Abstract
Downregulation of protein kinase C δ (PKC δ) by treatment with the tumor-promoting phorbol ester 12-O-tetradecanoylphorbol-13-acetate (TPA) transforms cells that overexpress the non-receptor class tyrosine kinase c-Src (Z. Lu et al., Mol. Cell. Biol. 17:3418–3428, 1997). We extended these studies to cells overexpressing a receptor class tyrosine kinase, the epidermal growth factor (EGF) receptor (EGFR cells); like c-Src, the EGF receptor is overexpressed in several human tumors. In contrast with expectations, downregulation of PKC isoforms with TPA did not transform the EGFR cells; however, treatment with EGF did transform these cells. Since TPA downregulates all phorbol ester-responsive PKC isoforms, we examined the effects of PKC δ- and PKC α-specific inhibitors and the expression of dominant negative mutants for both PKC δ and α. Consistent with a tumor-suppressing function for PKC δ, the PKC δ-specific inhibitor rottlerin and a dominant negative PKC δ mutant transformed the EGFR cells in the absence of EGF. In contrast, the PKC α-specific inhibitor Go6976 and expression of a dominant negative PKC α mutant blocked the transformed phenotype induced by both EGF and PKC δ inhibition. Interestingly, both rottlerin and EGF induced substantial increases in phospholipase D (PLD) activity, which is commonly elevated in response to mitogenic stimuli. The elevation of PLD activity in response to inhibiting PKC δ, like transformation, was dependent upon PKC α and restricted to the EGFR cells. These data demonstrate that PKC isoforms α and δ have antagonistic effects on both transformation and PLD activity and further support a tumor suppressor role for PKC δ that may be mediated by suppression of tyrosine kinase-dependent increases in PLD activity.
The conversion of a normal cell to a transformed cell is a progressive process whereby successive rounds of mutation (initiation) and selected amplification (promotion) of the initiated cells result in an amplified population of partially transformed cells. Within the amplified population of partially transformed cells additional mutations may occur that provide a further selective advantage to a single member of clonally amplified cells. This process selects for cells with more aggressive growth properties, resulting in the generation of cells with a more aggressive malignant phenotype. Substances that stimulate the amplification of incompletely transformed cells are known as tumor promoters, and while not inducing directly the genetic changes that ultimately result in a malignant tumor, they dramatically speed up the process by generating large populations of initiated cells that are subject to further mutation (6, 46–48). Epigenetic factors, such as diet, hormones, and cigarette smoke, have all been reported to have tumor-promoting properties and contribute in poorly understood ways to tumor progression.
Members of our group recently reported that rat fibroblasts overexpressing the non-receptor tyrosine kinase c-Src become transformed upon treatment with the tumor-promoting phorbol ester 12-O-tetradecanoylphorbol-13-acetate (TPA) (23). In this cell culture model, TPA was able to induce the amplification of cells containing an initiating mutation (c-Src overexpression) and therefore functioned very much like a tumor promoter in vitro. The tumor-promoting effect of TPA was determined to be due to the depletion of the δ isoform of protein kinase C (PKC) (23). Other studies also suggest that PKC δ negatively regulates cell division (4, 15–17, 19, 31, 44). PKC δ has been shown to negatively affect the Jak-Stat signaling pathway, which is frequently activated in response to cell division signals (34). Phospholipase D (PLD) activity, which is elevated in response to mitogenic signals, was stimulated by the downregulation of PKC in cells overexpressing c-Src (23), suggesting that PKC δ may also negatively regulate PLD activity. However, the activation of PLD by the mitogenic signals induced by both platelet-derived growth factor and epidermal growth factor (EGF) has been reported to be dependent on PKC (32, 45). PKC α has been implicated as an activator of PLD; however, it may be independent of its own kinase activity (36). Thus, a role for PLD in mitogenic signals may be complex and involve differential effects of different isoforms. Collectively, these data suggest that PKC δ negatively regulates cell division by suppressing mitogenic signals and are consistent with PKC δ having a tumor-suppressing effect that is lost when PKC δ is downregulated. In this report, we describe the antagonistic effects of PKC α and δ on both transformation and PLD activity in cells that overexpress the EGF receptor (EGFR cells); like c-Src, the EGF receptor is implicated in many human cancers (2, 27, 28).
MATERIALS AND METHODS
Cells and cell culture conditions.
Rat 3Y1 cells or rat 3Y1 EGFR cells were maintained in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% bovine calf serum (HyClone) as described previously (23, 49). Cell cultures were made quiescent by growing them to confluence and then replacing the medium with fresh medium containing 0.5% bovine calf serum for 1 day. For growth of cells in soft agar, 103 cells were suspended in top agar (DMEM, 20% calf serum, and 0.38% agar) and overlaid onto hardened bottom agar (DMEM, 20% calf serum, and 0.7% agar) as described previously (33).
Transfection.
Cells were plated at a density of 105/100-mm-diameter dish 18 h prior to transfection. Transfections were performed by using Lipofectamine reagent (GIBCO) according to the vendor’s instructions. Transfected cultures were selected with either puromycin (5 μg/ml) or hygromycin (200 μg/ml) for 10 to 14 days at 37°C. At that time antibiotic-resistant colonies were picked and expanded for further analysis under selective conditions.
Materials.
The PKC inhibitors rottlerin and Go6976 were obtained from Calbiochem. Monoclonal antibodies to the EGF receptor and PKC α were obtained from Transduction Laboratories, and a polyclonal antibody for PKC δ was obtained from Santa Cruz Biotechnology. pCEP4, which contains the hygromycin resistance gene, was obtained from Invitrogen.
Western analysis.
Extraction of proteins from cultured cells was performed as previously described (23, 24). Equal amounts of protein were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis with an 8% acrylamide separating gel, transferred to nitrocellulose, and blocked overnight at 4°C with 5% nonfat dry milk mixed with isotonic phosphate-buffered saline (PBS; 136 mM NaCl, 2.6 mM KCl, 1.4 mM KH2PO4, 4.2 mM Na2HPO4). The nitrocellulose filters were washed three times for 5 min in PBS and then incubated with antibodies as described below. Depending upon the origin of the primary antibodies, either anti-mouse or anti-rabbit immunoglobulin G was used for detection with the ECL system (Amersham).
PLD assays.
Confluent 35-mm-diameter culture dishes were prelabeled for 4 h with 3 μCi of [3H]myristate (40 Ci/mmol) in 3 ml of medium containing 0.5% newborn calf serum. PLD-catalyzed transphosphatidylation in the presence of 1% butanol was performed as described previously (37, 38). Extraction and characterization of lipids by thin-layer chromatography were performed as previously described (38).
RESULTS
3Y1 EGFR cells display a transformed phenotype upon treatment with EGF, but not TPA.
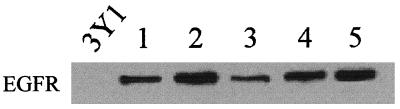
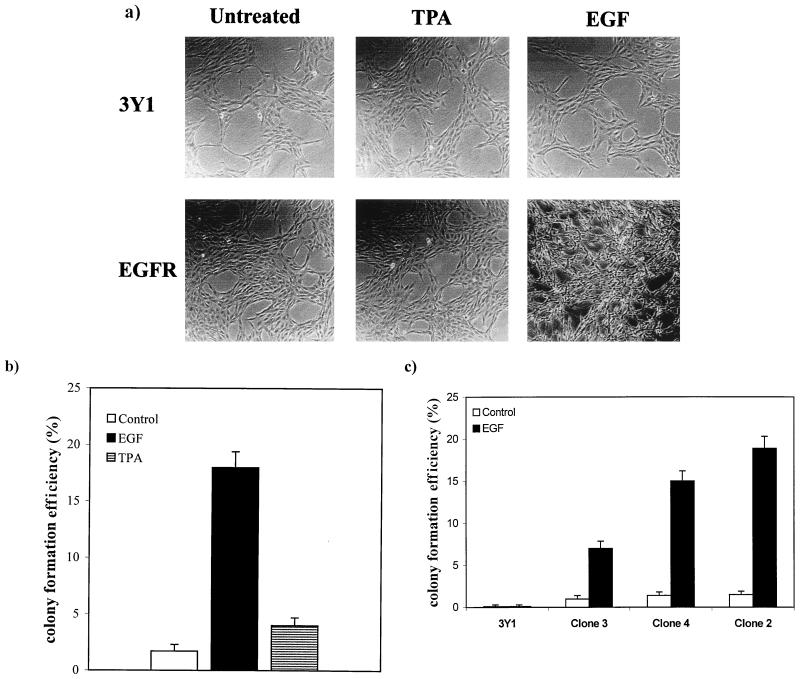
3Y1 rat fibroblasts were stably transfected with a plasmid (pPEGFr) containing a puromycin resistance marker gene and the EGF receptor gene under the control of the simian virus 40 promoter (5). EGF receptor overexpression was verified by Western analysis of several puromycin-resistant clones as shown in Fig. 1. Clone 2 expressed the highest levels of the EGF receptor and was used for most subsequent experiments. Upon establishment of EGFR cells, we examined the effects of both long-term TPA treatment and EGF on the morphology of these cells. In Fig. 2a, it is shown that the EGFR cells (clone 2) had a flat nontransformed morphology like that of the parental 3Y1 cells. In response to EGF, the EGFR cells took on a refractile morphology characteristic of transformation (Fig. 2a). This morphological change was observed in the other EGFR clones as well, indicating that the ability of EGF to induce this phenotype was not restricted to the clonal EGFR cell line shown (data not shown). However, in contrast with expectations, TPA treatment did not cause a transformed morphology as observed previously with c-Src-overexpressing cells (23). We next investigated the ability of EGFR cells to form colonies in soft agar, and as shown in Fig. 2b, EGF, but not TPA, induced anchorage-independent growth. The ability of the EGFR cells to form colonies in soft agar in the presence of EGF correlated well with the level of expression of the EGF receptor. Clone 3, which expressed low levels of the receptor, and clone 4, which expressed intermediate levels the receptor (Fig. 1), formed low and intermediate numbers of colonies in response to EGF relative to the numbers expressed by clone 2 (Fig. 2c). The inability of TPA to induce the transformed phenotype was not due to a lack of PKC isoform downregulation, since this treatment resulted in the same rapid degradation of PKC isoforms as reported previously (23, 24) (data not shown). These data indicate that downregulation of PKC isoforms in response to TPA does not have the same effect in EGFR cells as observed previously for cells overexpressing the non-receptor tyrosine kinase c-Src (23).
FIG. 1.
Establishment of 3Y1 EGFR cells. 3Y1 cells were transfected with pPEGFr, which expresses the EGF receptor from the simian virus 40 promoter and contains a puromycin resistance marker (5). Several puromycin-resistent colonies were picked and analyzed for levels of expression of the EGF receptor. Western blot analysis was performed on lysates from the parental 3Y1 cells (3Y1) and the puromycin-resistant colonies with an antibody raised against the EGF receptor.
FIG. 2.
EGFR cells display a transformed phenotype upon treatment with EGF but not TPA. (a) Parental 3Y1 or EGFR cells were either left untreated or treated with TPA (400 nM) or EGF (100 ng/ml) for 24 h, at which time the morphology of the cells was examined. (b) Anchorage-independent growth of the EGFR cells (clone 2) was examined in the presence or absence of either TPA (400 nM) or EGF (100 ng/ml) as shown. TPA and EGF were replenished every 4 days. A total of 103 cells were suspended in soft agar, and the percentage of cells that formed colonies was determined 3 weeks later. (c) Anchorage-independent growth of 3Y1 cells and 3Y1 EGFR cells (clones 2, 3, and 4) was examined in the presence of EGF (100 ng/ml) as described for panel b.
Differential effects of PKC α and δ on EGF receptor-mediated transformation.
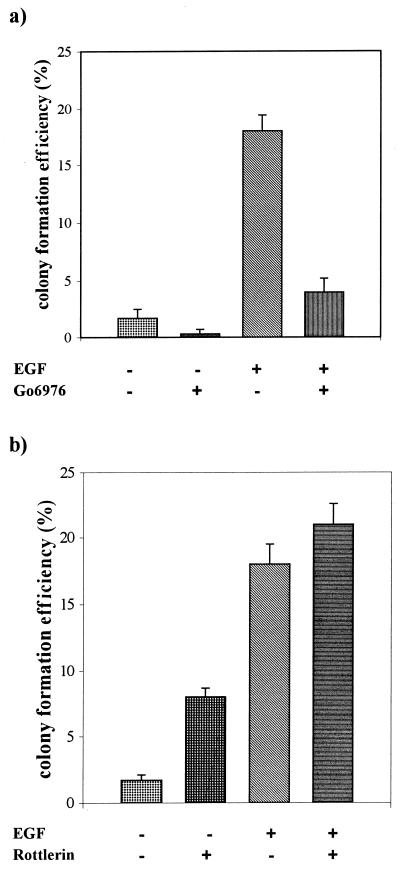
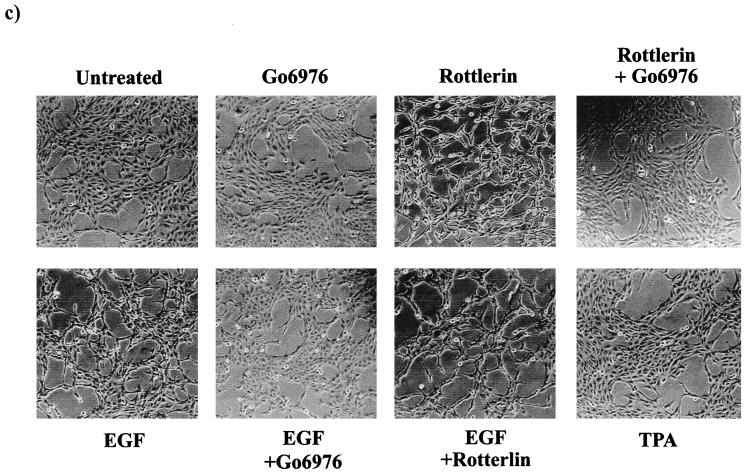
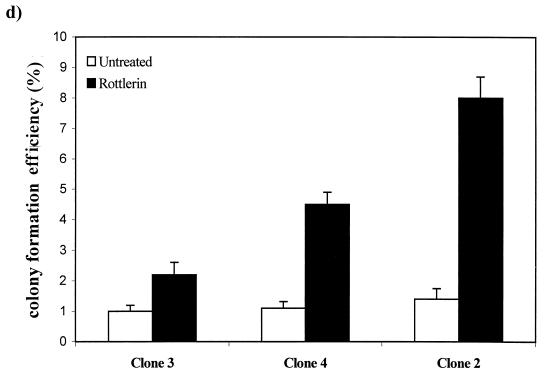
The inability of prolonged TPA treatment to induce a transformed phenotype in the EGFR cells could be due to a requirement for one of the PKC isoforms depleted by the TPA treatment for the EGF receptor to induce a mitogenic signal. To investigate this possibility, we employed inhibitors of PKC that were specific for PKC α and PKC δ. It was demonstrated previously that activation of PKC isoforms was required for ubiquitination and downregulation in 3Y1 cells and that Go6976 (29) specifically inhibited PKC α downregulation and rottlerin (18) specifically inhibited PKC δ downregulation (24). Thus, these two inhibitors were able to distinguish PKC α and δ requirements in these cells. We examined the effects of these two compounds on anchorage-independent growth in the EGFR and parental 3Y1 cells. Go6976 strongly inhibited both EGF-induced and background colony formation in the EGFR cells (Fig. 3a), indicating a PKC α requirement for EGF-induced mitogenic signals. Rottlerin, on the other hand, stimulated colony formation of the EGFR cells in the absence of EGF and increased the number of colonies formed in soft agar in the presence of EGF (Fig. 3b). Rottlerin did not induce colony formation in the parental 3Y1 cells (data not shown). Consistent with its inducing a transformed phenotype in the EGFR cells, rottlerin also caused a transformed morphology in the EGFR cells in the absence of EGF (Fig. 3c). The effect of rottlerin on colony formation was also seen in EGFR clones 3 and 4, which expressed low and intermediate levels of the EGF receptor, and the number of colonies correlated with the level of EGF receptor expression (Fig. 3d). Consistent with the inability of prolonged TPA treatment to induce a transformed phenotype in the EGFR cells, Go6976 inhibited the rottlerin-induced transformed morphology (Fig. 3c). These data indicate that inhibition of PKC δ specifically has a tumor-promoting effect similar to that reported previously for cells overexpressing c-Src (23). However, in contrast to c-Src, the EGF receptor has a PKC α requirement for mitogenesis that explains why TPA treatment, which downregulates both PKC α and δ, does not cause transformation of EGFR cells.
FIG. 3.
Effects of PKC α- and δ-specific inhibitors on EGFR cells. EGFR cells were treated with EGF (100 ng/ml) and either Go6976 (0.5 μM) or rottlerin (15 μM) and then examined for the ability to form colonies in soft agar as described for Fig. 2b. (c) The EGFR cells were treated with EGF (100 ng/ml), rottlerin (15 μM), Go6976 (0.5 μM), or TPA (400 nM) as shown, and the morphology of the cells was examined 24 h later as for Fig. 2a. (d) The ability of rottlerin to stimulate colony formation in EGFR clones 2, 3, and 4 was determined as described for panel a.
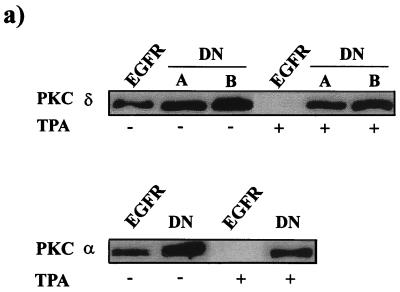
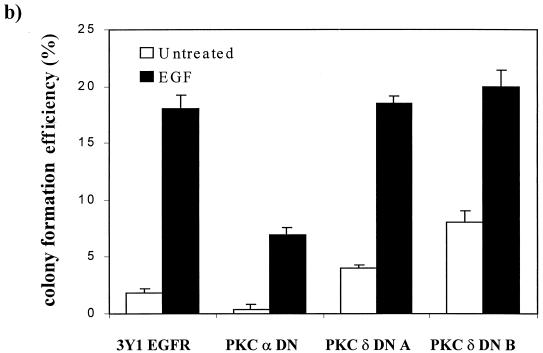
To further establish that the effects of the PKC-specific inhibitors Go6976 and rottlerin were due to effects on PKC α and PKC δ, respectively, we introduced dominant negative mutants of PKC α and δ into the EGFR cells. These mutants both have a conserved Lys in the ATP binding site converted to Ala and have been shown previously to act as dominant negative mutants for PKC α and δ (20, 22, 23, 42). Expression of the dominant negative PKC α and δ mutants was verified by taking advantage of the previous demonstration that PKC downregulation in response to TPA is dependent upon an active kinase (24). Two cell lines transfected with either the kinase-dead PKC α or δ were treated with TPA for 24 h, and the levels of PKC α and δ were then determined by Western blot analysis. As shown in Fig. 4a, both PKC α and δ were degraded by TPA treatment to below the level of detection in the parental EGFR cells. In contrast, PKC α and δ levels in the two cell lines expressing the dominant negative PKC δ were reduced only by about half, indicating that the kinase-dead dominant negative PKC mutants were expressed. Having established that the dominant negative PKC δ was expressed, we next examined the ability of cells to form colonies in soft agar in the presence and absence of EGF. As expected, the dominant negative PKC α inhibited EGF-induced colony formation and the cells expressing the dominant negative PKC δ now formed colonies in soft agar in the absence of EGF (Fig. 4b). There was also a correlation between colony number and the level of expression of the dominant negative PKC δ, with clone B expressing higher levels of the PKC δ mutant (Fig. 4a) and also forming more colonies. Expression of the dominant negative PKC δ mutant did not increase the number of colonies induced by EGF treatment (Fig. 4b), indicating that activation of the EGF receptor is able to overcome the inhibitory effects of PKC δ (see Discussion).
FIG. 4.
Effects of dominant negative (DN) mutants of PKC α and δ on the ability of EGFR cells to form colonies in soft agar. (a) EGFR cells were cotransfected with plasmids expressing either a dominant negative PKC α or δ mutant and pCEF4 (Invitrogen), which expresses a hygromycin resistance marker gene. One clone expressing the PKC α mutant and two clones expressing different levels of the PKC δ mutant were analyzed for expression of the mutants by Western blot analysis before and after treatment with TPA (400 nM, 24 h). TPA treatment downregulates the endogenous wild-type PKC α and δ, but not the kinase-dead mutants (24). (b) The ability of the EGFR cells and the EGFR cell lines expressing the dominant negative PKC α and δ mutants to form colonies in soft agar was determined as for Fig. 3 in the presence and absence of EGF (100 ng/ml) as shown.
Differential effects of PKC α and δ on PLD activity in EGFR cells.
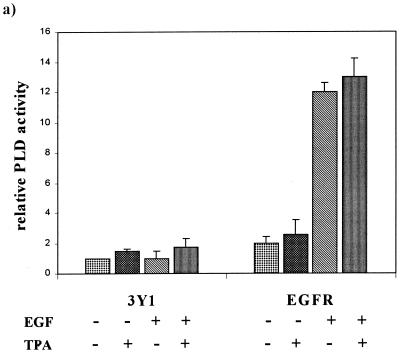
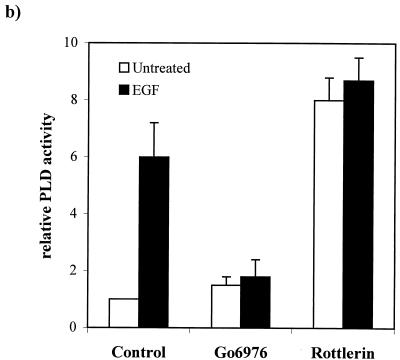
Members of our group reported previously that in cells overexpressing c-Src, downregulation of PKC isoforms with prolonged treatment with TPA resulted in an increase in PLD activity (23). We therefore examined the effect of PKC downregulation by TPA on PLD activity in EGFR and parental 3Y1 cells in the presence and absence of EGF. Activation of PLD by EGF has been reported previously (39, 45), and consistent with these reports, EGF strongly elevated PLD activity in the EGFR cells, between six- and eightfold (Fig. 5a). Downregulation of PKC isoforms with prolonged TPA treatment had no dramatic effect on the PLD activity in either EGF-treated or untreated 3Y1 or EGFR cells (Fig. 5a), suggesting that the EGF-induced PLD activity in these cells was independent of PKC. The role of PKC in the activation of PLD in response to EGF has been controversial. A dependence upon PKC was found in fibroblasts (45) but not in A431 human epidermoid carcinoma cells (39). The transformed phenotype induced by EGF was inhibited by the PKC α inhibitor Go6976 and enhanced by the PKC δ inhibitor rottlerin. Thus, it is possible that downregulation of all PKC isoforms by TPA treatment could have inhibitory and stimulatory effects on PLD activity that would neutralize each other. We therefore examined the effects of the PKC isoform-specific inhibitors Go6976 and rottlerin on the PLD activity in the EGFR cells. The PKC α-specific inhibitor Go6976 reduced the EGF-induced increase in PLD activity to approximately the level seen with the inhibitor alone (Fig. 5b). The PKC δ inhibitor rottlerin stimulated PLD activity in the EGFR cells to an even higher level than that observed in response to EGF (Fig. 5b). Rottlerin did not substantially elevate PLD activity in the parental 3Y1 cells (data not shown), indicating that the effect may be restricted to the partially transformed EGFR cells. Nor did EGF substantially further elevate PLD activity in the rottlerin-treated cells (Fig. 5b), indicating that the effect may be on the same population of PLD molecules activated in response to EGF. These data suggest that PKC α is a positive regulator of EGF-induced PLD activity and that PKC δ is a negative regulator of PLD activity.
FIG. 5.
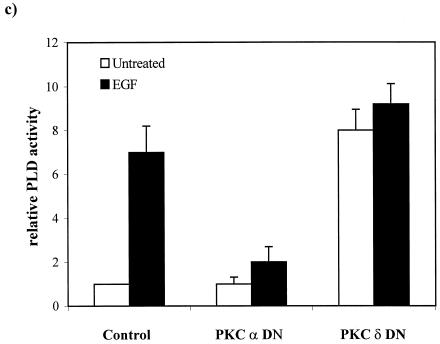
Downregulation of PKC δ elevates PLD activity in EGFR cells. (a) Parental 3Y1 and EGFR cells were treated with EGF (100 ng/ml, 5 min) and/or TPA (400 nM, 24 h) in the presence of 1% butanol, and PLD activity was determined by examining the levels of the PLD-generated transphosphatidylation product phosphatidylbutanol as described in Materials and Methods. The relative PLD activity was normalized to the PLD activity in the untreated 3Y1 cells. Error bars represent the standard deviations for two independent experiments performed in duplicate. (b) The effects of the PKC α- and δ-specific inhibitors Go6976 (0.5 μM) and rottlerin (15 μM) on EGF-induced PLD activity were determined as for panel a. The relative PLD activity was normalized to the PLD activity in the untreated EGFR cells. (c) EGFR cells expressing dominant negative (DN) mutants of PKC α and δ were examined for the effect on PLD activity in the presence and absence of EGF as shown. The relative PLD activity was normalized to the PLD activity in the untreated EGFR cells. Error bars represent the standard deviations for two independent experiments performed in duplicate, where duplicates varied by less than 10%.
We next examined the PLD activity in the EGFR cell lines expressing the dominant negative PKC mutants described for Fig. 4. If PKC α is required for EGF-induced PLD activity, as suggested by the data in Fig. 5b, then EGF-induced PLD activity should be reduced in the EGFR cells expressing the dominant negative PKC α, and as shown in Fig. 5c, that is exactly what was observed. EGF-induced PLD activity dropped from sevenfold to twofold. It was also anticipated that the EGFR cells expressing the dominant negative PKC δ would have an elevated basal level of PLD activity that could not be substantially elevated by EGF. And as shown in Fig. 5c, the basal PLD activity was elevated about eightfold relative to that of the parental EGFR cells and EGF did not significantly elevate the PLD activity further. These data are consistent with the results obtained with the PKC isoform-specific inhibitors and further indicate a PKC α requirement for EGF-induced PLD activity and an inhibitory effect for PKC δ on PLD activity in the EGFR cells that is lost upon downregulation or inhibition. The effects of the α and δ PKC isoforms on PLD activity in the EGFR cells mirror exactly the effects of these isoforms on transformation of these cells.
DISCUSSION
In this report, we have shown that in EGFR cells, inhibition of PKC δ results in a transformed phenotype. This suggests a tumor-suppressing effect for PKC δ that is lost upon inhibition. The addition of EGF to EGFR cells led to a transformed phenotype that was dependent upon PKC α. The ability of EGF to induce a transformed phenotype in the absence of PKC δ downregulation suggests that upon EGF treatment, the inhibitory effects of PKC δ can be overcome. In this regard, it was shown previously that in response to EGF, PKC δ becomes phosphorylated on tyrosine, leading to a reduction of PKC δ kinase activity (8). Thus, the ability of EGF to overcome the negative effects of PKC δ may be due to the ability to stimulate tyrosine phosphorylation of PKC δ. This is very similar to the differential abilities of v-Src and c-Src to transform cells. 3Y1 cells overexpressing c-Src are not transformed; however, downregulation of PKC δ results in the transformation of these cells (23). The same cells overexpressing v-Src are already transformed, but interestingly, PKC δ associates with v-Src in these cells and becomes phosphorylated on tyrosine and the tyrosine-phosphorylated PKC δ leads to reduced PKC δ kinase activity (50) and reduced PKC δ protein levels (3). A correlation between tyrosine phosphorylation of PKC δ and reduced levels of PKC δ kinase activity was also observed in cells transformed by v-Ras (7). Thus, there is apparently a mechanism whereby PKC δ can be downregulated through tyrosine phosphorylation in response to cell division signals that allows cells to overcome the negative regulatory effects of PKC δ.
It is possible that some of the effects of EGF receptor overexpression are mediated by c-Src. c-Src phosphorylates the EGF receptor at Tyr845, and this phosphorylation is required for EGF-induced DNA synthesis (41). The EGF receptor also phosphorylates c-Src. Thus, there is an intimate and complex relationship between the EGF receptor and c-Src, in which the EGF receptor functions both upstream and downstream of c-Src. Our previous studies with c-Src overexpression were done with cells that do not express detectable levels of the EGF receptor, indicating that the effect of overexpressed c-Src were not mediated by the EGF receptor. Thus, while c-Src may play some role in the effects mediated by EGF receptor overexpression, the EGF receptor did not play a role in the effects mediated by c-Src overexpression. Thus, inhibition of PKC δ has tumor-promoting effects in cells with different initiating mutations.
The data presented here and previously (23, 50) suggest a model where overexpression of c-Src or the EGF receptor primes a cell for division; however, cell division is blocked by PKC δ. This is similar conceptually to a model proposed several years ago by Stiles et al. where resting cells needed both “competence” and “progression” factors to leave the resting stage of the cell cycle (G0) and traverse G1 into S phase (40). Thus, overexpression of c-Src or the EGF receptor is postulated to act as a competence factor which either prevents entrance into the resting (G0) state or facilitates exit from G0. Inhibition of PKC δ, which allows for the traversing of G1, is then postulated to be a progression factor. In the tumor promotion model, inhibition or depletion of the tumor-suppressing PKC δ allows cell cycle progression and, therefore, the amplification of the initiated (c-Src- or EGF receptor-overexpressing) cells. In these two models for the regulation of cell proliferation, promotion and progression are similar in that they facilitate passage through G1 into S phase. Most of the characterized tumor suppressor genes also affect cell cycle progression through a G1/S cell cycle checkpoint. The genes encoding p53, p21cip, p27, members of the p16ink family, and retinoblastoma protein are the best studied in what is being called a tumor suppressor pathway (35). It has been suggested that a defect in this tumor-suppressing pathway, which blocks cell cycle progression in late G1, is essential for all human tumors (35). Members of our group have found that in serum-starved cells overexpressing c-Src, inhibition of PKC δ results in an increase in DNA synthesis that can be detected as soon as 3 h after treatment (reference 23 and our unpublished observations). This rapid induction of DNA synthesis by PKC δ inhibition suggests that, in the absence of serum, at least some of the c-Src-overexpressing cells are blocked in late G1 and that inhibiting PKC δ facilitates passage through this checkpoint into S phase. Consistent with this hypothesis, PKC δ was recently reported to suppress expression of the G1 cyclins D1 and E (15), which facilitate passage from G1 to S. Thus, PKC δ may be another regulator of this tumor-suppressing pathway that allows progression through the G1/S cell cycle checkpoint. Overexpression of PKC δ in CHO cells led to an accumulation of cells in G2/M upon TPA treatment (44), indicating that PKC δ may negatively regulate cell cycle progression at multiple points.
The role of PLD in cell cycle regulation is poorly understood; however, PLD activity is elevated in response to most, if not all, mitogenic stimuli (12, 13). RalA, a small GTPase which interacts directly with PLD1 (26), is required for v-Src-induced PLD activity (21, 26). RalA is also required for the transformation of fibroblasts in culture by Ras and Raf (43) and for the formation of tumors in mice (1). We have also found that RalA is required for the transformed phenotype induced by EGF in EGFR cells (25). The finding here that downregulation or inhibition of PKC δ in the EGFR cells leads to substantial increases in PLD activity is consistent with a role for PLD in the cell cycle progression stimulated by inhibition of PKC δ. It is not clear how PKC δ downregulation results in the elevation of PLD activity. However, since phosphorylation of the EGF receptor by PKC negatively regulates receptor function (10, 11, 14, 30), it is possible that the loss of PKC δ-mediated inhibition of the EGF receptor could lead to the observed increase in PLD activity that may in some as-yet-undetermined way contribute to cell cycle progression.
It was demonstrated previously (23) that cells overexpressing c-Src become transformed upon treatment with TPA, which downregulates both PKC α and δ. However, as demonstrated here, EGFR cells do not become transformed upon TPA treatment. This is presumably due to the requirement of PKC α for both EGF-induced transformation and PLD activity. Interestingly, the activation of PLD by Src, in contrast with the activation of PLD by EGF, is independent of PKC α (23, 38). Thus, the PKC α requirement for EGF-induced PLD activity may explain the differential effect of long-term TPA treatment on the transformed phenotype in cells overexpressing c-Src and in EGFR cells. The role that PKC α plays in the transformed phenotype and in PLD activity mediated by the EGF receptor is not clear. PKC α activates PLD directly in vitro and, surprisingly, is kinase independent (36). The dominant negative PKC α used in these studies to inhibit the PLD activity is kinase defective, suggesting that PKC α kinase activity is required. Additionally, several reports have described ligand-induced PLD activity that is sensitive to PKC inhibitors that block kinase activity (13). The role that PKC α plays in the activation of PLD observed here is apparently dependent upon the kinase activity of PKC α and is therefore not likely to act on PLD directly.
The differential effects of PKC α and δ upon PLD activity likely explain previously reported discrepancies in the dependence of EGF-induced PLD activity upon PKC (39, 45). Downregulation of all phorbol ester-responsive isoforms by TPA would be expected to have both inhibitory and stimulatory effects upon PLD activity in EGFR cells. And as reported here for EGFR fibroblasts and previously for human epidermoid carcinoma A431 cells, which also overexpress the EGF receptor (45), downregulation of PKC isoforms with TPA had little or no effect on PLD activity. However, as shown here, the PKC α-specific inhibitor Go6976 blocked EGF-induced PLD activity very effectively. These data are also consistent with previous reports implicating PKC α as an activator of PLD activity in vitro (36) and with a requirement for PKC in the activation of PLD by EGF as reported by Yeo and Exton (45). The apparent lack of a PKC requirement for EGF-induced PLD activity seen in the A431 cells was likely due to both stimulatory and inhibitory effects of downregulating all phorbol ester-responsive PKC isoforms with TPA.
Overexpression of a tyrosine kinase is a common genetic alteration in human cancers (9). This kind of genetic change does not by itself result in a tumor. This initiating event does, however, give the cell a selective growth advantage over other cells in the presence of promoting substances that suppress the proteins that prevent cell cycle progression. In this report, we have presented further evidence that inhibition of PKC δ is sufficient to stimulate anchorage-independent growth of cells overexpressing a receptor class tyrosine kinase, which is common in human tumors. Li et al. recently reported that PKC δ was required for transformation induced by the insulin-like growth factor I receptor (22). This suggests the possibility that PKC δ may also have positive roles in regulating cell division in response to other stimuli. However, the data presented here and previously (23) suggest that suppression of PKC δ by substances that either inhibit or downregulate PKC δ can enhance the promotion phase of tumor progression in cells in which either c-Src or the EGF receptor is overexpressed. It will be important to determine whether epigenetic events, such as diet, hormones, and cigarette smoke, are able to either inhibit or downregulate PKC δ such that cells containing mutations that elevate tyrosine kinase activity would be amplified and therefore subject to progression to a more cancerous phenotype.
ACKNOWLEDGMENTS
A.H. and Z.L. contributed equally to this work.
We thank Sergey Beychenok and Henghe Tian for comments on the manuscript. We thank Renato Baserga and Stuart Decker for providing EGF receptor expression plasmids and Shigeo Ohno for providing the vectors expressing the PKC α and PKC δ kinase-dead mutants.
This investigation was supported by grants from the National Institutes of Health (CA46677) and from the American Cancer Society (BE-243) (to D.A.F.) and by a Research Centers in Minority Institutions (RCMI) award from the Division of Research Resources, National Institutes of Health (RR-03037), to Hunter College.
REFERENCES
- 1.Aguirre Ghiso, J., P. Frankel, Z. Lu, H. Jiang, E. Farías, A. Olsen, L. A. Feig, E. Bal de Kier Joffé, and D. A. Foster. RalA requirement for v-Src- and v-Ras-induced tumorigenicity and overproduction of urokinase-type plasminogen activator and metalloproteases. Oncogene, in press. [DOI] [PubMed]
- 2.Biscardi J S, Belsches A P, Parsons S J. Characterization of human epidermal growth factor receptor and c-Src interactions in human breast tumor cells. Mol Carcinog. 1998;21:261–272. doi: 10.1002/(sici)1098-2744(199804)21:4<261::aid-mc5>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 3.Blake R A, Garcia-Paramio P, Parker P J, Courtneidge S A. Src promotes PKCδ degradation. Cell Growth Differ. 1999;10:231–241. [PubMed] [Google Scholar]
- 4.Borner C, Ueffing M, Jaken S, Parker P J, Weinstein I B. Two closely related isoforms of protein kinase C produce reciprocal effects on the growth of rat fibroblasts. J Biol Chem. 1995;270:78–86. doi: 10.1074/jbc.270.1.78. [DOI] [PubMed] [Google Scholar]
- 5.Collins K, Jacks T, Pavletich N P. The cell cycle and cancer. Proc Natl Acad Sci USA. 1997;94:2776–2778. doi: 10.1073/pnas.94.7.2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coppola D, Ferber A, Miura M, Sell C, D’Ambrosio C, Rubin R, Baserga R. A functional insulin-like growth factor I receptor is required for the mitogenic and transforming activities of the epidermal growth factor receptor. Mol Cell Biol. 1994;14:4588–4595. doi: 10.1128/mcb.14.7.4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Decker S J. Effects of epidermal growth factor and 12-O-tetradecanoylphorbol-13-acetate on metabolism of the epidermal growth factor receptor in normal human fibroblasts. Mol Cell Biol. 1984;4:1718–1724. doi: 10.1128/mcb.4.9.1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Denning M F, Dlugosz A A, Howett M K, Yuspa S H. Expression of an oncogenic rasHa gene in murine keratinocytes induces tyrosine phosphorylation and reduced activity of protein kinase C δ. J Biol Chem. 1993;268:26079–26081. [PubMed] [Google Scholar]
- 9.Denning M F, Dlugosz A A, Threadgill D W, Magnuson T, Yuspa S H. Activation of the epidermal growth factor receptor signal transduction pathway stimulates tyrosine phosphorylation of protein kinase C δ. J Biol Chem. 1996;271:5325–5331. doi: 10.1074/jbc.271.10.5325. [DOI] [PubMed] [Google Scholar]
- 10.Dickson R B, Salomon D S, Lippman M E. Tyrosine kinase receptor-nuclear protooncogene interactions in breast cancer. Cancer Treat Res. 1992;61:249–273. doi: 10.1007/978-1-4615-3500-3_13. [DOI] [PubMed] [Google Scholar]
- 11.Dingiovanni J. Multistage carcinogenesis in mouse skin. Pharmacol Ther. 1992;54:63–128. doi: 10.1016/0163-7258(92)90051-z. [DOI] [PubMed] [Google Scholar]
- 12.Downward J, Waterfield M D, Parker P J. Autophosphorylation and protein kinase C phosphorylation of the epidermal growth factor receptor. Effect on tyrosine kinase activity and ligand binding affinity. J Biol Chem. 1985;260:14538–14546. [PubMed] [Google Scholar]
- 13.Exton J H. Phospholipase D. Biochim Biophys Acta. 1998;1436:105–115. doi: 10.1016/s0005-2760(98)00124-6. [DOI] [PubMed] [Google Scholar]
- 14.Foster D A. Intracellular signalling mediated by protein-tyrosine kinases: networking through phospholipid metabolism. Cell Signal. 1993;5:389–399. doi: 10.1016/0898-6568(93)90078-z. [DOI] [PubMed] [Google Scholar]
- 15.Friedman B, Frackelton A R, Ross A H, Connors J M, Fujiki H, Sugimora T, Rosner M R. Tumor promoters block tyrosine-specific phosphorylation of the epidermal growth factor receptor. Proc Natl Acad Sci USA. 1984;81:3034–3038. doi: 10.1073/pnas.81.10.3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fukumoto S, Nishizawa Y, Hosoi M, Koyama H, Yamakawa K, Ohno S, Morii H. Protein kinase C δ inhibits the proliferation of vascular smooth muscle cells by suppressing G1 cyclin expression. J Biol Chem. 1997;272:13816–13822. doi: 10.1074/jbc.272.21.13816. [DOI] [PubMed] [Google Scholar]
- 17.Goode N T, Hajibagheri M A, Warren G, Parker P J. Expression of mammalian protein kinase C in Schizosaccharomyces pombe: isotype-specific induction of growth arrest, vesicle formation, and endocytosis. Mol Biol Cell. 1994;5:907–920. doi: 10.1091/mbc.5.8.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Griffiths G, Garrone B, Deacon E, Owen P, Pongracz J, Mead G, Bradwell A, Watters D, Lord J. The polyether bistratene A activates protein kinase C-δ and induces growth arrest in HL60 cells. Biochem Biophys Res Commun. 1996;222:802–808. doi: 10.1006/bbrc.1996.0830. [DOI] [PubMed] [Google Scholar]
- 19.Gschwendt M, Muller H-J, Kielbassa K, Zang R, Kittstein W, Rincke G, Marks F. Rottlerin, a novel protein kinase inhibitor. Biochem Biophys Res Commun. 1994;199:63–98. doi: 10.1006/bbrc.1994.1199. [DOI] [PubMed] [Google Scholar]
- 20.Hirai S, Izumi Y, Higa K, Kaibuchi K, Mizuno K, Osada S, Suzuki K, Ohno S. Ras-dependent signal transduction is indispensable but not sufficient for the activation of AP1/Jun by PKC δ. EMBO J. 1994;13:2331–2340. doi: 10.1002/j.1460-2075.1994.tb06517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang H, Luo J-Q, Urano T, Lu Z, Foster D A, Feig L. Involvement of Ral GTPase in v-Src-induced phospholipase D activation. Nature. 1995;378:409–412. doi: 10.1038/378409a0. [DOI] [PubMed] [Google Scholar]
- 22.Li W, Jiang Y-X, Zhang J, Soon L, Flechner L, Kapoor V, Pierce J H, Wang L-H. Protein kinase C-δ is an important signaling molecule in insulin-like growth factor I receptor-mediated cell transformation. Mol Cell Biol. 1998;18:5888–5898. doi: 10.1128/mcb.18.10.5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu Z, Hornia A, Jiang Y-W, Zang Q, Ohno S, Foster D A. Tumor promotion by depleting cells of protein kinase Cδ. Mol Cell Biol. 1997;17:3418–3428. doi: 10.1128/mcb.17.6.3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu Z, Liu D, Hornia A, Devonish W, Pagano M, Foster D A. Activation of protein kinase C triggers its ubiquitination and degradation. Mol Cell Biol. 1998;18:839–845. doi: 10.1128/mcb.18.2.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu, Z., A. Hornia, T. Sukezane, M. Zhong, T. Joseph, P. Frankel, L. A. Feig, and D. A. Foster. Ras-mediated activation of RalA/phospholipase D pathway is required for the growth-promoting effects of EGF. Submitted for publication.
- 26.Luo J-Q, Liu X, Hammond S M, Colley W C, Feig L A, Frohman M A, Morris A J, Foster D A. Ral interacts directly with the Arf-responsive PIP2-dependent phospholipase D1. Biochem Biophys Res Commun. 1997;235:854–859. doi: 10.1006/bbrc.1997.6793. [DOI] [PubMed] [Google Scholar]
- 27.Luttrell D K, Lee A, Lansing T J, Crosby R M, Jung K D, Willard D, Luther M, Rodriguez M, Berman J, Gilmer T M. Involvement of pp60c-src with two major signaling pathways in human breast cancer. Proc Natl Acad Sci USA. 1994;91:83–87. doi: 10.1073/pnas.91.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mao W, Irby R, Coppola D, Fu L, Wloch M, Turner J, Yu H, Garcia R, Jove R, Yeatman T J. Activation of c-Src by receptor tyrosine kinases in human colon cancer cells with high metastatic potential. Oncogene. 1997;15:3083–3090. doi: 10.1038/sj.onc.1201496. [DOI] [PubMed] [Google Scholar]
- 29.Martiny-Baron G, Kazanietz M G, Mischak H, Blumberg P M, Kochs G, Hug H, Marme D, Schachtele C. Selective inhibition of protein kinase C isozymes by the indolocarbazole Go 6976. J Biol Chem. 1993;268:9194–9197. [PubMed] [Google Scholar]
- 30.McCaffrey P G, Friedman B, Rosner M R. Diacylglycerol modulates binding and phosphorylation of the epidermal growth factor receptor. J Biol Chem. 1984;259:12502–12507. [PubMed] [Google Scholar]
- 31.Mischak H, Goodnight J A, Kolch W, Martiny-Baron G, Schachtle C, Kazanietz M G, Blumberg P M, Pierce J H, Mushinski J F. Overexpression of protein kinase C-δ and -ɛ in NIH 3T3 cells induces opposite effects on growth, morphology, anchorage dependence, and tumorigenicity. J Biol Chem. 1993;268:6090–6096. [PubMed] [Google Scholar]
- 32.Plevin R, Cook S G, Palmer S, Wakelam M J O. Multiple sources of sn-1,2-diacylglycerol in platelet-derived-growth factor-stimulated Swiss 3T3 fibroblasts. Biochem J. 1991;279:559–565. doi: 10.1042/bj2790559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qureshi S A, Joseph C K, Gupta R, Hendrickson M, Song J, Bruder J, Rapp U R, Foster D A. A dominant-negative Raf-1 mutant prevents v-Src-induced transformation. Biochem Biophys Res Commun. 1993;192:969–975. doi: 10.1006/bbrc.1993.1510. [DOI] [PubMed] [Google Scholar]
- 34.Saharinen P, Ekman N, Sarvas K, Parker P, Alitalo K, Silvennoinen O. The Bmx tyrosine kinase induces activation of the Stat signaling pathway, which is specifically inhibited by protein kinase C δ. Blood. 1997;90:4341–4353. [PubMed] [Google Scholar]
- 35.Sherr C J. Cancer cell cycles. Science. 1996;274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- 36.Singer W D, Brown H A, Jiang X, Sternweis P C. Regulation of phospholipase D by protein kinase C is synergistic with ADP-ribosylation factor and independent of protein kinase activity. J Biol Chem. 1996;271:4504–4510. doi: 10.1074/jbc.271.8.4504. [DOI] [PubMed] [Google Scholar]
- 37.Song J, Pfeffer L M, Foster D A. v-Src increases diacylglycerol levels via a type D phospholipase-mediated hydrolysis of phosphatidylcholine. Mol Cell Biol. 1991;11:4903–4908. doi: 10.1128/mcb.11.10.4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Song J, Foster D A. v-Src activates a phospholipase D activity that is distinguishable from phospholipase D activity activated by protein kinase C. Biochem J. 1993;294:711–717. doi: 10.1042/bj2940711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song J, Jiang Y-W, Foster D A. EGF induces the production of biologically distinguishable diglyceride species from phosphatidylinositol and phosphatidylcholine: evidence for the independent activation of type C and type D phospholipases. Cell Growth Differ. 1994;5:79–85. [PubMed] [Google Scholar]
- 40.Stiles C D, Capone G T, Scher C D, Antoniades H N, Van Wyk J J, Pledger W J. Dual control of cell growth by somatomedins and platelet-derived growth factor. Proc Natl Acad Sci USA. 1979;76:1279–1283. doi: 10.1073/pnas.76.3.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tice D A, Biscardi J S, Nickles A L, Parsons S J. Mechanism of biological synergy between cellular Src and epidermal growth factor receptor. Proc Natl Acad Sci USA. 1999;96:1415–1420. doi: 10.1073/pnas.96.4.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ueda Y, Hirai S, Osada S, Suzuku A, Mizuno K, Ohno S. Protein kinase C δ activates the MEK-ERK pathway in a manner independent of Ras and dependent on Raf. J Biol Chem. 1996;271:23512–23519. doi: 10.1074/jbc.271.38.23512. [DOI] [PubMed] [Google Scholar]
- 43.Urano T, Emkey R, Feig L A. Ral GTPases mediate a distinct downstream signaling pathway from Ras that facilitates cellular transformation. EMBO J. 1996;16:810–816. [PMC free article] [PubMed] [Google Scholar]
- 44.Watanabe T, Ono Y, Taniyama Y, Hazama K, Igarashi K, Ogita K, Kikkawa U, Nishizuka Y. Cell division arrest induced by phorbol ester in CHO cells overexpressing protein kinase C-δ subspecies. Proc Natl Acad Sci USA. 1992;89:10159–10163. doi: 10.1073/pnas.89.21.10159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yeo E J, Exton J H. Stimulation of phospholipase D by epidermal growth factor requires protein kinase C activation in Swiss 3T3 cells. J Biol Chem. 1995;270:3980–3988. doi: 10.1074/jbc.270.8.3980. [DOI] [PubMed] [Google Scholar]
- 46.Yuspa S H, Poirier M C. Chemical carcinogenesis: from animal models to molecular models in one decade. Adv Cancer Res. 1988;50:25–70. doi: 10.1016/s0065-230x(08)60434-0. [DOI] [PubMed] [Google Scholar]
- 47.Yuspa S H, Dlugosz A A, Cheng C K, Denning M F, Tennenbaum T, Glick A B, Weinberg W C. Role of oncogenes and tumor suppressor genes in multistage carcinogenesis. J Investig Dermatol. 1994;103:90S–95S. doi: 10.1111/1523-1747.ep12399255. [DOI] [PubMed] [Google Scholar]
- 48.Yuspa S H. The pathogenesis of squamous cell cancer: lessons learned from studies of skin carcinogenesis. J Dermatol Sci. 1998;17:1–7. doi: 10.1016/s0923-1811(97)00071-6. [DOI] [PubMed] [Google Scholar]
- 49.Zang Q, Frankel P, Foster D A. Selective activation of protein kinase C isoforms by v-Src. Cell Growth Differ. 1995;6:1367–1373. [PubMed] [Google Scholar]
- 50.Zang Q, Lu Z, Curto M, Barile N, Shalloway D, Foster D A. Interaction between v-Src and protein kinase C δ in v-Src-transformed fibroblasts. J Biol Chem. 1997;272:13275–13280. doi: 10.1074/jbc.272.20.13275. [DOI] [PubMed] [Google Scholar]