Abstract
Primary aldosteronism (PA) is a common cause of secondary hypertension. Recent technological advances in genetic analysis have provided a better understanding of the molecular pathogenesis of this disease. The application of next-generation sequencing has resulted in the identification of somatic mutations in aldosterone-producing adenoma (APA), a major subtype of PA. Based on the recent findings using a sequencing method that selectively targets the tumor region where aldosterone synthase (CYP11B2) is expressed, the vast majority of APAs appear to harbor a somatic mutation in one of the aldosterone-driver genes, including KCNJ5, ATP1A1, ATP2B3, CACNA1D, CACNA1H, and CLCN2. Mutations in these genes alter intracellular ion homeostasis and enhance aldosterone production. In a small subset of APAs, somatic activating mutations in the CTNNB1 gene, which encodes β-catenin, have also been detected. Accumulating evidence suggests that race and sex impact the somatic mutation spectrum of APA. Specifically, somatic mutations in the KCNJ5 gene, encoding an inwardly rectifying K+ channel, are common in APAs from Asian populations as well as women regardless of race. Associations between APA histology, genotype, and patient clinical characteristics have also been proposed, suggesting a potential need to consider race and sex for management of PA patients. Herein, we review recent findings regarding somatic mutations in APA and discuss potential roles of race and sex on the pathophysiology of APA as well as possible clinical implications.
Keywords: primary aldosteronism, CYP11B2, somatic mutation, race, sex
Introduction
Renin-independent autonomous aldosterone production from one or both adrenal glands causes hypertension and often hypokalemia. This condition is known as primary aldosteronism (PA) which was first reported by Dr. Jerome W. Conn in 1955 (1). PA is now recognized as a frequent cause of secondary hypertension, accounting for 6% of hypertensive patients in primary care practice (2). A recent study demonstrated a significant number of unrecognized yet biochemically confirmed PA even in normotensive subjects with a continuum of renin-independent aldosterone production that parallels hypertension severity (3). There are several subtypes of PA, including sporadic and rare familial forms. The vast majority of PA is classified as either sporadic aldosterone-producing adenoma (APA) or idiopathic hyperaldosteronism (IHA) (4). In general, patients with APA present with more severe phenotypes than those with IHA (4). To better understand this disease, significant efforts have been made to determine the pathogenesis of PA.
Recent advances in the genetic analysis using next-generation sequencing (NGS) have allowed the identification of the genetic causes of sporadic as well as familial PA. NGS has identified mutations in genes that control ion homeostasis of adrenocortical cells in PA patients. The affected genes include KCNJ5 (5), ATP1A1 (6), ATP2B3 (6), CACNA1D (7, 8), CACNA1H (9), and CLCN2 (10, 11). Mutations in these genes mostly stimulate aldosterone production via activation of the calcium signaling pathway by increasing intracellular calcium levels (12). Aldosterone-driver somatic mutations in these genes have been identified in the majority of APAs (13-17). The identification of somatic mutations in APA has provided new avenues for improved patient care such as steroid biomarkers and potential new treatment options for patients with APA harboring somatic KCNJ5 mutations, one of the most common genetic causes of APA (17-21). Emerging evidence indicates that there are racial and sex differences in the prevalence of aldosterone-driver somatic mutations. In this review, we summarize recent findings regarding the genetic causes of APA and discuss the impact of race and sex on the pathogenesis of APA as well as its clinical significance.
Identification of somatic mutations in APA
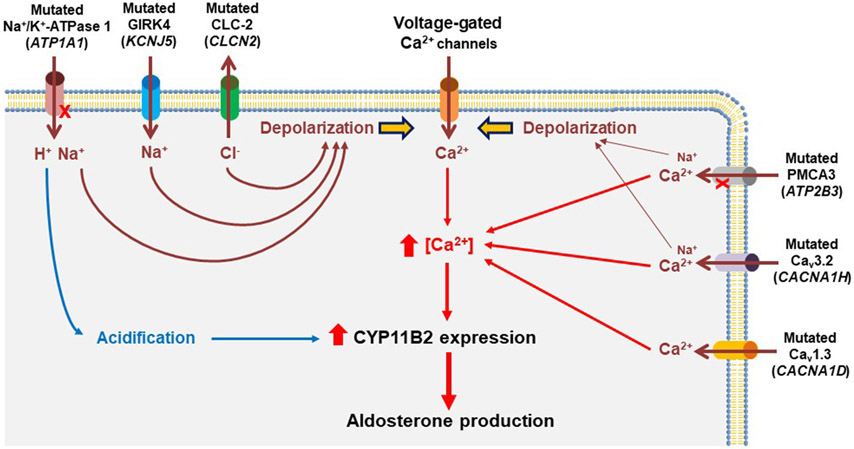
The genetic causes of sporadic APA remained poorly defined until the application of NGS. In 2011, the laboratory of Dr. Richard P. Lifton identified two recurrent heterozygous somatic mutations in KCNJ5 (p.G151R and p.L168R) in APAs using whole-exome sequencing (WES) (5). The group also identified a germline KCNJ5 variant (p.T158A) in a family with a Mendelian form of severe hyperaldosteronism with massive adrenal hyperplasia (5), which is now defined as familial hyperaldosteronism type III (FH-III). The KCNJ5 gene encodes an inwardly rectifying K+ channel (GIRK4) which is highly expressed in the zona glomerulosa (ZG) of adrenal cortex (5, 22, 23). Mutations in or near the ion selectivity filter of GIRK4 lead to increased Na+ conductance and cell membrane depolarization, resulting in increased intracellular calcium levels which enhances aldosterone synthase (CYP11B2) expression and aldosterone production (5, 24-26) (Figure 1).
Figure 1. Proposed mechanisms leading to autonomous aldosterone production in adrenal tumor cells harboring aldosterone-driver somatic mutations.
Over 90% of aldosterone-producing adenomas (APAs) harbor somatic mutations in KCNJ5, ATP1A1, ATP2B3, CACNA1D, CACNA1H, and CLCN2. Most of these somatic mutations cause directly or indirectly (via cell membrane depolarization) increased intracellular calcium levels that activate calcium signaling pathways, enhanced aldosterone synthase (CYP11B2) expression, and renin-independent aldosterone production. Notably, based on a cell-based study of mutant ATP1A1, no pathologic increase in cytosolic calcium levels was observed despite membrane depolarization. Alternative mechanisms including cellular acidification have been proposed for autonomous aldosterone production in ATP1A1-mutated APA.
Subsequently, somatic mutations in other genes, including ATP1A1 (6, 8), ATP2B3 (6), CACNA1D (7, 8), CACNA1H (27), and CLCN2 (28), have been identified in APA. The ATP1A1 gene encodes Na+/K+-ATPase α1 subunit. In vitro studies suggest that the loss of Na+/K+ pump function as a result of ATP1A1 mutation causes reduced affinity to K+, intracellular acidification due to abnormal H+ leak, and cell membrane depolarization, leading to excess aldosterone production with no pathologic alteration in cytosolic Ca2+ activity (29) (Figure 1). The ATP2B3 gene encodes a plasma membrane calcium ATPase (PMCA3). Cell-based studies indicate that mutations in ATP2B3 cause loss of its pump function and pathological cation permeability leading to increased intracellular calcium levels and enhanced aldosterone production (30) (Figure 1).
The CACNA1D gene encodes voltage-dependent L-type calcium channel subunit alpha-1D (Cav1.3). Unlike other aldosterone-driver genes, mutations in CACNA1D have been identified throughout the gene (31). Although few have been functionally characterized, mutations in CACN1D are thought to be gain-of-function mutations that induce increased Ca2+ entry as a result of channel activation at less depolarized potentials, leading to excess aldosterone production (7, 8) (Figure 1). Notably, de novo germline mutations in CACNA1D have also been identified in pediatric cases with PA and nerurodevelopmental abnormalities (7). This condition is currently defined as PA with seizures and neurologic abnormalities (PASNA) syndrome.
The CACNA1H gene encodes voltage-dependent T-type calcium channel subunit alpha-1H (Cav3.2). The association between this gene and PA has initially been explored by the identification of recurrent germline CACNA1H mutation (p.M1549V) in early-onset PA patients as the cause of FH-IV (9). Cell-based studies demonstrated that the CACNA1HM1549V induces a shift of activation to less depolarizing potentials, allowing increased Ca2+ entry and stimulation of aldosterone production (9, 32) (Figure 1). A recent study suggests that abnormal Na+ permeability of mutant Cav3.2 could also contribute to the depolarization of the membrane potential (33). Daniil et al. (34) identified four different additional heterozygous germline CACNA1H mutations in PA patients with different clinical phenotypes. We recently identified a recurrent somatic CACNA1H mutation (p.I1430T) in APAs without known aldosterone-driver mutations (27). The CACNA1H p.I1430T mutation was functionally characterized as having the ability to increase CYP11B2 mRNA expression and aldosterone production using a doxycycline-inducible HAC15 adrenal cell line (27).
Gain-of-function germline CLCN2 mutations in patients with FH-II and early-onset PA were identified by two groups in 2018 (10, 11). The CLCN2 gene encodes chloride voltage-gated channel 2 (ClC-2). Mutations in CLCN2 cause increased CYP11B2 expression and aldosterone production by membrane depolarization via enhanced chloride efflux (10, 11) (Figure 1). Dutta et al. (28) screened 80 APAs and found a somatic CLCN2 mutation, p.G24D, in one patient, which is identical to one identified and functionally characterized as a disease-causing de novo germline mutation that causes in an early-onset PA case (11). Besides the p.G24D mutation, we identified an additional CLCN2 somatic mutation (c.62-2_74del) in an APA although the clinical significance of this mutation is unclear until its functional effect on aldosterone production is determined (35). Notably, two different mouse models for PA due to CLCN2 mutations have recently been developed (36, 37). These mouse models will provide valuable tools to study PA pathogenesis and to develop new therapeutics for PA.
Somatic activating mutations in CTNNB1 (β-catenin) have been identified in adrenocortical tumors, including cortisol-producing adenoma (38-41), adrenocortical carcinoma (38, 42), and non-functioning adrenocortical adenoma (38, 43), suggesting a role of CTNNB1 mutation in adrenal tumorigenesis. Somatic CTNNB1 mutations have also been found in APAs with the prevalence of 2-5% (44-46). β-catenin is a key protein of the Wnt/β-catenin signaling pathway which plays an important role in adrenal cortex development and adrenal steroidogenesis (47-50). Constitutive activation of β-catenin in the mouse adrenal causes adrenal hyperplasia and hyperaldosteronism (51). A recent study demonstrated that gain-of-function mutation targeted to the mouse adrenal ZG caused progressive hyperplastic expansion of the ZG and elevated aldosterone production. Surprisingly no difference was observed in plasma renin activity between mice with and without β-catenin gain-of-function mutation (52). Further studies are needed to determine the molecular mechanisms of enhanced aldosterone production in CTNNB1-mutated APA.
Somatic mutation prevalence in APA using conventional approaches
Since the identification of aldosterone-driver somatic mutations in APA, significant efforts have been made to study the prevalence of these mutations. In the studies mainly conducted in European countries, the prevalence of KCNJ5 mutations have been reported to be approximately 40% (44, 53-58). The largest mutation prevalence study was conducted by Fernandes-Rosa et al. using material obtained through the European Network for the Study of Adrenal Tumors (ENS@T) (56). In that study, somatic mutations were identified in 257 out of 474 APAs (54.2%), including mutations in KCNJ5 (38.0%), CACNA1D (9.3%), ATP1A1 (5.3%), and ATP2B3 (1.7%).
The prevalence of KCNJ5 mutations appears to be much more common in East Asian and Southeast Asian countries, including China (59, 60), Taiwan (61), Japan (62, 63), South Korea (64), and Thailand (65), with the prevalence of approximately 70% although there are a few studies reporting a similar KCNJ5 mutation prevalence to that in European cohorts (66, 67). In Asian populations, mutations other than in KCNJ5 have only been rarely reported: ATP1A1 in 0-2.4%, ATP2B3 in 0-2.5%, and CACNA1D in 0-2.5% (46, 59-61, 64, 68). Overall, previous studies have identified somatic mutations in 54-80% of APAs, with higher detection rate in Asian populations compared with that in European populations. Of note, these prevalence studies have isolated DNA from macroscopically identified adrenal tumor tissue or formalin-fixed, paraffin-embedded (FFPE) tissue without consideration of tumor CYP11B2 expression and majority performed mutation hotspot-based Sanger-based sequencing (conventional approaches).
CYP11B2 IHC-guided sequencing analysis
CYP11B2 and 11β-hydroxylase (CYP11B1) are steroidogenic enzymes required for the final steps of aldosterone and cortisol production, respectively. CYP11B1 and CYP11B2 are encoded by two genes; each containing nine exons spread over approximately 7,000 base pairs of DNA. The encoded proteins are 93% identical in predicted amino acid sequence. Because of the high sequence similarity between CYP11B2 and CYP11B1, generation of specific antibodies against these enzymes was only accomplished in the past ten years (69). Subsequently the development of highly specific monoclonal antibodies against CYP11B2 and CYP11B1 significantly improved our understanding of the histopathology of adrenals with PA (70). CYP11B2 immunohistochemistry (IHC) clearly demonstrated positive expression in surgically resected adrenal tumors (APAs) from PA patients. At the same time, CYP11B2 IHC revealed minor histopathologic subgroups of PA adrenals, including cases with distinct intra-tumor CYP11B2 heterogeneity (71), with multiple APAs (13, 14, 72), as well as cases with a dominant CYP11B2-negative tumor with a satellite APA or aldosterone-producing micronodules (formerly known as aldosterone-producing cell clusters) in the adjacent adrenal tissue (13, 14, 73-75). The variations in PA adrenal presentation raised concerns regarding the accuracy of mutation prevalence studies that did not assess CYP11B2 expression of pathologic material prior to sequencing. To overcome this issue, we developed a CYP11B2-IHC guided sequencing method using DNA from FFPE tumor material (13, 14). Using this approach, aldosterone-driver somatic mutations have been identified in approximately 90% of APAs (13, 14, 15, 16, 17), which is significantly higher than that seen using conventional approaches (44, 56, 76). As mentioned earlier, many of the NGS-identified mutations especially in the CACNA1D gene have not yet been functionally characterized. Therefore, current studies may be overestimating the prevalence of somatic mutations responsible for APA aldosterone overproduction.
CYP11B2 IHC-guided sequencing basically allows the initial definition of sources of inappropriate aldosterone production followed by its capture in consecutive FFPE sections under a dissecting microscope. The scraped material is used for DNA isolation after deparaffinization. FFPE DNA sequencing can be performed with whole exome, gene targeted next-generation sequencing (NGS) panels, or traditional Sanger sequencing. Targeted NGS is a preferable method because of its high sensitively, ability to utilize small amounts of DNA, and especially for identifying mutations that span the CACNA1D gene which encodes more than 2,000 amino acids (31). Although CYP11B2 IHC-guided sequencing approach is time consuming and labor intensive, it has provided accurate and important findings as discussed later.
Impact of race on genetic causes of aldosterone-producing adenoma
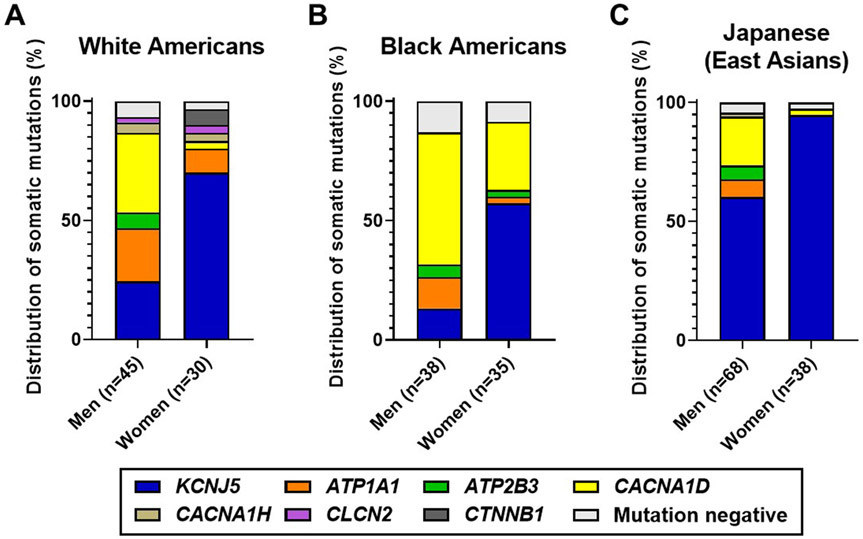
There is considerable evidence that supports the concept of racial differences in the prevalence of somatic mutations in APA. However, there were some concerns regarding the accuracy of the mutational analysis with conventional approaches that may impact the prevalence estimates. Using a CYP11B2 IHC-guided sequencing method, we have investigated the APA-related somatic mutation spectrum in cohorts of different races, including Americans of European descent (whites) (13), Americans of African descent (blacks) (14), and Japanese (East Asians) (15) (Figure 2). In the analysis of 75 APAs from whites, somatic KCNJ5 mutations were the most frequent genetic cause of APA (43%), followed by CACNA1D (21%), ATP1A1 (17%), ATP2B3 (4%), and CTNNB1 (3%) (13). The prevalence of KCNJ5 mutations appears to be similar to the results of the European multicenter collaborative study (56), while the CYP11B2 IHC-guided approach combined with deep gene sequencing improved the detection rate of mutations in genes such as CACNA1D and ATP1A1. Subsequent whole exome sequencing identified somatic mutations in CACNA1H and CLCN2 in small subsets of APA (27, 35). Large cohort studies will be needed to determine the prevalence of these rare APA mutations.
Figure 2. Race and sex differences in the genes affected by aldosterone-driver somatic mutations in APA based on CYP11B2 IHC-guided sequencing analysis.
A-C. Distribution of somatic mutations in APA from white Americans (A), black Americans (B), and Japanese (East Asian) patients (C). Data was adopted from our previous studies (13-15, 27, 35). APAs from black patients were not assessed for mutations in CACNA1H and CLCN2 genes due to limited sample availability.
In agreement with most of the previous reports from East Asian countries (59-63), KCNJ5 mutations were common in Japanese (73% of 106 APAs) when analyzed using the CYP11B2 IHC-guided method (15). While somatic mutations in other aldosterone-driver genes have been thought to be very rare events in Asian population, the CYP11B2 IHC-guided capture combined with deep sequencing methods successfully identified mutations in CACNA1D, ATP1A1, ATP2B3, and CACNA1H with the prevalence of 14%, 5%, 4%, and 1%, respectively (15). A meta-analysis of 1636 patients with APA from 13 studies indicates a possible association between the incidence of KCNJ5 mutation and sodium intake (77) although dedicated studies are needed to assess its causal relationship. This topic was further discussed in depth by Williams et al (78). Besides high sodium intake which may lead to a more severe phenotype and/or early detection of the disease, the authors proposed a possible selection bias due to diagnostic procedures as a potential factor for high incidence of KCNJ5 mutations in East Asians based on the evidence of a more pronounced phenotype of KCNJ5-mutated APA compared to that of non-KCNJ5-mutated APA (78). Other considerations would include the much higher circulating levels of phytoestrogens seen in East Asian men vs European men (79). Phytoestrogens can activate both traditional and cell surface estrogen receptors (80, 81). Differences in reproductive hormones and particularly circulating estrogens have been considered as a possible hormonal cause of the dominance of KCNJ5 mutations in APA from women vs men of all races (77, 82).
The high levels of hybrid steroids (18-hydroxycortisol and 18-oxocortisol), which are metabolites of cortisol, have been reported in patients with APA (83, 84) as well as rare familial forms of PA (FH-1 due to a chimeric CYP11B1/CYP11B2 gene and FH-III due to germline KCNJ5 mutations) (85, 86). Since these hybrid steroid levels are lower in patients with IHA than those with APA, the role of hybrid steroid measurement for PA subtype prediction (APA vs. IHA) has been studied (87-89). However, due to high variability in hybrid steroid values, a significant overlap between APA and IHA was observed (89). Recent steroid profiling studies using liquid chromatography-tandem mass spectrometry further revealed higher hybrid steroid production in KCNJ5-mutated sporadic APAs compared with non KCNJ5-mutated APAs (17-19). The somatic mutation distribution may partly explain the variability in hybrid steroid levels in APA patients. The high prevalence of somatic KCNJ5 mutations in APAs from East Asian patients may also explain the better utility of hybrid steroid measurement for subtype prediction (APA vs. IHA) in East Asians (90) compared with Europeans (89, 91). One goal for hybrid steroid measurement would be to decrease IHA patient adrenal venous sampling (AVS), which is an invasive procedure with a limited availability. However, for PA patients with high circulating hybrid steroids AVS would likely still be needed to lateralize disease. While cautiously optimistic about the utility of hybrid steroids as a PA diagnostic tool, the current complexity of hybrid steroid measurement and data interpretation requires standardization and therefore will remain unavailable for most clinical programs for the foreseeable future.
In contrast to whites and East Asians, CACNA1D mutations appear to be the most common genetic cause in blacks with the prevalence of 42% based on the analysis of 73 APAs using CYP11B2 IHC-guided sequencing approach (14). The second most prevalent genetic alteration in blacks was KCNJ5 mutations (34%), followed by ATP1A1 (8%) and ATP2B3 (4%) (14). In what appears to be a unique characteristic of black patients with PA, Zilbermint et al. (92) reported an association with germline variants in Armadillo repeat-containing protein 5 (ARMC5) gene, which is a putative tumor suppressor gene. Mutations in this gene were initially found as a genetic cause of primary bilateral macronodular adrenocortical hyperplasia, a rare form of Cushing syndrome (93). Although germline ARMC5 variants may predispose for certain types of aldosterone-driver mutations, it remains an open area of research.
Although the mechanisms leading to variation in the APA somatic mutation spectrum remain unclear, clarification of racial differences in the genetic causes of APA may help in the development of new diagnostics and personalized therapeutics for PA patients. To determine a more accurate somatic mutation prevalence, large multicenter prospective studies using a CYP11B2 IHC-guided sequencing approach would be desirable.
Impact of sex on genetic causes of aldosterone-producing adenoma
Sex differences in the type of aldosterone-driver mutations have been well documented particularly in the KCNJ5 gene. A predominant occurrence of somatic KCNJ5 mutations in APAs from women has been reported in many studies mainly conducted in European countries (44, 53, 54, 56, 57), while this sex difference has been debated in Asian populations where the prevalence of somatic KCNJ5 mutations is very high in both men and women (59-67). Our recent studies using the CYP11B2 IHC-guided sequencing method with targeted NGS demonstrated significantly higher incidences of KCNJ5 mutations in women than men in Japanese (15) as well as other races, including white (13) and black Americans (14) (Figure 2). The molecular mechanisms underlying sex differences in the prevalence of somatic KCNJ5 mutations in APA are largely unknown. Reproductive hormone differences between men and women represent a clear sex difference that could impact tumor growth and/or aldosterone production. The adrenal cortex expresses both conventional estrogen receptors (mainly ERβ) as well as G protein-coupled estrogen receptor 1 (GPER1) (82). In vitro studies have demonstrated that estradiol can stimulate aldosterone production via GPER1, which is expressed predominantly in APA (82). Estrogens therefore could play a role on PA pathogenesis in women although its association with APA genotype remains to be elucidated.
Besides sex, other clinical characteristics of patients with KCNJ5-mutated APA include young onset of the disease, high plasma aldosterone concentration, and large tumor size (77). The florid PA phenotype in patients with KCNJ5-mutated APA may explain early detection of the disease, resulting in the frequent observation of somatic KCNJ5 mutations in APA from young patients (54). Histologic analysis revealed that KCNJ5-mutated APAs contain a higher percentage of lipid-rich clear cells [zona fasciculata (ZF)-like cells] compared with non-KCNJ5-mutated APAs (17, 44, 94-97). Abundant expression of CYP17A1, which is required for cortisol biosynthesis, has also been observed in KCNJ5-mutated APAs (17, 35, 94, 97). Co-expression of CYP11B2 and CYP17A1 explains the production of hybrid steroids in KCNJ5-mutated APAs. Considering the high prevalence of somatic KCNJ5 mutations in women of all races examined to date, the measurement of hybrid steroids may provide an additional diagnostic tool for prediction of PA subtype (APA vs. IHA) in women although it does not provide any information regarding laterality of the disease. In contrast to the KCNJ5 gene, mutations in CACNA1D have been more often observed in men than women regardless of race (13-15, 56) and CACNA1D-mutated APAs tend to be smaller than KCNJ5-mutated APAs (8, 44, 56).
Somatic activating CTNNB1 mutations in APA appear to occur more frequently in women than men (45, 46). There is a case series report suggesting the possible association between somatic CTNNB1 mutations and enhanced tumor LHCGR and GNRHR expression in two pregnant women and one postmenopausal woman with PA (98). Aberrant G-protein-coupled receptors, including LHCGR and GNRHR, have been documented in a subset of PA patients (99-104). In vitro studies using H295R cell models transfected with LHCGR or GNRHR demonstrated that the corresponding agonist treatments resulted in a dose-dependent increase in CYP11B2 reporter activity (99, 103). Interestingly, in a recent study by Gagnon et al. (105), no CTNNB1 mutation was identified in 11 PA patients with positive or partial aldosterone response to GnRH. While the ectopic tumor expression of these receptors provides an intriguing explanation for hormonal activation of aldosterone production in a subgroup of APAs, the molecular mechanisms by which CTNNB1 mutations cause inappropriate aldosterone production in APA but not in other adrenal tumors remains unclear.
Conclusions
Recent technological advances in APA genetic analysis have led to the detection of aldosterone-driver somatic mutations in the vast majority of these tumors. Accumulating data suggests that there are clear race and sex differences in the distribution of somatic mutations in APAs. Although the mechanisms underlying these differences remain unknown, consideration of race and sex could be important for future personalized medicines in the management of PA patients.
Acknowledgements
We thank our collaborators from our previous APA somatic mutation prevalence studies; Drs. Debbie Cohen (University of Pennsylvania), Constantine A. Stratakis (National Institutes of Health), James M. Luther (Vanderbilt University Medical Center), Anand Vaidya (Brigham and Women’s Hospital and Harvard Medical School), Lester D.R. Thompson (Woodland Hills Medical Center), Fumitoshi Satoh (Tohoku University), Hironobu Sasano (Tohoku University), Celso E. Gomez-Sanchez (University of Mississippi Medical Center), Tobias Else (University of Michigan), Aaron M. Udager (University of Michigan), Scott A. Tomlins (University of Michigan), and Thomas J. Giordano (University of Michigan). We also thank Amy R. Blinder at the University of Michigan for her editorial assistance.
Funding
This work was supported by grants from National Institute of Diabetes and Digestive and Kidney Diseases (DK106618 and DK043140) to W.E.R and the American Heart Association (17SDG33660447) to K.N.
Footnotes
Declaration of interest
The authors have nothing to disclose.
References
- 1.Conn JW. Presidential address. I. Painting background. II. Primary aldosteronism, a new clinical syndrome. J Lab Clin Med. 1955;45(1):3–17. [PubMed] [Google Scholar]
- 2.Monticone S, Burrello J, Tizzani D, Bertello C, Viola A, Buffolo F, Gabetti L, Mengozzi G, Williams TA, Rabbia F, et al. Prevalence and Clinical Manifestations of Primary Aldosteronism Encountered in Primary Care Practice. J Am Coll Cardiol. 2017;69(14):1811–20. [DOI] [PubMed] [Google Scholar]
- 3.Brown JM, Siddiqui M, Calhoun DA, Carey RM, Hopkins PN, Williams GH, Vaidya A. The Unrecognized Prevalence of Primary Aldosteronism: A Cross-sectional Study. Ann Intern Med. 2020;173(1):10–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Young WF. Primary aldosteronism: renaissance of a syndrome. Clin Endocrinol (Oxf). 2007;66(5):607–18. [DOI] [PubMed] [Google Scholar]
- 5.Choi M, Scholl UI, Yue P, Bjorklund P, Zhao B, Nelson-Williams C, Ji W, Cho Y, Patel A, Men CJ, et al. K+ channel mutations in adrenal aldosterone-producing adenomas and hereditary hypertension. Science. 2011;331(6018):768–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beuschlein F, Boulkroun S, Osswald A, Wieland T, Nielsen HN, Lichtenauer UD, Penton D, Schack VR, Amar L, Fischer E, et al. Somatic mutations in ATP1A1 and ATP2B3 lead to aldosterone-producing adenomas and secondary hypertension. Nat Genet. 2013;45(4):440–4, 4e1-2. [DOI] [PubMed] [Google Scholar]
- 7.Scholl UI, Goh G, Stolting G, de Oliveira RC, Choi M, Overton JD, Fonseca AL, Korah R, Starker LF, Kunstman JW, et al. Somatic and germline CACNA1D calcium channel mutations in aldosterone-producing adenomas and primary aldosteronism. Nat Genet. 2013;45(9):1050–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Azizan EA, Poulsen H, Tuluc P, Zhou J, Clausen MV, Lieb A, Maniero C, Garg S, Bochukova EG, Zhao W, et al. Somatic mutations in ATP1A1 and CACNA1D underlie a common subtype of adrenal hypertension. Nat Genet. 2013;45(9):1055–60. [DOI] [PubMed] [Google Scholar]
- 9.Scholl UI, Stolting G, Nelson-Williams C, Vichot AA, Choi M, Loring E, Prasad ML, Goh G, Carling T, Juhlin CC, et al. Recurrent gain of function mutation in calcium channel CACNA1H causes early-onset hypertension with primary aldosteronism. Elife. 2015;4:e06315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scholl UI, Stolting G, Schewe J, Thiel A, Tan H, Nelson-Williams C, Vichot AA, Jin SC, Loring E, Untiet V, et al. CLCN2 chloride channel mutations in familial hyperaldosteronism type II. Nat Genet. 2018;50(3):349–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fernandes-Rosa FL, Daniil G, Orozco IJ, Goppner C, El Zein R, Jain V, Boulkroun S, Jeunemaitre X, Amar L, Lefebvre H, et al. A gain-of-function mutation in the CLCN2 chloride channel gene causes primary aldosteronism. Nat Genet. 2018;50(3):355–61. [DOI] [PubMed] [Google Scholar]
- 12.De Sousa K, Abdellatif AB, El Zein RM, Zennaro MC. Molecular mechanisms in primary aldosteronism. J Mol Endocrinol. 2019;242(3):R67–R79. [DOI] [PubMed] [Google Scholar]
- 13.Nanba K, Omata K, Else T, Beck PCC, Nanba AT, Turcu AF, Miller BS, Giordano TJ, Tomlins SA, Rainey WE. Targeted Molecular Characterization of Aldosterone-Producing Adenomas in White Americans. J Clin Endocrinol Metab. 2018;103(10):3869–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nanba K, Omata K, Gomez-Sanchez CE, Stratakis CA, Demidowich AP, Suzuki M, Thompson LDR, Cohen DL, Luther JM, Gellert L, et al. Genetic Characteristics of Aldosterone-Producing Adenomas in Blacks. Hypertension. 2019;73(4):885–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nanba K, Yamazaki Y, Bick N, Onodera K, Tezuka Y, Omata K, Ono Y, Blinder AR, Tomlins SA, Rainey WE, et al. Prevalence of Somatic Mutations in Aldosterone-Producing Adenomas in Japanese Patients. J Clin Endocrinol Metab. 2020;105(11) e4066–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Sousa K, Boulkroun S, Baron S, Nanba K, Wack M, Rainey WE, Rocha A, Giscos-Douriez I, Meatchi T, Amar L, et al. Genetic, Cellular, and Molecular Heterogeneity in Adrenals With Aldosterone-Producing Adenoma. Hypertension. 2020;75(4):1034–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo Z, Nanba K, Udager A, McWhinney BC, Ungerer JPJ, Wolley M, Thuzar M, Gordon RD, Rainey WE, Stowasser M. Biochemical, Histopathological, and Genetic Characterization of Posture-Responsive and Unresponsive APAs. J Clin Endocrinol Metab. 2020;105(9) e3224–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams TA, Peitzsch M, Dietz AS, Dekkers T, Bidlingmaier M, Riester A, Treitl M, Rhayem Y, Beuschlein F, Lenders JW, et al. Genotype-Specific Steroid Profiles Associated With Aldosterone-Producing Adenomas. Hypertension. 2016;67(1):139–45. [DOI] [PubMed] [Google Scholar]
- 19.Tezuka Y, Yamazaki Y, Kitada M, Morimoto R, Kudo M, Seiji K, Takase K, Kawasaki Y, Mitsuzuka K, Ito A, et al. 18-Oxocortisol Synthesis in Aldosterone-Producing Adrenocortical Adenoma and Significance of KCNJ5 Mutation Status. Hypertension. 2019;73(6):1283–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eisenhofer G, Duran C, Cannistraci CV, Peitzsch M, Williams TA, Riester A, Burrello J, Buffolo F, Prejbisz A, Beuschlein F, et al. Use of Steroid Profiling Combined With Machine Learning for Identification and Subtype Classification in Primary Aldosteronism. JAMA Netw Open. 2020;3(9):e2016209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scholl UI, Abriola L, Zhang C, Reimer EN, Plummer M, Kazmierczak BI, Zhang J, Hoyer D, Merkel JS, Wang W, et al. Macrolides selectively inhibit mutant KCNJ5 potassium channels that cause aldosterone-producing adenoma. J Clin Invest. 2017;127(7):2739–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Monticone S, Hattangady NG, Nishimoto K, Mantero F, Rubin B, Cicala MV, Pezzani R, Auchus RJ, Ghayee HK, Shibata H, et al. Effect of KCNJ5 mutations on gene expression in aldosterone-producing adenomas and adrenocortical cells. J Clin Endocrinol Metab. 2012;97(8):E1567–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang Y, Gomez-Sanchez CE, Jaquin D, Aristizabal Prada ET, Meyer LS, Knosel T, Schneider H, Beuschlein F, Reincke M, Williams TA. Primary Aldosteronism: KCNJ5 Mutations and Adrenocortical Cell Growth. Hypertension. 2019;74(4):809–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tauber P, Penton D, Stindl J, Humberg E, Tegtmeier I, Sterner C, Beuschlein F, Reincke M, Barhanin J, Bandulik S, et al. Pharmacology and pathophysiology of mutated KCNJ5 found in adrenal aldosterone-producing adenomas. Endocrinology. 2014;155(4):1353–62. [DOI] [PubMed] [Google Scholar]
- 25.Oki K, Plonczynski MW, Luis Lam M, Gomez-Sanchez EP, Gomez-Sanchez CE. Potassium channel mutant KCNJ5 T158A expression in HAC-15 cells increases aldosterone synthesis. Endocrinology. 2012;153(4):1774–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hattangady NG, Karashima S, Yuan L, Ponce-Balbuena D, Jalife J, Gomez-Sanchez CE, Auchus RJ, Rainey WE, Else T. Mutated KCNJ5 activates the acute and chronic regulatory steps in aldosterone production. J Mol Endocrinol. 2016;57(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nanba K, Blinder AR, Rege J, Hattangady NG, Else T, Liu CJ, Tomlins SA, Vats P, Kumar-Sinha C, Giordano TJ, et al. Somatic CACNA1H Mutation As a Cause of Aldosterone-Producing Adenoma. Hypertension. 2020;75(3):645–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dutta RK, Arnesen T, Heie A, Walz M, Alesina P, Soderkvist P, Gimm O. A somatic mutation in CLCN2 identified in a sporadic aldosterone-producing adenoma. Eur J Endocrinol. 2019;181(5):K37–K41. [DOI] [PubMed] [Google Scholar]
- 29.Stindl J, Tauber P, Sterner C, Tegtmeier I, Warth R, Bandulik S. Pathogenesis of Adrenal Aldosterone-Producing Adenomas Carrying Mutations of the Na(+)/K(+)-ATPase. Endocrinology. 2015;156(12):4582–91. [DOI] [PubMed] [Google Scholar]
- 30.Tauber P, Aichinger B, Christ C, Stindl J, Rhayem Y, Beuschlein F, Warth R, Bandulik S. Cellular Pathophysiology of an Adrenal Adenoma-Associated Mutant of the Plasma Membrane Ca(2+)-ATPase ATP2B3. Endocrinology. 2016;157(6):2489–99. [DOI] [PubMed] [Google Scholar]
- 31.Azizan EA, Brown MJ. Novel genetic determinants of adrenal aldosterone regulation. Curr Opin Endocrinol Diabetes Obes. 2016;23(3):209–17. [DOI] [PubMed] [Google Scholar]
- 32.Reimer EN, Walenda G, Seidel E, Scholl UI. CACNA1H(M1549V) Mutant Calcium Channel Causes Autonomous Aldosterone Production in HAC15 Cells and Is Inhibited by Mibefradil. Endocrinology. 2016;157(8):3016–22. [DOI] [PubMed] [Google Scholar]
- 33.Gurtler F, Jordan K, Tegtmeier I, Herold J, Stindl J, Warth R, Bandulik S. Cellular Pathophysiology of Mutant Voltage-Dependent Ca2+ Channel CACNA1H in Primary Aldosteronism. Endocrinology. 2020;161(10) bqaa135. [DOI] [PubMed] [Google Scholar]
- 34.Daniil G, Fernandes-Rosa FL, Chemin J, Blesneac I, Beltrand J, Polak M, Jeunemaitre X, Boulkroun S, Amar L, Strom TM, et al. CACNA1H Mutations Are Associated With Different Forms of Primary Aldosteronism. EBioMedicine. 2016;13:225–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rege J, Nanba K, Blinder AR, Plaska S, Udager AM, Vats P, Kumar-Sinha C, Giordano TJ, Rainey WE, Else T. Identification of Somatic Mutations in CLCN2 in Aldosterone-Producing Adenomas. J Endocr Soc. 2020;4(10):bvaa123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schewe J, Seidel E, Forslund S, Marko L, Peters J, Muller DN, Fahlke C, Stolting G, Scholl U. Elevated aldosterone and blood pressure in a mouse model of familial hyperaldosteronism with ClC-2 mutation. Nat Commun. 2019;10(1):5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goppner C, Orozco IJ, Hoegg-Beiler MB, Soria AH, Hubner CA, Fernandes-Rosa FL, Boulkroun S, Zennaro MC, Jentsch TJ. Pathogenesis of hypertension in a mouse model for human CLCN2 related hyperaldosteronism. Nat Commun. 2019;10(1):4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tissier F, Cavard C, Groussin L, Perlemoine K, Fumey G, Hagnere AM, Rene-Corail F, Jullian E, Gicquel C, Bertagna X, et al. Mutations of beta-catenin in adrenocortical tumors: activation of the Wnt signaling pathway is a frequent event in both benign and malignant adrenocortical tumors. Cancer Res. 2005;65(17):7622–7. [DOI] [PubMed] [Google Scholar]
- 39.Goh G, Scholl UI, Healy JM, Choi M, Prasad ML, Nelson-Williams C, Kunstman JW, Korah R, Suttorp AC, Dietrich D, et al. Recurrent activating mutation in PRKACA in cortisol-producing adrenal tumors. Nat Genet. 2014;46(6):613–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thiel A, Reis AC, Haase M, Goh G, Schott M, Willenberg HS, Scholl UI. PRKACA mutations in cortisol-producing adenomas and adrenal hyperplasia: a single-center study of 60 cases. Eur J Endocrinol. 2015;172(6):677–85. [DOI] [PubMed] [Google Scholar]
- 41.Ronchi CL, Di Dalmazi G, Faillot S, Sbiera S, Assie G, Weigand I, Calebiro D, Schwarzmayr T, Appenzeller S, Rubin B, et al. Genetic Landscape of Sporadic Unilateral Adrenocortical Adenomas Without PRKACA p.Leu206Arg Mutation. J Clin Endocrinol Metab. 2016;101(9):3526–38. [DOI] [PubMed] [Google Scholar]
- 42.Zheng S, Cherniack AD, Dewal N, Moffitt RA, Danilova L, Murray BA, Lerario AM, Else T, Knijnenburg TA, Ciriello G, et al. Comprehensive Pan-Genomic Characterization of Adrenocortical Carcinoma. Cancer Cell. 2016;29(5):723–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tadjine M, Lampron A, Ouadi L, Bourdeau I. Frequent mutations of beta-catenin gene in sporadic secreting adrenocortical adenomas. Clin Endocrinol (Oxf). 2008;68(2):264–70. [DOI] [PubMed] [Google Scholar]
- 44.Scholl UI, Healy JM, Thiel A, Fonseca AL, Brown TC, Kunstman JW, Horne MJ, Dietrich D, Riemer J, Kucukkoylu S, et al. Novel somatic mutations in primary hyperaldosteronism are related to the clinical, radiological and pathological phenotype. Clin Endocrinol (Oxf). 2015;83(6):779–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Akerstrom T, Maharjan R, Sven Willenberg H, Cupisti K, Ip J, Moser A, Stalberg P, Robinson B, Alexander Iwen K, Dralle H, et al. Activating mutations in CTNNB1 in aldosterone producing adenomas. Sci Rep. 2016;6:19546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu VC, Wang SM, Chueh SJ, Yang SY, Huang KH, Lin YH, Wang JJ, Connolly R, Hu YH, Gomez-Sanchez CE, et al. The prevalence of CTNNB1 mutations in primary aldosteronism and consequences for clinical outcomes. Sci Rep. 2017;7:39121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim AC, Reuter AL, Zubair M, Else T, Serecky K, Bingham NC, Lavery GG, Parker KL, Hammer GD. Targeted disruption of beta-catenin in Sf1-expressing cells impairs development and maintenance of the adrenal cortex. Development. 2008;135(15):2593–602. [DOI] [PubMed] [Google Scholar]
- 48.Heikkila M, Peltoketo H, Leppaluoto J, Ilves M, Vuolteenaho O, Vainio S. Wnt-4 deficiency alters mouse adrenal cortex function, reducing aldosterone production. Endocrinology. 2002;143(11):4358–65. [DOI] [PubMed] [Google Scholar]
- 49.Chen M, Hornsby PJ. Adenovirus-delivered DKK3/WNT4 and steroidogenesis in primary cultures of adrenocortical cells. Horm Metab Res. 2006;38(9):549–55. [DOI] [PubMed] [Google Scholar]
- 50.El Wakil A, Lalli E. The Wnt/beta-catenin pathway in adrenocortical development and cancer. Mol Cell Endocrinol. 2011;332(1-2):32–7. [DOI] [PubMed] [Google Scholar]
- 51.Berthon A, Sahut-Barnola I, Lambert-Langlais S, de Joussineau C, Damon-Soubeyrand C, Louiset E, Taketo MM, Tissier F, Bertherat J, Lefrancois-Martinez AM, et al. Constitutive beta-catenin activation induces adrenal hyperplasia and promotes adrenal cancer development. Hum Mol Genet. 2010;19(8):1561–76. [DOI] [PubMed] [Google Scholar]
- 52.Pignatti E, Leng S, Yuchi Y, Borges KS, Guagliardo NA, Shah MS, Ruiz-Babot G, Kariyawasam D, Taketo MM, Miao J, et al. Beta-Catenin Causes Adrenal Hyperplasia by Blocking Zonal Transdifferentiation. Cell Rep. 2020;31(3):107524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Akerstrom T, Crona J, Delgado Verdugo A, Starker LF, Cupisti K, Willenberg HS, Knoefel WT, Saeger W, Feller A, Ip J, et al. Comprehensive re-sequencing of adrenal aldosterone producing lesions reveal three somatic mutations near the KCNJ5 potassium channel selectivity filter. PLoS One. 2012;7(7):e41926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boulkroun S, Beuschlein F, Rossi GP, Golib-Dzib JF, Fischer E, Amar L, Mulatero P, Samson-Couterie B, Hahner S, Quinkler M, et al. Prevalence, clinical, and molecular correlates of KCNJ5 mutations in primary aldosteronism. Hypertension. 2012;59(3):592–8. [DOI] [PubMed] [Google Scholar]
- 55.Arnesen T, Glomnes N, Stromsoy S, Knappskog S, Heie A, Akslen LA, Grytaas M, Varhaug JE, Gimm O, Brauckhoff M. Outcome after surgery for primary hyperaldosteronism may depend on KCNJ5 tumor mutation status: a population-based study from Western Norway. Langenbecks Arch Surg. 2013;398(6):869–74. [DOI] [PubMed] [Google Scholar]
- 56.Fernandes-Rosa FL, Williams TA, Riester A, Steichen O, Beuschlein F, Boulkroun S, Strom TM, Monticone S, Amar L, Meatchi T, et al. Genetic spectrum and clinical correlates of somatic mutations in aldosterone-producing adenoma. Hypertension. 2014;64(2):354–61. [DOI] [PubMed] [Google Scholar]
- 57.Williams TA, Monticone S, Schack VR, Stindl J, Burrello J, Buffolo F, Annaratone L, Castellano I, Beuschlein F, Reincke M, et al. Somatic ATP1A1, ATP2B3, and KCNJ5 mutations in aldosterone-producing adenomas. Hypertension. 2014;63(1):188–95. [DOI] [PubMed] [Google Scholar]
- 58.Dutta RK, Welander J, Brauckhoff M, Walz M, Alesina P, Arnesen T, Soderkvist P, Gimm O. Complementary somatic mutations of KCNJ5, ATP1A1, and ATP2B3 in sporadic aldosterone producing adrenal adenomas. Endocr Relat Cancer. 2014;21(1):L1–4. [DOI] [PubMed] [Google Scholar]
- 59.Zheng FF, Zhu LM, Nie AF, Li XY, Lin JR, Zhang K, Chen J, Zhou WL, Shen ZJ, Zhu YC, et al. Clinical characteristics of somatic mutations in Chinese patients with aldosterone-producing adenoma. Hypertension. 2015;65(3):622–8. [DOI] [PubMed] [Google Scholar]
- 60.Wang B, Li X, Zhang X, Ma X, Chen L, Zhang Y, Lyu X, Tang Y, Huang Q, Gao Y, et al. Prevalence and characterization of somatic mutations in Chinese aldosterone-producing adenoma patients. Medicine (Baltimore). 2015;94(16):e708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu VC, Huang KH, Peng KY, Tsai YC, Wu CH, Wang SM, Yang SY, Lin LY, Chang CC, Lin YH, et al. Prevalence and clinical correlates of somatic mutation in aldosterone producing adenoma-Taiwanese population. Sci Rep. 2015;5:11396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kitamoto T, Suematsu S, Matsuzawa Y, Saito J, Omura M, Nishikawa T. Comparison of cardiovascular complications in patients with and without KCNJ5 gene mutations harboring aldosterone-producing adenomas. J Atheroscler Thromb. 2015;22(2):191–200. [DOI] [PubMed] [Google Scholar]
- 63.Okamura T, Nakajima Y, Katano-Toki A, Horiguchi K, Matsumoto S, Yoshino S, Yamada E, Tomaru T, Ishii S, Saito T, et al. Characteristics of Japanese aldosterone-producing adenomas with KCNJ5 mutations. Endocr J. 2017;64(1):39–47. [DOI] [PubMed] [Google Scholar]
- 64.Hong AR, Kim JH, Song YS, Lee KE, Seo SH, Seong MW, Shin CS, Kim SW, Kim SY. Genetics of Aldosterone-Producing Adenoma in Korean Patients. PLoS One. 2016;11(1):e0147590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Warachit W, Atikankul T, Houngngam N, Sunthornyothin S. Prevalence of Somatic KCNJ5 Mutations in Thai Patients With Aldosterone-Producing Adrenal Adenomas. J Endocr Soc. 2018;2(10):1137–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cheng CJ, Sung CC, Wu ST, Lin YC, Sytwu HK, Huang CL, Lin SH. Novel KCNJ5 mutations in sporadic aldosterone-producing adenoma reduce Kir3.4 membrane abundance. J Clin Endocrinol Metab. 2015;100(1):E155–63. [DOI] [PubMed] [Google Scholar]
- 67.Mohideen SK, Mustangin M, Kamaruddin NA, Muhammad R, Jamal ARA, Sukor N, Tan GC, Azizan EA. Prevalence and Histopathological Characteristics of KCNJ5 Mutant Aldosterone-Producing Adenomas in a Multi-Ethnic Malaysian Cohort. Front Endocrinol (Lausanne). 2019;10:666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kitamoto T, Suematsu S, Yamazaki Y, Nakamura Y, Sasano H, Matsuzawa Y, Saito J, Omura M, Nishikawa T. Clinical and Steroidogenic Characteristics of Aldosterone-Producing Adenomas With ATPase or CACNA1D Gene Mutations. J Clin Endocrinol Metab. 2016;101(2):494–503. [DOI] [PubMed] [Google Scholar]
- 69.Nishimoto K, Nakagawa K, Li D, Kosaka T, Oya M, Mikami S, Shibata H, Itoh H, Mitani F, Yamazaki T, et al. Adrenocortical zonation in humans under normal and pathological conditions. J Clin Endocrinol Metab. 2010;95(5):2296–305. [DOI] [PubMed] [Google Scholar]
- 70.Gomez-Sanchez CE, Qi X, Velarde-Miranda C, Plonczynski MW, Parker CR, Rainey W, Satoh F, Maekawa T, Nakamura Y, Sasano H, et al. Development of monoclonal antibodies against human CYP11B1 and CYP11B2. Mol Cell Endocrinol. 2014;383(1-2):111–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nanba K, Chen AX, Omata K, Vinco M, Giordano TJ, Else T, Hammer GD, Tomlins SA, Rainey WE. Molecular Heterogeneity in Aldosterone-Producing Adenomas. J Clin Endocrinol Metab. 2016;101(3):999–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fernandes-Rosa FL, Giscos-Douriez I, Amar L, Gomez-Sanchez CE, Meatchi T, Boulkroun S, Zennaro MC. Different Somatic Mutations in Multinodular Adrenals With Aldosterone-Producing Adenoma. Hypertension. 2015;66(5):1014–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nanba AT, Nanba K, Byrd JB, Shields JJ, Giordano TJ, Miller BS, Rainey WE, Auchus RJ, Turcu AF. Discordance between imaging and immunohistochemistry in unilateral primary aldosteronism. Clin Endocrinol (Oxf). 2017;87(6):665–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Volpe C, Hamberger B, Hoog A, Mukai K, Calissendorff J, Wahrenberg H, Zedenius J, Thoren M. Primary aldosteronism: functional histopathology and long-term follow-up after unilateral adrenalectomy. Clin Endocrinol (Oxf). 2015;82(5):639–47. [DOI] [PubMed] [Google Scholar]
- 75.Williams TA, Gomez-Sanchez CE, Rainey WE, Giordano TJ, Lam AK, Marker A, Mete O, Yamazaki Y, Zerbini MCN, Beuschlein F, et al. International Histopathology Consensus for Unilateral Primary Aldosteronism. J Clin Endocrinol Metab. 2021;106(1):42–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Akerstrom T, Willenberg HS, Cupisti K, Ip J, Backman S, Moser A, Maharjan R, Robinson B, Iwen KA, Dralle H, et al. Novel somatic mutations and distinct molecular signature in aldosterone-producing adenomas. Endocr Relat Cancer. 2015;22(5):735–44. [DOI] [PubMed] [Google Scholar]
- 77.Lenzini L, Rossitto G, Maiolino G, Letizia C, Funder JW, Rossi GP. A Meta-Analysis of Somatic KCNJ5 K(+) Channel Mutations In 1636 Patients With an Aldosterone-Producing Adenoma. J Clin Endocrinol Metab. 2015;100(8):E1089–95. [DOI] [PubMed] [Google Scholar]
- 78.Williams TA, Lenders JW, Burrello J, Beuschlein F, Reincke M. KCNJ5 Mutations: Sex, Salt and Selection. Horm Metab Res. 2015;47(13):953–8. [DOI] [PubMed] [Google Scholar]
- 79.Morton MS, Arisaka O, Miyake N, Morgan LD, Evans BA. Phytoestrogen concentrations in serum from Japanese men and women over forty years of age. J Nutr. 2002;132(10):3168–71. [DOI] [PubMed] [Google Scholar]
- 80.Molina L, Bustamante FA, Bhoola KD, Figueroa CD, Ehrenfeld P. Possible role of phytoestrogens in breast cancer via GPER-1/GPR30 signaling. Clin Sci (Lond). 2018;132(24):2583–98. [DOI] [PubMed] [Google Scholar]
- 81.Tanwar AK, Dhiman N, Kumar A, Jaitak V. Engagement of phytoestrogens in breast cancer suppression: Structural classification and mechanistic approach. Eur J Med Chem. 2020:113037. [DOI] [PubMed] [Google Scholar]
- 82.Caroccia B, Seccia TM, Campos AG, Gioco F, Kuppusamy M, Ceolotto G, Guerzoni E, Simonato F, Mareso S, Lenzini L, et al. GPER-1 and estrogen receptor-beta ligands modulate aldosterone synthesis. Endocrinology. 2014;155(11):4296–304. [DOI] [PubMed] [Google Scholar]
- 83.Chu MD, Ulick S. Isolation and identification of 18-hydroxycortisol from the urine of patients with primary aldosteronism. J Biol Chem. 1982;257(5):2218–24. [PubMed] [Google Scholar]
- 84.Ulick S, Chu MD, Land M. Biosynthesis of 18-oxocortisol by aldosterone-producing adrenal tissue. J Biol Chem. 1983;258(9):5498–502. [PubMed] [Google Scholar]
- 85.Lifton RP, Dluhy RG, Powers M, Rich GM, Cook S, Ulick S, Lalouel JM. A chimaeric 11 beta-hydroxylase/aldosterone synthase gene causes glucocorticoid-remediable aldosteronism and human hypertension. Nature. 1992;355(6357):262–5. [DOI] [PubMed] [Google Scholar]
- 86.Geller DS, Zhang J, Wisgerhof MV, Shackleton C, Kashgarian M, Lifton RP. A novel form of human mendelian hypertension featuring nonglucocorticoid-remediable aldosteronism. J Clin Endocrinol Metab. 2008;93(8):3117–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ulick S, Blumenfeld JD, Atlas SA, Wang JZ, Vaughan ED Jr. The unique steroidogenesis of the aldosteronoma in the differential diagnosis of primary aldosteronism. J Clin Endocrinol Metab. 1993;76(4):873–8. [DOI] [PubMed] [Google Scholar]
- 88.Hamlet SM, Gordon RD, Gomez-Sanchez CE, Tunny TJ, Klemm SA. Adrenal transitional zone steroids, 18-oxo and 18-hydroxycortisol, useful in the diagnosis of primary aldosteronism, are ACTH-dependent. Clin Exp Pharmacol Physiol. 1988;15(4):317–22. [DOI] [PubMed] [Google Scholar]
- 89.Mulatero P, di Cella SM, Monticone S, Schiavone D, Manzo M, Mengozzi G, Rabbia F, Terzolo M, Gomez-Sanchez EP, Gomez-Sanchez CE, et al. 18-hydroxycorticosterone, 18-hydroxycortisol, and 18-oxocortisol in the diagnosis of primary aldosteronism and its subtypes. J Clin Endocrinol Metab. 2012;97(3):881–9. [DOI] [PubMed] [Google Scholar]
- 90.Satoh F, Morimoto R, Ono Y, Iwakura Y, Omata K, Kudo M, Takase K, Seiji K, Sasamoto H, Honma S, et al. Measurement of peripheral plasma 18-oxocortisol can discriminate unilateral adenoma from bilateral diseases in patients with primary aldosteronism. Hypertension. 2015;65(5):1096–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Eisenhofer G, Dekkers T, Peitzsch M, Dietz AS, Bidlingmaier M, Treitl M, Williams TA, Bornstein SR, Haase M, Rump LC, et al. Mass Spectrometry-Based Adrenal and Peripheral Venous Steroid Profiling for Subtyping Primary Aldosteronism. Clin Chem. 2016;62(3):514–24. [DOI] [PubMed] [Google Scholar]
- 92.Zilbermint M, Xekouki P, Faucz FR, Berthon A, Gkourogianni A, Schernthaner-Reiter MH, Batsis M, Sinaii N, Quezado MM, Merino M, et al. Primary Aldosteronism and ARMC5 Variants. J Clin Endocrinol Metab. 2015;100(6):E900–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Assie G, Libe R, Espiard S, Rizk-Rabin M, Guimier A, Luscap W, Barreau O, Lefevre L, Sibony M, Guignat L, et al. ARMC5 mutations in macronodular adrenal hyperplasia with Cushing's syndrome. N Engl J Med. 2013;369(22):2105–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Azizan EA, Lam BY, Newhouse SJ, Zhou J, Kuc RE, Clarke J, Happerfield L, Marker A, Hoffman GJ, Brown MJ. Microarray, qPCR, and KCNJ5 sequencing of aldosterone-producing adenomas reveal differences in genotype and phenotype between zona glomerulosa- and zona fasciculata-like tumors. J Clin Endocrinol Metab. 2012;97(5):E819–29. [DOI] [PubMed] [Google Scholar]
- 95.Monticone S, Castellano I, Versace K, Lucatello B, Veglio F, Gomez-Sanchez CE, Williams TA, Mulatero P. Immunohistochemical, genetic and clinical characterization of sporadic aldosterone-producing adenomas. Mol Cell Endocrinol. 2015;411:146–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yamazaki Y, Nakamura Y, Omata K, Ise K, Tezuka Y, Ono Y, Morimoto R, Nozawa Y, Gomez-Sanchez CE, Tomlins SA, et al. Histopathological Classification of Cross-Sectional Image-Negative Hyperaldosteronism. J Clin Endocrinol Metab. 2017;102(4):1182–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ono Y, Yamazaki Y, Omata K, Else T, Tomlins SA, Rhayem Y, Williams TA, Reincke M, Carling T, Monticone S, et al. Histological Characterization of Aldosterone-producing Adrenocortical Adenomas with Different Somatic Mutations. J Clin Endocrinol Metab. 2020;105(3) e282–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Teo AE, Garg S, Shaikh LH, Zhou J, Karet Frankl FE, Gurnell M, Happerfield L, Marker A, Bienz M, Azizan EA, et al. Pregnancy, Primary Aldosteronism, and Adrenal CTNNB1 Mutations. N Engl J Med. 2015;373(15):1429–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Saner-Amigh K, Mayhew BA, Mantero F, Schiavi F, White PC, Rao CV, Rainey WE. Elevated expression of luteinizing hormone receptor in aldosterone-producing adenomas. J Clin Endocrinol Metab. 2006;91(3):1136–42. [DOI] [PubMed] [Google Scholar]
- 100.Ye P, Mariniello B, Mantero F, Shibata H, Rainey WE. G-protein-coupled receptors in aldosterone-producing adenomas: a potential cause of hyperaldosteronism. J Endocrinol. 2007;195(1):39–48. [DOI] [PubMed] [Google Scholar]
- 101.Zwermann O, Suttmann Y, Bidlingmaier M, Beuschlein F, Reincke M. Screening for membrane hormone receptor expression in primary aldosteronism. Eur J Endocrinol. 2009;160(3):443–51. [DOI] [PubMed] [Google Scholar]
- 102.Albiger NM, Sartorato P, Mariniello B, Iacobone M, Finco I, Fassina A, Mantero F. A case of primary aldosteronism in pregnancy: do LH and GNRH receptors have a potential role in regulating aldosterone secretion? Eur J Endocrinol. 2011;164(3):405–12. [DOI] [PubMed] [Google Scholar]
- 103.Nakamura Y, Hattangady NG, Ye P, Satoh F, Morimoto R, Ito-Saito T, Sugawara A, Ohba K, Takahashi K, Rainey WE, et al. Aberrant gonadotropin-releasing hormone receptor (GnRHR) expression and its regulation of CYP11B2 expression and aldosterone production in adrenal aldosterone-producing adenoma (APA). Mol Cell Endocrinol. 2014;384(1–2):102–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kishimoto R, Oki K, Yoneda M, Gomez-Sanchez CE, Ohno H, Kobuke K, Itcho K, Kohno N. Gonadotropin-Releasing Hormone Stimulate Aldosterone Production in a Subset of Aldosterone-Producing Adenoma. Medicine (Baltimore). 2016;95(20):e3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gagnon N, Caceres-Gorriti KY, Corbeil G, El Ghoyareb N, Ludwig N, Latour M, Lacroix A, Bourdeau I. Genetic Characterization of GnRH/LH-Responsive Primary Aldosteronism. J Clin Endocrinol Metab. 2018;103(8):2926–35. [DOI] [PubMed] [Google Scholar]