To the Editor:
The decision on the optimal time of invasive mechanical ventilation (IMV) onset in coronavirus disease (COVID-19) acute respiratory distress syndrome (ARDS) has been debated during the current pandemic (1, 2). Although affected by the availability of resources, timing of intubation may also affect the outcome for patients with COVID-19. In this study we investigate whether factors related to respiratory distress (RD) are associated with the driving pressure (DP) in patients with COVID-19 ARDS.
Methods
We prospectively enrolled all consecutive patients with COVID-19 ARDS who were admitted to the ICU of a tertiary hospital (April to December 2020). Patients were included if they were on IMV and had positive PCR for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and ARDS. The local ethics committee (protocol number 53398/2020) approved the study with a waiver for informed consent. The primary outcome was the identification of predictors independently associated with patients’ respiratory mechanics upon ICU admission.
In all patients, a lung protective protocol (Vt of 6–8 ml/kg of ideal body weight and proning [if PaO2/FiO2 < 150 mm Hg]) was adopted. Positive end-expiratory pressure was set after a recruitment maneuver at the level of the lowest DP.
PaO2/FiO2, airway pressures, Vt, respiratory system compliance (Vt/DP) and elastance (Ers: DP/Vt/ideal body weight), Acute Physiology and Chronic Health Evaluation II (APACHE II), Sequential Organ Failure Assessment score, vasopressors, laboratory tests (on ICU admission), and COVID-19 treatment received before ICU admission were recorded.
RD before intubation was defined as the time (hours) spent with hypoxemia (PaO2/FiO2 <150 mm Hg, PaO2/FiO2 <100 mm Hg, PaO2/FiO2 <50 mm Hg) and tachypnea (respiratory rate [RR] >25/min and RR >30/min). FiO2 values before intubation (oxygen delivery by face masks) were estimated on the basis of tables published in previous investigations (3), whereas in high-flow nasal cannula cases the value that was delivered by the device (myAirvo2, Fisher & Paykel Healthcare) was recorded. The monitoring protocol of patients with COVID-19 ARDS before ICU admission included 1) recordings of the RR, FiO2, SpO2, and other vital signs (i.e., arterial blood pressure, pulse rate, temperature, urine output) once every 3 hours ; 2) arterial blood gases once every 8 hours per protocol—unless the patients’ condition required more frequent sampling (as decided by the attending physician); and 3) continuous digital monitoring (arterial blood pressure, ECG, SpO2, RR, hourly updates by the nursing staff) for all severe cases. During their ICU stay, patients had continuous digital monitoring of all clinical variables of interest (arterial blood pressure, ECG, SpO2, RR, temperature), whereas arterial blood gases were measured once every 3 hours or more often.
The cutoff values for PaO2/FiO2 and RR used in the analysis were chosen on the basis of their frequency distribution and their clinical relevance (4).
The patients were classified in two groups according to the lowest DP achieved during the first 24 hours in ICU (after recruitment maneuvers and/or prone position): DP ⩽14 cm H2O and DP >14 cm H2O (5). This cutoff value was chosen on the basis of its association with mortality (5, 6).
Data are presented as mean (SD). Variables significantly associated with DP (continuous variable) in univariate analysis were entered in multivariate analyses. The linear regression model was used. Analyses were performed using RD indices and repeated using its components (time with hypoxemia and time with tachypnea) separately. Ancillary analysis was performed using Ers as a dependent variable. Receiver operating characteristic analysis evaluated the diagnostic performance of RD indices and its components to identify patients with DP >14 cm H2O. Two-tailed P values <0.05 were considered significant. SPSS version 26.0 was used (SPSS Inc.).
Results
Eighty-five patients with COVID-19 were admitted in the ICU during the study period and were included (one patient was excluded because he was admitted because of hemorrhagic shock but did not present ARDS). Measurements (per patient/day) available for SpO2, PaO2, FiO2, and RR were 24, 3, 24, and 8, respectively; no difference was found between the two groups (DP >14 cm H2O and DP ⩽14 cm H2O) in terms of the number of assessments. Table 1 presents baseline characteristics of participants and variables associated with DP >14 cm H2O.
Table 1.
Baseline Characteristics
| DP ⩽14 cm H2O (n = 57) | DP >14 cm H2O (n = 26) | P Value | |
|---|---|---|---|
| Age, yr | 68.1 ± 12.2 | 68.1 ± 10.9 | 0.987 |
| Sex, M, n (%) | 37 (64.9) | 19 (73.1) | 0.468 |
| BMI, kg/m2 | 27.4 ± 0.8 | 27.5 ± 1.1 | 0.907 |
| COPD, n (%) | 4 (7.0) | 3 (11.5) | 0.429 |
| Hypertension, n (%) | 35 (61.4) | 20 (76.9) | 0.122 |
| Diabetes mellitus, n (%) | 23 (40.3) | 6 (23.1) | 0.06 |
| Smoking, n (%) | 21 (36.8) | 5 (19.2) | 0.081 |
| COVID-19 symptoms’ duration, d | 9.5 ± 4.7 | 10.9 ± 5.5 | 0.23 |
| PaO2/FiO2 at hospital admission, mm Hg | 237.8 ± 109.2 | 211.8 ± 121.9 | 0.335 |
| Length of hospital stay before IMV, days | 4.2 ± 0.5 | 7.9 ± 1.2 | 0.001 |
| RD, h (n) | |||
| RD150/25 | 18.7 ± 19.2 (41) | 67.2 ± 78.5 (23) | <0.0001 |
| RD150/30 | 5.2 ± 7.9 (28) | 35.3 ± 49.8(23) | <0.0001 |
| RD100/25 | 9.3 ± 14.4 (33) | 63.3 ± 72.5 (23) | <0.0001 |
| RD100/30 | 4.4 ± 7.5 (28) | 36.9 ± 49.5 (23) | <0.0001 |
| HFNC use*, h (n) | 1.8 ± 7.2 (7) | 40.8 ± 77.9 (11) | 0.007 |
| NIV use, h (n) | 0.6 ± 3.7 (2) | 2.2 ± 6.2 (4) | 0.165 |
| Intubation time (min before ICU admission) | 29.8 ± 6.2 | 39.8 ± 12.3 | 0.423 |
| IMV onset | |||
| APACHE II† | 14.5 ± 5.5 | 20.2 ± 8.1 | <0.0001 |
| SOFA | 7.2 ± 1.8 | 8.7 ± 2.1 | 0.001 |
| PaO2/FiO2, mm Hg (before intubation) | 103.1 ± 41.5 | 61.1 ± 23.4 | <0.0001 |
| PaO2/FiO2, mm Hg (after intubation) | 121.2 ± 45.2 | 95.4 ± 41.1 | 0.01 |
| Ventilatory ratio | 1.9 ± 0.6 | 2.7 ± 1.1 | <0.0001 |
| Vt, ml | 462.3 ± 55.5 | 442.3 ± 54.7 | 0.131 |
| Vt/IBW, ml/kg | 7.1 ± 0.7 | 6.9 ± 0.8 | 0.360 |
| RR (on IMV) | 21.9 ± 3.1 | 25.1 ± 3.8 | <0.001 |
| Minute ventilation | 10.2 ± 1.8 | 11 ± 1.8 | 0.048 |
| DP, cm H2O | 11.5 ± 2.1 | 18.3 ± 3.6 | <0.0001 |
| Elastance, cm H2O/ml/kg | 1.6 ± 0.4 | 2.6 ± 0.7 | <0.0001 |
| PEEP, cm H2O | 11.6 ± 3.1 | 11.2 ± 2.9 | 0.581 |
| Pplat, cm H2O | 23.2 ± 4.2 | 29.1 ± 4.9 | <0.0001 |
| Crs, ml/cm H2O | 41.8 ± 10.9 | 25.1 ± 6.4 | <0.0001 |
| Prone positioning | 11 (19.3) | 11 (42.3) | 0.002 |
| Noradrenaline, μg/kg/min | 0.2 ± 0.3 | 0.6 ± 0.5 | <0.0001 |
| Vasopressin, n | 0 | 5 | 0.001 |
| CRP, mg/dl (<0.5) | 8.8 ± 9.1 | 11.1 ± 10.6 | 0.327 |
| D-dimers, ng/ml (<300) | 1,138.3 ± 1,365.5 | 2,035.2 ± 1,836.9 | 0.015 |
| Ferritin, ng/ml (24–336) | 1,710.9 ± 3,231.8 | 4,862 ± 12,785.3 | 0.089 |
| WBC × 106/L (4,000–10,000) | 8,914.1 ± 4,686.9 | 12,319.2 ± 6,327.9 | 0.008 |
| Lymph × 106/L (1,000–4,800) | 678.8 ± 611.5 | 587.5 ± 292.3 | 0.472 |
| Superinfections upon ICU admission | |||
| Bloodstream infection | 1 C. albicans 1 S. maltophilia |
1 A. baumannii | |
| Lower respiratory tract infection | 1 S. aureus (MSSA) | ||
| Outcomes | |||
| IMV duration, d | 14.5 ± 8.0 | 11.2 ± 7.6 | 0.079 |
| 28-d survival, n (%) | 29 (50.9) | 6 (23.1) | 0.01 |
Definition of abbreviations: A. baumannii = Acinetobacter baumannii; APACHE II = Acute Physiology and Chronic Health Evaluation II; BMI = body mass index; C. albicans = Candida albicans; COPD = chronic obstructive pulmonary disease; COVID-19 = coronavirus disease; CRP = C-reactive protein; Crs = respiratory system compliance; DP = driving pressure; elastance = respiratory system elastance; HFNC = high flow nasal cannula; IBW = ideal body weight; IMV = invasive mechanical ventilation; Lymph = lymphocytes; MSSA = methicillin-resistant Staphylococcus aureus; NIV = noninvasive ventilation; PEEP = positive end-expiratory pressure; Pplat = plateau pressure; RR = respiratory rate; RD = respiratory distress; S. aureus = Staphylococcus aureus; S. maltophilia = Stenotrophomonas maltophilia; SOFA = Sequential Organ Failure Assessment; WBC = white blood cells.
Data are presented as mean ± SD unless otherwise noted.
Note that three patients in the DP ⩽ 14 cm H2O group and two in the DP > 14 cm H2O group did not have PaO2/FiO2 <150 mm Hg before admission. These patients were intubated because of tachypnea and tachycardia. All patients were admitted in the ICU after intubation.
APACHE II score was calculated upon ICU admission.
Multiple linear regression analysis revealed that only RD100/25 (time with PaO2/FiO2 <100 mm Hg and RR >25/min) was independently associated with DP (R2 = 0.265, P < 0.001; β = 0.044, P = 0.009). When RD components (time with hypoxemia and time with tachypnea) were evaluated separately, only the duration (hours) with PaO2/FiO2 <100 mm Hg was independently associated with DP (R2 = 0.381, β = 0.044, P < 0.0001). Figure 1 presents correlations of RD components with DP.
Figure 1.
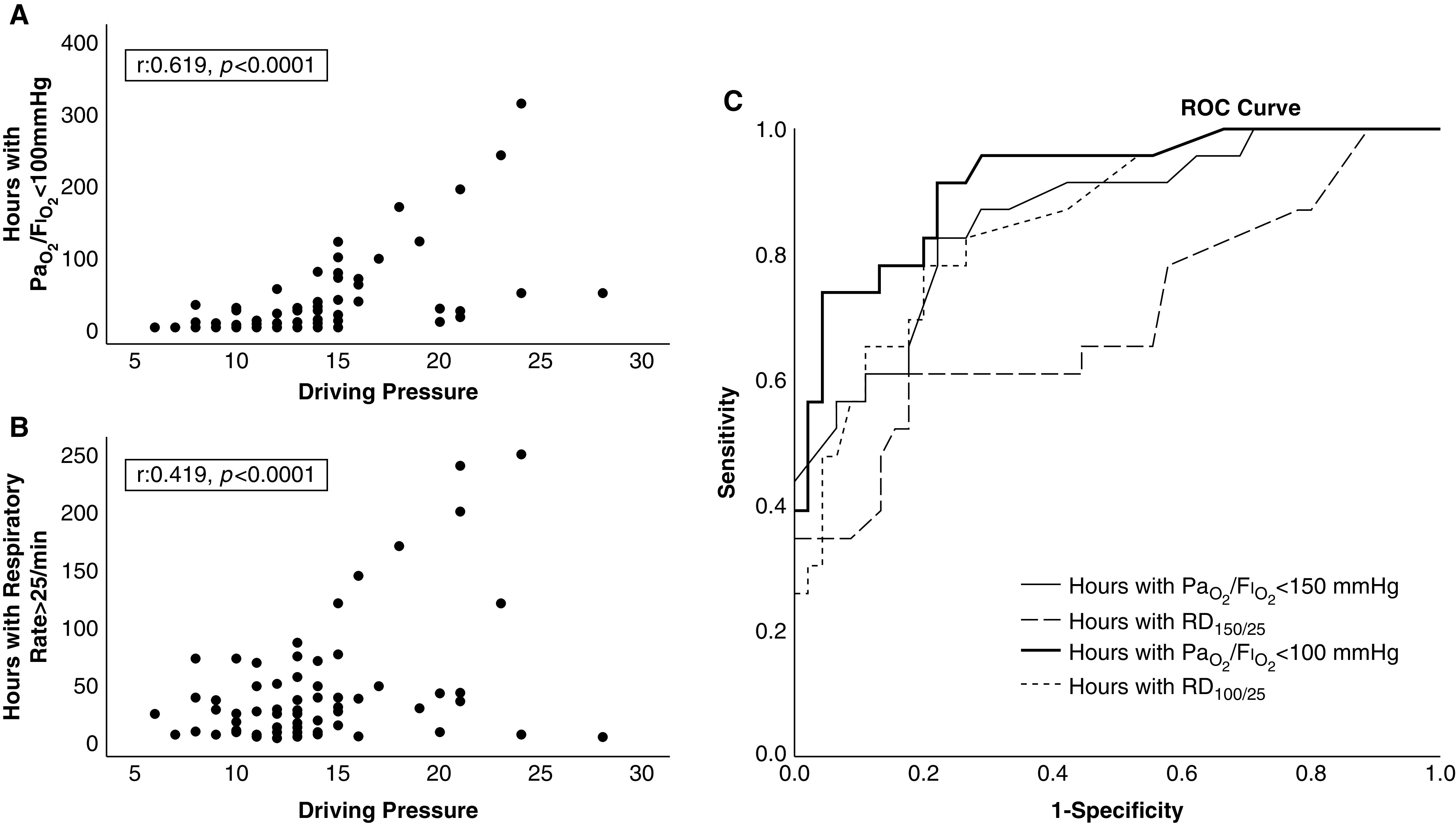
(A) Pearson correlation between hours with PaO2/FiO2 <100 mm Hg and driving pressure (r = 0.619, P < 0.0001). (B) Pearson correlation between hours with respiratory distress (RD)100/25 and driving pressure (r = 0.518, P < 0.0001). (C) ROC analysis of factors associated with driving pressure >14 cm H2O. The solid bold line depicts hours with PaO2/FiO2 <100 mm Hg (area under the curve [AUC], 0.916; 95% confidence interval [CI], 0.846–0.986; P < 0.0001). The solid line depicts hours with PaO2/FiO2 <150 mm Hg (AUC, 0.861; 95% CI, 0.768–0.954; P < 0.0001). The dotted line depicts hours with RD100/25 (AUC, 0.855; 95% CI, 0.764–0.946; P < 0.0001). The dashed line depicts hours with RD150/25 (AUC, 0.700; 95% CI, 0.557–0.842; P < 0.0001). ROC = receiver operating characteristic.
Ers at ICU admission was significantly correlated with both RD100/25 (0.513, P < 0.0001) and PaO2/FiO2 (baseline values) (r = −0.256, P = 0.019), but it was independently associated only with RD100/25 (R2 = 0.263, β = 0.017, P < 0.0001).
Receiver operating characteristic analysis showed that duration of hypoxemia (expressed as either hours with RD100/25 or hours with PaO2/FiO2 <100 mm Hg) could identify patients with DP >14 cm H2O; specifically, RD100/25 longer than 7 hours presented 83% sensitivity and 73% sensitivity to identify patients with DP >14 cm H2O (area under the curve [AUC],0.855; 95% confidence interval [CI], 0.764–0.946), whereas PaO2/FiO2 <100 mm Hg for more than 21.5 hours presented 83% sensitivity and 80% specificity (AUC, 0.916; 95% CI, 0.846–0.986) (Figure 1).
Discussion
This study demonstrates that 1) DP was above 14 cm H2O in 32.6% of patients with COVID-19 ARDS; 2) DP and Ers were independently associated with the duration of RD; and 3) duration of PaO2/FiO2 <100 mm Hg longer than 21.5 hours was the most significant factor predicting DP >14 cm H2O.
To our knowledge, this is the first study to evaluate differences between patients with COVID-19 ARDS according to DP and factors that are associated with DP. We chose DP as it represents the distending pressure applied to the lung during a tidal inflation. Moreover, DP reflects respiratory compliance and provides useful information when a lung protective ventilation protocol is adopted (5, 6). In this respect, we found it reasonable to target and analyze patients according to the best DP that could be achieved during the first 24 hours of ICU admission.
The factors that best correlated to DP >14 cm H2O were the duration of hypoxemia (PaO2/FiO2 <100 mm Hg) and its combination with tachypnea, the RD100/25. Baseline characteristics and infection duration did not have any impact. One explanation could be the increased disease severity leading to decreased compliance and tachypnea and hypoxemia. On the other hand, prolonged RD may further compound the lung injury induced by COVID-19 pathology. Our observation is in line with clinical data suggesting that vigorous, spontaneous ventilatory efforts for extended periods can exacerbate the lung damage (7), inducing a secondary insult (patient self-induced lung injury) (1). Experimental data show that in cases of acute lung injury, spontaneous breathing may provoke increased volumetric strain and heterogeneity in lung units (8).
Hypoxemia duration correlated with respiratory mechanics. The majority of patients with DP ⩽14 cm H2O presented hypoxemia and tachypnea, yet for a significantly shorter time interval than patients with DP >14 cm H2O. A duration of PaO2/FiO2 <100 mm Hg longer than 21.5 hours or RD100/25 longer than 7 hours predicted a DP >14 cm H2O. These findings could help identify thresholds for the degree and duration of hypoxemia/increased work of breathing to decide the optimal time for intubation in COVID-19 ARDS (1, 2, 9).
Moreover, patients with DP >14 cm H2O presented advanced multiorgan involvement in terms of APACHE II, Sequential Organ Failure Assessment scores, and vasopressors compared with patients with earlier IMV initiation.
Limitations of the study are its single-center and observational character. Therefore, the association of RD with a DP >14 cm H2O does not necessarily imply causality. Second, intrathoracic pressure swings were not directly evaluated; yet, esophageal pressure monitoring is not always feasible, especially in conscious patients with RD, and the interpretation of measurements is not always straightforward and simple (10). RD was arbitrarily defined as it has never been definitely quantified. One might certainly argue that the optimal time for intubation/ICU admission may also impact outcomes. Such decisions during a pandemic depend on clinical assessment, end-of-life issues, and resources/bed availability. We acknowledge that these parameters were not assessed and could be the scope of a future study.
Conclusions
In the present study, RD and hypoxemia duration in patients with COVID-19 ARDS were significantly associated with a DP >14 cm H2O on ICU admission.
Acknowledgments
Acknowledgment
The authors thank Dr. George Vavougios for his help in the statistical analyses and Ross J. Robertson for editing the manuscript.
Footnotes
Author Contributions: V.S.T., D.A.M., G.E.Z., and K.D.M.: conception and design, data analysis and interpretation, and manuscript drafting. V.S.T, D.A.M, G.E.Z., I.N.P., Ε.Ζ., K.V.D, M.-E.E.P., E.E.M, and E.S.G: data acquisition and interpretation and revision of the manuscript for important intellectual content. All authors: final approval of the version submitted for publication and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Originally Published in Press as DOI: 10.1164/rccm.202101-0234LE on June 15, 2021
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1. Marini JJ, Gattinoni L. Management of COVID-19 respiratory distress. JAMA . 2020;323:2329–2330. doi: 10.1001/jama.2020.6825. [DOI] [PubMed] [Google Scholar]
- 2. Tobin MJ, Laghi F, Jubran A. Caution about early intubation and mechanical ventilation in COVID-19. Ann Intensive Care . 2020;10:78. doi: 10.1186/s13613-020-00692-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vincent JL, Rello J, Marshall J, Silva E, Anzueto A, Martin CD, et al. EPIC II Group of Investigators. International study of the prevalence and outcomes of infection in intensive care units. JAMA . 2009;302:2323–2329. doi: 10.1001/jama.2009.1754. [DOI] [PubMed] [Google Scholar]
- 4. Darreau C, Martino F, Saint-Martin M, Jacquier S, Hamel JF, Nay MA, et al. Use, timing and factors associated with tracheal intubation in septic shock: a prospective multicentric observational study. Ann Intensive Care . 2020;10:62. doi: 10.1186/s13613-020-00668-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Amato MB, Meade MO, Slutsky AS, Brochard L, Costa EL, Schoenfeld DA, et al. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med . 2015;372:747–755. doi: 10.1056/NEJMsa1410639. [DOI] [PubMed] [Google Scholar]
- 6. Goligher EC, Costa ELV, Yarnell CJ, Brochard LJ, Stewart TE, Tomlinson G, et al. Effect of lowering Vt on mortality in acute respiratory distress syndrome varies with respiratory system elastance. Am J Respir Crit Care Med . 2021;203:1378–1385. doi: 10.1164/rccm.202009-3536OC. [DOI] [PubMed] [Google Scholar]
- 7. Hurtado DE, Erranz B, Lillo F, Sarabia-Vallejos M, Iturrieta P, Morales F, et al. Progression of regional lung strain and heterogeneity in lung injury: assessing the evolution under spontaneous breathing and mechanical ventilation. Ann Intensive Care . 2020;10:107. doi: 10.1186/s13613-020-00725-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Retamal J, Bergamini BC, Carvalho AR, Bozza FA, Borzone G, Borges JB, et al. Non-lobar atelectasis generates inflammation and structural alveolar injury in the surrounding healthy tissue during mechanical ventilation. Crit Care . 2014;18:505. doi: 10.1186/s13054-014-0505-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hernandez-Romieu AC, Adelman MW, Hockstein MA, Robichaux CJ, Edwards JA, Fazio JC, et al. Emory COVID-19 Quality and Clinical Research Collaborative. Timing of intubation and mortality among critically ill coronavirus disease 2019 patients: a single-center cohort study. Crit Care Med . 2020;48:e1045–e1053. doi: 10.1097/CCM.0000000000004600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gulati G, Novero A, Loring SH, Talmor D. Pleural pressure and optimal positive end-expiratory pressure based on esophageal pressure versus chest wall elastance: incompatible results. Crit Care Med . 2013;41:1951–1957. doi: 10.1097/CCM.0b013e31828a3de5. [DOI] [PubMed] [Google Scholar]


