Abstract
Rationale: Delirium is common in the ICU and portends worse ICU and hospital outcomes. The effect of delirium in the ICU on post–hospital discharge mortality and health resource use is less well known.
Objectives: To estimate mortality and health resource use 2.5 years after hospital discharge in critically ill patients admitted to the ICU.
Methods: This was a population-based, propensity score–matched, retrospective cohort study of adult patients admitted to 1 of 14 medical–surgical ICUs from January 1, 2014, to June 30, 2016. Delirium was measured by using the 8-point Intensive Care Delirium Screening Checklist. The primary outcome was mortality. The secondary outcome was a composite measure of subsequent emergency department visits, hospital readmission, or mortality.
Measurements and Main Results: There were 5,936 propensity score–matched patients with and without a history of incident delirium who survived to hospital discharge. Delirium was associated with increased mortality 0–30 days after hospital discharge (hazard ratio, 1.44 [95% confidence interval, 1.08–1.92]). There was no significant difference in mortality more than 30 days after hospital discharge (delirium: 3.9%, no delirium: 2.6%). There was a persistent increased risk of emergency department visits, hospital readmissions, or mortality after hospital discharge (hazard ratio, 1.12 [95% confidence interval, 1.07–1.17]) throughout the study period.
Conclusions: ICU delirium is associated with increased mortality 0–30 days after hospital discharge.
Keywords: critical care, delirium, mortality
At a Glance Commentary
Scientific Knowledge on the Subject
Delirium is common in the ICU and leads to worse ICU and hospital outcomes. Although small, single-center studies on mortality have been conducted, the effect of delirium in the ICU on post–hospital discharge mortality and health resource use is not well understood.
What This Study Adds to the Field
We used population-based data from 5,936 propensity-matched critically ill adults with and without a history of delirium to estimate the mortality and health resource use 2.5 years after hospital discharge. Delirium was associated with increased mortality 0–30 days after hospital discharge, but this association was not present at later time points. There was a persistent increased risk of emergency department visits, hospital readmissions, or mortality up to 2.5 years after hospital discharge.
Delirium, an acute state of confusion characterized by a fluctuating course, attention deficits, and severe disorganization of behavior (1), may affect over half of patients admitted to ICUs (2–9). Risk factors for delirium in the ICU include age, the severity of illness, and the need for mechanical ventilation (10). Delirium in critically ill patients is associated with adverse outcomes, including longer hospital stays (11), an increased risk of cognitive impairment, and ICU and hospital mortality (12–14).
Most studies on delirium in the ICU have reported on ICU or hospital mortality (9). Few studies have reported outcomes after hospital discharge (9); those that have were conducted in single centers with up to 1 year of follow-up, focused on ICU subpopulations, and reported contradictory results (12, 15–18). There is a growing body of literature suggesting that critical illness and ICU care have long-term consequences (19, 20). Given the high prevalence of delirium in critically ill patients and its well documented consequences in the ICU and hospital (21), understanding if and how delirium influences the health of patients recovering from critical illness is important. Therefore, we followed a large, multicenter, population-based cohort of critically ill patients admitted to the ICU for up to 2.5 years after hospital discharge to examine the association between delirium in the ICU and post–hospital discharge mortality and health resource use.
Methods
Study Design
We employed a multicenter, propensity score–matched, population-based retrospective cohort design to determine the association between delirium in the ICU (exposure) and 1) measures of health outcomes (mortality) and 2) a composite measure of emergency department visits, hospital readmission, or mortality (outcomes). This study is reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology statement (22).
Study Setting and Population
Consecutive adult patients (age ⩾18 yr) admitted to 14 medical–surgical ICUs (within 14 hospitals) with electronic medical records in Alberta, Canada (population 4.4 million), from January 1, 2014, to June 30, 2016, formed the study cohort. Patients were excluded if they had not undergone at least one assessment of delirium in the ICU or at least one ICU admission with an ICU length of stay (LOS) ⩾24 hours or if their home residence was located outside of Alberta (determined by using the provincial healthcare number) to ensure that any hospital readmission, emergency department visit, or mortality data after the ICU stay were available. If a patient had been admitted to the ICU more than once during the study period, only the first admission with an ICU LOS ⩾24 hours and at least one Intensive Care Delirium Screening Checklist (ICDSC) assessment was used to measure exposure to delirium. The primary cohort was based on patients surviving to hospital discharge, with follow-up starting from hospital discharge and therefore excluding patients who died in the ICU or in the hospital. All medical–surgical ICUs are closed units staffed by accredited intensive care physicians and provide mechanical ventilation, vasoactive medications, and invasive monitoring. Data for mortality, emergency department visits, and hospital readmissions were available until December 31, 2019.
Data Sources
We used data from five databases previously employed for research purposes (23–25). eCritical Alberta is integrated in a bedside electronic medical record (MetaVision) that prospectively captures demographic and clinical data for all patients admitted to every ICU in Alberta (24). The Discharge Abstract Database captures hospital outcomes and diagnoses (25 diagnostic codes based on the International Classification of Diseases, 10th Revision, Canadian Enhancement) on all hospitalized patients. Mortality after an ICU and/or hospital stay is recorded in a vital statistics database. The National Ambulatory Care Reporting System captures data for all emergency department visits. Data were linked by using a unique provincial healthcare number.
Exposure Measures
Delirium was assessed twice daily (once per shift) by the bedside registered nurse as part of standard care and in accordance with published recommendations by using the ICDSC (4) in all patients with a Richmond Agitation–Sedation Scale (26) score of ⩾−3. The ICDSC is a well-validated, 8-item delirium assessment tool for use in the ICU (4).
The primary exposure was ever having had versus never having had delirium. Exploratory analyses were conducted by using four additional categorizations of delirium: 1) days with delirium (0, 1, and 2 d because of the skewed distribution), 2) delirium severity (ICDSC scores of 0, 1–3, 4–6, and 7–8), 3) subsyndromal delirium (ICDSC scores of 1–3), and 4) the percentage of time (in days) with delirium (0%, 1–24%, 25–49%, 50–74%, and 75–100%). Patients were considered as ever having had delirium if an ICDSC score ⩾4 was recorded at least once during their ICU stay. If an ICDSC score ⩾4 was recorded at any point during a 24-hour period, that calendar day was considered a day with delirium. The number of days with delirium from ICU admission to ICU discharge were summed for each patient. Delirium severity was measured by using the continuous measure of delirium symptoms from the ICDSC, ranging from 0–8, with the most severe delirium measurement during the ICU stay being used for analyses. Subsyndromal delirium was defined as the patient’s highest ICDSC score being greater than 0 but below the diagnostic threshold for delirium (i.e., all ICDSC scores are ⩽3 with at least one ICDSC score >0). The percentage of time with delirium was defined as the number of days with delirium divided by the number of days in the ICU.
Outcome Measures
The main outcome measure was mortality, and the secondary outcome was a composite measure of subsequent emergency department visits, readmission to the hospital, or mortality.
Mortality was defined as any death after hospital discharge. The time to death was considered as the number of days that a person survived after hospital discharge. Readmission to the hospital was defined as any subsequent admission to any Alberta hospital after hospital discharge.
Covariate Measures
Patient and hospital factors that might affect the relationship between delirium and the selected outcomes were determined a priori on the basis of clinical experience and previous studies (12, 15, 17, 18, 27–29). Patient factors included age, sex, comorbidities (categorized as 0, 1, or ⩾ 2; derived by using the Deyo classification of Charlson comorbidities), illness severity on ICU admission (Sequential Organ Failure Assessment score and Acute Physiology and Chronic Health Evaluation II [APACHE II] score in the first 24 h of the ICU stay), use of invasive mechanical ventilation, use of vasoactive medications, use of continuous renal replacement therapy, ICU LOS, hospital LOS, and hospital discharge disposition (categorized as transferred to an acute care inpatient institution, transferred to continuing care, discharged to home or a home setting with support services or transferred to other, and discharged home with no support service from an external agency being required). Hospital factors included ICU occupancy (number of beds occupied at discharge divided by the total number of beds in the specific ICU), the number of hospital and ICU beds, and teaching hospital status.
Statistical Analysis
A propensity score–matched cohort of patients with and without a history of delirium was created. Propensity scores were based on age, sex, admission type (no surgery, elective surgery, or emergent surgery), the presence or absence of each of the individual Charlson comorbidities (diabetes, chronic lung disease, chronic kidney disease, liver disease, cancer, chronic heart or peripheral vascular disease, or neurological disease), admission APACHE II score, use of invasive mechanical ventilation (yes/no), use of vasoactive medications (yes/no), use of continuous renal replacement therapy (yes/no), and ICU LOS (<3, 3 to <7, or ⩾7 d). The cohort was based on 1:1 nearest-neighbor matching without replacement by using the logit of the propensity score and a specified caliper width equal to 0.05 of the SD of the logit of the propensity score. The analyses were repeated for the overall sample (i.e., non–propensity score–matched cohort). The time to mortality from hospital discharge among the overall cohort was compared among patients with and without a history of delirium by using the Kaplan-Meier method to determine the cumulative incidence. Because mortality is a competing risk for emergency department visits and hospital readmission, we considered a composite measure of the time to the first hospital readmission, the first emergency department visit, or mortality. Follow-up was 2.5 years or until death, with censoring for loss to follow-up (n = 1). Because of the proportional hazard assumption not being satisfied for the overall cohort, mortality analyses were stratified by specified time intervals (0 to 30 d, >30 to 90 d, and >90 d), which were selected on the basis of a review of spline plots (see Figures E1 and E2 in the online supplement), with nonproportionality being shown early after hospital discharge (see the online supplement for further details). Cox proportional hazard regression models accounting for clustering of patients within ICUs (by using robust sandwich variance estimators) were used to examine the association between delirium and the time to the first hospital readmission, the first emergency department visit, or mortality. All analyses were adjusted for the same variables included in the propensity score, with the addition of hospital LOS (<7, 7 to <14, ⩾14 d) and the hospital discharge disposition. A two-sided P value of <0.05 was considered to indicate statistical significance. Analyses used R (version 3.5.1, R Foundation for Statistical Computing) and the R packages “survival” (version 2.44–1.1), “cmprsk” (version 2.2–7), and “survRM2” (version 1.0–3). Propensity score matching was performed using the R package “MatchIt” (version 3.0.2) (30). Additional details on the methods and data analysis are provided in the online supplement.
Ethics Approval
This study was approved by the Conjoint Health Research Ethics Board at the University of Calgary (REB17-0389). The ethics approval allowed linkage of all databases and a waiver of individual patient consent.
Results
Study Population
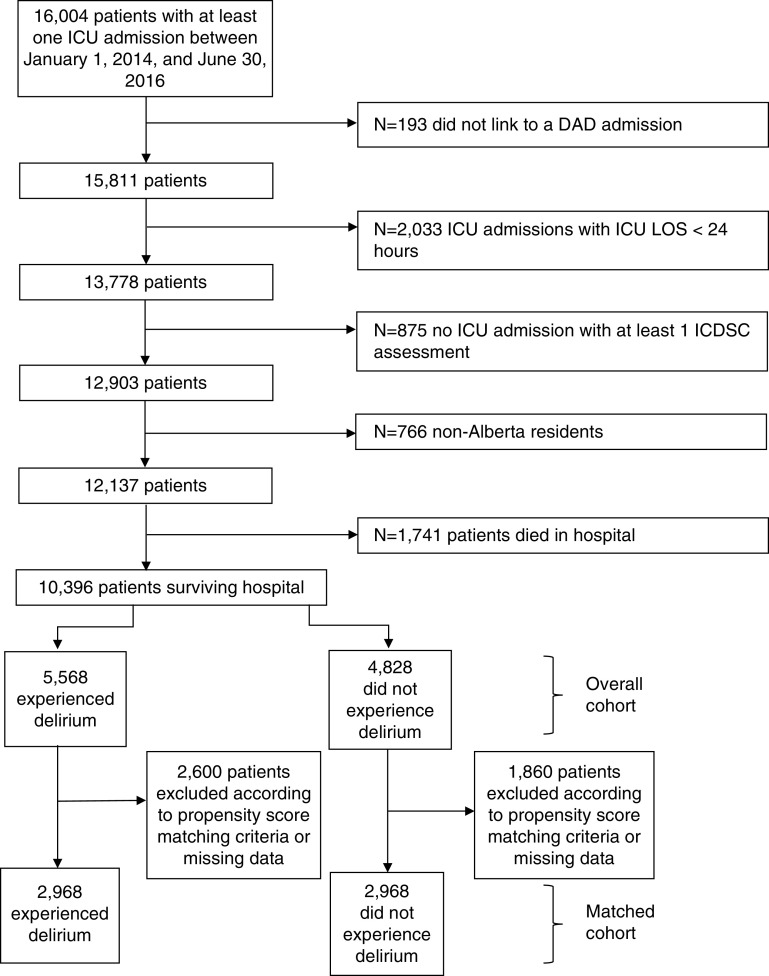
There were 12,137 eligible patients admitted to 1 of 14 mixed medical–surgical ICUs (Figure 1). After excluding those who died in the ICU or hospital (n = 2,079 with a history of delirium and n = 644 without a history of delirium), the overall study cohort for analysis consisted of 10,396 patients who survived to hospital discharge. Approximately half of patients (n = 5,568 [53.6%; 95% confidence interval (CI), 52.6–54.5]) had experienced delirium during their ICU stay. Because of a missing admission type for 145 patients (1.4%) from the overall cohort, propensity scores were calculated for 5,536 patients (99.4%) who had experienced delirium and 4,715 patients (97.7%) who had not experienced delirium. Of those with complete data, 2,600 patients who had experienced delirium and 1,860 patients who had not experienced delirium could not be matched with outcome data within the specified caliper width of 0.05 (Figure 1; see Table E1 in the online supplement). This resulted in a matched cohort of 5,936 patients (Table 1).
Figure 1.
Study cohort diagram. DAD = Discharge Abstract Database; ICDSC = Intensive Care Delirium Screening Checklist; LOS = length of stay.
Table 1.
Baseline Characteristics among Patients Surviving to Hospital Discharge
| Characteristic | All Patients Surviving to Hospital Discharge (n = 10,396) |
Propensity Score–matched Cohort (n = 5,936) |
||
|---|---|---|---|---|
| Delirium (n = 5,568) | No Delirium (n = 4,828) | Delirium (n = 2,968) | No Delirium (n = 2,968) | |
| Patient characteristics on ICU admission | ||||
| Age,* yr, median (IQR) | 57 (45–68) | 59 (45–69) | 58 (44–69) | 59 (44–69) |
| Sex, M,* n (%) | 3,330 (59.8) | 2,678 (55.5) | 1,710 (57.6) | 1,706 (57.5) |
| Comorbidities, n (%) | ||||
| Diabetes* | 1,455 (26.1) | 1,269 (26.3) | 793 (26.7) | 780 (26.3) |
| Chronic lung disease* | 1,017 (18.3) | 887 (18.4) | 541 (18.2) | 557 (18.8) |
| Chronic kidney disease* | 301 (5.4) | 253 (5.2) | 166 (5.6) | 158 (5.3) |
| Liver disease* | 526 (9.4) | 275 (5.7) | 202 (6.8) | 218 (7.3) |
| Cancer* | 519 (9.3) | 715 (14.8) | 348 (11.7) | 355 (12.0) |
| Chronic heart or peripheral vascular disease* | 1,011 (18.2) | 876 (18.1) | 513 (17.3) | 538 (18.1) |
| Neurological disease | 543 (9.8) | 215 (4.5) | 199 (6.7) | 184 (6.2) |
| Charlson category, n (%) | ||||
| 0 | 1,979 (35.5) | 1,723 (35.7) | 1,135 (38.2) | 1,044 (35.2) |
| 1 | 1,212 (21.8) | 1,041 (21.6) | 616 (20.8) | 669 (22.5) |
| ⩾2 | 2,377 (42.7) | 2,064 (42.8) | 1,217 (41.0) | 1,255 (42.3) |
| Admission type,* † n (%) | ||||
| Elective surgery | 277 (5.0) | 543 (11.5) | 226 (7.6) | 241 (8.1) |
| Emergent surgery | 917 (16.6) | 970 (20.6) | 597 (20.1) | 591 (19.9) |
| No surgery | 4,342 (78.4) | 3,202 (67.9) | 2,145 (72.3) | 2,136 (72.0) |
| Admission reason,‡ n (%) | ||||
| Medical | 3,535 (63.9) | 2,844 (60.4) | 1,783 (60.2) | 1,869 (63.0) |
| Neurological | 413 (7.5) | 162 (3.4) | 198 (6.7) | 129 (4.3) |
| Surgical | 1,049 (19.0) | 1,490 (31.6) | 730 (24.6) | 801 (27.0) |
| Trauma | 535 (9.7) | 213 (4.5) | 253 (8.5) | 167 (5.6) |
| Location before ICU admission, n (%) | ||||
| Emergency | 2,959 (53.1) | 2,126 (44.0) | 1,488 (50.1) | 1,355 (45.7) |
| Interfacility | 155 (2.8) | 135 (2.8) | 77 (2.6) | 86 (2.9) |
| Operating or recovery room | 1,006 (18.1) | 1,339 (27.7) | 698 (23.5) | 736 (24.8) |
| Other | 218 (3.9) | 193 (4.0) | 106 (3.6) | 114 (3.8) |
| Hospital ward | 1,230 (22.1) | 1,035 (21.4) | 599 (20.2) | 677 (22.8) |
| SOFA score, median (IQR) | 7 (5–10) | 5 (3–7) | 6 (4–8) | 5 (3–8) |
| APACHE II score,* median (IQR) | 20 (16–26) | 16 (11–21) | 18 (14–23) | 18 (13–23) |
| Interventions received in ICU | ||||
| Invasively ventilated,* n (%) | 4,389 (78.8) | 2,379 (49.3) | 1,963 (66.1) | 1,934 (65.2) |
| Noninvasive mechanical ventilation, n (%) | 764 (13.7) | 657 (13.6) | 336 (11.3) | 439 (14.8) |
| Vasoactive medications,* n (%) | 2,839 (51.0) | 1,579 (32.7) | 1,196 (40.3) | 1,151 (38.8) |
| Continuous renal replacement therapy,* n (%) | 389 (7.0) | 79 (1.6) | 95 (3.2) | 75 (2.5) |
| Patient characteristics on ICU discharge | ||||
| Discharge SOFA score, median (IQR) | 1 (0–3) | 1 (0–3) | 1 (0–3) | 1 (0–3) |
| ICU length of stay, d,* median (IQR) | 5.9 (3.3–11.0) | 2.8 (1.9–4.7) | 3.9 (2.4–6.1) | 3.7 (2.2–5.8) |
| Patient characteristics on hospital discharge | ||||
| Hospital length of stay, d, median (IQR) | 18.2 (9.3–38.5) | 9.7 (5.2–19.1) | 13.9 (7.0–28.8) | 11.4 (6.0–21.9) |
| Hospital discharge disposition,§ n (%) | ||||
| Transferred to acute care inpatient institution | 1,241 (22.3) | 730 (15.1) | 589 (19.9) | 516 (17.4) |
| Transferred to continuing care | 461 (8.3) | 158 (3.3) | 223 (7.5) | 111 (3.7) |
| Transferred to otherǁ | 48 (0.9) | 25 (0.5) | 22 (0.7) | 16 (0.5) |
| Discharged to home or a home setting with support services | 836 (15.0) | 712 (14.8) | 441 (14.9) | 455 (15.4) |
| Discharged home with no support service from an external agency required | 2,971 (53.5) | 3,198 (66.3) | 1,689 (57.0) | 1,866 (63.3) |
| Hospital characteristics | ||||
| Teaching hospital, n (%) | 4,805 (86.3) | 3,768 (78.0) | 2,531 (85.3) | 2,397 (80.8) |
| ⩾600 hospital beds, n (%) | 3,670 (65.9) | 2,732 (56.6) | 1,909 (64.3) | 1,754 (59.1) |
| ⩾20 ICU beds, n (%) | 3,318 (59.6) | 2,408 (49.9) | 1,731 (58.3) | 1,544 (52.0) |
| ICU occupancy ⩾80%, n (%) | 4,388 (78.8) | 3,489 (72.3) | 2,301 (77.5) | 2,193 (73.9) |
Definition of abbreviations: APACHE II = Acute Physiology and Chronic Health Evaluation II; IQR = interquartile range; SOFA = Sequential Organ Failure Assessment.
Variable included in propensity score.
145 patients missing data.
155 patients missing data.
Data unknown for 16 patients.
Includes emergency and ambulatory care (day surgery and clinics) in another facility or within the same reporting facility, palliative care facility or hospice, addiction treatment center, jails, and infants/children discharged to a social agency.
Baseline characteristics were balanced in the propensity score–matched cohort (Table 1). Most patients in the matched cohort were admitted to the ICU from the emergency department and had a medical diagnosis, with median APACHE II score on admission of 18 (interquartile range [IQR], 13–23). The most common comorbidities among patients were diabetes, chronic lung disease, and cancer. After propensity score matching, the median ICU LOS in patients with a history of delirium (3.9 d [IQR, 2.4–6.1 d]) was similar to that of those without a history of delirium (3.7 d [IQR, 2.2–5.8 d]). The median hospital LOS in the propensity score–matched cohort was longer in those with a history of delirium (13.9 d [IQR, 7.0–28.8 d]) than in those without a history of delirium (11.4 d [IQR, 6.0–21.9 d]). Most patients were discharged home from hospital with no support services from an external agency (Table 1). Among those with a history of delirium, the median number of days with delirium documented was 2 days (IQR, 1–3 d), and the median most severe ICDSC score throughout the ICU stay was 6 (IQR, 5–7).
Mortality Outcomes
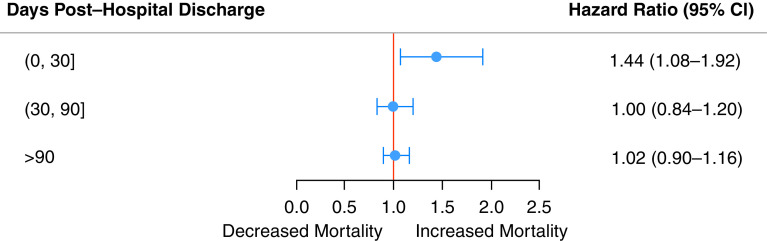
Mortality after hospital discharge was increased in those who had experienced delirium compared with those who had not (Figure 2A). At 30 days after hospital discharge, 3.9% of patients who had ever experienced delirium had died, whereas 2.6% of those who had never had delirium had died (Table E2). By 2.5 years, 23.0% of patients who had ever experienced delirium during their ICU stay had died, whereas 20.9% of those who had never had delirium had died. An increased risk of mortality was seen in the interval of 0–30 days after hospital discharge, as demonstrated by a hazard ratio (HR) of 1.44 (95% CI, 1.08–1.92) (Figure 3), and there were no significant differences in the other time intervals. A delirium duration of 2 or more days in those who had experienced delirium compared with those who had not experienced delirium (Figure 2B, Table E4) was associated with increased mortality 0–30 days after hospital discharge. There was no relationship between subsyndromal delirium (compared with those with only ICDSC scores of 0) and mortality after hospital discharge (Figure 2C, Table E3). At 0–30 days after hospital discharge, there was increased mortality in those who had experienced delirium for 25–49% and 50–74% of their ICU stay compared with those who had never experienced delirium (Figure 2D, Table E6).
Figure 2.
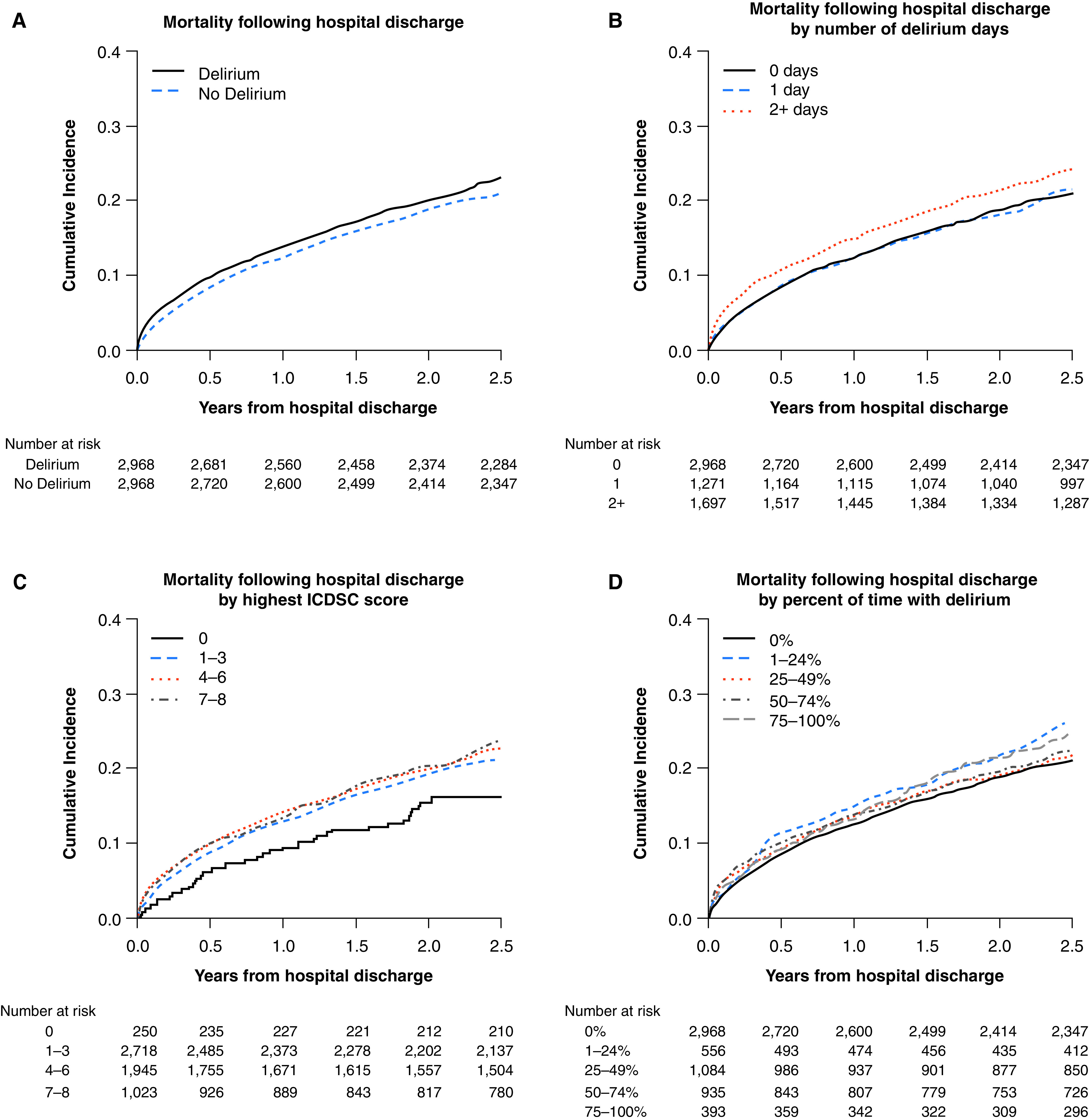
Delirium and cumulative incidence of mortality (A) after hospital discharge among all patients, (B) stratified by number of delirium days, (C) stratified by highest ICDSC score, and (D) stratified by percentage of time with delirium for the propensity score–matched cohort. ICDSC = Intensive Care Delirium Screening Checklist.
Figure 3.
Forest plot of hazard ratios for mortality after hospital discharge by time for the propensity score–matched cohort. CI = confidence interval.
Healthcare Use
There was an increased association with emergency department visits, hospital readmission, or mortality after hospital discharge among those who had ever experienced delirium (n = 2,556; 86.1%) compared with those who had not (n = 2,429; 81.5%) (HR, 1.12 [95% CI, 1.07–1.17]) during the study period. Among those who had experienced delirium in the ICU, 79.6% (n = 2,362) visited the emergency room at least once during the study period after hospital discharge, compared with 76.8% (n = 2,280) of those who had not experienced delirium. During the study period, 60.3% of patients with a history of delirium in the ICU (n = 1,789) were readmitted to hospital after hospital discharge, compared with 58.3% (n = 1,729) of those without a history of delirium.
Multivariable analyses of the overall cohort (Tables E3–E6 and Figure E3) were similar to those of the propensity score–matched cohort.
Discussion
In this multicenter, propensity score–matched, population-based cohort study, we found that ever having had delirium in the ICU was associated with an increased risk of mortality in the first 30 days after hospital discharge. Delirium was also associated with increased emergency department visits, hospital readmissions, or mortality up to 2.5 years after hospital discharge. Our findings suggest that when examining the outcomes of ICU delirium for research or quality-improvement purposes, follow-up for mortality to 30 days after hospital discharge is likely sufficient. However, longer periods of follow-up are needed when also examining emergency department visits and hospital readmissions.
Our multicenter, population-based study that followed almost 6,000 ICU patients for 2.5 years is the largest and longest follow-up of the health and health resource use of patients after ICU delirium. Before this study, the literature on long-term mortality outcomes after ICU delirium comprised a total of 1,785 patients followed for durations between 6 and 12 months after ICU discharge (12, 15, 17, 18).This literature was reviewed by Salluh and colleagues (9), who identified four studies that reported conflicting results. Two single-center studies reported an increased risk of mortality by 6 months in those who had experienced ICU delirium, and another reported an increased association between days of delirium and 1-year mortality (12, 17). Ely and colleagues (12) reported an increased risk of mortality (HR, 3.2 [95% CI, 1.4–7.7]) at 6 months in 275 mechanically ventilated ICU patients who had experienced delirium; the proportion of mechanically ventilated patients in our study was 65%, compared with 100% in the study by Ely and colleagues (12), which may explain the higher risk of mortality at 6 months. In 105 nonintubated ICU patients, Van Rompaey and colleagues (15) reported increased odds of mortality at 6 months in those who had experienced ICU delirium (odds ratio, 3.80 [95% CI, 1.11–13.05]; adjusted for age and sex); this is in contrast to the current study, although the study by Van Rompaey and colleagues(15) included all patients rather than just including those surviving to hospital discharge, which may explain the strength of the odds ratio reported. Pisani and colleagues (18) found a relationship between days with delirium and mortality (HR, 1.10 [95% CI, 1.02–1.18]) in an older (>60 yr) ICU population. A more recent single-center study of 1,101 ICU patients reported no relationship between delirium and mortality at 1 year. The previous studies on outcomes of ICU delirium varied in their ability to control for potential confounders, including the admission type and illness severity, which may further explain the discrepancy in findings (9). The sample sizes (between 105 and 1,101 patients; 1,785 in total) of the previous investigations resulted in wide CIs, which may also account for the observed differences.
Subsyndromal delirium (i.e., exhibiting symptoms of delirium that do not reach the diagnostic threshold) has received less attention in the literature (31). A systematic review by our team found an inconclusive association between subsyndromal delirium and mortality (31); three single-center studies (with sample sizes ranging from 162 to 537 patients) reported no association between subsyndromal delirium using the ICDSC and mortality in univariate (32, 33) and multivariable (34) analyses. In the current study, there was also no dose–response relationship observed between delirium severity and mortality. That is, the relationship between mortality and delirium was no different between patients who had shown subthreshold scores of delirium (i.e., ICDSC scores of 1–3) and patients who had shown threshold scores of delirium (i.e., ICDSC scores ⩾4). However, at up to 30 days after hospital discharge, both the number of days with delirium (i.e., 2 d or more) and the percentage of time with delirium (i.e., between 25% and 75% of the ICU stay) were associated with increased mortality. It appears that the amount of time spent with delirium (measured in both days and the percentage of time) may portend a worse outcome than delirium severity in ICU patients who survive to hospital discharge.
Our investigation into the association between ICU delirium and subsequent health resource use in ICU patients after hospital discharge is novel. We found an association between ICU delirium and subsequent emergency department visits, hospital readmissions, or mortality up to 30 days after hospital discharge. In line with previous studies, we also found an increased ICU and hospital LOS among patients who had experienced delirium. The 30-day cumulative cost associated with ICU delirium attributable to increased resource use is reported to be over $17,000 in U.S. dollars per patient (35). This suggests that the long-term burden of delirium is costly not only for the patient but also for the healthcare system.
Our study had notable strengths, including the number of patients included and the duration of follow-up. Importantly, delirium was measured by using the same validated instrument with the same timing and frequency for all participants. We were able to control for many potential confounders at the individual patient level and conducted a propensity score–matched analysis. Even with propensity score matching, it is possible that residual confounding exists—an inherent risk of observational studies. For example, given the nature of the data used, we were unable to control for the presence of Alzheimer disease and related dementias, which may be associated with both delirium and our outcomes of interest (36). It is difficult to disentangle the effect of ICU LOS on the association between delirium and long-term outcomes; control of variables associated with both the exposure and outcomes (e.g., severity of illness, receipt of mechanical ventilation) are essential to understand this relationship. The use of propensity scores introduces the risk of selection bias because no outcome data are provided for those individuals who had experienced delirium who were not matched and individuals with more comorbidities and a higher severity of illness. This suggests that the estimates of mortality and healthcare use associated with delirium are likely conservative. Our study did not assess patient- and family member–reported post–ICU discharge outcomes, including cognitive impairment, depression, and post-traumatic stress disorder (13, 37, 38). These established effects of critical illness may also confer an increased risk of mortality and health resource use in patients who have had delirium. The linked administrative data used did not provide information on primary care visits or private supports (e.g., psychologist, family caregiver) used after hospital discharge. There were missing ICDSC assessment data; however, the percentage of days with no ICDSC score was similar between groups with and without a history of delirium. We were unable to determine the cause of death for people dying within the first 30 days of hospital discharge, but the proximity to the ICU admission suggests that mortality may be modifiable by enacting targeted processes at discharge. This population-based, multicenter study conducted in a single-payer health system allowed for comprehensive follow-up of all patients. Jurisdictions with different patient populations and health systems may have different experiences.
Delirium in the ICU is associated with an increased risk of mortality in critically ill patients up to 30 days after hospital discharge. ICU delirium is also associated with increased emergency department visits, hospital readmissions, or mortality after hospital discharge. Delirium is a common, life-threatening complication of critical illness that places patients at risk of negative clinical outcomes and increased health resource use after an ICU admission.
Footnotes
Author Contributions: Substantial contributions to the conception or design of the work (K.M.F., A.S., C.H.L., D.J.N., E.W.E., C.J.D., and H.T.S.) or the acquisition (A.S. and C.H.L.), analysis (A.S. and C.H.L.), or interpretation of data for the work (K.M.F., A.S., C.H.L., D.J.N., E.W.E., C.J.D., and H.T.S.); drafting the work (K.M.F., A.S., and H.T.S.) or revising it critically for important intellectual content (K.M.F., A.S., C.H.L., D.J.N., E.W.E., C.J.D., and H.T.S.); final approval of the version to be published (K.M.F., A.S., C.H.L., D.J.N., E.W.E., C.J.D., and H.T.S.); and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved (K.M.F., A.S., C.H.L., D.J.N., E.W.E., C.J.D., and H.T.S.)
This article has an online supplement, which is accessible from this issue's table of content online at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202002-0320OC on April 6, 2021
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- 2. Ely EW, Inouye SK, Bernard GR, Gordon S, Francis J, May L, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU) JAMA . 2001;286:2703–2710. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 3. Dubois MJ, Bergeron N, Dumont M, Dial S, Skrobik Y. Delirium in an intensive care unit: a study of risk factors. Intensive Care Med . 2001;27:1297–1304. doi: 10.1007/s001340101017. [DOI] [PubMed] [Google Scholar]
- 4. Bergeron N, Dubois MJ, Dumont M, Dial S, Skrobik Y. Intensive Care Delirium Screening Checklist: evaluation of a new screening tool. Intensive Care Med . 2001;27:859–864. doi: 10.1007/s001340100909. [DOI] [PubMed] [Google Scholar]
- 5. Girard TD, Kress JP, Fuchs BD, Thomason JW, Schweickert WD, Pun BT, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet . 2008;371:126–134. doi: 10.1016/S0140-6736(08)60105-1. [DOI] [PubMed] [Google Scholar]
- 6. Pandharipande PP, Pun BT, Herr DL, Maze M, Girard TD, Miller RR, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA . 2007;298:2644–2653. doi: 10.1001/jama.298.22.2644. [DOI] [PubMed] [Google Scholar]
- 7. Pandharipande P, Cotton BA, Shintani A, Thompson J, Pun BT, Morris JA, Jr, et al. Prevalence and risk factors for development of delirium in surgical and trauma intensive care unit patients. J Trauma . 2008;65:34–41. doi: 10.1097/TA.0b013e31814b2c4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guenther U, Popp J, Koecher L, Muders T, Wrigge H, Ely EW, et al. Validity and reliability of the CAM-ICU Flowsheet to diagnose delirium in surgical ICU patients. J Crit Care . 2010;25:144–151. doi: 10.1016/j.jcrc.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 9. Salluh JI, Wang H, Schneider EB, Nagaraja N, Yenokyan G, Damluji A, et al. Outcome of delirium in critically ill patients: systematic review and meta-analysis. BMJ . 2015;350:h2538. doi: 10.1136/bmj.h2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zaal IJ, Devlin JW, Peelen LM, Slooter AJ. A systematic review of risk factors for delirium in the ICU. Crit Care Med . 2015;43:40–47. doi: 10.1097/CCM.0000000000000625. [DOI] [PubMed] [Google Scholar]
- 11. Thomason JWW, Shintani A, Peterson JF, Pun BT, Jackson JC, Ely EW. Intensive care unit delirium is an independent predictor of longer hospital stay: a prospective analysis of 261 non-ventilated patients. Crit Care . 2005;9:R375–R381. doi: 10.1186/cc3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ely EW, Shintani A, Truman B, Speroff T, Gordon SM, Harrell FE, Jr, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA . 2004;291:1753–1762. doi: 10.1001/jama.291.14.1753. [DOI] [PubMed] [Google Scholar]
- 13. Pandharipande PP, Girard TD, Jackson JC, Morandi A, Thompson JL, Pun BT, et al. BRAIN-ICU Study Investigators. Long-term cognitive impairment after critical illness. N Engl J Med . 2013;369:1306–1316. doi: 10.1056/NEJMoa1301372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brummel NE, Jackson JC, Pandharipande PP, Thompson JL, Shintani AK, Dittus RS, et al. Delirium in the ICU and subsequent long-term disability among survivors of mechanical ventilation. Crit Care Med . 2014;42:369–377. doi: 10.1097/CCM.0b013e3182a645bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pisani MA, Kong SY, Kasl SV, Murphy TE, Araujo KL, Van Ness PH. Days of delirium are associated with 1-year mortality in an older intensive care unit population. Am J Respir Crit Care Med . 2009;180:1092–1097. doi: 10.1164/rccm.200904-0537OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lin SM, Liu CY, Wang CH, Lin HC, Huang CD, Huang PY, et al. The impact of delirium on the survival of mechanically ventilated patients. Crit Care Med . 2004;32:2254–2259. doi: 10.1097/01.ccm.0000145587.16421.bb. [DOI] [PubMed] [Google Scholar]
- 17. Van Rompaey B, Schuurmans MJ, Shortridge-Baggett LM, Truijen S, Elseviers M, Bossaert L. Long term outcome after delirium in the intensive care unit. J Clin Nurs . 2009;18:3349–3357. doi: 10.1111/j.1365-2702.2009.02933.x. [DOI] [PubMed] [Google Scholar]
- 18. Wolters AE, van Dijk D, Pasma W, Cremer OL, Looije MF, de Lange DW, et al. Long-term outcome of delirium during intensive care unit stay in survivors of critical illness: a prospective cohort study. Crit Care . 2014;18:R125. doi: 10.1186/cc13929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Needham DM, Davidson J, Cohen H, Hopkins RO, Weinert C, Wunsch H, et al. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders’ conference. Crit Care Med . 2012;40:502–509. doi: 10.1097/CCM.0b013e318232da75. [DOI] [PubMed] [Google Scholar]
- 20. McPeake J, Mikkelsen ME. The evolution of post intensive care syndrome. Crit Care Med . 2018;46:1551–1552. doi: 10.1097/CCM.0000000000003232. [DOI] [PubMed] [Google Scholar]
- 21. Hashem MD, Nallagangula A, Nalamalapu S, Nunna K, Nausran U, Robinson KA, et al. Patient outcomes after critical illness: a systematic review of qualitative studies following hospital discharge. Crit Care . 2016;20:345. doi: 10.1186/s13054-016-1516-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet . 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 23. Chiasson TC, Manns BJ, Stelfox HT. An economic evaluation of venous thromboembolism prophylaxis strategies in critically ill trauma patients at risk of bleeding. PLoS Med . 2009;6:e1000098. doi: 10.1371/journal.pmed.1000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brundin-Mather R, Soo A, Zuege DJ, Niven DJ, Fiest K, Doig CJ, et al. Secondary EMR data for quality improvement and research: a comparison of manual and electronic data collection from an integrated critical care electronic medical record system. J Crit Care . 2018;47:295–301. doi: 10.1016/j.jcrc.2018.07.021. [DOI] [PubMed] [Google Scholar]
- 25. Stelfox H, Soo A, Niven D, Fiest K, Wunsch H, Rowan K, et al. The safety of discharging select patients directly home from the intensive care unit: a multicenter population-based cohort study. JAMA Intern Med . 2018;178:1390–1399. doi: 10.1001/jamainternmed.2018.3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sessler CN, Gosnell MS, Grap MJ, Brophy GM, O’Neal PV, Keane KA, et al. The Richmond Agitation–Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med . 2002;166:1338–1344. doi: 10.1164/rccm.2107138. [DOI] [PubMed] [Google Scholar]
- 27. Patel SB, Poston JT, Pohlman A, Hall JB, Kress JP. Rapidly reversible, sedation-related delirium versus persistent delirium in the intensive care unit. Am J Respir Crit Care Med . 2014;189:658–665. doi: 10.1164/rccm.201310-1815OC. [DOI] [PubMed] [Google Scholar]
- 28. Quach S, Hennessy DA, Faris P, Fong A, Quan H, Doig C. A comparison between the APACHE II and Charlson Index Score for predicting hospital mortality in critically ill patients. BMC Health Serv Res . 2009;9:129. doi: 10.1186/1472-6963-9-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Soo A, Zuege DJ, Fick GH, Niven DJ, Berthiaume LR, Stelfox HT, et al. Describing organ dysfunction in the intensive care unit: a cohort study of 20,000 patients. Crit Care . 2019;23:186. doi: 10.1186/s13054-019-2459-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ho D, Imai K, King G, Stuart EA. MatchIt: nonparametric preprocessing for parametric causal inference. J Stat Softw . 2011;42:1–28. [Google Scholar]
- 31. Rosgen BK, Krewulak KD, Stelfox HT, Ely EW, Davidson JE, Fiest KM. The association of delirium severity with patient and health system outcomes in hospitalised patients: a systematic review. Age Ageing . 2020;49:549–557. doi: 10.1093/ageing/afaa053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tomasi CD, Grandi C, Salluh J, Soares M, Giombelli VR, Cascaes S, et al. Comparison of CAM-ICU and ICDSC for the detection of delirium in critically ill patients focusing on relevant clinical outcomes. J Crit Care . 2012;27:212–217. doi: 10.1016/j.jcrc.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 33. Breu A, Stransky M, Metterlein T, Werner T, Trabold B. Subsyndromal delirium after cardiac surgery. Scand Cardiovasc J . 2015;49:207–212. doi: 10.3109/14017431.2015.1041423. [DOI] [PubMed] [Google Scholar]
- 34. Ouimet S, Riker R, Bergeron N, Cossette M, Kavanagh B, Skrobik Y. Subsyndromal delirium in the ICU: evidence for a disease spectrum. Intensive Care Med . 2007;33:1007–1013. doi: 10.1007/s00134-007-0618-y. [DOI] [PubMed] [Google Scholar]
- 35. Vasilevskis EE, Chandrasekhar R, Holtze CH, Graves J, Speroff T, Girard TD, et al. The cost of ICU delirium and coma in the intensive care unit patient. Med Care . 2018;56:890–897. doi: 10.1097/MLR.0000000000000975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hapca S, Guthrie B, Cvoro V, Bu F, Rutherford AC, Reynish E, et al. Mortality in people with dementia, delirium, and unspecified cognitive impairment in the general hospital: prospective cohort study of 6,724 patients with 2 years follow-up. Clin Epidemiol . 2018;10:1743–1753. doi: 10.2147/CLEP.S174807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Herridge MS, Moss M, Hough CL, Hopkins RO, Rice TW, Bienvenu OJ, et al. Recovery and outcomes after the acute respiratory distress syndrome (ARDS) in patients and their family caregivers. Intensive Care Med . 2016;42:725–738. doi: 10.1007/s00134-016-4321-8. [DOI] [PubMed] [Google Scholar]
- 38. Cameron JI, Chu LM, Matte A, Tomlinson G, Chan L, Thomas C, et al. RECOVER Program Investigators (Phase 1: towards RECOVER); Canadian Critical Care Trials Group. One-year outcomes in caregivers of critically ill patients. N Engl J Med . 2016;374:1831–1841. doi: 10.1056/NEJMoa1511160. [DOI] [PubMed] [Google Scholar]