Abstract
Background:
This study compared the analytical performance of the Elecsys 602 (Roche Diagnostics) system with the I2000 (Abbott laboratories) system for the quantitative measurement of squamous cell carcinoma antigen (SCCA) to assess its role as an indicator in pan squamous cell carcinoma.
Methods:
435 serum samples included pan squamous cell cancer group (n = 318) and healthy subjects (n = 52) and non-squamous cell group (n = 41) and benign diseases group (n = 24) were measured by 2 systems and compared.
Results:
The within-run precision coefficient of variation (CV) for Abbott and Roche systems were 3.34-4.88% and 0.95 -1.96%, and the total precision CV were 2.89-9.48% and 3.97-5.38%, respectively. Good correlation was showed in Abbott and Roche systems (slopes = 0.749, r = 0.9658). Serum SCCA in the groups of nasopharyngeal carcinomas, lung squamous cell carcinoma, esophageal squamous cell carcinoma, bladder cancer and cervical squamous cell carcinoma under the curve area (AUC) was more than 0.5, while the AUC in the non- nasopharyngeal carcinomas head and neck squamous cell carcinoma was less than 0.5. The AUC of 2 systems was statistically different in lung squamous cell carcinoma and nasopharyngeal carcinomas (P < 0.05). The levels of SCCA of 2 systems were similarities in esophageal squamous cell carcinoma(stage IV vs. stage 0a-II)and bladder cancer(stage I vs. stage Oa)and cervical squamous cell carcinoma(stage IIB-III vs. stage I-IIA), which advanced stage had higher level of SCCA than early stage. But the SCCA levels of 2 systems were inconsistent in bladder cancer (stage II-IV vs. stage Oa in Abbott), head and neck squamous cell carcinoma (stage IV vs. stage Oa-I in the Roche) and lung squamous cell carcinoma (stage III vs. stage I-II in the Roche). (P < 0.05)
Conclusions:
2 systems correlated well in SCCA detection of squamous cell carcinoma, but there were individual differences. Serum SCCA may also contribute to the diagnosis of bladder cancer.
Keywords: squamous cell carcinoma (SCC), squamous cell carcinoma antigen (SCCA), esophageal squamous cell carcinoma, cervical squamous cell carcinoma, bladder cancer
Introduction
Squamous cell carcinoma antigen (SCCA), originally discovered in the squamous cell carcinoma of the cervix,1 is associated with the occurrence and development of squamous cell carcinomas (SCC) and expressed in normal squamous cell epithelia. Previous studies have found that its serum concentration is elevated in a variety of tumors including lung cancer,2 cervical cancer,3 nasopharyngeal carcinoma,4 esophageal squamous cell carcinoma.5 Moreover, SCCA has been confirmed to be closely related to the prognosis of lung cancer and cervical cancer.3
SCCA in the serum contains 2 isoforms, SCCA1 (SERPINB3) and SCCA2 (SERPINB4), are members of the serine protease inhibitor (serpin) superfamily and highly homologous proteins, 91% identity at the amino acid level.6 These 2 isoforms are co-expressed in squamous epithelium of tongue, esophagus and cervix, as well as in moderately and well differentiated SCC of lung and head and neck.7 But the isoforms may have different proportions of expression in different tumors and skin diseases. Yasumatsu shown that combined measurements of both serum SCCA1 and SCCA2 concentrations can be useful for distinguishing sinonasal inverted papilloma from SCC.8 Several studies were reported that differing expressions of SCCA1 and SCCA2 at the mRNA level and tissue level in various cancer.9-11 Therefore, expression of SCCA1 and SCCA2 differs in various cancers may apply the difference to clinical diagnosis and prognosis.
Automated immunoassay analyzers for the quantification of human SCCA in serum are currently widely available. Available assays in China are Architect i2000 or i1000 from Abbott Laboratories and Elecsys 411 or Elecsys 602 from Roche Diagnostics. They both measured serum SCCA1 and SCCA2 levels, the total SCCA, but the ratio of SCCA1 and SCCA2 that they detected is still unknown. This discordance between different antibody responses assays which may affect clinical judgment. Whether the differences in SCCA results with different systems remains a cause of considerable concern. Test results of the 2 systems may be different in some cancers and normal people.12 Although Stefan’s analyzed of the correlation between the results of the cobas e 411 versus Architect system in 193 serum samples (Pearson’s correlation was r = 0.937).13 The study did not analyze the situation in various squamous cell carcinomas. Until now, there is no report on the complete comparison of these 2 detection systems in pan squamous cell carcinomas. The correlation and difference between 2 detection systems in pan squamous cell cancer and normal people is still unknown. Therefore, in order to understand whether the difference between the 2 detection systems will exist in different squamous carcinomas, non-squamous carcinomas, benign diseases and healthy people, we conducted a large-scale comparative study of the 2 systems.
The present study evaluated the clinical usefulness of serum SCCA as a part of routine detection of patients with pan squamous cell cancer and normal people. We also investigated whether there is a difference between serum SCCA concentration detected by the Elecsys 602 system and the Abbott I2000 system in different cancer, and assessed whether these differences can be better used for disease diagnosis.
Materials and Methods
Study Population
Patients and control subjects were enrolled between June 2018 and May 2020 in Sun Yat-sen University Cancer Center. The population included a group of pan squamous cell cancer patients as well as a control group. The pan squamous cell cancer group comprised 318 newly diagnosed patients enrolled prior to any treatment included nasopharyngeal carcinoma (NPC, n = 49), non-nasopharyngeal carcinoma head and neck squamous cell carcinomas (HNSCC, n = 39) included laryngeal cancer (n = 21), oral squamous cell carcinoma (n = 9), tongue cancer (n = 9), esophageal squamous cell carcinoma (ESCC, n = 75), lung squamous cell carcinoma (LSCC, n = 53), cervical squamous cell carcinoma (CSCC, n = 43), bladder cancer (BC, n = 52), Penile cancer (PC, n = 6), Skin squamous cell carcinoma (n = 1).
The control group included 3 subgroups: healthy subjects (HC, n = 52); non- squamous cell patients (n = 41) included adenocarcinoma of esophagogastric junction (n = 18), lung adenocarcinoma (n = 4), B-cell non-Hodgkin’s lymphoma (n = 1), cervical adenocarcinoma (n = 14), antrum adenocarcinoma (n = 1), cutaneous lipoma (n = 1) lymphoepithelioma-like carcinoma (n = 1), ovarian cancer (n = 1); and subjects with benign diseases (n = 24) included cervical intraepithelial neoplasias, CIN (CIN I, n = 3; CIN II, n = 1; CIN III, n = 14), chronic inflammation of soft palate mucosa (n = 1), Iaryngopharyngitis (n = 2), myoma of uterus (n = 3).
All cancer patients diagnosis was histologically confirmed by biopsy and further tests, including MRI or CT. Stages of progression were classified according to the UICC-8th TNM Classification of Malignant Tumors. Healthy controls received blood pressure, routine blood tests, chest X-ray, and electrocardiogram to exclude the subjects with cancer, skin disease and any chronic medical illness including liver, kidney, lung, heart, and metabolic diseases. Benign diseases were diagnosed on the basis of endoscopy, computed tomography (CT)/positron emission tomography (PET), or pathologic examination.
Assay Systems
Two different assay systems for the measurement of SCCA, I2000 and Elecsys 602, were used. Assays were performed according to the manufacturers’ specifications.
Abbott ARCHITECT I2000 system
The ARCHITECT SCC assay is a 2-step immunoassay for the quantitative determination of SCC Ag in human serum and plasma using CMIA technology with flexible assay protocols, referred to as Chemiflex. The resulting chemiluminescent reaction is measured as relative light units (RLUS). There is a direct relationship between the amount of SCC Ag in the sample and the RLUS detected by the ARCHITECT system optics.
Roche Cobas e 602 system
The Elecsys SCC assay is an electrochemiluminescence immunoassay (ECLIA) that measures SCCA levels from serum samples and is used on the cobas e 602 analyzers. The assay uses 2 SCC-specific monoclonal antibodies that recognize both SCCA1 and SCCA2 human isoforms in an equimolar manner. It utilizes a biotin–streptavidin sandwich principle and the read-out is via electrochemiluminescence.
Precision
The precision was evaluated according to the EP15-A2 protocol of the Clinical and Laboratory Standards Institute (CLSI).14 The quality controls were analyzed 3 times per day during a period of 5 consecutive workdays (n = 15 per level). Precision was evaluated as the coefficient of variation (CV), which was calculated from the data series mean and standard deviation.
Method Comparisons and Differential Diagnosis
The main focus of the clinical part of this study was to check for consistency between SCCA levels using 2 systems, and provide the best scheme selected for clinical detection. The SCC assay was compared between the commercially available Abbott Architect and Roche Elecsys assays. For comparison, correlation coefficient analysis was used to estimate the agreement between the 2 systems. Areas under the receiver operating characteristic (ROC)curve (AUCs) were calculated for the differential diagnosis analyses. Subgroup analyses were conducted with studies grouped by each different pan squamous Cell Carcinoma and control groups for showcasing the advantages and disadvantages of 2 systems.
Statistical Analysis
Correlation between serum SCCA level in 2 system was analyzed by correlation coefficient analysis using MedCalc statistical software(v19.5.1). A receiver operating characteristic (ROC) curve was constructed by plotting sensitivity versus 1-specificity, and the areas under the curve (AUC) was analyzed according to SPSS19.0 statistical software. All statistical tests were non-parametric tests and a P value <0.05 was considered statistically significant. Values are given with 95% confidence interval (95% CI) if applicable. All statistical analyses were performed using IBM SPSS Statistics for Windows, version19.0 (IBM Corp.), MedCalc, version 19.5 and GraphPad Prism for Windows, version 8.0.
Results
Precision Performance
The results of the within-run and total precision studies were summarized in Table 1. The CV, as determined using the control samples, was within the specified range, i.e. 0–10%, for both instruments. It is clear from the results that the Roche system gave more precise results, with a low CV value of 0.95% to 1.96% for the within-run precision and 3.97% to 5.38% for the total precision, as compared with the Abbott system, which showed a relatively high CV value of 3.34% to 4.88% for within-run precision and 2.89% to 9.48% for total precision.
Table 1.
Precision and Reproducibility of SCCA Detection on Abbott and Roche Using the Controls Provided by the Manufacturers.
| Instrument | Controls | Mean ng/mL | Within run | Total | ||
|---|---|---|---|---|---|---|
| SD | CV% | SD | CV% | |||
| Abbott | Low | 2.13 | 0.10 | 4.86 | 0.20 | 9.48 |
| Medium | 10.95 | 0.53 | 4.88 | 0.54 | 4.95 | |
| High | 52.47 | 1.75 | 3.34 | 1.52 | 2.89 | |
| Roche | PCTM1 | 1.84 | 0.04 | 1.96 | 0.10 | 5.38 |
| PCTM2 | 18.82 | 0.18 | 0.95 | 0.75 | 3.97 | |
Mean is of 15 samples.
Comparison of Abbott I2000 and Roche E602
Total 435 subjects include pan squamous cell cancer patients (n = 318), healthy subjects (HC, n = 52); non- squamous cell patients (n = 41), and benign diseases subjects (n = 24) were investigated to establish the correlation of 2 system. The slope between the Abbott versus Roche was calculated as 0.749, and the Pearson’s correlation was r = 0.9658 (Figure 1). We observed that concentration value of SCCA tested by Roche system was higher than Abbott.
Figure 1.
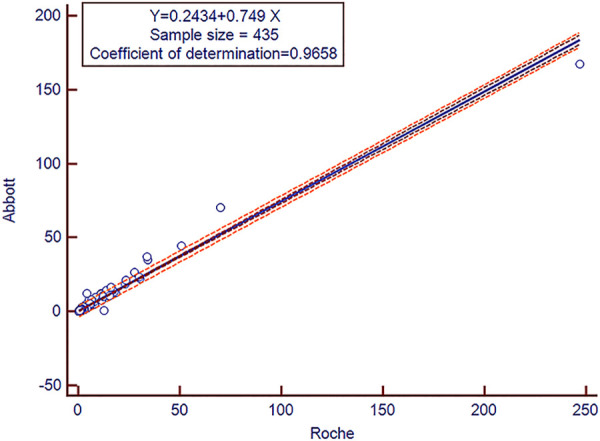
Squamous cell carcinoma antigen (SCCA) assay method comparison results. The cobas e602 versus Architect system using serum samples (n = 435).
Diagnostic Value Analysis of Roche and Abbott in Patients With Various Squamous Cell Carcinomas
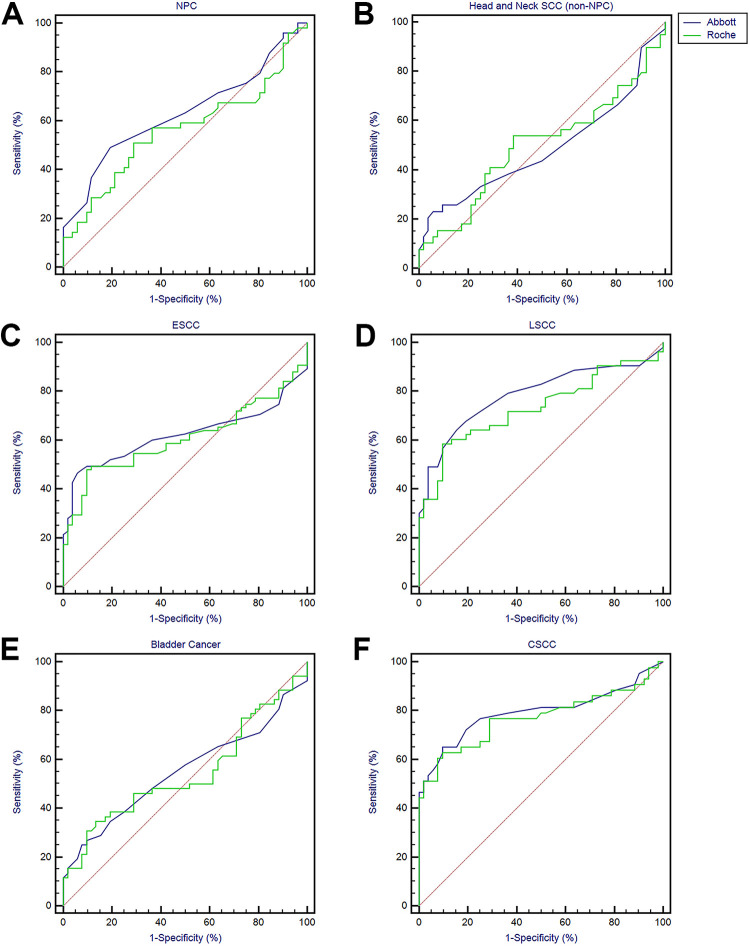
The performance of the 2 systems in measuring SCCA is showed in Table 2 and Figure 2. ROC analysis showed that the AUCs of ESCC, LSCC, BC and CSCC were all greater than 0.5, especially the area of LSCC and CSCC is greater than 0.7, indicating higher diagnostic value (Table 2 and Figure 2C-F). In addition to HNSC, the diagnostic value of Abbott is higher than that of Roche, and the difference between NPC and LSCC was statistically significant (63.1% vs. 56.1%, p = 0.0051; 78.7% vs. 73.2%, p = 0.0066). SCCA sensitivity and specificity for NPC versus apparently healthy patients was 48.98% and 80.77% by Abbott, 51.02% and 71.15% by Roche, respectively, while that for HNSCC (non-NPC) was 23.08% and 94.23% by Abbott, 53.85% and 61.54% by Roche, respectively. What’s more, the sensitivity for ESCC was 46.67% and 48.00%, and the specificity was 94.23% and 90.38%, respectively; the sensitivity for LSCC was 64.15% and 58.49%, and the specificity was 84.62% and 90.38%, respectively; the sensitivity for BC was 25.00% and 34.62%, and the specificity was 92.31% and 86.54%, respectively; the sensitivity for CSCC was 64.15% and 58.49%, and the specificity was 84.62% and 90.38%, respectively (Table 2).
Table 2.
ROC Analysis AUC, Sensitivity and Specificity Results.
| Cut-off (ng/ml) | AUC, % (95%CI) | Sensitivity, % (95% CI) | Specificity, % (95% CI) | P | ||
|---|---|---|---|---|---|---|
| NPC | Abbott | 0.60 | 63.1 (52.9-72.5) | 48.98 (34.4-63.7) | 80.77 (67.5-90.4) | 0.0051 |
| Roche | 1.09 | 56.1 (45.9-66.0) | 51.02 (36.3-65.6) | 71.15 (56.9-82.9) | ||
| HNSC | Abbott | 1.60 | 48.7 (38.1-59.4) | 23.08 (11.1-39.3) | 94.23 (84.1-98.8) | 0.9275 |
| Roche | 1.20 | 49.8 (39.2-60.5) | 53.85 (37.2-69.9) | 61.54 (47.0-74.7) | ||
| ESCC | Abbott | 1.60 | 62.2 (53.2-70.6) | 46.67 (35.1-58.6) | 94.23 (84.1-98.8) | 0.4688 |
| Roche | 1.87 | 60.7 (51.7-69.3) | 48.00 (36.3-59.8) | 90.38 (79.0-96.8) | ||
| LSCC | Abbott | 1.20 | 78.7 (69.6-86.1) | 64.15 (49.8-76.9) | 84.62 (71.9-93.1) | 0.0066 |
| Roche | 1.87 | 73.2 (63.7-81.4) | 58.49 (44.1-71.9) | 90.38 (79.0-96.8) | ||
| BC | Abbott | 1.50 | 55.0 (44.9-64.8) | 25.00 (14.0-38.9) | 92.31 (81.5-97.9) | 0.8093 |
| Roche | 1.74 | 54.3 (44.2-64.1) | 34.62 (22.0-49.1) | 86.54 (74.2-94.4) | ||
| CSCC | Abbott | 1.30 | 79.4 (69.9-87.0) | 65.12 (49.1-79.0) | 90.38 (79.0-96.8) | 0.2264 |
| Roche | 1.87 | 77.0 (67.3-85.0) | 62.79 (46.7-77.0) | 90.38 (79.0-96.8) |
Figure 2.
ROC analysis of SCCA levels as a diagnostic biomarker for differentiating squamous cell carcinoma from other groups.
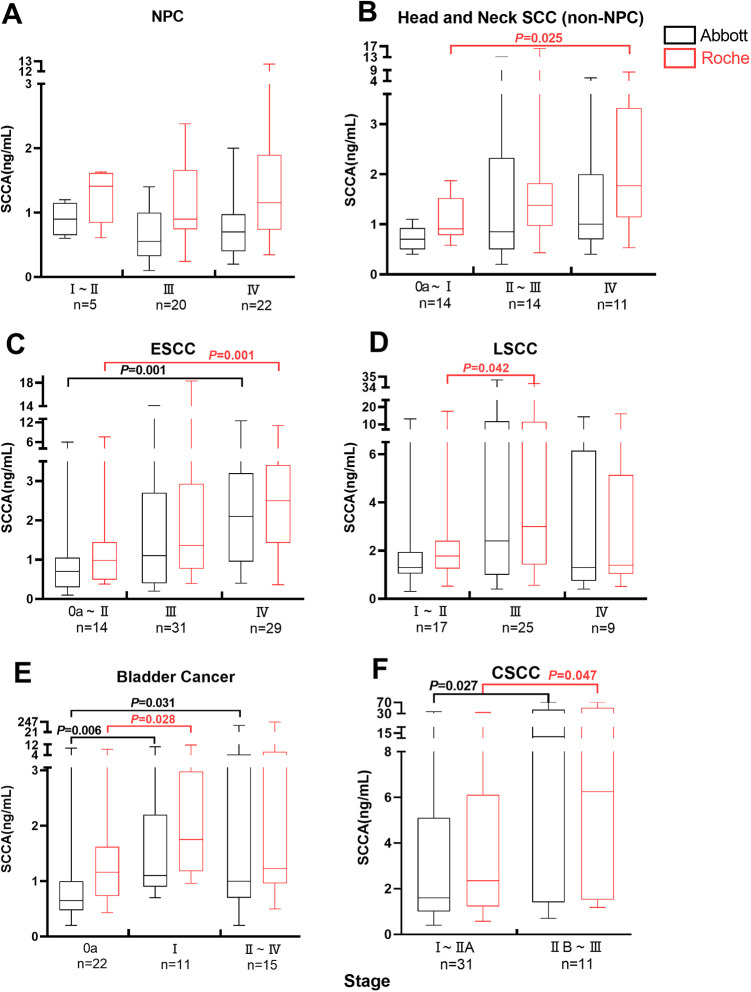
Serum SCCA Concentration and Clinical Stage of Pan Squamous Cell Cancer
Median SCCA levels were observed in Roche system was higher than Abbott. Higher median SCCA levels were observed in the earlier TMN stages of NPC, particularly for stages I-II, but there was no significant difference (Figure 3A). Significantly higher median SCCA levels were observed in the more advanced stages (IV) of HNSCC (non- NPC) compared with the earlier stages (Oa–I) (Roche p = 0.025; Figure 3B). Higher SCCA levels were observed in the more advanced stages of ESCC, particularly for stages IV (p = 0.001; Figure 3C). A similar situation prevails in LSCC, median SCCA levels in the stages III higher than the earlier stages (I–II) (Roche p = 0.042; Figure 3D). The same phenomenon was also observed in the earlier stages of BC compared with the median stages and advanced stages (Oa vs. I, Abbott p = 0.006, Roche p = 0.028; Oa vs. II–IV, Abbott p = 0.031; Figure 3E). Significantly higher median SCCA levels were observed in the more advanced stages (IIB–III) of CSCC compared with the earlier stages (I–IIA)(Abbott p = 0.027, Roche p = 0.047; Figure 3F).
Figure 3.
Box plot of serum SCCA levels in 6 kinds of squamous cell carcinoma: relation to clinical stage (TNM). The bottoms and tops of the boxes and the bottom ends of the lower whiskers and top ends of the upper whiskers represent the 25th, 75th, 10th, and 90th percentiles, respectively. The transverse lines in the boxes are the median values.
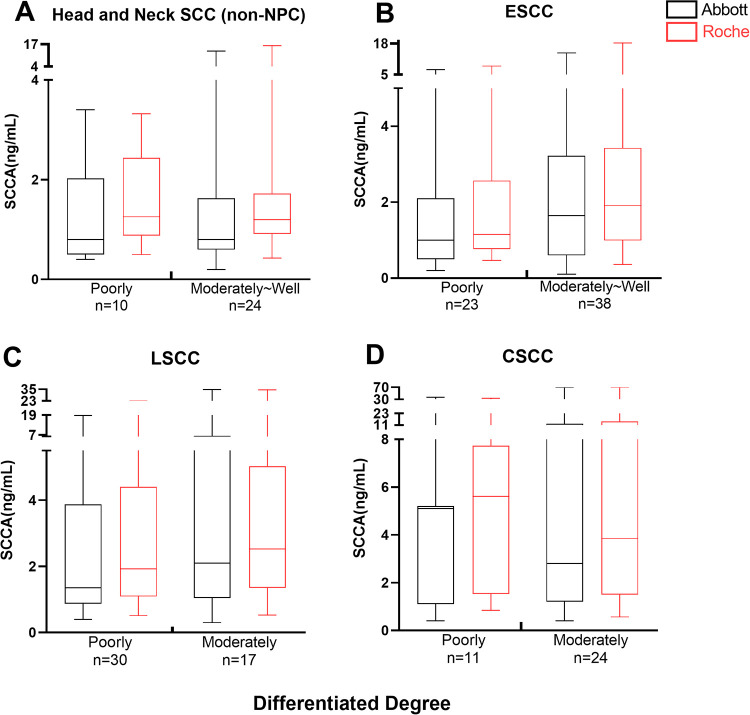
Serum SCCA Concentration and Differentiation of Tumors
The median SCCA level in the moderately and well differentiated were generally higher than those in the poorly differentiated in HNSCC (non-NPC), ESCC and LSCC, while higher median SCCA levels were observed in the poorly differentiated of CSCC compared with moderately and well differentiated, but there was no significant difference in these median values (p > 0.05)(Figure 4).
Figure 4.
Box plot of serum SCCA levels in 6 kinds of squamous cell carcinoma: relation to differentiated degree. The bottoms and tops of the boxes and the bottom ends of the lower whiskers and top ends of the upper whiskers represent the 25th, 75th, 10th, and 90th percentiles, respectively. The transverse lines in the boxes are the median values.
Discussion
SCCA is a serological marker for SCC of the uterine cervix, lung, head and neck, vulva and esophagus, and has been suggested to be correlated with the clinical stage, outcome and the degree of histological differentiation of the tumor.15-17 At present, few studies of serum SCCA have been reported in various types of pan squamous cell cancer and the difference between different detect system remains to be clarified. Although Holdenrieder et al study13 evaluated the clinical value of biomarker for the differential diagnosis of squamous cell carcinoma of cervical, lung, head and neck, this study did not analyze other squamous cell carcinomas. In the present study, Abbott I2000 and Roche e 602 detection systems were the first time compared among various squamous cell carcinomas, including squamous cell carcinoma of NPC, head and neck, esophageal, lung, bladder and cervical.
As a first step, we evaluated the method’s precision using the controls provided by the manufacturer. The precision studies showed the accuracy of both instruments with the control values within the specified range (<10%). But the Roche system gave more precise results, whether in within-run precision or total precision (Table 1).
To evaluate whether there were differences between this 2 systems, 435 serum samples included pan squamous cell cancer (n = 318) and controls (n = 117) were measured. Despite the detection methods and reagents of the 2 systems are different, the results showed a good correlation between the data produced using the Abbott I2000 and Roche e 602. It is worth noting that the Roche system detected higher SCCA concentration values than the Abbott system in the same sample. This factor should be taken into consideration when comparing results from these 2 systems, especially when it is monitoring of treatment progress. For convenience, this study was provided a simple conversion formula (Y = 0.749X+0.2434) for result between these 2 systems.
To further evaluate diagnostic performance of SCCA in different squamous carcinoma, ROC curves were plotted to test and compare the performance of 2 systems. The AUC area of Abbott system was higher than Roche system in NPC and LSCC (p<0.05). When using Abbott system as SCCA test in LSCC, the sensitivity, specificity and AUC were better in our study. Previous studies have shown that SCCA level in patients with head and neck cancer is increased,18,19 and higher in patients with severe disease.20,21 Our study also found that the median SCCA level in patients with advanced stage (IV) HNSCC (non-NPC) was significantly higher than that in patients with earlier stage (Oa-I), which was consistent with VIVIAN’s study’s prediction.21 However, there was no significant correlation between SCCA level and TNM staging in NPC, which may be caused by the fact that there were fewer stage I-II specimens in NPC. The ability of SCCA to discriminate HNSCC from apparently healthy patients was un-satisfactory (Table 2). These results suggest that the SCCA as a single marker is unsuitable for screening of head and neck cancer.
Although the specificity of SCCA (90.38% -94.23%) was better for the ESCC, the sensitivity(46.67%-48.00%) remained insufficient. Our findings are similar to those of Nakamura et al. and Kosugi et al, who found a significant correlation between serum SCCA level and TNM stage in patients with esophageal cancer.22,23 Higher SCCA levels were observed in the more advanced stages of ESCC, particularly for stages IV. Abnormally elevated serum SCC antigen levels may be an effective predictor of advanced ESCC. Thus, serum SCCA level can be used as one of the indicators for ESCC diagnosis and differentiation of ESCC stages.
In LSCC, SCCA’s ability to distinguish SCC from apparently healthy was well, with 64.15% and 58.49% sensitivity, 84.62% and 90.38% specificity, respectively. Holdenrieder et al. found that SCCA levels are high in advanced LSCC, especially stage III–IV.13 Our results showed that SCCA levels were higher in the median stage (III) of LSCC compared with the earlier stages (I–II) (P<0.05), but decreased in the stage IV (P > 0.05), which may be due to the small number of the individuals in the stage IV (n = 9).
Patients with CSCC had significantly higher levels of SCCA than healthy people, as has been seen previously.24 In terms of the use of SCCA as a differential diagnostic tool, CSCC had the most obvious results, with AUC > 0.77, 65.12% and 62.79% sensitivity, 90.38% specificity, respectively. Our study found that significantly higher median SCCA levels were observed in the more advanced stages (IIB–III) of CSCC compared with the earlier stages (I–IIA), which is consistent with previous studies that found significant differences between IIA and IIB.13,24-26 Therefore, SCCA could also be a sensitive tool for the differential diagnosis of CSCC.
So far there is no study evaluating an association between serum SCCA concentration and BC. BC exists as several distinct subtypes, including urothelial carcinoma (UCa), squamous cell carcinoma (SCCa), adenocarcinoma and small cell carcinoma. Ehdaie B’s study showed that SCC was associated with worse clinical outcomes compared with urothelial carcinoma after adjusting for stage and other prognostic factors.27 In our study, the AUC area of BC was more than 0.5, the sensitivity was 25.00% and 34.62%, the specificity was 92.31% and 86.54%. In addition, lower median SCCA levels were observed in the stage Oa of BC compared with stages I and stages II-IV. These results suggested that in BC, SCCA may also be a potential tumor marker for detecting and monitoring the course of the disease when used in conjunction with other biomarkers.
To sum up, there are some inconsistencies between serum SCCA detected by 2 systems in different tumors and stages. The use of different methods may produce individual differences. Possible causes may be due to the different characteristics of the antibody, their titers, affinity and specificity.28-30 We speculate that these different results might be caused by the different components of SCCA1 and SCCA2. This is also one of the necessities in this analysis. The results in Ryuji et al. are also reflected our views. The serum SCCA2 level was significantly higher in the HNSCC patients than in control group, whereas there were no significant differences in the serum SCCA1 level.8 The Elecsys SCC is the method to detect SCCA1 and SCCA2 human isoforms in an equimolar manner.13 However, the proportion of SCCA1 and SCCA2 in Abbott is unknown. In addition, Kawaguchi et al. found no correlation between serum SCC antigen levels and TNM stage,31 which was inconsistent with our findings and Nakamura et al. and Kosugi et al.22,23 One of the reasons may be that the study using radioimmunoassay, and the subtypes SCCA is unknown.
Conclusion
In summary, this study is the first comparative study of 2 systems to detect SCCA in the differential diagnosis of nasopharyngeal, head and neck, esophageal, lung, bladder and neck SCC. Our results shown a good correlation between Roche and Abbott in SCCA detection, but Abbott system is more suitable for LSCC screening. In the future, it will be better to separate different subtypes of SCCA detection reagent and use in different tumor detection. In addition, serum SCCA were first applied for BC patients and confirmed to be a potential serum tumor marker.
Acknowledgments
We thank the following: all members of the Guangdong Provincial Anticancer Association and Southern China Tumor Markers Standardization Alliance for their support and the patients who participated in this study.
Abbreviations
- AUC
Area Under The Curve
- BC
Bladder cancer
- CSCC
Cervical Squamous Cell Carcinoma
- ESCC
Esophageal Squamous Cell Carcinoma
- HNSCC
Head And Neck Squamous Cell Carcinomas (non-NPC)
- LSCC
Lung Squamous Cell Carcinoma
- NPC
Nasopharyngeal Carcinoma
- ROC
receiver operating characteristic
- SCC
Squamous cell carcinoma
- SCCA
Squamous cell carcinoma antigen
Authors’ Note: Hao Chen and Liru Tian and Jiahong Chen contributed equally to this article. This study was approved by the Institutional Review Committee of Sun Yat-sen University Cancer Center. All patients provided written-informed consent for the collection and publication of medical information at the first visit to our center, which was recorded in their medical records and approved by the Ethics Committee. The authenticity of this article has been verified by uploading the key raw data onto the Research Data Deposit public platform (www.researchdata.org.cn), with the approval RDD number as RDDA2020001686.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Natural Science Foundation of Guangdong Province (2018A030313622).
ORCID iD: Xingping Wu, BM  https://orcid.org/0000-0003-4922-1173
https://orcid.org/0000-0003-4922-1173
References
- 1.Izuhara K, Ohta S, Kanaji S, Shiraishi H, Arima K. Recent progress in understanding the diversity of the human ov-serpin/clade B serpin family. Cell Mol Life Sci. 2008;65(16):2541–2553. doi:10.1007/s00018-008-8049-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao W, Yu H, Han Z, Gao N, Xue J, Wang Y.Clinical significance of joint detection of serum CEA, SCCA, and bFGF in the diagnosis of lung cancer. Int J Clin Exp Pathol. 2015;8(8):9506–9511. [PMC free article] [PubMed] [Google Scholar]
- 3.Markovina S, Wang S, Henke LE, et al. Serum squamous cell carcinoma antigen as an early indicator of response during therapy of cervical cancer. Br J Cancer. 2018;118(1):72–78. doi:10.1038/bjc.2017.390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee JK, Hsieh JF, Tsai SC, Ho YJ, Sun SS, Kao CH. Comparison of CYFRA 21-1 and squamous cell carcinoma antigen in detecting nasopharyngeal carcinoma. Ann Otol Rhinol Laryngol. 2001;110(8):775–778. doi:10.1177/000348940111000814 [DOI] [PubMed] [Google Scholar]
- 5.Zheng X, Xing S, Liu XM, et al. Establishment of using serum YKL-40 and SCCA in combination for the diagnosis of patients with esophageal squamous cell carcinoma. BMC Cancer. 2014;14:490. doi:10.1186/1471-2407-14-490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Izuhara K, Yamaguchi Y, Ohta S, et al. Squamous cell carcinoma antigen 2 (SCCA2, SERPINB4): an emerging biomarker for skin inflammatory diseases. Int J Mol Sci. 2018;19(4). doi:10.3390/ijms19041102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cataltepe S, Gornstein ER, Schick C, et al. Co-expression of the squamous cell carcinoma antigens 1 and 2 in normal adult human tissues and squamous cell carcinomas. J Histochem Cytochem. 2000;48(1):113–122. doi:10.1177/002215540004800112 [DOI] [PubMed] [Google Scholar]
- 8.Yasumatsu R, Nakano T, Hashimoto K, Kogo R, Wakasaki T, Nakagawa T. The clinical value of serum squamous cell carcinoma antigens 1 and 2 in head and neck squamous cell carcinoma. Auris Nasus Larynx. 2019;46(1):135–140. doi:10.1016/j.anl.2018.07.010 [DOI] [PubMed] [Google Scholar]
- 9.Stenman J, Hedstrom J, Grenman R, et al. Relative levels of SCCA2 and SCCA1 mRNA in primary tumors predicts recurrent disease in squamous cell cancer of the head and neck. Int J Cancer. 2001;95(1):39–43. doi:10.1002/1097-0215(20010120)95:1<39::aid-ijc1007>3.0.co;2-n [DOI] [PubMed] [Google Scholar]
- 10.Hsu KF, Huang SC, Shiau AL, et al. Increased expression level of squamous cell carcinoma antigen 2 and 1 ratio is associated with poor prognosis in early-stage uterine cervical cancer. Int J Gynecol Cancer. 2007;17(1):174–181. doi:10.1111/j.1525-1438.2006.00663.x [DOI] [PubMed] [Google Scholar]
- 11.Takeda A, Kajiya A, Iwasawa A, Nakamura Y, Hibino T. Aberrant expression of serpin squamous cell carcinoma antigen 2 in human tumor tissues and cell lines: evidence of protection from tumor necrosis factor-mediated apoptosis. Biol Chem. 2002;383(7-8):1231–1236. doi:10.1515/BC.2002.136 [DOI] [PubMed] [Google Scholar]
- 12.Passerini R, Cassatella MC, Boveri S, et al. The pitfalls of CA19-9: routine testing and comparison of two automated immunoassays in a reference oncology center. Am J Clin Pathol. 2012;138(2):281–287. doi:10.1309/AJCPOPNPLLCYR07 H [DOI] [PubMed] [Google Scholar]
- 13.Holdenrieder S, Molina R, Qiu L, et al. Technical and clinical performance of a new assay to detect squamous cell carcinoma antigen levels for the differential diagnosis of cervical, lung, and head and neck cancer. Tumour Biol. 2018;40(4):1010428318772202. doi:10.1177/1010428318772202 [DOI] [PubMed] [Google Scholar]
- 14.Wu X, Chao Y, Wan Z, et al. A comparative evaluation of the analytical performances of Capillarys 2 Flex Piercing, Tosoh HLC-723 G8, Premier Hb9210, and Roche Cobas c501 Tina-quant Gen 2 analyzers for HbA1c determination. Biochem Med (Zagreb). 2016;26(3):353–364. doi:10.11613/BM.2016.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Snyderman CH, D’Amico F, Wagner R, Eibling DE. A reappraisal of the squamous cell carcinoma antigen as a tumor marker in head and neck cancer. Arch Otolaryngol Head Neck Surg. 1995;121(11):1294–1297. doi:10.1001/archotol.1995.01890110068012 [DOI] [PubMed] [Google Scholar]
- 16.de Bruijn HW, Duk JM, van der Zee AG, et al. The clinical value of squamous cell carcinoma antigen in cancer of the uterine cervix. Tumour Biol. 1998;19(6):505–516. doi:10.1159/000030044 [DOI] [PubMed] [Google Scholar]
- 17.Kato H. Squamous cell carcinoma antigen. In: Sell S, ed. Serological Cancer Markers. 1992;(11):437–451. doi:10.1007/978-1-4612-0401-5_21 [Google Scholar]
- 18.Imai R, Takenaka Y, Yasui T, et al. Prognostic significance of serum squamous cell carcinoma antigen in patients with head and neck cancer. Acta Otolaryngol. 2015;135(3):295–301. doi:10.3109/00016489.2014.951454 [DOI] [PubMed] [Google Scholar]
- 19.Koch T, Eiffert H, Spindler MB. Relevance of the new tumor marker SCC (squamous cell carcinoma antigen) for the diagnosis and follow-up control of squamous epithelial carcinoma of the head and neck [in German]. HNO. 1989;37(11):454–459. Die Relevanz des neuen Tumormarkers SCC (Squamous Cell Carcinoma Antigen) fur die Diagnostik und Verlaufskontrolle von Plattenepithelkarzinomen im Kopf-Hals-Bereich. [PubMed] [Google Scholar]
- 20.Molina R, Filella X, Torres MD, et al. SCC antigen measured in malignant and nonmalignant diseases. Clin Chem. 1990;36(2):251–254. [PubMed] [Google Scholar]
- 21.Barak V, Meirovitz A, Leibovici V, et al. The diagnostic and prognostic value of tumor markers (CEA, SCC, CYFRA 21 -1, TPS) in head and neck cancer patients. Anticancer Res. 2015;35(10):5519–5524. [PubMed] [Google Scholar]
- 22.Kosugi S, Nishimaki T, Kanda T, Nakagawa S, Ohashi M, Hatakeyama K. Clinical significance of serum carcinoembryonic antigen, carbohydrate antigen 19-9, and squamous cell carcinoma antigen levels in esophageal cancer patients. World J Surg. 2004;28(7):680–685. doi:10.1007/s00268-004-6865-y [DOI] [PubMed] [Google Scholar]
- 23.Nakamura T, Ide H, Eguchi R, Hayashi K, Takasaki K, Watanabe S. CYFRA 21-1 as a tumor marker for squamous cell carcinoma of the esophagus. Dis Esophagus. 2017;11(1):35–39. doi:10.1093/dote/11.1.35 [DOI] [PubMed] [Google Scholar]
- 24.Gadducci A, Tana R, Cosio S, Genazzani AR. The serum assay of tumour markers in the prognostic evaluation, treatment monitoring and follow-up of patients with cervical cancer: a review of the literature. Crit Rev Oncol Hematol. 2008;66(1):10–20. doi:10.1016/j.critrevonc.2007.09.002 [DOI] [PubMed] [Google Scholar]
- 25.Sturgeon CM, Duffy MJ, Hofmann BR, et al. National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines for use of tumor markers in liver, bladder, cervical, and gastric cancers. Clin Chem. 2010;56(6):e1–48. doi:10.1373/clinchem.2009.133124 [DOI] [PubMed] [Google Scholar]
- 26.Yoon SM, Shin KH, Kim JY, et al. The clinical values of squamous cell carcinoma antigen and carcinoembryonic antigen in patients with cervical cancer treated with concurrent chemoradiotherapy. Int J Gynecol Cancer. 2007;17(4):872–878. doi:10.1111/j.1525-1438.2007.00878.x [DOI] [PubMed] [Google Scholar]
- 27.Ehdaie B, Maschino A, Shariat SF, et al. Comparative outcomes of pure squamous cell carcinoma and urothelial carcinoma with squamous differentiation in patients treated with radical cystectomy. J Urol. 2012;187(1):74–79. doi:10.1016/j.juro.2011.09.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cole LA, Kardana A. Discordant results in human chorionic gonadotropin assays. Clin Chem. 1992;38(2):263–270. [PubMed] [Google Scholar]
- 29.Franek M. Structural aspects of steroid-antibody specificity. J Steroid Biochem. 1987;28(1):95–108. doi:10.1016/0022-4731(87)90130-0 [DOI] [PubMed] [Google Scholar]
- 30.O’Connor T, Gosling JP. The dependence of radioimmunoassay detection limits on antibody affinity. J Immunol Methods. 1997;208(2):181–189. doi:10.1016/s0022-1759(97)00148-8 [DOI] [PubMed] [Google Scholar]
- 31.Kawaguchi H, Ohno S, Miyazaki M, et al. CYFRA 21 -1 determination in patients with esophageal squamous cell carcinoma: clinical utility for detection of recurrences. Cancer. 2000;89(7):1413–1417. doi:10.1002/1097-0142(20001001)89:7<1413::aid-cncr1>3.0.co;2-i [DOI] [PubMed] [Google Scholar]