Abstract
Objective
In this study, we investigated the usability of atherogenic indices of patients who underwent coronary artery bypass surgery (CABG) due to coronary artery disease and patients without CAD as risk factors and markers for cardiovascular disease (CVD).
Methods
The data of 150 patients who underwent CABG, and 155 patients who underwent coronary angiography and was not diagnosed with CAD were analysed retrospectively. Demographic data and plasma lipid values were collected. The relationship between these ratios and CVD was investigated via univariate logistic regression analysis performed by creating atherogenic indices.
Results
The data of 125 patients who underwent CABG between May 2018 and May 2020 (90 males, 35 females; mean age 64,94 ± 9,61), and 155 patients who had coronary angiography between the same dates and found to have no CAD (64 males, 91 females; mean age 60,12 ± 11,6) were analysed retrospectively. The atherogenic index of plasma (AIP), atherogenic coefficient (AC) and lipoprotein combined index (LCI) ratios were found to be significantly higher in the CABG group compared to the control group (p < 0.001). CABG applied patients were divided into three groups according to their SYNTAX Score-I values. There was no statistical difference in the AIP (p = 0.434), AC (p = 0.715) and LCI (p = 0.891) ratios between the groups. In the ROC analysis of the CABG group, it was found that the AC value was the highest in terms of sensitivity with a value of 74.4% (AUC = 0.669, p < 0.001), and the LCI was the highest in terms of specificity with a value of 65.8% (AUC = 0.634, p < 0.001). In the univariate logistic regression analysis created, it was seen that all three indices had a significant effect in the CABG group (AIP; OR 0.493 p = 0,002, AC; OR 0.298 p < 0,001, LCI; OR 0.358 p = 0,001).
Conclusion
The use of atherogenic indices in daily practice can be recommended in the process of monitoring the risk of CVD in CAD patients, along with determining those patients’ lipid profiles.
Keywords: Atherogenic index of plasma, Atherogenic coefficient, Lipoprotein combined index, Dyslipidaemia, Atherosclerosis
1. Introduction
Cardiovascular diseases (CVDs) are considered to be one of the most important causes of morbidity and mortality in Turkey as well as all over the world [1,2]. CVD is a complex disease with various risk factors such as dyslipidaemia, hypertension (HT), diabetes mellitus (DM), obesity, and metabolic syndrome [3,4]. Atherosclerosis, which is the most common cause of cardiovascular diseases, is a complex, inflammatory, fibroproliferative response developing due to accumulation of the atherogenic lipoproteins in the arterial intima. Numerous studies have shown that low levels of high-density lipoprotein cholesterol (HDLc), high levels of total cholesterol (TC), low-density lipoprotein cholesterol (LDLc) and triglyceride (TG) contribute to the progression of atherosclerosis [5–7]. Among all these lipid parameters, the first target in treatment is LDLc. Even if LDLc levels are reduced to the recommended level with medical treatment and lifestyle changes, CVD risk in individuals continues, albeit at a diminishing pace [8]. Therefore, new CVD risk markers with atherogenic components (e.g.LDLc) in the numerator and antiatherogenic components (e.g. HDLc) in the denominator have been introduced to better reflect lipoprotein metabolism.
Traditionally, the atherogenic lipid profile consists of an increased level of TC, LDLc, TG, and decreased HDLc. Currently, the atherogenic index of plasma (AIP; Log TG/HDLc), the atherogenic coefficient (AC; Non-HDLc/HDLc) and lipoprotein combine index (LCI; TC*TG*LDL/HDL) parameters are used for a better prognosis in cases of atherosclerosis and CVD [9–12]. The ratios can comprehensively reflect the balance between the atherogenic and antiatherogenic potentials in an individual.
Although the Syntax score gives a clue about the complex coronary anatomy, parameters used in scoring such as the number of lesion vessels, the region and length of the lesion, the degree of calcification, and total occlusion also indicate the severity and prevalence of coronary atherosclerosis. Syntax score increases in the presence of these parameters [13,14]. In this study, we investigated the effects of AIP, AC and LCI rates on coronary artery disease (CAD) development in patients without CAD and patients who underwent coronary artery bypass grafting (CABG) due to CAD. We aimed to evaluate the possible relationship of these values with the high SYNTAX-I score in patients that underwent CABG.
2. Subject and methods
After obtaining permission from Süleyman Demirel University ethics committee with the number 22.10.2020/337, the data of 150 patients who underwent CABG in our hospital between May 2018 and May 2020 were reviewed retrospectively. As the control group, 155 patients randomly selected among the patients who underwent coronary angiography (CAG) between the same date as a result of the CVD suspicion due to findings such as effort dyspnoea, angina pectoris, ischemic changes in electrocardiography, and positivity of effort test, and found to have no stenosis in any coronary artery, were examined retrospectively and included in the study. Patients who took statins before CABG or CAG were excluded from the study. In total, 125 patients that underwent CABG were included in the study.
The written consent form was obtained from all the patients. Detailed medical history review, physical examination and routine blood tests, echocardiogram, electrocardiogram, carotid doppler ultrasonography (CDU), chest radiograms and respiratory function tests, body measurement index (BMI), and European System for Cardiac Operative Risk Evaluation (EuroSCORE) were performed on all the patients that were planned to have an open-heart surgery. Patients that were smoking on the day of coronary angiography were evaluated as smokers. Internal medicine consultation was requested for the patients who had a previous diagnosis of DM, and the patients who did not have a diagnosis of DM but had fasting blood glucose of>126 mg/dl; and the diagnosis of DM was confirmed. Patients who had previously received antihypertensive treatment and those who had >130/85 mm/Hg blood pressure during clinical follow-up were considered as hypertensive patients. All patients that had Chronic Obstructive Pulmonary Disease (COPD) were evaluated by a chest physician with a pulmonary function test or an arterial blood gas test, and those who could not perform a pulmonary function test were evaluated through physical examination.
2.1. SYNTAX score calculation
The SS for each patient was calculated retrospectively by scoring all coronary lesions with a diameter stenosis ≥50%, in vessels ≥1.5 mm, using the SS algorithm, which is described in full elsewhere and is available on the SS website [13,14]. The subjects were categorized according to the level of their SS: low (0–22), intermediate (23–32), or high (≥33) [15].
2.2. Biochemical parameters and calculation of lipid indices
Blood samples were taken after a fast of 12 h. Lipid parameters’ levels were measured by using Advia 2400 Chemistry System (Siemens Healthcare Diagnostics, Tokyo, Japan), and AIP, LCI and AC were calculated as log(TG/HDLc), TC*TG*LDLc/ HDLc, and non-HDLc/HDLc respectively.
2.3. Statistical analyses
The statistical analyses of the study were performed via SPSS 20.0 (IBM Inc, Chicago, IL, USA). The descriptive statistics were presented as frequency (percentage) for categorical variables and mean ± SD (median, min, max where necessary) for numerical variables. The continuous variables were checked by Kolmogorov–Smirnov test. Independent sample t-test and one-way ANOVA were used for the comparison of study/control groups and SYNTAX groups. Chi-square test and Monte Carlo method were used to determine the relationship between the categorical variables. In all analyses, p < .05 value was considered as a statistically significant result for 5% type-I error.
2.4. Power analysis
The power analysis was performed by GPower 9.1.2 (Universitaet Kiel, Germany). The analysis was based on one-tailed t-test, and the effect size was calculated as 0.48 and 0.41 using a priori atherogenic index of plasma and lipoprotein combined index values, obtained in the pilot study, respectively for patient groups. The requirements of the analysis were considered as 5% error rate, 0.95 (actual power 0.961) power and allocation ratio (N1:N2) = 1. The minimum sample sizes were calculated as 95 and 117 for each group. Therefore, the sample size was considered as the bigger ones, and the study was conducted by 125 patients in CABG group considering the SYNTAX classes.
3. Results
A total of 280 patients, 125 of whom underwent CABG (90 males, 35 females, age: 63.94[9.61]) and 155 of whom (64 males, 91 females, age: 60.12[11.6]) were the control group, were included in the study. The majority of patients that underwent CABG had 3-vessel CAD (79.2%). The median value of the number of bypasses applied to these patients was found 3.0 (1.0–5.0).
The demographic and clinical characteristics of the cases in the CABG and control groups were compared (Table 1). Age (p = .003) and ratio of male patients (p < .001) were found significantly higher in the CABG group. DM (p < .001), COPD (p < .001) and PAD (p = .001) were also found significantly higher in the CABG group. In the CABG group, all lipid parameters and AIP, AC and LCI ratios were found to be significantly higher than the control group, except for the HDLc value, which was significantly lower than the control group.
Table 1.
Characteristics of patients between CABG and Control groups.
| Variables | CABG (n = 125) | Control (n = 155) | p | |
|---|---|---|---|---|
|
| ||||
| Mean ± SD | ||||
| Age | year | 63.94 ± 9.61 | 60.12 ± 11.60 | 0.003 * |
| EF | 54.01 ± 9.73 | – | N/A | |
| EuroScore | 1.62 ± 1.19 | – | N/A | |
| BMI | kg/m 2 | 29.06 ± 4.33 | – | N/A |
| TC | mg/dL | 198.67 ± 44.34 | 187.21 ± 42.66 | 0.029 |
| Triglyceride | mg/dL | 194.32 ± 119.94 | 154.66 ± 77.12 | 0.002 * |
| LDLc | mg/dL | 121.06 ± 40.33 | 108.54 ± 34.71 | 0.006 * |
| HDLc | mg/dL | 41.26 ± 10.56 | 47.25 ± 13.85 | <0.001 * |
| Atherogenic Index of Plasma | 0.62 ± 0.30 | 0.48 ± 0.28 | <0.001 * | |
| Atherogenic Coefficient | 4.04 ± 1.50 | 3.20 ± 1.25 | <0.001 * | |
| Lipoprotein Combined Index | 125,665 ± 119,927 | 83,315 ± 77,951 | <0.001 * | |
| SYNTAX score-I | 24.10 ± 9.30 | – | N/A | |
| Gender n (%) | Male | 90 (72.0) | 64 (41.3) | <0.001 ** |
| Female | 35 (28.0) | 91 (58.7) | ||
| DM n (%) | None | 60 (48.0) | 127 (81.9) | <0.001 ** |
| Yes | 65 (52.0) | 28 (18.1) | ||
| HT n (%) | None | 75 (60.0) | 76 (49.0) | 0.067 |
| Yes | 50 (40.0) | 79 (51.0) | ||
| COPD n (%) | None | 71 (56.8) | 141 (91.0) | <0.001 ** |
| Yes | 54 (43.2) | 14 (9.0) | ||
| Smoking n (%) | None | 99 (79.2) | 126 (81.3) | 0.662 |
| Yes | 26 (20.8) | 29 (18.7) | ||
| PAD n (%) | None | 111 (88.8) | 151 (97.4) | 0.001 ** |
| Lower extremity PAD | 4 (3.2) | 3 (1.9) | ||
| Carotid artery stenosis | 10 (8.0) | 1 (0.6) | ||
| SYNTAX score-I n (%) | Low | 58 (46.4) | ….. | N/A |
| Intermediate | 49 (39.2) | ….. | ||
| High | 18 (14.4) | ….. | ||
CABG: Coronary artery bypass grafting, EF: Ejection fraction, BMI: Body mass index, TC: Total cholesterol, LDLc: Low density lipoprotein c, HDLc: High density lipoprotein c, DM: Diabetes mellitus, HT: Hypertension, COPD: Chronic obstructive pulmonary disease, PAD: Peripheral arterial disease.
Significant at 0.05 level according to Independent sample t-test.
Significant at 0.05 level according to Chi-square test.
The patients who underwent CABG were divided into three classes according to their SYNTAX score-I values as low, medium, and high. A comparison of AIP, AC and LCI ratios was made between the classes (Table 2). However, none of these index values differed significantly between the classes (p > .005).
Table 2.
Index values according to SYNTAX score-I classes.
| Variables | Low (n = 58) | Intermediate (n = 49) | High (n = 18) | P * |
|---|---|---|---|---|
|
| ||||
| Mean ± SD | ||||
| Atherogenic Index of Plasma | 0.644 ± 0.303 | 0.606 ± 0.295 | 0.542 ± 0.289 | 0.434 |
| Atherogenic Coefficient | 4.13 ± 1.70 | 3.90 ± 1.21 | 4.07 ± 1.55 | 0.715 |
| Lipoprotein Combined Index | 130,929 ± 118,240 | 122,919 ± 134,517 | 116,614 ± 83,139 | 0.891 |
Comparisons according to One-way ANOVA.
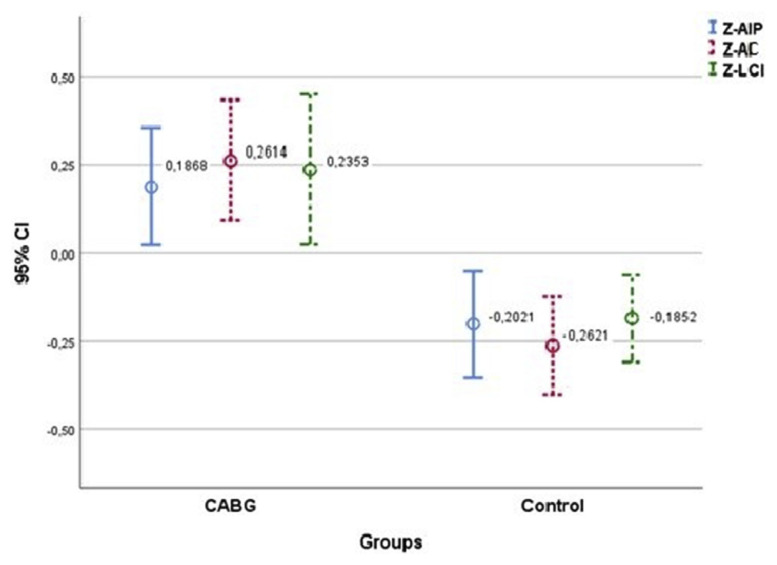
The effect of AIP, AC and LCI values on the CABG group was examined. As a result of the ROC analyses, all three index values were found significant (Table 3). Among these indices, the sensitivity was found to be the highest with 74.4% in the 3.043 cutoff value taken for AC (AUC = 0.669, p < .001). LCI was found to have the highest specificity among these indices with 65.8% (AUC = 0.634, p < .001). The effects of index values on the prognosis of CAD requiring CABG were investigated by taking the control group as reference. In the univariant logistic regression model, which was created by taking the index values as an independent variable, it was observed that all three indices had a significant effect on the progression to CAD that would require CABG. Standardized average values of the indices are shown in Fig. 1. AC was determined as the index with the highest risk increase with OR = 1.607 (1.139–2.267) (Table 3).
Table 3.
Univariate logistic regression analyses for untraditional lipid parameters with coronary artery disease.
| Variables | AUC | p | Cut Off | Sensitivity | Specificity |
|---|---|---|---|---|---|
| Atherogenic Index of Plasma | 0.617 | 0.001 * | 0.541 | 56.80% | 61.90% |
| Atherogenic Coefficient | 0.669 | <0.001 * | 3.043 | 74.40% | 53.50% |
| Lipoprotein Combined Index | 0.634 | <0.001 * | 78,830.70 | 58.20% | 65.80% |
|
| |||||
| Beta | p | OR*** | 95% CI | ||
|
| |||||
| Atherogenic Index of Plasma | −0.707 | 0.002 ** | 0.493 (2.026) | 0.283 | 0.670 |
| Atherogenic Coefficient | −1.210 | <0.001 ** | 0.298 (3.350) | 0.089 | 0.476 |
| Lipoprotein Combined Index | −1.027 | 0.001 ** | 0.358 (2.792) | 0.167 | 0.513 |
Significant at 0.05 level according to ROC analysis.
Significant at 0.05 level according to Univariate Logistic Regression (−2LL = 370.31, R2(Nagelkerke) = 0.268, Hosmer-Lemeshow χ 2 = 11.90 (p = 0.255)).
OR’s should be taken as reciprocal (1/OR) since ‘Beta’s are negative.
Fig. 1.
Index values between CABG and control groups
4. Discussion
In this study, the relationship between CVD and AIP, and AC and LCI rates were investigated in the CABG group of patients. Our results revealed that AIP, AC and LCI were significant and independent predictors for CVD risk and might be better than traditional lipid parameters.
The complex mechanism of atherosclerosis has been studied in many experimental and epidemiological studies [3–7]. Many risk factors such as gender, dyslipidaemia, HT, DM, and smoking have been defined for CVD that develops due to atherosclerosis [5,7,16,17]. In particular, the incidence between traditional lipid measurements such as TC, LDLc, HDLc and TG and CVD has been shown in some studies [6,7,18]. In our study, similar to the literature, traditional lipid parameters were found to be significantly higher and HDLc level was found to be lower in the CABG group compared to the control group. Also, the presence of male gender (p < .001), age (p = .003), DM (p < .001), COPD (p < .001), and PAD (p < .001) were found to be significantly higher in the CABG group.
Mortality in the diabetic CVD group is higher than it is in the non-diabetic CVD group [19,20]. In addition, in the studies conducted in the diabetic CVD group, it was found that there was a significantly higher rate of major adverse cardiac and cerebrovascular events such as myocardial infarction and cardiac death [20,21]. Although serum LDLc levels are the first target of treatment in CVD patients, it has been shown in some studies that there is no significant difference in LDLc levels between diabetic and non-diabetic patients who have undergone percutaneous coronary intervention [8,9,18,21].
Apolipoprotein B100 (apoB100) is the major apolipoprotein found in all atherogenic lipoproteins. Therefore, plasma apoB100 concentrations reflect the total atherogenic potential. In contrast, apolipoprotein AI (apoAI) is a major component of antiatherogenic HDLc, and the plasma content of apoAI represents the total of antiatherogenic potential. High apoB100/apoAI ratios have been identified as a risk factor for CVD in many studies [22–25]. In practice, however, it is not cheap or easy to measure apolipoprotein levels. Non-HDLc is a indicator of all the cholesterol contained in atherogenic lipoproteins, such as small density lipoprotein (sdLDLc), intermediary density lipoprotein (IDLc), LDLc and Lp(a). Therefore, in clinical practice, non-HDLc value reflects plasma apoB100 and HDLc value reflects plasma apoAI levels. Therefore, instead of laboratory measurements that require complex examinations, researchers have begun to introduce new CVD risk markers that have ratios having atherogenic components (e.g.LDLc), which can better reflect lipid profile, atherogenic balance and lipoprotein metabolism, in the numerator and antiatherogenic components (e.g. HDLc) in the denominator.
In case control study of Zhu et al. including 738 CAD and 157 control patients, they found the rate of AC to be higher in the CAD group (p = .001). In the univariate logistic regression analysis performed, they found that the elevation of AC increased the risk of CAD with OR and 95% CI 1.135 (1.019–1.265) [23]. Cai et al. conducted a study with 5387 patients, 2935 of whom had CAD and 2452 of whom were in the control groups, and found that the rates of AIP, AC and LCI in the CAD group were significantly higher than they were in the control group (p < .001). In univariate logistic regression analysis, they found OR 1.782 (95% CI:1.490–2.131) for AIP, OR 1.235 (95% CI:1.181–1.292) for AC and OR 1.007 (95% CI:1.005–1.009) for LCI [12]. Onat et al. stated in another study involving 2676 patients that high AIP values are a predictor risk factor for CAD. In the same study, AIP was detected as a significant predictor for DM and HT in both genders [26]. Similar to the literature, in our study, we also identified all three indices as predictor risk factors for CAD. Among these, it was determined that OR 1.607 (95% CI:1.139–2.267) is the strongest marker for AC. In the ROC analysis, AC had the highest sensitivity (AUC = 0.669, p < .001) with 74.4%, and LCI had the highest specificity (AUC = 0.634, p < .001) with a value of 65.8%.
Having lower particle sizes compared to LDLc, sdLDLc invades and accumulates more easily in the artery wall. In addition, sdLDLc is oxidized more easily than LDLc. As a result, it is phagocytosed by macrophages and transformation into foam cells occurs. This situation causes the development of atherosclerosis and CVD. sdLDLc is an important marker for the prediction of atherosclerosis, and its clinical use has been recommended in some studies [27]. However, because of the complicated detection method and expensive cost, the detection of sdLDLc is limited in clinical application. Dobiasova and Frohlich defined AIP in 2001. They suggested indeed that AIP values of ≥0.3 to 0.1 may be associated with low, 0.1 to 0.24 with medium and above 0.24 with high risk of CVD [9]. Another previous study showed that the value of AIP was inversely associated with the diameter of LDLc particles and indicated the sdLDL particle size [28]. After that, it has been shown with many studies that AIP is effective in atherosclerosis and CVD development, along with being effective as a prognostic indicator compared to traditional lipid parameters [10,12,18,29]. In our study, we found that the value of AIP in the CABG group was higher than it was in the control group (0.62[0.30] vs 0.48[0.28]). The univariate logistic regression analysis revealed that AIP was a lipid parameter strongly associated with CABG, with an unadjusted OR of 1.155 (95% CI:1.069–1.304, p = .038) for an increase of 1-SD.
Although untraditional lipid parameters such as AC, AIP and LCI have been reported to be risk factors for CVD in many studies so far, not enough studies have been conducted to examine the possible effects of these parameters on SYNTAX score. In the statistical analysis we made by grouping CABG patients according to low, medium, and high SYNTAX scores, we did not find any significant difference between the index values and the groups. We think that this might be due to the relatively low number of our patients.
Our study has several limitations. First, this study is a retrospective study. Second, the present study was not designed to examine all the risk factors associated with CVD. Rather, the specific goal was to examine whether the lipoprotein ratios were superior to conventional lipid parameters as predictors for CVD. Thirdly, the patients were not chosen from general population. The patients reflect a single centre experience.
4.1. Conclusion
We believe that in the follow-up of the patients that have undergone CABG surgery or have been diagnosed with CAD or already have risk factors, parameters such as AIP, AC and LCI, which can be calculated more easily and that can better reflect the complex lipid metabolism, should be taken into account in daily practice in addition to follow-up of traditional lipid parameters and target therapy.
In-depth studies are needed to determine and standardize cut-off and risk values for each parameter.
Abbreviation list
- CVDs
Cardiovascular diseases
- HT
hypertension
- DM
diabetes mellitus
- HDLc
high-density lipoprotein cholesterol
- TC
total cholesterol
- LDLc
low-density lipoprotein cholesterol
- TG
triglyceride
- AIP
atherogenic index of plasma
- AC
the atherogenic coefficient
- LCI
lipoprotein combine index
- CAD
coronary artery disease
- CABG
coronary artery bypass grafting
- CAG
coronary angiography
- CDU
carotid doppler ultrasonography
- BMI
body measurement index
- EuroSCORE
European System for Cardiac Operative Risk Evaluation
- COPD
Chronic Obstructive Pulmonary Disease
- apoB100
Apolipoprotein B100
- apoAI
apolipoprotein AI
- sdLDLc
small density lipoprotein
- IDLc
intermediary density lipoprotein
Footnotes
Disclosure of funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contribution
Conception and design of Study: Ersin Çelik, Ahmet Rıfkı Çora, Kadir Burhan Karadem. Literature review: Ersin Çelik, Ahmet Rıfkı Çora, Kadir Burhan Karadem. Acquisition of data: Ersin Çelik, Ahmet Rıfkı Çora, Kadir Burhan Karadem. Analysis and interpretation of data: Ersin Çelik, Ahmet Rıfkı Çora, Kadir Burhan Karadem. Research investigation and analysis: Ersin Çelik, Ahmet Rıfkı Çora, Kadir Burhan Karadem. Data collection: Ersin Çelik, Ahmet Rıfkı Çora, Kadir Burhan Karadem. Drafting of manuscript: Ersin Çelik, Ahmet Rıfkı Çora, Kadir Burhan Karadem. Revising and editing the manuscript critically for important intellectual contents: Ersin Çelik, Ahmet Rıfkı Çora, Kadir Burhan Karadem. Data preparation and presentation: Ersin Çelik, Ahmet Rıfkı Çora, Kadir Burhan Karadem. Supervision of the research: Ersin Çelik. Research coordination and management: Ersin Çelik, Ahmet Rıfkı Çora.
Conflict of interest
None declared.
References
- 1.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Onat A, Ugur M, Cicek G, Ayhan E, Dogan Y, Kaya H, Can G. The Turkish adult risk factors survey 2009: similar cardiovascular mortality in rural and urban areas. Arch Turk Soc Cardiol. 2010;38(3):159–63. [PubMed] [Google Scholar]
- 3.Libby P, Theroux P. Pathophysiology of coronary artery disease. Circulation. 2005;111(25):3481–8. doi: 10.1161/CIRCULATIONAHA.105.537878. [DOI] [PubMed] [Google Scholar]
- 4.Wei Y, Guo H, The E, Che W, Shen J, Hou L, et al. Persistent lipid abnormalities in statin-treated coronaryartery disease patients with and without diabetes in China. Int J Cardiol. 2015;182:469–75. doi: 10.1016/j.ijcard.2015.01.024. [DOI] [PubMed] [Google Scholar]
- 5.Carmena R, Duriez P, Fruchart JC. Atherogenic lipoprotein particles in atherosclerosis. Circulation. 2004;109:2–7. doi: 10.1161/01.CIR.0000131511.50734.44. [DOI] [PubMed] [Google Scholar]
- 6.Goliasch G, Wiesbauer F, Blessberger H, Demyanets S, Wojta J, Huber K, et al. Premature myocardial infarction is strongly associated with increased levels of remnant cholesterol. J Clin Lipidol. 2015;9:801–6. doi: 10.1016/j.jacl.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 7.Sniderman AD, Williams K, Contois JH, Monroe HM, McQueen MJ, de Graaf J, et al. A meta-analysis of low-density lipoprotein cholesterol, non-low-density lipoprotein cholesterol, and apolipoprotein B as markers of cardiovascular risk. Circ Cardiovasc Qual OutComes. 2011;4:337–45. doi: 10.1161/CIRCOUTCOMES.110.959247. [DOI] [PubMed] [Google Scholar]
- 8.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557–65. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 9.Dobiasova M, Frohlich J. The plasma parameter log (TG/ HDLC) as an atherogenic index: correlation with lipoprotein particle size and esterification rate in apolipoprotein depleted plasma (FER (HDL)) Clin Biochem. 2001;34:583–8. doi: 10.1016/S0009-9120(01)00263-6. [DOI] [PubMed] [Google Scholar]
- 10.Fernández-Macías JC, Ochoa-Martínez AC, Varela-Silva JA, Pérez-Maldonado IN. Atherogenic index of plasma: novel predictive biomarker for cardiovascular illnesses. Arch Med Res. 2019 Jul;50(5):285–94. doi: 10.1016/j.arcmed.2019.08.009. [DOI] [PubMed] [Google Scholar]
- 11.Lu W, Resnick HE, Jablonski KA, Jones KL, Jain AK, Howard WJ, et al. Non-HDL cholesterol as a predictor of cardiovascular disease in type 2 diabetes: the strong heart study. Diabetes Care. 2003;26:16–23. doi: 10.2337/diacare.26.1.16. [DOI] [PubMed] [Google Scholar]
- 12.Cai G, Shi G, Xue S, Lu W. The atherogenic index of plasma is a strong and independent predictor for coronary artery disease in the Chinese Han population. Medicine (Baltim) 2017;96:e8058. doi: 10.1097/MD.0000000000008058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sianos G, Morel MA, Kappetein AP, Morice MC, Colombo A, Dawkins K, et al. SYNTAX Score: an angiographic tool grading the complexity of coronary artery disease. Euro-Interv. 2005;1:219–27. [PubMed] [Google Scholar]
- 14.Serruys PW, Onuma Y, Garg S, Sargo G, van den Brand M, Kappetein AP, et al. Assessment of the SYNTAX score in the Syntax study. Euro-Interv. 2009;5:50–6. doi: 10.4244/eijv5i1a9. [DOI] [PubMed] [Google Scholar]
- 15.Serruys PW, Morice MC, Kappetein P, Colombo A, Holmes DR, Mack MJ, et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360:961–72. doi: 10.1056/NEJMoa0804626. [DOI] [PubMed] [Google Scholar]
- 16.Liao J, Farmer J. Arterial stiffness as a risk factor for coronary artery disease. Curr Atherosclerosis Rep. 2014;16:387. doi: 10.1007/s11883-013-0387-8. [DOI] [PubMed] [Google Scholar]
- 17.Lloyd-Jones DM, Larson MG, Beiser A, Levy D. Lifetime risk of developing coronary heart disease. Lancet. 1999;353:89–92. doi: 10.1016/S0140-6736(98)10279-9. [DOI] [PubMed] [Google Scholar]
- 18.Quin Z, Zhou K, Li Y, Cheng W, Wang Z, Wang J, et al. The atherogenic index of plasma plays an important role in predicting the prognosis of type 2 diabetic subject under going percutaneous coronary intervention: results from an observational cohort study in China. Cardiovasc Diabetol. 2020;19:23. doi: 10.1186/s12933-020-0989-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.American Diabetes Association. Cardiovascular disease an risk managment. Diabetes Care. 2015;38(Suppl):49–57. doi: 10.2337/dc15-S011. [DOI] [PubMed] [Google Scholar]
- 20.Koskinas KC, Siontis GC, Piccolo R, Franzone A, Hagnes A, Rat-Wirtzler J, et al. Impact of diabetic status on outcomes after revascularization with drug-eluting stents in relation to coronary artery disease complexity: patient-level pooled analysis of 6081 patients. Circ Cardiovasc Interv. 2016;9:e003255. doi: 10.1161/CIRCINTERVENTIONS.115.003255. [DOI] [PubMed] [Google Scholar]
- 21.Qin Z, Zhou K, Li YP, Wang JL, Cheng WJ, Hu CP, et al. Remnant lipoproteins play an important role of in-stent restenosis in type 2 diabetes undergoing percutaneous coronary intervention: a single-centre observational cohort study. Cardiovasc Diabetol. 2019;18:11. doi: 10.1186/s12933-019-0819-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tamang HK, Timilsina U, Singh KP, Shrestha S, Raman RK, Panta P, et al. Apo B/Apo A-I ratio is statistically A better predictor of cardiovascular disease (CVD) than Conventional Lipid profile: a Study from Kathmandu Valley, Nepal. Clin Diagn Res. 2014;8:34–6. doi: 10.7860/JCDR/2014/7588.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu L, Lu Z, Zhu L, Ouyang X, Yang Y, He W, et al. Lipoprotein ratios are better than conventional lipid parameters in predicting coronary heart disease in Chinese Han people. Kardiol Pol. 2015;73(10):931–8. doi: 10.5603/KP.a2015.0086. [DOI] [PubMed] [Google Scholar]
- 24.Liu Y, Jia SD, Yuan DS, Xu N, Jiang L, Gao Z, et al. Apolipoprotein B/A-I ratio predicts lesion severity and outcomes in diabetic patients with acute coronary syndrome. Circ J. 2020;25(7):1132–9. doi: 10.1253/circj.CJ-19-1097.84. [DOI] [PubMed] [Google Scholar]
- 25.Forte F, Calcaterra I, Lupoli R, Orsini RC, Chiurazzi M, Tripaldella M, et al. Association of aoplipo protein levels with peripheral arterial disease: a meta-analysis of literature studeies. Eur J Prev Cardiol. 2020:zwaa029. doi: 10.1093/eurjpc/zwaa029. [DOI] [PubMed]
- 26.Onat A, Can G, Kaya H, Hergenç G. ‘Atherogenic index of plasma’ (log10 triglyceride/high-density lipoprotein-cholesterol) predicts high blood pressure, diabetes, and vascular-events. J Clin Lipidol. 2010;4:89–98. doi: 10.1016/j.jacl.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 27.National Cholesterol Education Program (NCEP) Expert panel of detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). Third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- 28.Frohlich J, Dobiasova M. Fractional esterification rate of cholesterol and triglyceridesto HDL-cholesterol are powerful predictors of positive findigs on coronary angiography. Clin Chem. 2003;4911:1873–80. doi: 10.1373/clinchem.2003.022558. [DOI] [PubMed] [Google Scholar]
- 29.Hartopo AB, Arso IA, Setianto BY. Low plasma atherogenic index associated with poor prognosis in hospitalized patients with acute myocardial infarction. Acta Med Indones. 2016;48:106–13. [PubMed] [Google Scholar]