Abstract
Objectives:
Numerous studies have suggested that an increase in neutrophil-to-lymphocyte ratio (NLR) before treatment is associated with worse survival in pancreatic adenocarcinoma (PAC). The aim of this study was to investigate the prognostic value of treatment-induced NLR change among PAC patients so as to better identify the characteristics of those who can benefit more from treatment.
Methods:
This meta-analysis was undertaken using the PRISMA statement. Previously published studies between the correlation of NLR change and patients’ survival were searched in Pubmed, Embase, and Web of Science databases. RevMan 5.3 was used to conduct statistical analysis.
Results:
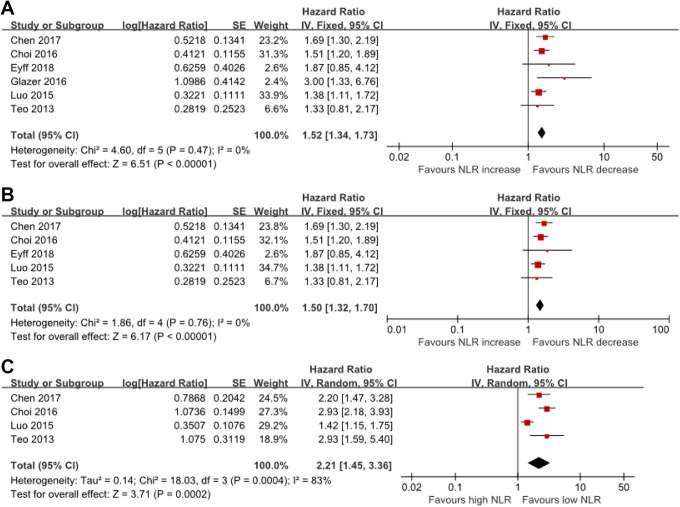
A total of 1213 patients with PAC from 6 retrospective studies were included in this meta-analysis. Four studies investigated the HR of pre-treatment NLR, demonstrating its prognostic impact on overall survival (OS) (HR = 2.21, 95%CI: 1.45-3.36). One study reported that an elevated post-treatment NLR was associated with poorer OS (HR = 1.28, 95%CI = 1.08-1.52). Pooled analysis indicated that NLR reduction might predict favorable survival in both the overall population (HR = 1.52, 95% CI: 1.34–1.73) and the subgroup treated with chemotherapy (HR = 1.50, 95% CI: 1.32-1.70).
Conclusion:
Treatment-induced NLR change can act as an early predictor for PAC. Patients with reduced NLR after chemotherapy are expected to have better survival.
Keywords: pancreatic adenocarcinoma, neutrophil-to-lymphocyte ratio, systemic inflammatory response, prognosis, meta-analysis
Introduction
Pancreatic adenocarcinoma (PAC) is one of the most aggressive malignancies with high incidence. PAC is the fourth leading cause of cancer-related death worldwide.1 The 5-year survival for PAC is less than 8%, while the 5-year survival for those who undergo radical resection is much higher (up to 27%).2 However, only 20% of patients at the time of diagnosis are eligible for surgery.3,4 Chemotherapy and neoadjuvant chemoradiation are other vital strategies in the multimodality treatment for PAC.2,5,6 Yet, the efficacy and long-term outcome are still unsatisfactory.6,7 Thus, identifying new prognostic factors for PAC is crucial for a better selection of patients that will benefit most from this treatment.
Previous studies have revealed that systemic inflammation response participates in tumorigenesis by promoting angiogenesis, cell proliferation, tissue invasion, and metastatic dissemination.8 So far, many systemic inflammation markers have shown predictive value in PAC, including neutrophil-to-lymphocyte ratio (NLR), platelet to lymphocyte ratio (PLR), C-reactive protein to albumin ratio (CRP/Alb), Glasgow Prognostic Score (GPS), and modified Glasgow Prognostic Score (mGPS). Yet, most of the studies are focusing on investigating the correlation of pre-treatment systemic inflammation markers and prognosis after treatment. Whether the marker changes in response to treatment can be predictive remains unclear.
NLR is considered the most valuable marker for predicting PAC prognosis.9,10 Thus, the aim of this meta-analysis was to investigate the prognostic value of pre- and post-treatment NLR change in patients with pancreatic adenocarcinoma, especially among the patients treated with chemotherapy.
Methods
This study was designed in conformity with the guidelines of the 2009 Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement.
Literature Search
Pubmed, Web of Science, and Embase databases were searched for relevant articles published before May 2020 using the following search terms: “(neutrophil to lymphocyte ratio OR neutrophil-lymphocyte ratio OR neutrophil/lymphocyte ratio OR NLR) AND (pancreatic adenocarcinoma OR pancreatic cancer)”, “AB = (neutrophil to lymphocyte ratio OR neutrophil-lymphocyte ratio OR neutrophil/lymphocyte ratio OR NLR) AND TI = (pancreatic adenocarcinoma OR pancreatic cancer)”, and “(neutrophil to lymphocyte ratio or neutrophil-lymphocyte ratio or neutrophil lymphocyte ratio or NLR).ab. and (pancreatic adenocarcinoma or pancreatic cancer).ti.”, respectively. Moreover, references of retrieved studies and reviews were also searched. Only studies published in the English language were reviewed.
Inclusion and Exclusion Criteria
The inclusion criteria were the following: (1) PAC diagnosed with pathologic methods; (2) NLR was tested at least 2 times: at baseline (at diagnosis, at admission, or right before treatment), and after treatment; (3) the correlation of NLR change (ΔNLR) and survival was investigated; (4) the values of hazard ratios (HR) and 95% confidence interval (CI) could be directly extracted or calculated with the method reported by Tierney et al.11
The exclusion criteria were: (1) other pathologic types of pancreatic cancer or neoplasms; (2) reviews, case reports, letters, conference abstracts, or laboratory studies; (3) indirect analysis between ΔNLR and survival; (4) insufficient information for pooled HRs.
Data Extraction
The following information was collected: first author’s surname, research region, publication year, study design (prospective or retrospective), sample size, tumor type, and staging, treatment, outcome measure, the timing of NLR tests, classification of NLR change, and their distribution; if the multivariate analysis were conducted and whether the results were positive or negative, and eventually HR, 95%CI. The results of multivariate analyses were predominantly used; otherwise, the results of univariate analyses could be extracted. Two investigators independently assessed articles and extracted all data; consensuses were reached by discussion. If there were further disagreements, a third reviewer was invited.
Quality Assessment
The quality assessment of included studies was evaluated by 2 reviewers (X Luo and N Jiang) independently by the Newcastle-Ottawa Scale (NOS) for cohort studies. This tool consists of 3 dimensions, including the selection of the study groups (0-4 points), the comparability of the groups (0-2 points), and the ascertainment of or outcome (0-2 points). NOS scores range from 0 to 9; a higher score indicates better quality.
Statistical Analysis
This meta-analysis was conducted using RevMan (version 5.3; The Cochrane Collaboration). The heterogeneity of pooled results was measured by Cochrane’s Q test and Higgins I-squared statistic. Significant heterogeneity was defined as p < 0.1 or I2 > 50%; thus, the random effect model was applied; otherwise, the fixed-effect model was used for included studies without significant heterogeneity. Next, the software was used to generate a forest plot so as to demonstrate pooled results. In addition, the funnel plot was constructed for testing publication bias.
Results
Selection and Characteristics of Included Studies
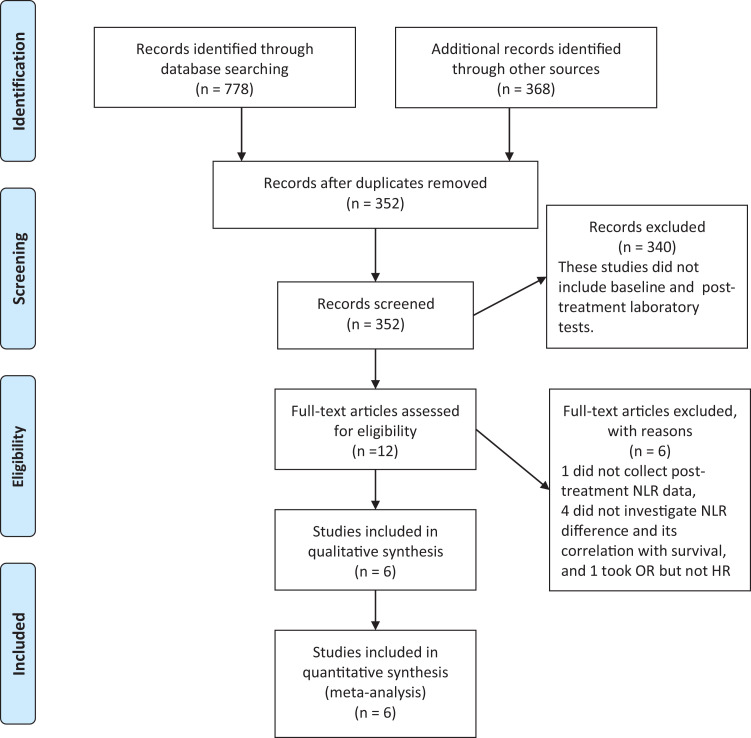
A total of 12 studies were identified since they seemed to have explored the impact of laboratory tests before and after a period of therapy. After the full-text screening, 1 article, which did not collect post-treatment NLR value,12 4 articles, which did not calculate NLR difference and did not analyze the correlation between NLR change and long-term survival,13-16 and 1 article that took odds ratio (OR) to reflect the prognostic value of NLR change on pancreatic adenocarcinoma treatment17 were excluded. Finally, 6 studies were included in this meta-analysis18-23 (Figure 1). The characteristics of the included studies are summarized in Table 1.
Figure 1.
Flow chart of selection and inclusion of studies.
Table 1.
Characteristics and Quality of Included Studies.
| Study | Country | Study design | Sample size | Disease | Disease stage | Treatment | Outcome measure | Sampling time 1 | Sampling time 2 | Grouping | Distribution | Multivariate analysis | Results | NOS Score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chen 201718 | China | retrospective | 132 | PDAC | locally advanced and metastatic | palliative chemotherapy | OS | baseline | after 2 cycles of chemo | Increased(ΔNLR>0) Decreased(ΔNLR≤0) |
50 82 |
yes | positive | 9 |
| Choi 201619 | Korea | retrospective | 396 | PAC | metastatic | palliative chemotherapy | OS | baseline | after 1 cycle of chemo | Increased(ΔNLR≥0) Decreased(ΔNLR<0) |
120 261 |
yes | positive | 9 |
| Eyff 201820 | Brazil | retrospective | 135 | PAC | StageⅠ-Ⅳ | palliative chemotherapy (subgroup, n = 49) |
OS | baseline | after 2 cycles of chemo | Increased(ΔNLR>0) Decreased(ΔNLR<0) |
21 10 |
yes | negative | 9 |
| Glazer 201621 | America | retrospective | 62 | PDAC | borderline resectable | neoadjuvant chemoradiation | OS | baseline | after neoadjuvant therapy | Increased* Stable |
19 43 |
yes | positive | 9 |
| Luo 201522 | China | retrospective | 403 | PAC | locally advanced and metastatic | palliative chemotherapy | OS | baseline | after 1 cycle of chemo | Increased Decreased |
211 146 |
yes | positive | 9 |
| Teo 201323 | Ireland | prospective | 85 | PDAC | locally advanced and metastatic | palliative chemotherapy | OS | baseline | after 4 weeks of chemo | Increased(ΔNLR≥0) Decreased(ΔNLR<0) |
38 27 |
yes | negative | 7 |
* Increased NLR in this study was defined as an increase of standard deviation of baseline NLR, and the remaining were classified as stable NLR. Abbreviations: PAC-pancreatic adenocarcinoma; PDAC-pancreatic ductal adenocarcinoma.
Prognostic Value of NLR Change
Since the heterogeneity test revealed minor heterogeneity (I2 = 0%, P = 0.45) between the studies, a fixed-effects model was chosen for the analysis. A pooled HR of 1.52 (95% CI: 1.34–1.73) showed that elevated post-treatment NLR was correlated with shorter OS in patients (Figure 2A). Since our focus was on the role of NLR change in patients treated with chemotherapy, subgroup analysis was conducted among chemotherapy-specific studies regardless of the significant heterogeneity (Figure 2B). Pooled data of the subgroup treated with chemotherapy (HR = 1.50, 95% CI: 1.32-1.70) was consistent with that of the overall population.
Figure 2.
Forest plot of HR and 95%CI for the association between (A) NLR change and OS, (B) NLR change and OS in the subgroup of patients who receive chemotherapy, (C) pre-treatment NLR and OS.
Prognostic Value of Pre-Treatment and Post-Treatment NLR
HR of baseline NLR could be extracted from 4 out of the 6 included studies.18,19,22,23 Considering that the heterogeneity was significant (I2 = 83%, P < 0.01), the random-effects model was used. Meta-analysis results showed that elevated baseline NLR was associated with poorer OS (HR = 2.21, 95% CI: 1.45-3.36) (Figure 2C). Moreover, one study investigated the association between post-treatment NLR and OS, as well as the prognostic impact on survival of post-treatment NLR (HR = 1.28, 95% CI = 1.08-1.52).20
Publication Bias
The absence of studies in the lower left quadrant was noticed by visual inspection, indicating a potential bias in this meta-analysis, despite only 6 studies were eventually selected (Figure 3).
Figure 3.
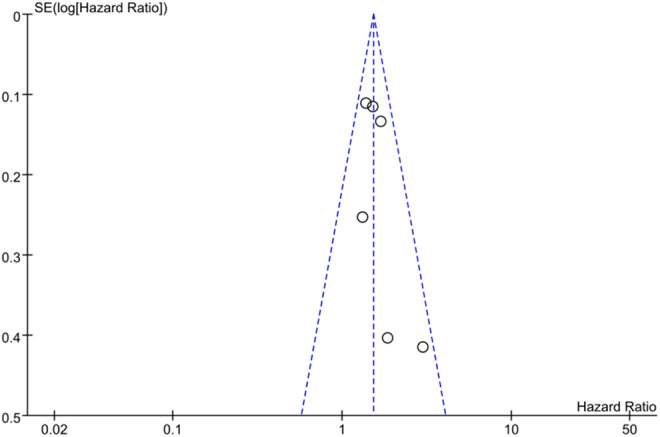
Funnel plot of included studies.
Discussion
To the best of our knowledge, this is the first systemic review assessing NLR change in response to treatment in PAC patients. Based on our meta-analysis, we found the prognostic significance of NLR change in PAC. Patients with elevated NLR (measured within 1 or 2 months after the initiation of treatment) had a shorter survival. Similarly, patients with increased NLR, who were treated with chemotherapy alone, had a higher HR of death. Several clinical studies have indicated that elevated baseline NLR is correlated with poorer survival in pancreatic cancer treated with surgery,24 chemotherapy, radiotherapy,25 and mixed therapy,26 while some other studies reported controversial conclusion.27 Moreover, 3 meta-analyses investigated the effect of pre-treatment NLR for the prediction of pancreatic cancer patients.9,28,29 In addition, elevated NLR at baseline has been identified as a prognostic factor of poorer clinical outcomes in some other cancer types, including non-small cell lung cancer,30,31 esophageal cancer,32 gastric cancer,33 ampullary cancer,34 colorectal cancer,35 and breast cancer.36 Thus, in solid tumors, the prognostic value of pre-treatment NLR has been generally recognized.
By comparing the prognostic value of NLR change and pre-treatment NLR by pooled HRs, it was not possible to come to a final conclusion. Many studies assessing pre-treatment NLR’s role were excluded as we only included papers that reported both pre-treatment and post-treatment NLR, which additionally limited the interpretation of results. We found 3 meta-analyses that reported on pre-treatment NLR in pancreatic cancer.9,28,29 The cut-off values of elevated pre-treatment NLR were 2.0 in one study,9 and 2-528 and 2.3-529 in the other 2. The pooled HRs of 3 studies were 1.147 (95%CI: 0.147-1.180) by fixed effect model, 1.737 (95%CI: 1.502-2.009) by random effect model,9 1.59 (95%CI: 1.41-1.78) by fixed-effect model,28 and 2.61 (95% CI: 1.68-4.06) by random effect model,29 respectively. Moreover, Formica et al found that high NLR helps select metastatic pancreatic cancer patients benefitting from oxaliplatin and gemcitabine.37 In addition, the difference between pre-treatment and pos-treatment NLR may reflect treatment response. We hypothesized that patients who experienced NLR decrease might benefit more from the current regimen. Validation studies are being designed.
Most of the included studies had 1 or 2 cycles of monitoring for the collection of post-treatment data. Besides systemic inflammation markers, timing has shown to be a predictive factor for prognosis. Neutropenia, as well as NLR decrease, indicate systemic inflammation receding.38,39 The timing of neutropenia is an independent predictor of prognosis in metastatic colon cancer patients who received mFOLFOX6. Moreover, patients with early-onset and late-onset chemotherapy-induced neutropenia (CIN) had longer survival than patients without CIN.38 The earlier detection and subgroup identification are of greater clinical significance.
The prognostic significance of systemic inflammation markers warrants future studies on the cluster of markers. A combination of NLR and CRP might have a predictive effect on patients with gastric cancer.40 A novel systemic inflammation response index (SIRI) was reported for predicting PAC patients who receive chemotherapy, defined as the multiplication of neutrophil count and monocyte count divided by lymphocyte (SIRI = N*M/L).41 The dynamic change of CA19-9 has been found to be associated with pancreatic cancer in several studies.42,43 Combined CA19-9 and NLR are better prognostic marker than either alone in metastatic pancreatic cancer patients.44
This study has a few limitations. First, all of the included studies were retrospective and thereby more prone to bias, i.e. publication bias and language bias (see asymmetric funnel plots). Second, only 6 studies were included, indicating the prognostic value of NLR change; thus, more prospective clinical studies are required to validate its predictive effect. Third, the criteria for elevated NLR in response to treatment deserve more attention. Usually, it is calculated by latter NLR minus prior one, but Glazer et al took the standard deviation of pre-treatment NLR into consideration.21 To determine a definition of NLR change in response to treatment would help to better understand its prognostic value. Last but not least, confounding factors in this study cannot be ignored, e.g. the role of neutrophil counts38,39 or disease stage. Selected studies examined different disease stages (resectable, borderline resectable, locally advanced, and metastatic tumor), which leads to different treatment choices.
In conclusion, we found that reduced NLR in response to treatment is associated with improved OS in pancreatic adenocarcinoma patients. Neutrophil and lymphocyte counts were both routinely measured; thus, inexpensive laboratory tests could be used for an easily accessible identification of pancreatic patients’ prognosis.
Acknowledgments
We thank all of staff members of the Department of Pharmacy Fudan University Shanghai Cancer Center, and Department of Pancreatic Surgery of Fudan University Shanghai Cancer Center. We also thank SAGE journals Editorial Board for the editing service.
Footnotes
Author Contribution: Xin Luo, Bo Yu and Nan Jiang are authors contributed equally to this work.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the National Natural Science Foundation of China (81872366), the Outstanding Academic Leader Program of the “Technological Innovation Action Plan” of Shanghai Science and Technology Commission (18XD1401200), and the Young Talented Specialist Training Program of Shanghai.
ORCID iD: Xin Luo, PhD  https://orcid.org/0000-0002-4592-6734
https://orcid.org/0000-0002-4592-6734
Previous Communication of Work: This work has not been previously communicated to a society or meeting.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A.Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.McGuigan A, Kelly P, Turkington RC, Jones C, Coleman HG, McCain RS. Pancreatic cancer: a review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol. 2018;24(43):4846–4861. doi:10.3748/wjg.v24.i43.4846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ilic M, Ilic I. Epidemiology of pancreatic cancer. World J Gastroenterol. 2016;22(44):9694–9705. doi:10.3748/wjg.v22.i44.9694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet. 2011;378(9791):607–620. doi:10.1016/s0140-6736(10)62307-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350(12):1200–1210. doi:10.1056/NEJMoa032295 [DOI] [PubMed] [Google Scholar]
- 6.Springfeld C, Jager D, Buchler MW, et al. Chemotherapy for pancreatic cancer. Presse Med. 2019;48(3 Pt 2):e159–e174. doi:10.1016/j.lpm.2019.02.025 [DOI] [PubMed] [Google Scholar]
- 7.Ducreux M, Seufferlein T, Van Laethem JL, et al. Systemic treatment of pancreatic cancer revisited. Semin Oncol. 2019;46(1):28–38. doi:10.1053/j.seminoncol.2018.12.003 [DOI] [PubMed] [Google Scholar]
- 8.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi:10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 9.Oh D, Pyo JS, Son BK. Prognostic roles of inflammatory markers in pancreatic cancer: comparison between the neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio. Gastroenterol Res Pract. 2018;2018:9745601. doi:10.1155/2018/9745601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asama H, Suzuki R, Takagi T, et al. Evaluation of inflammation-based markers for predicting the prognosis of unresectable pancreatic ductal adenocarcinoma treated with chemotherapy. Mol Clin Oncol. 2018;9(4):408–414. doi:10.3892/mco.2018.1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi:10.1186/1745-6215-8-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miyamoto R, Oda T, Hashimoto S, et al. Platelet x CRP multiplier value as an indicator of poor prognosis in patients with resectable pancreatic cancer. Pancreas. 2017;46(1):35–41. doi:10.1097/mpa.0000000000000697 [DOI] [PubMed] [Google Scholar]
- 13.Ichikawa K, Mizuno S, Hayasaki A, et al. Prognostic nutritional index after chemoradiotherapy was the strongest prognostic predictor among biological and conditional factors in localized pancreatic ductal adenocarcinoma patients. Cancers (Basel). 2019;11(4):514. doi:10.3390/cancers11040514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qi Q, Zhuang L, Shen Y, et al. A novel systemic inflammation response index (SIRI) for predicting the survival of patients with pancreatic cancer after chemotherapy. Cancer. 2016;122(14):2158–2167. doi:10.1002/cncr.30057 [DOI] [PubMed] [Google Scholar]
- 15.Tsujita E, Ikeda Y, Kinjo N, et al. Postoperative neutrophil-to-lymphocyte ratio as a predictor of long-term prognosis after pancreatectomy for pancreatic carcinoma: a retrospective analysis. Am Surg. 2017;83(6):610–616. [PubMed] [Google Scholar]
- 16.Xue P, Kanai M, Mori Y, et al. Neutrophil-to-lymphocyte ratio for predicting palliative chemotherapy outcomes in advanced pancreatic cancer patients. Cancer Med. 2014;3(2):406–415. doi:10.1002/cam4.204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao Y, Wang WJ, Zhi Q, et al. Neutrophil/lymphocyte ratio is a more sensitive systemic inflammatory response biomarker than platelet/lymphocyte ratio in the prognosis evaluation of unresectable pancreatic cancer. Oncotarget. 2017;8(51):88835–88844. doi:10.18632/oncotarget.21340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Y, Yan H, Wang Y, Shi Y, Dai G. Significance of baseline and change in neutrophil-to-lymphocyte ratio in predicting prognosis: a retrospective analysis in advanced pancreatic ductal adenocarcinoma. Sci Rep. 2017;7(1):753. doi:10.1038/s41598-017-00859-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi Y, Oh DY, Park H, et al. More accurate prediction of metastatic pancreatic cancer patients’ survival with prognostic model using both host immunity and tumor metabolic activity. PLoS One. 2016;11(1):e0145692. doi:10.1371/journal.pone.0145692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eyff TF, Bosi HR, Toni MS, et al. The role of immunoinflammatory markers in the prognosis and resectability of pancreatic adenocarcinoma. Arq Bras Cir Dig. 2018;31(2):e1366. doi:10.1590/0102-672020180001e1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glazer ES, Rashid OM, Pimiento JM, Hodul PJ, Malafa MP. Increased neutrophil-to-lymphocyte ratio after neoadjuvant therapy is associated with worse survival after resection of borderline resectable pancreatic ductal adenocarcinoma. Surgery. 2016;160(5):1288–1293. doi:10.1016/j.surg.2016.04.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luo G, Guo M, Liu Z, et al. Blood neutrophil-lymphocyte ratio predicts survival in patients with advanced pancreatic cancer treated with chemotherapy. Ann Surg Oncol. 2015;22(2):670–676. doi:10.1245/s10434-014-4021-y [DOI] [PubMed] [Google Scholar]
- 23.Teo M, Mohd Sharial MS, McDonnell F, Conlon KC, Ridgway PF, McDermott RS. Prognostic role of neutrophil-to-lymphocyte ratio in advanced pancreatic ductal adenocarcinoma: impact of baseline fluctuation and changes during chemotherapy. Tumori. 2013;99(4):516–522. doi:10.1700/1361.15104 [DOI] [PubMed] [Google Scholar]
- 24.Abe T, Nakata K, Kibe S, et al. Prognostic value of preoperative nutritional and immunological factors in patients with pancreatic ductal adenocarcinoma. Ann Surg Oncol. 2018;25(13):3996–4003. doi:10.1245/s10434-018-6761-6 [DOI] [PubMed] [Google Scholar]
- 25.Alagappan M, Pollom EL, von Eyben R, et al. Albumin and neutrophil-lymphocyte ratio (NLR) predict survival in patients with pancreatic adenocarcinoma treated with SBRT. Am J Clin Oncol. 2018;41(3):242–247. doi:10.1097/coc.0000000000000263 [DOI] [PubMed] [Google Scholar]
- 26.Bhatti I, Peacock O, Lloyd G, Larvin M, Hall RI. Preoperative hematologic markers as independent predictors of prognosis in resected pancreatic ductal adenocarcinoma: neutrophil-lymphocyte versus platelet-lymphocyte ratio. Am J Surg. 2010;200(2):197–203. doi:10.1016/j.amjsurg.2009.08.041 [DOI] [PubMed] [Google Scholar]
- 27.Chawla A, Huang TL, Ibrahim AM, Hardacre JM, Siegel C, Ammori JB. Pretherapy neutrophil to lymphocyte ratio and platelet to lymphocyte ratio do not predict survival in resectable pancreatic cancer. HPB (Oxford). 2018;20(5):398–404. doi:10.1016/j.hpb.2017.10.011 [DOI] [PubMed] [Google Scholar]
- 28.Cheng H, Long F, Jaiswar M, Yang L, Wang C, Zhou Z. Prognostic role of the neutrophil-to-lymphocyte ratio in pancreatic cancer: a meta-analysis. Sci Rep. 2015;5:11026. doi:10.1038/srep11026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang JJ, Hu ZG, Shi WX, Deng T, He SQ, Yuan SG. Prognostic significance of neutrophil to lymphocyte ratio in pancreatic cancer: a meta-analysis. World J Gastroenterol. 2015;21(9):2807–2815. doi:10.3748/wjg.v21.i9.2807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scilla KA, Bentzen SM, Lam VK, et al. Neutrophil-lymphocyte ratio is a prognostic marker in patients with locally advanced (Stage IIIA and IIIB) non-small cell lung cancer treated with combined modality therapy. Oncologist. 2017;22(6):737–742. doi:10.1634/theoncologist.2016-0443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gu X-B, Tian T, Tian X-J, Zhang X-J. Prognostic significance of neutrophil-to-lymphocyte ratio in non-small cell lung cancer: a meta-analysis. Sci Rep. 2015;5:12493–12493. doi:10.1038/srep12493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sharaiha RZ, Halazun KJ, Mirza F, et al. Elevated preoperative neutrophil: lymphocyte ratio as a predictor of postoperative disease recurrence in esophageal cancer. Ann Surg Oncol. 2011;18(12):3362–3369. doi:10.1245/s10434-011-1754-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shimada H, Takiguchi N, Kainuma O, et al. High preoperative neutrophil-lymphocyte ratio predicts poor survival in patients with gastric cancer. Gastric Cancer. 2010;13(3):170–176. doi:10.1007/s10120-010-0554-3 [DOI] [PubMed] [Google Scholar]
- 34.Demirci NS, Erdem GU. Prognostic role of neutrophil-to-lymphocyte ratio (NLR) in patients with operable ampullary carcinoma. Bosn J Basic Med Sci. 2018;18(3):268–274. doi:10.17305/bjbms.2017.2530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chua W, Charles KA, Baracos VE, Clarke SJ. Neutrophil/lymphocyte ratio predicts chemotherapy outcomes in patients with advanced colorectal cancer. Br J Cancer. 2011;104(8):1288–1295. doi:10.1038/bjc.2011.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu J, Ni C, Ma C, et al. Association of neutrophil/lymphocyte ratio and platelet/lymphocyte ratio with ER and PR in breast cancer patients and their changes after neoadjuvant chemotherapy. Clin Transl Oncol. 2017;19(8):989–996. doi:10.1007/s12094-017-1630-5 [DOI] [PubMed] [Google Scholar]
- 37.Formica V, Morelli C, Ferroni P, et al. Neutrophil/lymphocyte ratio helps select metastatic pancreatic cancer patients benefitting from oxaliplatin. Cancer Biomark. 2016;17(3):335–345. doi:10.3233/cbm-160645 [DOI] [PubMed] [Google Scholar]
- 38.Chen Y, Wang Y, Shi Y, Dai G. Timing of chemotherapy-induced neutropenia predicts prognosis in metastatic colon cancer patients: a retrospective study in mFOLFOX6-treated patients. BMC Cancer. 2017;17(1):242. doi:10.1186/s12885-017-3240-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamada Y, Fujii H, Watanabe D, et al. Severe neutropenia is associated with better clinical outcomes in patients with advanced pancreatic cancer who receive modified FOLFIRINOX Therapy. Cancers (Basel). 2018;10(11):454. doi:10.3390/cancers10110454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu X, Sun X, Liu J, et al. Preoperative c-reactive protein/albumin ratio predicts prognosis of patients after curative resection for gastric cancer. Transl Oncol. 2015;8(4):339–345. doi:10.1016/j.tranon.2015.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jomrich G, Paireder M, Kristo I, et al. High systemic immune-inflammation index is an adverse prognostic factor for patients with gastroesophageal adenocarcinoma. Ann Surg. 2019. doi:10.1097/sla.0000000000003370 [DOI] [PubMed] [Google Scholar]
- 42.Chen Y, Wang YR, Deng GC, Dai GH. CA19-9 decrease and survival according to platelet level in patients with advanced pancreatic cancer. BMC Cancer. 2019;19(1):860. doi:10.1186/s12885-019-6078-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ko AH, Hwang J, Venook AP, Abbruzzese JL, Bergsland EK, Tempero MA. Serum CA19-9 response as a surrogate for clinical outcome in patients receiving fixed-dose rate gemcitabine for advanced pancreatic cancer. Br J Cancer. 2005;93(2):195–199. doi:10.1038/sj.bjc.6602687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Song JY, Chen MQ, Guo JH, Lian SF, Xu BH. Combined pretreatment serum CA19-9 and neutrophil-to-lymphocyte ratio as a potential prognostic factor in metastatic pancreatic cancer patients. Medicine (Baltimore). 2018;97(4):e9707. doi:10.1097/md.0000000000009707 [DOI] [PMC free article] [PubMed] [Google Scholar]