Abstract
The occurrence of bovine ketosis involves the accumulation of β-hydroxybutyric acid (BHBA), which contributes to the initiation and acceleration of hepatic metabolic stress and inflammation. Metformin has other beneficial effects apart from its medical intervention for diabetes, such as prevention of laminitis and hyper-triglyceridemic. AMPK maintains energy homeostasis and is the intracellular target of metformin action. This study aims to uncover the role of metformin in modulating BHBA-induced inflammatory responses through the activation of AMPK signaling. The hepatocytes were isolated from the liver tissue of mid-lactation multiparous Holstein cows (~160 d postpartum). Treatments were conducted as follows: treated with PBS for 18 h (control); pretreated with PBS for 12 h followed by treatment of 1.2 mM BHBA for 6 h (BHBA); pretreated with 1.5 mM or 3 mM metformin for 12 h followed by the BHBA treatment (1.2 mM) for 6 h (M(1.5)+B; M(3)+B). The inhibitor of AMPK, Compound C, at a concentration of 10 μM, was applied to substantiate the AMPK-dependent responses. RT-qPCR were applied for the mRNA expression while Western-blots and immunofluorescence were conducted for the target proteins expression. Among dose-dependent assays for BHBA, the concentration of BHBA at 1.2 mM activated NF-κB signaling by upregulating the expression of phosphorylated NF-κB and pro-inflammatory cytokines compared with the control cells (P < 0.05). Along with the upregulation of phosphorylated AMPKα and ACCα, metformin at 1.5 and 3 mM inactivated NF-κB signaling components (p65 and IκBα) and the inflammatory genes (TNFA, IL6, IL1B and COX-2) which were activated by BHBA. Additionally, BHBA inhibited cells staining intensity in EdU assay were increased by pretreatment with metformin. The activation of AMPK resulted in the increased gene and protein expression of SIRT1, along with the deacetylation of H3K9 and H3K14. However, the AMPK inhibitor compound C blocked this effect. Compared with BHBA treated cells, the protein expression of COX-2 and IL-1β were decreased by the pretreatment with metformin, and the inhibitory effect of metformin was released by compound C. The bound of NF-κB onto IL1B promoter displayed higher in BHBA group and this was suppressed by pretreatment with metformin (P < 0.05). Altogether, metformin attenuates the BHBA-induced inflammation through the inactivation of NF-κB as a target for AMPK/SIRT1 signaling in bovine hepatocytes.
Keywords: bovine hepatocytes, β-hydroxybutyric acid, inflammation, AMPK signaling, NF-κB signaling
Introduction
Elevated β-hydroxybutyric acid (BHBA) in blood circulation is regarded as one of the leading indicators of bovine ketosis (Xia et al., 2012). Unlike monogastric animals, ruminants suffer from physiological stress, pregnancy, and early lactation are exposed to the incidence of negative energy balance (NEB). The NEB caused by the decreased dry matter intake and increased demand for energy to support milk production is often suggested to induce mobilization of non-esterified fatty acids (NEFA) and BHBA(McArt et al., 2013; Song et al., 2014). The occurrence of bovine ketosis is related to the inflammatory response as well as oxidative stress along with metabolic disorders due to the over production of BHBA(Hammon et al., 2006). It has been reported that plasma concentration of BHBA over 1.2 mM is defined as subclinical ketosis (Iwersen et al., 2013). Furthermore, previous studies have highlighted that ketosis and infectious disease are positively correlated (Shi et al., 2014; Song et al., 2016). Among the various types of signaling that regulates inflammation, the NF-κB complex of transcription factors acts as a master switch in regulating a wealth of immune genes, including pro-inflammatory cytokines like tumor necrosis factor-α (TNFA), interleukin-1β (IL1B), and -6 (IL6) (Hayden and Ghosh, 2011). The existing literature suggests that BHBA induces the secretion of pro-inflammatory factors through the activation of nuclear factor kappa B (NF-κB) signaling pathway in calf hepatocytes (Shi et al., 2014).
Metformin (1, 1-dimethylbiguanide hydrochloride) was originally developed from natural compounds found in the plant Galega officinalis, known as French lilac or goat’s rue. For decades, it has been the first-line therapy for the treatment of type 2 diabetes. An increasing number of studies has suggested that metformin-induced activation of AMPK blocks the inflammatory response via the inhibition of NF-κB signaling pathway in rat aortic smooth muscle cells or human umbilical vein endothelial cells (Hattori et al., 2006; Xiaorui et al., 2013). Recent research has shown that sirtuin 1 (SIRT1) mediates the effect of AMPK-dependent regulation on inflammation and apoptosis in bovine retinal capillary endothelial cells (BREC) and the retina of diabetic rats(Zheng et al., 2012). Since the activity of SIRT1 is NAD-dependent, innate immunity and energy metabolism are connected through the crosstalk between NF-κB and SIRT1 signaling pathways (Kauppinen et al., 2013). Our recent research also revealed that the supply of metformin assists in reducing inflammation via AMPK/NF-κB signaling in LPS-challenged bovine mammary epithelial cells (Xu et al., 2021). Furthermore, studies have demonstrated that AMPK signaling regulates bovine hepatic lipid metabolism in BHBA-mediated disorders (Deng et al., 2015). These studies reveal that the signaling crosstalk among the AMPK, NF-κB and SIRT1 pathways controls the homeostasis of inflammatory phases and energy metabolic supply. However, the role of AMPK signaling in the regulation of BHBA-induced inflammation of bovine hepatocytes remains unclear. The current study aims to uncover metformin’s suppressive effect on BHBA-induced inflammation through the AMPK/SIRT1/NF-κB signaling pathway in bovine hepatocytes.
Materials and Methods
All experimental procedures were approved by the Animal Experiment Committee of Yangzhou University, in accordance with the Regulations for the Administration of Affairs Concerning Experimental Animals (The State Science and Technology Commission of China, 1988) published by the Ministry of Science and Technology, China, in 2004. All of the experimental protocols were performed in accordance with the approved guidelines and regulations.
Chemicals
BHBA was purchased from Sigma-Aldrich (St. Louis, MO). The 10-mM BHBA stock solution was prepared in distilled water and sterilized by filtration. Metformin was purchased from Sigma (D150959, Sigma-Aldrich, St. Louis, USA) with a purity of more than 97%. Then, 1 mM of metformin was dissolved in PBS as a stock solution. Compound C (an AMPK inhibitor) was purchased from MedChemExpress (BML-275, MCE, NJ), and 10 mM was prepared in DMSO.
Cell culture conditions
The bovine hepatocytes used in this study were obtained and cultured in conditions described in the previous publication (Xu et al., 2018). The hepatocytes were briefly isolated from the liver tissue of mid-lactation multiparous Holstein cows (~160 d postpartum). All experiments were performed with cells at the 4 to 6 passage. Cells (2 × 105) were seeded in 6-well plates with overnight incubation in complete medium (90% RPMI 1640, 8119417 Gibco, CA, 10% fetal bovine serum) (Gibco, CA) and antibiotics (penicillin 100 IU/mL; streptomycin 100 μg/mL). Finally, the cells were maintained at 37 °C in a humidified 5% CO2 incubator until reaching confluence.
Experimental design
The scale of the BHBA concentration in this study was based on the blood concentration of dairy cows with ketosis. The hepatocytes were isolated from the liver tissue of mid-lactation multiparous Holstein cows (~160 d postpartum). For the pre-experimental treatment, the bovine primary hepatocytes were precultured with serum-free medium for 12 h. In the dose-dependent experiment for metformin, cells were treated with metformin for 12 h at concentrations of 0 mM, 0.5 mM, 1.5 mM, 3 mM, 5 mM, and 10 mM. In the dose-dependent experiment for BHBA, cells were treated with BHBA for 6 h at concentrations of 0 mM, 0.6 mM, 1.2 mM, 2.4 mM, 10 mM, and 20 mM. Treatments of cells were conducted as follows: treated with PBS for 18 h (control); pretreated with PBS for 12 h followed by treatment of 1.2 mM BHBA for 6 h (BHBA); pretreated with 1.5 mM or 3 mM metformin for 12 h followed by the BHBA treatment (1.2 mM) for 6 h (M(1.5)+B; M(3)+B). The inhibitor of AMPK, Compound C, at a concentration of 10 μM, was applied to substantiate the AMPK-dependent responses.
Flow cytometry
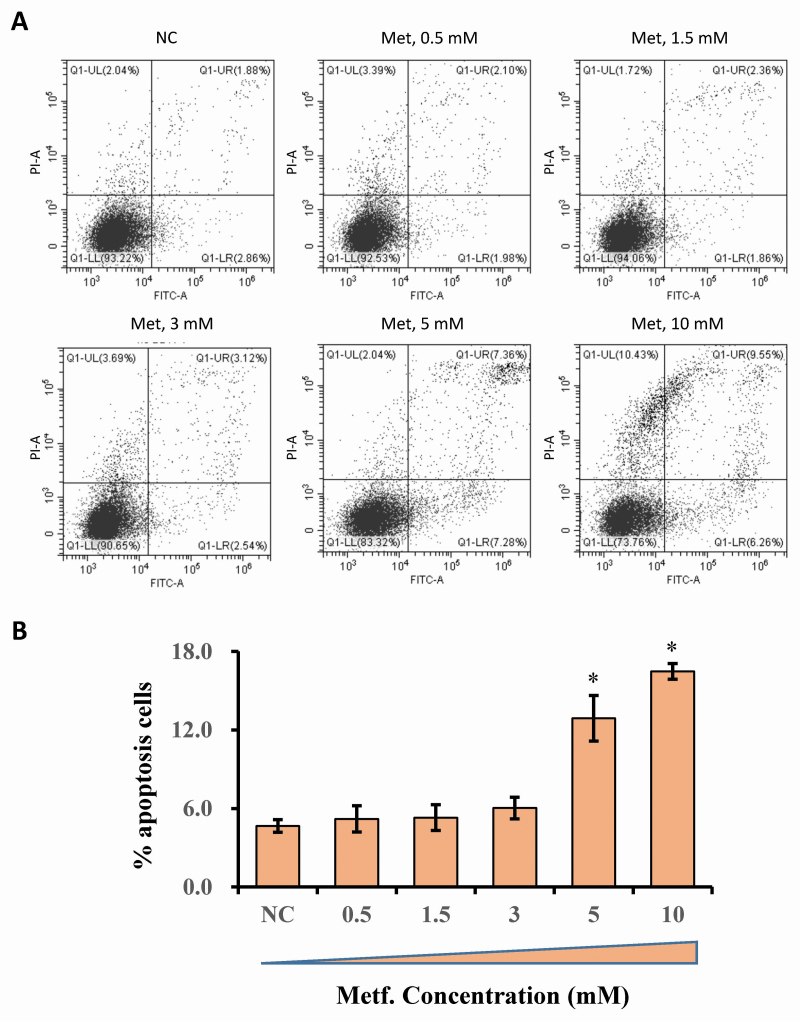
Bovine hepatocytes were seeded into 6-well plates (2 × 105 cells/well). Cells were maintained in medium with 10% (v/v) fetal bovine serum and the various treatments selected for this study. Cells were treated with metformin at concentrations of 0 mM, 1 mM, 2 mM, 3 mM, 5 mM, and 10 mM. The apoptotic effect of metformin on differentiating hepatocytes was evaluated using an Annexin V staining kit (#11858777001, Sigma-Aldrich). Cells were collected via trypsinization and stained. The flow cytometry was analyzed using the FACSCalibur platform (BD Biosciences, Franklin Lakes, NJ).
5-ethynyl-2’-deoxyuridine (EdU) detection
Following the manufacturer’s protocols, the BeyoClick EdU cell proliferation kit with Alexa Fluor 555 (Beyotime, Shanghai, China) was used to measure the ability of bovine hepatocytes to proliferate under different treatments. Cells were incubated with 1X Edu (10 μM) solution for 2 h, subsequently fixed with 4% paraformaldehyde for 20 min at room temperature (RT), and permeabilized with 0.3% Triton X-100 in PBS for 15 min at RT. For nuclear staining, cells were incubated with 1X Hoechst for 10 min at RT protected from light. Finally, cells were imaged with a fluorescence microscope DMi8 Microsystems GmbH (Leica, Wetzlar, Germany) at 200× magnification.
RNA extraction and real-time quantitative PCR analysis
Total RNA was isolated with the RNA Isolater Total RNA Extraction Reagent (R401-01, Vazyme, Nanjing, China) according to the manufacturer’s instructions. Then, cDNA was synthesized using HiScript III RT SuperMix (R323-01, Vazyme, Nanjing, China) and then purified with a purification kit (Axygen, Tewksbury, MA). In line with a previous study, qRT-PCR was performed using AceQ Universal SYBR qPCR Master Mix (Vazyme, Nanjing, China) on an Applied Biosystems QuantStudio 5 Real-Time PCR System (Applied Biosystems, Foster City, CA) (Xu et al., 2018). Furthermore, primers were designed with the Premier 6.0 software (Premier Biosoft International, Palo Alto, CA), as illustrated in earlier publications (Xu et al. 2015, 2017). The selected genes, specifically GAPDH, RPS9, and UXT, were used as internal control genes. The geometric mean of the internal control genes was utilized to normalize the target gene expression data. Previous reports have validated the use of internal control genes as references for normalizing gene expression in hepatocytes samples (Zhou et al., 2018). The 2−ΔΔCt method was adopted for relative quantification (Pfaffl, 2001).
NAD+ measurement
Using the NAD+/NADH assay kit with WST-8 according to the manufacturer’s instructions (Beyotime, S0175, Beijing, China), after the treatments, hepatocytes (1 × 106 cells/sample) were harvested, and intracellular NAD+ levels were determined. Briefly, cell lysis was collected with 200 μL of pre-cold lysis buffer. In order to measure the total NAD+/NADH, 20 μL of cell lysates was added to a 96-well plate. Then, measuring NADH required the incubation of the lysed cell suspension at 60 °C for 30 min, and 20 μL was added to a 96-well plate. Subsequently, 90 μL of alcohol dehydrogenase was added and incubated at 37 °C for 10 min. Finally, 10 μL of the chromogenic solution was added to the plate, and the mixture was incubated at 37 °C for 30 min. The standard curve was generated and measured with the samples simultaneously. The absorbance was obtained at 450 nm and analyzed on a microplate reader (SPARK, TECAN, Switzerland). By subtracting NADH from the total NAD+/NADH, the amount of NAD+ was derived.
Western-blotting analysis
Western blot was performed using the protocols described previously (Chen et al., 2019). Equal amounts of protein isolated from pbMEC by RIPA lysis buffer (Beyotime, Shanghai, China) were briefly separated on 10% SDS polyacrylamide gels (GenScript ExpressPlus PAGE Gels, Nanjing, China). Then, proteins were transferred onto nitrocellulose membranes (Millipore, Billerica, MA), which were incubated with primary antibodies overnight at 4 °C. After washing for six consecutive times, the blots were incubated with horseradish peroxidase-coupled secondary antibody. Differences in protein transfer efficiency between blots were normalized with GAPDH quantification. The gray values of the bands of each target protein were quantified using the ImageJ system analysis software. Primary antibodies for p-P65, IL1β, TNFα, acetyl-H3K14, acetyl-H3K9, histone H3, p-AMPKα, AMPKα, p-ACCα, ACCα, COX-2, and SIRT1 were purchased from Cell Signaling Technology (Danvers, MA) (#3033, #15101, #3866, #7627, #9649S, #4499, #2535, #5831, #11818, #3676, #12282S, and # 2496S), and p65 was purchased from Abcam (ab16502) and diluted 1:1,000 for incubation. The primary antibody for GAPDH was purchased from Abcam Corporation (ab8245) and diluted 1:5,000 for incubation.
Immunofluorescence
After the treatment, bovine hepatocytes (2 × 104 cells/well) were seeded onto 12-well plates accordingly. Then, the cells were fixed with 4% paraformaldehyde for 15 min, washed with PBS, and incubated with 0.5% Triton X-100 for 15 min at room temperature to increase the permeability. Incubation with 5% BSA at 37 °C for 1 h was needed for blocking. The cells were incubated at 4 °C overnight with the primary antibody (targeting the proteins interested) in PBS containing 1% BSA and 0.3 Triton X-100 (T9284, Sigma-Aldrich). After three PBS washes, the cells were incubated for 1 h with the secondary antibody in a dark 37 °C room and then washed three times with PBS. DAPI (1 μg/mL) (D8417, Sigma-Aldrich) was used for nuclear counterstaining for 5 min, and then the cells were washed three times. Finally, the cells were imaged using the DMi8 Microsystems GmbH (Leica, Wetzlar, Germany).
Chromatin immunoprecipitation assay
The preparation of samples and experiment was performed based on the protocols described previously (Xu et al., 2017). The cells were briefly seeded into six-well plates for treatment and harvested using the PBS containing protease inhibitor cocktail (Cat. #11697498001; Roche, Basel, Switzerland). Formaldehyde at a concentration of 1% was added for the cross-link of protein and DNA. After shaking for 10 min, glycine was used to stop the reaction. The mix was then centrifuged at 4 °C with 4,000 × g for 5 min. Chromatin preparations were fragmented at 200–500 bp in length with sonication on ice and then incubated with 4 µg of primary antibody (Anti-P65, ab16502, Abcam) at 4 °C for 16 h. Rabbit IgG was incubated with the sample as a negative control. Protein A/G agarose beads (40 µL, 50% slurry, sc-2003; Santa Cruz Biotechnology) were utilized to capture immunoprecipitated chromatin complexes, and 200 µL of chromatin preparation served as the input. Promoter fragments harvested during chromatin immunoprecipitation were quantified with qPCR using primers specific to the respective areas of IL1B promoter (forward 5’ GGCT CAGCTTGTAAAGAATC 3’ and reverse 5’ GAATGCACGAAAGTC ATCC 3’).
Statistics
The data were expressed as the means ± standard deviation (mean ± SD) and analyzed using one-way ANOVA with Dunnett’s post-test by SAS Statistics (v 9.2, SAS Institute Inc., Cary, NC). Normalized mRNA and protein expression data were log2 transformed before statistical analysis. Differences with P-values <0.05 were considered statistically significant. All experiments were carried out in triplicate, with three replicates in each experiment.
Results
Determination of metformin’s cytotoxic effect under different doses
Metformin’s cytotoxicity was determined using flow cytometry and results are shown in Figure 1. At doses ranging from 0 to 3 mM, no cytotoxic effect of metformin was observed. Significant cytotoxicity was found at doses of 5 and 10 mM with increased apoptotic cells compared with that in the control cells (P < 0.05). Based on the cytotoxic study of metformin, we selected the specific doses of 1.5 and 3 mM for use in the following experiments.
Figure 1.
Flow cytometric analysis of hepatocytes treated with doses ranging from 0 to 10 mM of metformin. Cells were treated with metformin for 12 h at concentrations of 0 mM, 0.5 mM, 1.5 mM, 3 mM, 5 mM, and 10 mM. (A) X-axis indicates the absorbance of FITC-A; Y-axis indicates the absorbance of fluorescein isothiocyanate−Annexin V. (B) The apoptosis percentages in cultures exposed to doses ranging from 0 to 10 mM of metformin. Results were expressed as the mean ± SD. *P < 0.05 vs. the NC group. These data are representative of three independent experiments.
Determination of BHBA-induced inflammatory responses and AMPK activation by metformin
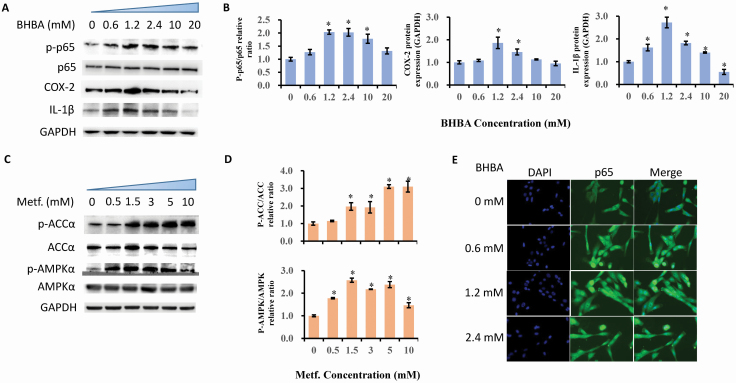
To verify the BHBA-induced inflammatory responses, we examined the protein expression in terms of the phosphorylation levels of p65, COX-2, and IL-1β with an increasing amount of BHBA in bovine hepatocytes. The significant upregulation of phosphorylated p65 appeared at the BHBA concentrations of 1.2, 2.4, and 10 mM compared with the 0 mM BHBA group (P = 0.001, P = 0 .003, and P = 0.014, respectively). Consistently, BHBA at doses of 1.2 and 2.4 induced a dramatic increase in staining and translocation of p65 into nuclear. COX-2 concentration exhibited elevation in the cells treated with 1.2 and 2.4 mM of BHBA compared with the 0 mM group (P = 0.031 and P = 0.036). Additionally, the level of IL-1β protein expressed was proximately 2-folds higher in 1.2 mM BHBA treated cells than that of the cells in the 0 mM BHBA group (P < 0.001). Interestingly, BHBA at a concentration of 20 mM decreased the IL-1β expression, though the cells treated with 2.4 and 10 mM of BHBA displayed upregulation of IL-1β compared with the 0 mM BHBA group (P = 0.002). Figure 2A, B, and E illustrates the data.
Figure 2.
Determination of BHBA induced inflammatory responses and AMPK activation by metformin. Results were expressed as the mean ± SD. (A and B) Immunoblotting and intensity from the respective blots. Cells were treated with BHBA for 6 h at concentrations of 0 mM, 0.6 mM, 1.2 mM, 2.4 mM, 10 mM, and 20 mM. *P < 0.05 vs. the cell without treatment with BHBA at 0 mM. (C and D) Immunoblotting and intensity from the respective blots. Cells were treated with metformin for 12 h at concentrations of 0 mM, 0.5 mM, 1.5 mM, 3 mM, 5 mM, and 10 mM. *P < 0.05 vs. the cell without treatment with metformin at 0 mM. The proteins expression was normalized by the respective abundance of GAPDH. (E) Effect of BHBA on the predominant location of NF-κB p65 protein in bovine hepatocytes nuclei with doses at 0 mM, 0.6 mM 1.2 mM, and 2.4 mM of BHBA. Cells were treated with BHBA (0, 0.6, 1.2, 2.4 mM) for 6 h. Immunofluorescence for NF-κB p65 (green) was performed using FITC labeled secondary antibody, and the nuclear dye DAPI (blue) was used for the indication of nuclei.
In order to confirm the role of metformin in activating AMPK signaling in bovine hepatocytes, we incorporated the test for phosphorylation of AMPKα and ACCα using western blots, as shown in Figure 2C and D. After increasing the amount of metformin added to the cells, the phosphorylation of AMPKα was upregulated at doses ranging from 1.5 to 10 mM compared with the 0 mM metformin-treated cells (P < 0.05). After adding metformin, the concentration of phosphorylated ACCα increased from 0.5 mM all the way to 10 mM (P < 0.05). Based on the results of metformin’s significant cytotoxicity at doses higher than 5 mM, both 1.5 and 3 mM were deemed optimal doses for use in the succeeding experiments.
Metformin reversed the BHBA-induced pro-inflammatory genes expression
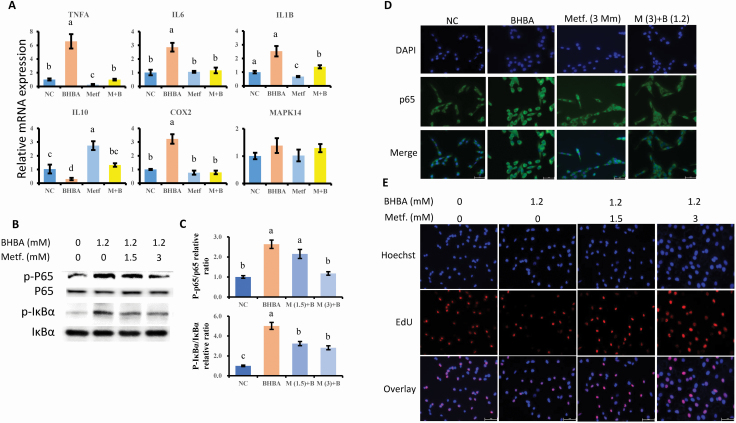
Figure 3A presents the expression of genes related to inflammatory responses. BHBA induced the increase of pro-inflammatory genes expression at the dose of 1.2 mM, as a result of the upregulation of TNFA, IL6, IL1B, and COX-2, compared with the NC group (P = 0.001, P = 0.001, P = 0.003, P = 0.006, respectively). In addition to the downregulation of TNFA and IL1B by metformin treatment, the expressions of the BHBA-induced upregulated inflammatory genes (TNFA, IL6, IL1B, and COX-2) were also suppressed by the pretreatment with metformin following BHBA induction (P = 0.001, P = 0.002, P = 0.008, P = 0.004, respectively). Moreover, as an anti-inflammatory gene, the expression level of IL10 in the cells pretreated with metformin at 3 mM following BHBA treatment was significantly upregulated when compared with that in the BHBA-primed cells (P < 0.001).
Figure 3.
The suppressive effect of metformin on the BHBA induced pro-inflammatory responses. (A) The expression of genes associated with pro- and anti-inflammation, normalized by the geometric mean of the internal control genes (GAPDH, UXT, and RPS9). The expression of genes in NC group was set as 1.0. (B and C) Protein levels of phosphorylated p65 and IκBα in each group of bovine hepatocytes. The expression of proteins in NC group was set as 1.0. The letters in superscript indicate that the difference between groups was significant (P < 0.05). (D) Immunofluorescence for detecting p65 activity. After fixation and permealization steps, cells were stained with antibody against subunit p65 of NF-κB (FITC labeled secondary antibody) and counterstained with DAPI (blue) for nuclear staining. (E) EdU assay for determination of cell proliferation. Cells were pretreated with or without metformin at doses of 1.5 and 3 mM for 12 h, followed by the induction of 1.2 mM BHBA for 6 h. Results were expressed as the mean ± SD. Metf is stand for metformin; BHBA is stand for β-hydroxybutyric acid;
Metformin affected the BHBA-induced activation of NF-κB signaling pathway and inhibition of cells proliferation
As shown in the BHBA-induced activation of NF-κB signaling, metformin is effective in the suppression of BHBA-activated NF-κB signaling. Figure 3B–D illustrates the data. Compared with the NC group, the addition of BHBA upregulated the protein expression of phosphorylated IκBα and NF-κB subunit p65 (P < 0.001, P = 0.002). However, significant inactivation of NF-κB signaling with regards to the decreased level of phosphorylated IκBα and p65 was observed in the cells pretreated with metformin compared with the BHBA group (P = 0.006, P = 0.003). We further studied metformin’s effect on BHBA-induced NF-κB translocation using immunofluorescence (Figure 3D). Compared with control cells, the higher expression and the predominant nuclear location of activated NF-κB (p65) in pbMEC confirmed the inflammatory response after BHBA addition. However, the BHBA-induced activation of NF-κB (p65) was dampened by pretreatment with metformin, leading to lower staining of NF-κB protein in the mammary cell nuclei.
As a result of the inhibited EdU staining, a BHBA dose of 1.2 mM affected the cells proliferation, compared with the control group. However, cells pretreated with metformin at a concentration of 3 mM blocked the effect of BHBA-mediated suppression of cells proliferation (Figure 3E).
SIRT1 is involved in the AMPK-mediated regulation of histone deacetylation
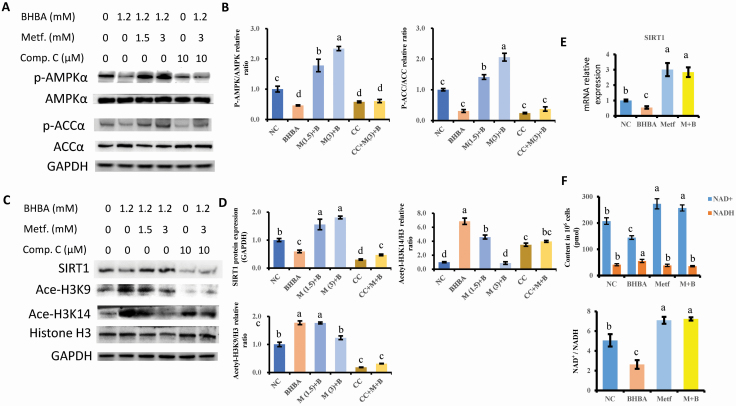
The levels of phosphorylated AMPKα and ACCα were lower in BHBA-primed bovine hepatocytes than those in the NC group (P = 0.006 and P < 0.001). Cells pretreated with metformin significantly upregulated the activity of AMPK signaling with regards to the increased expression of phosphorylated AMPKα and ACCα (P < 0.001). The AMPK inhibitor compound C blocked the effect of metformin on the AMPK signaling pathway activation, resulting in the equal levels of AMPKα and ACCα phosphorylation with the NC group (P = 0.06 and P = 0 .48).
Afterwards, we examined the expressions of SIRT1 and acetylated histone H3 at the positions of K9 and K14. The decreased gene and protein expression of SIRT1 induced by BHBA was upregulated by pretreatment with metformin (P < 0.001). Compound C significantly blocked metformin’s effect on the dampened expression of SIRT1 induced by BHBA (P < 0.001). As a histone deacetylase, the changes in SIRT1 also altered the histone H3 deacetylation level. BHBA induced a dramatic hyperacetylation of H3K9 and H3K14 compared with the control cells (P = 0.002 and P < 0.001). However, this increasing acetylation of both H3K9 and H3K14 was suppressed by the pretreatment with metformin (P = 0.005 and P < 0.001). It is worth noting that the treatment of cells with the AMPK inhibitor compound C led to the interruption of H3K14 deacetylation that resulted from metformin pretreatment, but not to the level of H3K9 deacetylation. Figure 4C–E presents the data.
Figure 4.
The involvement of AMPK signaling in regulating SIRT1 expression and Histone H3 deacetylation. (A and B) Immunoblotting and intensity from the respective blots. The proteins expression were normalized by the respective abundance of GAPDH. The expression of proteins in NC group was set as 1.0. (C and D) Immunoblotting and intensity from the respective blots. Compound C were represented for the inhibitory effect of AMPK activity. The proteins expression were normalized by the respective abundance of GAPDH. The expression of proteins in NC group was set as 1.0. (E) mRNA expression of SIRT1 normalized with geometric mean of GAPDH, UXT, and RPS9. The expression of SIRT1 in NC group was set as 1.0. (F) The concentration of NAD+, NADH, and the ratio of NAD+/NADH using WST-8 assay. Cells were pretreated with or without metformin at doses of 1.5 and 3 mM for 12 h, followed by the induction of 1.2 mM BHBA for 6 h. The inhibitor of AMPK, Compound C, at a concentration of 10 μM, was applied to substantiate the AMPK-dependent responses. The letters in superscript indicate that the difference between groups was significant (P < 0.05). Results were expressed as the mean ± SD. These data are representative of three independent experiments.
Since AMPK mediates the activity of NAD+-dependent histone deacetylase SIRT1, we investigated the concentration of NAD+/NADH in hepatocytes subjected to BHBA and metformin treatment. According to the results, BHBA led to the reduction of NAD+ and elevation of NADH compared with the control cells (P = 0.002 and P = 0.026). However, the pretreatment of metformin significantly reversed the alteration of BHBA-affected production of NAD+ and NADH (P < 0.001 and P = 0.006). Moreover, the BHBA-induced decrease in the ratio of NAD+/NADH was increased by the pretreatment with metformin (P < 0.001). Figure 4 presents the data.
Inhibitory effect of metformin on inflammatory responses is driven by AMPK signaling
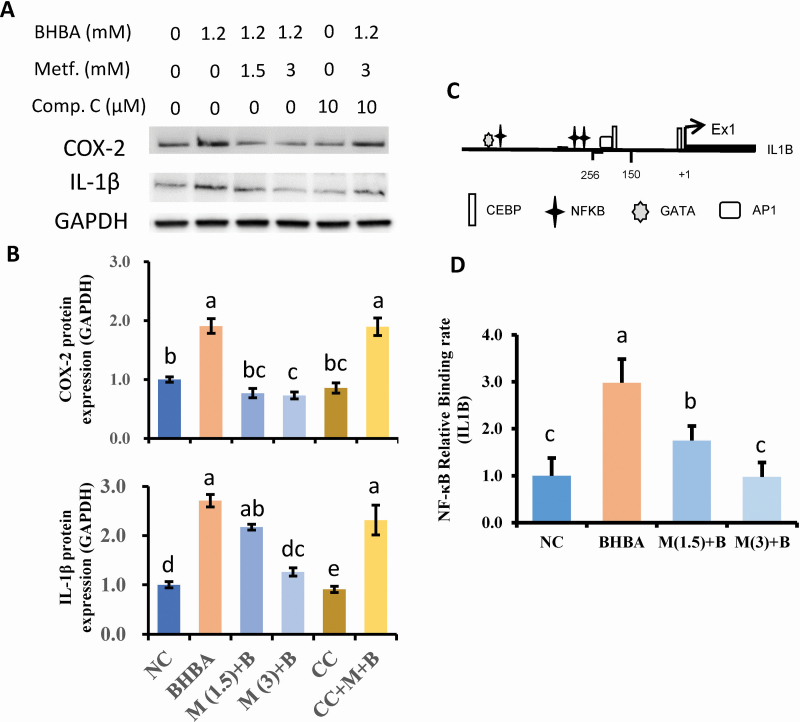
To further demonstrate the anti-inflammatory effect of metformin through an AMPK-dependent way, we tested the blockade of AMPK, which may result in the abrogation of metformin regulating immune responses in bovine hepatocytes. As shown in Figure 5A and B, the increased protein expression of COX-2 and IL-1β induced by BHBA were dampened by the pretreatment with metformin (P = 0.001 and P < 0.001). Compound C blocked preventive effect of metformin on the regulation of COX-2 and IL-1β protein expression.
Figure 5.
AMPK is required for the metformin mediated inhibition of pro-inflammatory proteins expression. (A and B) Immunoblotting and intensity from the respective blots. Compound C were represented for the inhibitory effect of AMPK activity. The proteins expression was normalized by the respective abundance of GAPDH. The expression of proteins in NC group was set as 1.0. (C) Numbers refer to the position relative to the transcriptional starting site, indicated by black arrows. The position of the transcription factors is indicated by the respective symbols. The positions of primers used for chromatin immunoprecipitation assays are denoted by dark lines surround the symbol of NF-κB. The identification for target promoter regions of candidate genes were determined by BLAST analysis as DNA-sequences that are 5’-upstream of the mRNA sequences deposited in the NCBI: NM_174093.1 (IL1B). (D) Level of NF-κB binding to IL1B promoter. Six individual samples in each group were involved in generating chromatin immunoprecipitation analysis. The letters in superscript indicate that the difference between groups was significant (P < 0.05). Cells were pretreated with or without metformin at doses of 1.5 and 3 mM for 12 h, followed by the induction of 1.2 mM BHBA for 6 h. Results were expressed as the mean ± SD. These data are representative of three independent experiments.
Since the acetylation of histone H3 may contribute to the difficulties associated with chromatin accessibility for DNA transcription initiation and subsequent protein expression, we incorporated the test for the NF-κB binding ability onto the IL1B promoter. The putative binding site for NF-κB on IL1B promoter was analyzed using the online PROMO software (http://alggen.lsi.upc.es/cgi-bin/promo_v3/ promo/promoinit.cgi?dirDB=TF_ 8.3). Filters ≥ 0.90 was established as the threshold for the similarity of the core sequence. The result showed that BHBA could drive the binding activity of NF-κB onto the IL1B promoter compared with the control group (P < 0.001). In contrast, pretreatment with metformin interdicted the binding ability of NF-κB compared with that in the BHBA treated cells (P = 0.001), which is in line with the decreased translocation of p65 into nuclear (Figure 5C and D).
Discussion
As one of the primary ketones, BHBA is overproduced during the excessive NEB-induced lipid mobilization from the adipose tissue(Zhang et al., 2013). The occurrence of bovine ketosis accompanied with BHBA elevation induces metabolic stress in the liver. Consequently, higher BHBA levels in the serum lead to metabolic disorders, reproductive failure, and inflammatory responses in lactating cows(Herdt, 2000; Song et al., 2021). In this study, we found that BHBA at a concentration of 1.2 mM results in the inflammation of primary bovine hepatocytes. However, cells pretreated with metformin attenuate the BHBA-induced inflammation through the regulation of AMPK/SIRT1/NF-κB signaling pathway.
The NF-κB transcription factor significantly contributes to the central regulation of mammalians inflammatory process (Lawrence, 2009; Hayden and Ghosh, 2011). It is worth noting that BHBA has been proposed to elicit the increase of pro-inflammatory factors (TNF-α, IL-6, and IL-1β) through the activation of NF-κB signaling in calf hepatocytes (Shi et al., 2014). Hence, the activation of NF-κB contributes to hepatic inflammation induced by both pathogenic infections and metabolic stress. Consistently, we found that increasing the BHBA amount led to the upregulation of phosphorylated NF-κB subunit p65. Along with the increase of the predominant location of NF-κB into nucleus, the increased secretion of COX-2 and IL-1β also indicates that BHBA functions to stimulate the hepatic stress due to overaccumulation of ketone and drive the cells into an inflammatory response.
Although studies have reported numerous concerns regarding metformin’s role in the regulation of inflammation, the underlying mechanism of the anti-inflammatory effects is still not fully understood. Some have revealed that AMPK contributes to the crucial mechanisms underlying metformin’s pleiotropic effects on different types of tissues (Huang et al., 2009; Jian et al., 2013; Ye et al., 2018; Yang et al., 2020). Nevertheless, the existing literature suggests that metformin mediates the anti-inflammatory effects through both AMPK dependent and independent way in human retinal microvascular endothelial cells (Jing et al., 2018). Although the activity of ACCα is linked to the lipogenesis and bovine liver is considered not a lipogenic tissue when compared with those non-ruminant animals, the investigation of phosphorylated ACCα is still an indication of the AMPK activity(Bergen and Mersmann, 2005). In this study, metformin at a concentration of 1.5 mM appeared to activate AMPK signaling by increasing the levels of phosphorylated AMPKα and phosphorylated ACCα. However, the inhibitor of AMPK (Compound C) blocked the metformin-induced activation of AMPK signaling in bovine hepatocytes. These results confirmed that AMPK signaling activation is involved in the effect of metformin and brought insights into the AMPK-dependent way of metformin in bovine hepatocytes.
Studies have put forward that AMPK could dampen inflammatory responses by modulating NF-κB, MAPK, and JAK/STAT signaling pathways. In this study, we investigated metformin’s inhibitory effect on the BHBA-induced inflammation response in bovine hepatocytes. Interestingly, cells pretreated with metformin lowered the BHBA-induced expression of pro-inflammatory genes (IL1B, TNFA, IL6, COX-2) and the expression of proteins (IL-1β and COX-2). Moreover, our study found that AMPK contributes to the anti-inflammatory effect with regards to the activation of IL10 expression. Concurrently, our results demonstrated that metformin decreased the BHBA-induced expression of phosphorylated p65 and the translocation of p65 in the nucleus of bovine hepatocytes along with the blocked phosphorylation of IκBα. Since NF-κB could be activated and acts as a master switch in regulating the expression of genes concerned with the inflammation in nuclear, our outcomes indicated that metformin might conduct the modulation of immune genes through NF-κB inactivation. Although metformin was suggested to reduce cells proliferation, like its role in anti-cancer properties, no effect was found in normal cells, such as the HEK293/TLR4 cells in a recent study (Queiroz et al., 2014; Brodowska et al., 2014; Zhihui et al., 2017). This supports that metformin may affect cells with a high proliferative and metabolic rate like tumor cells, but not the normal tissue cells (Zhihui et al., 2017). Similar to what was observed in cells proliferation, our results demonstrated that pretreatment with metformin enhanced the hepatocytes proliferation that was dampened by BHBA. Collectively, these outcomes imply that the inhibition of NF-κB by activation of AMPK is pivotal for metformin’s anti-inflammatory action.
Although its underlying mechanism has not been completely uncovered, the reduction of inflammatory responses by AMPK and SIRT1 activation might be involved (Yang et al., 2015). To determine by which way AMPK activation could attenuate NF-κB activity induced by BHBA in bovine hepatocytes, we investigated SIRT1-regulated histone deacetylation. Emerging pieces of evidence have suggested that SIRT1 mimics AMPK’s numerous effects on energy sensing and metabolism (Dasgupta and Milbrandt, 2007). Additionally, with the elevation of NAD+/NADH, the activation of SIRT1 represents both NF-κB deacetylation and histone deacetylation (Gillum et al., 2011; Kauppinen et al., 2013). Several mechanisms take place through the inhibitory effect of SIRT1 on specific target gene by modulating the chromatin structure, such as histone modification, acetylation of transcription factors, or DNA methylation (Zhang and Kraus, 2010). The reversed histone deacetylation at both K9 and K14 in the pretreatment of metformin following BHBA-induction in this study indicates that activation of SIRT1 may contribute to the inhibitory expression of immune genes through histone deacetylation. Furthermore, AMPK inhibitor compound C blocks the metformin-induced activation of SIRT1, and deacetylation of histone at lysine 14 may also evidence the role of AMPK/SIRT1 in the downregulation of pro-inflammatory genes induced by BHBA.
COX‐2 is expressed in many cell types like non-inflammatory cells and responds to inflammation through the synthesis of prostaglandins in monocytes and macrophages (Dannenberg et al., 2001; Howe and Dannenberg, 2002). Along with the decrease of phosphorylated p65 in cells pretreated with metformin, we found that Compound C also reduces the effects of AMPK-mediated suppression of COX-2 and IL-1β protein expression. Moreover, the decreased binding ability of NF-κB p65 onto the IL1B promoter in cells pretreated with metformin followed by BHBA stimulation also substantiates the lower levels of IL1B gene expression. However, further studies are required to verify the direct regulation of metformin on the modulation of proteins, such as protein deacetylation by SIRT1 activation.
Altogether, these results indicate that BHBA induces inflammatory responses through the activation of NF-κB signaling, while pretreatment with metformin dampens the pro-inflammatory cytokines through the activation of AMPK. The regulation of histone deacetylation by SIRT1 may illustrate the mechanism of the downregulated cytokines expression. Therefore, AMPK/SIRT1/NF-κB signaling may point to the new mechanism of bovine hepatocytes inflammatory response and a potential target for the development of a ketosis therapy.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (31872324).
Glossary
Abbreviations
- AMPK
adenosine 5‘-monophosphate (AMP)-activated protein kinase
- BHBA
β-hydroxybutyric acid
- RT-qPCR
real-time quantitative polymerase chain reaction
- ACC,
acetyl CoA carboxylase
- NF-κB
nuclear factor kappa B
- IκBα,
inhibitor of kappa B alpha
- TNF
tumor necrosis factor
- IL
interleukin
- COX
cyclooxygenase
- SIRT1
silent information regulator 1
- NEB
negative energy balance
- NEFA
non-esterified fatty acids
- EdU
5-Ethynyl-2’-deoxyuridine
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- RPS9
ribosomal protein S9
- UXT
ubiquitously-expressed transcript
- NADH
nicotinamide adenine dinucleotide
Authors’ Contributions
Xu and Lu analyzed and interpreted the data. Arbab performed the experiment. Xu, Loor, and Yang were major contributors in writing the manuscript. Yang supported financially throughout the experiment and generated ideas. All authors read and approved the final manuscript.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflict of interest statement. The authors declare no real or perceived conflicts of interest.
Literature Cited
- Bergen, W. G., and Mersmann H. J.. . 2005. Comparative aspects of lipid metabolism: impact on contemporary research and use of animal models. J. Nut. 135:2499–2502. doi: 10.1093/jn/135.11.2499 [DOI] [PubMed] [Google Scholar]
- Brodowska, K., Theodoropoulou S., Melissa M. Z. H. R., Paschalis E., Takeuchi K., Scott G., Ramsey D., Kiernan E., Hoang M., and Cichy J.. . 2014. Effects of metformin on retinoblastoma growth in vitro and in vivo. Int. J. Oncol. 45:2311–2324. doi: 10.3892/ijo.2014.2650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Z., Chu S., Wang X., Sun Y., Xu T., Mao Y., Loor J. J., and Yang Z.. . 2019. MiR-16a regulates milk fat metabolism by targeting large tumor suppressor kinase 1 (LATS1) in bovine mammary epithelial cells. J. Agric. Food Chem. 67:11167–11178. doi: 10.1021/acs.jafc.9b04883 [DOI] [PubMed] [Google Scholar]
- Dannenberg, A. J., Altorki N. K., Boyle J. O., Dang C., Howe L. R., Weksler B. B., and Subbaramaiah K.. . 2001. Cyclo-oxygenase 2: a pharmacological target for the prevention of cancer. Lancet. Oncol. 2:544–551. doi: 10.1016/S1470-2045(01)00488-0. [DOI] [PubMed] [Google Scholar]
- Dasgupta, B., and Milbrandt J.. . 2007. Resveratrol stimulates AMP kinase activity in neurons. Proc. Natl Acad. Sci. U.S.A. 104: 7217–7222. doi: 10.1073/pnas.0610068104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, Q., Liu G., Liu L., Zhang Y., Yin L., Shi X., Wang J., Yuan X., Sun G., Li Y., . et al. 2015. BHBA influences bovine hepatic lipid metabolism via AMPK signaling pathway. J. Cell Biochem. 116:1070–1079. doi: 10.1002/jcb.25062 [DOI] [PubMed] [Google Scholar]
- Gillum, M. P., Kotas M. E., Erion D. M., Kursawe R., Chatterjee P., Nead K. T., Muise E. S., Hsiao J. J., Frederick D. W., Yonemitsu S., . et al. 2011. SirT1 regulates adipose tissue inflammation. Diabetes 60:3235–3245. doi: 10.2337/db11-0616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammon, D. S., Evjen I. M., Dhiman T. R., Goff J. P., and Walters J. L.. . 2006. Neutrophil function and energy status in Holstein cows with uterine health disorders. Vet. Immunol. Immunopathol. 113:21–29. doi: 10.1016/j.vetimm.2006.03.022. [DOI] [PubMed] [Google Scholar]
- Hattori, Y., Suzuki K., Hattori S., and Kasai K.. . 2006. Metformin inhibits cytokine-induced nuclear factor kappaB activation via AMP-activated protein kinase activation in vascular endothelial cells. Hypertension 47:1183–1188. doi: 10.1161/01.HYP.0000221429.94591.72. [DOI] [PubMed] [Google Scholar]
- Hayden, M. S., and Ghosh S.. . 2011. NF-κB in immunobiology. Cell Res. 21:223–244. doi: 10.1038/cr.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herdt, T. H. 2000. Ruminant adaptation to negative energy balance. Influences on the etiology of ketosis and fatty liver. Vet. Clin. North Am. Food Anim. Pract. 16:215–30, v. doi: 10.1016/s0749-0720(15)30102-x. [DOI] [PubMed] [Google Scholar]
- Howe, L. R., and Dannenberg A. J.. . 2002. A role for cyclooxygenase-2 inhibitors in the prevention and treatment of cancer. Semin. Oncol. 29(3 Suppl 11):111–119. doi: 10.1053/sonc.2002.34063. [DOI] [PubMed] [Google Scholar]
- Huang, N. L., Chiang S. H., Hsueh C. H., Liang Y. J., Chen Y. J., and Lai L. P.. . 2009. Metformin inhibits TNF-α-induced IκB kinase phosphorylation, IκB-α degradation and IL-6 production in endothelial cells through PI3K-dependent AMPK phosphorylation. Int. J. Cardiol. 134:0–175. doi: 10.1016/j.ijcard.2008.04.010 [DOI] [PubMed] [Google Scholar]
- Iwersen, M., Klein-Jöbstl D., Pichler M., Roland L., Fidlschuster B., Schwendenwein I., and Drillich M.. . 2013. Comparison of 2 electronic cowside tests to detect subclinical ketosis in dairy cows and the influence of the temperature and type of blood sample on the test results. J. Dairy Sci. 96:7719–7730. doi: 10.3168/jds.2013-7121. [DOI] [PubMed] [Google Scholar]
- Jian, M. Y., Alexeyev M. F., Wolkowicz P. E., Zmijewski J. W., and Creighton J. R.. . 2013. Metformin-stimulated AMPK-α1 promotes microvascular repair in acute lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 305:L844–L855. doi: 10.1152/ajplung.00173.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing, H., Yue L., Xiuli L., Tongrong Z., Haijing S., Paul E., Hua G., Fu-Shin Y., Xiaoxi Q., and Jing C.. . 2018. Metformin suppresses retinal angiogenesis and inflammation in vitro and in vivo. PloS one 13:e0193031. doi: 10.1371/journal.pone.0193031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauppinen, A., Suuronen T., Ojala J., Kaarniranta K., and Salminen A.. . 2013. Antagonistic crosstalk between NF-κB and SIRT1 in the regulation of inflammation and metabolic disorders. Cell. Signal. 25:1939–1948. doi: 10.1016/j.cellsig.2013.06.007. [DOI] [PubMed] [Google Scholar]
- Lawrence, T. 2009. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 1:a001651–a001651. doi: 10.1101/cshperspect.a001651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArt, J. A., Nydam D. V., Oetzel G. R., Overton T. R., and Ospina P. A.. . 2013. Elevated non-esterified fatty acids and β-hydroxybutyrate and their association with transition dairy cow performance. Vet. J. 198:560–570. doi: 10.1016/j.tvjl.2013.08.011. [DOI] [PubMed] [Google Scholar]
- Pfaffl, M. W. 2001. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acid. Res. 29(9):9. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queiroz, E. A., Puukila S., Eichler R., Sampaio S. C., Forsyth H. L., Lees S. J., Barbosa A. M., Dekker R. F., Fortes Z. B., and Khaper N.. . 2014. Metformin induces apoptosis and cell cycle arrest mediated by oxidative stress, AMPK and FOXO3a in MCF-7 breast cancer cells. PloS One. 9:e98207. doi: 10.1371/journal.pone.0098207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, X., Li X., Li D., Li Y., Song Y., Deng Q., Wang J., Zhang Y., Ding H., and Yin L.. . 2014. β-Hydroxybutyrate activates the NF-κB signaling pathway to promote the expression of pro-inflammatory factors in calf hepatocytes. Cell. Physiol. Biochem. 33:920. doi: 10.1159/000358664. [DOI] [PubMed] [Google Scholar]
- Song, Y., Li N., Gu J., Fu S., Peng Z., Zhao C., Zhang Y., Li X., Wang Z., Li X., and Liu G.. . 2016. β-Hydroxybutyrate induces bovine hepatocyte apoptosis via an ROS-p38 signaling pathway. J. Dairy Sci. 99:9184–9198. doi: 10.3168/jds.2016-11219 [DOI] [PubMed] [Google Scholar]
- Song, Y., Li X., Li Y., Li N., Shi X., Ding H., Zhang Y., Li X., Liu G., and Wang Z.. . 2014. Non-esterified fatty acids activate the ROS-p38-p53/Nrf2 signaling pathway to induce bovine hepatocyte apoptosis in vitro. Apoptosis 19:984–997. doi: 10.1007/s10495-014-0982-3. [DOI] [PubMed] [Google Scholar]
- Song, Y., Loor J., Li C., Liang Y., Li N., Shu X., Yang Y., Feng X., Du X., and Wang Z.. . 2021. Enhanced mitochondrial dysfunction and oxidative stress in the mammary gland of cows with clinical ketosis. J. Dairy. Sci. 104:6909–6918. doi: 10.3168/jds.2020-19964. [DOI] [PubMed] [Google Scholar]
- Xia, C., Wang Z., Xu C., and Zhang H. Y.. . 2012. Concentrations of plasma metabolites, hormones, and mRNA abundance of adipose leptin and hormone-sensitive lipase in ketotic and nonketotic dairy cows. J. Vet. Intern. Med. 26:415–417. doi: 10.1111/j.1939-1676.2011.00863.x. [DOI] [PubMed] [Google Scholar]
- Xiaorui, C., Huan L., Huiren T., Ning W., Lifeng Y., Dawei Z., Xiaozhao L., Jinyu Z., Zifan L., and Qingsheng Z.. . 2013. Metformin inhibits vascular calcification in female rat aortic smooth muscle cells via the AMPK-eNOS-NO pathway. Endocrinology 10:3680–3689. doi: 10.1210/en.2013-1002 [DOI] [PubMed] [Google Scholar]
- Xu, T., Ma N., Wang Y., Shi X., Chang G., Loor J. J., and Shen X.. . 2018. Sodium butyrate supplementation alleviates the adaptive response to inflammation and modulates fatty acid metabolism in lipopolysaccharide-stimulated bovine hepatocytes. J. Agric. Food Chem. 66:6281–6290. doi: 10.1021/acs.jafc.8b01439. [DOI] [PubMed] [Google Scholar]
- Xu, T. L., Seyfert H. M., and Shen X. Z.. . 2017. Epigenetic mechanisms contribute to decrease stearoyl-CoA desaturase 1 expression in the liver of dairy cows after prolonged feeding of high-concentrate diet. J. Dairy. Sci. 101:2506–2518. doi:S0022030217311670. [DOI] [PubMed] [Google Scholar]
- Xu, T., Tao H., Chang G., Zhang K., Xu L., and Shen X.. . 2015. Lipopolysaccharide derived from the rumen down-regulates stearoyl-CoA desaturase 1 expression and alters fatty acid composition in the liver of dairy cows fed a high-concentrate diet. BMC Vet. Res. 11:52. doi: 10.1186/s12917-015-0360-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, T., Wu X., Lu X., Liang Y., and Yang Z.. . 2021. Metformin activated AMPK signaling contributes to the alleviation of LPS-induced inflammatory responses in bovine mammary epithelial cells. BMC Vet. Res. 17:97. doi: 10.1186/s12917-021-02797-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, S., Chen X., Xu Y., Hao Y., and Meng X.. . 2020. Effects of metformin on lipopolysaccharide-induced depressive-like behavior in mice and its mechanisms. Neuroreport 31:305–310. doi: 10.1097/WNR.0000000000001401. [DOI] [PubMed] [Google Scholar]
- Yang, H., Bi Y., Xue L., Wang J., Lu Y., Zhang Z., Chen X., Chu Y., Yang R., Wang R., . et al. 2015. Multifaceted modulation of SIRT1 in cancer and inflammation. Crit. Rev. Oncog. 20:49–64. doi: 10.1615/critrevoncog.2014012374 [DOI] [PubMed] [Google Scholar]
- Ye, J., Zhu N., Sun R., Liao W., and Fan S.. . 2018. Metformin inhibits chemokine expression through the AMPK/NF-kappa B signaling pathway. J. Interferon Cytokine Res. 38:363–369. doi: 10.1089/jir.2018.0061 [DOI] [PubMed] [Google Scholar]
- Zheng, Z., Chen H., Li J., Li T., Zheng B., Zheng Y., Jin H., He Y., Gu Q., and Xu X.. . 2012. Sirtuin 1-mediated cellular metabolic memory of high glucose via the LKB1/AMPK/ROS pathway and therapeutic effects of metformin. Diabetes 61:217–228. doi: 10.2337/db11-0416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H., Wu L., Xu C., Xia C., Sun L., and Shu S.. . 2013. Plasma metabolomic profiling of dairy cows affected with ketosis using gas chromatography/mass spectrometry. BMC Vet. Res. 9:186. doi: 10.1186/1746-6148-9-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, T., and Kraus W. L.. . 2010. SIRT1-dependent regulation of chromatin and transcription: linking NAD+ metabolism and signaling to the control of cellular functions. Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics 1804:1666–1675. doi: 10.1016/j.bbapap.2009.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhihui, X., Wenjun W., and Vladimir P.. . 2017. Metformin suppressed CXCL8 expression and cell migration in HEK293/TLR4 cell line. Mediat. Inflamm. 2017:1–11. doi: 10.1155/2017/6589423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, Y., Zhou Z., Peng J., and Loor J. J.. . 2018. Methionine and valine activate the mammalian target of rapamycin complex 1 pathway through heterodimeric amino acid taste receptor (TAS1R1/TAS1R3) and intracellular Ca(2+) in bovine mammary epithelial cells. J. Dairy Sci. 101:11354–11363. doi: 10.3168/jds.2018-14461 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflict of interest statement. The authors declare no real or perceived conflicts of interest.