) in outcome -score per unit increase in perfluoroalkyl substance concentration estimated in separate linear regression models adjusted for maternal age at delivery, midpregnancy BMI, race/ethnicity, household income, parity, prenatal vitamin use, average blood lead concentration, and child age at follow-up. Models for the overall population are additionally adjusted for child sex and child sex by child age at follow-up. Indirect and direct effects were estimated using structural equation models, maximum likelihood estimation, and bias-corrected and accelerated bootstrap confidence intervals. Missing covariate information accounted for using full-information maximum likelihood. BMC, bone mineral content; BMI, body mass index; NDE, natural direct effect; NIE, natural indirect effect; PFHxS, perfluorohexane sulfonic acid; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonic acid.
Sensitivity Analyses
In models additionally adjusted for Tanner stage at age 12 y, estimates of association between maternal serum PFAS concentrations and bone outcome -scores were similar to estimates from primary models (Tables S13 and S14).
Discussion
In this prospective cohort study, we found that higher maternal serum concentrations during pregnancy of several PFAS and their mixture were associated with lower bone mineral content and density in their children at age 12 y. Most associations were strongest for the two perfluorocarboxylic acids (PFOA and PFNA) and at cortical bone sites (forearm and total hip). For PFOA, there was some evidence that associations with trabecular bone sites (femoral neck, ultradistal forearm, and spine) were modified by child’s sex, with lower bone outcome -scores among males but not females. BMC and aBMD -score differences were as large as 0.3 SD per doubling of maternal serum PFAS concentrations. For context, each hour of vigorous physical activity per day was associated with a 0.05 SD greater -score for whole-body less head BMC and total hip aBMD in the Bone Mineral Density in Childhood Study (Mitchell et al. 2016). Each 1 SD change in BMC or aBMD is associated with 1.3–1.4 times the odds of forearm fracture in children (Kalkwarf et al. 2011).
Similar to our findings, three previous studies of early life PFAS exposures reported adverse associations with childhood bone outcomes. We are aware of only one other prospective study, which also relied on maternal PFAS serum concentrations measured during pregnancy and assessed 257 17-y-old females in the Avon Longitudinal Study of Parents and Children (ALSPAC) (Jeddy et al. 2018). Greater maternal serum PFAS concentrations were associated with lower whole-body less head BMC, aBMD, and bone area (Jeddy et al. 2018). PFAS concentrations were not significantly associated with total hip or femoral neck aBMD measured in a subset of girls with a DXA scan of the hip () (Jeddy et al. 2018). In a cross-sectional study of 576 8-y-old children in the Project Viva cohort, higher plasma concentrations of a mixture of six PFAS was associated with lower whole-body less head aBMD -score ( per interquartile range difference in all PFAS: , 95% CI: , ), with PFOA having the strongest individual chemical associations (Cluett et al. 2019). Associations were weaker for whole-body BMC in comparison with aBMD -scores, and there were no statistically significant differences by child’s sex (Cluett et al. 2019). In a small cross-sectional study of 23 children with obesity age 8–12 y, serum concentrations of PFOA, PFOS, PFNA, and PFHxS were inversely correlated with stiffness and speed of sound assessed by quantitative ultrasound of the heel, measures that are correlated with bone density (Khalil et al. 2018).
In the ALSPAC study, most associations were attenuated after adjustment for lean mass (Jeddy et al. 2018). We formally tested whether lean mass mediates associations of PFAS with bone outcomes and found that LBMI partially mediated associations of PFNA with all bone outcomes among females but did not mediate associations with other PFAS or the PFAS mixture. In the same HOME Study cohort, we previously reported that higher maternal PFOA concentrations during pregnancy were associated with higher BMI, whereas PFOS and PFHxS concentrations were associated with lower BMI, at age 12 y (Braun et al. 2020b). Given that LBMI and BMI -scores were highly correlated in our sample, these findings suggest that observed associations of maternal PFAS concentrations assessed during pregnancy on bone outcomes are independent of their effects on body composition. The previous study in ALSPAC did not conduct formal mediation analyses, adjusted for a more limited set of covariates (child age at follow-up, maternal education, and gestational age at sample collection), measured bone outcomes among older girls, and analyzed bone outcomes measures that were not height-adjusted (Jeddy et al. 2018), all of which may contribute to differences in results.
Like the cross-sectional Project Viva study (Cluett et al. 2019), we did not find significant EMM by sex in associations of any PFAS with whole-body less head aBMD. However, we observed modest evidence of sex differences at skeletal sites that were not measured in the previous study, particularly those composed more of trabecular than cortical bone. Greater maternal PFOA was associated with lower bone density at the femoral neck, ultradistal forearm, and spine among males but not females, whereas higher percentiles of the PFAS mixture were associated with greater femoral neck aBMD among females but not males. Cortical bone comprises the hard, outer shell and protects the bone marrow and trabecular bone within. Trabecular bone is a spongelike matrix, which increases markedly during puberty (Weaver et al. 2016) and may be more affected than cortical bone by disruption of sex steroid hormone receptors during development (Khosla and Monroe 2018). Sex differences may also reflect the timing of our follow-up visit, given that bone mineral accretion rates peak around age 12.0–12.5 y for females and 14 y for males (Bailey et al. 1999; Jones et al. 2002; McCormack et al. 2017). Finally, our sample sizes within sex were small and differences may be due to random error.
We compared two approaches for estimating effects of PFAS mixtures on bone outcomes. Results for quantile g-computation and BKMR were generally similar in the overall population where both approaches suggested that the concentration–response associations were approximately linear. However, sex-stratified associations exhibited nonlinearity in BKMR models, whereas our implementation of quantile g-computation assumed a linear relationship. Notably, the magnitudes of associations observed using both quantile g-computation and BKMR were generally larger than those observed for individual PFAS. A increase is approximately equivalent to the interquartile ranges of PFAS concentrations in our population, which is comparable to about a two-quartile increase in the PFAS mixture estimated with quantile g-computation or an increase from the 25th to 75th percentile of the PFAS mixture estimated with BKMR.
The only animal study of developmental PFOA exposure and bone morphology reported effects on cortical but not trabecular bone measures (Koskela et al. 2016). Mice were exposed to of PFOA during pregnancy and lactation, and female offspring were studied at 13 and 17 months of age using micro-computed tomography imaging (Koskela et al. 2016). Compared with controls, exposed offspring had lower tibial cortical BMD at 13 and 17 months, but there were no differences between treatment groups for trabecular BMD (Koskela et al. 2016). These results are consistent with our findings of stronger associations at sites composed of greater cortical bone. Koskela et al. (2016) did not study male offspring, and therefore it is unclear whether there could be sex-dependent effects on trabecular bone as suggested by our finding that maternal PFOA concentrations were associated with lower bone density at trabecular sites among males but not females.
Although the potential mechanisms are unknown, PFAS may affect bone morphogenesis, mineralization, or remodeling via several pathways. PFAS have varying abilities to act as peroxisome proliferator–activated receptor ( or peroxisome proliferator–activated receptor ( agonists (Fang et al. 2009; Mahapatra et al. 2017; Rosen et al. 2008; Vanden Heuvel et al. 2006; Wan Ibrahim et al. 2013; Watkins et al. 2015; Zhang et al. 2014). Although activation increases BMD, activation suppresses osteogenic differentiation of mesenchymal stem cells; therefore, effects of individual PFAS may depend on which PPAR it activates more strongly (Cluett et al. 2019). Bone metabolism is regulated by multiple hormonal processes (Bassett and Williams 2016; Khosla and Monroe 2018) and may be susceptible to effects of endocrine disruptors, including PFAS (Agas et al. 2013, 2018; Jensen and Leffers 2008). Endocrine disrupting chemicals may affect vitamin D status due to the structural similarity of 1,25-dihydroxyvitamin D to classical steroid hormones (Johns et al. 2016). PFOA competes with 1,25-dihydroxyvitamin D on the same binding site of the vitamin D receptor, leading to altered response of vitamin D–related genes in osteoblasts (Di Nisio et al. 2020b). In human osteoblast cells, coexposure to PFOA significantly reduced 1,25-dihydroxyvitamin D associated mineralization (Di Nisio et al. 2020b). In a cross-sectional study of 7,040 individuals in the 2003–2010 National Health and Nutrition Examination Survey with measured serum PFAS and 25-hydroxyvitamin D concentrations, we previously found that 25-hydroxyvitamin D concentrations were positively associated with PFHxS, were inversely associated with PFOS, and were not associated with PFOA or PFNA (Etzel et al. 2019). Still, the importance of the vitamin D pathway for effects of gestational PFAS exposures on bone health remains unknown. Finally, two small studies reported the detection of certain PFAS in bone (Koskela et al. 2017; Pérez et al. 2013). In one study measuring multiple PFAS in autopsied bone from 20 individuals, PFOA was detected in 55% of samples (mean: wet weight), whereas PFHxS was detected in 5% (mean: wet weight), and PFOS and PFNA were not detected (Pérez et al. 2013). These studies should be interpreted with caution, however, because concentrations were measured in postmortem samples and the studies lacked detail on sampling and quality control measures. Additional research is needed to determine the extent to which PFAS may accumulate in human bone.
Our study has several strengths and limitations. We assessed BMC and BMD at multiple skeletal sites using DXA, a standard clinical tool for assessing bone health in children (Weaver et al. 2016). We estimated effects of PFAS mixtures using two state-of-the-art approaches, BKMR and quantile g-computation, and formally evaluated whether LBMI mediates associations of individual PFAS or their mixture with bone outcomes. We accounted for a wide array of potential confounders, including maternal lead concentrations during pregnancy. Limitations of this study include the moderate sample size resulting from loss to follow-up, which reduced our statistical power for estimating sex-specific effects. Because children in our study were at various stages of puberty, we accounted for age, growth, and bone size using height-adjusted and age-, sex-, and population ancestry-specific -scores and conducted sensitivity analyses adjusting for Tanner stage at age 12 y. Pubertal timing may mediate associations of PFAS concentrations with bone outcomes given that some studies have reported differences in pubertal development in relation to maternal PFAS concentrations during pregnancy (Ernst et al. 2019; Rappazzo et al. 2017). We were unable to assess potential mediation given that males and females had attained different stages of sexual maturation at age 12 y, and we lacked sufficient sample sizes within sex. Although adjusting for Tanner stage did not appreciably change effect estimates in our analyses, larger future studies with longer follow-up could assess the potential for associations to be mediated by pubertal timing. An additional limitation of the timing of our bone outcome measurements is that midpuberty aBMD does not predict future aBMD as well as measures after sexual maturity (Kalkwarf et al. 2010; Wren et al. 2014). We focused on gestation as a plausible period of heightened susceptibility to PFAS and did not examine postnatal exposures, though childhood may be another important period given the ongoing and dynamic nature of bone growth and remodeling. Because 12% of serum samples analyzed for PFAS were collected at 26 wk gestation or at delivery rather than early pregnancy, there is potential for exposure misclassification due to changes in glomerular filtration rate and hemodynamics during pregnancy (Verner et al. 2015). However, this concern is mitigated by the very good to excellent reproducibility of serum PFAS concentrations during pregnancy in this cohort (PFOA, 0.76; PFNA, 0.68; PFHxS, 0.78; PFOS, 0.76) (Braun et al. 2020b). Finally, we conducted many statistical tests given our goal to evaluate both cortical and trabecular bone sites.
In this cohort, we found that maternal serum concentrations of PFOA, PFNA, and the PFAS mixture during pregnancy were associated with lower child bone mineral content and density in early adolescence, particularly at cortical bone sites. For PFOA, we observed evidence that associations with trabecular bone sites may be sex-dependent. If confirmed in future studies, our findings suggest that reducing maternal PFAS exposures during pregnancy may improve skeletal health in adolescence with possible long-term implications for risk of fractures and osteoporosis later in life.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institute of Environmental Health Sciences of the National Institutes of Health (NIH, R01 ES030078, R01 ES025214, R01ES020349, R01ES027224, P01ES011261). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. We are grateful to the participants for the time they have given to the HOME Study.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the U.S. CDC. Use of trade names is for identification only and does not imply endorsement by the U.S. CDC, the Public Health Service, or the U.S. Department of Health and Human Services.
References
- Agas D, Lacava G, Sabbieti MG. 2018. Bone and bone marrow disruption by endocrine-active substances. J Cell Physiol 234(1):192–213, PMID: 29953590, 10.1002/jcp.26837. [DOI] [PubMed] [Google Scholar]
- Agas D, Sabbieti MG, Marchetti L. 2013. Endocrine disruptors and bone metabolism. Arch Toxicol 87(4):735–751, PMID: 23192238, 10.1007/s00204-012-0988-y. [DOI] [PubMed] [Google Scholar]
- Bailey DA, McKay HA, Mirwald RL, Crocker PR, Faulkner RA. 1999. A six-year longitudinal study of the relationship of physical activity to bone mineral accrual in growing children: the University of Saskatchewan bone mineral accrual study. J Bone Miner Res 14(10):1672–1679, PMID: 10491214, 10.1359/jbmr.1999.14.10.1672. [DOI] [PubMed] [Google Scholar]
- Bassett JH, Williams GR. 2016. Role of thyroid hormones in skeletal development and bone maintenance. Endocr Rev 37(2):135–187, PMID: 26862888, 10.1210/er.2015-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobb JF, Valeri L, Claus Henn B, Christiani DC, Wright RO, Mazumdar M, et al. 2015. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics 16(3):493–508, PMID: 25532525, 10.1093/biostatistics/kxu058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM. 2017. Early-life exposure to EDCs: role in childhood obesity and neurodevelopment. Nat Rev Endocrinol 13(3):161–173, PMID: 27857130, 10.1038/nrendo.2016.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Buckley JP, Cecil KM, Chen A, Kalkwarf HJ, Lanphear BP, et al. 2020a. Adolescent follow-up in the Health Outcomes and Measures of the Environment (HOME) Study: cohort profile. BMJ Open 10(5):e034838, PMID: 32385062, 10.1136/bmjopen-2019-034838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Eliot M, Papandonatos GD, Buckley JP, Cecil KM, Kalkwarf HJ, et al. 2020b. Gestational perfluoroalkyl substance exposure and body mass index trajectories over the first 12 years of life. Int J Obes (Lond) 45(1):25–35, PMID: 33208860, 10.1038/s41366-020-00717-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Kalloo G, Chen A, Dietrich KN, Liddy-Hicks S, Morgan S, et al. 2017. Cohort profile: the Health Outcomes and Measures of the Environment (HOME) Study. Int J Epidemiol 46(1):24, PMID: 27006352, 10.1093/ije/dyw006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck RC, Franklin J, Berger U, Conder JM, Cousins IT, de Voogt P, et al. 2011. Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integr Environ Assess Manag 7(4):513–541, PMID: 21793199, 10.1002/ieam.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley JP, Doherty BT, Keil AP, Engel SM. 2017. Statistical approaches for estimating sex-specific effects in endocrine disruptors research. Environ Health Perspect 125(6):067013, PMID: 28665274, 10.1289/EHP334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC (U.S. Centers for Disease Control and Prevention). 2019. Fourth national report on human exposure to environmental chemicals, updated tables, January, 2019.
- Clark EM, Ness AR, Bishop NJ, Tobias JH. 2006. Association between bone mass and fractures in children: a prospective cohort study. J Bone Miner Res 21(9):1489–1495, PMID: 16939408, 10.1359/jbmr.060601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cluett R, Seshasayee SM, Rokoff LB, Rifas-Shiman SL, Ye X, Calafat AM, et al. 2019. Per- and polyfluoroalkyl substance plasma concentrations and bone mineral density in midchildhood: a cross-sectional study (Project Viva, United States). Environ Health Perspect 127(8):87006, PMID: 31433236, 10.1289/EHP4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree NJ, Arabi A, Bachrach LK, Fewtrell M, El-Hajj Fuleihan G, Kecskemethy HH, et al. 2014. Dual-energy X-ray absorptiometry interpretation and reporting in children and adolescents: the revised 2013 ISCD Pediatric Official Positions. J Clin Densitom 17(2):225–242, PMID: 24690232, 10.1016/j.jocd.2014.01.003. [DOI] [PubMed] [Google Scholar]
- Devick KL, Bobb JF, Mazumdar M, Henn BC, Bellinger DC, Christiani DC, et al. 2018. Bayesian kernel machine regression-causal mediation analysis. arXiv:1811.10453. Version 2. Preprint posted online August 16, 2019. [Google Scholar]
- Di Nisio A, De Rocco Ponce M, Giadone A, Rocca MS, Guidolin D, Foresta C. 2020a. Perfluoroalkyl substances and bone health in young men: a pilot study. Endocrine 67(3):678–684, PMID: 31565782, 10.1007/s12020-019-02096-4. [DOI] [PubMed] [Google Scholar]
- Di Nisio A, Rocca MS, De Toni L, Sabovic I, Guidolin D, Dall’Acqua S, et al. 2020b. Endocrine disruption of vitamin D activity by perfluoro-octanoic acid (PFOA). Sci Rep 10(1):16789, PMID: 33033332, 10.1038/s41598-020-74026-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority). 2008. Perfluoroctane sulfonate (PFOS), perfluorooctanoic acid (PFOA) and their salts: scientific opinion of the panel on contaminants in the food chain. EFSA J 653:1–131, 10.2903/j.efsa.2008.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders CK. 2001. The impact of nonnormality on full information maximum-likelihood estimation for structural equation models with missing data. Psychol Methods 6(4):352–370, PMID: 11778677. [PubMed] [Google Scholar]
- Ernst A, Brix N, Lauridsen LLB, Olsen J, Parner ET, Liew Z, et al. 2019. Exposure to perfluoroalkyl substances during fetal life and pubertal development in boys and girls from the Danish National Birth Cohort. Environ Health Perspect 127(1):17004, PMID: 30628845, 10.1289/EHP3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etzel TM, Braun JM, Buckley JP. 2019. Associations of serum perfluoroalkyl substance and vitamin D biomarker concentrations in NHANES, 2003–2010. Int J Hyg Environ Health 222(2):262–269, PMID: 30503928, 10.1016/j.ijheh.2018.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X, Feng Y, Shi Z, Dai J. 2009. Alterations of cytokines and mapk signaling pathways are related to the immunotoxic effect of perfluorononanoic acid. Toxicol Sci 108(2):367–376, PMID: 19196829, 10.1093/toxsci/kfp019. [DOI] [PubMed] [Google Scholar]
- Fernández I, Gavaia PJ, Laizé V, Cancela ML. 2018. Fish as a model to assess chemical toxicity in bone. Aquat Toxicol 194:208–226, PMID: 29202272, 10.1016/j.aquatox.2017.11.015. [DOI] [PubMed] [Google Scholar]
- Fromme H, Tittlemier SA, Völkel W, Wilhelm M, Twardella D. 2009. Perfluorinated compounds–exposure assessment for the general population in Western countries. Int J Hyg Environ Health 212(3):239–270, PMID: 18565792, 10.1016/j.ijheh.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Haentjens P, Magaziner J, Colón-Emeric CS, Vanderschueren D, Milisen K, Velkeniers B, et al. 2010. Meta-analysis: excess mortality after hip fracture among older women and men. Ann Intern Med 152(6):380–390, PMID: 20231569, 10.7326/0003-4819-152-6-201003160-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrast SD, Kalkwarf HJ. 1998. Effects of gestational age, maternal diabetes, and intrauterine growth retardation on markers of fetal bone turnover in amniotic fluid. Calcif Tissue Int 62(3):205–208, PMID: 9501952, 10.1007/s002239900418. [DOI] [PubMed] [Google Scholar]
- Hu Y, Liu G, Rood J, Liang L, Bray GA, de Jonge L, et al. 2019. Perfluoroalkyl substances and changes in bone mineral density: a prospective analysis in the pounds-lost study. Environ Res 179(pt A):108775, PMID: 31593837, 10.1016/j.envres.2019.108775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeddy Z, Tobias JH, Taylor EV, Northstone K, Flanders WD, Hartman TJ. 2018. Prenatal concentrations of perfluoroalkyl substances and bone health in British girls at age 17. Arch Osteoporos 13(1):84, PMID: 30076472, 10.1007/s11657-018-0498-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen AA, Leffers H. 2008. Emerging endocrine disrupters: perfluoroalkylated substances. Int J Androl 31(2):161–169, PMID: 18315716, 10.1111/j.1365-2605.2008.00870.x. [DOI] [PubMed] [Google Scholar]
- Johns LE, Ferguson KK, Meeker JD. 2016. Relationships between urinary phthalate metabolite and bisphenol A concentrations and vitamin D levels in U.S. adults: National Health and Nutrition Examination Survey (NHANES), 2005–2010. J Clin Endocrinol Metab 101(11):4062–4069, PMID: 27648964, 10.1210/jc.2016-2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DR, Jarrett JM, Tevis DS, Franklin M, Mullinix NJ, Wallon KL, et al. 2017. Analysis of whole human blood for Pb, Cd, Hg, Se, and Mn by ICP-DRC-MS for biomonitoring and acute exposures. Talanta 162:114–122, PMID: 27837806, 10.1016/j.talanta.2016.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones IE, Williams SM, Dow N, Goulding A. 2002. How many children remain fracture-free during growth? A longitudinal study of children and adolescents participating in the Dunedin Multidisciplinary Health and Development Study. Osteoporos Int 13(12):990–995, PMID: 12459942, 10.1007/s001980200137. [DOI] [PubMed] [Google Scholar]
- Kalkwarf HJ, Gilsanz V, Lappe JM, Oberfield S, Shepherd JA, Hangartner TN, et al. 2010. Tracking of bone mass and density during childhood and adolescence. J Clin Endocrinol Metab 95(4):1690–1698, PMID: 20194709, 10.1210/jc.2009-2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkwarf HJ, Laor T, Bean JA. 2011. Fracture risk in children with a forearm injury is associated with volumetric bone density and cortical area (by peripheral QCT) and areal bone density (by DXA). Osteoporos Int 22(2):607–616, PMID: 20571770, 10.1007/s00198-010-1333-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, Wong LY, Jia LT, Kuklenyik Z, Calafat AM. 2011. Trends in exposure to polyfluoroalkyl chemicals in the U.S. population: 1999–2008. Environ Sci Technol 45(19):8037–8045, PMID: 21469664. [DOI] [PubMed] [Google Scholar]
- Keil AP, Buckley JP, O’Brien KM, Ferguson KK, Zhao S, White AJ. 2020. A quantile-based g-computation approach to addressing the effects of exposure mixtures. Environ Health Perspect 128(4):47004, PMID: 32255670, 10.1289/EHP5838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil N, Chen A, Lee M, Czerwinski SA, Ebert JR, DeWitt JC, et al. 2016. Association of perfluoroalkyl substances, bone mineral density, and osteoporosis in the U.S. population in NHANES 2009–2010. Environ Health Perspect 124(1):81–87, PMID: 26058082, 10.1289/ehp.1307909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil N, Ebert JR, Honda M, Lee M, Nahhas RW, Koskela A, et al. 2018. Perfluoroalkyl substances, bone density, and cardio-metabolic risk factors in obese 8-12 year old children: a pilot study. Environ Res 160:314–321, PMID: 29040951, 10.1016/j.envres.2017.10.014. [DOI] [PubMed] [Google Scholar]
- Khosla S, Monroe DG. 2018. Regulation of bone metabolism by sex steroids. Cold Spring Harb Perspect Med 8(1):a031211, PMID: 28710257, 10.1101/cshperspect.a031211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindler JM, Kalkwarf HJ, Lappe JM, Gilsanz V, Oberfield S, Shepherd JA, et al. 2020. Pediatric reference ranges for ultradistal radius bone density: results from the bone mineral density in childhood study. J Clin Endocrinol Metab 105(10):e3529–e3539, PMID: 32561914, 10.1210/clinem/dgaa380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindler JM, Lappe JM, Gilsanz V, Oberfield S, Shepherd JA, Kelly A, et al. 2019. Lumbar spine bone mineral apparent density in children: results from the bone mineral density in childhood study. J Clin Endocrinol Metab 104:1283–1292, PMID: 30265344, 10.1210/jc.2018-01693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koskela A, Finnilä MA, Korkalainen M, Spulber S, Koponen J, Håkansson H, et al. 2016. Effects of developmental exposure to perfluorooctanoic acid (PFOA) on long bone morphology and bone cell differentiation. Toxicol Appl Pharmacol 301:14–21, PMID: 27068293, 10.1016/j.taap.2016.04.002. [DOI] [PubMed] [Google Scholar]
- Koskela A, Koponen J, Lehenkari P, Viluksela M, Korkalainen M, Tuukkanen J. 2017. Perfluoroalkyl substances in human bone: concentrations in bones and effects on bone cell differentiation. Sci Rep 7(1):6841, PMID: 28754927, 10.1038/s41598-017-07359-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Fletcher T, Mucs D, Scott K, Lindh CH, Tallving P, et al. 2018. Half-lives of PFOS, PFHxS and PFOA after end of exposure to contaminated drinking water. Occup Environ Med 75(1):46–51, PMID: 29133598, 10.1136/oemed-2017-104651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin LY, Wen LL, Su TC, Chen PC, Lin CY. 2014. Negative association between serum perfluorooctane sulfate concentration and bone mineral density in US premenopausal women: NHANES, 2005–2008. J Clin Endocrinol Metab 99(6):2173–2180, PMID: 24606077, 10.1210/jc.2013-3409. [DOI] [PubMed] [Google Scholar]
- Liu Y, Li N, Papandonatos GD, Calafat AM, Eaton CB, Kelsey KT, et al. 2020. Exposure to per- and polyfluoroalkyl substances and adiposity at age 12 years: evaluating periods of susceptibility. Environ Sci Technol 54(24):16039–16049, PMID: 33269902, 10.1021/acs.est.0c06088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackinnon DP, Lockwood CM, Williams J. 2004. Confidence limits for the indirect effect: distribution of the product and resampling methods. Multivariate Behav Res 39(1):99, PMID: 20157642, 10.1207/s15327906mbr3901_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahapatra CT, Damayanti NP, Guffey SC, Serafin JS, Irudayaraj J, Sepúlveda MS. 2017. Comparative in vitro toxicity assessment of perfluorinated carboxylic acids. J Appl Toxicol 37(6):699–708, PMID: 27917506, 10.1002/jat.3418. [DOI] [PubMed] [Google Scholar]
- Mamsen LS, Björvang RD, Mucs D, Vinnars MT, Papadogiannakis N, Lindh CH, et al. 2019. Concentrations of perfluoroalkyl substances (PFASs) in human embryonic and fetal organs from first, second, and third trimester pregnancies. Environ Int 124:482–492, PMID: 30684806, 10.1016/j.envint.2019.01.010. [DOI] [PubMed] [Google Scholar]
- McCormack SE, Cousminer DL, Chesi A, Mitchell JA, Roy SM, Kalkwarf HJ, et al. 2017. Association between linear growth and bone accrual in a diverse cohort of children and adolescents. JAMA Pediatr 171(9):e171769, PMID: 28672287, 10.1001/jamapediatrics.2017.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JA, Chesi A, Elci O, McCormack SE, Roy SM, Kalkwarf HJ, et al. 2016. Physical activity benefits the skeleton of children genetically predisposed to lower bone density in adulthood. J Bone Miner Res 31(8):1504–1512, PMID: 27172274, 10.1002/jbmr.2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris NM, Udry JR. 1980. Validation of a self-administered instrument to assess stage of adolescent development. J Youth Adolesc 9(3):271–280, PMID: 24318082, 10.1007/BF02088471. [DOI] [PubMed] [Google Scholar]
- Muthén B, Asparouhov T. 2015. Causal effects in mediation modeling: an introduction with applications to latent variables. Struct Equ Modeling 22(1):12–23, 10.1080/10705511.2014.935843. [DOI] [Google Scholar]
- Olsen GW, Burris JM, Ehresman DJ, Froehlich JW, Seacat AM, Butenhoff JL, et al. 2007. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ Health Perspect 115(9):1298–1305, PMID: 17805419, 10.1289/ehp.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez F, Nadal M, Navarro-Ortega A, Fàbrega F, Domingo JL, Barceló D, et al. 2013. Accumulation of perfluoroalkyl substances in human tissues. Environ Int 59:354–362, PMID: 23892228, 10.1016/j.envint.2013.06.004. [DOI] [PubMed] [Google Scholar]
- Rappazzo KM, Coffman E, Hines EP. 2017. Exposure to perfluorinated alkyl substances and health outcomes in children: a systematic review of the epidemiologic literature. Int J Environ Res Public Health 14:, PMID: 28654008, 10.3390/ijerph14070691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen MB, Lee JS, Ren H, Vallanat B, Liu J, Waalkes MP, et al. 2008. Toxicogenomic dissection of the perfluorooctanoic acid transcript profile in mouse liver: evidence for the involvement of nuclear receptors PPAR alpha and CAR. Toxicol Sci 103(1):46–56, PMID: 18281256, 10.1093/toxsci/kfn025. [DOI] [PubMed] [Google Scholar]
- Sayers A, Tobias JH. 2010. Fat mass exerts a greater effect on cortical bone mass in girls than boys. J Clin Endocrinol Metab 95(2):699–706, PMID: 20008022, 10.1210/jc.2009-1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd JA, Wang L, Fan B, Gilsanz V, Kalkwarf HJ, Lappe J, et al. 2011. Optimal monitoring time interval between DXA measures in children. J Bone Miner Res 26(11):2745–2752, PMID: 21773995, 10.1002/jbmr.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer A, Exuzides A, Spangler L, O’Malley C, Colby C, Johnston K, et al. 2015. Burden of illness for osteoporotic fractures compared with other serious diseases among postmenopausal women in the United States. Mayo Clin Proc 90(1):53–62, PMID: 25481833, 10.1016/j.mayocp.2014.09.011. [DOI] [PubMed] [Google Scholar]
- Taylor A, Konrad PT, Norman ME, Harcke HT. 1997. Total body bone mineral density in young children: influence of head bone mineral density. J Bone Miner Res 12(4):652–655, PMID: 9101377, 10.1359/jbmr.1997.12.4.652. [DOI] [PubMed] [Google Scholar]
- Taylor SJ, Whincup PH, Hindmarsh PC, Lampe F, Odoki K, Cook DG. 2001. Performance of a new pubertal self-assessment questionnaire: a preliminary study. Paediatr Perinat Epidemiol 15(1):88–94, PMID: 11237120, 10.1046/j.1365-3016.2001.00317.x. [DOI] [PubMed] [Google Scholar]
- Valeri L, Vanderweele TJ. 2013. Mediation analysis allowing for exposure-mediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros. Psychol Methods 18(2):137–150, PMID: 23379553, 10.1037/a0031034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zwol-Janssens C, Trasande L, Asimakopoulos AG, Martinez-Moral MP, Kannan K, Philips EM, et al. 2020. Fetal exposure to bisphenols and phthalates and childhood bone mass: a population-based prospective cohort study. Environ Res 186:109602, PMID: 32668547, 10.1016/j.envres.2020.109602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanden Heuvel JP, Thompson JT, Frame SR, Gillies PJ. 2006. Differential activation of nuclear receptors by perfluorinated fatty acid analogs and natural fatty acids: a comparison of human, mouse, and rat peroxisome proliferator-activated receptor-alpha, -beta, and -gamma, liver X receptor-beta, and retinoid X receptor-alpha. Toxicol Sci 92(2):476–489, PMID: 16731579, 10.1093/toxsci/kfl014. [DOI] [PubMed] [Google Scholar]
- Verner MA, Loccisano AE, Morken NH, Yoon M, Wu H, McDougall R, et al. 2015. Associations of perfluoroalkyl substances (PFAS) with lower birth weight: an evaluation of potential confounding by glomerular filtration rate using a physiologically based pharmacokinetic model (PBPK). Environ Health Perspect 123(12):1317–1324, PMID: 26008903, 10.1289/ehp.1408837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Ibrahim WN, Tofighi R, Onishchenko N, Rebellato P, Bose R, Uhlen P, et al. 2013. Perfluorooctane sulfonate induces neuronal and oligodendrocytic differentiation in neural stem cells and alters the expression of PPARγ in vitro and in vivo. Toxicol Appl Pharmacol 269(1):51–60, PMID: 23500012, 10.1016/j.taap.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Watkins AM, Wood CR, Lin MT, Abbott BD. 2015. The effects of perfluorinated chemicals on adipocyte differentiation in vitro. Mol Cell Endocrinol 400:90–101, PMID: 25448844, 10.1016/j.mce.2014.10.020. [DOI] [PubMed] [Google Scholar]
- Weaver CM, Gordon CM, Janz KF, Kalkwarf HJ, Lappe JM, Lewis R, et al. 2016. The national osteoporosis foundation’s position statement on peak bone mass development and lifestyle factors: a systematic review and implementation recommendations. Osteoporos Int 27(4):1281–1386, PMID: 26856587, 10.1007/s00198-015-3440-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber DR, Moore RH, Leonard MB, Zemel BS. 2013. Fat and lean BMI reference curves in children and adolescents and their utility in identifying excess adiposity compared with BMI and percentage body fat. Am J Clin Nutr 98(1):49–56, PMID: 23697708, 10.3945/ajcn.112.053611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wren TA, Kalkwarf HJ, Zemel BS, Lappe JM, Oberfield S, Shepherd JA, et al. 2014. Longitudinal tracking of dual-energy X-ray absorptiometry bone measures over 6 years in children and adolescents: persistence of low bone mass to maturity. J Pediatr 164(6):1280–1285.E2, PMID: 24485819, 10.1016/j.jpeds.2013.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright NC, Looker AC, Saag KG, Curtis JR, Delzell ES, Randall S, et al. 2014. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res 29(11):2520–2526, PMID: 24771492, 10.1002/jbmr.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemel BS, Kalkwarf HJ, Gilsanz V, Lappe JM, Oberfield S, Shepherd JA, et al. 2011. Revised reference curves for bone mineral content and areal bone mineral density according to age and sex for black and non-black children: results of the bone mineral density in childhood study. J Clin Endocrinol Metab 96(10):3160–3169, PMID: 21917867, 10.1210/jc.2011-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Ren XM, Wan B, Guo LH. 2014. Structure-dependent binding and activation of perfluorinated compounds on human peroxisome proliferator-activated receptor γ. Toxicol Appl Pharmacol 279(3):275–283, PMID: 24998974, 10.1016/j.taap.2014.06.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


