Abstract
Introduction:
Cryotherapy is a cold-based ablative therapy used primarily as second line therapy in patients with Barrett’s esophagus (BE) who have persistent dysplasia after undergoing endoscopic treatment with radiofrequency ablation (RFA). Few studies have described the use of cryotherapy as a primary treatment modality for dysplastic or neoplastic BE.
Aim:
To evaluate the efficacy of cryotherapy as primary treatment of dysplastic and/or neoplastic BE by conducting a systemic review and meta-analysis.
Methods:
A systematic search of Medline, Embase, and Web of Science was performed from January 2000 through March 2020. Articles included were observational studies and clinical trials which included patients who had biopsy confirmed dysplastic or neoplastic BE (i.e., high grade dysplasia (HGD), low grade dysplasia (LGD) or intramucosal adenocarcinoma (ImCA)), underwent ≥1 session of cryotherapy, and had a follow-up endoscopy. Primary outcomes were pooled proportions of patients achieving complete eradication of dysplasia (CE-D) and/or intestinal metaplasia (CE-IM) by using a random effects model.
Results:
Fourteen studies making up 405 patients with follow-up ranging from 3-54 months were included. In 13 studies, a total of 321/405 patients achieved CE-D with a pooled proportion of 84.8% (95% confidence interval [CI] 72.2-94.4), with substantial heterogeneity (I2 = 88.3%). In 13 studies, a total of 321/405 patients achieved CE-D with a pooled proportion of 84.8% (95% confidence interval [CI] 72.2-94.4), with substantial heterogeneity (I2 = 88.3%). Subgroup analysis of only high-quality studies revealed a pooled proportion of CE-D 91.3% (95% CI, 83.0-97.4, I2 = 69.5%) and pooled proportion of CE-IM of 71.6% (95% CI, 59.0-82.9, I2 = 80.9%). Adverse events were reported in 12.2% patients.
Conclusion:
Cryotherapy is a safe and effective primary therapy for dysplastic/early neoplastic BE. CE-D and CE-IM rates are comparable to those for other ablation modalities, including RFA. Cryotherapy should be considered for primary therapy of dysplastic BE and early esophageal neoplasia.
Introduction
Barrett’s esophagus (BE) is a histologic diagnosis referring to metaplastic columnar epithelium which replaces the stratified squamous mucosa that normally lines the distal esophagus.1,2 Proposed pathophysiology is chronic esophageal injury, mediated at least in part by gastric contents in gastroesophageal reflux disease (GERD), causing the damaged squamous cells to be replaced by mucus-secreting columnar cells, with contribution from genetic and other risk factors.2 BE is the strongest known risk factor for development of esophageal adenocarcinoma (EAC).3 Studies have proposed a multistep pathway, the metaplasia-dysplasia-adenocarcinoma sequence, for the development of esophageal cancer.4
The increasing incidence of EAC over the last few decades is worrisome. This rise, particularly among the Caucasian population in the US, has been estimated to have increased at least 6 folds over the last 3-4 decades.5 These numbers have also been paralleled by an increase in disease related mortality.5 EAC generally has a poor prognosis given the late onset of symptoms and rapid progression of the tumor. Unfortunately, despite increasing efforts to improve surveillance, diagnosis, and treatment, the overall 5-year survival remains less than 15-20%.5,6 These statistics further re-enforce the need to identify risk factors for BE and the need for early endoscopic detection, followed by tailored surveillance strategies for early detection of dysplasia or EAC. Patients with EAC limited to the mucosa (T1a) appear to have better outcomes with both endoscopic and surgical treatment, with 5-year survival rates greater than 80%.7 Endoscopic ablation techniques are frequently used to eliminate dysplastic BE and therefore decrease the risk of progression to EAC.8,9
Endoscopic treatment modalities in use today include multipolar electrocoagulation (MPEC), argon plasma coagulation (APC), radiofrequency ablation (RFA), and cryotherapy. Currently, the most commonly utilized first-line treatment is RFA, with studies reporting CE-D to be as high as 90.5-95% in LGD1,10 and 81% in HGD.1 In the last decade, endoscopic cryotherapy has emerged as yet another ablative technique,11 first reported for endoscopic use with a liquid nitrogen spray based device in 2005.12 Another device using compressed carbon dioxide has proven to be effective therapy for elimination or treatment of Barrett’s neoplasia,13,14 and a third most recently developed system utilizing a nitrous-oxide based (the cryo-balloon) ablation system has also demonstrated promising results.15,16
A meta-analysis of 11 studies suggested cryotherapy as a safe and efficacious second-line therapy for patients who were previously treated with RFA and have persistent dysplasia or intestinal metaplasia (IM).17 The study reported that cryotherapy was able to successfully achieve CE-IM and CE-D in approximately 50% and 75% of the patients, respectively.17 A small number of studies have evaluated the use of cryotherapy as a primary treatment modality, given its remarkable safety profile. One study utilizing cryoablation treatment reported CE-IM of 41% (p = 0.02) and CE-D of 79% (p = 0.15), with as high as 88% of patients with HGD achieving CE-D (p = 0.99).18 A recent meta-analysis of 6 studies found the efficacy of cryotherapy as a first line therapy to be 69% for CE-IM and 97% for CE-D. However, the meta-analysis only included full text studies and inadvertently missed some key studies. Based on high reported rates of CE-IM and CE-D with cryotherapy, and its encouraging safety profile, we aimed to perform an updated systematic review and meta-analysis to evaluate the efficacy of cryotherapy as primary treatment for BE.
Methods
All procedures used in this meta-analysis were consistent with the PRISMA (Meta-analysis Of Observational Studies in Epidemiology) criteria for observational studies.19
Selection Criteria
The studies considered in this meta-analysis were case-control studies, cohort studies, or clinical trials of use of cryotherapy for BE. Only the studies that included biopsy confirmed dysplastic or neoplastic BE (LGD, HGD, ImCA) who underwent ≥1 session of cryotherapy (either liquid nitrogen, carbon dioxide gas, or balloon-based or focal liquid nitrous oxide) with follow-up endoscopy were included. We included patients with initial treatment for ImCA with endoscopic mucosal resection (EMR). We excluded patients that were treated with RFA, MPEC, APC, chemoradiation or other ablative therapies prior to cryotherapy. We also excluded patients that were treated for non-dysplastic BE. Studies with published full text or abstract form were included to prevent reporting bias. If more than 1 publication from a study or institution was identified, the most recent publication with relevant information was included for meta-analysis to avoid duplicate data.
Data Sources and Search Strategy
We conducted a comprehensive search of Ovid MEDLINE In-Process & Other Non-Indexed Citations, Ovid MEDLINE, Ovid Embase, Ovid Cochrane Central Register of Controlled Trials, Ovid Cochrane Database of Systematic Reviews, Web of Science, and Scopus from January 2000 to March 2020. The search strategy was designed and conducted by study investigators (R.T. and V.K.) and experienced library staff, independently. The search was limited to studies in the English language. Controlled vocabulary supplemented with keywords was used to search for studies of cryotherapy use for BE. Main keywords used in the search were the following: barrett,* esophag,* oesophag,* barrett esophagus, esophageal diseases, dysplas,* metaplas,* columnar,* esophagitis, peptic/or esophageal stenosis, cryosurgery,* cryother,* cryoablat,*cryogen,* freeze,* cryo,* cancer*, carcinoma* or adenocarcinoma*. The detailed search strategy is described Online Appendix 1.
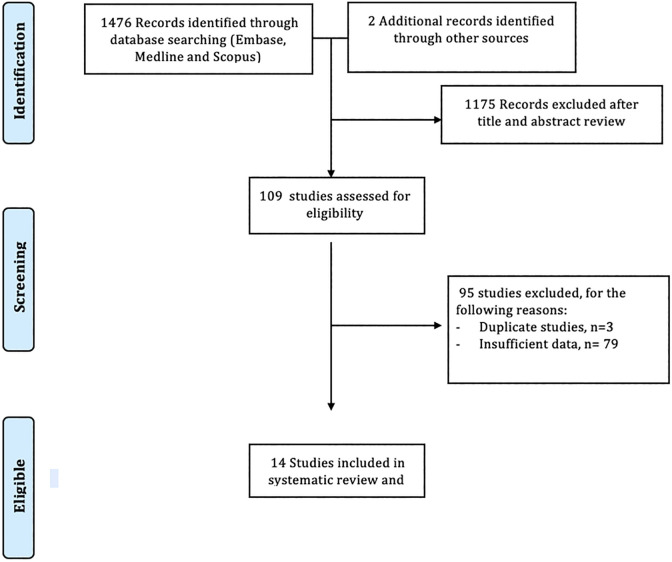
Two authors (R.T. and V.K.) independently reviewed the titles and abstracts of the identified studies, and those that did not answer the research question of interest were excluded. The abstracts and full texts of the remaining articles were reviewed to determine inclusion criteria fulfillment. The reference lists of articles with information on the topic were also reviewed for additional pertinent studies. We then manually searched the abstracts from major gastroenterology conferences from 2000 to 2020. A flow diagram of included studies is shown in Figure 1.
Figure 1.
Flow diagram of study selection process.
The modified version of Newcastle-Ottawa scale was used to assess the methodologic quality of the included studies by 2 investigators (R.T. and M.H.).20,21 Using this scale, studies were accessed by 6 questions (representative of community, cohort size, EMR before cryotherapy, number of cryotherapy sessions, verification of no prior ablative treatment before cryotherapy, adequacy of follow up) (Supplementary Table 1). Studies with a cumulative score >3 were considered high quality. Any discrepancies were addressed by a joint re-evaluation of the original article.
Data Abstraction
Data was independently abstracted to a predetermined collection form by 2 investigators (R.T. and M.H.). Data collected for each study included study setting and design, year of publication, location, patient characteristics, histological pathology before cryotherapy, length of BE segment and follow up period. Conflicts in data abstraction were resolved by consensus, referring to the original article.
Outcomes Assessed
Our primary analysis focused on calculating pooled proportions of patients achieving CE-D and/or CE-IM. We also performed subgroup analyses to calculate pooled proportion of CE-D and CE-IM for full text studies, high quality studies and studies with liquid nitrogen cryotherapy, separately. Adverse events, when reported, were also extracted.
Statistical Analyses
Our primary outcome of the pooled analysis was clinical cure rates. The random-effects model described by DerSimonian and Laird22 was used to calculate weighted pooled resolution rate (WPR). We calculated WPR with corresponding 95% CIs for the overall analysis as well as subgroup analyses. Data was weighted on the basis of sample size in each study to calculate WPR. Freeman-Tukey double arcsine transformation was used to avoid giving more weight to studies with prevalence estimates that are too large or too small. We assessed heterogeneity within groups with the I 2 statistic, which estimates the proportion of total variation across studies that is due to heterogeneity in study patients, design, or interventions rather than chance; I 2 values greater than 50% suggest substantial heterogeneity.23 All P values reported were 2-tailed and considered statistically significant if < 0.05. For all tests (except for heterogeneity), a probability level less than .05 was considered statistically significant. Publication bias was assessed by visual inspection of funnel plots and numerically using LFK (Luis Furuya-Kanamori) estimate on a Doi plot (No asymmetry: LFK index within ±; minor asymmetry: LFK index exceeds ±1 but within ±2; major asymmetry: LFK index exceeds ±2). Publication bias was considered if the given analyses had major asymmetry on the inspection of funnel plots. If publication bias was found on funnel plot, we used the trim and fill for adjusting publication bias.23,24 OpenMetaAnalyst version 10.10 was used for all statistical calculations.25
Results
Search Results
The described search strategy revealed 1478 potentially relevant studies; titles and abstracts were screened and full manuscripts were obtained for relevant articles (Figure 1). In all, 109 articles were reviewed, of which 95 were excluded for various reasons (Figure 1). A total of 14 studies were included in this meta-analysis.13,18,26-37 Of those, 9 were full text articles and 5 were abstracts.
Quality of Included Studies
The quality of included studies is presented in Supplementary table 1. In all, 8 studies were high quality and 6 were low quality. Median NOS score was 3.5 (range 1.5-4.5).
Characteristics of Included Studies
Among the 14 included studies, 6 were retrospective and 8 were prospective. 11 studies were single center and 3 were multicenter. Cryotherapy was most commonly performed using liquid nitrogen (n = 11), however carbon dioxide gas (n = 2) and nitrous oxide balloon treatment (n = 1) were also included. There was a median of 4 cryotherapy sessions per patient. Most patients were older (mean age range 60.5 to 70.9), male, and had long-segment BE (median of 4.6 cm). The median follow-up period was 22.45 months or 1.8 patient years for CE-D (range 3-54 months, Table 1).
Table 1.
Study and Patient Characteristics.
| Study, Publication year | Publication type | Study design, period | Study location | Total number of patients | Number of Males | Mean age | Type of cryotherapy | Median number of cryotherapy sessions | Histology before cryotherapy | Treatments before cryotherapy | Mean BE length | Median follow up |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Canto 2015 |
Full text | Retrospective, SC, 2006-2013 | USA | 20 | 19 | 70.9 | Carbon dioxide | 4 | HGD 18 ImCA 2 |
EMR | 5.9 cm | 54 months |
| Canto 2018 |
Full text | Prospective, SC, 2015-2016 | USA | 22 | 19 | 65.5 | Nitrous oxide | 3 | LGD 7 HGD 11 ImCA 4 |
EMR | Prague C 2.2 | 20.9 months |
| Cheng 2013 |
Abstract | Retrospective, SC, 2010-2012 | USA | 52 | 41 | 66.8 | Liquid nitrogen | 6.3 | LGD 7 HGD 20 imCA 25 |
None | NR | 3 months |
| Eluri 2017 |
Abstract | Prospective, MC, 2013-2017 | USA | 67 | 63 | 68 | Liquid nitrogen | 3 | LGD 15 HGD 37 ImCA 15 |
None | NR | 11 months |
| Goldberg 2012 |
Abstract | Prospective, SC, NR | USA | 13 | 12 | 60.5 | Liquid nitrogen | 4.7 | HGD 4 LGD 5 IND 4 |
None | 3.6 cm | 45 months |
| Gosaine 2013 |
Full text | Prospective, SC, 2006-2009 | USA | 32 | 29 | 60.5 | Liquid nitrogen | 4 | HGD 32 | EMR | 3 cm | 37 months |
| Greenwald 2010 |
Full text | Prospective, MC, 2005-2007 | USA | 24 | NR | 69 | Liquid nitrogen | 4 | HGD 17 ImCA7 |
None | 4 cm | 9 months |
| Halsey 2011 |
Full text | Prospective, SC, 2006-2010 | USA | 36 | 33 | 62 | Liquid nitrogen | 4 | HGD 36 | None | 3 cm | 6.5 months |
| Johnston 2013 |
Abstract | Retrospective, SC, NR | USA | 20 | NR | NR | Liquid nitrogen | 4 | NR | NR | 5 cm | 24 months |
| Ramay 2017 | Full text | Retrospective, SC, 2006-2012 | USA | 50 | 47 | 61.9 | Liquid nitrogen | 3 | NR | EMR | 3.5cm | 36months |
| Trindade 2017 | Full text | Retrospective, MC, 2007-2015 | USA | 27 | 24 | 68 | Liquid Nitrogen | 3 | HGD 22 LGD 5 |
EMR | 3cm | 24 months |
| Thota 2018 |
Full text | Retrospective, SC, 2006-2011 | USA | 81 | 65 | 69.1 | Liquid nitrogen | 3 | LGD 11 HGD 49 ImCA 21 |
EMR | 5.2 cm | 31.8 months |
| Verbeek 2015 |
Full text | Prospective, SC, 2011 | Netherlands | 9 | NR | NR | Carbon dioxide | 2.5 | LGD 5 HGD 1 IM 4 |
EMR | 5 cm | 12 months |
| Wani 2012 |
Abstract | Prospective, SC, NR | USA | 19 | NR | NR | Liquid nitrogen | 4.5 | NR | EMR | 4.6 cm | 3 months |
BE: Barrett’s esophagus; EAC: esophageal adenocarcinoma; ImCA: intramucosal carcinoma; LGD: low grade dysplasia; HGD: high grade dysplasia; IND: indefinite for dysplasia; IM: intestinal metaplasia; EMR: endoscopic mucosal resection; APC: Argon; SC: single center; MC: multicenter; NR: not recorded.
Pooled Rate of CE-D
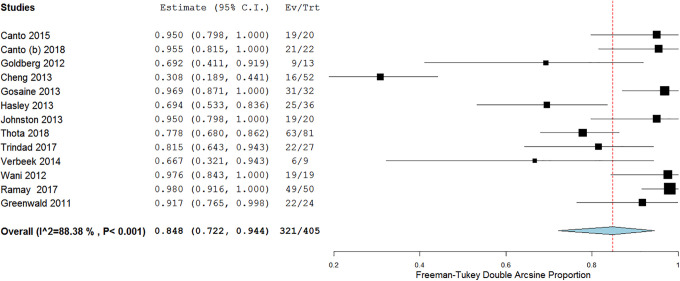
In 13 studies, a total of 321/405 patients achieved CE-D with a pooled proportion of 84.8% (95% confidence interval [CI] 72.2-94.4), with substantial heterogeneity (I 2 = 88.3%) (Figure 2). No publication bias was seen on Doi plot (Supplementary Figure 1).
Figure 2.
Efficacy of cryotherapy as primary treatment for complete eradication of dysplasia.
Pooled Rate of CE-IM
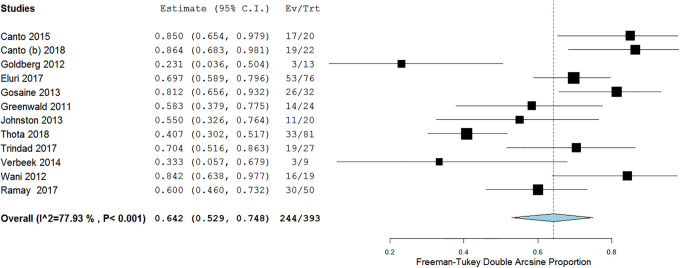
In 12 studies, a total of 244/393 patients achieved CE-IM with a pooled proportion of 64.2% (95% confidence interval [CI] 52.9-74.8), with substantial heterogeneity (I 2 = 77.9%, Figure 3). Mild asymmetry was seen on the doi plot (Supplementary Figure 2).
Figure 3.
Efficacy of cryotherapy as primary treatment for complete eradication of intestinal metaplasia.
Pooled Rate of CE-D With Liquid Nitrogen Cryotherapy
A total of 10 studies reported the rates of CE-D with liquid nitrogen cryotherapy. On meta-analysis, the pooled proportion of CE-D was 83.6% (95% CI, 68.2-94.9, I 2 = 90.58%).
Pooled Rate of CE-IM With Liquid Nitrogen Cryotherapy
A total of 9 studies reported the rates of CE-IM with liquid nitrogen cryotherapy. On meta-analysis, the pooled proportion of CE-IM was 61.4% (95% CI, 49.5-72.8, I 2 = 77.33%).
Pooled Recurrence Rates
A total of 4 studies assessed the rate of recurrence after cryotherapy. Follow-up period was variable among those. Of a total of 85 patients, 15 had recurrence of HGD with pooled rate of 17.6%. Four studies evaluated for recurrence rates of IM. Of those, 41 of 116 patients developed recurrence with a pooled rate of 35.3%.
Pooled Rate of Persistent Intestinal Metaplasia, Dysplasia and Progression to Cancer
A total of 6 studies evaluated the rates of persistent IM and dysplasia. The pooled rate of persistent IM was 13.7% and for persistent dysplasia was 7.3%. A total of 2 studies evaluated for progression of cancer with rate of 3.8% (3/77 patients).
Subgroup Analyses
Full Text Studies
A total of 9 full text studies reported the rates of CE-D. Subgroup analysis of only full text studies revealed a pooled proportion of CE-D 88.4% (95% CI, 79.6-95.2, I 2 = 72.7%).
A total of 8 full text studies reported the rates of CE-IM. Subgroup analysis of only full text studies revealed a pooled proportion of CE-IM 65.9% (95% CI, 51.4-79.1, I 2 = 80%).
High Quality Studies
A total of 8 studies reporting CE-D were considered high quality. Subgroup analysis of only high-quality studies revealed a pooled proportion of CE-D 91.3% (95% CI, 83.0-97.4, I 2 = 69.5%).
A total of 8 studies reporting CE-IM were considered high quality. Subgroup analysis of only high-quality studies revealed a pooled proportion of CE-IM 71.6% (95% CI, 59.0-82.9, I 2 = 80.9%).
Adverse Events
Of the included studies, 11 studies reported individualized adverse events. One study was excluded from the analysis, because the study reported adverse effects in proportion to the number of procedures performed rather than patients.27 The rate of adverse events was 12.2% (45/367). Most common adverse events were esophageal strictures (n = 27) followed by post procedural pain (n = 10). Gastric perforation was reported in 2 patients in the cohort, one following carbon dioxide gas delivery and the other with liquid nitrogen-based spray therapy. Both patients were successfully managed with surgical intervention. Bleeding was reported in 2 patients but the severity of the bleed was not stated. Two patients from the cohort developed esophagitis as a post-procedural side effect (4.4%; 2/45). No deaths related to cryotherapy treatment were reported in any of the studies.
Discussion
Endoscopic ablative therapy is a widely accepted treatment modality for dysplastic BE and/or early esophageal neoplasia. Techniques such as RFA and cryotherapy have been shown to be effective at downgrading dysplasia and reversing IM to neosquamous epithelium in several prior studies. However, cryotherapy has been primarily reported as second line therapy for patients who do not achieve CE-D or CE-IM with RFA. Our primary aim through this systematic review and meta-analysis was to determine the efficacy of cryotherapy as first-line treatment in patients with dysplastic BE and/or early esophageal neoplasia. The reported efficacy of RFA in the literature is comparable to the efficacy of cryotherapy in our study. One meta-analysis of 18 studies with 3802 patients reported CE-D in 91% and CE-IM in 78% with RFA.10 Our analysis of shows that cryotherapy is an effective modality for treatment of dysplastic BE with a pooled rate of achieving CE-D of 84% and a pooled rate of achieving CE-IM of 64%. This suggests cryotherapy may be an effective endoscopic ablative modality for first-line therapy in patients with dysplastic and early neoplastic BE.
There may be several advantages to using cryotherapy over RFA for first line treatment of dysplastic BE and/or early esophageal neoplasia. RFA is technically challenging in patients who have a tortuous esophagus, significant esophageal stricturing or nodular BE,11 whereas cryotherapy is not significantly limited by these anomalies. Cryotherapy penetrates deeper into tissue with less injury to the tissue architecture and subsequently has a lower risk of stricture formation and post-procedural discomfort compared to heat-based ablative modalities.11 Cryotherapy has been successfully performed on patients with bleeding diathesis or anticoagulation.18
The first pilot study for use of cryotherapy in Barrett’s esophagus was reported by Johnston et al in 2005 and included 11 patients who were treated with liquid nitrogen-based spray cryotherapy. Of the 9 patients who completed the study, all achieved CE-IM at the end of treatment.12 Over the years, 2 additional endoscopic cryotherapy platforms have been developed, one utilizing compressed carbon dioxide spray and the other utilizing a nitrous-oxide balloon-based ablation system. Studies utilizing any of these 3 cryotherapy treatment modalities were included in our analysis.
There are important differences between the 3 modalities of cryotherapy, including delivery platform/gas used, freezing temperature, and dosimetry. The most largely represented delivery method in our analysis is liquid nitrogen-based spray cryotherapy (G2 system from 2007 to 2012 and the truFreeze device from 2013 to present; CSA Medical, Lexington, MA). In this system, liquid nitrogen is delivered at −196 °C via a 7F flexible catheter which is introduced through the biopsy channel of a standard endoscope. Several freeze-thaw cycles are performed. A decompression tube is utilized to vent the esophagus and the stomach to reduce the risk of perforation due to the rapid expansion of nitrogen gas. Dosimetry ranges from 20 seconds x 3 applications (with intermittent thawing between sprays) or 30 seconds x 2 applications for flat dysplastic BE.
Subgroup analysis of studies that use liquid nitrogen cryotherapy revealed efficacy of 83% for CE-D and 61% for CE-IM. A recent retrospective analysis published last year compared RFA and liquid nitrogen spray cryotherapy and concluded that patients who received cryotherapy had comparable rates of achieving CE-D (78.8% vs 87.5%, p = 0.15) but lower rate of achieving CE-IM (66.7% vs 41.3%, P = 0.002) compared to RFA.18 Similar rates of achieving CE-D and CE-IM have been shown in prior studies as well. In 2010, Greenwald et al published their results of 17 patients treated with liquid nitrogen-based spray cryotherapy. They reported complete eradication of HGD (CE-HGD), CE-D, and CE-IM of 94%, 88%, and 53%, respectively.27 A larger, multi-center, retrospective cohort which included 60 patients with high grade dysplasia, reported similar findings with 97% of patients having achieved CE-HGD, 87% achieved CE-LGD, and 57% achieved CE-IM.38
Two studies in our meta-analysis used a liquid carbon dioxide-based cryotherapy system (Polar Wand; GI Supply, Camp Hill, PA). This is a through the scope system which utilizes multiple freeze-thaw cycles and also requires continuous gastric decompression. It freezes the mucosa at −80 oC. The catheters for this device were discontinued in March 2016 by the manufacturer and it is no longer in use.
The third method of delivery included in our analysis involves the use of a new contact cryoballoon focal ablation system (C2 Therapeutics, Inc, Redwood City, CA). This is a portable, battery-powered system in which liquid nitrous oxide is converted to gas within a single-use low pressure compliant balloon freezing targeted mucosa to −85 oC. The cryogen can be directed toward the targeted mucosa by rotating the catheter and/or sliding it up and down within the balloon. It is applied as a single application of 6-12 seconds. A decompression tube is not necessary in this system. Canto et al reported their results from 22 treatment naïve patients. CE-HGD, CE-LGD, and CE-IM rates were 100%, 100%, and 86%, respectively,19 which are very comparable to the CE-D and CE-IM rates of RFA.10
There were no significant differences in the calculated rates of CE-D and CE-IM in our subgroup analysis. Our calculated adverse-events rate was 12.2%. Due to limited data on the reported adverse events, we did not attempt to analyze their pooled rates. The most common adverse events reported were esophageal stricture and chest pain. There were no deaths reported in any of the studies.
The strengths of our study include a comprehensive literature review which presents data from all available papers on this topic. Our meta-analysis includes well-defined inclusion criteria (carefully excluding redundant studies), estimation of CE-IM and CE-D rates, with detailed extraction of adverse events and rigorous evaluation of study quality. We excluded all the studies which had included patients who were previously treated with another modality of ablation.
Our study has some limitations. The individual studies included in our meta-analysis varied in several ways including study design, differences in delivery of cryotherapy (liquid nitrogen vs. CO2 vs. nitrous oxide), number of sessions of cryotherapy and follow up period. There is therefore significant heterogeneity but that is to be expected when different technologies are at play over a period of time with different dosimetry and treatment algorithms. Additionally, all the included studies are observational in nature, hence the quality of evidence available from the current body of literature is low.
Conclusion
Cryotherapy is a well-established endoscopic ablative modality which induces tissue injury through mucosal freezing with liquid nitrogen, carbon dioxide gas, or balloon-based nitrous oxide. This systematic review and meta-analysis demonstrates cryotherapy as primary treatment for dysplastic BE and/or early esophageal neoplasia can achieve rates of CE-D and CE-IM similar to those reported for RFA, with an acceptable adverse event profile.
In addition, Cryotherapy is increasingly being used as a “salvage” therapy in patients who do not achieve CE-IM or CE-D with RFA and for palliative endotherapy in patients with locally advanced esophageal cancer. It is also to be noted that Cryotherapy is an effective option for first line treatment, especially in patients with esophageal anatomic challenges that make RFA treatment technically challenging.
In our meta-analysis, we found endoscopic Cryotherapy to be effective for treatment of dysplastic BE and associated early esophageal neoplasia in patients naïve to ablative therapy with >80% achieving CE-D and 63% achieving CE-IM. Therefore, Cryotherapy could also be offered to patients as first line treatment for dysplastic BE and early esophageal neoplasia, when discussing treatment options. Further studies with standardized treatment protocols and long-term follow-up to demonstrate durability will further clarify the role and efficacy of the currently available cryotherapy modalities and how they compare to the non-cryoablation platforms used in BE endotherapy.
Supplemental Material
Supplemental Material, sj-pdf-1-ccx-10.1177_1073274820976668 for Efficacy of Cryotherapy as a Primary Endoscopic Ablation Modality for Dysplastic Barrett’s Esophagus and Early Esophageal Neoplasia: A Systematic Review and Meta-Analysis by Raseen Tariq, Sarah Enslin, Maham Hayat and Vivek Kaul in Cancer Control
Supplemental Material, sj-pdf-2-ccx-10.1177_1073274820976668 for Efficacy of Cryotherapy as a Primary Endoscopic Ablation Modality for Dysplastic Barrett’s Esophagus and Early Esophageal Neoplasia: A Systematic Review and Meta-Analysis by Raseen Tariq, Sarah Enslin, Maham Hayat and Vivek Kaul in Cancer Control
Supplemental Material, sj-pdf-3-ccx-10.1177_1073274820976668 for Efficacy of Cryotherapy as a Primary Endoscopic Ablation Modality for Dysplastic Barrett’s Esophagus and Early Esophageal Neoplasia: A Systematic Review and Meta-Analysis by Raseen Tariq, Sarah Enslin, Maham Hayat and Vivek Kaul in Cancer Control
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Vivek Kaul is a consultant for CSA Medical (Now Steris Corp) and Cook Medical.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Vivek Kaul, MD  https://orcid.org/0000-0002-7978-7517
https://orcid.org/0000-0002-7978-7517
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Spechler S.Barrett esophagus and risk of esophageal cancer: a clinical review. JAMA. 2013;310(6):627–636. [DOI] [PubMed] [Google Scholar]
- 2.Spechler SJ, Souza RF. Barrett’s esophagus. N Engl J Med. 2014;371(9):836–845. [DOI] [PubMed] [Google Scholar]
- 3.Rajendra S, Sharma P. Barrett esophagus and intramucosal esophageal adenocarcinoma. Hematol Oncol Clin North Am. 2017;31(3):409–426. [DOI] [PubMed] [Google Scholar]
- 4.Jankowski JA, Wright NA, Meltzer SJ, et al. Molecular evolution of the metaplasia-dysplasia-adenocarcinoma sequence in the esophagus. Am J Pathol. 1999;154(4):965–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coleman HG, Xie S-H, Lagergren J. The epidemiology of esophageal adenocarcinoma. Gastroenterology. 2018;154(2):390–405. [DOI] [PubMed] [Google Scholar]
- 6.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. [DOI] [PubMed] [Google Scholar]
- 7.Prasad GA, Wu TT, Wigle DA, et al. Endoscopic and surgical treatment of mucosal (T1a) esophageal adenocarcinoma in Barrett’s esophagus. Gastroenterology. 2009;137(3):815–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lagergren J, Smyth E, Cunningham D, et al. Oesophageal cancer. Lancet. 2017;390(10110):2383–2396. [DOI] [PubMed] [Google Scholar]
- 9.Kroep S, Heberle CR, Curtius K, et al. Radiofrequency ablation of Barrett’s esophagus reduces esophageal adenocarcinoma incidence and mortality in a comparative modeling analysis. Clin Gastroenterol Hepatol. 2017;15(9):1471–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Orman ES, Li N, Shaheen NJ. Efficacy and durability of radiofrequency ablation for Barrett’s esophagus: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2013;11(10):1245–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lal P, Thota PN. Cryotherapy in the management of premalignant and malignant conditions of the esophagus. World J Gastroenterol. 2018;24(43):4862–4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnston MH, Eastone JA, Horwhat JD, Cartledge J, Mathews JS, Foggy JR. Cryoablation of Barrett’s esophagus: a pilot study. Gastrointest Endosc. 2005;62(6):842–848. [DOI] [PubMed] [Google Scholar]
- 13.Canto MI, Shin EJ, Khashab MA, et al. Safety and efficacy of carbon dioxide cryotherapy for treatment of neoplastic Barrett’s esophagus. Endoscopy. 2015;47(7):582–591. [DOI] [PubMed] [Google Scholar]
- 14.Xue HB, Tan HH, Liu WZ, et al. A pilot study of endoscopic spray cryotherapy by pressurized carbon dioxide gas for Barrett’s esophagus. Endoscopy. 2011;43(5):379–385. [DOI] [PubMed] [Google Scholar]
- 15.Committee AT, Parsi MA, Trindade AJ, et al. Cryotherapy in gastrointestinal endoscopy. VideoGIE. 2017;2(5):89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scholvinck DW, Friedland S, Triadafilopoulos G, et al. Balloon-based esophageal cryoablation with a novel focal ablation device: dose-finding and safety in porcine and human models. Dis Esophagus. 2017;30(11):1–8. [DOI] [PubMed] [Google Scholar]
- 17.Visrodia K, Zakko L, Singh S, Leggett CL, Iyer PG, Wang KK. Cryotherapy for persistent Barrett’s esophagus after radiofrequency ablation: a systematic review and meta-analysis. Gastrointest Endosc. 2018;87(6):1396–1404.e1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thota PN, Arora Z, Dumot JA, et al. Cryotherapy and radiofrequency ablation for eradication of Barrett’s esophagus with dysplasia or intramucosal cancer. Dig Dis Sci. 2018;63(5):1311–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. [DOI] [PubMed] [Google Scholar]
- 20.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Accessed May 5, 2012. http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm
- 21.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. [DOI] [PubMed] [Google Scholar]
- 22.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. [DOI] [PubMed] [Google Scholar]
- 23.Easterbrook PJ, Berlin JA, Gopalan R, Matthews DR. Publication bias in clinical research. Lancet. 1991;337(8746):867–872. [DOI] [PubMed] [Google Scholar]
- 24.Duval S, Tweedie R.Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–463. [DOI] [PubMed] [Google Scholar]
- 25.Wallace BC, Dahabreh IJ, Trikalinos TA, Lau J, Trow P, Schmid CH. Closing the gap between methodologists and end-users: R as a computational back-end. J Stat Softw. 2012;49(5):1–15. [Google Scholar]
- 26.Canto MI, Shaheen NJ, Almario JA, Voltaggio L, Montgomery E, Lightdale CJ. Multifocal nitrous oxide cryoballoon ablation with or without EMR for treatment of neoplastic Barrett’s esophagus (with video). Gastrointest Endosc. 2018;88(3):438–446.e432. [DOI] [PubMed] [Google Scholar]
- 27.Greenwald BD, Dumot JA, Horwhat JD, Lightdale CJ, Abrams JA. Safety, tolerability, and efficacy of endoscopic low-pressure liquid nitrogen spray cryotherapy in the esophagus. Dis Esophagus. 2010;23(1):13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheng J, Bouhaidar D, Chambers K, Willingham R, Zfass A. Cryoablation therapy for Barrett’s esophagus and esophageal cancer: a single center experience: 52. Am J Gastroenterol. 2013;108:S19. [Google Scholar]
- 29.Eluri S, Cotton CC, Kaul V, Joshi V, Hoffman BJ, Shaheen NJ. Tu1166 liquid nitrogen spray cryotherapy effectively eradicates Barrett’s esophagus irrespective of severity of baseline histology: results of a US Multicenter Registry. Gastrointest Endosc. 2017;85(5):AB564–AB565. [Google Scholar]
- 30.Goldberg ME, Horwhat D, Cash BD. Tu1609 long-term analysis of outcomes associated with endoscopic spray cryotherapy for Barrett’s esophagus with and without dysplasia. Gastrointest Endosc . 2012;75(4): AB463. [Google Scholar]
- 31.Gosain S, Mercer K, Twaddell WS, Uradomo L, Greenwald BD. Liquid nitrogen spray cryotherapy in Barrett’s esophagus with high-grade dysplasia: long-term results. Gastrointest Endosc. 2013;78(2):260–265. [DOI] [PubMed] [Google Scholar]
- 32.Halsey K, Chang J, Waldt A, Greenwald BD. Recurrent disease following endoscopic ablation of Barrett’s high-grade dysplasia with spray cryotherapy. Endoscopy. 2011;43(10):844–848. [DOI] [PubMed] [Google Scholar]
- 33.Johnston MH. Sa1388 low pressure cryospray ablation of high grade dysplasia in Barrett’s esophagus: 2 year follow-up. Gastrointest Endosc. 2013;77(5): AB187. [Google Scholar]
- 34.Verbeek RE, Vleggaar FP, Fiebo J, van Baal JW. Cryospray ablation using pressurized CO2 for ablation of Barrett’s esophagus with early neoplasia: early termination of a prospective series. Endosc Int Open. 2015;3(02): E107–E112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wani S, Heif M, Fukami N. Tu1591 efficacy of endoscopic spray cryotherapy with Endoscopic Mucosal Resection (EMR) or Submucosal Dissection (ESD) in patients with Barrett’s esophagus (BE) related neoplasia. Gastrointest Endosc. 2012;75(4): AB457. [Google Scholar]
- 36.Ramay FH, Cui Q, Greenwald BD. Outcomes after liquid nitrogen spray cryotherapy in Barrett’s esophagus-associated high-grade dysplasia and intramucosal adenocarcinoma: 5-year follow-up. Gastrointest Endosc. 2017;86(4):626–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trindade AJ, Pleskow DK, Sengupta N, et al. Efficacy of liquid nitrogen cryotherapy for Barrett’s esophagus after endoscopic resection of intramucosal cancer: a multicenter study. J Gastroenterol Hepatol. 2018;33(2):461–465. [DOI] [PubMed] [Google Scholar]
- 38.Shaheen NJ, Greenwald BD, Peery AF, et al. Safety and efficacy of endoscopic spray cryotherapy for Barrett’s esophagus with high-grade dysplasia. Gastrointest Endosc. 2010;71(4):680–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-pdf-1-ccx-10.1177_1073274820976668 for Efficacy of Cryotherapy as a Primary Endoscopic Ablation Modality for Dysplastic Barrett’s Esophagus and Early Esophageal Neoplasia: A Systematic Review and Meta-Analysis by Raseen Tariq, Sarah Enslin, Maham Hayat and Vivek Kaul in Cancer Control
Supplemental Material, sj-pdf-2-ccx-10.1177_1073274820976668 for Efficacy of Cryotherapy as a Primary Endoscopic Ablation Modality for Dysplastic Barrett’s Esophagus and Early Esophageal Neoplasia: A Systematic Review and Meta-Analysis by Raseen Tariq, Sarah Enslin, Maham Hayat and Vivek Kaul in Cancer Control
Supplemental Material, sj-pdf-3-ccx-10.1177_1073274820976668 for Efficacy of Cryotherapy as a Primary Endoscopic Ablation Modality for Dysplastic Barrett’s Esophagus and Early Esophageal Neoplasia: A Systematic Review and Meta-Analysis by Raseen Tariq, Sarah Enslin, Maham Hayat and Vivek Kaul in Cancer Control