Abstract
The binding of microorganisms to each other and oral surfaces contributes to the progression of microbial infections in the oral cavity. Candida dubliniensis, a newly characterized species, has been identified in human immunodeficiency virus-seropositive patients and other immunocompromised individuals. C. dubliniensis phenotypically resembles Candida albicans in many respects yet can be identified and differentiated as a unique Candida species by phenotypic and genetic profiles. The purpose of this study was to determine oral coaggregation (CoAg) partners of C. dubliniensis and to compare these findings with CoAg of C. albicans under the same environmental conditions. Fifteen isolates of C. dubliniensis and 40 isolates of C. albicans were tested for their ability to coaggregate with strains of Fusobacterium nucleatum, Peptostreptococcus micros, Peptostreptococcus magnus, Peptostreptococcus anaerobius, Porphyromonas gingivalis, and Prevotella intermedia. When C. dubliniensis and C. albicans strains were grown at 37°C on Sabouraud dextrose agar, only C. dubliniensis strains coaggregated with F. nucleatum ATCC 49256 and no C. albicans strains showed CoAg. However, when the C. dubliniensis and C. albicans strains were grown at 25 or 45°C, both C. dubliniensis and C. albicans strains demonstrated CoAg with F. nucleatum. Heating the C. albicans strains (grown at 37°C) at 85°C for 30 min or treating them with dithiothreitol allowed the C. albicans strains grown at 37°C to coaggregate with F. nucleatum. CoAg at all growth temperatures was inhibited by mannose and α-methyl mannoside but not by EDTA or arginine. The CoAg reaction between F. nucleatum and the Candida species involved a heat-labile component on F. nucleatum and a mannan-containing heat-stable receptor on the Candida species. The CoAg reactions between F. nucleatum and the Candida species may be important in the colonization of the yeast in the oral cavity, and the CoAg of C. dubliniensis by F. nucleatum when grown at 37°C provides a rapid, specific, and inexpensive means to differentiate C. dubliniensis from C. albicans isolates in the clinical laboratory.
Candida dubliniensis has recently been added to the growing list of potential opportunistic pathogens colonizing the immunocompromised population and is associated with human immunodeficiency virus (HIV)-seropositive individuals (3, 6, 23, 38, 41, 49–53). To date, however, little is known concerning its epidemiology, pathogenesis, and colonization mechanisms. Despite the growing concerns regarding the clinical significance of this novel Candida species, efforts to detect it in clinical laboratories have been hampered due to the phenotypic characteristics it shares with Candida albicans. Although there are some clear differences between C. dubliniensis and C. albicans, there is currently no convenient, reliable test that can be used to differentiate between these two species. Rather, a battery of tests are used, and genetic testing is often used for final confirmation (2, 3, 6, 23, 28, 43–47, 50–52, 54).
Intergeneric coaggregations (CoAgs) have been observed between C. albicans and Fusobacterium species (14) as well as several other oral microorganisms (1, 15, 21, 22, 24). These interactions may be an important factor in the microbial colonization and progression of infections in the oral cavity (10, 29). Fusobacterium nucleatum, an anaerobic gram-negative nonsporeforming bacillus, is reported to be the most frequently isolated microbe from the subgingival plaque of periodontal lesions (39, 40), and it has been reported that F. nucleatum plays an important role in microbial colonization in the oral cavity via its extensive ability to attach to human cells (7) and to coaggregate with many other oral microorganisms (5, 9, 32).
Experiments were performed to determine CoAg partners of C. dubliniensis and to compare the conditions and characteristics of CoAg with those of C. albicans.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
F. nucleatum (ATCC 49256), Peptostreptococcus micros (ATCC 33270), Peptostreptococcus magnus (ATCC 15794), Peptostreptococcus anaerobius (ATCC 27337), Porphyromonas gingivalis (ATCC 33277), and Prevotella intermedia (ATCC 25611) strains were used in this study. All organisms were grown on Brucella blood agar (BBAHK) or in brain heart infusion broth (BBL Microbiology Systems, Cockeysville, Md.), both media supplemented with hemin (5 μg/ml) and menadione (1 μg/ml) in a Coy anaerobic chamber (Coy Laboratory Products, Ann Arbor, Mich.) at 37°C for 2 to 4 days. Gram stain and phase-contrast microscopy were used to check for purity. Organisms were harvested by scraping the plates or centrifuging the broth cultures for 10 min at 3,000 × g and washed twice in CoAg buffer (20 mM Tris-HCl [pH 7.8], 0.1 mM CaCl2, 0.1 mM MgCl2, 0.15 M NaCl, 0.02% NaN3) (14). Suspensions of bacterial cells were adjusted to approximately 120 Klett units (approximately 109 bacterial cells/ml) by using a Klett Summerson photoelectric colorimeter (Klett Manufacturing Co., New York, N.Y.), or the packed cells after centrifugation at 3,000 × g for 10 min were resuspended into a 1% (vol/vol) cell suspension with CoAg buffer. All bacterial cells were used immediately or stored at 4°C until used.
Yeast strains, culture conditions, and identification.
Forty isolates collected between January and March of 1998 were identified as C. albicans by the clinical laboratories at the University of Maryland. They were reevaluated by growth on Sabouraud dextrose agar (SDA; Difco Laboratories, Detroit, Mich.) at 45°C, chlamydospore production on TOC agar (Remel, Lenexa, Kans.) and germ tube formation in serum.
Fifteen strains of C. dubliniensis including reference strains (CD36 NCPF 3949) and an Irish clinical isolate (JP1) (both kindly supplied by Derek Sullivan), 9 isolates recovered from HIV-seropositive patients managed at the University of Maryland Dental School, and 4 isolates recovered from patients at the University of Maryland Hospital were also tested for CoAg. C. dubliniensis strains were identified by using published criteria including germ tube production in serum after 3 h of incubation at 37°C, amount and arrangement of chlamydospores produced on TOC agar, inability to grow at 45°C, colony color on CHROMagar Candida medium (CHROMagar, Paris, France), and sugar assimilation profiles, using the API 20C AUX yeast identification system (bioMerieux Vitek, Inc., Hazelwood, Mo.). For all of the C. dubliniensis isolates, further genetic testing was performed by an electrophoretic karyotyping method based on the method of King et al. (27).
Suspensions were prepared for C. albicans strains from colonies grown at 25, 37, or 45°C for 24 or 48 h on SDA plates and for C. dubliniensis from colonies grown at 25 or 37°C for 24 or 48 h. Plates grown at 37°C were also left at room temperature for up to 7 days, and suspensions of the yeast cells were tested for CoAg with F. nucleatum. Also, suspensions were made of strains after the induction of germ tubes or hyphae. Suspensions were made by removing colonies from the surface of the agar plate. After washing the colonies in cold CoAg buffer, 10% suspensions were prepared in CoAg buffer by the centrifugation procedure previously described for the bacteria. Cells were used immediately or stored at 4°C for use within 3 days.
Visual CoAg assay.
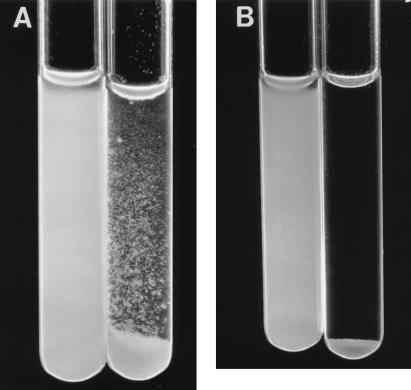
CoAg ability of microorganisms was screened by the visual CoAg assay (9). A 100-μl aliquot of yeast suspension (10%) was mixed with 200 μl of the bacterial suspension (1%) and 100 μl of CoAg buffer or, as controls, each CoAg partner with just CoAg buffer. The mixtures were vortexed for 10 s, shaken on a rotary platform shaker for 3 min, and left undisturbed at room temperature for 2 additional min. The degree of CoAg was recorded on a scale of 0 to 4+ as follows: a score of 0 for no visible aggregates in the cell suspension, 1+ for small uniform coaggregates in the suspension, 2+ for coaggregates that are easily seen but no immediate settling of coaggregates, 3+ for large coaggregates which settle rapidly and leave some turbidity in the supernatant fluid, and 4+ for large coaggregates which settle immediately and leave clear supernatant fluid (Fig. 1). All CoAg reactions were performed in duplicate.
FIG. 1.
Visual CoAg testing of C. albicans or C. dubliniensis with F. nucleatum. For each pair of tubes, the left tube contains C. albicans grown at 37°C plus F. nucleatum, and the right tube contains C. dubliniensis grown at 37°C plus F. nucleatum. The tubes are shown 45 s (A) or 5 min (B) after mixing (CoAg score of 4+).
Turbidimetric monitoring assay.
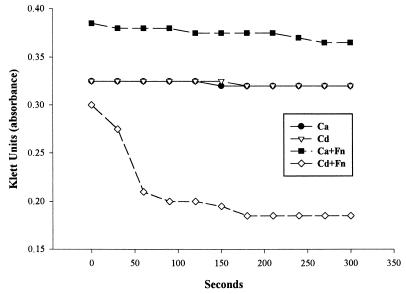
A turbidimetric monitoring assay was also performed to determine the degree of CoAg during a 5-min period. The method was similar to that of the visual CoAg assay but required 1 ml of the yeast suspension, 2 ml of bacterial suspension, and 1 ml of CoAg buffer followed by a 20-s vortexing step (9). The reading of Klett units was taken at 1-min time intervals.
Inhibition of CoAg by sugars, amino acids, and EDTA.
By using the visual CoAg assay described above, the following sugars or amino acids prepared in CoAg buffer were screened for their ability to inhibit CoAg: d-(+)-galactose, lactose, d-mannose, d-(+)-glucosamine, α-methyl mannoside, l-arginine, l-lysine, and l-alanine. Inhibition experiments used the visual CoAg assay with the addition of 100 μl of the sugar or amino acid tested as an inhibitor at a final concentration of 100 mM in the CoAg mixture. EDTA was tested in the same manner at a final concentration of 100 mM. Tests for autoaggregation included only suspensions of each microorganism with CoAg buffer.
Inhibition of CoAg by heat and dithiothreitol (DTT) treatment of bacterial cells.
Heating was performed by incubating the Candida or F. nucleatum cell suspensions at 85°C for 30 min or at 45°C for up to 3 days. The visual CoAg assay was performed on either heated and unheated cell suspensions of each microorganism with the heterologous CoAg partner.
DTT extraction was performed on the Candida strains as previously described (20), and the treated cells were then used in the CoAg assay. Briefly, C. albicans yeast cells grown at 37°C were prepared for DTT treatment by first washing the cells with cold deionized water and then adjusting the cell concentration to 2 × 108 cells per ml in deionized water. Two milliliters of yeast cell suspension was mixed with 2 ml of a 50 mM DTT solution in 0.1 M Tris-HCl–0.5 mM EDTA buffer, pH 8.6. The mixture was incubated for 60 min at 37°C with occasional shaking. Following incubation, cells were pelleted and washed three times with CoAg buffer (4°C) to remove all DTT. Cells were then resuspended in CoAg buffer (4°C) to a 10% concentration and used in the CoAg assay with F. nucleatum. All strains were also treated as described above except CoAg buffer was added instead of DTT.
RESULTS
CoAg of selected oral bacteria with C. dubliniensis and C. albicans.
Suspensions in CoAg buffer of 15 strains of C. dubliniensis and 40 strains of C. albicans, grown at 37°C on SDA plates, were tested with six oral anaerobic microorganisms for coaggregating ability. CoAg was observed by the visual (Fig. 1) and turbidimetric assays (Fig. 2) only with the 15 C. dubliniensis strains and F. nucleatum. No CoAg occurred with the 15 C. dubliniensis strains and the other oral microorganisms or with F. nucleatum and 40 C. albicans strains grown at 37°C (Table 1). However, germ tubes and hypha-displaying cells of C. albicans grown at 37°C showed a 4+ CoAg reaction with F. nucleatum. The CoAg reaction between the 15 C. dubliniensis strains (yeast cells, germ tube, and hypha-displaying cells) and F. nucleatum was 4+, and suspensions of F. nucleatum remained active in CoAg reactions when stored for several weeks in CoAg buffer at 4°C. Suspensions of C. dubliniensis stored at 4°C also remained positive in the CoAg reaction for several weeks. No autoaggregation was observed when each suspension of microorganism was incubated only with CoAg buffer (Fig. 2).
FIG. 2.
Turbidimetric CoAg assay of C. dublieniensis (Cd) and C. albicans (Ca) with and without F. nucleatum (Fn).
TABLE 1.
CoAg of C. dubliniensis or C. albicans with F. nucleatum
| Yeast | CoAga
|
||||||
|---|---|---|---|---|---|---|---|
| 37°C
|
25°C
|
45°C
|
|||||
| No treatment | Heated at 85°C for 30 min | After DTT extraction | With 0.1 M mannose | No treatment | With 0.1 M mannose | No treatment | |
| C. dubliniensis | |||||||
| CD-36b | 4+ | 4+ | 4+ | 0 | 4+ | 0 | 4+c |
| 14 clinical isolates | 4+ | 4+ | 4+ | 0 | 4+ | 0 | 4+c |
| C. albicans | |||||||
| ATCC 18804 | 0 | 4+ | 3–4+ | 0 | 3–4+ | 0 | 2–3+ |
| 34 clinical isolates | 0 | 4+ | 3–4+ | 0 | 3–4+ | 0 | 2–3+ |
| 4 clinical isolates | 0 | 4+ | 3–4+ | 0 | 0 | 0 | 0 |
CoAg scores of suspensions of C. dubliniensis or C. albicans strains and F. nucleatum when the strains were subjected to different treatments and growth temperatures. CoAg was scored as described in Materials and Methods (4+ for maximum CoAg and 0 for no CoAg).
C. dubliniensis type strain provided by Derek Sullivan.
Cells were grown at 37°C; the cell suspensions were incubated at 45°C for up to 3 days.
Effects of heat, DTT, and growth at different temperatures on CoAg activity.
In an attempt to determine what type of CoAg receptor was present on the C. dubliniensis and F. nucleatum organisms, suspensions of each were heated at 85°C for 30 min and then the heated and unheated partners were placed in the CoAg reaction mixture. It was observed that when the F. nucleatum strain was heated and then allowed to react with the 15 unheated C. dubliniensis strains, no CoAg was observed, whereas when the 15 C. dubliniensis strains were heated and then allowed to react with the unheated F. nucleatum CoAg partner, CoAg remained intact. Therefore, it appeared that a heat-labile binding site was present on the F. nucleatum strain which was interacting with a heat-stable receptor on the C. dubliniensis strains.
As mentioned earlier, 40 C. albicans strains grown at 37°C were negative in the CoAg assay. However, after being heated at 85°C for 30 min, they all demonstrated a CoAg score of 4+ with F. nucleatum (Table 1). After DTT extraction, suspensions of all 40 C. albicans CoAg-negative strains (grown at 37°C) had CoAg scores of 3 to 4+ with F. nucleatum (Table 1), whereas extraction with CoAg buffer resulted in no change in pretreatment CoAg ability. When the C. albicans strains were grown at 37°C and the agar plates were left on the bench for 4 days at room temperature, all 40 suspensions demonstrated CoAg with F. nucleatum. When the C. dubliniensis or C. albicans strains were grown at 25°C for 24 or 48 h, all C. dubliniensis and all but four C. albicans strains demonstrated a CoAg reaction of 4+ with F. nucleatum (Table 1). When all C. albicans strains were grown at 45°C for 24 h, a CoAg with F. nucleatum was scored at 2 to 3+ (Table 1).
Testing of sugars, amino acids, and EDTA for CoAg inhibitory activity.
Sugars, amino sugars, and EDTA were tested to see whether they would inhibit CoAg. Mannose and α-methyl mannoside inhibited CoAg of C. dubliniensis (grown at 25 and 37°C) or C. albicans (the strains heated or grown at 25°C) as CoAg partners with F. nucleatum (Table 1). The CoAg between F. nucleatum and C. dubliniensis or C. albicans (heated or grown at 25°C) was not inhibited by any of the other sugars, amino acids, or EDTA.
DISCUSSION
Interactions among oral microorganisms and microbial attachment to mucosal surfaces have been shown to be important steps in infectious disease processes in the oral cavity. The importance of intergeneric CoAg in microbial colonization and accretion of bacterial cells has been well documented (10, 29). As we know very little of these processes, knowledge of Candida species adhesins and CoAg partners may help shed light on their involvement in colonization and disease processes.
The results of this study established that a specific CoAg reaction exists between F. nucleatum and C. dubliniensis, but not C. albicans when grown on SDA at 37°C. The results from the visual CoAg assay were confirmed by the turbidimetric method, the latter also used to determine the optimal time for CoAg over a 5-min period between the C. dubliniensis strains and F. nucleatum. These results as well as earlier investigations support F. nucleatum as an extremely important intergeneric bridge in microbial colonization, as fusobacteria constitute the largest portion of the microbial population in dental plaque samples taken from most diseased sites (39, 40). The multigeneric CoAg ability of F. nucleatum has been observed with a wide variety of oral microorganisms (5, 9, 31, 32).
A variety of methods have been utilized in an attempt to characterize the mediators of CoAg reactions, including the addition of potential inhibitors such as sugars, amino acids, chelators (EDTA), and ions (13, 25, 30, 31, 33, 42, 57). CoAg was characterized as a heat labile-heat stable receptor interaction, suggesting that F. nucleatum possesses a protein receptor and the C. dubliniensis strains possess either a heat-stable or polysaccharide component. The binding of F. nucleatum strains to C. albicans as observed by Grimaudo and Nesbitt (14) was also inhibited by mannose and α-methyl mannoside. The presence of mannan in the cell walls of Candida species and inhibition of CoAg by mannose and α-methyl mannoside would suggest a group on mannan as the site of binding of the heat-labile component on F. nucleatum. Inhibition of CoAg of F. nucleatum strains among certain gram-negative microorganisms has involved a lactose-galactose-mediated interaction (8, 25, 26, 31), whereas among gram-positive microorganisms, an arginine-mediated mechanism was demonstrated when arginine inhibited F. nucleatum ATCC 10953 CoAg with strains of oral streptococci (55). EDTA did not inhibit CoAg between F. nucleatum ATCC 49256 and C. dubliniensis, although it was reported that EDTA caused total disaggregation of other F. nucleatum strains and C. albicans pairs of cells (14).
Suspensions of 40 strains of C. albicans grown at 37°C for 24 to 48 h on SDA would not coaggregate with F. nucleatum. However, when these 40 C. albicans strains were grown at 25°C, all but four of the isolates showed CoAg, whereas when they were grown at 45°C on the same medium, when germ tubes and hyphae were induced or when they were treated with heat or DTT, CoAg would occur with all 40 isolates. It would appear that growth of C. albicans on SDA at 37°C results in the presence of a heat-labile, DTT-extractable material on the surface of the yeast cells which interferes with CoAg with F. nucleatum. This heat-labile material may be the hydrophilic outer layer described by previous investigators as a fibrillar coat consisting largely of mannoproteins (4, 16–20, 34, 37, 48, 56). The fibers of this outermost layer are responsible for the initial loose attachment of C. albicans to epithelial cells and other surfaces. This initial association is followed by a tight adhesin-receptor interaction, with the C. albicans adhesin being a mannoprotein (17, 56). Hydrophobic proteins in the polysaccharide matrix of the C. albicans cell wall contribute to the strength of this adhesin-receptor bond, in turn contributing to the virulence and pathogenesis of the yeast (12, 16–18, 20, 35, 36). These hydrophobic proteins are present in blastoconidia when the cells were grown at both 25 and 37°C (16, 18). In contrast to the tight association of the hydrophobic proteins to the cell wall of C. albicans, the hydrophilic masking layer is loosely associated and is easily destroyed by heat and removed by extraction with DTT (4, 20). Thus, DTT treatment rapidly converts hydrophilic cells into hydrophobic cells by removing the outer fibrillar layer and exposing hydrophobic proteins (18, 20). Once yeast cells become hydrophobic, they may rapidly produce germ tubes and become invasive (18). Previous investigations have shown that germ tubes and hyphae of C. albicans are highly and invariably hydrophobic, regardless of whether the mother cell displays cell surface hydrophobicity (16, 17, 19, 35), and that hydrophobic sites are particularly abundant at the hyphal apex (18). In this study, growing C. albicans at 25 or 45°C may have resulted in total inhibition of or considerable reduction in the production of the fibrillar layer, as suggested by enhanced CoAg. The reduced presence of this layer in the C. albicans strains due to growth at 25 or 45°C, heat treatment, DTT extraction, or leaving the plates grown at 37°C at room temperature for 4 days, allowed for the CoAg of C. albicans strains with F. nucleatum. The four C. albicans strains that did not coaggregate when grown at 25°C may be hydrophilic variants as described by Hazen (17) in that they are hydrophilic at growth temperatures of 25 and 37°C. The production of the outer fibrillar layer in these four variants does not seem to be affected by growth at 25°C, although it is diminished at 45°C as CoAg was then observed. C. dubliniensis may have a reduced outermost fibrillar layer as demonstrated by its ability to coaggregate with F. nucleatum at all growth temperatures. A reduction in the hydrophilic layer resulting in a hydrophobic cell surface may give this species a greater ecological advantage in allowing enhanced attachment to oral microbes and buccal epithelial cells (11, 38).
Many questions remain to be answered regarding the role C. dubliniensis plays in oral diseases, the factors that encourage its colonization and growth, its occurrence in healthy individuals and patient groups other than the HIV-seropositive population, and the relationship of its coexistence with C. albicans and other microorganisms in the oral cavity. The concern over the occurrence of fluconazole resistance in clinical isolates, as observed in readily inducible fluconazole resistance in vitro (23, 41), suggests that patients who have received multiple antimicrobial treatments for fungal infections throughout their HIV infection may have a higher risk of harboring C. dubliniensis in their oral cavity. In order to further study the role of C. dubliniensis in disease processes, the first step is for clinical microbiology laboratories to be able to rapidly, inexpensively, and accurately distinguish C. dubliniensis isolates from typical C. albicans in patient samples. We propose that CoAg of C. dubliniensis with F. nucleatum provides a simple, inexpensive, and rapid method to identify C. dubliniensis strains in clinical or research laboratories. After a 24-h growth period on SDA at 37°C, followed by a 3-h germ tube test, a suspension of yeast cells from the original culture plate would then undergo a 5-min CoAg test with F. nucleatum ATCC 49256. If no CoAg results, C. albicans would be indicated, whereas CoAg-positive isolates would be further tested as presumptive C. dubliniensis. The use of this regimen in clinical laboratories receiving large volumes of yeast isolates from immunocompromised patients will greatly expedite the rate of turnover by allowing laboratory personnel to confidently release all germ tube-positive noncoaggregating yeast isolates that could grow at 37°C as C. albicans. All germ tube-positive isolates demonstrating CoAg after growth at 37°C would be considered suspicious for C. dubliniensis and would then undergo further testing for confirmation.
This novel partnership between F. nucleatum and C. dubliniensis provides an alternative avenue to study the colonization of this potentially pathogenic yeast in the oral cavity.
REFERENCES
- 1.Bagg J, Silverwood R W. Coagglutination reactions between Candida albicans and oral bacteria. J Med Microbiol. 1986;22:165–169. doi: 10.1099/00222615-22-2-165. [DOI] [PubMed] [Google Scholar]
- 2.Bikandi J, Millan R S, Moraguew M D, Cebas G, Clarke M, Coleman D C, Sullivan D J, Quindos G, Ponton J. Rapid identification of Candida dubliniensis by indirect immunofluorescence based on differential localization of antigens on C. dubliniensis blastospores and Candida albicans germ tubes. J Clin Microbiol. 1998;36:3007–3012. doi: 10.1128/jcm.36.9.2428-2433.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boerlin P, Loerlin-Petzold F, Durussel C, Addo M, Pagani J-L, Chave J-P, Bille J. Cluster of oral atypical Candida albicans isolates in a group of human immunodeficiency virus-positive drug users. J Clin Microbiol. 1995;33:1129–1135. doi: 10.1128/jcm.33.5.1129-1135.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calderone R A, Braun P C. Adherence and receptor relationships of Candida albicans. Microbiol Rev. 1991;55:1–20. doi: 10.1128/mr.55.1.1-20.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cisar J O, Kolenbrander P E, McIntire F C. Specificity of coaggregation reactions between human oral streptococci and strains of Actinomyces viscosus or Actinomyces naeslundii. Infect Immun. 1979;24:742–752. doi: 10.1128/iai.24.3.742-752.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coleman D C, Sullivan D J, Bennett D E, Moran G P, Barry H J, Shanley D B. Candidiasis: the emergence of a novel species, Candida dubliniensis. AIDS. 1997;11:557–567. doi: 10.1097/00002030-199705000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Dehazya P, Coles R S., Jr Agglutination of human erythrocytes by Fusobacterium nucleatum: factors influencing hemagglutination and some characteristics of the agglutinin. J Bacteriol. 1980;143:205–211. doi: 10.1128/jb.143.1.205-211.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Falkler W A, Jr, Burger B S. Microbial surface interactions: reduction of the hemagglutination activity of the oral bacterium Fusobacterium nucleatum by absorption with Streptococcus and Bacteroides. Arch Oral Biol. 1981;26:1015–1025. doi: 10.1016/0003-9969(81)90112-6. [DOI] [PubMed] [Google Scholar]
- 9.George K S, Falkler W A., Jr Coaggregation studies of the Eubacterium species. Oral Microbiol Immunol. 1992;7:285–290. doi: 10.1111/j.1399-302x.1992.tb00590.x. [DOI] [PubMed] [Google Scholar]
- 10.Gibbons R J, Nygaard M. Interbacterial aggregations of plaque bacteria. Arch Oral Biol. 1970;15:1397–1400. doi: 10.1016/0003-9969(70)90031-2. [DOI] [PubMed] [Google Scholar]
- 11.Gilfillan G D, Sullivan D J, Haynes K, Parkinson T, Coleman D C, Gow N A R. Candida dubliniensis: phylogeny and putative virulence factors. Microbiology. 1998;144:829–838. doi: 10.1099/00221287-144-4-829. [DOI] [PubMed] [Google Scholar]
- 12.Glee P M, Sundstrom P, Hazen K C. Expression of surface hydrophobic proteins by Candida albicans in vivo. Infect Immun. 1995;63:1373–1379. doi: 10.1128/iai.63.4.1373-1379.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grenier D, Mayrand D. Functional characterization of extracellular vesicles produced by Bacteroides gingivalis. Infect Immun. 1987;55:111–117. doi: 10.1128/iai.55.1.111-117.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grimaudo N J, Nesbitt W E. Coaggregation of Candida albicans with oral Fusobacterium species. Oral Microbiol Immunol. 1997;12:168–173. doi: 10.1111/j.1399-302x.1997.tb00374.x. [DOI] [PubMed] [Google Scholar]
- 15.Grimaudo N J, Nesbitt W, Clark W. Coaggregation of Candida albicans with oral Actinomyces species. Oral Microbiol Immunol. 1996;11:59–61. doi: 10.1111/j.1399-302x.1996.tb00337.x. [DOI] [PubMed] [Google Scholar]
- 16.Hazen B W, Hazen K C. Dynamic expression of cell surface hydrophobicity during initial yeast cell growth and before germ tube formation of Candida albicans. Infect Immun. 1988;56:2521–2525. doi: 10.1128/iai.56.9.2521-2525.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hazen K C. Participation of yeast cell surface hydrophobicity in adherence of Candida albicans to human epithelial cells. Infect Immun. 1989;57:1894–1900. doi: 10.1128/iai.57.7.1894-1900.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hazen K C, Brawner D L, Riesselman M H, Jutila M A, Cutler J E. Differential adherence of hydrophobic and hydrophilic Candida albicans yeast cells to mouse tissues. Infect Immun. 1991;59:907–912. doi: 10.1128/iai.59.3.907-912.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hazen K C, Hazen B W. Hydrophobic surface protein masking by the opportunistic fungal pathogen Candida albicans. Infect Immun. 1992;60:1499–1508. doi: 10.1128/iai.60.4.1499-1508.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hazen K C, Lay J G, Hazen B W, Fu R C. Partial biochemical characterization of cell surface hydrophobicity and hydrophilicity of Candida albicans. Infect Immun. 1990;58:3469–3476. doi: 10.1128/iai.58.11.3469-3476.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holmes A R, Gopal P K, Jenkinson H F. Adherence of Candida albicans to a cell surface polysaccharide receptor on Streptococcus gordonii. Infect Immun. 1995;63:1827–1834. doi: 10.1128/iai.63.5.1827-1834.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsu L Y, Minah G, Peterson D E, Wingard J R, Merz W G, Altomonte V, Tylenda C A. Coaggregation of oral Candida isolates with bacteria from bone marrow transplant recipients. J Clin Microbiol. 1990;28:2621–2626. doi: 10.1128/jcm.28.12.2621-2626.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jabra-Rizk M A, Baqui A A M A, Kelley J I, Falkler W A, Jr, Merz W G, Meiller T F. Identification of Candida dubliniensis in a prospective study of patients in the United States. J Clin Microbiol. 1999;37:321–326. doi: 10.1128/jcm.37.2.321-326.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jenkinson H F, Lala H C, Shepherd M G. Coaggregation of Streptococcus sanguis and other streptococci with Candida albicans. Infect Immun. 1990;58:1429–1436. doi: 10.1128/iai.58.5.1429-1436.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kinder S A, Holt S C. Characterization of coaggregation between Bacteroides gingivalis T22 and Fusobacterium nucleatum T18. Infect Immun. 1989;57:3245–3248. doi: 10.1128/iai.57.11.3425-3433.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kinder S A, Holt S C. Localization of the Fusobacterium nucleatum T18 adhesin activity mediating coaggregation with Porphyromonas gingivalis T22. J Bacteriol. 1993;175:840–850. doi: 10.1128/jb.175.3.840-850.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.King D, Rhine-Chalberg J, Pfaller M A, Moser S A, Merz W G. Comparison of four DNA-based methods for strain delineation of Candida lusitaniae. J Clin Microbiol. 1995;33:1467–1470. doi: 10.1128/jcm.33.6.1467-1470.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kirkpatrick W R, Revankar S G, McAtee R K, Lopez-Ribot J L, Fothergill A W, McCarthy D I, Sanche S E, Cantu R A, Rinaldi M G, Patterson T F. Detection of Candida dubliniensis in oropharyngeal samples from human immunodeficiency virus-infected patients in North America by primary CHROMagar Candida screening and susceptibility testing of isolates. J Clin Microbiol. 1998;36:3007–3012. doi: 10.1128/jcm.36.10.3007-3012.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kolenbrander P E. Surface recognition among oral bacteria: multigeneric coaggregations and their mediators. Crit Rev Microbiol. 1989;17:137–159. doi: 10.3109/10408418909105746. [DOI] [PubMed] [Google Scholar]
- 30.Kolenbrander P E, Anderson R N. Multigeneric aggregations among oral bacteria: a network of cell-to-cell interactions. J Bacteriol. 1986;168:851–859. doi: 10.1128/jb.168.2.851-859.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kolenbrander P E, Andersen R N. Inhibition of coaggregation between Fusobacterium nucleatum and Porphyromonas (Bacteroides) gingivalis by lactose and related sugars. Infect Immun. 1989;57:3204–3209. doi: 10.1128/iai.57.10.3204-3209.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kolenbrander P E, Anderson R N, Moore L V H. Coaggregation of Fusobacterium nucleatum, Selenomonas fluegei, Selenomonas infelix, Selenomonas noxia, and Selenomonas sputigena, with strains from 11 genera of oral bacteria. Infect Immun. 1989;57:3194–3203. doi: 10.1128/iai.57.10.3194-3203.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kolenbrander P E, Williams B L. Lactose-reversible coaggregation between oral actinomycetes and Streptococcus sanguis. Infect Immun. 1981;33:95–102. doi: 10.1128/iai.33.1.95-102.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kusamichi M, Monodane T, Tokunaga M, Koike H. Influence of surrounding media on preservation of cell wall ultrastructure of Candida albicans revealed by low temperature scanning electron microscopy. J Electron Microsc. 1990;39:477–486. [PubMed] [Google Scholar]
- 35.Lopez-Ribot J L, Casanova M, Martinez J P, Sentandreu R. Characterization of cell wall proteins of yeast and hydrophobic mycelial cells of Candida albicans. Infect Immun. 1991;59:2324–2332. doi: 10.1128/iai.59.7.2324-2332.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Masuoka J, Hazen K C. Cell wall protein mannosylation determines Candida albicans cell surface hydrophobicity. Microbiology. 1997;143:3015–3021. doi: 10.1099/00221287-143-9-3015. [DOI] [PubMed] [Google Scholar]
- 37.McCourtie J, Douglas L J. Relationship between cell surface composition, adherence, and virulence of Candida albicans. Infect Immun. 1984;45:6–12. doi: 10.1128/iai.45.1.6-12.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCullough M, Ross B, Reade P. Characterization of genetically distinct subgroup of Candida albicans strains isolated from oral cavities of patients infected with human immunodeficiency virus. J Clin Microbiol. 1995;33:696–700. doi: 10.1128/jcm.33.3.696-700.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moore W E C, Holdeman L V, Cato E P, Smibert R M, Burmeister J A, Palcanis K C, Ranney R R. Comparative bacteriology of juvenile periodontitis. Infect Immun. 1985;48:507–519. doi: 10.1128/iai.48.2.507-519.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moore W E C, Holdeman L V, Cato E P, Smibert R M, Burmeister J A, Ranney R R. Bacteriology of moderate (chronic) periodontitis in mature human adults. Infect Immun. 1983;42:510–515. doi: 10.1128/iai.42.2.510-515.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moran G P, Sanglard D, Donnelly S M, Shanley D B, Sullivan D J, Coleman D C. Identification and expression of multidrug transporters responsible for fluconazole resistance in Candida dubliniensis. Antimicrob Agents Chemother. 1998;42:1818–1830. doi: 10.1128/aac.42.7.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nishikata M, Yoshimura F, Nodasaka Y. Possibility of Bacteroides gingivalis hemagglutinin possessing protease activity revealed by inhibition studies. Microbiol Immunol. 1989;33:75–80. doi: 10.1111/j.1348-0421.1989.tb01499.x. [DOI] [PubMed] [Google Scholar]
- 43.Odds F C, Bernaerts R. CHROMagar Candida, a new differential isolation medium for presumptive identification of clinically important Candida species. J Clin Microbiol. 1994;32:1923–1929. doi: 10.1128/jcm.32.8.1923-1929.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Odds F C, Van Nuffel L, Dams G. Prevalence of Candida dubliniensis isolates in a yeast stock collection. J Clin Microbiol. 1998;36:2869–2873. doi: 10.1128/jcm.36.10.2869-2873.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pinjon E, Sullivan D, Salkin I, Shanley D, Coleman D. Simple, inexpensive, reliable method for differentiation of Candida dubliniensis from Candida albicans. J Clin Microbiol. 1998;36:2093–2095. doi: 10.1128/jcm.36.7.2093-2095.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Salkin I F, Pruitt W R, Padhye A A, Sullivan D, Coleman D, Pincus D H. Distinctive carbohydrate assimilation profiles used to identify the first clinical isolates of Candida dubliniensis recovered in the United States. J Clin Microbiol. 1998;36:1467. doi: 10.1128/jcm.36.5.1467-1467.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schoofs A, Odds F C, Colebunders R, Ieven M, Goosens H. Use of specialised isolation media for recognition and identification of Candida dubliniensis isolates from HIV-infected patients. Eur J Clin Infect Dis. 1997;16:296–300. doi: 10.1007/BF01695634. [DOI] [PubMed] [Google Scholar]
- 48.Shepherd M G. Cell envelope of Candida albicans. Clin Rev Microbiol. 1987;15:7–25. doi: 10.3109/10408418709104445. [DOI] [PubMed] [Google Scholar]
- 49.Sullivan D, Bennett D, Henman M, Harwood P, Flint S, Mulcahy F, Shanley D, Coleman D. Oligonucleotide fingerprinting of isolates of Candida species other than C. albicans and of atypical Candida species from human immunodeficiency virus-positive and AIDS patients. J Clin Microbiol. 1993;31:2124–2133. doi: 10.1128/jcm.31.8.2124-2133.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sullivan D, Coleman D. Candida dubliniensis: characteristics and identification. J Clin Microbiol. 1998;36:329–334. doi: 10.1128/jcm.36.2.329-334.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sullivan D, Haynes K, Bille J, Boerlin P, Rodero L, Lloyd S, Henman M, Coleman D. Widespread geographic distribution of oral Candida dubliniensis strains in human immunodeficiency virus-infected individuals. J Clin Microbiol. 1997;35:960–964. doi: 10.1128/jcm.35.4.960-964.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sullivan D, Westerneng T, Haynes K A, Bennett D, Coleman D. Candida dubliniensis sp. nov.: phenotypic and molecular characterization of a novel species associated with oral candidiasis in HIV-infected individuals. Microbiology. 1995;141:1507–1521. doi: 10.1099/13500872-141-7-1507. [DOI] [PubMed] [Google Scholar]
- 53.Sullivan D J. Abstracts of the 98th General Meeting of the American Society for Microbiology 1998. Washington, D.C: American Society for Microbiology; 1998. Presentation: molecular epidemiological analysis of Candida dubliniensis, a newly described pathogen; p. 26. [Google Scholar]
- 54.Sullivan D J, Henman M C, Moran G P, O’Neill L C, Benett D E, Shanley D B, Coleman D C. Molecular genetic approaches to identification, epidemiology and taxonomy of non-albicans Candida species. J Med Microbiol. 1996;44:399–408. doi: 10.1099/00222615-44-6-399. [DOI] [PubMed] [Google Scholar]
- 55.Takemoto T O, Shirakawo M, Hino T, Okamoto H. Purification of arginine sensitive hemagglutinin from Fusobacterium nucleatum and its role in coaggregation. J Periodontal Res. 1993;28:21–26. doi: 10.1111/j.1600-0765.1993.tb01046.x. [DOI] [PubMed] [Google Scholar]
- 56.Tokunaga M, Nimi M, Kusamichi M, Koike H. Initial attachment of Candida albicans cells to buccal epithelial cells. Mycopathologia. 1990;111:61–66. doi: 10.1007/BF02277306. [DOI] [PubMed] [Google Scholar]
- 57.Weiss E I, London J, Kolenbrander P E, Kagermeier A S, Andersen R N. Characterization of lectinlike surface components on Capnocytophaga ochracea ATCC 33596 that mediate coaggregation with gram-positive oral bacteria. Infect Immun. 1987;55:1198–1202. doi: 10.1128/iai.55.5.1198-1202.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]